POLITECNICO DI MILANO
Scuola di Ingegneria Industriale e dell’Informazione
Corso di Laurea Magistrale in Ingegneria Biomedica
Bioreactor-based engineered liver
systems for disease modelling
Lisa Sassi
ID number 878078
Supervisor: Prof. Sara Mantero (Politecnico di Milano)
Supervisor: Dr. Luca Urbani (Institute of Hepatology, King's College London)
Supervisor: Dr. Alessandro F. Pellegata (Great Ormond Street Institute of Child
Health, UCL)
3
Abstract
Liver disease accounts for 2 million deaths per year worldwide. Liver fibrosis represents an inflammatory state resulting from liver injury, where excessive extracellular matrix (ECM) is produced by activated hepatic stellate cells (HSCs) compromising anatomy and function of the liver. This condition can evolve into cirrhosis and hepatocellular carcinoma. The gold standard treatment for end-stage liver disease is transplantation, but it is associated with severe limitations, such as organ availability and immune-suppressive therapy. Therefore, new therapeutic strategies are required and since recent findings have shown reversibility of the fibrogenic mechanism, new approaches consist in targeting liver fibrosis progression to avoid a more chronic uncontrolled wound healing process.
This work describes the development of bioreactor systems to support the generation of humanized 3D bioengineered liver tissues that recapitulate features of liver fibrosis, combining decellularized scaffolds, patient-derived HSCs and circulating immune cells (peripheral blood mononuclear cells - PBMCs) and chemical and mechanical signals using tissue engineering technology. Two bioreactors were optimized and produced: a bioreactor for whole-liver perfusion and culture (the WL) and a bioreactor to culture smaller scaffolds, increasing numerosity and decreasing the amount of media and cells used per experiment (the Poppins). A perfusion decellularization process was used to generate a rat whole-liver scaffold, which showed preservation of the vasculature network and the ECM architecture of the native organ. The vascular access was used to repopulate scaffolds with HSCs and to maintain the seeded scaffold in dynamic culture conditions for up to 6 days, achieving higher cell infiltration and activation to a fibrotic state compared to static culture conditions and conventional 2D cultures. The bioreactor systems also supported dynamic perfusion of PBMCs, producing circulation of live immune cells through the vasculature of the decellularized scaffold. Effects of fluid-dynamic stress and viscosity on cell viability and phenotype were tested to identify the best dynamic culture conditions. These results suggested that the bioreactors represent relevant tools to support 3D cultures to generate bio-engineered liver disease models.
4
Sommario
Le malattie epatiche causano 2 milioni di morti all'anno in tutto il mondo. La fibrosi epatica é caratterizzata da uno stato infiammatorio derivante da un danno a carico del fegato: ció innesca l’attivazione delle cellule stellate, che provoca una sbilanciamento nel processo fisiologico di turn-over della matrice extracellulare verso una sintesi eccessiva. Questa sovra-produzione incontrollata compromette l’architettura e la funzionalità del fegato. Se il danno persiste nel tempo, lo stadio fibrotico puo’ evolvere in cirrosi epatica e carcinoma epatocellulare.
Nonostante, dal punto di vista terapeutico, il trapianto di fegato sia il gold standard per le patologie epatiche in stadio avanzato-terminale, questo approccio chirurgico é caratterizzato da due gravi limitazioni: la ridotta disponibilitá di organi e la necessitá di una costante terapia immunosoppressiva. Risulta quindi di vitale importanza lo sviluppo di nuove strategie terapeutiche per le malattie epatiche. Poiché recenti studi hanno dimostrato una possibile regressione del processo fibrogenico, nuovi approcci mirano direttamente alla reversione o al blocco della fibrosi come obiettivo chiave per rallentare od evitare la progressione della patologia verso condizioni croniche.
Lo scopo di questo lavoro di tesi é lo sviluppo di due bioreattori in grado di supportare la generazione di un tessuto epatico tri-dimensionale (3D) bioingegnerizzato, che mimi le caratteristiche più importanti della fibrosi epatica. Tramite un approccio di ingegneria tessutale, questo modello di malattia epatica é stato realizzato combinando scaffold ottenuti da decellularizzazione di fegati di ratto, cellule stellate derivate da pazienti, cellule immunitarie da sangue periferico da pazienti (peripheral blood mononuclear cells - PBMCs) e specifici segnali di natura chimica e biomeccanica.
Nello specifico, gli obiettivi di questo studio sono stati lo sviluppo e ottimizzazione di due bioreattori: un bioreattore per la perfusione dinamica e coltura dell’intero organo epatico (WL bioreactor) e un bioreattore per la perfusione e coltura di scaffold di ridotte dimensioni (Poppins bioreactor). Quest’ultimo risponde alla necessitá di ridurre la quantitá dei reagenti e delle cellule utilizzati, aumentando allo stesso tempo la numerositá dei replicati e dei parametri testabili per eperimento. Lo scaffold utilizzato per lo sviluppo del tessuto 3D bioingegnerizzato é stato ottenuto tramite un processo di decellularizzazione per perfusione di fegati di ratto, con cui é
5
possibile rimuovere la componente cellulare conservando la rete vascolare e l'architettura della matrice extracellulare dell'organo nativo. Il network vascolare è stato sfruttato come accesso sia per la ripopolazione dello scaffold con cellule stellate, sia per realizzare una coltura dinamica del costrutto fino a 6 giorni. La coltura dinamica ha promosso una piu` efficace infiltrazione e distribuzione delle cellule all’interno dello scaffold rispetto alle coltura statica. La coltura dinamica si é dimostrata inoltre superiore nel riprodurre tratti caratteristici della fibrosi epatica: la perfusione prolungata ha indotto una significativa attivazione nelle cellule stellate, corrispondente ad uno stato fibrotico che non risulta essere riproducibile nelle convenzionali colture statiche e bidimensionali.
Inoltre, i bioreattori sviluppati hanno supportato la perfusione dinamica di cellule immunitarie (PBMCs), cellule che fisiologicamente circolano nell’organismo e non necessitano di un substrato a cui aderire. L’impatto dello stress fluidodinamico e della viscosità sulla vitalità cellulare e sul fenotipo delle PBMCs é stato analizzato testando diversi parametri, per identificare le migliori condizioni di coltura dinamica.
I risultati ottenuti hanno evidenziato che i bioreattori prodotti in questo lavoro di tesi rappresentano strumenti essenziali per supportare lo sviluppo e l’utilizzo di tessuti 3D bioingegnerizzati per lo studio di stadi patologici della fibrosi epatica.
7
Chapter 1
Introduction
1.1 Framework
Liver disease accounts for 2 million deaths per year worldwide, 1 million due to complications related to cirrhosis and 1 million due to viral hepatitis and hepatocellular carcinoma (HCC). Cirrhosis is currently the 11th most common cause of death globally and liver cancer is the 16th leading cause of death: combined, they account for 3.5% of all deaths worldwide.
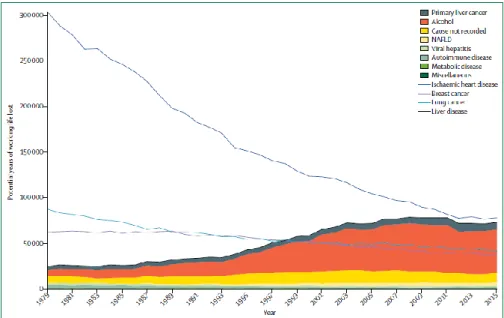
Roughly 75 million people are diagnosed with alcohol-use disorders and are at risk of developing alcohol-associated liver disease (ALD). Approximately 2 billion adults are obese or overweight and over 400 million have diabetes, both of which are risk factors for non-alcoholic fatty liver disease (NAFLD) and HCC [1].In the UK, mortality associated with liver diseases has increased by 400% since the 1970s. Moreover, when compared to cancer, as of 1999 liver disease surpassed lung cancer and breast cancer as the leading cause of years of working life
lost [2] (Figure 1.1). From a clinical point of view the picture gets even worse; the gold standard therapy currently available for end-stage liver disease is transplantation, and although the field
Figure 1.1 Potential years of working life lost (before 65 years of age) estimated with Office for National Statistics mortality data for 1979–2015.
8
of transplantation has improved with better patient survival, there are still several limitations, such as:
1. organ availability: the demand for organs continues to exceed supply, we are going through a transplant crisis and the gap between demand and supply is progressively widening. Organ waiting lists increase, together with the waiting time and as a result, a progressively higher number of patients die because they are not transplanted in time (Figure 1.2). Liver transplantation is the second most common organ transplantation, even with the option of split liver transplant, in other words a single donated liver is transplanted into two recipients, less than 10% of global transplantation needs are met at current rates;
2. in chronic conditions, such as cirrhosis and HCC, the transplanted organ can be attacked by the pathology, thus chronic patients cannot be put on the waiting list;
3. to limit transplanted organ rejection, a chronic immune-suppressive therapy is prescribed, which decreases the body’s defence;
Figure 1.2 'Waiting list length increases with time’(optn.transplant.hrsa.gov)
Although these numbers are sobering, they highlight the urgent need to find valid and reliable alternatives to liver transplantation, by deeply investigating the pathogenesis of the liver injury and the disease progression.
9
1.2 The liver
1.2.1 The anatomy
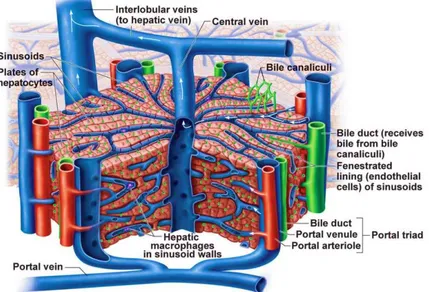
The liver is the largest gland in the body, a spongy mass of wedge-shaped lobes that plays a variety of essential metabolic and secretory functions.
It’s characterized by a dual vascular supply (Figure 1.3):
− Inlet 1: the hepatic artery brings oxygenated blood from the aorta;
− Inlet 2: the hepatic portal vein carries blood enriched with digested nutrients, processed by the gastrointestinal tract as well as products synthesized by the spleen and the pancreas;
− Outlet 1: the hepatic vein conveys venous blood to the inferior vena cava;
− Outlet 2: bile duct, that conveys bile to the small intestine, at the duodenum level; Oxygen is provided from both sources: approximately half of the liver’s oxygen demand is met by the hepatic portal vein and half is met by the hepatic arteries.
Figure 1.3 The hepatic circulatory system [115].
The liver is surrounded by a connective capsule, the Glisson’s capsule and is subdivided into four lobes: the left, right, caudate, and quadrate. The lobes are further divided into lobules, which represent the functional units of the liver (Figure 1.4). Each lobule is typically hexagonal in cross section and has a dimension of approximately 100 microns. At the corners, between
10
adjacent lobules, are the so-called portal areas, regions of connective tissue, which include branches of the bile duct, the portal vein and the hepatic artery.
Figure 1.4 The functional unit of the liver: the lobule.
The blood enters the lobule from terminal branches of the hepatic artery and portal vein located at the lobule vertices, it flows within a vascular network, called sinusoids, while facilitates exchange of nutrients with hepatic cells through the sinusoidal endothelium. The processed blood exits the lobule through the central vein, from which it flows into the hepatic vein and out of the liver. The lobule is formed by two cell populations: the parenchymal cells and non-
11
parenchymal cells (Figure 1.5). The parenchymal cells are described below:
− Hepatocytes occupy almost 80% of the total liver volume and play a critical role in synthesis, metabolism, and detoxification. They are involved in carbohydrate metabolism (glycogen synthesis/glycogenolysis and gluconeogenesis), protein and fat metabolism (cholesterol synthesis), biosynthesis of non-essential amino acids and the production of bile, an isotonic solution containing cholesterol, bile salts and bilirubin, which aids the digestion of lipids. They also process potentially toxic molecules, by increasing their solubility, through the addition or substitution of organic groups. A critical mass of functioning hepatocytes is essential to meet the daily demands of homeostasis: this is ensured by a regenerative capacity, which enables the replacement of lost hepatocytes through proliferation of healthy adult cells.
− Cholangiocytes area heterogeneous, dynamic population of epithelial cells. They show a cuboidal shape and are responsible for the secretion of bile into the bile ducts. Bile modification occurs through coordinated transport of ions and solutes, across the apical and baso-lateral plasma membranes. It is modulated by hormones, peptides, nucleotides, neurotransmitters and bile acids, through intracellular signalling pathways and regulatory cascades. Cholangiocytes have been shown to possess a regenerative capacity and their proliferation represents a key mechanism of response o liver damage and, as such, plays a role in determining the evolution of injury [3].
The non-parenchymal cells are:
− Sinusoidal endothelial cells (SEC): SECs constitute the wall of the hepatic sinusoid and perform a filtration function, due to the presence of small fenestrae that allow free diffusion of substances below the size of chylomicrons, between the blood and the hepatocyte surface. Particularly, this diffusion takes place in the perisinusoidal space, the space of Disse, located between the sinusoids and the hepatocytes: hepatocyte icrovilli extend into this area, allowing plasma components from the sinusoids to be absorbed by the hepatocytes. SEC show remarkable endocytic capacity for different ligands including glycoproteins, components of the extracellular matrix (ECM), such as hyaluronate, collagen fragments, fibronectin, or chondroitin sulphate proteoglycan, and immune complexes [4]. SEC may function as antigen-presenting cells (APC) in the context of both MHC-I and MHC-II restriction with the resulting development of
12
antigen-specific T-cell tolerance. They are also active in the secretion of cytokines, eicosanoids, nitric oxide and specific ECM components.
− Kupffer cells (KC): KCs are intra-sinusoidal located tissue macrophages with endocytic and phagocytic capacity. Hepatic macrophages secrete potent inflammatory mediators (reactive oxygen species, eicosanoids, nitric oxide, carbon monoxide, TNF-alpha and other cytokines) and thus control the early phase of liver inflammation, playing an important role in the innate immune defence. They are in constant contact with soluble bacterial products: a high exposure to these bacterial products, (especially endotoxin such as lipopolysaccharide), can lead to the intensive production of inflammatory mediators and ultimately to liver injury. Moreover, during liver injury and inflammation, KC secrete enzymes and cytokines that may damage hepatocytes and actively remodel the ECM [3].
− Stellate cells (HSC) are present in the perisinusoidal space and are characterized by an abundance of intra-cytoplasmic fat droplets and the presence of well-branched cytoplasmic processes. In the healthy liver, HSC store vitamin A, control turnover of ECM and regulate the contractility of sinusoids.
1.2.2 Liver diseases: fibrosis
Hepatic fibrosis is a dynamic process represented by a wound-healing response to liver injury and characterized by the accumulation of ECM. Fibrosis does not follow one specific pathway, different patterns of fibrosis are associated with different aetiologies, and are influenced by the anatomical area affected and the predominant mechanism of fibrosis. (Figure 1.6). Moreover, step wise accumulation of multiple events of genetic nature (including gene rearrangements, somatic mutations, epigenetic effects and growth factor pathway alterations) eventually leads to HCC (Figure 1.7).
The incidence of HCC varies depending on aetiology, race, ethnicity, gender, age and geographic area, but it has been shown that the presence of fibrosis is a common factor in each of these risks. Indeed, 90% of HCC cases arise from cirrhotic liver, for which progressive fibrosis is a common pre-cursor. [6].
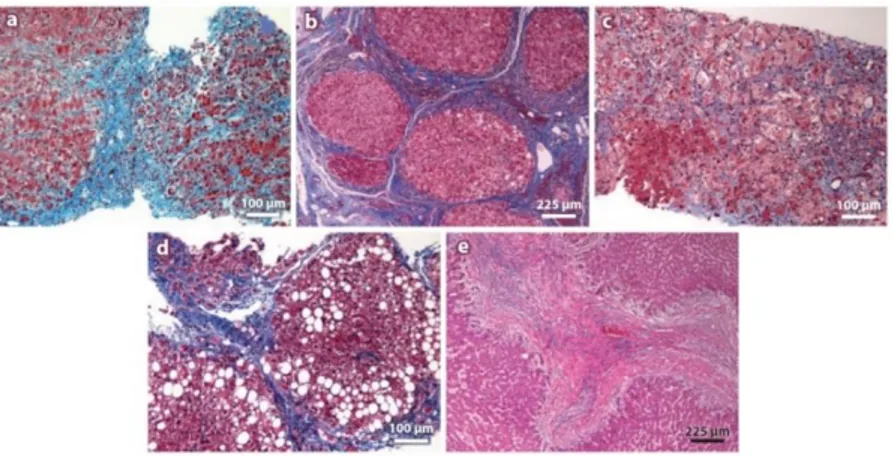
13
Figure 1.6 Fibrosis patterns in different aetiologies of liver disease. (a) Autoimmune hepatitis. Portal central vein necrosis. (b)Chronic viral hepatitis C. Trichrome staining showing portal-central fibrotic septa (c) Acute
alcoholic hepatitis. Deposition of ECM and ballooning degeneration of hepatocytes. (d) Non-alcoholic steatohepatitis. Trichrome staining showing vesicular steatosis and pericellular fibrosis. (e)Biliary cirrhosis[4].
The ECM is a dynamic architecture of both structural and functional proteins (collagen, proteoglycans, laminin, fibronectin and matricellular proteins) and in the healthy liver it is maintained by a precisely regulated equilibrium between synthesis and degradation [7].
Figure 1.7 Chronic exposure of the liver to injury from viral hepatitis, alcohol abuse or non-alcohol related factor causes repeated hepatocyte damage and sets up a vicious cycle of cell death and proliferation, which results in fibrotic condition and consequently in cirrhosis. The resultant genomic instability brings to HCC [5].
In homeostasis, the low-density basement membrane–like matrix of the space of Disse is mainly formed of collagens IV and VI: after liver injury, disruption of this matrix and replacement by fibrillar collagens takes place and the matrix is invaded by collagens I and III and fibronectin [9, 10]. The deposition of ECM interferes with the endothelial fenestrations and hepatocyte
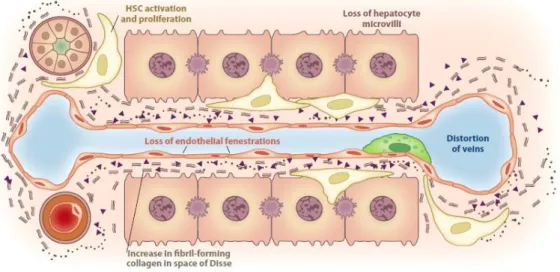
14
microvilli, which provokes the impairment of bidirectional metabolic exchange between the bloodstream and the hepatocytes (a phenomenon called capillarization of the sinusoids) (Figure 1.8). Although fibrosis is a pathological state perpetuated by different cells (such as fibroblasts, bone marrow derived cells and epithelial cells), fibrogenesis is chiefly driven by the differentiation of resident mesenchymal cells, HSCs, into contractile myofibroblast-like cells (MF).
Figure 1.8 Deposition of ECM in the space of Disse leads to the loss of both endothelial fenestrations and hepatocyte microvilli, which results in both the impairment of normal bidirectional metabolic exchange between
portal venous flow and hepatocytes and the development of portal hypertension. [4]
Upon liver injury, HSCs become activated, which leads to their transition from a quiescent cell enriched in vitamin A to a MF phenotype: the latter is characterized by the absence of vitamin A droplets, a remarkable proliferation and contraction capability, increased secretion of ECM proteins - particularly collagen type I [117], and secretion of proinflammatory, profibrogenic and promitogenic cytokines, which in turn leads to accumulation of ECM [11]. HSC activation involves a variety of signalling molecules and pathways and is mediated by a complex crosstalk between the liver cells, the ECM and the immune system. (Figure 1.9).
One of the key HSC activators are cytokines, a family of proteins including transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEFG) and connective tissue growth factor (CTGF).
15
Figure 1.9 The different signalling pathways involved in initiating the fibrosis and perpetuating the HSC activation: the secretion of proinflammatory and fibrogenic molecules, the activation of immune cells, the role of transcription factors, which can promote or inhibit the recruitment of RNA polymerase and consequently control the rate of gene expression and changes in genes expression, which can also occur through epigenetic processes
(histone deacetylation DNA methylation and silencing by miRNAs). REF
PDGF is a potent mitogen which induces HSC proliferation and activation, it has been shown that its genetic depletion causes a reduction in fibrosis in a mouse model [12]. VEGF is secreted by LSECs and HSCs and is an angiogenic modulator, driving HSC proliferation and stimulating collagen production [13, 14]. It represents a good example of the intrinsic paradox of this disease: on the one hand it induces fibrosis; but on the other it is suggested to be essential for fibrosis resolution and its antagonism could lead to a harmful inhibition of tissue regeneration, cytokine signalling and physiological angiogenesis [15, 16]. The major cytokine implicated in activating HSCs is TGF-β: recent studies reveal that other activators of HSCs (such as ROS) apply their effect via TGF-β [17]. TGF-β induces fibrogenesis by promoting trans-differentiation of HSCs and production of ECM proteins [18]: the expression level of TGF-β is remarkably higher in fibrotic liver and reaches its maximum in cirrhosis [19, 20]. Furthermore, TGF-β stimulates HSC proliferation and growth which contributes to the maintenance of their MF phenotype [21].
Epigenetics has also been suggested to play a role in HSC activation. Recent findingsperformed in a primary rat HSC cell-culture model have identified Trichostatin A as a hystone deacytalase inhibitor of collagen I and α-SMA gene transcription [22, 23], correlated with the activation of MFs. Interestingly, this is supported by the ethanol effect: chronic consumption of which is well established as a potent risk factor for developing fibrosis and has been shown to directly cause acetylation of histone H3 lysine [24].
16
The liver is supplied with blood from the gut and, as such, is constantly in contact with gut-derived microbial antigens, therefore, it represents a crucial line of defence. The adaptive and innate immune systems of the liver are highly involved in the fibrogenesis and play a role in triggering HSC activation. The link between the immune system and the development of fibrosis is multifaceted and involves a variety of cells from both the innate immune system; neutrophils, dendritic cells, monocyte-derived macrophages, KC and the adaptive immune system, T and B cells. For example, macrophages produce cytokines and chemokines that activate HSCs. In vivo, induced depletion of macrophages in a mouse model results in decreased HSC activation after CCL4- induced liver injury, suggesting that removal of macrophages might
lead to reduced fibrosis [25 - 27]. On the other hand, macrophages also express apoptosis-related ligands that promote HSC apoptosis [28] and a sub-set of macrophages, which have a pro-healing, regenerative phenotype, have been identified and are implicated in the resolution of fibrosis. This arises from a switch from a profibrogenic to a pro-regenerative phenotype, which orchestrates an overall regression of the disease in a murine model [29].
In addition, the ECM plays a specific role in HSC activation: the accumulation of ECM is not simply a by-product of HSC activation but is implicated in a feed-forward loop to further drive HSC activation and thus drive progression of disease [30]. The ECM harbours a range of growth factors, enzymes and matrix metalloproteinases, bound and stored in latent forms, it modulates the availability of these molecules which in turn influences cellular activity. Indeed, binding of survival factors to the ECM is essential for preventing apoptosis in the damaged liver and for growth factor proteolysis [8]. ECM also provides cells with signals for polarization, adhesion, migration, proliferation, survival and differentiation.
It has generally been accepted that the micro-environment plays a fundamental role in modulating the evolution of a disease [31-34]: following this trend, studies on fibrosis conducted in different tissues, such as lung and pancreas, highlight the relevance of the role of the ECM in disease. In an environment devoid of exogenous cytokines, it has been shown that fibrotic lung ECM mediates a profibrotic positive-feedback loop, by inducing healthy lung fibroblasts to become MF and therefore perpetuating fibrosis [35]. In another study conducted on decellularized ECM scaffold, obtained from patient biopsy from fibrotic lung tissue, it was seen that fibrotic ECM activates a profibrotic response [36]. More recently, it has been shown in pancreatic fibrosis, that increased stiffness of ECM induces pancreatic stellate cell (PSC) activation: quiescent PSCs seeded onto soft matrices maintain their resting-like phenotype,
17
conversely, PSC proliferation and fibronectin expression increases with increasing matrix stiffness- mimicking progressive fibrosis [37].
A common dogma in scientific literature is that the mechanisms driving fibrosis are irreversible: but recent findings and analyses based on biopsies, contradict this long-lasting belief, highlighting the bidirectionality of this process. Regression of fibrosis was initially proposed in 2000 through pathological analysis of 52 isolated livers [38], where histological features were identified to support the regression hypothesis. This has set an exciting scenario for clinical trials to test anti-fibrotic drugs capable to effectively promoting fibrosis regression and thus reducing pathological evolution towards more severe conditions [4, 11, 39, 40].
1.2.3 Diagnosis and treatment
The increasing evidence of the potential reversibility of fibrogenesis has opened the door to the search for anti-fibrotic therapies. But the absence of accurate and resolutive diagnostic techniques able to provide a reliable diagnosis and evaluation of the fibrosis level and its potential evolution, still represents a major obstacle for the validation of effective anti-fibrotic therapy.
Liver biopsy, performed percutaneously, trans-venously or surgically (open or laparoscopic operations), is still regarded as the gold standard for assessing the severity of fibrosis. However, this is an invasive technique with potential complications including, intraperitoneal haemorrhage, hypotension, haemobilia and, less commonly, uncontrolled bleeding and sepsis. The limited size of the biopsy makes it subject to sampling variability and therefore it might not be an accurate or realistic representation of the overall histological condition and moreover, the interpretation is intrinsically affected by an inter-operator variability [41]. For these reasons, ongoing effort in the field is still focussed on the establishment of less invasive techniques, with high sensitivity and specificity for the evaluation of disease stage and potential evolution. Currently, studies for future diagnostic strategies are conducted on:
1) Serum markers. The identification of biomarkers able to reflect variations in ECM metabolism, such as collagen and laminin level (direct biomarkers) or that reveal alterations in liver function, for example indicators of oxidative stress, interferon and ferritin expression (indirect biomarkers) [43]. Limitations of biomarker models include
18
a significant indeterminate range and a predictive ability limited to broad levels of fibrosis.
2) Imaging techniques [41, 42], such as MRI-based technologies and MRE (propagating behaviour of the wave in the tissue, according to stiffness). Limitations include subjectivity, post-processing software and cost and represent time-consuming techniques.
Table 1. Completed trials of antifibrotic agents in patients with liver disease [42].
The field of the development of anti-fibrotic agents has followed various strategies, such as eliminating the cause of injury, reducing inflammation and modulating the immune response, inhibiting matrix synthesis or promoting matrix degradation. Unfortunately, completed clinical trials of antifibrotic agents have shown there is still significant work to be done: some reveal the absence of reduction of fibrosis, an equal short-term mortality and even worse, an increase in fibrosis; pancreatic fibrosis was higher compared to the placebo group (Table 1).
19
The results highlight the urgent need to deeply investigate the mechanisms governing the disease and the underlying crosstalk events between the ECM, liver cells and the immune system, to establish effective diagnostic and therapeutic techniques. To achieve this, it is crucial to establish reliable models to study the disease.
1.2.4 Models of liver fibrosis
Ideally, a disease model should reflect the molecular features, the synergistic tissue-specific interactions between cells, the microenvironment and the signalling pathways perpetuated during pathogenesis, in order to allow us to interrogate the mechanisms driving fibrosis and identify new targetable pathways.
Various models of liver fibrosis are currently available: 2D mono-cellular and multi-cellular cultures, sandwich cultures, organoids, precision-cut liver slices and animal models [44 - 46], which are characterized by different strengths, but are not free of severe limitations. Some characteristics are summarized below:
1) 2D culture: cell culture models are relatively inexpensive and reproducible; however, they do not provide a physiologically relevant environment, lack cell-ECM interactions and the immune system and expose cells to supra-physiological levels of mechanical stress. The mechanical stress affects cell behaviour [37], for example by inducing a trans-differentiation from a quiescent HSCs into a pro-fibrotic phenotype. The latter limitation can be overcome by culturing quiescent HSCs on soft matrigel or on soft hydrogels, where HSCs show suppression of pro-fibrotic genes [37, 47 - 49]. Moreover, successful and consistent isolation of primary human hepatocytes still represents a challenge for therapeutic transplantation and laboratory research: in the absence of a 3D environment, hepatocytes rapidly dedifferentiate and down-regulate synthesis of albumin and metabolic enzymes within 24 hours in culture [50].
2) Animal models offer more complexity than cell culture models, for example they provide cell-cell, cell-matrix interactions and often have a functioning immune system. However, they have a different physiology, metabolism and immunity to humans. Traditional animal models of liver disease aim to trigger liver fibrosis by employing different injury stimuli, such as toxic agents, diet-based models, auto-immune mechanisms, alcohol administration and genetic tools. The widely used toxic agent is represented by the organic compound CCl4; nevertheless the efficiency and reliability
20
of this strategy is critically affected by the variability of the parameters’ involved, on one hand correlated to the administration strategy (the route of injection, the dosage, the frequency) and on other, due to the animal specific susceptibility, which depends on its immunologic background [45, 47]. At present, no animal model has been able to recapitulate all of the characteristics of alcoholic liver disease [51, 52]. This is due to rapid alcohol metabolism, which prevents high blood alcohol levels. Even animals fed alcohol by intragastric infusion (Tsukamoto-French model), do not develop severe liver fibrosis, suggesting a different alcohol toxicity mechanism between animals and humans [53]. Moreover, several liver diseases do not occur in rodents, such as HCV: chimpanzees and Tupaia belangeri (a tree shrew) are the only animals that can be affected by HCV, although they do not develop fibrosis [54].
3) Precision-cut liver slices (PCLS) are used as an ex-vivo culture model to study hepatic drug metabolism and fibrosis. They show the remarkable advantage of maintaining the 3D structure and the physiological ECM components allowing cell-cell and cell-ECM interactions, however, they lack the immune cell axis. The system is not stand-alone: since under static condition and normoxia it shows a limited lifespan of 48 hours, which results in a disruption of tissue architecture and a critical reduction in albumin levels, dynamic perfusion or high level of oxygen are required [55].
1.3 Tissue engineering
In the field of regenerative medicine, tissue engineering has emerged as a suitable tool to face the therapeutic need of technologies promoting regeneration of damaged tissues and organs. Tissue engineering describes the combination of biomaterials, cells and biochemical and physical stimuli, to design biological constructs which are able to perpetuate, restore or improve the function of their healthy or pathological counterpart in vivo. Tissue engineering can also be exploited as powerful tool for disease modelling, by designing 3D bioengineered construct which exhibit pathological features: these constructs can be used as physiological relevant model to perform drug test. The rationale behind tissue engineering is to generate a bioartificial
21
construct, through the combination of cells, isolated from autologous or allogenic sources, seeded on a scaffold.
Figure 1.10 Generic tissue engineering process diagram [56].
is stimulated in different ways depending on the target tissue to induce cell attachment, proliferation and differentiation, and the resulting construct is transplanted (Figure 1.10) [56]. Over the past decade, the field of tissue engineering has accomplished substantial progress in terms of [57]:
1) cell sources, including the cutting-edge field of induced-pluripotent stem cells (iPSCs), which overcomes ethical issues related to the use of embryonic stem cells and paves the way for personalized medicine;
2) delivery mechanisms of biochemical compounds;
3) a deeper understanding of the interaction between implanted bioartificial constructs and the immune system towards the development of biomimetic materials;
4) fabrication technologies, including self-assembly techniques, 3D bioprinting and decellularization strategy.
Major effort in the field has been carried out toward the development of techniques which can be translated into clinical practice and some technologies are already positively affecting the lives of patients. One example is represented by Holoclar®, the first stem cell-based treatment
22
approved in the European Union, developed to treat limbal stem cell deficiency due to, for example, physical and chemical burn [58]. Patient limbal stem cells are isolated and expanded
in vitro then seeded on a fibrin substrate before transplantation into the affected eye (Figure
1.11). These key components of tissue engineered constructs and their roles are described in the following chapter.
Figure 1.11 Holoclar technology: the phases from the biopsy to the transplantation [58].
1.3.1 Tissue Engineering components: cells
Cells are the protagonists of tissue engineering and the selection of the cellular source depends on the specific application. This can be primary cells, derived directly from biopsy, or immortalized cells, a population of cells that lack senescence due to a natural or experimentally induced mutation, via genetic tools, chemical agents or irradiation.
These cells can be classified according to:
a) Immunogenicity (when the engineered construct needs to be transplanted into a recipient): 1) Autologous: the donor corresponds to the recipient.
i. Cells isolated from the patient’s tissue;
ii. Cells isolated from the patient’s cryo-preserved umbilical cord or placenta;
23
iii. Induced-pluripotent stem cells (iPSC) derived from the patient;
The autologous source represents the optimum choice in terms of histocompatibility but shows limited availability and expandability and for iPSCs there is a risk of teratoma formation.
2) Syngeneic: the donor and the recipient show identical genotypes. i. Cells isolated from tissue donated by a patients’ twin;
ii. Cells isolated from an embryo cloned from the patient’s somatic cells; Although there is a risk of teratoma formation for embryonic cells and ethical, technical and regulatory limitations for cloned cells, this cell source shows high histocompatibility.
3) Allogenic: the donor and the recipient belong to the same species. i. Cells isolated from a live or deceased donor;
ii. Human embryonic stem cells; iii. Immortalized cells lines;
The use of immortalized cell lines is associated with the risk of immune rejection and tumorigenesis.
4) Xenogenic: the donor and the recipient belong to different species. There is the risk of acute immune rejection and transmission of xeno-zoonoses from viruses (such as the HIV from non-human primates and EBOLA), endogenous retroviruses (such as porcine endogenous retroviruses) or prions (such as bovine spongiform encephalopathy).
b)Tissue origin.
1) Germ cells: sexual or reproductive cells such as the ovum cell, the sperm cell and their developmental precursors;
2) Somatic cells;
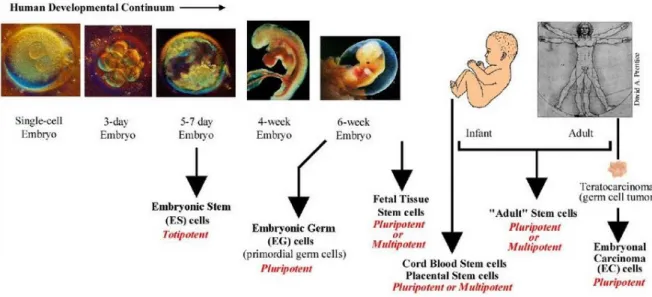
c) Cell potency, which describes the ability of cells to differentiate into any cell type (totipotency), multiple cell types (pluripotency), closely related types of cells (multipotency) or into a single cell type (unipotency) (Figure 1.12). Major differences occur between these cell sources. For example, some of the major differences between primary cells and cell lines are highlighted in table 2.
24
Figure 1.12 Cell potency at different developmental stages [59].
Primary cells Immortalized cell lines
Source Isolated from tissue or blood Cell bank
Characterization Complex Established
Homogeneity Heterogeneous population Homogeneous population
Differentiation Can de-differentiate Limited differentiation
Proliferation potential Limited passage number Unlimited passage number
Reproducibility Low High
Culture condition Complex Established and easier
In vitro response Physiologically relevant Less physiologically relevant
Application Clinical field Research field
25
1.3.2 Tissue Engineering components: the scaffold
Progress in biomaterial sciences combined with an increasing understanding of the crucial role of micro-environmental factors in the mechanism of tissue regeneration, have led to the development of scaffolds tailored to guarantee an appropriate 3D micro-environment. Ideally, the combination of biochemical and mechanical properties of the biomaterial should guide target tissue regrowth according to the so-called contact-guidance theory, for which the cell behaviour is strongly influenced by the geometrical patterns, architecture and surface topography of the scaffold.
The properties of a biomaterial depend on its’ specific application, but they can be generally described in terms of:
1) Biocompatibility. The capability to induce a favourable interplay with the human body; 2) Topography and porosity;
3) Biodegradability and biostability. The kind of support provided by the biomaterial can be: a) Permanent: the construct is implanted in the human body and should maintain
its’ designated performance for the life expectancy of the patient. An example of a permanent construct is in regeneration of the anterior cruciate ligament driven by a bioengineered ligament constituted of collagen and fibrin [60]. b) Transient: it’s characterized by customized degradation kinetics which is
exploited to provide initial support to the damaged tissue after which it degrades, leaving to the new regrowth tissue. This strategy is also adopted to deliver biomolecules, aimed at reducing adverse immune response and promoting tissue regeneration. The biodegradable biomaterial used for the fabrication of scaffolds should:
i. exhibit appropriate degradation kinetics, be accurately designed to be compatible with the tissue-specific healing/regeneration mechanism; ii. have adequate biochemical and mechanical properties, without inducing
an excessive inflammatory response or toxic effects after implantation; iii. generate non-toxic products, capable of being metabolized and excreted; iv. be sterilizable;
4) Surface chemistry;
26
Figure 1.13 Biomaterials currently employed for the generation of constructs in tissue engineering.
Biomaterials employed in the field of the tissue engineering include (Figure 1.13):
a) Natural-derived polymers, including polypeptides (for example, collagen) and polysaccharides (for example, hyaluronic acid). These have been extensively exploited for the development of tissue engineering constructs for cartilage repair and pancreatic diseases [61, 62]. Although natural polymers present an innate biocompatibility and capability of promoting cell survival [63, 64], the difficulties in controlling degradation kinetics and limited mechanical properties represent major drawbacks.
b) Synthetic polymers exhibit the advantage of being reproducibly manufactured, conferring a wide spectrum of properties and degradation mechanisms, to allow the generation of application-specific scaffolds. For example, scaffolds composed by poly-lactic-co-glycolic acid (PLGA) have been developed for promoting the regeneration of blood vessels and bone [61]. They do not show the same intrinsic capability of favouring cell attachment and survival as natural polymers, but they can be functionalised by incorporating biomolecules, drugs and active domains.
c) Decellularized ECM: scaffolds are obtained from harvested tissues treated with a series of chemical and/or mechanical processes, which effectively remove the cellular components, leaving behind a structure which retains overall architecture, composition and the
Biomaterials Natural polymers Fibrin, silk, fibroin, elastin, hyaluloran, collagen Synthetic polymers PLA, PGA, PCL, PGS Decellularized matrix Human and animal source Hybrid scaffolds
27
biomechanical properties of the native ECM. This technique will be described in detail in the following chapter.
d) A hybrid approach characterized by the combination of natural-derived and synthetic material. For example, a recent study combined synthetic polymers with bladder decellularized matrix to generate vascularized bladder in murine and porcine pre-clinical models [65].
.
Decellularization
The generation of a 3D scaffold with appropriate properties to support cell engraftment and tissue functionality is technically challenging and the lack of adequate materials has represented a major obstacle for the generation of bioengineered organs. Decellularization is a recently well-established technique, based on the removal of native cells from discarded donor organs to generate a 3D scaffold, which retains its tissue-specific architecture and composition and which can be subsequently recellularized and eventually transplanted. This technology has been adopted for the fabrication of a variety of 3D scaffolds including, heart [66], lung [67], liver [68-69], kidney [70], bladder [71], artery [72], oesophagus [73] skin [74], and trachea [75] and at present many products available on the market are based on decellularized biological tissues (Table 3). This technique is attracting the interest of the scientific community because of the advantages presented by decellularized scaffolds:
1) Immunogenicity. Since the cellular component is removed from the ECM, this technology could overcome the requirement of the administration of an immune-suppressor therapy, necessary to ensure an effective organ transplantation and reduce the risk of rejection. In a recent study, decellularized porcine and murine liver scaffolds were seeded with hepatoblastoma cells and transplanted into rodents: cytotoxicity analyses showed no altered immune response with allogeneic (rat scaffold into rat) or xenogeneic (pig scaffold into rat) transplants up to 28 days, compared with sham control [76]. Moreover, a further study demonstrated that in decellularized liver scaffold seeded with liver sinusoidal endothelial cells (LSEC), distributed along the vascular tree, a net decrease in platelet deposition is observed,
28
Table 3. Products available on the market based on the decellularized of biological tissues [82]
indicating a reduction in the risk of blood clot formation and an improved tolerance of the construct by the organism [77].
2) Maintenance of the 3D architecture and composition of the native ECM provides a tissue-specific micro-environment ideal for promoting cellular survival, engraftment, and differentiation and for performing long-term culture. Gene expression analysis conducted on foetal hepatocytes, seeded and culture for 3 days on liver decellularized ECM, showed that levels of hepatocyte-specific proteins; albumin, alpha-1 antitrypsin (A1AT), glucose 6-phosphatase (G6P) and transferrin, were increased in the decellularized scaffold, compared to 2D culture, suggesting the proactive role of this scaffold towards maturation of foetal hepatocytes [78].
3) Conservation of the vasculature network, which can be exploited as a route for scaffold recellularization and to deliver nutrients. It is well-established that a cell has a limited autonomy
29
after a certain distance from a nutrient source: it can generally survive within an area of 1 mm away from a vessel [79]. This feature assumes paramount importance in the field of bioengineered liver: because of the high rate of the hepatocyte oxygen consumption, any hepatic mass should contain an extensive micro-vascular network to ensure constant nutrient supply and avoid any ischemic damage [80-81]. The presence of the vascular tree provides different natural injection routes, to allow the generation of a construct with high cell density: for the decellularized liver ECM, the most commonly used vascular inlets are the portal vein and the bile duct. Although both are characterized by high engraftment efficiency, seeding through the bile duct shows a better cell distribution in the parenchyma [78]. The effectiveness of the decellularization process is dependent on different factors, including the cellular population, the cellular density, the lipid concentration, the dimension of the specific organ. The decellularization itself can be performed through different strategy, such as perfusion via vascular network or immersion into solutions.Different decellularization agents have been studied, which exhibit various effects: some of which are reported in (table 4) [82].
The 3D ECM scaffold is considered to be effectively decellularized when the following two requirements are met:
1) there remains < 50 ng dsDNA per mg ECM dry weight; 2) any residual DNA is < 200 base pairs in length;
In order to evaluate the efficiency of decellularization, the scaffold is assessed for absence of native genetic material (cells and residual DNA) and maintenance of the structural elements characteristic of the ECM. This is done by:
• optical inspection: a preliminary and qualitative evaluation, by observing if the scaffold has achieved a translucent appearance;
• histological analyses: DAPI staining for nuclei, Haematoxylin & Eosin (H&E) to evaluate the integrity of the tissue, Masson's Trichrome (MT) for distinguishing cells and surrounding connective tissue and Picrosirius Red (PR) for collagen;
• vascular network imaging, to ensure the maintenance of the vascular networks; • quantitative analyses of major ECM proteins (such as elastin, collagen and
30
31
Decellularization represents a versatile strategy to obtain 3D ECM-like scaffolds. Some of the challenges associated with this process include the requirement for large quantities of solutions and problems with reproducibility, which depends on many factors, but can be better controlled by making educated decisions in terms of choice of decellularization agent and protocol.
1.3.3 Tissue Engineering components: biochemical and physical signals
In the physiological environment, cell behaviour is controlled by a complex and wide network of signals. These signals can be classified as chemical signals, such as growth factors, hormones, neurotransmitters and extracellular matrix components; and physical signals, such as mechanical forces, electric field and compression. It’s therefore essential, in order to generate a functional bioengineered tissue, to provide cells with signals which recapitulate the target tissue environment. Some of these signals are provided by the bioreactor and will be described in more detail in the following chapter.
1.4 Bioreactors
Bioreactors are devices which provide a suitable environment for the development of biological systems, under tightly controlled conditions and close monitoring of the variables which are well known to affect cell behaviour, such as pH, temperature, mechanical stimuli, nutrients and waste removal [83].
The appearance on the world scientific scene of the bioreactors tool can be considered in the late ’80, when a NASA team of engineers and biologists was aiming the development of a cylindrical device able to overcome the limitations of the 2D culture, by providing a suitable environment for the growth of human kidney cells. In a more physiological-like environment, the cells would have produced erythropoietin, a hormone that modulates red blood cell production and can be exploited to treat anaemia. But there was a major barrier: the bioreactor was supposed to move the media to avoid stagnating condition, but this gave rise to turbulent
32
fluid-dynamic conditions, harmful for the fragile cells, causing a massive cell death. They overcame the issue though the idea, that rotating the wall of the reactor would solve fluid mechanical problems, by eliminating the velocity gradient of the fluid media and creating a constant free fall condition within the fluid and the suspending cells. The first result, when they run the test with the cells, was not encouraging at all: the cells were all dead. Through following chemical analysis, Wolf and his colleagues realized the cells had died for a different reason: they had run out of nutrients, because they grew too fast. The new bioreactor was, in a sense, too effective. This micro-gravity effect, beneficial for the cells, was then strongly demonstrated through following experiments conducted in the space (Figure 1.14) [84].
Figure 1.14. Astronaut David Wolf performs maintenance on a NASA bioreactor unit on board the Mir space station. Experiments conducted by Wolf demonstrated that the bioreactor produces even more effective cell
growth results in space.
A high level of reproducibility, control and automation combined with the potential versatility of bioreactors, makes them widely applicable in a range of different settings. There is an urgent need of reliable technologies providing a controlled environment for the development, stimulation and preservation of 3D constructs. But this demand doesn’t just concern the clinical community: Google spent €250,000 to support scientists to develop a bioreactor to grow bovine stem-cell-derived muscle cells to make hamburgers, with the aim of reducing the environmental impact of greenhouse gases produced by farming and to improve animal welfare [86]. These newperspectives highlight the fundamental role performed by the bioreactor and its intrinsic potential.
In the field of tissue engineering, bioreactors are widely used to:
1) Increase mass transport: to create bioengineered tissues, diffusion of nutrients and gasses to the cells contained in the biological construct is a key design criterion. The presence of an intact vasculature can be exploited to allow diffusion of nutrients but also the geometry and dimension of the scaffold should be considered.
33
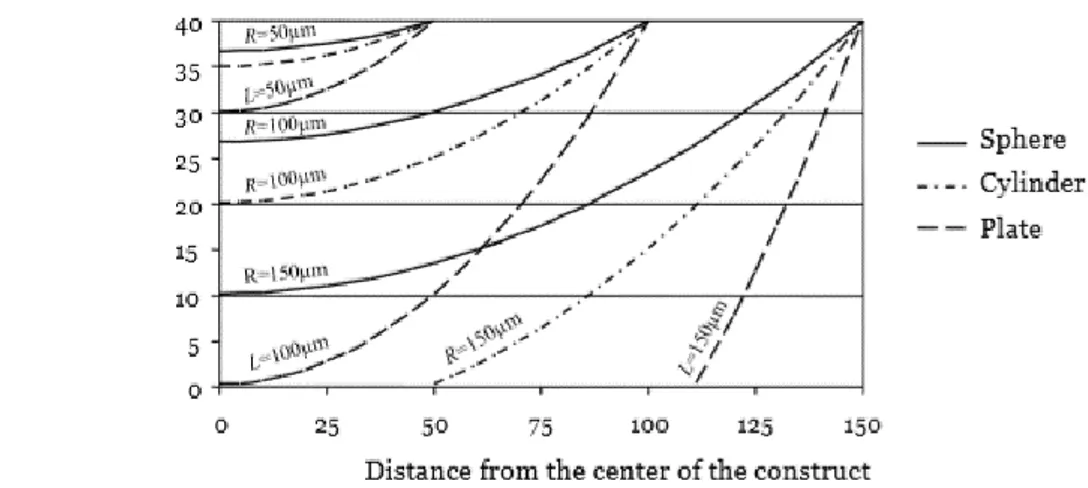
The oxygen concentration in subcutaneous tissues is approximately 40 mmHg, as in the venous circulation. In bioengineered constructs without vascularization, the oxygen concentration profile, estimated by the mass transport equation in stationary conditions, in the presence of diffusion and constant consumption, shows different behaviours depending chiefly on the construct’s geometry and size.
Figure 1.14. Example of possible geometries of bioengineered constructs.
For example (Figure 1.14), a change in oxygen concentration was measured in three construct designs; sheet-like, spherical or cylindric. The greatest drop in oxygen concentration was observed in the flat, sheet-like scaffold, while spherical constructs showed the least change (Figure 1.15) [56].
Figure 1.15 Oxygen concentration profile arising from different construct geometries: a greater drop in solute concentration can be observed in the flat scaffold, while the lowest one within the spheroid geometry
34
This observation is supported by an early study showing hypoxic and necrotic centre of 1 mm cellular spheroids [87]. The bioreactor can provide a more effective solute diffusion, as has been demonstrated in a glycosaminoglycan (GAG) staining of a cartilage tissue in a comparison study between static culture, spinner-flask culture and rotating-wall vessel bioreactor (RWV). The staining shows a negligible GAG deposition in the central region of the cartilage cultured in static, a more pronounce GAG presence but encapsulated by fibrous tissue with the spinner flask culture and a remarkable intense and homogeneous GAG presence in cartilage cultures in the RWV. The authors speculated that the observed differences were due to differences in the laminar flow (Figure 1.16) [83,88].
Figure 1.16. GAG staining of a cartilage tissue between different culture conditions: a) Static culture : there is a negligible deposition in the central region; b) Spinner-flask culture more pronounce GAG presence, but a
fibrotic capsule has formed around the inner area; c) RWV bioreactor culture shows a remarkable and homogeneous GAG deposition along the tissue [83, 88]
2) Perform long term culture, up to several weeks. This specification is guaranteed by closed hydraulic circuits, protocols for sterile assembly and culture medium replacement [56].
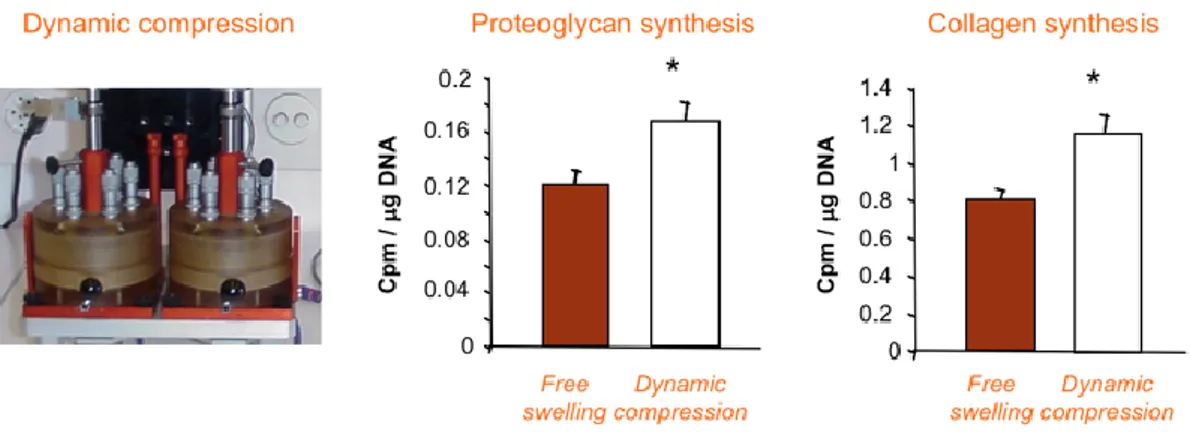
3) Adequate stimulation of cells. Bioreactors can provide a variety of different stimuli: compression, tension, bending, interstitial flow, superficial flow, hydrodynamic shear stress, electric field force, magnetic force, microgravity, acoustic wave and heat. It has become evident that a complex crosstalk exists between tissue growth and homeostasis and the complex network of stimuli provided by the micro-environment surrounding the cells: by considering synergistic interaction of both mechanical and biochemical elements, we can define tailored culture conditions for the development of biologically functional bioengineered tissues [89]. For example, it has been shown that chondrocytes, cells composing the cartilage tissue, synthesize higher levels of proteoglycans and collagen when stimulated by dynamic compression [90] (Figure 1.17).
35
Figure 1.17. Chondrocytes synthesize higher level of proteoglycan and collagen, under dynamic compression stimulus [90].
Another outstanding example is represented by the Camelot, a bioreactor able to generate mechanical and electrical stresses, promoting differentiation and maturation of muscular tissue constructs. Analysis of Myosin Heavy Chain (MHC) production showed a remarkably higher production of myosin in the muscular tissue after 10 days culture in the bioreactor system compared to static culture (Figure 1.18).
Figure 1.18. Camelot bioreactor is able to stimulate the construct through mechanical and electrical signals,
enhancing differentiation and maturation of the muscular tissue. [90]
Mizuno and Heyland found that the cyclic application of hydrostatic pressure is a driving factor that remarkably increases the synthesis of ECM [91, 92]. In a recent study on spinal cord injury, pulsatile electrical stimulation has been demonstrated as a potent promotor in enhancing in vitro and in vivo sensory axon growth [93]. Similarly, by combining hypoxia and hydrodynamic forces, Correia at al. enhanced iPSC differentiation toward cardiomyocytes [94]. In the field of the liver, Nishii et al. showed that shear stress upregulates the regeneration-related genes in
36
ECM-like microenvironments [95] and specific values of this mechanical force are known to induce HSC migration [96] and sinusoid formation [97].
4) Manufacture engineered grafts. One of the major obstacles in translating engineered grafts from low-scale production (such as those used in research), into a large-scale commercially available products is the lack of clinically effective and economically sustainable manufacturing technologies, according to the GMP rules. Dermagraft, developed by Advanced Tissue Sciences (ATS) represents a cutting-edge example of how bioreactors can be employed in large-scale production of tissue-engineered products: ATS created a completely automatic media substitution system and a cell culture bioreactor system, able to seed and culture 96 tissue-engineered skin grafts [83].
5) Perfusion and cell seeding in 3D scaffolds. Ideally, the engineered construct should house high density cell populations, since this has been correlated with a remarkable tissue formation. Even considering a small scaffold, it is extremely challenging to distribute a high density of cells uniformly throughout the scaffold structure. Static loading of cells, by surface-seeding or injection, still represents the most common technique. However, it is associated with low seeding efficiency, non-homogeneous distribution and intra-operator variability. Moreover, after the seeding, the cells should receive the appropriate amount of nutrients present in the media and diffused through the scaffold porosity, but the traditional 2D culture does not allow media diffusion through thick constructs. Different bioreactors have been developed to improve both seeding and media perfusion: as examples, the U-tube bioreactor pushes the media and the cells through the scaffold, providing the so-called confined perfusion or the T-cup bioreactor, where the scaffold is moved through the media (Figure 1.19).
The specific requirements of a bioreactor are dependent on its application, however, some general requirements are presented below:
• The materials must be biocompatible in areas of the bioreactor which come into contact with cells and medium. This an essential specification for bioreactors used in both a clinical and research setting;
• Reliability and versatility;
• Sterilization and sterility: the sterilization process should not alter the materials properties or affect the bioreactor’s performances;
37
• No cross-contamination: for research applications, it would be useful to have a bioreactor able to manage different experiments with different cell lines in parallel to optimize time and provide replicates in a short-time period. In clinical practice, on the other hand, cross-contamination must be avoided at all costs. One possible solution would be to use disposable bioreactors.
• Automatic seeding, to limit inter-operator variability and risk of contamination;
• Increased mass transport of nutrients and gases from the culture medium to engineered constructs;
• Physical and biochemical stimulation of the biological constructs; • Small footprint in a cost-saving perspective;
• Automation and control; • Stand alone.
Figure 1.19. The U-tube bioreactor and T-cup bioreactor allow the cell seeding through the scaffold, by exploiting different strategy. The U-tube bioreactor pushes the suspension of media and cells through the scaffold, providing the so-called confined perfusion and the T-cup bioreactor moves the scaffold through the
39
Chapter 2
Materials and methods
Bioreactor Poppins design
The technical drawings of the chamber and the insert were designed with SolidWorks. The files were converted into a 3D project with ArtCAM Software. The bioreactor was manufactured phase at the Institute of Making, University College London. The Computer Numerical Control machine (CNC, Roland, Jap) was used to create the chamber and the insert. All of the components were made using Teflon and Nylon 6.6 (RS, UK). For the manufacturing of the chamber sheets of material were cut with the cutter machine (Saws, UK), to obtain a piece with the appropriate dimension to fit the CNC. The sheet was cut on the surface (3 mm) to obtain a more precise flat surface. The sheet of material was fixed to the CNC platform with screws. The CNC was programmed to initially create a chamber consisting of 6 wells, followed by 6 internal cavities.
To create the insert the CNC machine was used to cut the surface of the material, until the height of the insert was reached, followed by the creation of 6 cylinders. Next, the square cavity in each cylinder was made. Two lateral holes for each insert were made using a drill (Pillar Drill, UK). All parts were produced according to the Good Manufacture Practice (GMP).
Perfusion system
The perfusion system was designed to be compatible with the Poppins chamber and the WL chamber. The hydraulic system consisted of one syringe pump, (World Precision Instruments , UK), a Bubble Trap (BT; Kinesis ScientificR , UK) linked to a Vacuum Assistance (VA; Cole Parmer , UK), Pharmed BPTR silicone tubes (Internal Diameter: 1.6 mm for the Poppins design and 3.2 for the WL), connectors and o-rings (Cole Parmer , UK),) three ways (Vygon, UK) and two check valves (Cole ParmerR , UK) to allow for flow in one specific direction. The device was assembled, autoclaved and tested with the Dulbecco's Medium Eagle Medium (DMEM; Life Technologies, UK) with 1% of penicillin and streptomycin (P/S; SigmaR, UK) for 72 hours in the incubator in the absence of cells and scaffold. The syringe program was set as following:
40
PH:02 function (RATE) direction: withdrawing
PH:03 Lp: en (loop of the previous instructions)
PH:04 STOP
Organ Harvesting
All surgical procedures and animal husbandry were carried out in accordance with the recommendations in the Animal (Scientific Procedures) Act 1986 and the local ethics committee.
Wild type healthy adult Sprague-Dawley rats of 280 – 300g were euthanized by CO2 inhalation, and the livers were isolated and harvested as described by Maghsoudlou et al. 2016 [1]. Briefly, the abdomen of the rat was sterilized with 70% Ethanol (EtOH; VWRR, UK), the abdominal-pelvic cavity was exposed and the inferior vena cava (IVC) and portal vein (PV) were identified. The PV was cannulated with a 24G cannula (TERUMO, Fisher Scientific, UK) and the IVC was ligated with silk sutures (FST, UK). The whole liver was released from the supporting tissue using the diaphragm as a holding point. Sterile phosphate buffered saline (PBS) with 1U/ml heparin (Sigma, UK) was perfused to remove excess blood and check for leaks.
Decellularization of the rat liver
The decellularization was performed directly after rat liver harvesting. This technique involves treating the liver with a series of chemical solutions and detergents, perfused through the vasculature network, to remove residual blood and genetic material. The protocol is described by Maghsoudlou et al. 2016 [1]. The cannulated PV was connected to a peristaltic pump (iPumps, UK) to perfuse solutions. A bubble trap (Kinesis Scientific, UK) was exploited to ensure that no bubbles were perfused into the vasculature of the liver: the absence of bubbles has been found to be a key factor for effective decellularization. The liver was perfused with MilliQ water for 18 hours at a flow rate of 4.5ml/min at room temperature, followed by 4% sodium deoxycholate (SDC; Sigma, UK) for 5 hours at 6.5ml/min. Next, the rat liver was perfused at 6.5ml/min with PBS for 1 hour, then 3 hours with 25 mg/L DNase-I (Sigma, UK) in saline solution (0.15M NaCl/10mM CaCl2 Sigma, UK), both pre-warmed and maintained at 37 ̊C. DNAse treatment was followed by perfusion of warm PBS for 1 hour and finally PBS
41
overnight at 1ml/min at room temperature. The day after, the scaffold was sterilized by perfusion with 0.1% PAA/4%Ethanol in milliQ water for 90 minutes, followed by 30 minutes of PBS with 1% Penicillin-Streptomycin (Sigma, UK) and 50ng /ml Primocin (Invitrogen, UK): this step was performed twice. Decellularized livers were gamma irradiated and stored at 4 ̊C in sterile PBS with 1% Penicillin-Streptomycin (Sigma, UK) and 50ng /ml Primocin (Invivogen, UK) until use.
Vascular network imaging
The liver was manually perfused with trypan blue (SigmaR, UK) diluted 10% in PBS solution. The experiment was performed under the hood at room temperature.
H & E staining
Fresh rat liver and decellularized liver were analysed by histology. The samples were fixed in 4 % paraformaldehyde (PFA; SigmaR, UK), dehydrated in 30 % sucrose O/N and embedded in 7.5 % Gelatin/ 15 % Sucrose, then sectioned using a Cryostat. Prior to staining, slides were de-gelatinzed in 1 X PBS in a 37℃ water bath for 30 minutes. The sections are left in haematoxylin (Richard Allan Scientific, US) for 5 minutes, rinsed in tap water two times and then in alcoholic acid (ETOH 70 %-HCl 1 % solution). The sections were counterstained in eosin (Microbiology, UK) for 5 minutes and washed for 5 minutes in tap water. The sections were rehydrated in graded EtOH (70%; 90%; 100%). The sections were washed for 6 minutes in histoclear and mounted with D.P.X. (SigmaR , UK).
Immunofluorescent staining
Fresh rat liver and decellularized liver were analysed for remaining cells by DAPI staining and re-seeded scaffolds (dynamic culture of HSCs) were processed and stained for alpha smooth muscle actin (α- SMA), a marker of stellate cell activation. Briefly, samples were fixed in 4% PFA and then rehydrated in a solution of 30% sucrose in PBS (SigmaR, UK), embedded in gelatin (15% sucrose + 7.5% gelatin in PBS), frozen and sectioned at 12 μm using a Cryostat (Bright, UK). The resulting sections were incubated for 1 hour in PBS at 37 ℃ to remove gelatin then prepared for immunofluorescent and/or DAPI staining. For α- SMA staining; sections were blocked with PBS + Triton X-100 0.1 % (PBS-T) for 1 hour, room temperature, then stained with primary antibody, anti- α- SMA (1/500) overnight at 4 ℃. The following day, sections
42
were washed with PBS-T then stained with DAPI (1/1000 in block solution, above) and secondary antibody (anti-rabbit-488), (1/1000 in block solution, above) for 1 hour at room temperature. Sections were washed in PBS-T then mounted with vectashield (Vector, US) and covered with a coverslip. For DAPI staining; the same protocol was followed, however, sections were stained only with DAPI. Images were taken using a fluorescence microscope (Olympus, UK).
DNA Quantification
Total DNA of fresh liver and decellularized scaffold samples was isolated using DNeasy Blood and Tissue kit (Qiagen, UK) as per the manufacturer’s instructions. The yield and purity of the purified DNA samples were measured spectrophotometrically using Nanodrop (Thermo Scientific, US).
Collagen
Total collagen content of fresh and decellularized liver samples were measured using a commercial collagen assay kit (QuickZyme, Biosciences, NL) as per the manufacturer’s instructions. Briefly, the liver samples were hydrolyzed with 6M HCl for 20 hours at 95˚C, and the hydrolysates were incubated with a chromogen solution for 1 hour at 60 °C to develop color. The color intensity was directly proportional to the amount of hydroxyproline within the acid-hydrolyzed sample, which could be a direct measure of the total collagen. The resulting solutions were measured spectrophotometrically at 555nm, and the collagen quantity was calculated from a standard curve plotted with known collagen hydrolysate concentrations.
Elastin Quantification
Elastin content in the decellularized and fresh liver samples was quantified using the Fastin Elastin Assay Kit (Biocolor, UK) according to the manufacturer’s instructions. The kit measured soluble tropoelastins, lathyrogenic elastins and ĸ-elsatin.
![Table 1. Completed trials of antifibrotic agents in patients with liver disease [42].](https://thumb-eu.123doks.com/thumbv2/123dokorg/7516355.105665/18.892.114.773.346.827/table-completed-trials-antifibrotic-agents-patients-liver-disease.webp)
![Figure 1.10 Generic tissue engineering process diagram [56].](https://thumb-eu.123doks.com/thumbv2/123dokorg/7516355.105665/21.892.181.712.225.593/figure-generic-tissue-engineering-process-diagram.webp)
![Figure 1.11 Holoclar technology: the phases from the biopsy to the transplantation [58]](https://thumb-eu.123doks.com/thumbv2/123dokorg/7516355.105665/22.892.195.713.347.627/figure-holoclar-technology-phases-biopsy-transplantation.webp)