Author’s Proof
Carefully read the entire proof and mark all corrections in the appropriate place, using the Adobe Reader commenting tools (Adobe Help). Do not forget to reply to the queries.
We do not accept corrections in the form of edited manuscripts.
In order to ensure the timely publication of your article, please submit the corrections within 48 hours.
If you have any questions, please [email protected].
Author Queries Form
Q1 The citation and surnames of all of the authors have been highlighted. Please check all of the names carefully and indicate if any are incorrect. Please note that this may affect the indexing of your article in repositories such as PubMed.
Q2 Confirm that the email address in your correspondence section is accurate.
Q3 Please ask the following authors toregisterwith Frontiers (at https:// www.frontiersin.org/Registration/Register.aspx) if they would like their names on the article abstract page and PDF to be linked to a Frontiers profile. Please ensure to provide us with the profile link(s) when submitting the proof corrections. Non-registered authors will have the default profile image displayed.
“Marco Diani” “Andrea Altomare” “Paola Secchiero” “Giuseppe Banfi.”
Q4 If you decide to use previously published,copyrighted figuresin your article, please keep in mind that it is your responsibility, as the author, to obtain the appropriate permissions and licenses and to follow any citation instructions requested by third-party rights holders. If obtaining the reproduction rights involves the payment of a fee, these charges are to be paid by the authors.
Q5 Ensure that all the figures, tables and captions are correct.
Q6 Verify that all the equations and special characters are displayed correctly.
Q7 Ensure to add all grant numbers and funding information, as after publication this is no longer possible.
Q8 Ensure, if it applies to your study, the ethics statement is included in the article.
00529/full#supplementary-material (you may need to copy-paste the link directly in your browser).
Please provide new files if you have any corrections and make sure all Supplementary files are cited. Note that ALL supplementary files will be deposited to FigShare and receive a DOI. Notify us of any previously deposited material.
Q10 Could you please confirm if all author affiliations are fine as listed?
Q11 Please provide the name of the department for “Università Vita-Salute San Raffaele, Milan, Italy.”
Q12 Please confirm whether the heading levels in the article have been identified correctly.
Q13 Please confirm that the Data Availability statement is accurate.
Q14 Kindly confirm if the details appearing in the “Author Contributions” section are correct and make sure all authors listed in the Author’s list are mentioned there.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 ORIGINAL RESEARCH published: xx March 2020 doi: 10.3389/fimmu.2020.00529 Edited by: Carlo Riccardi, University of Perugia, Italy Reviewed by: Michio Tomura, Osaka Ohtani University, Japan James Edward Pease, Imperial College London,
Q2 United Kingdom *Correspondence: Eva Reali [email protected] Specialty section: This article was submitted to Autoimmune and Autoinflammatory Disorders, a section of the journal Frontiers in Immunology Received: 15 November 2019 Accepted: 09 March 2020 Published: xx March 2020 Citation: Casciano F, Diani M, Altomare A, Granucci F, Secchiero P, Banfi G and Reali E (2020) CCR4+Skin-Tropic
Phenotype as a Feature of Central Memory CD8+T Cells in Healthy
Subjects Psoriasis Patients. Front. Immunol. 11:529. doi: 10.3389/fimmu.2020.00529
CCR4
+
Skin-Tropic Phenotype as a
Feature of Central Memory CD8
+
T Cells in Healthy Subjects Psoriasis
Patients
Q1 Q3
Fabio Casciano1, Marco Diani2, Andrea Altomare2, Francesca Granucci3, Paola Secchiero1, Giuseppe Banfi2,4and Eva Reali2*
1Department of Morphology, Surgery and Experimental Medicine and LTTA Centre, University of Ferrara, Ferrara, Italy,
2IRCCS Istituto Ortopedico Galeazzi, Milan, Italy,3Department of Biotechnology and Biosciences, University of Q10
Milano-Bicocca, Milan, Italy,4Università Vita-Salute San Raffaele, Milan, Italy Q11
The chemokine receptor CCR4 has emerged as a T cell skin-homing molecules important for the migration of T cells from the blood to the dermis. From our previous data on psoriasis patients, CCR4+memory T cells emerged as a putative recirculating population between skin and blood. Here we focused our attention on the expression of CCR4 and skin-tropic molecules in the different stages of memory T cell differentiation. We analyzed the chemokine receptor profile in CD8+ and CD4+ CD45RA−CCR7+ (T
CM) and CD45RA−CCR7−(TEM) cells. Subpopulations were further divided on the basis of CD62L expression, and the distribution among the subsets of the skin-homing molecule CLA (Cutaneous Leukocyte Antigen) was evaluated. The characterization was performed on peripheral blood mononuclear cells isolated from 21 healthy subjects and 24 psoriasis patients. The results indicate that (i) the skin-homing CCR4 marker is mainly expressed in TCM cells, (ii) CCR4+ TCM cells also express high level of CLA and that (iii) the more differentiated phenotype TEM expresses CXCR3 and CCR5 but lower level of CCR4 and CLA. This indicates that progressive stages of memory T cell differentiation have profoundly different chemokine receptor patterns, with CD8 TCM displaying a marked skin-tropic phenotype CLA+CCR4+. Differential skin-tropic phenotype between T
CM and TEM cells was observed in both healthy subjects and psoriasis patients. However, patients showed an expanded circulating population of TCMCD8+cells with phenotype CCR4+CXCR3+that could play a role in the pathophysiology of psoriasis and possibly in disease recurrence.
Keywords: Psoriatic disease, effector memory, central memory, tissue immunosurveillance, skin, T cells
INTRODUCTION
Memory T cell subpopulations were first classified on the basis of their phenotype and functional
Q12 Q6
features. The classical definition refers to cells with phenotype CCR7+CD45RA− as central
memory (TCM), CCR7−CD45RA− as effector memory (TEM) and CCR7−CD45RA+ as effector
115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228
stages of memory T cell differentiation (4, 5). TCM cells
have phenotype, functional features and molecular signature intermediate between naïve T cells (CCR7+CD45RA+) and TEMcells, they express CD62L and home mainly to secondary
lymphoid organs. TEM and TEMRA, by contrast, are mainly
recruited to the inflamed tissues (2, 6–8). TCM cells show
high capacity of self-renewal as indicated by high basal and cytokine-induced STAT-5 phosphorylation levels, whereas TEM
and TEMRA express higher level of effector molecules and have
minimal capacity of self-renewal (9). In recent years, other subsets of memory T cells have entered into the classification. These include memory stem cells (TSCM), antigen-experienced
circulating T cells with maximal capability of self-renewal and tissue-resident memory T cells (TRM), which can persist
for long in tissues without exiting into the bloodstream (5). In human skin, they express cutaneous lymphocyte antigen (CLA) and chemokine receptors such as CCR4 and CCR10 (10,11).
The recent advances in the characterization of tissue resident memory T cells has raised the question of the origin of TRM cells and their developmental relationship with the other
subpopulations of memory T cells. The evidence of a common clonal origin shown for TCMand TRMprovided by Gaide et al.
now supports the concept that TCM patrolling the tissues can
seed antigen-experienced cells that can finally develop into TRM
(12–16).
Memory T cells are very heterogeneous in regard to tissue-homing properties and cytokine production. Different chemokine receptor expression profiles have been characterized in association with T cell polarization. According to the current classification, Th1 cells acquire the capacity to produce IFNγ and the expression of chemokine receptors CXCR3, CCR5, and CXCR6, whereas Th2 cells acquire the capacity to produce IL-4 and express receptor CRTh2 and CCR4 (8, 17). Th17 cells have been characterized by the expression of CCR6 as well as CCR4 and Th22 for the expression of both CCR4 and CCR10 (4, 18). Despite these indications, the role of CCR4 as a marker of polarized T cells is still unclear, whereas it is emerging its role in directing T cell migration to the skin.
In a previous study we showed that, in patients with cutaneous psoriasis, CCR4+cells were expanded in the circulating memory
(CD45RA−) CD4 and CD8 compartments and their percentage
positively correlated with the severity of the disease (19). This has led to the hypothesis that CCR4+cells may represent a key recirculating population between skin and blood.
This study has the aim to test the hypothesis that skin-tropic chemokine receptors such as CCR4 are differentially distributed among the memory differentiation stages. In addition, we analyzed the skin-tropic phenotype of patients diagnosed with cutaneous psoriasis.
MATERIALS AND METHODS
Study Design
The study had as a first aim the characterization of the chemokine expression profile in memory T cell subpopulations
TCM and TEM. Specifically, CCR4, CXCR3, CCR5, and
CCR6 were analyzed on CD8+ and CD4+ CD45RA− CCR7+ (TCM) and CD45RA−CCR7− (TEM) cells. In order
to define the skin- tropic phenotype within the memory T cell subpopulations, we analyzed the distribution of the skin-homing molecule CLA among the subsets of memory T cells. The characterization was performed on peripheral blood mononuclear cells (PBMCs) isolated from 21 healthy subjects and then compared with a group of 24 psoriasis patients.
In some experiments TCM and TEM cells were further
divided on the basis on CD62L expression. CCR4 and CLA were evaluated on CCR7+CD62L+CD45RA− and CCR7+CD62L−CD45RA−cells.
Human Subjects and Patients Recruitment
Healthy control subjects with negative family and personal anamnesis for psoriasis and patients with psoriasis were recruited by the Department of Dermatology, Istituto di Ricovero e Cura a Carattere Scientifico Istituto Ortopedico Galeazzi (Milan, Italy) within the clinical study approved by the local Ethical Committee (Comitato Etico dell’Ospedale San Raffaele, Milan, Italy) and registered on ClinicalTrials.gov (NCT03374527).
Subjects undergoing treatment with cyclosporin A, methotrexate, systemic corticosteroids or any other immunosuppressant or biotechnological agents within at least 3 weeks prior to the collection of blood samples were excluded from the study. Systemic autoimmune diseases such as type 1 diabetes, neoplastic diseases, chronic or acute infections were used as exclusion criteria. (Some demographic and clinical characteristics are summarized in Supplemental Table 1).
Peripheral venous blood samples were collected from each patient and healthy subjects into BD Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA) containing EDTA for flow cytometry analysis.
T Cell Isolation and FACS Analysis
Peripheral blood mononuclear cells (PBMCs) were prepared from whole blood from healthy subjects and patients by Ficoll gradient centrifugation (Lympholyte R, Cederlane R
Hornby, Ontario, Canada) as previously described (20). For the phenotypic characterization, unstimulated PBMCs were stained with different combinations of fluorochrome-conjugated antibodies. We used combinations of fluorochrome-conjugated antibodies against: CD4 (RPA-T4), CD8 (SK1), CD45RA (HI100), CCR4 (1G1), CXCR3 (1C6), CCR5 (2D7/CCR5), CCR6 (11A9) (all from BD Biosciences,) and CD3 (REA613), CCR7 (REA546), CD62L (145/15) and the skin-tropic CLA molecule (REA1101) (all from Miltenyi Biotec GmbH). To automatically assess fluorescence compensation, MACS Comp Bead Kits (Miltenyi Biotec) as well as the fluorochrome-conjugated antibodies were used. To evaluate non-specific fluorescence when defining positive events, we used Fluorescence Minus One (FMO) controls which contains the multicolor staining combination except the antibody in
229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327 328 329 330 331 332 333 334 335 336 337 338 339 340 341 342
Casciano et al. Skin-Tropic Central Memory T Cells
the detector of interest (Supplemental Figures 1, 2) (21–23). As additional control, samples were stained with isotype controls antibodies to confirm the threshold for the marker of interest.
Samples were acquired using FACSAriaII (BD biosciences) flow cytometer (19,24) and analyzed with the FlowJo software (Tree Star, Ashland, OR). A representative gating strategy is shown in Supplemental Figure 3.
Statistical Analysis
The Gaussian distribution of overall data was evaluated using the Shapiro–Wilk test. Statistical comparisons between each subpopulation in individual subjects was performed by paired or unpaired analysis calculated with non-parametric tests (Wilcoxon signed-rank test or Mann–Whitney non-parametric U-test, as appropriated) when no Gaussian distribution was found, otherwise Student’s t-test was used. Statistical analysis was performed using GraphPad Prism 6 software.
RESULTS
Differential Expression of CCR4 in CD8
+T
CMand T
EMCells
From our previous data on psoriasis patients, CCR4+ memory
T cells emerged as a putative recirculating population between skin and blood. Here we focused our attention on the expression of CCR4 in different subsets of memory T cells.
We analyzed the chemokine receptor expression in circulating CD8+and CD4+memory T cells with CD45RA−CCR7+(T
CM)
and CD45RA−CCR7− (TEM) phenotype in 21 healthy subjects.
Specifically, we evaluated the expression of chemokine receptors CCR4, CCR6, CXCR3, and CCR5.
The results of the analysis revealed that the skin-homing CCR4 receptor was expressed at markedly higher level in TCM than in TEM cells. Indeed, as shown in Figure 1A, in
CD8+T cells the percentage of CCR4+CCR5−cells significantly
decreased from TCM to TEM cells (p < 0.0001). By contrast,
CCR4+CCR5 -CCR4+CCR5+ CCR4-CCR5+ CCR4-CCR5 -0 20 40 60 80 100 % C C R 4 +C C R 5 - c e ll s < 0.000 Exact **** 0 20 40 60 80 100 % C C R 4 -C C R 5 + c e ll s A B
FIGURE 1 | Differential expression of CCR4 in CD8+T
CMand TEMcells. PBMCs isolated from healthy control subjects were stained for CD8, memory T cell
Q4
Q5 phenotype markers (CD45RA and CCR7) and for chemokine receptors CCR4 and CCR5. (A) CD8+T cells gated as CD45RA−CCR7+T
CMand CD45RA−CCR7−
TEMwere analyzed for the expression of CCR4 and CCR5. Representative analysis is shown in figure. The axis scales for fluorescence are reported as log. Statistical
analysis of the differences was performed by Mann–Whitney test. P-values < 0.05 were considered significant: ****p < 0.0001. (B) Mean values of the percentage of CCR4/CCR5 subpopulations among TCMand TEMwere shown in pie charts.
343 344 345 346 347 348 349 350 351 352 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374 375 376 377 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399 400 401 402 403 404 405 406 407 408 409 410 411 412 413 414 415 416 417 418 419 420 421 422 423 424 425 426 427 428 429 430 431 432 433 434 435 436 437 438 439 440 441 442 443 444 445 446 447 448 449 450 451 452 453 454 455 456
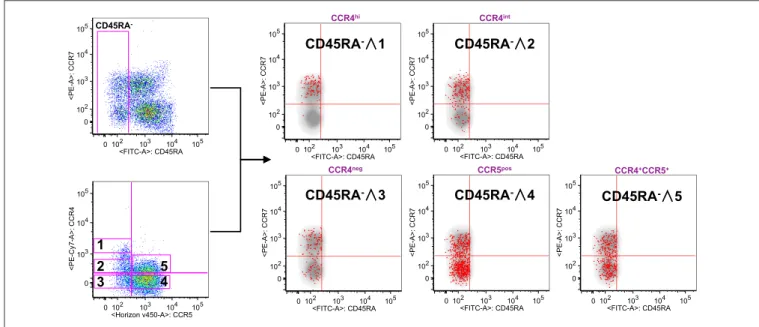
FIGURE 2 | CCR4+expression characterize T
CMcompartment. PBMCs isolated from healthy control subjects were stained for CD8, memory T cell phenotype
markers (CD45RA and CCR7) and for chemokine receptors CCR4 and CCR5. (A) CD8+T cells were analyzed for the memory phenotype according to CD45RA,
CCR7 expression and for the expression of the chemokine receptors CCR4 and CCR5. On the basis of the chemokine receptor expression we identified five subsets CCR4hi(gate 1), CCR4int(gate 2), CCR4−CCR5−(gate 3), CCR4−CCR5+(gate 4) CCR4+CCR5+(gate 5). These five subsets were superimposed to the density plot
of the CD45RA−gated cells. Each red dot identifies cells from the corresponding subset as reported in figure. The axis scales for fluorescence are reported as log.
CCR5+CCR4−cells that were present at low frequency in TCM
strongly augmented in the TEMcompartment.
Representing the chemokine receptor profiles in the different subsets of memory T cells (Figure 1B), we evidenced that CD8+
TCM cells contained a high percentage of CCR4+CCR5− cells
(34.6 ± 11.0%; mean ± SD) whereas they contained 16.7 ± 15.2% of cells with phenotype CCR5+CCR4−. The chemokine receptor
profile dramatically changed in TEMcells where the percentage
of CCR4+CCR5−cells lowered down to 11.8 ± 5.2% whereas the
percentage of CCR5+CCR4−cells increased to 43.8 ± 13.7% in
the TEMcompartment.
These results led to the hypothesis that CCR4 could represent a specific feature of CD8+ T cells with central
memory phenotype.
To verify this possibility, we used the reverse approach (Figure 2). CD8+ gated T cells were analyzed on the basis of CCR7 and CD45RA expression or for the expression of CCR4 and CCR5. Total CD8+ gated
cells were divided into five subpopulations: CCR4 highly expressing cells (CCR4hi), cells expressing
intermediate level of CCR4 (CCR4int), CCR4 and CCR5 double negative cells (CCR4neg), cells expressing CCR5 (CCR5pos) and cells co-expressing CCR4 and CCR5 (CCR4+CCR5+). Overlay analysis of these selected areas
with CD45RA− CD8+ T cells showed that CCR4hiCCR5−
cells were almost entirely central memory (p < 0.0001, Supplemental Table 2).
CCR4int CD8+ T cells had a trend toward an accumulation in the TCMpopulation whereas the CCR4−CCR5+cells, though
being detectable in all the selected CD45RA− populations were
for the vast majority in the TEM compartment (p < 0.0001,
Supplemental Table 2).
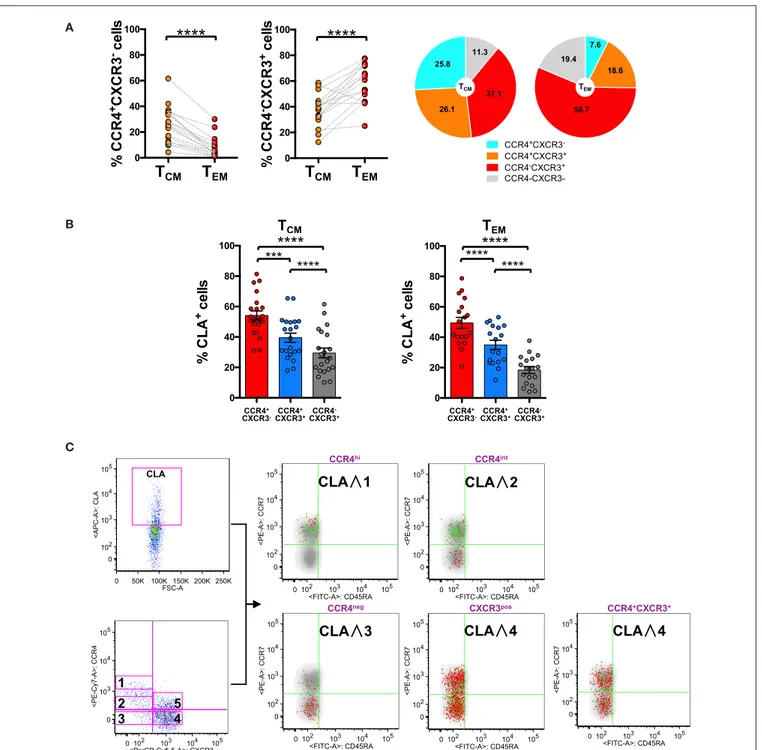
Analysis of CCR4 and CXCR3 expression and distribution in CD8 TCM and TEM compartments evidenced that
CCR4+CXCR3− cells also accumulated in TCM (25.8 ±
14.0% in TCM vs. 7.6 ± 7.9% in TEM). CXCR3+CCR4− cells
conversely represented the 37.1 ± 12.0% of TCM cells and
increased to 58.7 ± 14.3% in the TEMcompartment (Figure 3A).
Interestingly, a considerable fraction of cells in the TCM compartment showed a double positive phenotype
(CCR4+CXCR3+ 26.1 ± 12.7%), that was reduced in the TEM
compartment (18.6 ± 10.0%, p < 0.05).
These data evidenced a selective accumulation of the skin-tropic CCR4 chemokine receptor in TCM cells and a
clear shift toward a CXCR3+ and CCR5+ phenotype in the
TEMcompartment.
Similar results were obtained in the CD4+ subpopulation (Supplemental Figure 4). In this case the percentage of CCR4+CCR5− was 39.9 ± 9.1% in the T
CM and was
lowered to 26.0 ± 7.5% in the TEM compartment. Conversely,
CCR5+CCR4−cells that were minimally represented in TCM(4.7
±3.3%) increased to 27.6 ± 11.8% in TEMcells. Analyzing the
CCR4+CXCR3−subset, a significant difference was still observed
between TCM(35.8 ± 7.7%) and TEM(29.9 ± 8.7%) cells even if
the difference was less pronounced (Supplemental Figure 5). The analysis of the distribution of CCR6+CXCR3−,
CCR6+CXCR3+, and CXCR3+CCR6−in the two compartments showed that CCR6+CXCR3− cells were represented with similar frequencies in the two subsets of memory T cells (Supplemental Figure 6).
457 458 459 460 461 462 463 464 465 466 467 468 469 470 471 472 473 474 475 476 477 478 479 480 481 482 483 484 485 486 487 488 489 490 491 492 493 494 495 496 497 498 499 500 501 502 503 504 505 506 507 508 509 510 511 512 513 514 515 516 517 518 519 520 521 522 523 524 525 526 527 528 529 530 531 532 533 534 535 536 537 538 539 540 541 542 543 544 545 546 547 548 549 550 551 552 553 554 555 556 557 558 559 560 561 562 563 564 565 566 567 568 569 570
Casciano et al. Skin-Tropic Central Memory T Cells
A
B
C
FIGURE 3 | CLA expression is maximal in CCR4+cells and considerably decreases in CXCR3+cells. PBMCs isolated from healthy control subjects were stained for
CD8, memory T cell phenotype markers (CD45RA and CCR7), for chemokine receptors CCR4, CXCR3, and for CLA. (A) CD8+T cells gated as T
CMand TEMwere
analyzed for the expression of CCR4 and CXCR3. Statistical analysis of the differences was performed by Mann-Whitney test. P-values < 0.05 were considered significant: ****p < 0.0001. Mean value for CCR4/CXCR3 subpopulation among TCMand TEMwere shown in pie charts. (B) TCMand TEMcells were analyzed for
differences in the percentage of CLA cells in the CCR4+CXCR3−, CCR4+CXCR3+, and CXCR3+subpopulations using by Mann-Whitney test. P-values < 0.05 were
considered significant: ****p < 0.0001. (C) Reverse analysis is shown in the panel. Total memory CD45RA−CD8+T cells were gated and analyzed for the expression
of CLA and for chemokine receptors CCR4/CXCR3. On the basis of the chemokine receptor expression we identified five subsets CCR4hi(gate 1), CCR4int(gate 2),
CCR4−CXCR3−(gate 3), CCR4−CXCR3+(gate 4), CCR4+CXCR3+(gate 5). The five subsets of CCR4/CXCR3 expressing cells were overlaid to the density plot of
CD45RA−cells. Each red dot represents a cell from corresponding subset. Among those dots, green dots identify cells of the gated subsets (CCR4hi, CCR4dim,
Q15
571 572 573 574 575 576 577 578 579 580 581 582 583 584 585 586 587 588 589 590 591 592 593 594 595 596 597 598 599 600 601 602 603 604 605 606 607 608 609 610 611 612 613 614 615 616 617 618 619 620 621 622 623 624 625 626 627 628 629 630 631 632 633 634 635 636 637 638 639 640 641 642 643 644 645 646 647 648 649 650 651 652 653 654 655 656 657 658 659 660 661 662 663 664 665 666 667 668 669 670 671 672 673 674 675 676 677 678 679 680 681 682 683 684
To confirm this first part of the results, we searched for gene expression data in individual immune cell populations using ImmGen Browser (http://www.immgen.org/databrowser/ index.html) (25). Dataset analysis confirmed that CCR4 gene is expressed at markedly higher level in the TCMthan in TEMsubset
whereas CCR5 gene is expressed by TEMcells and at markedly
lower level by TCM(Supplemental Figure 7).
These data finally confirm that the subsets of memory T cells have markedly different patterns of chemokine receptor profile.
CLA Expression Is Maximal in CCR4
+Subset and Considerably Decreases in
CXCR3
+Subset
To investigate further the skin-tropic phenotype, we analyzed CLA expression in the different memory T cell differentiation stages. As shown in Figure 3B, in the CD8 compartment, the expression of the skin-homing molecule CLA was maximal in CCR4+ T cells and progressively decreases toward the
CXCR3+phenotype.
Analyzing the localization of the CLA+ fraction among the CD8+memory T cells together with the CCR4high, CCR4int, and CXCR3 expression using the reverse approach, we found that CLA co-localized with CCR4 (green dots) mainly in the TCM
cells (Figure 3C). Indeed CCR4hiCXCR3−co-express cutaneous
lymphocytes antigen CLA mainly in the TCMcompartment (p
= 0.0017, Supplemental Table 2) whereas in CCR4−CXCR3+ cells CLA expression was more abundant in the effector memory subset (p < 0.0001, Supplemental Table 2).
Accumulation of CLA+cells in CCR4+subsets was observed
also in the total memory CD4 compartment, however, CLA expression was not significantly different in CCR4 TCM and
TEM subsets (data not shown). This difference underlines that
skin-tropic features of TCM cells are more evident in the
CD8 compartment.
CD62L
+CCR7
+Central Memory T Cells
Selectively Express a Skin-Tropic
Phenotype
In a recent study two distinct populations of recirculating memory T cells have been described within the CCR7+memory
T cell compartment and were distinguished on the basis of the expression of CD62L (26). We therefore analyzed the frequency of CCR4+ cells among CD62L+ and CD62L− fractions of
CD45RA−CCR7+cells (Figure 4A).
We found that CCR4+ cells were actually present at significantly higher percentage in CD62L+T
CMfraction whereas
CXCR3+ cells accumulated mainly in the CD62L− T CM
compartment (Figure 4B). Notably CLA was found to be expressed at a higher level in the CD62L+ fraction of TCMcells
(40.40 ± 1.6) compared to CD62L−(22.64 ± 3.1) (p < 0.001, data not shown). A significant increase in CLA+cells was observed in
CCR4+CXCR3+ and CCR4−CXCR3+subsets of CD62L+T CM
cells compared to the same subsets of CD62L− T
CMcells. No
differences were observed in the CCR4+CXCR3− cells where
CLA was expressed at high level in both CD62L+and CD62L− TCMsubsets (Figure 4C).
This finding further restricts the subset of skin-tropic T cells within the CD8+ TCM compartment and defines
CLA+CCR4+CD62L+ CD8+ TCM cells as a highly
skin-tropic population.
Skin-Tropic Phenotype of Circulating CD8
+T Cells in Psoriasis Patients
To explore the possibility of a role of the skin-tropic subset of CD8+ T
CM cells in skin immunopathology we performed
a comparison of chemokine receptor expression in both CD8 and CD4 TCMand TEMcompartments of psoriasis patients and
healthy subjects.
In the psoriasis patient cohort there was the same difference in the chemokine receptor percentage between TCM and TEM
cells as observed in healthy subjects (Supplemental Figure 8). However, the comparison of the phenotype of circulating CD8+ T cells between psoriasis patients and healthy subjects evidenced a significant increase in the percentage of CCR4+CXCR3+CD8+
TCM cells in the circulation of psoriasis patients (Figure 5A).
In these patients we also evidenced a significantly higher CLA expression in the CD8+ CCR4+CXCR3− T
CM but not in the
TEM compartment (Figure 5B). Correlation analysis with the
PASI (Psoriasis Area and Severity Index) score indicates that the circulating levels of these subsets did not directly correlate with the severity of the cutaneous disease (data not show). Nevertheless, they could be involved in recirculating events associated with disease recurrence or systemic manifestation.
DISCUSSION
From the results of this study it emerged that the skin-homing CCR4 marker is mainly expressed in TCMcells. CCR4+TCMcells
also express high level of CLA (Cutaneous Leukocyte Antigen) whereas the more differentiated phenotype TEM expresses
CXCR3 and CCR5 but lower level of CCR4 and CLA.
This indicates that progressive stages of memory T cell differentiation have different chemokine receptor profiles, with TCMdisplaying a marked skin-tropic phenotype CLA+CCR4+.
It is important to note that skin-tropic features are progressively lost in more advanced stages of memory T cell differentiation and in CXCR3+phenotype.
The evidence of a selective accumulation of the skin-tropic CCR4 chemokine receptor in the central memory compartment suggests a preferential migration of the small population of CCR7+ CD8+ memory T cells toward the skin. By contrast expression of the receptors CXCR3 and CCR5 for the inflammatory chemokines CXCL10 and CCL5 is clearly confirmed as a feature of cells in a more advanced stage of differentiation (8,27). This underlines a clear distinction between TCM and TEM migratory properties enlightening skin-tropic
features for CCR7+ memory T cells and migratory properties
toward inflamed tissues for TEMcells.
A prevalence of CCR4 phenotype in TCM cells has been
occasionally reported in previous works (28,29), however more controversial findings have been reported in recent studies thus not strengthening this evidence (4).
685 686 687 688 689 690 691 692 693 694 695 696 697 698 699 700 701 702 703 704 705 706 707 708 709 710 711 712 713 714 715 716 717 718 719 720 721 722 723 724 725 726 727 728 729 730 731 732 733 734 735 736 737 738 739 740 741 742 743 744 745 746 747 748 749 750 751 752 753 754 755 756 757 758 759 760 761 762 763 764 765 766 767 768 769 770 771 772 773 774 775 776 777 778 779 780 781 782 783 784 785 786 787 788 789 790 791 792 793 794 795 796 797 798
Casciano et al. Skin-Tropic Central Memory T Cells
A
B
C
FIGURE 4 | CD62L+CCR7+central memory T cells selectively express skin-tropic phenotype. (A) Representative analysis of chemokine receptor expression in
CD62L+/−gated CD45RA−CCR7+(T
CM) CD8+T cells. Each subset (CCR4+CXCR3−, CCR4+CXCR3+, and CCR4−CXCR3+) in CD62L+and CD62L−gates was
also analyzed for the expression of cutaneous lymphocytes antigen CLA. The axis scales for fluorescence are reported as log, the axis scales for FSC are reported as linear. (B) CD45RA−CCR7+(TCM) CD8+T cells were gated on the basis of the expression of CD62L and analyzed for the expression of chemokine receptors
(CCR4/CXCR3). The percentage of positive cells for each phenotype in CD62L+and CD62L−gated cells was represented as Tukey’s boxplot. Significance of the
differences was calculated using Student’s t-test for paired samples. P-values < 0.05 were considered significant: *p < 0.05. (C) Difference in the CLA+cells in
subpopulations of CCR4/CXCR3 expressing cells in the CD8+T
CMgated on the basis of the expression of CD62L were represented as Tukey’s boxplot. Significance
799 800 801 802 803 804 805 806 807 808 809 810 811 812 813 814 815 816 817 818 819 820 821 822 823 824 825 826 827 828 829 830 831 832 833 834 835 836 837 838 839 840 841 842 843 844 845 846 847 848 849 850 851 852 853 854 855 856 857 858 859 860 861 862 863 864 865 866 867 868 869 870 871 872 873 874 875 876 877 878 879 880 881 882 883 884 885 886 887 888 889 890 891 892 893 894 895 896 897 898 899 900 901 902 903 904 905 906 907 908 909 910 911 912 A B
FIGURE 5 | Skin-tropic phenotype of circulating CD8+T cells in psoriasis
patients. (A) CD8+cells from patients with cutaneous psoriasis (PsO) and
healthy subjects were analyzed for the percentage of cells expressing chemokine receptors CCR4/CXCR3 in the CD45RA−CCR7+T
CM
compartment. Statistical analysis of the differences was performed by Mann–Whitney test. P-values < 0.05 were considered significant: **p < 0.01. (B) CD8+cells gated on the basis of the memory T
CMor effector TEM
phenotype, from patients with cutaneous psoriasis (PsO) and healthy subjects were analyzed for the percentage of CLA expressing cells in the
CCR4+CXCR3−subpopulation. Statistical analysis of the differences was
performed by Mann–Whitney test. P-values <0.05 were considered significant: *p < 0.05.
Our results strongly reinforce the evidence of the first reports and enlighten a novel classification of chemokine receptors and skin-tropic molecules as a function of memory T cell differentiation.
CCR4 is important for the mechanism by which T cells migrate into the dermis from the blood and has been defined as a skin-homing receptor that is up-regulated by memory T cells primed in skin-draining lymph nodes (2, 30). To enter non-inflamed skin, these T cells must interact with the low constitutive levels of homing molecules expressed on resting endothelium such as CLA ligand E-selectin and the CCR4 ligand CCL17 (31).
Under physiological conditions CCR4 has been reported as a no redundant, necessary component of skin-specific lymphocyte trafficking (32). Its role could however change under inflammatory conditions, where other chemokines could play a major role in the recruitment of effector memory/effector T cells (27, 33). Consistent with this view, different groups evidenced that T cell mediated skin-inflammation is largely independent of CCR4 and rather requires CXCR3 (34, 35).
Along this line in psoriatic patients both gene expression analysis and circulating T cell phenotype suggest recruitment of CXCR3+and CCR5+cells to the inflamed skin associated with upregulation of the skin expression of their ligand CXCL10 and CCL5 (19,24).
CCR4 could therefore play a major role in patrolling the skin by central memory T cells rather than in mediating the recruitment of effectors cells to the inflamed skin. Consistently, in a recent study it has emerged that TCMcells plays a role in
tissue immunosurveillance. In line with this concept, the skin compartment had been previously suggested as an important site for lymphocyte differentiation and antigen encounter and was proposed for the definition of “peripheral lymphoid organ” (15,36).
Our results strengthen the role of the skin compartment as a preferential trafficking site for TCM cells, where it is
possible that antigen encounter occurs. It also defines the limited subpopulation of circulating CD8+ TCM cells as a subset with
high skin-tropic features. These cells after antigen encounter and under appropriate environmental conditions could possibly give rise to non-circulating TRM cells. In immunopathological
skin conditions such as psoriasis the expanded subset of TCM
cells expressing CCR4 and CXCR3 could play a role in disease recurrence or redistribution to distant sites such as joint synovial tissues and enthesis.
Our data therefore add a new evidence to the concept of physiological skin trafficking and immunosurveillance, focusing the attention on a specific subpopulation of cells with central memory and CCR4+phenotype. It also opens the question of the physiological role of this phenomenon and its possible alterations in pathological conditions.
DATA AVAILABILITY STATEMENT
The datasets generated for this study are available on request to
the corresponding author. Q13
ETHICS STATEMENT
The study was approved by the local Ethical Committee
(Comitato Etico dell’Ospedale San Raffaele, Milan, Italy) (30IOG Q9
17/07/2014), and written informed consent was obtained from all patients and healthy subjects before they entered the study, which was performed in accordance with the Declaration of Helsinki. The study was registered on ClinicalTrials.gov, Identifier: NCT03374527.
AUTHOR CONTRIBUTIONS
FC performed the flow cytometry experiments, analyzed the Q14
data, prepared the figures, and contributed to the writing of the manuscript. MD and AA selected the patients and control subjects to be recruited in the study, collected samples, and clinical data. FG participated in the supervision of the research activities, in the data interpretation. PS participated in data interpretation and contributed to the final version of the manuscript. GB participated in the coordination of the activities
913 914 915 916 917 918 919 920 921 922 923 924 925 926 927 928 929 930 931 932 933 934 935 936 937 938 939 940 941 942 943 944 945 946 947 948 949 950 951 952 953 954 955 956 957 958 959 960 961 962 963 964 965 966 967 968 969 970 971 972 973 974 975 976 977 978 979 980 981 982 983 984 985 986 987 988 989 990 991 992 993 994 995 996 997 998 999 1000 1001 1002 1003 1004 1005 1006 1007 1008 1009 1010 1011 1012 1013 1014 1015 1016 1017 1018 1019 1020 1021 1022 1023 1024 1025 1026
Casciano et al. Skin-Tropic Central Memory T Cells
between clinical and research groups. ER designed the study, coordinated the research activities, and wrote the final version of the manuscript.
FUNDING
The first part of this work was funded by the National Psoriasis Foundation Discovery Grant 2014. The final part was
Q7
supported by Grant from Fondazione Natalino Corazza Onlus, Bologna, Italy.
SUPPLEMENTARY MATERIAL
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.
2020.00529/full#supplementary-material Q9
REFERENCES
1. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. (1999) 401:708–12. doi: 10.1038/44385
2. Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. (2013) 31:137– 61. doi: 10.1146/annurev-immunol-032712-095954
3. Tian Y, Babor M, Lane J, Schulten V, Patil VS, Seumois G, et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat Commun. (2017) 8:1473. doi: 10.1038/s41467-017-01728-5
4. Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. (2013) 43:2797–809. doi: 10.1002/eji.201343751
5. Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr Opin Immunol. (2013) 25:556–63. doi: 10.1016/j.coi.2013.09.003 6. Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of
memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. (2000) 251:167–71. doi: 10.1007/978-3-642-57276-0_21
7. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. (2004) 22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702 8. Sallusto F. Heterogeneity of human CD4(+) T cells
against microbes. Annu Rev Immunol. (2016) 34:317–34. doi: 10.1146/annurev-immunol-032414-112056
9. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. (2014) 14:24–35. doi: 10.1038/nri3567
10. Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol. (2013) 14:978–85. doi: 10.1038/ni.2680
11. Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat Rev Immunol. (2019) 19:490–502. doi: 10.1038/s41577-019-0162-3
12. Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. (2013) 34:27–32. doi: 10.1016/j.it.2012.08.008
13. Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, et al. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. (2015) 21:647–53. doi: 10.1038/nm.3860
14. Thome JJ, Farber DL. Emerging concepts in tissue-resident T cells: lessons from humans. Trends Immunol. (2015) 36:428–35. doi: 10.1016/j.it.2015.05.003
15. Gehad A, Teague JE, Matos TR, Huang V, Yang C, Watanabe R, et al. A primary role for human central memory cells in tissue immunosurveillance. Blood Adv. (2018) 2:292–8. doi: 10.1182/bloodadvances.20170 11346
16. Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, et al. Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to candida albicans after skin infection. J Allergy Clin Immunol. (2018) 142:647–62. doi: 10.1016/j.jaci.2017. 09.042
17. Sallusto F, Cassotta A, Hoces D, Foglierini M, Lanzavecchia A. Do memory CD4 T cells keep their cell-type programming: plasticity
versus fate commitment? T-cell heterogeneity, plasticity, and selection in humans. Cold Spring Harb Perspect Biol. (2018) 10:a029421. doi: 10.1101/cshperspect.a029421
18. Lugli E, Hudspeth K, Roberto A, Mavilio D. Tissue-resident and memory properties of human T-cell and NK-cell subsets. Eur J Immunol. (2016) 46:1809–17. doi: 10.1002/eji.201545702
19. Sgambelluri F, Diani M, Altomare A, Frigerio E, Drago L, Granucci F, et al. A role for CCR5(+)CD4 T cells in cutaneous psoriasis and for CD103(+) CCR4(+) CD8 Teff cells in the associated systemic inflammation. J Autoimmun. (2016) 70:80–90. doi: 10.1016/j.jaut.2016. 03.019
20. Voltan R, Rimondi E, Melloni E, Rigolin GM, Casciano F, Arcidiacono MV, et al. Ibrutinib synergizes with MDM-2 inhibitors in promoting cytotoxicity in B chronic lymphocytic leukemia. Oncotarget. (2016) 7:70623–38. doi: 10.18632/oncotarget. 12139
21. Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. (2000) 243:77–97. doi: 10.1016/S0022-1759(00)00229-5
22. Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. (2001) 45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::AID-CYTO1163>3.0.CO;2-C
23. Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. (2006) 69:1037–42. doi: 10.1002/cyto.a.20333
24. Diani M, Galasso M, Cozzi C, Sgambelluri F, Altomare A, Cigni C, et al. Blood to skin recirculation of CD4(+) memory T cells associates with cutaneous and systemic manifestations of psoriatic disease. Clin Immunol. (2017) 180:84–94. doi: 10.1016/j.clim.2017.04.001
25. Ma CS, Wong N, Rao G, Nguyen A, Avery DT, Payne K, et al. Unique and shared signaling pathways cooperate to regulate the differentiation of human CD4+ T cells into distinct effector subsets. J Exp Med. (2016) 213:1589–608. doi: 10.1084/jem.20151467
26. Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. (2015) 7:279ra239. doi: 10.1126/scitranslmed.30 10302
27. Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am J Pathol. (2002) 160:347–55. doi: 10.1016/S0002-9440(10)64378-7
28. Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. (2003) 101:4260–6. doi: 10.1182/blood-2002-11-3577
29. Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. (2010) 116:767–71. doi: 10.1182/blood-2009-11-251926
30. Tubo NJ, McLachlan JB, Campbell JJ. Chemokine receptor requirements for epidermal T-cell trafficking. Am J Pathol. (2011) 178:2496–503. doi: 10.1016/j.ajpath.2011.02.031
31. Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in
1027 1028 1029 1030 1031 1032 1033 1034 1035 1036 1037 1038 1039 1040 1041 1042 1043 1044 1045 1046 1047 1048 1049 1050 1051 1052 1053 1054 1055 1056 1057 1058 1059 1060 1061 1062 1063 1064 1065 1066 1067 1068 1069 1070 1071 1072 1073 1074 1075 1076 1077 1078 1079 1080 1081 1082 1083 1084 1085 1086 1087 1088 1089 1090 1091 1092 1093 1094 1095 1096 1097 1098 1099 1100 1101 1102 1103 1104 1105 1106 1107 1108 1109 1110 1111 1112 1113 1114 1115 1116 1117 1118 1119 1120 1121 1122 1123 1124 1125 1126 1127 1128 1129 1130 1131 1132 1133 1134 1135 1136 1137 1138 1139 1140
normal skin. J Immunol. (2006) 176:4431–9. doi: 10.4049/jimmunol.17 6.7.4431
32. Campbell JJ, O’Connell DJ, Wurbel MA. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. (2007) 178:3358–62. doi: 10.4049/jimmunol.178.6.3358
33. Casciano F, Pigatto PD, Secchiero P, Gambari R, Reali E. T cell hierarchy in the pathogenesis of psoriasis and associated cardiovascular comorbidities. Front Immunol. (2018) 9:1390. doi: 10.3389/fimmu.2018.01390
34. Sells RE, Hwang ST. Paradoxical increase in skin inflammation in the absence of CCR4. J Invest Dermatol. (2010) 130:2697–9. doi: 10.1038/jid.2010.292 35. Gehad A, Al-Banna NA, Vaci M, Issekutz AC, Mohan K, Latta M, et al.
Differing requirements for CCR4, E-selectin, and alpha4beta1 for the migration of memory CD4 and activated T cells to dermal inflammation. J Immunol. (2012) 189:337–46. doi: 10.4049/jimmunol.1102315
36. Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol. (2011) 131:2178–85. doi: 10.1038/jid.2011.198
Conflict of Interest:The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Copyright © 2020 Casciano, Diani, Altomare, Granucci, Secchiero, Banfi and Reali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.