DOCTORAL SCHOOL IN BIOLOGY
Biology Applied to Human Health
XXVII Cycle
DUAL RNA-SEQUENCING APPROACH FOR DISSECTING
NONTYPEABLE HAEMOPHILUS INFLUENZAE AND HOST CELL
TRANSCRIPTOMES
Presented by:
Buket Baddal
Supervisors:
Dr. Alfredo Pezzicoli
Dr. Marco Soriani
Director:
II
Characterization of host-pathogen interactions is critical for the development of next-generation therapies and vaccines. Classical approaches involve the use of transformed cell lines and/or animal models which may not reflect the complexity and response of the human host. The Gram-negative bacterium nontypeable
Haemophilus influenzae (NTHi) commonly resides as a commensal in the human nasopharynx from where it
can disseminate to local organs to cause a wide spectrum of diseases including otitis media, chronic obstructive pulmonary disease, cystic fibrosis and bronchitis. Successful colonization by NTHi depends on its ability to adhere and adapt to the respiratory tract mucosa, which serves as a frontline defense against respiratory pathogens. In opportunistic infections, colonization is followed by either a paracellular route across the epithelial barrier or invasion of non-phagocytic and epithelial cells. However, the temporal events associated to a successful colonization are far from being fully characterized.
Recent improvements in tissue engineering techniques including the development of differentiated primary cell cultures and organotypic 3D cellular models have significantly increased our understanding of microbial pathogenesis by providing physiologically relevant representations of human upper airway tissue. Bridging of these techniques with the currently available next-generation sequencing technologies is a conceptually novel approach for studying infection-linked transcriptome alterations in such systems. Massively parallel cDNA sequencing (RNA-seq) offers the possibility of comprehensive and simultaneous whole genome transcriptional profiling of both host and invading pathogen, and overcomes the existing technical and economical limitations of probe-dependent methods.
Taking advantage of the technological advances, we reconstituted the ciliated human bronchial epithelium in
vitro using primary bronchial epithelial cells to simultaneously monitor the infection-linked global changes in
NTHi and infected host epithelia gene expression by dual RNA-seq. Acquisition of a total of nearly 2,5 billion sequences allowed construction of high-resolution strand-specific transcriptome maps of NTHi during infection of host mucosal surface, and monitoring of metabolic as well as stress-induced host-adaptation strategies of this pathogen. The initial stage of colonization was characterized by the binding of NTHi to cilia. Temporal analysis of host mRNA signatures revealed consequent remodeling of target cell cytoskeleton and junction complexes elicited by bacterial infection, with a profound effect on intermediate filament network of bronchial epithelium. At later stage of infection when bacteria start to internalize, NTHi down-regulated the central metabolism and increased the expression of transporters indicating alterations in the bacterial metabolic regime due to the evolving substrate availability. Concurrently, the oxidative environment generated by infected cells instigated bacterial expression of stress-induced defense mechanisms including the transport of exogenous glutathione and the activation of the toxin-antitoxin system.
Notably, as part of our screening for novel signatures of infection, we identified a global profile of noncoding transcripts that are candidate small RNAs regulated during human host infection in Haemophilus species. Our data by providing a robust and comprehensive catalogue of regulatory and adaptive responses reflecting the complex crosstalk between the host and invading pathogen, may provide important insights into NTHi pathogenesis and the development of efficacious preventive strategies.
IV
RIASSUNTO
La caratterizzazione delle interazioni ospite-patogeno è fondamentale per lo sviluppo di nuove terapie e vaccini di ultima generazione. Approcci classici prevedono l'utilizzo di linee cellulari trasformate e/o modelli animali che non possono riflettere la complessità e la risposta dell'ospite umano. Il batterio Gram-negativo nontypeable Haemophilus influenzae (NTHi) risiede comunemente come commensale nel rinofaringe umano da dove può diffondere ai tessuti adiacenti provocando un ampio spettro di malattie, tra cui otite media, COPD, fibrosi cistica e bronchite. La capacità di colonizzazione di NTHi dipende dalla sua abilità di aderire ed adattarsi alla mucosa delle vie respiratorie, che ha la funzione di prima linea difensiva contro gli agenti patogeni del tratto respiratorio. Durante le infezioni opportunistiche, la colonizzazione è seguita da un attraversamento paracellulare della barriera epiteliale o dall’invasione di cellule non fagocitiche ed epiteliali. Tuttavia gli eventi temporali associati ad una colonizzazione produttiva non sono stati ancora completamente caratterizzati.
Recenti miglioramenti delle tecniche di ingegneria tissutale in vitro, tra cui lo sviluppo di colture cellulari primarie differenziate e modelli cellulari 3D organotipici, hanno aumentato significativamente la nostra comprensione della patogenesi microbica fornendo rappresentazioni fisiologicamente rilevanti di tessuto delle vie aeree superiori umani. La combinazione di queste tecniche con le tecnologie di sequenziamento di nuova generazione è un approccio concettualmente innovativo per studiare le alterazioni del trascrittoma durante un’infezione. Il sequenziamento tramite RNA-seq offre la possibilità di analizzare simultaneamente il trascrittoma di ospite e patogeno, superando le limitazioni tecniche relative alla separazione dei campioni eucariotici da quelli procariotici.
In questo studio l'epitelio ciliato bronchiale umano è stato ricostituito in vitro utilizzando cellule epiteliali bronchiali primarie ed usato come modello di infezione per NTHi. Successivamente, tramite dual-RNA sequencing, sono state analizzate le variazioni dell’espressione genica di entrambi ospite e patogeno. L’acquisizione di un totale di quasi 2,5 miliardi di sequenze ha permesso la costruzione di mappe ad alta risoluzione del trascrittoma di NTHi durante l'infezione dell’epitelio, la caratterizzazione dell’adattamento metabolico nonché dei pathways di adattamento allo stress cellulare. Nella fase iniziale della colonizzazione è stata osservata l’adesione di NTHi alle cellule ciliate. L’analisi temporale del trascrittoma dell’epitelio ha evidenziato il rimodellamento del citoscheletro, in particolare come riarrangiamento della rete dei filamenti intermedi. Nelle fasi successive dell’infezione, quando i batteri iniziano ad essere internalizzati, NTHi down-regola il metabolismo centrale e aumenta l'espressione di trasportatori specifici per alcuni substrati disponibili nel nuovo ambiente. Contemporaneamente l'ambiente ossidativo generato dalle cellule infettate induce l’espressione batterica di meccanismi di difesa dallo stress fra cui il trasporto di glutatione esogeno e l'attivazione dei sistemi tossina/anti-tossina.
Abbiamo inoltre identificato una serie di nuovi small RNAs batterici fortemente regolati durante l'infezione. Questo lavoro fornisce la caratterizzazione dei trascrittomi dell’ospite e del patogeno nelle prime fasi d’infezione, e suggerisce lo sviluppo di strategie mirate per prevenire le infezioni di NTHi.
This work was conducted at Novartis Vaccines, Siena in In Vitro Cell Biology Group, headed by Dr. Marco Soriani. I am very thankful for his welcoming in the lab, providing me with the opportunity to work at the frontier of current vaccine development and cutting-edge technologies, but most importantly for always being there to help. I equally thank Prof. Christoph Tang who has awarded me with Marie Curie Fellowship and allowed me to perform my thesis project under the framework ofEuropean Community's Seventh Framework Programme “EIMID ITN” [European Institute of Microbiology and Infectious Diseases Initial Training Network, FP7-PEOPLE-2010-264388], as well as his enriching collaboration and hosting me in this laboratory in University of Oxford.
I am extremely grateful to my supervisor, Dr. Alfredo Pezzicoli for his guidance over the course of my PhD, for his never-wavering optimism, giving me the initiative to grow as an independent research scientist and above all, for teaching me how much fun microscopy can be. I also thank Prof. Paolo Visca from University of Roma Tre for all the support and valuable advice he has given me during my PhD.
I would like to acknowledge all my lab members (past and present) of the In Vitro Cell Biology group at Novartis, particularly Lucia Lapazio, Maria Valeri, Pasquale Marrazzo, Silvia Rossi Paccani and Alessandra Greco for the friendly atmosphere they created in the lab,their scientific feedback, and moral support during tough days. I am especially grateful to my precious friends Christina Merakou, Tanja Dapa, Cristina Faralla, Magdalena Kasendra, Riccardo Barrile and Giacomo Golfieri for their kindhearted help and care, all the fun we had together, keeping me company in the lab at the weekends and over summer, and for making my PhD experience thoroughly enjoyable. Special thanks to Giuseppe Lofano for his valuable support, as for his inestimable encouragement to me and my work.
I would like to equally thank to my doctoral schools at University Roma Tre and PhD Academy at Novartis Vaccines for their active drive to provide a dynamic, interdisciplinary and stimulating science education. I also feel the urge to thank Rino Rappuoli for being such a pioneering and inspiring leader,for theconstructive discussions we had, and his encouragements to improve my scientific approaches.
To the members of my thesis defense committee, I am thankful for kindly accepting to spend your precious time to judge my work.
Lastly, I really want to thank my special family; my father Zafer, my mother Nevres, and my sister Nukhet, for their continuous and unconditional support, starting from the beginning of my studies to the end of my PhD, for making me to believe in myself and for teaching to give always the best of me.
VI
TABLE OF CONTENTS
ABSTRACT
... III
ACKNOWLEDGEMENTS
... V
LIST OF ABBREVIATIONS
... X
LIST OF FIGURES
... VIII
LIST OF TABLES
... IX
RIASSUNTO
... IV
TABLE OF CONTENTS
... VI
1
INTRODUCTION ... 1
1.1 HAEMOPHILUS INFLUENZAE ... 11.1.1 NTHi: emergence of a significant human pathogen ... 2
1.1.2 Otitis Media (OM) ... 4
1.1.3 Chronic Obstructive Pulmonary Disease (COPD) ... 5
1.1.4 NTHi: host-pathogen interactions ... 6
1.2 TISSUE ENGINEERING FOR RECONSTITUTING IN VITRO CORRELATES OF HUMAN TISSUE ... 11
1.2.1 Structure and cellular organization of human tracheo-bronchial epithelium ... 12
1.2.2 In vitro tissue culture systems to study pathogen interactions with human airway epithelium ... 14
1.3 HOST-PATHOGEN INTERACTIONS ... 16
1.3.1 Monitoring interactions at host-pathogen interface: an emerging theme ... 16
1.3.2 Transcriptomic approaches for studying host-pathogen cross-talk ... 17
1.3.2.1 Array-based methodologies ... 18
1.3.2.2 Next-generation sequencing ... 22
AIMS OF THE STUDY ... 25
2
EXPERIMENTAL PROCEDURES ... 26
2.1 CALU-3CELL-LINE ... 26
2.2 PRIMARY AIRWAY EPITHELIAL CELLS ... 27
2.3 BACTERIAL CULTURE AND TIME-COURSE INFECTION OF CALU-3 AND WD-NHBECELLS... 28
2.4 ISOLATION OF TOTAL RNA FROM INFECTED CELLS ... 29
2.6.1 Bioinformatics ... 34
2.6.2 Clustering and Enrichment Analysis ... 34
2.7 REAL-TIME QUANTITATIVE REVERSE-TRANSCRIPTION-PCR ... 34
2.8 IMMUNOFLUORESCENCE MICROSCOPY... 35
2.9 ELECTRON MICROSCOPY ... 35
2.10 WESTERN BLOTTING ... 35
2.11 DETERMINATION OF CYTOKINE/CHEMOKINE CONCENTRATION ... 36
2.12 STATISTICAL ANALYSIS ... 36
3
RESULTS ... 37
3.1 NTHI INTERACTIONS WITH HUMAN BRONCHIAL EPITHELIUM IN VITRO ... 37
3.1.1 NTHi forms biofilms on airway epithelia ... 37
3.1.2 NTHi preferentially targets ciliated cells of primary human bronchial epithelium ... 38
3.2 DUAL RNA-SEQUENCING OF NTHI-INFECTED WD-NHBE CELLS ... 41
3.3 TRANSCRIPTOME ANALYSIS OF BRONCHIAL EPITHELIUM DURING NTHI INFECTION ... 42
3.3.1 The human host cell response to NTHi infection ... 42
3.3.2 Functional characterization of host transcriptome signatures ... 49
3.4 NTHI WHOLE-TRANSCRIPTOME ANALYSIS DURING INFECTION OF BRONCHIAL EPITHELIUM ... 53
3.4.1 Genome-wide NTHi transcriptome map during infection ... 53
3.4.2 Gene repertoire of NTHi during host infection ... 54
4
DISCUSSION ... 62
5
CONCLUDING REMARKS ... 65
6
BIBLIOGRAPHY... 66
VIII
LIST OF FIGURES
FIGURE 1.MORPHOLOGICAL CHARACTERISTICS OF NONTYPEABLE HAEMOPHILUS INFLUENZAE. ... 2
FIGURE 2.EPIDEMIOLOGY AND DISTRIBUTION OF H. INFLUENZAE SEROTYPES POST TYPE B VACCINATION. ... 3
FIGURE 3.SIGNALING PATHWAYS BY WHICH NTHI INDUCES INFLAMMATORY RESPONSES FROM EPITHELIAL CELLS. ... 8
FIGURE 4.MODEL OF NTHI COLONIZATION AND INVASION OF EPITHELIAL CELLS... 10
FIGURE 5.CELL TYPES COMPRISING THE HUMAN TRACHEAL AND BRONCHIAL AIRWAY EPITHELIUM. ... 13
FIGURE 6.A COMMON HOST-TRANSCRIPTIONAL RESPONSE TO PATHOGENS. ... 21
FIGURE 7.COMPARISON BETWEEN PROBE-BASED TECHNIQUES AND RNA-SEQ APPROACH FOR TRANSCRIPTOMICS. ... 23
FIGURE 8.ESTIMATION OF MINIMAL SEQUENCING DEPTH REQUIRED FOR DUAL HOST-PATHOGEN RNA-SEQ. .... 24
FIGURE 9.IN VITRO CALU-3 MODEL OF POLARIZED EPITHELIUM. ... 26
FIGURE 10.DIFFERENTIATION SCHEDULE OF PRIMARY NHBE CELLS AT AIR-LIQUID INTERFACE. ... 27
FIGURE 11.MUCOCILIARY PHENOTYPE MARKERS ARE PRESENT IN THE RECONSTITUTED IN VITRO HUMAN BRONCHIAL EPITHELIUM. ... 28
FIGURE 12.OVERVIEW OF THE SCRIPTSEQ V2RNA-SEQ LIBRARY PREPARATION METHOD. ... 30
FIGURE 13.ILLUMINA'S SOLEXA SEQUENCING TECHNOLOGY. ... 32
FIGURE 14.DATA ANALYSIS STRATEGY FOR DUAL RNA-SEQ. ... 33
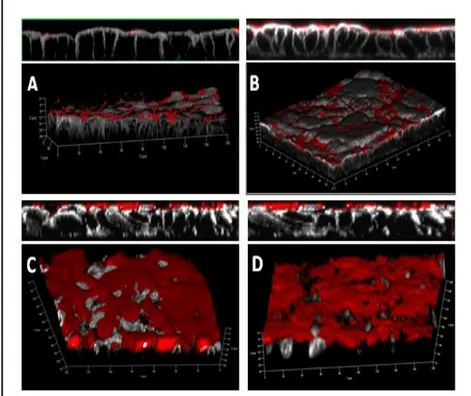
FIGURE 15.POLARIZED AIRWAY EPITHELIAL CULTURES INFECTED WITH NTHI SHOW PROGRESSIVE BIOFILM FORMATION OVER TIME. ... 37
FIGURE 16.SEM ANALYSIS REVEALS THE PRESENCE OF A MATRIX STRUCTURE EMBEDDING BIOFILM -ASSOCIATED BACTERIA. ... 38
FIGURE 17.NTHI PREFERENTIALLY TARGETS CILIATED CELLS OF PRIMARY HUMAN BRONCHIAL EPITHELIUM. . 39
FIGURE 18.ELECTRON MICROSCOPY ANALYSIS CONFIRMS NTHI CILIARY BINDING. ... 40
FIGURE 19.CELLULAR FITNESS DECREASES IN A TIME-DEPENDENT MANNER DURING NTHI INFECTION. ... 40
FIGURE 20.MAPPING ANALYSIS OF NON-RRNA READS OBTAINED FROM RNA-SEQ AGAINST NTHI AND HUMAN GENOMES. ... 41
FIGURE 21.ANALYSIS OF HOST MRNA CHANGES DURING NTHI INFECTION. ... 43
FIGURE 22.SEM MICROGRAPHS REVEAL MORPHOLOGICAL CHANGES IN INFECTED HOST CELL AS INDICATED BY TRANSCRIPTOME SIGNATURES. ... 46
FIGURE 23.HEAT-MAP PROFILES OF NOVEL SNORNAS, MICRORNAS AND SCARNAS IDENTIFIED TO BE DIFFERENTIALLY EXPRESSED DURING NTHI INFECTION IN HOST CELLS ... 48
FIGURE 24.FUNCTIONAL CHARACTERIZATION OF HOST TRANSCRIPTOME SIGNATURES. ... 50
FIGURE 25.BASOLATERAL CYTOKINE/CHEMOKINE SECRETIONS INDUCED FOLLOWING NTHI INFECTION. ... 51
FIGURE 26.VALIDATION OF COMPARATIVE RNA-SEQ ANALYSIS. ... 52
FIGURE 27.GENOME-WIDE NTHI TRANSCRIPTOME MAP DURING 72 H OF HOST INFECTION. ... 53
FIGURE 28.TEMPORAL EXPRESSION DYNAMICS OF NTHI DURING HOST INFECTION. ... 55
FIGURE 29.EXPRESSION PROFILE OF SELECTED VIRULENCE FACTORS AND VACCINE CANDIDATES. ... 57
FIGURE 30.EXPRESSION DYNAMICS OF SELECTED NTHI TRANSPORT MACHINERIES. ... 58
FIGURE 31.INCREASED EXPRESSION OF STRESS-INDUCED BACTERIAL FACTORS DURING INFECTION. ... 60
FIGURE 32.ARTEMIS VIEWER OF HIS LEADER ELEMENT AND GCVB... 61
FIGURE 33:PER BASE SEQUENCE ILLUMINA QUALITY CONTROL ... 93
TABLE 1.PREDOMINANT DISEASES AND THEIR BURDEN CAUSED BY NTHI ... 4
TABLE 2.COMMON CELL TYPES USED IN THE GENERATION OF LUNG TISSUE MODELS ... 14
TABLE 3.EXPERIMENTAL APPROACHES FOR MICROARRAY ANALYSIS ... 19
TABLE 4.SUMMARY OF ILLUMINA RNA-SEQ MAPPING DATA. ... 42
TABLE 5.INGENUITY PATHWAY ANALYSIS – TOP MOLECULAR AND CELLULAR FUNCTIONS SUMMARY ... 44
TABLE 6.INGENUITY PATHWAY ANALYSIS – TOP CANONICAL PATHWAYS SUMMARY ... 45
TABLE 7.SELECTED PAIRED INGENUITY PATHWAY ANALYSIS OF HOST FUNCTIONS SHOWING CHANGES IN CELLULAR ASSEMBLY AND ORGANIZATION MODULE. ... 46
TABLE 8.SELECTED NTHI CANDIDATE SMALL NON-CODING REGULATORY RNAS WITH HIGH TRANSCRIPTIONAL ACTIVITY DURING HOST INFECTION IDENTIFIED BY RNA-SEQ ... 61
TABLE 9.PRIMER SEQUENCES USED IN QRT-PCR ... 78
TABLE 10.ANTIBODIES USED IN CLSM AND WB ANALYSIS ... 79
TABLE 11.ILLUMINA HISEQ FLOWCELL DESIGN,2-LANE RAPID RUN ... 89
TABLE 12.ILLUMINA HISEQ FLOWCELL DESIGN,8-LANE FLOWCELL FULL-RUN ... 89
TABLE 13.SEQUENCE ANALYSIS PRIMARY RUN METRICS-ILLUMINA SEQUENCING RAW DATA (FULL RUN) ... 91
TABLE 14.SEQUENCE ANALYSIS PRIMARY RUN METRICS-ILLUMINA SEQUENCING RAW DATA (RAPID RUN) ... 92
TABLE 15.RAW DATA FOR EUKARYOTIC ANALYSIS ... 95
X
LIST OF ABBREVIATIONS
AECII alveolar epithelial cells type II
ALI air-liquid interface
AOM acute otitis media
AP1 activator protein-1
ARPC2 actin related protein 2/3 complex ATCC american type culture collection
BAL bronchoalveolar lavage
BEBM bronchial epithelial basal growth medium
BH Benjamini-Hochberg
BHI brain heart infusion
Bis-Tris 2,2-Bis(hydroxymethyl)-2,2',2"-nitrilotriethanol
BSA bovine serum albumin
CAM cell-adhesion molecule
cAMP cyclic adenosine monophosphate
CDH cadherin
CEACAM1 carcinoembryonic antigen family 1
CF cystic fibrosis
ChoP phosphorylcholine
CILP cartilage intermediate layer protein
CK cytokeratin
CLDN claudin
CLSM confocal laser scanning microscopy
CO2 carbon dioxide
COL7A1 collagen type VII
COPD chronic obstructive pulmonary Disease
CRP C-reactive protein
CXCL chemokine (C-X-C motif) ligand DAPI 4',6-diamidino-2-phenylindole
DAVID database for annotation, visualization and integrated discovery DE differentially expressed
DMEM Dulbecco's modified Eagle's medium DNABII DNA-binding family II
ECL enhanced chemiluminescence
ECM extracellular matrix
EEA1 early endosomal antigen 1 ENaC epithelial sodium channel
EPPK epiplakin
ER endoplasmic reticulum
Erk extracellular-signal-regulated kinases
ET eustachian tube
FBN2 fibrillin 2
FBS fetal bovine serum
FLG filaggrin
FoM Figure of Merit
GAPDH glyceraldehyde 3-phosphate dehydrogenase GCSF granulocyte-colony stimulating factor GEO gene expression omnibus
GO gene ontology
H hour
HARV high-aspect rotating vessel
Hib Haemophilus influenzae type b
HLA human leukocyte antigen
HMCN1 hemicentin
HMW high-molecular-weight protein ICAM-1 intercellular adhesion molecule-1
IF intermediate filaments
IFN interferon
IgA immunoglobulin A
K-cells Kulchitsky’s cells
kDa kilodaltons
KEGG Kyoto encyclopedia of genes and genomes
KRT keratin
LAMB3 laminin β3
LAMP lysosomal-associated membrane protein3
LDH lactate dehydrogenase
LOS lipooligosaccharide
LPS lipopolysaccharide
MAPK mitogen activated protein kinase
MEV multiexperiment viewer
MMP matrix metalloproteinase
mRNA messenger RNA
MUC mucin
MYL myosin light chain
NAD+ nicotinamide adenine dinucleotide Neu5Ac N-acetylneuraminic acid NGS next generation sequencing NHBE normal human bronchial epithelial NTHi nontypeable Haemophilus influenzae OAS oligoadenylate synthetase
OD optical density
OMP outer membrane protein
OMV outer membrane vesicle
O/N overnight
ORF open reading frame
PCR polymerase chain reaction
Pg picogram
PKCϵ protein kinase C epsilon
PKR protein kinase R
PML promyelocytic leukaemia protein PRP polyribitol ribose phosphate
PTS fructose-specific phosphotransferase system qRT-PCR quantitative real-time PCR
RNA-seq RNA (deep) sequencing
RPKM kilobase of target per million mapped reads
RPTN repetin
rRNA ribosomal RNA
RSV respiratory syncytial virus
RWV rotating wall vessel
scaRNA small Cajal-body specific RNA SEM scanning electron microscopy SMS single-molecule sequencing snoRNA small nucleolar RNA
SPON2 spondin 2
SPRR small proline rich protein
sRNA small RNA
STLV slow- turning lateral vessel
TA toxin-antitoxin
TEER transepithelial electrical resistance TEM transmission electron microscopy TGF-β transforming growth factor-beta
TJs tight junctions
TLR toll-like receptor
TNF-α tumour necrosis factor-alpha
TRAP tripartite ATP-independent periplasmic
tRNA transfer RNA
TTSS type III secretion system
TUNEL terminal transferase-mediated dUTP nick end labeling
CHAPTER I: INTRODUCTION
1
1 INTRODUCTION
1.1 Haemophilus influenzae
H. influenzae is a Gram-negative, coccobacillary, facultatively anaerobic bacterium belonging to the
Pasteurellaceae family (Fig. 1). The name of the genus, Haemophilus is derived from the Greek words meaning ‘blood-loving’ and refers to the fastidious nature of this organism with specific dependence on haeme-related molecules for growth under aerobic conditions. Indeed, laboratory in vitro growth requires the availability of nicotinamide adenine dinucleotide (NAD+) and haemin provided through lysed blood cells. H.
influenzae strains are serologically classified on the basis of their distinct polysaccharide capsular antigens.
These include encapsulated strains a, b, c, d, e, f and the non-encapsulated strain, nontypeable H. influenzae (NTHi). This bacterium commonly resides in the nasopharynx of most of the healthy human population as a part of the commensal flora, however under certain circumstances such as underlying viral infections, secondary bacterial infections or where host mucosal clearance mechanisms are compromised or impaired, it can disseminate to local organs to cause a wide spectrum of diseases. Of the capsulated strains, H. influenzae
type b (Hib) is clinically the most important. In infants and young children, Hib is responsible for
life-threatening conditions including pneumonia, bacteremia and acute bacterial meningitis. Prior to the development and use of Hib conjugate vaccines, Hib was the most common cause of invasive bacterial infection and bacterial meningitis in children in the United States. 20-30% of children who suffer from these devastating infections have permanent sequelae, ranging from mild hearing loss to mental retardation. Although the majority of cases of Hib-related morbidity and mortality are due to meningitis and pneumonia, other severe invasive infections caused by Hib include epiglottitis, osteomyelitis, septic arthritis, septicaemia, cellulitis, and pericarditis [1].
Hib conjugate vaccine was introduced in the late 1980s, the routine use being established in 1985. Hib vaccination has been largely effective as the widespread use have nearly eradicated invasive Hib disease in children in countries where the vaccine is widely used. The conjugate vaccines induce bactericidal antibodies to capsular polysaccharide [polyribitol ribose phosphate [(PRP)], a critical virulence factor that facilitates hematogenous dissemination. Like other polysaccharides, the PRP of the Hib capsule shares the common immunological property of T-independent B-cell activation, which is associated with poor or absent immunogenicity when administered as a vaccine in infancy and a failure to induce immunological memory at any age. Therefore, a key step in the development of the vaccine was the conjugation of PRP to a protein carrier, facilitating T-cell recruitment and enabling the induction of antibody responses in infants at the peak age incidence of Hib infection [2]. As well as inducing protective humoral immune responses, vaccination also markedly reduced circulating strains of Hib in the population by reducing nasopharyngeal carriage. The reduction of circulating strains in the population results in a herd effect, contributing significantly to the
efficacy of the vaccine. However, these alterations in the nasopharyngeal colonization patterns had a profound impact on the ecology of respiratory tract colonization by H. influenzae and the epidemiology of related infections.
Figure 1. Morphological characteristics of nontypeable Haemophilus influenzae. A) Scanning electron micrograph showing coccobacillary cells, scale bar = 0.5 µm and B) transmission electron micrograph demonstrating the biofilm formed by H. influenzae, scale bar = 2 µm (Sources: Ohio State University Center for Clinical and Translational Science; Gallaher et al, 2006).
1.1.1
NTHi: emergence of a significant human pathogen
In recent years, a large number of surveys have focused on monitoring invasive H. influenzae disease in the Hib conjugate vaccine era by means of different study designs throughout the world. During these studies, infections caused by encapsulated non-type b serotypes, especially serotypes a and f, have been observed in selected geographic regions such as Canada, Germany and Sweden [4-12]. Of high importance,these surveys have revealed a shift in the distribution of capsular serotypes with nontypeable strains replacing type b strains as the most common bloodstream isolates, identifying NTHi strains as the predominant cause of invasive infection in the post vaccination era (Fig. 2). The shift in serotype distribution resulting from prevention of Hib disease in infants and children was accompanied by a shift in the peak age incidence. This was reflected in the cases of most common disease manifestation of invasive H. influenzae infection being bacteremia caused by nontypeable strains in adult population.
The heightened surveillance of NTHi have also led to the understanding of the increasing importance of NTHi as a pathogen in infections of the upper and lower respiratory tract. Considering the niche replacement taking place, continued surveillance procedures to carefully track the incidence, strain distribution, and clinical manifestations of H. influenzae disease remains critical to health intervention strategies.
CHAPTER I: INTRODUCTION
Figure 2. Epidemiology and distribution of H. influenzae serotypes post type b vaccination. NTHi is replacing H. influenzae type b niche in the post vaccination era - all cases (Source: Health Protection Agency, UK. September 2011)
NTHi is a common commensal of the human respiratory tract mucosa and occupies this niche as its natural habitat. The rate of nasopharyngeal colonization increases from approximately 20% during the first year of life to over 50% by the age of 5-6 years, and remains high through adulthood. The mechanism of pathogenesis of infection by NTHi is predominantly by contiguous spread, with migration of bacteria from the nasopharynx to adjacent structures, including sinuses, the middle ear, the trachea, and lower airways. NTHi is responsible for a wide spectrum of diseases including sinusitis, otitis media, exacerbations of chronic obstructive pulmonary disease (COPD), cystic fibrosis and bronchitis. The global burden of these non-invasive infections with NTHi and non-bacteraemic pneumonia is reported to be very high (Table 1). Transmission generally occurs by airborne droplets or by direct contact with respiratory secretions. Colonization and infection are mediated by multiple virulence factors, including adhesins, nutrient uptake systems, molecules that resist host factors, and others. Biofilm formation by NTHi in the middle ear and the airways of cystic fibrosis patients is important for the pathogenesis of infection, particularly chronic and recurrent infections that characterize these clinical settings.
0 100 200 300 400 500 600 700 800 900 1000 b NTHi a, c, d, e, f Num b er o f ca se s fo r 1 0 0 ,0 0 0 p o p u la ti o n
Table 1. Predominant diseases and their burden caused by NTHi (Modified from: Van Eldere et al, 2014) )
BURDEN Non-invasive disease
Otitis media 55-95% cases in children
Bacterial conjunctivitis 44-68% of cases in children, 25% of cases in adults
Bacterial sinusitis 41% of cases
Exacerbations of COPD >90% during an acute exacerbation Persistent bacterial bronchitis 81% of cases in children
Cystic fibrosis Up to 30% of sputum samples
Lower respiratory infections BAL: 20–94% of cases with community-acquired
pneumonia*; lung aspirate: 15–40% of cases with pneumonia; blood culture: 2–10% of cases with bacteraemic pneumonia Invasive disease†
Neonatal infections (sepsis and meningitis) 1.6-4.9 per 100 live births
Epiglottis and bone, joint, skin, and soft tissue infections have been reported but at a low incidence. BAL= bronchoalveolar lavage.*Geographically variable. †Usually associated with underlying comorbidity (40–70% in children; 60–80% in adults).
1.1.2
Otitis Media (OM)
Otitis media is the most frequently diagnosed childhood disease requiring medical assistance and clinic visits, affecting more than 75% of children younger than 3 years [14]. Acute otitis media (AOM) is the inflammation of the middle ear (the cavity between the eardrum and the inner ear), and episodes are characterized by fever, ear pain, and in severe cases, discharge from the ear. The disease presents as AOM or chronic infections, depending on the host’s ability to thoroughly clear the infection. Pathogenesis is a result of pathogen-induced inflammatory damage of the middle ear which can lead to significant or in some cases total hearing loss. The gold standard for an etiologic diagnosis is culture of middle ear fluid and requires tympanocentesis which is an invasive procedure and therefore is not routinely performed.
OM is a complex, multifactorial and polymicrobial disease caused by a number of viruses and bacteria [15]. Three bacteria are isolated in the majority of clinically-presented cases of OM: Streptococcus pneumoniae is isolated from approximately 40% of cases, NTHi from approximately 20-30% of cases, and Moraxella
catarrhalis from 10-20% of cases. Based on results of middle ear fluid cultures obtained by tympanocentesis
as part of clinical trials, NTHi is one of the most common causes of otitis media, accounting for 25 to 35% of episodes of acute OM [16]. Globally, up to 330 million people suffer from recurrent and chronic otitis media, and in developing countries complications, including chronic suppurative OM, are commonly observed [17].
CHAPTER I: INTRODUCTION
Biofilms are increasingly recognized as contributing factors to various disease pathogenesis including OM, cystic fibrosis and other respiratory tract diseases associated with chronic bacterial infections. The hypothesis that NTHi grows as biofilms emerged from the initial observations in which monthly sputum cultures from COPD patients revealed intermittent negative cultures despite continuous colonization by the same isolate proven by molecular typing, suggesting that the organism is present despite the negative sputum cultures [18]. Similarly, Rayner and colleagues have reported the presence of H. influenzae mRNA in a significant percentage of culturally sterile middle ear aspirates of children with OM, establishing the presence of viable, metabolically active, intact organisms in some cases of culture-negative OM with effusion [19]. These observations indicated that the culture methods designed to detect planktonic growth may be less sensitive for detecting slow-growing bacteria in biofilms. Indeed, in later studies NTHi biofilms were reported to be directly visualized in middle ear aspirates from children with OM [20], and additionally biofilms structures were demonstrated to form in the middle-ear chamber of experimentally infected chinchillas [21]. Biofilms serve as an immune evasion mechanism of pathogenic bacteria confirming protection against antibody-mediated targeting as well as protection from antibiotic therapy, hence promoting persistent and chronic infections. Interestingly, there are several lines of evidence that indicate that these biofilms are polymicrobial and are constituted by the bacterial communities colonizing the airway mucosal surfaces [22].1.1.3
Chronic Obstructive Pulmonary Disease (COPD)
NTHi is the most common bacterial pathogen associated with airway infection in COPD, both in stable disease and during exacerbations. COPD is characterized by the progressive development of airflow limitation that is not fully reversible [23]. The term COPD encompasses chronic obstructive bronchitis, with obstruction of small airways, and emphysema, with enlargement of air spaces and destruction of lung parenchyma, loss of lung elasticity, and closure of small airways [24]. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles and gases, most frequently cigarette smoke, as well as to infecting respiratory pathogens. COPD is commonly observed in adults aged 40 years or older, particularly in the smoking population. It is the second most prevalent respiratory illness after asthma, and the fourth leading cause of mortality in the USA.
As one of the leading aetiological agents, NTHi is the predominant bacterium colonizing the airways in COPD patients and is found in the lower respiratory tract of ~30% of individuals with COPD at any time [25-28]. In addition to colonization during clinically stable periods, acquisition of new strains of NTHi is an important cause of lower respiratory tract infection, resulting in exacerbations of COPD [29]. Together, these findings suggest that persistent or repetitive exposure of the airway to NTHi may contribute to airway inflammation in COPD. Defective immune responsiveness and impaired phagocytosis by alveolar macrophages might provide an immunologic basis for persistence in the airways of adults with COPD [30]. Cigarette smoke induces mucus dysfunction by several mechanisms such as shortening of the airway cilia,
impaired respiratory epithelial ciliogenesis and ciliary abnormalities [31-33], ultimately increasing mucin production, reducing mucus hydration, and decreasing mucus clearance, which might also contribute to airway colonization in COPD patients [34].Several other mechanisms may also allow the infection to persist in the lower airways of patients including tissue invasion [35], antigenic alteration [36] and biofilm formation [37]. On the other hand, NTHi can contribute to COPD progression by inducing neutrophilic influx into the airways, neutrophil necrosis with release of neutrophil elastase and other matrix metalloproteinases, and production of oxygen radicals [38,39]. These mediators can overwhelm the anti-proteinase barrier of the lung and damage airway and alveolar structures, thereby amplifying smoking-induced lung damage.
1.1.4
NTHi: host-pathogen interactions
Successful colonization by NTHi depends on its ability to adhere and adapt to the respiratory tract mucosa, which serves as a frontline defense against respiratory pathogens. Upon entering the respiratory tract, NTHi interacts initially with mucus and are largely eliminated by the mucociliary escalator, which consists of ciliated respiratory epithelial cells and the associated mucus layer. Studies using chinchilla eustachian tube (ET) and middle ear mucosa sections demonstrate that NTHi mediates ascension of the ET from the nasopharynx primarily via adherence to and growth in mucus overlying the floor region of the tubal lumen. The outer membrane protein (OMP) P5-homologous fimbriae were shown to contribute to this binding as mutants lacking P5 exhibit reduced binding to respiratory mucus [40]. Interestingly, OMPs P2 and P5 were reported to mediate bacterial attachment to a complement of sialylated oligosaccharides within respiratory mucins [41]. More recently, it has been established that P5 binds to a member of the carcinoembryonic antigen family (CEACAM1), a glycoprotein expressed by respiratory epithelial cells and up-regulated during inflammation [42]. In vitro studies using isolated epithelial cells indicate that a range of other bacterial factors contribute to NTHi adherence. These include type IV pilus, expressed by selected NTHi isolates and in particular the major pilin subunit protein, PilA, which mediates adherence to respiratory epithelium [43,44]. Surface-exposed high-molecular-weight proteins HMW1 and HMW2 are major factors involved in facilitating the colonization of cultured human epithelial cells and the expression of HMW proteins is regulated by phase variation suggesting the ability of the organism to vary between states associated with efficient adherence versus effective immune evasion [45,46]. Experiments involving modification of the surface of Chang conjunctival cells indicate that HMW1 recognizes a glycoprotein receptor containing N-linked oligosaccharide chains with terminal sialic acid in an α-2,3 configuration, whereas the receptor of HMW2 remains uncharacterized [47]. Approximately 25% of nontypeable strains lack proteins belonging to the HMW1/HMW2 family of adhesins, however, nearly all such strains remain capable of efficient adherence to cultured human epithelial cells. This binding is commonly mediated by Hia protein which has sequence similarity to another H. influenzae adhesin, Hsf, associated with the formation of short pilus-like structures termed fibrils [48]. On the other hand, mutants deficient in expression of HMW1 and HMW2 or Hia still
CHAPTER I: INTRODUCTION
remain capable of low-level adherence to cultured epithelial cells, and this is attributed to the presence of Hap protein. Hap is an autotransporter protein that undergoes autoproteolytic cleavage, with release of the adhesive passenger domain, Hap(s), from the bacterial cell surface. Hap has been demonstrated to promote bacterial adherence to extracellular matrix proteins fibronectin, laminin, and collagen IV and Hap-mediated adherence is enhanced by inhibition of autoproteolysis [49].The molecular mechanisms underlying the pathogenesis of NTHi-induced infections involve activation of NF-κβ, a transcriptional activator of multiple host defense genes involved in immune and inflammatory responses (Fig. 3). This is mediated by binding of OMP P6 to cell surface TLR2 (Toll Like Receptor 2) and lipooligosaccharide (LOS) to the LPS receptor TLR4 (Toll Like Receptor 4) [50,51]. TLR ligation activates the transcription factor to translate various cytokine and mucin genes [52]. NTHi strongly activates NF-κβ in human epithelial cells via two distinct signaling pathways; NF-κβ translocation-dependent and -independent pathways. The NF-κβ translocation-dependent pathway involves activation of NIK (NF-κβ Inducing Kinase)-IKKα/β (I-κβ Kinases)-complex leading to I-κβ-α phosphorylation and degradation, whereas the NF-κβ translocation-independent pathway involves activation of MKK3/6-p38MAPK (Mitogen Activated Protein Kinase) pathway. MKK6 is a common activator of p38-αa and p38-β, whereas MKK3 activates only p38-α [53]. Bifurcation of NTHi-induced NIK-IKK-α/β- I-κβ-α and MKK3/6-p38MAPK signaling may occur at TAK1 (TGF-Beta Activated Kinase-1). TLR2 is required for NTHi-induced NF-κβ activation. In addition, several key inflammatory mediators including IL-β, IL-8, and TNF-α (Tumor Necrosis Factor-β) are upregulated by NTHi.
NTHi utilizes the TGF-β (Transforming Growth Factor-Beta)-SMAD signaling pathway together with the TLR2-MyD88-TAK1-NIK-IKK-β/ɣ-I-κβ-α pathway to mediate NF-κβ-dependent Muc2, mucin transcription. TGF-β initiates signaling through the ligand-dependent activation of a heteromeric complex of TGF-β RII and TGF-β RI (Type-II and Type-I Receptors). The TGF-β RII kinase then phosphorylates the TGF-β RI in a conserved GS domain (Glycine-Serine domain), resulting in activation of the TGF-β RI. The activated Type-I receptor subsequently recognizes and phosphorylates the R-SMAD (Receptor-activated SMADs), including SMAD3. This causes dissociation of R-SMAD from the receptor, stimulates the assembly of a heteromeric complex between the phosphorylated R-SMAD and the Co-SMAD, SMAD4, and consequently induces the translocation of the SMAD complex to the nucleus, where the SMAD complex regulates the expression of target genes by direct interaction and functional cooperation with other transcription factors, such as NF-κβ [54]. The functional cooperation of NF-KappaB (p65/p50) with SMAD3/4 positively mediates NF-κβ-dependent Muc2 transcription. Overproduction of mucin and the strong inflammatory response induced by NTHi damage the epithelium, and highly contribute to the airway pathology observed in COPD and otitis media.
Figure 3. Signaling pathways by which NTHi induces inflammatory responses from epithelial cells. Schematic representation of NTHi-induced signal transduction pathways involved in NF-kB activation in human epithelial cells (Source: Pathway Central, Qiagen)
As a pathogen colonizing the ciliated respiratory epithelium, NTHi is capable of inducing ciliotoxicity on mucosal surfaces. At least two H. influenzae factors are known to influence ciliotoxicity and these include lipopolysaccharide (referred to as lipooligosaccharide or LOS in H. influenzae due to lacking O-side chains) and protein D. Purified LOS has been reported to causes ciliostasis, loss of cilia, and eventual sloughing of cells [55]. On the other hand, protein D is a highly conserved, surface-exposed 42 kDa lipoprotein. Interestingly, Janson and coworkers compared isogenic protein D-expressing and protein D-deficient strains in assays with human adenoid tissue in culture and demonstrated that protein D was essential for maximal impairment of ciliary activity and damage to ciliated cells [56]. Recently, with the use primary cultures of ciliated bovine bronchial epithelial cells Bailey and colleagues have shown that NTHi decreases cilia beating via protein kinase C epsilon (PKCϵ) activation and auto-down-regulation of PKCϵ leads to detachment of ciliated cells [57].
CHAPTER I: INTRODUCTION
Once established on the host mucosal surface, bacteria face the challenge of persisting. Persistence requires evasion of the immune system, including both non-specific and specific host responses. The predominant immunoglobulin produced by mucosal tissues is IgA, a molecule that participates in host defense by inhibiting microbial adherence and invasion, inactivating bacterial toxins, and mediating antibody dependent cytotoxicity [58]. Along with several other bacterial species, NTHi harbors an extracellular endopeptidase called IgA1 protease, which cleaves the hinge region of the serum and secretory forms of IgA1, and releases the antigen-binding Fab domains from the Fc portion of the molecule. As a result of cleavage, the agglutination activity of both free and antigen-bound IgA1 is eliminated [59,60]. As previously described in Section 1.1.2, formation of bacterial aggregates and microcolonies that mature into a biofilm is an important and intensely studied form of NTHi persistence in vitro and in vivo [61-64]. Biofilm state of growth is considered to be a significant virulence factor, conferring resistance to natural bacteriostatic compounds such as lactoferrin, lysozyme, and peroxidases, which are present in human respiratory secretions and are important components of the innate immune system. In addition, bacterial aggregates block the access of antibodies to individual organisms, thereby hindering antibody-dependent killing and clearance by phagocytosis.Although initially considered as an extracellular pathogen, numerous studies suggest that NTHi is able to pass between cells and invade the subepithelial space [65,66]. In opportunistic infections, colonization is followed by either a paracellular route across the epithelial barrier or invasion of non-phagocytic and epithelial cells (Fig. 4). Indeed, wild-type NTHi clinical isolates have been demonstrated in vitro and in vivo to adhere and invade a number of cell types mainly by macropinocytosis [67-72]. Garmendia and colleagues have demonstrated that NTHI in mouse alveolar macrophages, in human alveolar epithelial cells, and in primary normal human bronchial epithelial cells were trafficked by the endolysosomal pathway [73,74]. NTHi was detected within vesicles positive for early endosomal antigen 1 (EEA1), a marker of early endosomes, and in vesicles positive for LAMP1, LAMP2, or CD63, which are markers of late endosomes and lysosomes. In addition, few studies have examined the role of autophagy in NTHi infection. These studies suggest that NTHi does not co-localize with autophagy marker LC3 in alveolar epithelial cells, or in cells treated with rapamycin, a known autophagy inducer. Eukaryotic secretion is the pathway by which proteins and lipids are modified and packaged through the endoplasmic reticulum (ER) and Golgi for intracellular or extracellular destinations. Certain bacterial pathogens engage this pathway to reach their destination or to form a replicative intracellular niche [75]. Although little is known about the role of eukaryotic secretion in NTHi infections, Morey et al. has shown that NTHi do not co-localize with resident Golgi proteins GM130 and TGN46 in alveolar epithelial cells.
Figure 4. Model of NTHi colonization and invasion of epithelial cells. NTHi adheres to mucus and unidentified epithelial cells. NTHi aggregates mature into a biofilm composed of bacterial and host components. Bacteria are observed within, between, and beneath epithelial cells in vitro and ex vivo. NTHi is internalized by macropinocytosis and is trafficked to vesicles that are positive for endolysosomal markers. It is unclear what role(s) other host internalization and trafficking pathways (indicated by question marks) play, or how these pathways affect NTHI viability (Source: Clementi and Murphy, 2011).
On the other hand, H. influenzae has evolved to employ maximal phase variation, commonly involving structures that facilitate the pathogenesis of disease and serve as targets of the immune response such as LOS, pili, HMW1 and HMW2, and heme receptors. The biosynthesis of LOS involves multiple enzymatic steps and a number of genes, of which lic1A, lic2A, lic3A, lex-2, and lgtC contain long stretches of four-base-pair repeats within their 5’ coding region, that undergo frameshift mutation. Such frame shifts result in production of a protein with a different N-terminus or eliminate protein production altogether. Changes in lic2A and lic3A influence glycotransferase activity hence altering the reactivity with monoclonal antibodies directed against specific LOS oligosaccharide epitopes [77]. Variation in the number of repeat units in lic1A by slipped-strand mispairing alters the alignment of initiation codons with the licA open reading frame creating a translational switch that results in spontaneous phase variation in expression of the choline kinase responsible for addition of phosphorylcholine (ChoP) to the LOS molecule [78]. Variations in the ChoP epitope contributes to persistence, in which ChoP+ variants of NTHi are more sensitive to the bactericidal activity of human serum through binding of C-reactive protein (CRP). Therefore, the ability of NTHi to vary expression of ChoP epitopes is considered as an immune evasion mechanism through which the bacterium can persist on the mucosal surface (ChoP+ phenotype) and to cause invasive infection by evading innate immunity mediated by CRP (ChoP− phenotype) [79]. Added complexity comes from variable expression and availability of certain host cell receptors and matrix proteins. These bacterial and host variations likely promote bacterial evasion of host clearance mechanisms. The Sap transporter and LOS ChoP appear to protect NTHi against
CHAPTER I: INTRODUCTION
human antimicrobial peptides β-defensin and cathelicidin LL-37, respectively, which are important respiratory defense molecules [80,81]. NTHi LOS glycosyltransferase Lic2B activity and the ability to bind host complement inhibitors C4 binding protein, factor H, and vitronectin promote NTHi evasion of complement-mediated killing [82-84].On the host side, the respiratory epithelium is capable of expression a range of inflammatory mediators which stimulate the activation and influx of immune cells including neutrophils, monocytes, macrophages and eosinophils. Mucosal inflammation is beneficial to the host, limiting the spread and facilitating the clearance of pathogenic invading organisms. Yet, this response can also have detrimental effects in certain conditions. For example, influx of neutrophils results in release of neutrophil elastase which can damage epithelial cells, impairing of opsonophagocytosis and stimulating the production of mucus. This can also lead to the impairment of the mucociliary clearance allowing further replication and persistence of colonizing bacteria with a further flux of inflammatory cells [85].
1.2 Tissue engineering for reconstituting in vitro correlates of human tissue
To understand fully how tissues form and function, as well as their pathophysiology, it is crucial to study how cells and tissues behave as parts of whole living organs that are composed of multiple, tightly opposed tissue types that are highly dynamic and variable in terms of their 3D structure, mechanical properties and biochemical microenvironment [86]. Tissue engineering has evolved from the field of biomaterials development and refers to the practice of combining scaffolds, cells, and biologically active molecules into functional tissues. The goal of tissue engineering is to assemble functional constructs that restore, maintain, or improve damaged tissues or whole organs. In recent years, this approach has been applied by various fields of scientific research including pharmaceutical studies for drug development, regenerative medicine and importantly to the study of infectious diseases.
Animal models are undoubtedly indispensable tools in the current scientific setting, in particular for microbiology and immunobiology studies as they provide an in vivo milieu and an immune cell repertoire that significantly enhance our understanding in the context of clinical background and therapeutical interventions. Yet, animal models usually fail to reflect the complexity and response of the human host, and majority of the pre-clinical data produced in animal models are usually not supported by efficacy/clinical trials. In addition, animal models have the disadvantages of related ethical issues and high costs. In the last decade, there has been a plethora of studies with various cellular models with the unique aim of recapitulating the in vivo microenvironment of the modeled organ system to study microbial pathogenesis and ultimately reduce dependency on animal testing. Depending on the pathogen and the stage of pathogenesis under investigation, this can be a skin model, an intestinal, lung, liver, vaginal, nasopharyngeal or neuronal tissue.Most of these studies have relied on analysis of cells, usually transformed/immortalized cell-lines, grown in 2D cell culture
models which involve growing cells as monolayers on solid, impermeable surfaces (plastic or glass) or in uniform suspension. Indeed, 2D monolayers have contributed greatly to our understanding of infectious-disease processes, including the host immune and physiological mechanisms used to defend against viral, bacterial, fungal and parasitic infections [87-93]. Despite these tremendous efforts, it is becoming increasingly evident that the ‘flat biology’ approach with conventional 2D cell culture, in which key phenotypic and functional characteristics are often lost, is not predictive of in vivo tissue responses [94]. One key reason for the loss of differentiation that occurs in monolayers is the dissociation from the native in vivo 3D structure to 2D propagation on flat, impermeable substrates in vitro, which also prevents cells responding to chemical and molecular gradients in three dimensions; reflecting the apical, basal and lateral cell surfaces. Due to the lack of the complexity, and often physiological relevance, of the tissues that are encountered by a pathogen during the natural course of infection in vivo, 2D monolayers may fail to predict the course of infection.
To overcome the inherent limitations associated with 2D monolayers, there has been a shift towards the use of 3D tissue models as high fidelity tools to facilitate the transition from basic cellular research to clinical applications, given that tissues and organs are 3D structures. In contrast to 2D monolayers, 3D cell culture models are modular, and adaptable biomedical systems that range in complexity from a monotypic (single cell type), representing the minimum unit of undifferentiated in vivo tissue, to complex co-culture models that recapitulate both 3D architecture and the multicellular complexity of the parental tissue. The establishment of complex 3D tissue equivalents is principally based on primary (untransformed) cells and organ cultures which retain the ability to differentiate into different cell types when grown in in vitro conditions, and their establishment can be achieved via several methodologies, including spontaneous aggregation in a suspension culture, implantation into 3D matrix scaffolds and culture in transwell systems or in a rotating culture bioreactors [95].
1.2.1
Structure and cellular organization of human tracheo-bronchial epithelium
The human airway epithelium represents a primary site for contact between microbes and their hosts. It is the prime target of various respiratory bacterial pathogens e.g. H. influenzae, S. pneumoniae, M. catarrhalis, N.
meningitides, particularly during colonization, as well many viruses such as influenzae viruses and respiratory syncytial virus (RSV). Several morphologically distinct and specialized cell types comprise the
human trachea-bronchial epithelium, which represents as a pseudo-stratified columnar epithelium. Ciliated cells are terminally differentiated columnar cells and as a component of the mucociliary escalator system, their main function is to remove inhaled particulate matter, including viruses and bacteria which have been trapped in the mucus layer. With a smaller percentage, there are also ciliated-secretory cells that bear fully developed cilia and contain mucus granules. Secretory cells comprise 15-25% of the bronchial epithelium and are present in several types including goblet cells, the main producers of airway mucus and predominant
CHAPTER I: INTRODUCTION
secretory cell type in the larger airways and neuroendocrine cells (Kulchitsky’s cells or K-cells) that contain amines and peptide hormones. Clara cells are the predominant cell type in the bronchioles and produce the surfactant apoproteins A and B, as well as secretory leukoprotease inhibitors. Whereas all the mentioned cell types form the epithelial surface of the airways, basal cells reside deep in the trachea-epithelium and are considered as the stem cell or progenitor cells of the bronchial epithelium (Fig. 5).Figure 5. Cell types comprising the human tracheal and bronchial airway epithelium. A) Graphical representation of the structure and cellular organization of bronchial epithelium, constituted by highly specialized cell types including ciliated cells with beating activity and goblet cells which secrete mucus, and is decorated by junction complexes that exhibit host defense activities (Source: Wadsworth et al, 2012) B) Light microscopy of hematoxylin and eosin stained bronchial airway epithelium showing the abundance of goblet cells (G), ciliated cells (C), basal cells (B). Bar = 50 µm (Source: Oakland et al, 2012).
Bronchial epithelial cells constitute part of the non-specific immune system by accomplishing a physical barrier with secretory and ciliary functions, and hence the integrity of the epithelial function is crucial for host defense. In term of integrity, the bronchial epithelium forms a continuous later, with distinct apical and basolateral surfaces maintained by several cell-cell adhesion mechanisms. The desmosomes (macula adherens) and the intermediate junctions (adherence junctions, AJs or zonula adherens) are involved in cell-cell adhesion. The tight junctions, TJs (zonula occludens, ZO) located at the apical-most region of the lateral surface and define the boundary between the apical and basolateral domains. Tight junctions play pivotal roles in tissue integrity and maintenance of cell polarity, regulating the paracellular passage of molecules as well as pathogens [97]. The epithelial cells are anchored to the basement membrane by hemidesmosomes and other focal adhesion molecules (e.g. integrins, vinculin, talin, radixin) that form focal-adhesion complexes. Inhaled particles and pathogens transmitted via secreted air droplets are cleared from airways through trapping in mucus upon deposition and subsequent clearance of the trapped particles mediated by propelling of the coordinated beating of cilia, termed mucociliary escalator system. In addition to these defense factors, the bronchial epithelium secretes a large number of mediators including antibacterial substances (lactoferrin,
lysozyme), anti-proteases (α1-protease inhibitor, α1-anti-chymotrypsin, α2-macroglobulin, tissue inhibitors of
metalloproteinases) and anti-oxidant/redox-regulatory molecules (glutathione, superoxide dismutase, transferrin and catalase). Bronchial epithelial cells are also capable of transporting secretory immunoglobulin A into the bronchial lumen.
1.2.2
In vitro tissue culture systems to study pathogen interactions with human airway
epithelium
Throughout the years, various models have been developed to study physiological responses of the lungs and pathological changes in lung disease and infections. The differences between models depend upon what region of the lungs the engineering system is attempting to mimic, and the pathological condition and infectious agents being studied. Although there exists a broad range of lung tissue models used, the most commonly used systems in generation of bronchial tissue are summarized in Table 2, indicating advantages and disadvantages of each model.
Table 2. Common cell types used in the generation of lung tissue models (Modified fromNichols et al, 2014)
Cell type Derivation Advantages Disadvantages
Calu-3 [99,100] Submucosal adeno-carcinoma of the bronchus
- Readily available and well characterized
- Forms confluent monolayer of polarized cells
- Immortalized - Express mucin gene - Develop cilia & microvilli
- Variation in TJ formation
BEAS-2B [101,102] Transformed bronchial epithelium
- Immortalized
- Forms confluent monolayers - Secrete cytokines - Express antioxidants - No mucin secretion - Lack TJs 16HBE14o- [103-105] Transformed bronchial epithelium - Immortalized
- Differentiated & multilayered - Develop cilia & microvilli - Secrete cytokines
- No mucin secretion
A549 [106,107] Human lung adenocarcinoma alveolar basal cell line
- Immortalized
- Produce confluent monolayers with AECII morphology and lamellar bodies present - Consistent AECII
metabolic/transport properties
- Monolayers lack TJs - Exhibit very low
trans-epithelial electrical resistance values
Primary AECs
[108,109]
Human alveolar epithelial type I and II
- Accurately demonstrate in vivo behavior
- Time consuming isolation
CHAPTER I: INTRODUCTION
mix (primary cells) - Controllable cell division- Capable of TGF-β1 induced epithelial mesenchymal transition in culture
- Harbor necessary cell surface receptors for signaling
- High risk for bacterial or fungal contamination - Cells cannot be passaged
repeatedly
- High cost for reagents used in isolation NHBE [92,110-112] Normal human
bronchial epithelium (primary cells)
- Not transformed and long lifespan
- Differentiated and multilayered - Form cilia, TJs, secrete mucus - Availability of various donors
e.g. pediatric, COPD, CF - Serum-free medium
- Can be used up to maximum passage 3 (not immortal)
- Labour intensive - Potential for microbial
contamination MatTek EpiairwayTM [66,113] Human tracheal/bronchial epithelium (primary cells)
- Commercially available for purchase in differentiated form - Not transformed and long
lifespan
- Differentiated and multilayered - Form cilia, TJs, secrete mucus - Serum-free medium
- Not immortal - Cost to purchase
As previously mentioned, organotypic 3D tissue culture systems are becoming increasingly recognized as human tissue equivalents in replacement of 2D cultures. Various systems are available as tools for engineering 3D models. Rotating wall vessel (RWV) is one of these platforms that has been used for studying the cellular and molecular responses of both hosts and pathogens. There are two different RWV designs, the high-aspect rotating vessel (HARV) and the slow-turning lateral vessel (STLV), which differ mainly in aeration source. The design of RWV bioreactor is highly physiological as it provides a low-fluid-shear growth environment similar to that encountered by pathogens in certain regions of the body (such as the part between the brush border microvilli of epithelial cells and in utero); which represents a biomechanical force known to influence cellular differentiation and development in mammals. The dynamic culture conditions in RWV allows cells to grow in 3D, to aggregate based on natural cellular affinities and to differentiate into 3D tissue-like assemblies. The spinner flask is also a cost-effective method used for generation of suspension cultures of 3D spheroids, with the disadvantage of having very high levels of fluid shear in culture which can damage cells and interfere with differentiation schedule.
Another common technique involved implanting cells into a 3D matrix scaffold composed of collagen, extract of extracellular matrix, synthetic or semi-synthetic materials (such as hyaluronan hydrogels), or a combination of these materials. This approach has been proven successful in recapitulating the 3D architecture of various tissue types and has been particularly beneficial in cancer and stem cell research. However, the major challenge for studying host-pathogen interactions using this system is the hindering of host cell exposure to pathogens due to the matrix in a non-physiologically relevant manner. These systems are thoroughly reviewed in [114]. Finally, the next wave of 3D cell-culture models relies on micro-engineering techniques and microfluidics. Microfluidics is the manipulation of small amounts of fluids (10-9
to 10-8 L) in micro-fabricated hollow channels, a core microsystem technology which is used to generate and precisely tune dynamic fluid flows and spatio-temporal gradients, deliver nutrients and other chemical cues to cells in a controlled manner. Also referred to as ‘organs-on-chips’, this technology integrated microfluidics with living cells cultured within 3D devices (microchips), to create tissue-tissue interfaces to mimic organ microarchitecture and to study human physiology in an organ-specific context (Reviewed in detail in [86]). Although still in a preliminary phase, the implementation of ‘organs-on-chips’ to biomedical research carry the potential to serve as replacements for animals used in drug development and toxin testing.
1.3 Host-pathogen interactions
1.3.1
Monitoring interactions at host-pathogen interface: an emerging theme
In recent years, it is becoming increasingly clear that bacterial pathogens have evolved complex functional interfaces with their hosts, particularly during long-standing associations allowing evolutionary forces to shape the molecular machines and strategies that can be characterized by their refinement. The study of the cell biology and immunobiology of these interactions is a fruitful area of research, as they not only yield remarkable aspects of the host-pathogen functional interface but also provide a unique window into the basic aspects of cellular functions and the innate immune system. This approach is no longer restricted to few model organisms but rather is applicable to diverse group of pathogens with unique and diverse mechanisms to engage their hosts.
Modulation of the host-cell actin cytoskeleton by bacterial pathogens to enter into cells, to move within the cells and spread from cell to cell, avoid uptake by phagocytic cells, and to promote intimate attachment is a well-known phenomenon of host-pathogen interaction field. The main focus of this spatial and temporal coordination of actin dynamics is a subset of small molecular weight GTP-binding proteins of the Rho family, in particular Cdc42, Rac1 and Rho. Various pathogens have evolved the capability to modulate the activity of these proteins as a means to subvert the actin nucleating machinery to mediate their own uptake into host cells. Examples include; Yersinia spp protein invasin which binds α5-β1 integrins in a tight manner, generating signals from the receptor that lead to the activation of Rac1, misregulation promoted by translocation of YopE and YopT proteins into target cell [115]; Salmonella enterica effector proteins (SopE/E2, SopB, SipA, SipC and SptP) delivered to host cells via type III secretion system (TTSS) which can activate Cdc42 and Rac1, leading to profuse actin cytoskeleton rearrangements and membrane ruffling [116]; and enteropathogenic E.
coli which utilizes a TTSS to deliver a number of bacterial effector proteins whose coordinated action results
in the formation of well-organized actin ‘pedestals’ facilitating the intimate attachment of bacteria to mucosal surfaces. Similarly, many other pathogens such as Listeria, Shigella, Rickettsia, Clostridium, and mycobacterial species such as Mycobacterium marinum and Burkholderia pseudomallei evolved different strategies to subvert the actin cytoskeleton to move within and between cells [117].