DOTTORATODIRICERCAINBIOLOGIAAMBIENTALEEDEVOLUZIONISTICA
XXXICICLO
M
OVING IN A CROWDED WORLD:
ECOLOGICAL AND HUMAN-RELATED FACTORS AFFECTING BROWN BEAR SPACE
-
USE PATTERNSP
H.D.
C
ANDIDATE:
D
ANIELED
EA
NGELISTUTOR:DR.PAOLO CIUCCI CO-TUTOR:PROF.JOSIP KUSAK
FACOLTÀ DI SCIENZE MATEMATICHE, FISICHE E NATURALI DIPARTIMENTO DI BIOLOGIA E BIOTECNOLOGIE “CHARLES DARWIN”
CURRICULUM IN BIOLOGIA ANIMALE
3
Table of contents
EXECUTIVE SUMMARY ... 7
Overview ... 7
Chapter I - Determinants of brown bear home ranges in a landscape of food and fear ... 8
Chapter II - Partial migrations of brown bears in a south-eastern European population ... 9
Chapter III – Seasonal habitat selection by Dinaric brown bears ... 10
Chapter IV – Seasonal corridors for brown bear movements: an integrated modelling approach ... 10
INTRODUCTION ... 12
Conservation of Large Carnivores in Europe ... 13
Brown bears (Ursus arctos) in Europe ... 14
Aims of the Thesis ... 15
Data on bear movements and Study Area ... 16
Study design ... 17
CHAPTER I ... 26
BROWN BEAR SPACE-USE PATTERNS IN A LANDSCAPE OF FOOD AND FEAR ... 26
ABSTRACT ... 27
INTRODUCTION ... 28
METHODS ... 30
Study Areas ... 30
Live-trapping and telemetry ... 31
Home range analyses ... 31
RESULTS ... 34
DISCUSSION ... 35
References ... 41
Tables and figures ... 47
Supplementary Material ... 55
CHAPTER II ... 59
PARTIAL MIGRATIONS OF BROWN BEARS IN A SOUTH-EASTERN EUROPEAN POPULATION ... 59
ABSTRACT ... 60
INTRODUCTION ... 61
METHODS ... 63
RESULTS ... 69
Classification of individual movements ... 69
4
Summer versus stopovers habitat use ... 70
DISCUSSION ... 71
References ... 79
Tables and figures ... 86
Supplementary Material ... 94
CHAPTER III ... 95
SEASONAL HABITAT SELECTION BY DINARIC BROWN BEARS IN A HUMAN-ALTERED LANDSCAPE ... 95
ABSTRACT ... 96
INTRODUCTION ... 97
METHODS ... 100
Study area ... 100
Animal captures and bear telemetry data ... 101
Environmental data ... 101
Study design ... 102
Exploratory analysis: K-select analysis ... 103
Seasonal RSF ... 103 RESULTS ... 105 K-select analysis ... 105 Seasonal RSF ... 106 DISCUSSION ... 107 References ... 112
Tables and figures ... 121
Supplementary Material ... 126
CHAPTER IV ... 131
SEASONAL CORRIDORS FOR BROWN BEARS: AN INTEGRATED MODELLING APPROACH ... 131
ABSTRACT ... 132
INTRODUCTION ... 133
METHODS ... 136
Study Area ... 136
Bear GPS Data ... 137
Modelling summer and fall functional patches ... 138
Modelling landscape permeability through step selection functions ... 140
Modelling potential paths for bear seasonal migrations ... 142
RESULTS ... 143
Selection of summer and fall habitat patches ... 143
Movement-based estimation of permeability and potential bear paths ... 144
DISCUSSION ... 145
Seasonal patches distribution and overall connectivity ... 145
5
Modelled bear paths ... 147
Supplementary Material ... 171
CONCLUSIONS ... 175
7
Executive Summary
Overview
Space is one of the most disputed resources between humans and other wildlife species in modern human-dominated landscapes. Successful wildlife management and conservation requires a deep understanding of the interactions between a species and the space where it lives (Groom et al. 2006), including direct and indirect effects of both natural and human-related factors of fundamental ecological processes (Johnson et al. 2004). The high spatio-temporal resolution of Global Positioning System (GPS) tracking data turns tagged animals into in situ sensors of the environment, and allows investigating how environmental changes affect species’ distribution and ecological function (Kays et al. 2015). Large carnivores, in particular, with their wide movement ranges and large spatial requirements are highly susceptible to disturbance from infrastructure development; as such, they can represent an ideal case study to investigate the effects of expansion of human activities on species’ spatial ecology at multiple levels, spanning from patch to landscape scales (Ripple et al. 2014).
In this thesis, I investigated space-use patterns of a south-eastern European population of brown bears (Ursus arctos), whose distribution is shared among more than five countries, from Slovenia to Northern Greece, with its core between Slovenia and Croatia. Despite being the third largest brown bear population in Europe (Chapron et al. 2014), only few studies have focussed on the spatial ecology of the Dinaric-Pindos bear population (Huber and Roth 1993; Krofel et al. 2010). I investigated how environmental factors and proxies of human activities (e.g., roads, human settlements, hunting sites) influenced patterns of bear space use, movements and habitat selection. I used this knowledge to develop a movement-based modelling approach, aimed at mapping patch connectivity for seasonal movements of bears within the study area. In addition to enhancing our knowledge of bear ecology at broader scale across Europe, the results from this thesis are also of practical value, as they inform current and future management and conservation scenarios in the light of ongoing development projects throughout the countries inhabited by the study population.
8
Chapter I - Determinants of brown bear home ranges in a landscape of food
and fear
“Everything in the biology of mammals from the skin out or the skin in, from birth to death, is linked to home ranges” Powell (2012). Home range is that area across which an individual routinely travel to carry out all its most fundamental biological activities of searching for food and water, breeding, giving birth to and raising its offspring, net of occasional exploratory sallies (Burt 1943, Powell 2012). In this chapter, I investigated ecological and human-related factors affecting home-range size and inner configuration of Dinaric brown bears (Ursus arctos) contrasting three areas, one located in the North (n= 5 bears, 1 females, 4 males) and two in the South (n= 5 bears, 2 females, 3 males), which differed in terms of road and human density, as well as in the availability of supplementary feeding sites. I used Brownian bridge movement models (BBMMs) (Horne et al. 2007) to estimate home-range size and configuration, and used linear mixed-effect models (LMMs) to investigate the effects of gender, time of the day, season and study area on home-range size. To investigate the internal use of the home range, I used an individual-based method (Vander Wal and Rodgers 2012) to avoid arbitrary thresholds to define core seasonal areas and assessed their internal configuration using Environmental Niche Factor Analysis (ENFA; Hirzel et al. 2002). Although I failed to find a gender effect on home-range size, probably due to a limited sample size, time of day was an important predictor of home-range size. Nocturnal home ranges (103.3 ±72.8 km2) were larger
than diurnal ones (62.3 ±16.6 km2). Limited to areas where bears had relatively lower accessibility to
artificial feeding sites, I also detected a seasonal effect on home-range size, with fall ranges smaller (25.0 ±7.0 km2) than those in other seasons (spring 92.5 ±55.0 km2; summer 61.6 ±13.6 km2). Where
anthropogenic features (i.e., densities of roads, human settlements, and artificial feeding sites) were highest, these were avoided by bears when establishing their core areas. Overall, this study revealed that even within the same population range, bears can show behavioural plasticity and adapt to local conditions to buffer human disturbance. Although behavioural plasticity may contribute to ensure carnivore persistence in human-dominated areas by means of their adaptation, the changes in home range patterns that we detected can be also interpreted as a warning sign of on-going environmental
9 degradation. As this is the first study to estimate space-use patterns in this bear population, the results represent a baseline upon which to assess potential changes in the future.
Chapter II - Partial migrations of brown bears in a south-eastern European
population
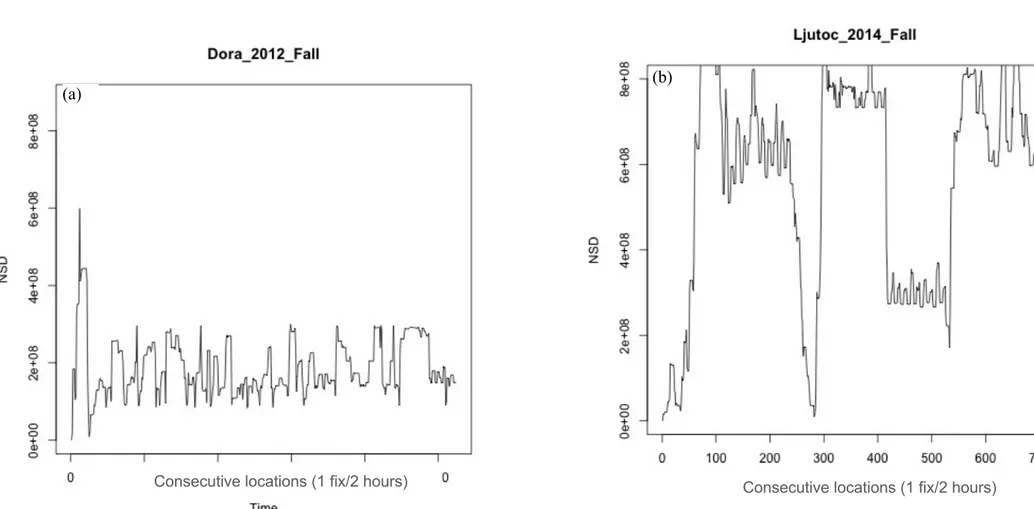
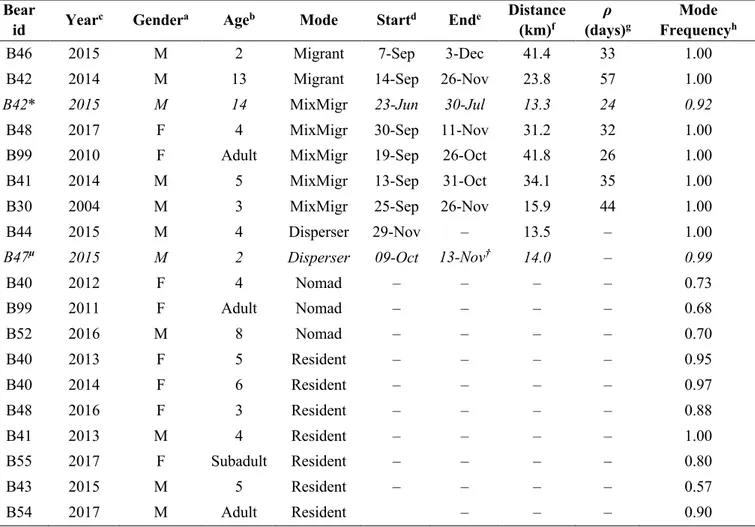
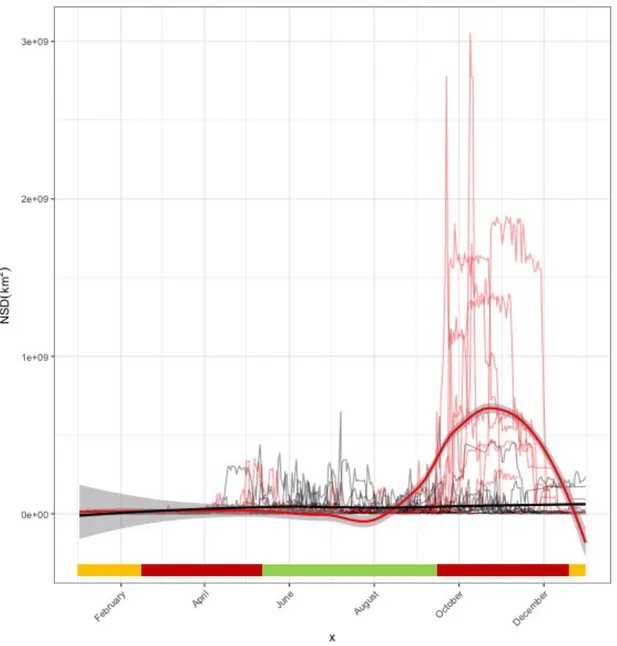
The analysis of space-use patterns by bears illustrated in Chapter 1 allowed me to detect dislocated seasonal ranges for some of the bears in our sample. Accordingly, I investigated to a greater extent space-use patterns by these bears in order to: i) describe seasonal migratory movements in terms of frequency, period, duration, and length; ii) investigate the environmental drivers and correlates of such migratory movements. I classified individual movement patterns by means of non-linear Net Squared Displacement (NSD) models (Bunnefeld et al. 2011). By means of canonical Overlying Mean Index analysis (OMI; Doledec et al. 2000), I identified habitat descriptors of pre-migratory and post-pre-migratory ranges at the landscape scale. I then quantified the strength of variation in bear habitat use at summer versus fall ranges using a Latent Selection Difference analysis (LSD; Latham et al. 2011), at both the population and the individual level. Findings revealed that 6 out of 12 individuals showed facultative and partial seasonal migrations (Dingle and Drake 2007) between disjointed seasonal ranges. Migratory patterns were markedly seasonal, with all departures occurring between mid-September and mid-October (median= 19th September), and returns occurring before the wintering period (median= 18th November). Migratory movements connected seasonal ranges up to >40 km apart (mean = 28.9 km). Most bears migrated from areas characterised by coniferous and mixed forests to lower areas with high proportion of deciduous forest, forest edge and shrubs. Compared to pre-migratory ranges, within migration ranges bears increased both their distance to anthropogenic structures (i.e. paved roads, settlements, artificial feeding sites, cultivated lands) and their selection for highly productive areas (i.e. deciduous forest, forest edge and shrubs). Due to lack of data on fine-scale forest productivity and on hunters’ visits to hunting sites within bear ranges, the ultimate cause that triggered the observed bear migrations remains to be assessed. However, these results contribute to improve our understanding of bears ecology in European settings, as similar migration patterns have never been reported before in any other European bear population.
10
Chapter III – Seasonal habitat selection by Dinaric brown bears
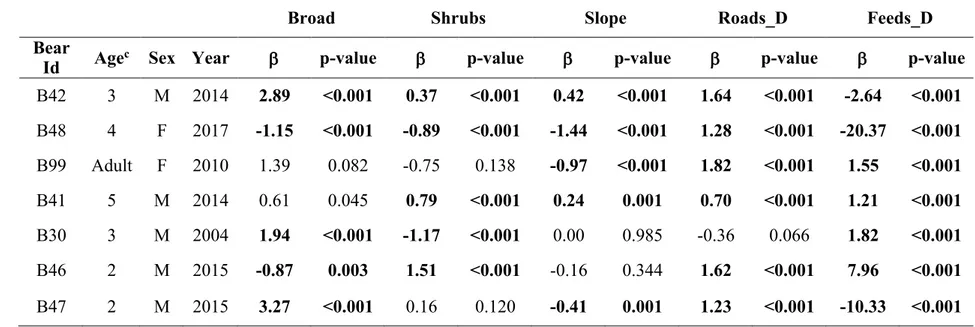
In this chapter, I investigated seasonal habitat selection in 11 bears (4 females and 7 males), limiting the analysis to resident bears that did not perform seasonal migrations (see Ch. 2). In particular, I investigated seasonal changes in bear selection or avoidance of human infrastructures such as highways, paved roads, railway, forest roads, human settlements and supplemental feeding sites. The working hypothesis was that bears display stronger avoidance towards proxies of human disturbance during periods of increased hunting pressure (i.e., spring and fall). I therefore first explored general patterns of seasonal habitat selection using K-select analysis, aimed at identifying the principal components of bear seasonal habitat preferences accounting for individuals (Calenge et al. 2005); successively, I used resource selection functions (RSF, Manly et al. 2002) in a use/availability design contemplating availability within each individual bear’s home ranges. In agreement with my hypothesis, the K-select analysis showed a common pattern of habitat selection during spring and fall for the majority of individuals, characterized by avoidance to forest roads and selection for areas featuring steeper slopes. Conversely, during summer, bears showed more variable patterns of habitat selection, reflecting higher individual variability. The results of the RSF analysis confirmed these patterns, with distance to forest roads (bspring= 0.33 ±0.01; bfall = 0.30 ±0.04) and use
of slopes (bspring= 0.12 ±0.01; bfall = 0.20 ±0.02) playing a major role in shaping habitat selection
patterns during hunting seasons, and a significant use of supplementary food (i.e., decreased distance from artificial feeding sites) during hyperphagy (bfall = -0.30 ±0.04). Overall, these findings suggest
that hunting is perceived by bears as a form of predation risk that forces them to increase their concealment, influencing within-home-range habitat use.
Chapter IV – Seasonal corridors for brown bear movements: an integrated
modelling approach
In this chapter I integrated classical habitat selection studies and cutting-edge movement algorithms (Panzacchi et al. 2016) to model bear movements among suitable resource patches at the study area scale. To this aim, I used a movement-based modelling approach to project potential
11 corridors connecting summer and fall habitat patches. First, I modelled habitat patches associated with habitat use during summer and fall using Resource Selection Functions (RSF; Manly et al. 2002) and accounting for behavioural state (i.e., considering non-moving only GPS bear relocations indicating stationary behaviour (i.e. feeding or resting). Then, based on bear trajectories representing bear travelling, I used Step Selection Functions (SSF; Fortin et al. 2005) to estimate bears’ responses to natural and anthropogenic features, whose results were used to estimate a spatial-explicit map of landscape permeability to bear movement. Finally, I used a Randomised Shortest Path (RSP) algorithm (Panzacchi et al. 2016) to project potential bear corridors between summer and fall habitat patches within our study area. Compared to more classical algorithms used to model animal movements (i.e. least-cost path, circuit theory; McRae et al. 2008; Pinto and Keitt 2009), the RSP does not make the assumption that animals move either randomly or totally optimally, rather allowing exploring different intermediate strategies in animal movement (Panzacchi et al. 2016). According to my findings, bears could successfully travel across sub-optimal habitat to reach suitable patches in the fall, although the presence of anthropogenic structures such as highways, main paved roads, railways, and cultivated fields strongly decreased the probability of a bear traveling or traversing the area. Model predictions correlated moderately with the frequency of bears killed by vehicle collisions (0.68≤rs≤0.79), suggesting that modelled corridors might indicate areas along road infrastructures
where bears are more likely to cross. Overall the degree of inter-patch connectivity was rather high across the study area, with many potential bear paths connecting suitable summer and fall habitat patches. However, these findings warn that improvement of mitigation measures should be evaluated at the intersection between modelled corridors and linear infrastructures. This modelling approach might be applied also to other ecological contexts where habitat fragmentation represents a major threat to the long-term persistence of wide-ranging mammalian populations.
12
Introduction
We live on a rapidly changing human-dominated planet, where humans became an earth-modifying force that caused transformation of land and marine ecosystems for their use (Crutzen & Stoermer, 2000). To capture this dramatic shift in the relationship between humans and the environment, the above authors coined the term “Anthropocene” to distinguish the current epoch from the former Holocene (Crutzen & Stoermer, 2000; Waters et al. 2016). Seen from above, Earth’s landscapes appear transfigured by the presence of humans and their activities, with always fewer places left untouched. Natural systems have faced unprecedented challenges in recent decades that have accelerated habitat loss and fragmentation, with effects on biodiversity and ecosystem function (Fahrig 2003). Habitat loss it is indeed still recognized as one of the major threats to biodiversity across the globe (Dirzo et al. 2014), and the extinction of ~75% of the species is expected in the next few centuries if current trends will continue (Barnosky et al. 2011). Under this scenario of human expansion and land use change, one resource emerges as being particularly disputed between humans and other wildlife species: space.
Europe is one of the most crowded continents on Earth, with up to 80% of its territory occupied by road infrastructures, production systems (e.g. cultivated lands) and urban areas (European Environment Agency 2008). Since the past years, the “land sharing” versus “land sparing” debate has gathered scientists in the attempt to address important questions about how biodiversity should be conserved in our day and age (Phalan et al. 2011; Kremen 2015). Land sharing consists of integrating biodiversity conservation and food production on the same land, for example, using wildlife-friendly farming methods; whereas land sparing involves separating land for conservation and land for crops (Phalan et al. 2011). Should biodiversity be preserved in a dedicated space, segregated from human activities, or are there any chances for humans and biodiversity to coexist in functional and dynamic ecosystems? Addressing this question is beyond the scope of this thesis.
13 However, given the lack of true wilderness areas compared to North American landscapes, in Europe the most probable scenario will be characterized by a dynamic interspersion of both wildlife and humans. Under this premise, understanding how and how much humans affect the ecology of wildlife species will be necessary to determine a successful strategy for term coexistence on the long-term.
Conservation of Large Carnivores in Europe
Large carnivores have been particularly challenged by human expansion during the last centuries, facing a drastic reduction of their distribution (Di Marco et al. 2014). These species occur at low densities, and necessitate large home ranges to fulfil their biological needs in the wild (Dahle and Swenson 2003b; Herfindal et al. 2005; Persson et al. 2010; Mattisson et al. 2013). These aspects of their ecology and their protein-rich diet, contribute to make large carnivores one of the groups of species most affected by habitat loss, fragmentation, human activities and direct persecution (Woodroffe 2000; Treves and Karanth 2003). Currently, four species of large carnivores inhabit European landscapes: wolf (Canis lupus), lynx (Lynx lynx), brown bear (Ursus arctos) and wolverine (Gulo gulo). During past decades, land abandonment and the introduction of several protection policies have allowed carnivores to re-expand over a large portion of their former range in Europe (Chapron et al. 2014). Except wolverine, whose distribution is partly shaped by climatic constraints (Saether et al. 2005), large carnivores occur across nearly the entire latitudinal range of the European continent and have adapted to various climatic conditions and ecological contexts (Chapron et al. 2014). Humans can play a central role in shaping carnivores’ spatial patterns, not only by directly affecting their survival through hunting or lethal control (Woodroffe 2000; Treves and Karanth 2003), but also indirectly influencing their behaviour by jeopardising habitat quality (Cozzi et al. 2016; Krofel et al. 2016). Much of the research conducted on large carnivores’ spatial behaviour in Europe has highlighted their capability to adjust their behaviour in relation to different sources of anthropogenic disturbance (Kaczensky et al. 2006; Martin et al. 2010; Ordiz et al. 2012; Zimmermann et al. 2014; Cozzi et al. 2016; Mancinelli et al. 2018). Because large carnivores play a fundamental role in maintaining the integrity of biodiverse ecosystems, human-caused changes in their behaviour
14 might have unexpected effects on entire ecosystem dynamics (Beschta and Ripple 2009; Ripple et al. 2014). As a consequence, understanding large carnivores’ responses to different types of human pressure, local management and ecological contexts could provide important insights for conserving biodiversity in human-dominated landscapes (Carroll et al. 2001; Beschta and Ripple 2009).
Brown bears (Ursus arctos) in Europe
With about 17,000 individuals, the brown bear (Ursus arctos) is the most abundant large carnivore inhabiting the human-dominated landscapes of Europe (Chapron et al. 2014). In Europe, brown bears are clustered into 10 populations: the Carpathian, Scandinavian and Dinaric-Pindos populations are large and connected, whereas bears in Spain, France, Greece, Italy and Austria are small and fragmented (Zedrosser et al. 2001). The brown bear is a forest-dwelling, opportunistic omnivore, which can utilize all easily accessible foods, both from natural and anthropogenic sources (Kavčič et al. 2013, 2015; Ciucci et al. 2014; Cozzi et al. 2016). Indeed, in some cases bears can occur in proximity to humans, which often leads to the insurgence of conflicts (Elfström et al. 2014b). Most conflicts arise from bears causing damage to humans and their activities, such as livestock depredation (Rigg et al. 2011; Kavčič et al. 2013) or attacks on humans (Herrero et al. 2005). Other conflicts are mutually problematic, such as traffic accidents (Huber et al. 1998; Kaczensky et al. 2003). Across Europe, the main human-related factors that threaten bear populations are fragmentation and habitat loss (Ciucci and Boitani 2008; Falcucci et al. 2009; Kopatz et al. 2012; Mateo-Sánchez et al. 2014), disturbance (Martin et al. 2010; Støen et al. 2015), low acceptance (Rigg et al. 2011), road mortality (Huber et al. 1998; Kaczensky et al. 2003) and direct persecution (Zedrosser et al. 2001). Moreover, some populations are subject to hunting for management and/or recreational purposes (Kaczensky et al. 2004; Majić et al. 2011; Bischof et al. 2012). Despite these threats, in many cases bears have proven successful in competing for space across European landscapes (Chapron et al. 2014).
Bears constitute an ideal case study to investigate questions concerning the influence of humans on wildlife. Bears are behaviourally plastic animals that, in some cases, can even take advantage of the coexistence with humans (Elfström et al. 2014b; Cozzi et al. 2016). Previous
15 research conducted in Europe illustrated how bear behaviour and life-history traits can be influenced by several sources of human-related disturbance, such as hunting (Ordiz et al. 2012; Støen et al. 2015), recreational activities (Kaczensky et al. 2006), road infrastructures (Frąckowiak et al. 2014; Ordiz et al. 2014) and supplementary feeding (Krofel et al. 2016). These factors can affect bear circadian rhythms (Kaczensky et al. 2006; Ordiz et al. 2014), habitat selection (Martin et al. 2010; Frąckowiak et al. 2014), diet (Kavčič et al. 2015) and movement patterns (Selva et al. 2017). Prolonged exposure to persecution perpetrated by humans is thought to have affected behaviour and life-history traits of European bears, as compared to the wilder areas of North America (Kaczensky et al. 2006; Zedrosser et al. 2011). Although being a warning sign of human-caused habitat degradation, these behavioural adaptations likely played an important role for the survival of European brown bear populations from past persecution and will prove crucial to ensure its long-term persistence in future scenarios of human expansion (Zedrosser et al. 2011).
Aims of the Thesis
The main objective of my research project was to gain better insights into the spatial ecology of the brown bear in a population that shares the landscape with humans. In particular, this thesis aimed at investigating both ecological factors and proxies of human activities (e.g. roads, human settlements, hunting sites) influencing bear home range characteristics, spatial arrangement, movements and habitat selection. I then used the information gathered at this stage to develop a movement-based approach, aimed at modelling patch connectivity for bear seasonal movements.
To conduct my research, I focussed on the following research questions:
1. Do different levels of human disturbance affect brown bear home range characteristics and home range internal configuration ?
2. Which environmental drivers contribute the most in shaping the observed patterns of bear seasonal movements?
3. How does habitat selection of brown bears change over the seasons? And, more specifically, does habitat selection reflect different levels of human-avoidance during hunting seasons?
16 4. Can movement-based connectivity models indicate areas that should be prioritized for
reducing bear traffic mortality?
Data on bear movements and Study Area
This study has been entirely conducted using telemetry locations obtained from Global Positioning System (GPS) collected from brown bears belonging to the Dinaric-Pindos population. Bears were live-captured in Croatia and Bosnia-Herzegovina between 2004 and 2018, under the framework of several conservation projects coordinated by the team of the Croatian Large Carnivore Project, affiliated to the Veterinary Faculty of the University of Zagreb. Although being the third largest population in Europe (Chapron et al. 2014), few studies have focussed on the spatial ecology of Dinaric-Pindos brown bears (Roth and Huber 1986; Huber and Roth 1993; Kaczensky et al. 2006; Krofel et al. 2010; Ćirović et al. 2015).
With about 2100-2800 estimated individuals, the Dinaric-Pindos population spreads from Slovenia to Northern Greece, with the population core area shared between Slovenia and Croatia (Kaczensky et al. 2012). The area where bears were monitored represented a promising ecological contexts to assess how recent trends of human development might affect the life history traits of this wide-ranging species. Indeed, the study area in part encompasses one of the largest forest systems of South-Central Europe, the Dinaric mixed mountain forests (Huber et al. 2008; Klopcic et al. 2010). Human density is generally lower than European standards, ranging from about 27 inhabitants per km-2 in the north to 13 inhabitants per km-2 in the south (Kaczensky et al. 2006). However, several
human infrastructures are present in our study area, such as highway, roads, villages and urban areas. Recently Croatia has assisted to a remarkable improvement of its road infrastructures, with the construction of more than 218 km of new highway, of which 68 km were located within the bear core area (Botrić et al. 2006; Kusak et al. 2009). Bear mortality linked to traffic incidents represents the second most important cause of bear death in Croatia (Kaczensky et al. 2003; Ličina et al. 2015) Bears in Croatia are also subject to seasonal hunting from 16th September to 15th December and from
16th February to 15th May (Bišćan et al. 2016), whereas a continuous hunting period in Bosnia Herzegovina spans 1st October-15th May. Across the entire area, hunters use numerous artificial
17 feeding sites refilled year-round to lure bears at hunting sites, which can represent attractive food sources (Huber et al. 2008b; Kavčič et al. 2015).
Study design
Home-range size is one of the most fundamental ecological parameters in animal ecology (Powell and Mitchell 2012). Because it delimits the area an animal needs to accomplish its biological needs (Burt 1943), it can provide insights on the trade-off animals choose between resource access and energetic costs (Mattisson et al. 2013). In the first phase of my research (Chapter I) I investigated ecological and human-related factors affecting bear seasonal home-range size and inner configuration. I contrasted home ranges of animals moving in distinct areas, which differed in terms of road and human density, as well as in the availability of supplementary feeding sites. I investigated the effects of gender, time of the day, season and study area on home-range size. By means of an individual-based time-maximizing method (Vander Wal and Rodgers 2012) I depicted seasonal core areas and assessed whether the internal configuration of seasonal home ranges differed among study areas through an Environmental Niche Factor Analysis (ENFA; Hirzel et al. 2006).
The analysis of bear home ranges assessed in Chapter I helped identify peculiar movement patterns for some of the bears in our sample, which moved between dislocated seasonal ranges. The specific objectives of Chapter II were i) describing seasonal migratory patterns of brown bears indicating frequency, period, duration and length of inter-seasonal range movements; ii) investigating potential environmental drivers of such migratory behaviour. I identified habitat descriptors of pre-migratory and post-pre-migratory ranges at the landscape scale. I then quantified the strength of variation in bear habitat use at summer versus fall ranges using a latent selection difference analysis (LSD; Latham et al. 2011), at both the population and the individual level.
Establishing the link between animals and their habitat provides pivotal knowledge for their conservation and ecological management (Boyce and McDonald 1999). In Chapter III I studied seasonal habitat selection for bears remaining resident. In particular, I investigated changes in bear use/avoidance of human infrastructures such as highway, paved roads, railway, forest roads, human settlements and supplementary feeding sites. The general working hypothesis of this chapter was that
18 bears might display stronger avoidance towards our proxies of human disturbance during periods of increased hunting pressure (i.e. spring and fall). To confirm my hypothesis, I integrated the use of K-select analysis and resource K-selection functions (RSF) to visualize seasonal patterns of habitat selection and quantify the effect of both ecological and human-related factors on bear seasonal space use.
In Chapter IV, I used a modelling approach to simulate bear movements among suitable resource patches, integrating classical habitat selection studies and cutting-edge movement algorithms. To this aim, I have used a movement-based modelling approach to project potential corridors connecting summer and fall habitat patches. I used Resource Selection Functions (RSF; Manly et al. 2002) based on bear relocations representing stationary behaviour (i.e. feeding or resting) to model habitat patches associated to intensive habitat use during summer and fall. Based on bear trajectories representing bear travelling, I then used Step Selection Functions (SSF; Fortin et al. 2005) to model spatial-explicit permeability. By means of Randomised Shortest Path (RSP) algorithm (Panzacchi et al. 2016) I projected potential bear corridors between summer and fall habitat patches within our study area. The RSP allowed testing different alternative strategies adopted by bears when moving across the landscape. Finally, I used an independent dataset of bear fatalities along roads to evaluate the capability of the modelled corridors to indicate areas that should be prioritized in future management actions aimed at reducing bear-vehicle collisions.
19
REFERENCES
Barnosky, A.D., Matzke, N., Tomiya, S., Wogan, G.O.U., Swartz, B., Quental, T.B., Marshall, C., McGuire, J.L., Lindsey, E.L., Maguire, K.C., Mersey, B., and Ferrer, E.A. 2011. Has the Earth’s sixth mass extinction already arrived? Nature 471: 51–57. doi:10.1038/nature09678.
Beschta, R.L., and Ripple, W.J. 2009. Large predators and trophic cascades in terrestrial ecosystems of the western United States. doi:10.1016/j.biocon.2009.06.015.
Bischof, R., Nilsen, E.B., Brøseth, H., Männil, P., Ozoliņš, J., and Linnell, J.D.C. 2012. Implementation uncertainty when using recreational hunting to manage carnivores. J. Appl. Ecol. 49(4): 824–832. doi:10.1111/j.1365-2664.2012.02167.x.
Botrić, V., Šišinački, J., and Škuflić, L. 2006. Road Infrastructure and Regional Development: Some Evidence From Croatia. Science: 11–12.
Boyce, M.S., and McDonald, L.L. 1999. Relating populations to habitats using resource selection functions. Trends Ecol. Evol. 14(7): 268–272. doi:10.1016/S0169-5347(99)01593-1.
Bunnefeld, N., Börger, L., Van Moorter, B., Rolandsen, C.M., Dettki, H., Solberg, E.J., and Ericsson, G. 2011. A model-driven approach to quantify migration patterns: Individual, regional and yearly differences. J. Anim. Ecol. 80(2): 466–476. doi:10.1111/j.1365-2656.2010.01776.x. Burt, W.H. 1943. Territoriality and home range concepts as applied to mammals. J. Mammal. 24(3):
346–352. doi:10.2307/1374834.
Calenge, C., Dufour, A.B., and Maillard, D. 2005. K-select analysis: A new method to analyse habitat selection in radio-tracking studies. Ecol. Modell. 186(2): 143–153. doi:10.1016/j.ecolmodel.2004.12.005.
Carroll, C., Noss, R.F., and Paquet, P.C. 2001. Carnivores as focal species for conservation planning in the rocky mountain region. Ecol. Appl. 11(4): 961–980. doi:10.2307/3061005.
Chapron, G., Kaczensky, P., Linnell, J.D.C., Arx, M. von, Huber, D., Andrén, H., López-Bao, J.V., Adamec, M., Álvares, F., Anders, O., Balčiauskas, L., Balys, V., Bedő, P., Bego, F., Blanco, J.C., Breitenmoser, U., Brøseth, H., Bufka, L., Bunikyte, R., Ciucci, P., Dutsov, A., Engleder, T., Fuxjäger, C., Groff, C., Holmala, K., Hoxha, B., Iliopoulos, Y., Ionescu, O., Jeremić, J., Jerina, K., Kluth, G., Knauer, F., Kojola, I., Kos, I., Krofel, M., Kubala, J., Kunovac, S., Kusak, J., Kutal, M., Liberg, O., Majić, A., Männil, P., Manz, R., Marboutin, E., Marucco, F., Melovski, D., Mersini, K., Mertzanis, Y., Mysłajek, R.W., Nowak, S., Odden, J., Ozolins, J., Palomero, G., Paunović, M., Persson, J., Potočnik, H., Quenette, P.-Y., Rauer, G., Reinhardt, I., Rigg, R., Ryser, A., Salvatori, V., Skrbinšek, T., Stojanov, A., Swenson, J.E., Szemethy, L., Trajçe, A., Tsingarska-Sedefcheva, E., Váňa, M., Veeroja, R., Wabakken, P., Wölfl, M., Wölfl, S., Zimmermann, F., Zlatanova, D., and Boitani, L. 2014. Recovery of large carnivores in Europe’s
20 modern human-dominated landscapes. Science 346(6216): 1517–1519. doi:10.1126/science.1257553.
Ćirović, D., de Gabriel Hernando, M., Paunović, M., and Karamanlidis, A.A. 2015. Home range, movements, and activity patterns of a brown bear in Serbia. Ursus 26(2): 79–85. doi:10.2192/URSUS-D-15-00010.
Ciucci, P., and Boitani, L. 2008. The Apennine brown bear: A critical review of its status and conservation problems. Ursus 19(2): 130–145. doi:10.2192/07PER012.1.
Ciucci, P., Tosoni, E., Di Domenico, G., Quattrociocchi, F., and Boitani, L. 2014. Seasonal and annual variation in the food habits of Apennine brown bears, central Italy. J. Mammal. 95(3): 572–586. doi:10.1644/13-MAMM-A-218.
Cozzi, G., Chynoweth, M., Kusak, J., Çoban, E., Çoban, A., Ozgul, A., and Şekercioğlu, H. 2016. Anthropogenic food resources foster the coexistence of distinct life history strategies: year-round sedentary and migratory brown bears. J. Zool. 300(2): 142–150. doi:10.1111/jzo.12365. Crutzen, P.J., and Stoermer, E.F. 2000. The “Anthropocene.” Glob. Chang. Newsl. 41: 17–18.
doi:10.1111/j.1398-9995.2007.01564.x.
Dahle, B., and Swenson, J.E. 2003. Home ranges in adult Scandinavian brown bears (Ursus arctos): effect of mass, sex, reproductive category, population density and habitat type. J. Zool. 260: 329–335. doi:10.1017/S0952836903003753.
Dingle, H., and Drake, V.A. 2007. What Is Migration ? 57(2): 113–121.
Dirzo, R., Young, H.S., Galetti, M., Ceballos, G., Isaac, N.J.B., and Collen, B. 2014. Defaunation in the Anthropocene. Science 345(6195): 401–406. doi:10.1126/science.1251817.
Doledec, S., Chessel, D., and Gimaret-Carpentier, C. 2000. Niche Separation in Community Analysis: A New Method. Ecology 81(10): 2914. doi:10.2307/177351.
Elfström, M., Zedrosser, A., Støen, O.G., and Swenson, J.E. 2014. Ultimate and proximate mechanisms underlying the occurrence of bears close to human settlements: Review and management implications. Mamm. Rev. 44(1): 5–18. doi:10.1111/j.1365-2907.2012.00223.x. European Environment Agency. 2008. Corine Land Cover. Available from
https://www.eea.europa.eu/themes/landuse/intro.
Fahrig, L. 2003. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 34(1): 487–515. doi:10.1146/annurev.ecolsys.34.011802.132419.
Falcucci, A., Ciucci, P., Maiorano, L., Gentile, L., and Boitani, L. 2009. Assessing habitat quality for conservation using an integrated occurrence-mortality model. J. Appl. Ecol. 46(3): 600–609. Blackwell Publishing Ltd. doi:10.1111/j.1365-2664.2009.01634.x.
Fortin, D., Beyer, H.L., Boyce, M.S., Smith, D.W., Duchesne, T., and Mao, J.S. 2005. Wolves Influence Elk Movements: Behavior Shapes a Trophic Cascade in Yellowstone National Park. Ecology 86(5): 1320–1330. doi:10.1890/04-0953.
21 Frąckowiak, W., Theuerkauf, J., Pirga, B., and Gula, R. 2014. Brown bear habitat selection in relation to anthropogenic structures in the Bieszczady Mountains, Poland. Biologia (Bratisl). 69(7): 926– 930. doi:10.2478/s11756-014-0386-4.
Groom, J, M., Meffe, K, G., and Carrol, C, R. 2006. Principles of Conservation Biology. In Third. Sinauer Associates, Sunderland, Massachusetts.
Herfindal, I., Linnell, J.D.C., Odden, J., Nilsen, E.B., and Andersen, R. 2005. Prey density, environmental productivity and home-range size in the Eurasian lynx (Lynx lynx). J. Zool. London 265: 63–71. doi:10.1017/S0952836904006053.
Herrero, S., Smith, T., Debruyn, T.D., Gunther, K., and Matt, C.A. 2005. From the Field: Brown bear habituation to people-safety, risks, and benefits. Wildl. Soc. Bull. 33(1): 362–373. doi:10.2307/3784879.
Hirzel, A.H., Hausser, J., Chessel, D., and Perrin, N. 2002. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 83(7): 2027–2036. doi:10.1890/0012-9658(2002)083[2027:ENFAHT]2.0.CO;2.
Hirzel, A.H., Le Lay, G., Helfer, V., Randin, C., and Guisan, A. 2006. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Modell. 199(2): 142–152. doi:10.1016/j.ecolmodel.2006.05.017.
Horne, S., Garton, O., and Lewis, S. 2007. Analyzing Animal Movements Using Brownian Bridges. Ecology 88(9): 2354–2363.
Huber, Đ., Jakšić, Z., Frković, A., Štahan, Ž., Kusak, J., Majnarić, D., Grubešić, M., Kulić, B., Sindičić, M., Majić Skrbinšek, A., Lay, V., Ljuština, M., Zec, D., Laginja, R., and Francetić, I. 2008a. Brown Bear Management Plan for the Republic of Croatia. 1: 86.
Huber, D., Kusak, J., and Frkovic, A. 1998. Traffic Kills of Brown Bears in Gorski Kotar, Croatia. Ursus 10: 167–171. doi:10.2307/3873124.
Huber, D., Kusak, J., Majić-Skrbinšek, A., Majnarić, D., Sindičić, M., Majić Skrbinšek, A., Majnarić, D., and Sindičić, M. 2008b. A multidimensional approach to managing the European brown bear in Croatia. Ursus 19: 22–32. doi:10.2192/1537-6176(2008)19[22:AMATMT]2.0.CO;2.
Huber, D., and Roth, H.U. 1993. Movements of european brown bears in croatia. Acta Theriol. (Warsz). 38(2): 151–159.
Johnson, C.J., Seip, D.R., and Boyce, M.S. 2004. A quantitative approach to conservation planning:using resource selection functions to map the distribution of mountain caribou at multiple spatial scales. J. Appl. Ecol. 41: 238–251.
Kaczensky, P., Blazic, M., and Gossow, H. 2004. Public attitudes towards brown bears (Ursus arctos) in Slovenia. Biol. Conserv. doi:10.1016/j.biocon.2003.10.015.
Kaczensky, P., Chapron, G., and Arx, M. Von. 2012. Status, management and distribution of large carnivores-bear, lynx, wolf & wolverine in Europe - Part 1. Eur. Comm. (December): 1–200.
22 Kaczensky, P., Gossow, H., Knauer, F., Krze, B., Jonozovic, M., and Adamic, M. 2003. The impact of high speed, high volume traffic axes on brown bears in Slovenia. Biol. Conserv. 111: 191– 204.
Kaczensky, P., Huber, D., Knauer, F., Roth, H., Wagner, A., and Kusak, J. 2006. Activity patterns of brown bears (Ursus arctos) in Slovenia and Croatia. J. Zool. 269(4): 474–485. doi:10.1111/j.1469-7998.2006.00114.x.
Kavčič, I., Adami, M., Kaczensky, P., Krofel, M., and Jerina, K. 2013. Supplemental feeding with carrion is not reducing brown bear depredations on sheep in Slovenia. Ursus 24(2): 111–119. doi:10.2192/URSUS-D-12-00031R1.1.
Kavčič, I., Adamič, M., Kaczensky, P., Krofel, M., Kobal, M., and Jerina, K. 2015. Fast food bears: brown bear diet in a human-dominated landscape with intensive supplemental feeding. Wildlife Biol. 21(1): 1–8. doi:http://dx.doi.org/10.2981/wlb.00013.
Kays, R., Crofoot, M.C., Jetz, W., and Wikelski, M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348(6240): aaa2478. doi:10.1126/science.aaa2478.
Klopcic, M., Jerina, K., and Boncina, A. 2010. Long-term changes of structure and tree species composition in Dinaric uneven-aged forests: Are red deer an important factor? Eur. J. For. Res. 129(3): 277–288. doi:10.1007/s10342-009-0325-z.
Kopatz, A., Eiken, H.G., Hagen, S.B., Ruokonen, M., Esparza-Salas, R., Schregel, J., Kojola, I., Smith, M.E., Wartiainen, I., Aspholm, P.E., Wikan, S., Rykov, A.M., Makarova, O., Polikarpova, N., Tirronen, K.F., Danilov, P.I., and Aspi, J. 2012. Connectivity and population subdivision at the fringe of a large brown bear (Ursus arctos) population in North Western Europe. Conserv. Genet. 13(3): 681–692. doi:10.1007/s10592-012-0317-2.
Kremen, C. 2015. Reframing the land-sparing/land-sharing debate for biodiversity conservation. Ann. N. Y. Acad. Sci. 1355(1): 52–76. doi:10.1111/nyas.12845.
Krofel, M., Filacorda, S., and Jerina, K. 2010. Mating-related movements of male brown bears on the periphery of an expanding population. Ursus 21(1): 23–29. doi:10.2192/09SC015.1.
Krofel, M., Špacapan, M., and Jerina, K. 2016. Winter sleep with room service: denning behaviour of brown bears with access to anthropogenic food. J. Zool.: 1–7. doi:10.1111/jzo.12421.
Kusak, J., Huber, D., Gomerčić, T., Schwaderer, G., and Gužvica, G. 2009. The permeability of highway in Gorski kotar (Croatia) for large mammals. Eur. J. Wildl. Res. 55(1): 7–21. doi:10.1007/s10344-008-0208-5.
Latham, A.D.M., Latham, M.C., and Boyce, M.S. 2011. Habitat selection and spatial relationships of black bears ( Ursus americanus ) with woodland caribou ( Rangifer tarandus caribou ) in northeastern Alberta. Can. J. Zool. 89(4): 267–277. doi:10.1139/z10-115.
Ličina, T., Krofel, M., Reljić, S., Huber, Đ., Jonozovič, M., Stergar, M., and Jerina, K. 2015. Impact of bear-vehicle collisions on Slovenian-Croatian brown bear population and its expansion into
23 the Alps. In LIFE DINALP BEAR (LIFE13 NAT/SI/000550) Report. Available from http://dinalpbear.eu/wp-content/uploads/2014/12/A4_Bear-vehicle-collisions_FINAL-report-ENG.pdf.
Majić, A., Marino Taussig de Bodonia, A., Huber, duro, and Bunnefeld, N. 2011. Dynamics of public attitudes toward bears and the role of bear hunting in Croatia. Biol. Conserv. 144(12): 3018– 3027. doi:10.1016/j.biocon.2011.09.005.
Mancinelli, S., Boitani, L., and Ciucci, P. 2018. Determinants of home range size and space use patterns in a protected wolf (Canis lupus) population in central Apennines, Italy. Can. J. Zool.: cjz-2017-0210. doi:10.1139/cjz-2017-0210.
Manly, B.F.J., Mcdonald, L.L., Thomas, D.L., and Erickson, W.P. 2002a. Resource selection by animals: statistical analysis and design for field studies, 2nd edn. Kluwer Academic Publishers, Boston, Massachusetts, USA. doi:10.1017/CBO9781107415324.004.
Manly, B.F.J.J., Mcdonald, L.L., Thomas, D.L., Erickson, W.P., McDonald, T.L., and Erickson, W.P. 2002b. Resource selection by animals: statistical design and analysis for field studies. Kluwer Academic Publishers, Boston, Massachusetts, USA. doi:10.1017/CBO9781107415324.004. Di Marco, M., Boitani, L., Mallon, D., Hoffmann, M., Iacucci, A., Meijaard, E., Visconti, P.,
Schipper, J., and Rondinini, C. 2014. A Retrospective evaluation of the global decline of carnivores and ungulates. Conserv. Biol. 28(4): 1109–1118. doi:10.1111/cobi.12249.
Martin, J., Basille, M., Van Moorter, B., Kindberg, J., Allaine, D., and Swenson, J. 2010. Coping with human disturbance: spatial and temporal tactics of the brown bear (Ursus arctos). Can. J. Zool. Can. Zool. 88(9): 875–883. doi:10.1139/Z10-053.
Mateo-Sánchez, M.C., Cushman, S.A., and Saura, S. 2014. Connecting endangered brown bear subpopulations in the Cantabrian Range (north-western Spain). Anim. Conserv. 17(5): 430–440. doi:10.1111/acv.12109.
Mattisson, J., Sand, H., Wabakken, P., Gervasi, V., Liberg, O., Linnell, J.D.C., Rauset, G.R., and Pedersen, H.C. 2013. Home range size variation in a recovering wolf population: Evaluating the effect of environmental, demographic, and social factors. Oecologia 173(3): 813–825. doi:10.1007/s00442-013-2668-x.
McRae, B.H., Dickson, B.G., Keitt, T.H., and Shah, V.B. 2008. Using circuit theory to model connectivity in ecology, evolution and conservation. 88(1): 36–59. doi:10.1002/ecm.1283. Ordiz, A., Kindberg, J., Sæbø, S., Swenson, J.E., and Støen, O.G. 2014. Brown bear circadian
behavior reveals human environmental encroachment. Biol. Conserv. 173: 1–9. doi:10.1016/j.biocon.2014.03.006.
Ordiz, A., Støen, O.G., Sæbø, S., Kindberg, J., Delibes, M., and Swenson, J.E. 2012. Do bears know they are being hunted? Biol. Conserv. 152: 21–28. doi:10.1016/j.biocon.2012.04.006.
24 and Boitani, L. 2016. Predicting the continuum between corridors and barriers to animal movements using Step Selection Functions and Randomized Shortest Paths. J. Anim. Ecol. 85(1): 32–42. doi:10.1111/1365-2656.12386.
Persson, J., Wedholm, P., and Segerström, P. 2010. Space use and territoriality of wolverines (Gulo gulo) in northern Scandinavia. Eur. J. Wildl. Res. 56(1): 49–57. doi:10.1007/s10344-009-0290-3.
Phalan, B., Onial, M., Balmford, A., and Green, R.E. 2011. Reconciling food production and biodiversity conservation Supporting Material. Science 333(September): 1289–1291. doi:10.1126/science.1208742.
Pinto, N., and Keitt, T.H. 2009. Beyond the least-cost path: Evaluating corridor redundancy using a graph-theoretic approach. Landsc. Ecol. 24(2): 253–266. doi:10.1007/s10980-008-9303-y. Powell, R. a. 2012. Diverse perspectives on mammal home ranges or a home range is more than
location densities. J. Mammal. 93(4): 887–889. doi:10.1644/12-MAMM-5-060.1.
Powell, R.A., and Mitchell, M.S. 2012. What is a home range? J. Mammal. 93(4): 948–958. doi:10.1644/11-MAMM-S-177.1.
Rigg, R., Find̂o, S., Wechselberger, M., Gorman, M.L., Sillero-Zubiri, C., and MacDonald, D.W. 2011. Mitigating carnivore-livestock conflict in Europe: Lessons from Slovakia. Oryx 45(2): 272–280. doi:10.1017/S0030605310000074.
Ripple, W.J., Estes, J. a, Beschta, R.L., Wilmers, C.C., Ritchie, E.G., Hebblewhite, M., Berger, J., Elmhagen, B., Letnic, M., Nelson, M.P., Schmitz, O.J., Smith, D.W., Wallach, A.D., and Wirsing, A.J. 2014. Status and ecological effects of the world’s largest carnivores. Science 343(6167): 1241484. doi:10.1126/science.1241484.
Roth, H.U., and Huber, D. 1986. Diel activity of brown bears in Plitvice Lakes National Park, Yugoslavia. Int. Conf. Bear Res. Manag. 6: 177–181.
Saether, B.E., Engen, S., Persson, J., Broseth, H., Landa, a, and Willebrand, T. 2005. Management strategies for the wolverine in Scandinavia. J. Wildl. Manage. 69(3): 1001–1014. doi:doi:10.2193/0022-541X(2005)069[1001:MSFTWI]2.0.CO;2.
Selva, N., Teitelbaum, C.S., Sergiel, A., Zwijacz-Kozica, T., Zięba, F., Bojarska, K., and Mueller, T. 2017. Supplementary ungulate feeding affects movement behavior of brown bears. Basic Appl. Ecol. 24: 68–76. doi:10.1016/j.baae.2017.09.007.
Støen, O.G., Ordiz, A., Evans, A.L., Laske, T.G., Kindberg, J., Fröbert, O., Swenson, J.E., and Arnemo, J.M. 2015. Physiological evidence for a human-induced landscape of fear in brown bears (Ursus arctos). Physiol. Behav. 152: 244–248. doi:10.1016/j.physbeh.2015.09.030. Treves, A., and Karanth, K.U. 2003. Human-Carnivore Conflict and Perspectives on Carnivore
Management Worldwide. In Conservation Biology. pp. 1491–1499. doi:10.1111/j.1523-1739.2003.00059.x.
25 Vander Wal, E., and Rodgers, A.R. 2012. An individual-based quantitative approach for delineating core areas of animal space use. Ecol. Modell. 224(1): 48–53. Elsevier B.V. doi:10.1016/j.ecolmodel.2011.10.006.
Waters, C.N., Zalasiewicz, J., Summerhayes, C., Barnosky, A.D., Poirier, C., Gałuszka, A., Cearreta, A., Edgeworth, M., Ellis, E.C., Ellis, M., Jeandel, C., Leinfelder, R., McNeill, J.R., Richter, D.D.B., Steffen, W., Syvitski, J., Vidas, D., Wagreich, M., Williams, M., Zhisheng, A., Grinevald, J., Odada, E., Oreskes, N., and Wolfe, A.P. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science. doi:10.1126/science.aad2622.
Woodroffe, R. 2000. Predators and people: using human densities to interpret declines of large carnivores. Anim. Conserv. 3(2): 165–173. doi:10.1111/j.1469-1795.2000.tb00241.x.
Zedrosser, A., Dahle, B., Swenson, J.E., and Gerstl, N. 2001. Status and Management of the Brown Bear in Europe. Ursus 12: 9–20.
Zedrosser, A., Steyaert, S.M.J.G., Gossow, H., and Swenson, J.E. 2011. Brown bear conservation and the ghost of persecution past. Biol. Conserv. 144(9): 2163–2170. Elsevier Ltd. doi:10.1016/j.biocon.2011.05.005.
Zimmermann, B., Nelson, L., Wabakken, P., Sand, H., and Liberg, O. 2014. Behavioral responses of wolves to roads: Scale-dependent ambivalence. Behav. Ecol. 25(6): 1353–1364. doi:10.1093/beheco/aru134.
26
Chapter I
Brown bear space-use patterns in a landscape of food and fear
Daniele De Angelis1, Djuro Huber2, Slaven Reljic2, Paolo Ciucci1, Josip Kusak2
1 Sapienza University of Rome, Dept. of Biology and Biotechnology “Charles Darwin”, Viale dell’Università
32, Roma 00185, Italy
27
ABSTRACT
Studying how animals interact with their environment is fundamental to address proper conservation and management actions, especially when dealing with space-demanding species living in human-affected landscapes such as large carnivores. In this study, we investigated seasonal and anthropogenic influences on home-range size and configuration of Dinaric brown bears (Ursus
arctos) inhabiting areas differing in terms of road and human density, as well as in the availability of
supplementary feeding sites. Between 2004 and 2016, we GPS-tracked 10 brown bears (3 females, 7 males) and used Brownian bridge movement models (BBMMs) to estimate circadian and seasonal home ranges. We used linear mixed-effect models (LMM) to investigate the effects of gender, time of the day, season and study area on home-range size. Using an individual-based method, we also depicted seasonal core areas and used Environmental Niche Factor Analysis (ENFA) to investigate possible habitat-mediated responses to human presence and activity. Although we failed to find a sex effect on home-range size, time of day was an important predictor of home-range size, with nocturnal home ranges larger than diurnal ones. We also detected a seasonal effect on home-range size, but this was limited to areas where bears had relatively lower accessibility to artificial feeding sites. In the northern area, characterized by higher densities of roads, human settlements, and artificial feeding sites, selection of core areas by bears was characterized by avoidance for anthropogenic features.
INTRODUCTION
Ensuring persistence of large carnivores in human-altered environments is pivotal for the conservation of functional ecosystems in future scenarios of human expansion (Carroll et al. 2001). During the last decades, large carnivores have recovered large part of their former range in Europe, fostered by countryside abandonment, legal protection and their intrinsic capability to adapt to human dominated landscapes (Chapron et al. 2014; Carter and Linnell 2016). However, large carnivores are a controversial group and their presence may cause substantial social conflict, far from being solved in many European countries (Liberg et al. 2012). Competing with humans for space is often the main challenge that such species must overcome to functionally persist in shared landscapes (Treves and Karanth 2003). Accordingly, large carnivores often adjust their space use patterns to human presence and activity, aiming at ensuring access to fundamental resources while reducing direct contacts with humans (Martin et al. 2010; Kertson et al. 2011; Mancinelli et al. 2018).
The brown bear (Ursus arctos) is one of the most successful large carnivores worldwide, and its populations in Europe are the most numerous (Chapron et al. 2014). Its widespread presence in a heavily human-altered continent suggests that the brown bear adopted efficient coexistence strategies, likely based on avoiding humans and their activities, while, in some cases, taking advantage of anthropogenic resources (Cozzi et al. 2016; Krofel et al. 2016; Selva et al. 2017). Past persecution in Europe (Treves and Karanth 2003) and current human disturbance are among the main factors explaining some of the behavioural differences observed between bear populations living in the old continent and those living in more pristine areas of North America (Woodroffe 2000; Munro et al. 2006; Martin et al. 2010; Ordiz et al. 2013, 2014). This can be observed by the combined adoption of space use patterns aimed at minimizing human-related disturbance and more nocturnal activity patterns compared to North American grizzlies (Klinka and Reimchen 2002; Kaczensky et al. 2006; Munro et al. 2006). Human-induced changes are sometimes observable even within the same population range, with bears adapting both space use and activity patterns. For example, bears can show more pronounced nocturnal activity in areas where road density is higher (Ordiz et al. 2014), or selecting more remote areas where human disturbance is more intense (Martin et al. 2010). Bear
29 space use patterns can also be largely affected by the presence of anthropogenic food (e.g. garbage dumps, supplemental feeding), leading to important management implications (see Cozzi et al. 2016; Selva et al. 2017).
Previous studies conducted on brown bears reported gender, reproductive status and, sometimes, age as the main determinants of home-range size in bears (Dahle and Swenson 2003a, c; Dahle et al. 2006). Seasonal variation in bear space use has been reported for bears living in wilderness areas of North America, with bears moving less when food availability increases (late summer-fall) (McLoughlin et al. 1999). However, little is known about bear home ranges and their seasonal variation in European populations (Clevenger et al. 1990; Dahle and Swenson 2003a; Mertzanis et al. 2005) and little information is available about human-related effects on variation in home-range size of large carnivores (Grinder and Krausman 2001). Indeed, anthropogenic factors (e.g. artificial feeding) might mitigate the effect of ecological drivers known to play a major role in shaping individual spatial patterns (e.g. sex, age-class), which in turn could produce unexpected effects at the population level (Cozzi et al. 2016).
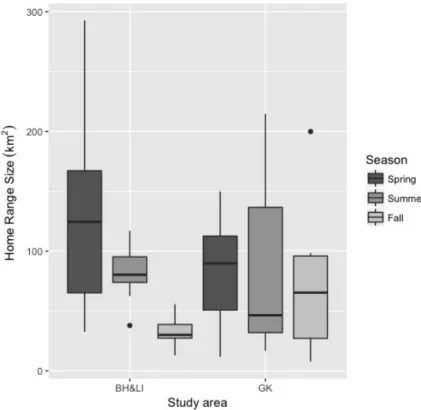
In this study, we investigated ecological and human-related factors possibly affecting space use patterns of Dinaric-Pindos brown bears. With about 3070 estimated individuals, the Dinaric-Pindos population is the third largest bear population in Europe (Chapron et al., 2014). Using the Dinaric-Pindos population as a case study, provided us with the opportunity to investigate space use patterns of bears in a region of Europe highly suitable for the presence of the species (Kusak and Huber 1998; Kusak et al. 2009), although substantial hunting activities and road development. Under the hypothesis that brown bear spatial patterns might be influenced by different degrees of human disturbance, we investigated seasonal home ranges and configuration of core areas of GPS-collared bears inhabiting sub-areas with similar environmental conditions but different density of human-related structures (i.e. settlements, roads, supplemental feeding sites). We formulated the following predictions: we expected a study-area dependent circadian response in bear ranges; where human disturbance is higher, bears are expected to be more nocturnal (prediction I); we expected no significant seasonal effect on home-range size in areas with high density of artificial feeding, as the
30 presence of year-round available artificial food might reduce seasonal variation in space use patterns (prediciton II); finally, we expected human-related variables to play a major role in the selection of core areas in more human-altered areas (prediction III).
METHODS
Study Areas
We GPS-tracked movements of brown bears inhabiting three distinct areas: Gorski kotar (GK), Lika (LI) and a transboundary area between Croatia and Bosnia Herzegovina (BH) (Figure 1). The GK area (≈1776 km2) is situated in northern Croatia and it is a core area for large carnivores (Kusak
et al. 2009). Elevations in GK reach 1500 m (median: 750), and about 88% of the whole area is covered by forests. Only <4% of GK is within protected areas (NP Risnjak), and it has a higher percentage of human settlements compared to LI and BH (≈2 km2 of settlements per 100 km2). Mean
paved road density in GK is 0.7 km/km2 (± 1 SD, range 0-6.0 km/km2), and it is crossed in its middle
by the Rijeka-Zagreb highway.The portion of Lika region where bears were tracked (hereafter “LI” ≈610 km2) is situated in the central part of Croatia. It has elevations up to 1270 m (median: 754) and
about 76% of its territory is covered by forest. About 48% of LI area is within the Plitvice Lakes National Park (PLNP). Human presence in this area increases during summer, with the increase of touristic activities. In BH area (≈1640 km2), elevations reach 1635 m (median: 672), forests covers
about 68% of the area and the Una National Park encompasses about 13% of the study area (if you have calculated this by yourself, then this should go in to results). Both LI and BH have lower road density (0.3 km/km2 ±0.6 SD range 0-4.2 and 0.3 km/km2 ±0.6, range 0-3.3, respectively) compared
to GK, and settlements density is <1 km2 per 100 km2. All three areas are located within the Dinaric
mountain range, a mountainous region characterized by karst topography (Huber & Roth, 1993; Kaczensky et al., 2006). Forests below 800 meters are dominated by a mixture of Fagus sylvatica,
Abies alba, Acer pseudoplatanus, Ulmus spp., with Picea abies growing on cooler places (e.g. high
mountain valleys, sink holes), whereas higher elevations (900-1300 m) are dominated by pure beech stands. The study areas are all inhabited by large- and meso-carnivore species, such as lynx (Lynx
31
lynx), wolf (Canis lupus), brown bear (Ursus arctos), badger (Meles meles), marten (Martes martes, Martes foina), red fox (Vulpes vulpes), and ungulates including roe deer (Capreolus capreolus), red
deer (Cervus elaphus) and wild boar (Sus scrofa) (Kusak and Krapinec 2010). In Croatia bear hunting is allowed from 16 September to 15 December and from 16 February to 15 May (Bišćan et al. 2016) outside protected areas and within predefined quotas, while in B&H bears are hunted form 1st October to 15th May (Huber et al., 2008). In all three study areas, supplemental feeding for ungulates (especially wild boars) and bears is practiced outside protected areas through year-round active artificial feeding sites, which are mostly represented by timer-activated corn dispensers (Huber et al., 2008; Krofel et al., 2016).
Live-trapping and telemetry
Bears were lured at trap sites with various baits (e.g. corn or animal carcasses), then live-captured using spring activated foot-snares (Aldrich snare). Captured bears were measured, sexed, and aged based on vestigial tooth analysis (Hensel and Sorensen 1980). Bears of all ages were equipped with Global Positioning System and Global System for Mobile Communications collars (GPS-GSM). Collar fix rates ranged 1 fix/15 mins to 1 fix/2 hours. For homogeneity, we subsampled all GPS data at one fix every 2 hours. We included only data from collars with GPS fix success rate >50% and obtained average collar success rate of 81.9% (±15.4 SD; range: 52.0–97.6%). We dropped all 2-D fixes with a value of Horizontal Dilution of Precision (HDOP) > 5 (Lewis et al., 2007), which corresponded on average to < 5% (±5.5 SD) of locations loss for each bear-season.
Home range analyses
To be consistent with the ecological concept of home range, we retained in the analysis only bears displaying resident-like space-use patterns through Net Squared Displacement (Bunnefeld et al. 2011). We then conducted home range analysis accounting for seasonal and circadian effects. We defined biological seasons considering mating period, and main annual dietary shifts previously reported for Dinaric bears (Krofel et al. 2010; Kavčič et al. 2015): spring period (15th March–14th
June) corresponded to post-denning hypophagia and mating, summer period (15th June–14th
32 period (15th September–14th November), corresponded to late hyperphagia and diet mostly based on
hard mast and fruit. We excluded winter due to hibernation or, for those bears that did not den, their highly-reduced activity. To investigate a circadian effect on movement ranges, we classified diurnal and nocturnal fixes using the solarpos function in the maptools R package (Lewin-koh and Bivand 2003), which differentiates day and night based on the position of the sun relative to the horizon for each location, time and date.
We estimated yearly and seasonal home-range sizes using Brownian Bridge movement models (BBMMs), as this method handles the spatio-temporal autocorrelation that characterizes GPS fixes by explicitly incorporating time between successive relocations, thus, predicting movement paths more realistically than classical kernel estimators (Horne et al. 2007; Walter et al. 2011). For each bear and season, we estimated σ1 using the maximum likelihood approach implemented in the
function liker of the adehabitatHR R package (Calenge, 2006), while we set at σ2 = 25 m the inherent
imprecision of GPS locations (Ćirović et al. 2015). Based on a grid size of 50x50m, we then quantified home-range size as the area encompassed by the 95% isopleth of the BBMM utilization distribution (UD).
Cumulative functions of home-range size against sampling period indicated that >6 consecutive months (>180 days) were necessary to approximate annual home ranges (FigureS1). We considered this threshold acceptable given that we included only resident bears, whose ranges are expected to increase asymptotically. Similarly, we included in seasonal analysis bears tracked for more than 7 weeks (>49 days) (Figure S2). We accepted to include one bear tracked during 47 days to increment the number of females in our dataset. Finally, we discarded years and seasons with GPS gaps >72 hours to avoid home range overestimation, which is an expected outcome when using BBMM (Walter et al., 2011).
We fitted linear mixed-effect models (LMMs) using lme4 R package (Bates et al. 2015) to examine variation in log-transformed home-range sizes (dependent variable) in response to study area, time of the day (i.e., day vs. night), season, bear sex and age class (Börger et al. 2006). Only for this analysis, we pooled bears in BH and LI given the lower road, human settlement and artificial
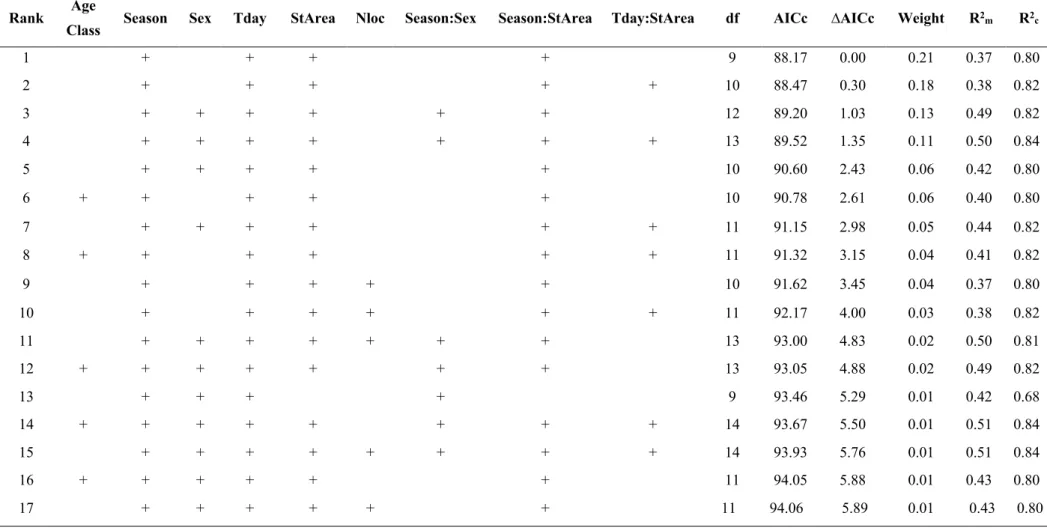
33 feeding sites densities in both areas in contrast to GK. We therefore obtained an almost balanced dataset, with similar sex and age class ratios both in GK and in BH & LI (Table 1). Then, we included three interactions: between time of the day and study area, to test for a stronger circadian effect in the area with higher human disturbance (prediction I); between season and study area, to test for an effect of supplemental feeding on home range seasonal variation (prediction II); and between sex and season, to account for sex-dependency on seasonal ranges (Dahle and Swenson 2003a). We also added the number of locations as a model covariate to account for differences due to sample size. Given the nested structure of our data and to account for data autocorrelation and individual variability, we fitted all models with ‘individual’ as random factor (Hebblewhite and Merrill 2008). We checked for correlation between the variables using Pearson’s correlation coefficient (r<0.6) and Variance Inflation Factor analysis (VIF<2) to check for collinearity (Zuur et al. 2010). Starting from a saturated model including the aforementioned interactions, we explored all possible models through an automated model selection (dredge function in MumIN R package; Barton, 2015) based on corrected Akaike’s information criterion (AICc) scores (Burnham and Anderson 2002). We finally performed conditional model averaging (model.avg function, MumIN R package; Barton, 2015), including only models with ∆AIC≤6 to minimize the risk of spurious results from parameter estimates of models with low weight (Richards et al. 2011; Mattisson et al. 2013). For each model, we measured the variance explained by only fixed factors, and by both fixed and random factors by computing marginal (Rm2) and conditional (Rc2) r-squared, respectively (Nakagawa and Schielzeth 2013).
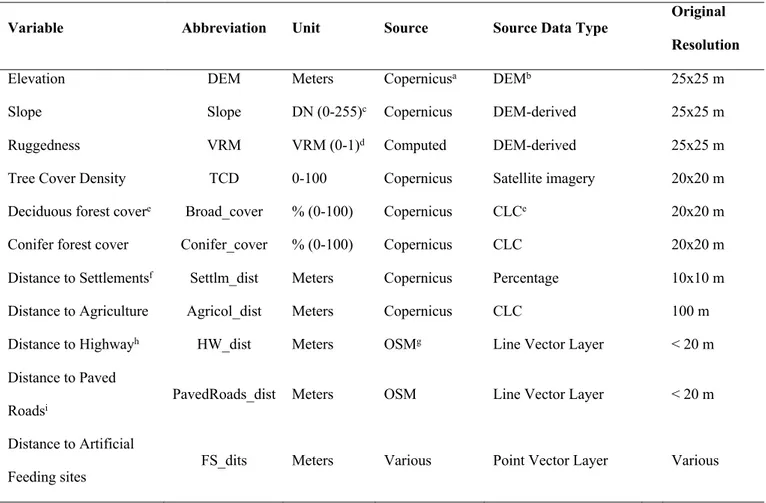
To assess factors affecting within-home range space use patterns, we calculated seasonal core areas by adopting the approach developed by Vander Wal & Rodgers (2012), which extracts the UD-volume proportion delimiting the area most intensively used by each individual (i.e. core area isopleth). Following Mancinelli et al. (2018), we determined the main habitat features discriminating seasonal core areas from the whole home range, should selection be operated by bears when selecting core areas within the home range. To achieve this, we conducted an Environmental Niche Factor Analysis for each seasonal home range (Hirzel et al. 2002) implemented in adehabitatHS R package (Calenge 2006). The main advantage offered by this method is represented by the opportunity of
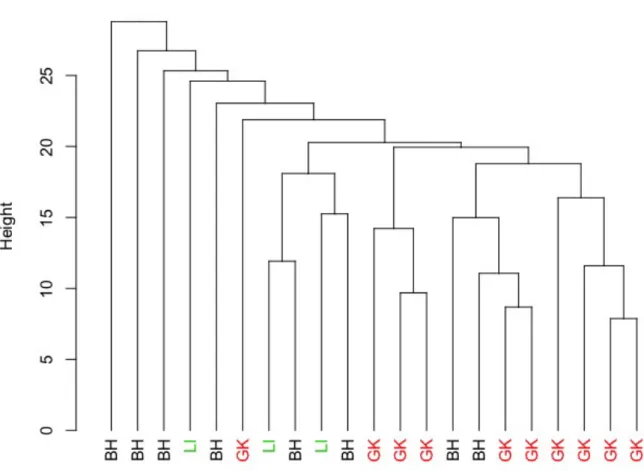
34 producing a good description of a species niche while dealing with presence-only autocorrelated data (Basille et al. 2008). We compared environmental conditions at each pixel within core areas with those in the entire home range. Therefore, we obtained marginality values indicating how eco-geographical characteristics within each seasonal core area differed from those available in the home range, whereas specialization values indicated the level of tolerance in respect to each variable (Hirzel et al. 2002). We chose topographic, vegetation type and distance to human-related variables that we thought might influence the selection of core areas (Table S2). All variables were resampled in a GIS environment (QGIS Development Team 2017) using a common origin and 50x50m pixel size. To visualize environmental features characterizing the selection of core areas within seasonal home ranges at the population level, we ranked marginality values within each bear-season (ranking range 1-11). Finally, to investigate similarities in the selection of core areas, we grouped seasonal core areas based on their marginality scores through a hierarchical clustering using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) algorithm (McGarigal et al. 2000). This method initially places each observation in its own cluster, and then successively joins clusters together based on their “closeness”. To measure “closeness” while accounting for autocorrelation in the environmental variables, we computed Mahalanobis distances between ENFA marginality values rescaled to range from -1 to 1 (McGarigal et al. 2000).
RESULTS
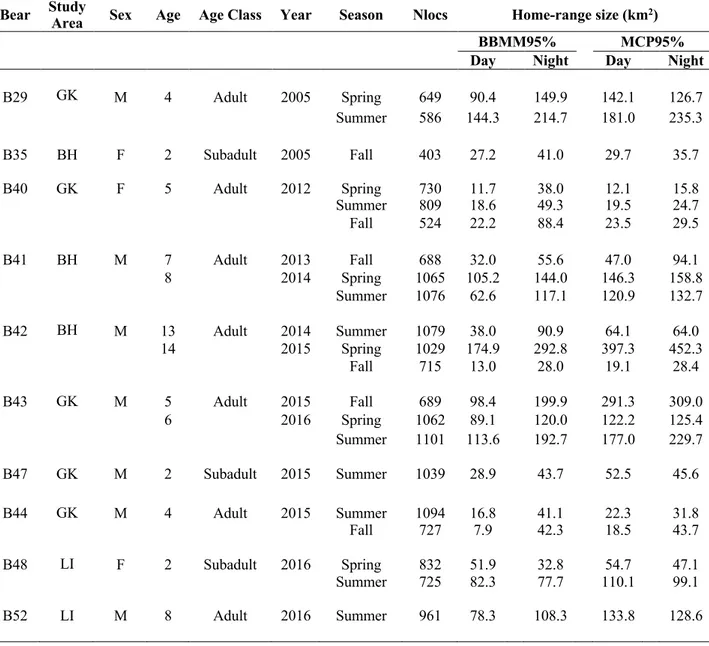
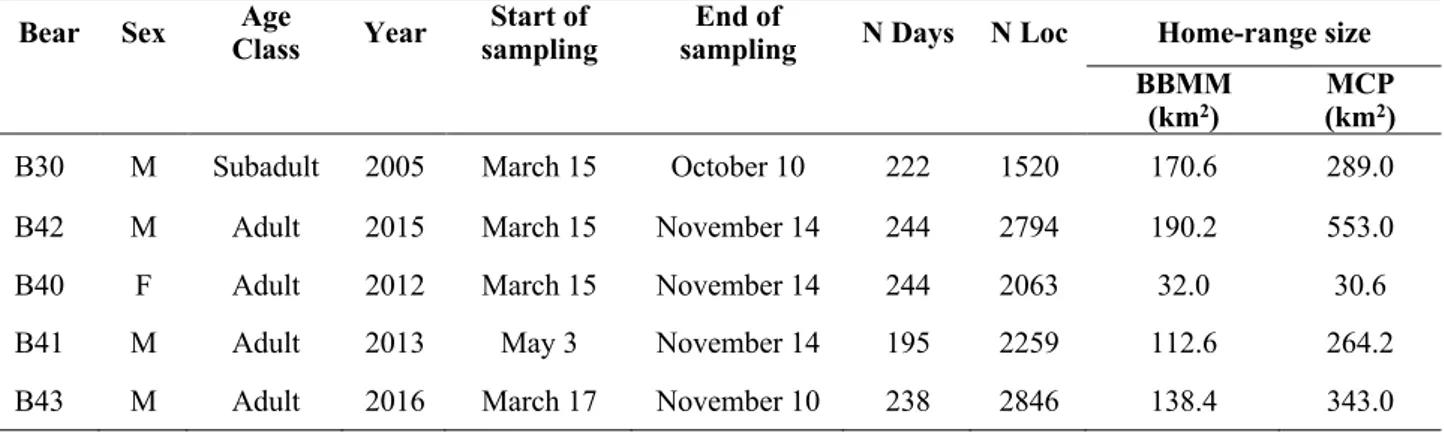
From 2004-2016, 19 bears were captured and equipped with GPS-collars. Nine bears were excluded from the analysis due to important collar failure or insufficient tracking period. We estimated seasonal home ranges for the remaining 10 bears: five in GK (4 males and 1 female) and other five in BH and LI (3 males and 2 females). Overall, we estimated 21 diurnal seasonal home ranges and 21 nocturnal ones (Table 1) We estimated annual home ranges for five bears, which averaged 128.8 (±61.7) km2 (Table 2).
All tracked bears crossed the boundaries of the National Parks within the study areas, except for a subadult female, whose annual home range was entirely comprised within the Plitvice Lakes NP