ContentslistsavailableatScienceDirect
Acta
Histochemica
j ou rn a l h o m e p a g e :w w w . e l s e v i e r . d e / a c t h i s
Melatonin
action
in
tumor
skeletal
muscle
cells:
an
ultrastructural
study
S.
Burattini
a,∗,
M.
Battistelli
a,
S.
Codenotti
b,
E.
Falcieri
a,
A.
Fanzani
b,1,
S.
Salucci
a,1aDepartmentofBiomolecularSciences(DiSB),UrbinoUniversityCarloBo,ViaCa’LeSuore2,61029Urbino,Italy bDepartmentofMolecularandTranslationalMedicine,BresciaUniversity,VialeEuropa11,25123Brescia,Italy
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received14October2015
Receivedinrevisedform19February2016 Accepted19February2016 Keywords: Apoptosis Melatonin Rhabdomyosarcoma
a
b
s
t
r
a
c
t
Melatonin(Mel),orN-acetyl-5-methoxytryptamine,isacircadianhormonethatcandiffusethroughall thebiologicalmembranesthankstoitsamphiphilicstructure,alsoovercomingtheblood–brainbarrier andplacenta.AlthoughMelhasbeenreportedtoexhibitstrongantioxidantpropertiesinhealthytissues, studiescarriedoutontumorculturesgaveadifferentpictureofitsaction,oftendescribingMelaseffective totriggerthecelldeathoftumorcellsbyenhancingoxidativestress.
Basedonthispremise, hereMeleffectwasinvestigatedusingatumorcelllinerepresentativeof thehumanalveolarrhabdomyosarcoma(ARMS),themostfrequentsofttissuesarcomaaffecting child-hood.Forthispurpose,Melwasgiveneitherdissolvedinethanol(EtOH)ordimethylsulfoxide(DMSO) atdifferentconcentrationsandtimeexposures.Cellviabilityassaysandultrastructuralobservations demonstratedthatMelwasabletoinduceadose-andtime-dependentcelldeathindependentlyonthe dissolutionsolvent.Microscopyanalyseshighlightedthepresenceofvariousapoptoticandnecrotic pat-ternscorrelatingwiththeincreasingMeldoseandtimeofexposure.ThesefindingssuggestthatMel, triggeringapoptosisinARMScells,couldbeconsideredasapromisingdrugforfuturemultitargeted therapies.
©2016PublishedbyElsevierGmbH.
1. Introduction
Humanrhabdomyosarcoma(RMS)isthemostcommon
pedi-atricsofttissuesarcomathatarisesfrommesenchymalprecursors
withthepotentialtodifferentiateintoskeletalmusclecells,but
failingtocompletemyogenesisbecauseofthepresenceof
chromo-somalaberrations(Zanolaetal.,2012).RMSareaggressivetumors
whose current chemotherapy is often unsuccessful(Dantonello
etal.,2015),especiallyforthealveolarrhabdomyosarcoma(ARMS)
thatischaracterizedbyachromosomaltranslocationthat
generat-ingthePax3-Foxo1fusionproteinconfersamoreaggressivetumor
phenotypeandresistancetoradiationtherapy(Jothietal.,2013).
Currently,theemploymentofamultimodaltherapyhasincreased
thesurvivalrateforpatientswithlocalizeddiseaseto70%albeit
withsignificanttoxicity(Soundararajanetal.,2012).Despitethat,
∗ Correspondingauthor.Fax:+390722304244. E-mailaddresses:[email protected]
(S.Burattini),[email protected](M.Battistelli),[email protected] (S.Codenotti),[email protected](E.Falcieri),[email protected] (A.Fanzani),[email protected](S.Salucci).
1 Theseauthorsequallycontributedtothiswork.
thetherapysuccessforhighriskpatientsisabout20–30%(Lietal.,
2013),suggestingtheneedofmoreeffectivedrugs.
Over thepast years, Mel,besideits strongand documented
antioxidanteffects (Saluccietal.,2014b;Ganieetal.,2015),has
been shown to act as an anticancer drug in different tumors
(Sánchez-Hidalgoetal.,2012;Batistaetal.,2014;Codenottietal.,
2015),forexampleviainductionofthemitochondrialapoptosis
(Bejaranoetal.,2009)orattenuationoftelomeraseactivitybothin
vivoandinvitro(Leon-Blancoetal.,2003).
Numerousstudieshavedescribeditsanticanceractioninsolid
tumorsand inleukemiathroughinductionoftheapoptoticcell
death,leadingtoreplacementofneoplasticcellswithhealthycells
(Sánchez-Hidalgo etal., 2012;Chuffa etal.,2015).Additionally,
Kubatkaetal.(2001)demonstratedtheanti-tumoractionofMelin
breastcancerinvivo,andthiseffectwasthenconfirmedinclinical
studiesshowingitsprotectiveeffectfrommammary
carcinogen-esis(Nowfaretal.,2002)andinotherneoplasticdiseases(Hong etal.,2014;Wuetal.,2015).
Herewehaveevaluatedtheultrastructuralchangesoccurring
inthehumanalveolarRH30cellsuponadministrationofMel
dis-solvedeitherinEtOH(Saluccietal.,2014a)orDMSO(Jardim-Perassi
etal.,2014)atdifferentdosesandexposuretimes.
http://dx.doi.org/10.1016/j.acthis.2016.02.004 0065-1281/©2016PublishedbyElsevierGmbH.
S.Burattinietal./ActaHistochemica118(2016)278–285 279 2. Materialsandmethods
2.1. Cellculture
Human RH30 cell line, purchased from the European
Col-lection of Cell Cultures (ECACC; Salisbury, UK), was routinely
tested for the expression of the Pax3-Foxo1 chimeric protein
byboth genesequencingand westernblotanalysisusing
poly-clonalanti-Foxo1(H-128)antibody(Rabbit,sc-11350,SantaCruz
Biotecnology, Dallas, USA). Cells were also routinely tested for
mycoplasmacontaminationusingtheLookOutMycoplasmaPCR
DetectionKit (MP0035 Lookout,Sigma), according tothe
man-ufacturer’sinstructions.RH30cellswereculturedinRPMI1640,
supplementedwith10%heat-inactivatedfetalbovineserum,2mM
glutamine,1%antibiotics(PenicillinandStreptomycin)and
main-tained at37◦Cin humidified airwith5% CO2 (Codenotti etal.,
2015).
Mel(Sigma,St.Louis,MO,USA)wasdissolvedin1%DMSOor1%
EtOHattheconcentrationof100mM.After1or2mMMel
treat-ment,thecellbehaviorwasmonitoredbymeansoftheinverted
microscopy.TrypanBlueexclusionassaywasemployedto
quan-tifycellviability.Therelativenumberofdeadandlivingcellswas
obtainedcountingthenumber of stained(dead)and unstained
(live)cellsusingaNeubauerchamberatrevertedmicroscope.
Stu-dent’st-testwasappliedforstatisticalanalysestocompareresults
obtainedincontrolversustreatedsamples;P<0.05wasconsidered
assignificancethreshold.
2.2. Scanningelectronmicroscopy(SEM)
RH30cells werecultured oncoverslipsin Petridishes. After
washing with0.1Mphosphate buffer, adherent and suspended
cells were fixed with 2.5% glutaraldehyde in 0.1M phosphate
bufferfor1h.Thesuspendedcellpopulationwasdepositatedon
polylysine-coatedcoverslipsandthesampleswerepost-fixedwith
1%osmiumtetroxide(OsO4)in 0.1Mphosphatebuffersolution
(PBS)for1h.Afteralcoholdehydration,thesampleswere
criti-calpointdried,goldsputteredandobservedusingaPhilips515
scanningelectronmicroscope(Codenottietal.,2015).
2.3. Transmissionelectronmicroscopy(TEM)
RH30-treatedcellswerewashedandfixedwith2.5%
glutaralde-hydein0.1MPBSfor15min.Thecellswerescrapedandcentrifuged
at300xgfor10min.Thepelletswerefixedin2.5%
glutaralde-hydeforanadditional30min.Thesuspendedcellswerecollected
inEppendorf,centrifugedandfixedfor45mininglutaraldehyde.
Thesampleswerepost-fixedin1%OsO4for1h,alcoholdehydrated
andembeddedinaraldite.Thinsectionswerestainedwithuranyl
acetateandleadcitrateandanalyzedusingaPhilipsCM10
trans-missionelectronmicroscope(Saluccietal.,2014b).
2.4. Acridineorange(AO)andpropidiumiodide(PI)nuclei stainingconfocallaserscanningmicroscope(CLSM)
Cells werefixed with4% paraformaldehydein PBS (pH 7.4)
for30min,andsuspendedcellsweredepositedonpoly-lysinated
coverslips in Petri dishes. Adherent and suspendend samples
werewashedtwiceusingPBSandthen pre-treatedwithRNase
A10g/mL inPBSfor 30min.AfterPBSwashing sampleswere
exposedtoanequalmixtureofPI(1g/mL,LifeTechnologies)and
AO(1g/mL,LifeTechnologies)dilutedinPBSatroomtemperature
(protectedfromlight)for10minandthenobservedatCLSM(Leica
MicrosystemsCMSGmbH);FICTandPIexcitationwereat488and
500nm,respectively,andtheiremissionsignalsweredetectedat
617and525nm.CLSMimagesarepresentedasmaximumintensity
projectionorsingle-planeimages(Saluccietal.,2014b).
2.5. Westernblotting
Proteinhomogenateswereobtainedbyharvestingcellsinacold
lysisbuffer,composedby20mMTris–HCl(pH7.6),1%NonidetP40,
0.5%sodiumdeoxycholate,0.1%SDS,50mMNaClandacocktail
ofproteaseinhibitors(Roche)plusphosphataseinhibitors(1mM
Na3VO4and4mMNaF).
Bradford reagent assay has been used to calculate protein
concentration. Equal amount of protein samples were
sepa-ratedbySDS-PAGEunderreducingconditionsandtransferredto
polyvinylidinefluoridemembranes.Samplewereincubatedwith
specific primaryantibodies, Rabbitanti-Bax, polyclonal (Rabbit,
sc-526,SantaCruzBiotecnology,Dallas,USA)orRabbit
anti-Bcl-2, polyclonal (Rabbit, sc-492, Santa Cruz Biotecnology, Dallas,
USA) followed by peroxidase-conjugated secondary antibodies
(anti-mouseIgGfromSantaCruzBiotechnology,Dallas,USA;
anti-rabbitIgGfromThermoScientific,Erembodegem,Belgium).The
immunocomplexeswerevisualizedusingtheenhanced
chemilu-minescencereagent(GeneSpin,Milan,Italy)andimmune-reactive
bandswerequantifiedusingdensitometryanalyses(SoftwareGel
ProAnalyzer,version4)(Codenottietal.,2015).
2.6. Insilicoanalysis
The primary mouse tumor cultures fromRMS-bearing mice
werepreviouslyobtainedbyKellerandcolleagues(Rubinetal.,
2011)andutilizedforgeneexpressionanalysisbyemployingthe
microarrayapproach(IlluminaMouseRef-8BeadChip,v1.1).Data
setsweredepositedintheNCBIGeneExpressionOmnibusdatabase
with the accession number GSE22520. To analyze Mel
recep-torexpression,datawereprocessedusingthePartek® Genomics
Suite software version 6.6 (Partek, St. Louis, USA). Briefly, the
microarrayrawdatasetwerereprocessedbythebackground
cor-rection, normalization and summarization of probe intensities,
using the robust multiarray average analysis to determine the
specifichybridizingsignalfor each probe set.Afterbackground
correction,thedataweretransformedinbase-2logarithm.
Qual-itycontrolwasalsoperformedtoidentifythepresenceofarrays
withpoorqualityandevaluatewhetherbatcheffectsignificantly
affectedthedata.
A one-way analysis of variance (ANOVA) was performed to
determinedifferentiallyexpressedgenesinmousealveolarRMS
incomparisontonormalmusclesamples.Foldchange>2and
p-values<0.05wereusedascriteriatoevaluatesignificantdifference
ingeneexpression.
3. Results
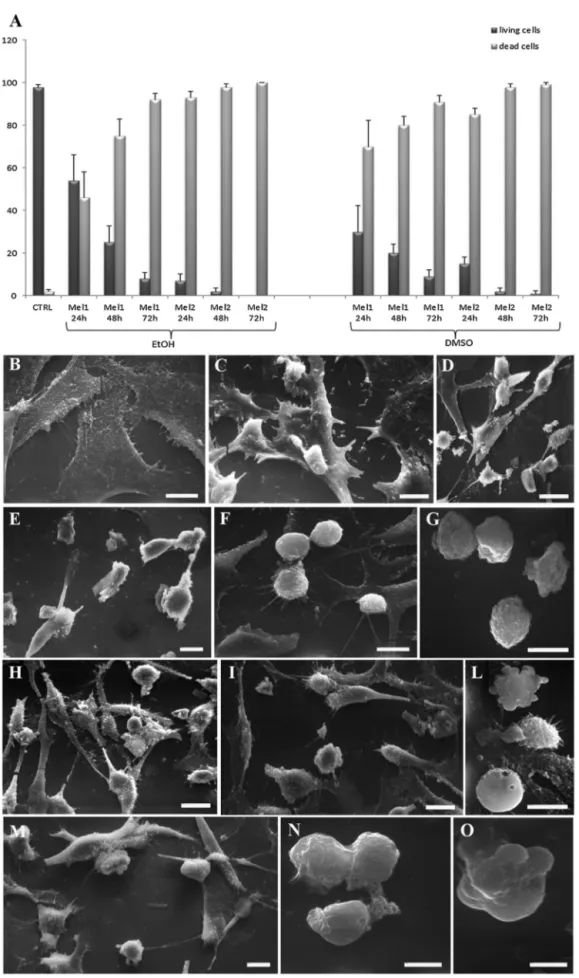
ToanalyzewhetherMelmight affectcellsurvival in human
alveolarRH30cells,TrypanBlueexclusionassaywasperformed,
revealingthatMeldissolvedeitherinEtOHorDMSOsignificantly
reducedtheviabilityofRH30cellsinatimeanddosedependent
manner(Fig.1A).
Insupportofthisevidence,SEM(Fig.1)andTEM(Figs.2and3)
analysesconfirmedthatMelinducedcelldeathofRH30cellsina
dose-andtime-dependentmannerandregardlessofthe
dissolu-tionsolvent.Inparticular,whilecontroladherentcellsappearedto
befusiformorstar-shaped(1B),1mMMel-treatedcellsshoweda
roundshapemorphologyalreadyafter24hoftreatment(1C,1H).
Thiseffectwasevenincreasedafter48h(1D,1I)or72h(1E,1L)of
Meltreatmentandaccompaniedbythemembraneblebbing
Fig.1.Trypanblueexclusionassay.Histogramshowingthepercentagesofliving(darkgreycolumn)anddead(lightgreycolumn)cells,underthedifferenttreatment conditions(A).Dataarefromthreeindependentexperimentsandareshownasmeanofvaluepercentage±standarddeviation.AllMeltreatmentsarestatisticallysignificant versuscontrolcondition(T-test,p<0.05).
SEManalysisofcontroluntreatedcells(B)versusMel-treatedcells(C-O);indetail,representativepicturesshowingcellstreatedwith1mMMel(inEtOH)for24-48–72h(C, D,Erespectively)and2mMMel(inEtOH)for24–48(F,Grespectively)or,alternatively,with1mMMel(inDMSO)for24-48–72h(H,I,L,respectively)and2mMMel(in DMSO)for24(M)-48h(N,O).Bars:10mB-O.
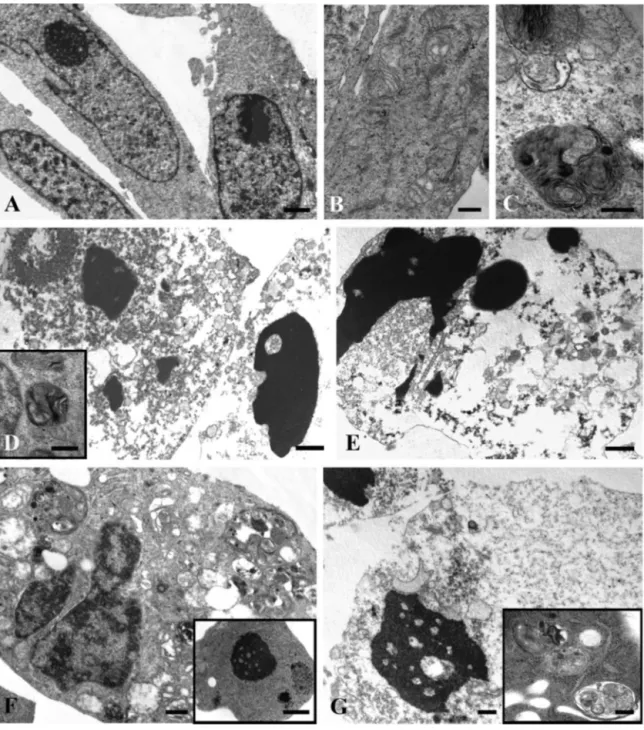
S.Burattinietal./ActaHistochemica118(2016)278–285 281
Fig.2. TEManalysisofcontroluntreatedcells(A)versusMel-treatedRH30cells(inEtOH)(B–G);indetail,representativepicturesshowingcellstreatedwith1mMMelfor 24h(B,C),48h(D,insetD),72h(E)and2mMMelfor24–48h(F,Grespectively)Bars:A,B,G,F,insetD,0.5m;C,insetG,0.25m;D,E,insetF,1m.
thediffusecelldamageoccurringafter24h(1F,1M)and48h(1G,
1N,1O)wascharacterizedbyroundingandblebbedcells.
TEMobservations(Fig.2)showedcontrolcellshavingan
elon-gatedshape andintactsubcellularstructures(2A).Instead,after
1mM Mel administration for 24h, cells showed altered
mito-chondria(2B)and autophagic vacuoles (2C).Occasionally, early
apoptoticnuclearfeatureswereobserved(datanotshown).After
48and72h,mostofthetreatedcellsappearedtobeinlate
apo-ptosis(2D,2E),whereassomeofthemshowedahighnumberof
autophagic-likestructures(inset2D).
Increasing Mel concentration up to 2mM for 24 and 48h
triggereddifferentcellular alterations, includinga diffuse
cyto-plasmicvacuolization(2F),chromatincondensation(insetF)and
autophagic features (inset G). In particular, cells in secondary
necrosiswereobservedafter2mMexposurefor48h(2G).
Con-sistentwiththeseresults,theeffectsproducedonRH30cellsby
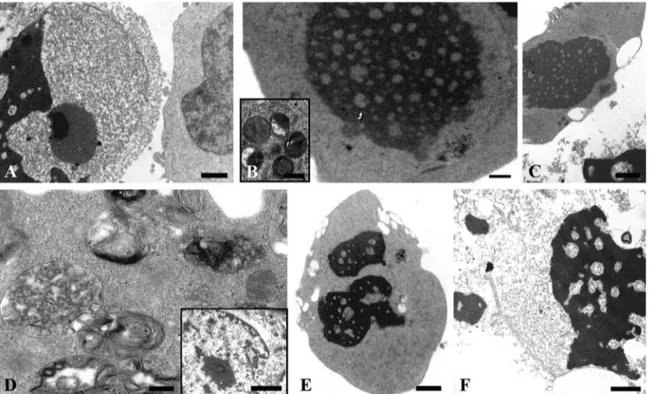
MeldissolvedinDMSOweresimilar(Fig.3).Earlyandlate
apop-toticpatternscouldbeobservedafter1mMMelat24h(3A),48h
(3B)and72h(3C).Secondarynecrosisaswellasautophagic
fea-turesappearedafter2mMMeltreatmentat24h(3D,insetD,3E)
and48h(3F).
CLSM evaluation of RH30 cells exposed to Mel dissolvedin
DMSOwascarriedoutbymeansofAO/PIdoublestaining(Fig.4).
InFig4A,theuniformgreenlabelingindicativeofcellularhealthy
structureswasvisibleincontrolcondition.AfterAOandPInuclear
staining,treatedcells(1mMMelfor24h,Fig.4B)showedagreen
stainsimilartocontrols,althoughdisplayingamorerounded
mor-phologycomparedtountreatedcells.After48and72h,thenumber
ofapoptoticcellsincreased(Fig.4C,insetCandD)andsomecells
withorangeareasduetoPIpermeabilitywereobserved(Fig.4C).
Cellstreated with2mMMelfor 24 h(Fig.4E, insetE)showed
orangenucleiduetolateapoptoticeventsandnecrosis.
Inaccordancewiththeobservedapoptoticresponseafter1mM
Fig.3.TEManalysisofMel–treatedRH30cells(inDMSO)(A–F);indetail,representativepicturesshowingcellstreatedwith1mMMelfor24–48–72h(A,B,Crespectively) and2mMMelfor24(D,E)and48h(F)Bars:A,C,E,1m;B,F,0.5m;insetB,D,0.25m;insetD,2m.
Fig.4.CLSManalysisofcontroluntreated(A)versusMel-treatedRH30cells(inDMSO)afterAO/PIdoublestaining(B–E).Indetail,representativepicturesshowingcells treatedwith1mMMelfor24–48–72h(B,C,Drespectively)and2mMMelfor24h(E).Bars:A–E,10m.(Forinterpretationofthereferencestocolorinthetext,thereader isreferredtothewebversionofthisarticle.)
WesternBlotanalysisshowingexpressionlevelsofBaxandBcl-2invehicle-treatedversus1mMMel-treatedRH30cellsattheindicatedtime-points(F).Tubulinwasused asproteinloadingcontrol.
bothanincreaseinthepro-apoptoticBaxexpressionandadecrease
inthelevelsofanti-apoptoticBcl-2incomparisontountreatedcells
(Fig.4F),thereforeconfirmingthatMelinducedapoptosisinRH30
cells.
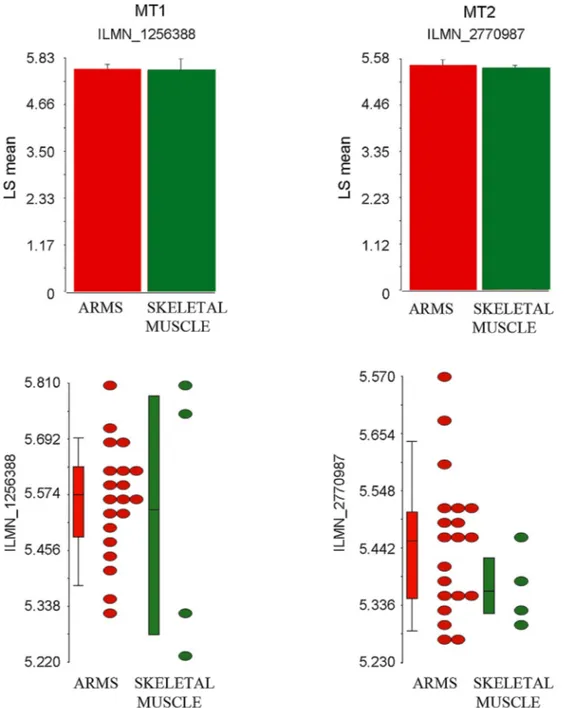
Finally,aninsilicoanalysisofmicroarraysdatageneratedfrom
primarymouseRMStumorcultures(Kelleretal.,2004;Kellerand
Capecchi,2005)revealedthatARMStumorscouldbesensitiveto
S.Burattinietal./ActaHistochemica118(2016)278–285 283
Fig.5.DotplotgraphsandrelativemeanhistogramsrepresentingthetranscriptionallevelsofMT1(ILMN1256388probenumber)andMT2(ILMN2770987probenumber), asdetectedinsilicobyperformingacomparisonbetweentwentyARMSsamplesandfourskeletalmusclesamples.pvaluewasnotsignificant.
receptorsMT1and MT2weredetectableinARMS subsets with
expressionlevelscomparabletothoseobservedinskeletalmuscle
(Fig.5).
4. Discussion
Epidemiologicalstudiescarriedout overthepastyears have
indicated that patients affected by some types of cancer have
reducedbloodylevelsofMelcomparedwithhealthypeople(Mills
etal.,2005;Lee,2006).Sincethen,thispinealneurohormoneMel
hasbeenrepeatedlydescribedasapotentialanti-cancer
molecu-lartoolgivenitsdemonstratedpro-apoptoticeffectandoncostatic
action towards differentcancercell lines (Bizzarriet al., 2013;
Perdomoetal.,2013;Rodriguezetal.,2013;Hongetal.,2014),
thereforecontributingtocancerprotection(Hrusheskyetal.,2009;
Maoetal.,2010;Bukowska,2011;Santoroetal.,2012).
Basedonthispremise,herewefocusedontheultrastructural
effectsinducedbyMelonahumanARMScelllinecarryingaunique
molecularsignaturefoundamongsarcomas,thePax3-Foxo1fusion
proteinarisingfroma specificchromosomal translocation(Jothi
etal.,2013).RMSneoplasmisthemostcommonsofttissuesarcoma
ofchildhood.AlthoughaggressivetreatmentofRMScouldprovide
long-termbenefit,resistancetoprolongedtherapiesisarecurrent
problem(Fayeetal.,2015).Thelastdecadehasseenan
extraordi-naryincreaseofstudiesonRMSforunderstandinghowapoptotic
mechanismsmaycontributeoncancereradication.Inthisregard,
theintegrityofapoptosispathwaysinRMSconstitutesacritical
determinantofthesensitivitytomostcurrenttreatmentstrategies
(Fulda,2013).
Inthisworkwehavefirstconfirmedpreviousfindingsshowing
the ability of Mel administered at pharmacological
concentra-tions(Rodriguezetal.,2013)intriggeringcelldeathofRMScells
(Codenottietal.,2015);moreover,sincethedissolution solvent
representsanimportantvariablepotentiallyinfluencingtheMel
action,typicallytheabsoluteEtOH(Saluccietal.,2014a;Salucci
etal.,2014),herewe haveestablishedthatboth thesesolvents
didnotinfluencetheefficacyofneurohormoneactivityasdeath
inducerinRH30cellline.
IncreasingbothMeldosesandexposuretimeleadtolate
apo-ptosisandsecondarynecrosisaconsistentnumberofRH30cells.
In this regard, ourultrastructural analysesrevealed that 1mM
Meldoseforupto48and72hrepresentedtheidealconditions
fortriggeringclassicalapoptosis-related morphologicaland
bio-chemicalchanges,suchassurfaceblebbing,chromatinmargination
andcondensation,mitochondriaalteration,cytoplasmic
vacuoliza-tion, autophagic vacuoles accompanied by Bax activation and
Bcl-2downregulation;inthiscase,RH30cellsmaintaineda
mor-phological plasma membrane integrity and necrotic cells were
detectedonlyoccasionally.Ontheotherside,secondarynecrosis
andnecroticcelldeathappearedtobethemajordeathresponseat
veryhighMeldose(2mM).Fromatherapeuticpointofview,killing
cancercellsviaapoptosisratherthannecrosiscouldbeconsidered
amorepreferableway,giventhattheappearanceofnecrosisinvivo
mightbeaccompaniedbyaninflammatoryresponsetypically
asso-ciatedtoamoreunfavorableoutcome.Hence,theoptimizationof
dosagesforinvivotrialsshouldtakeintoaccountthesepreliminary
invitroobservations.
ThemechanismsbywhichMelinfluencesapoptosishavenot
beenyetfullyclarified(Sainzetal.,2003;Wölfleretal.,2001;Gong
etal.,2003),althoughseveralreportshaveputforwardtheability
ofMeltoelicitapoptoticeffectsthroughchangesintheoxidative
status.Inthisregard,acorrelationbetweentheincreaseinROS
productionand theinductionofMel-drivenapoptosishasbeen
describedindifferentcelllines(Büyükavcietal.,2006).
Melaction isclassicallymediatedbytheinteractionwithits
membrane receptors(Radognaet al., 2009), i.e., MT1and MT2,
whichseemedtobeexpressedalsoinanumberofmouseARMS
tumors,asinferredthroughaninsilicoanalysisthatstillawaits
furtherinvitroandinvivoconfirmation.Hence,thepro-apoptotic
MeleffectonRH30cellscouldbereceptordependent,althoughit
isalsopossiblethathighdosesofMelmayfavoritssimple
diffu-sionacrossthecellmembraneinareceptor-independentmanner.
Onthewhole, understandingwhetherMelactionin ARMScells
maybe mediated or not (orat least partially)by its receptors
willbeimportantforidentifyingthepathwaysunderlyingits
pro-apoptoticaction;inaddition,thesynthesisandselectionofmimetic
compoundswithincreasedactivitycouldprovideafurther
phar-macologicaltoolforovercomingthegrowthofthissolidtumor.
5. Conclusions
ThedatareportedinthisworkindicatethatMel,when
adminis-teredatpharmacologicaldoses,canprofoundlyaffectcellsurvival
inRMS,themostfrequentmyogenicsofttissuesarcomaaffecting
childrenandadolescents(Ognjanovicetal.,2009;MeyerandSpunt,
2004).Inparticular,Melwasabletotriggeranapoptoticprogram,
independentlyonthedissolutionsolvent,thereforerepresentinga
promisingdrugforcounteractingRMStumorprogression.
References
Batista,A.P.,daSilva,T.G.,Teixeira,A.A.,deMedeiros,P.L.,Teixeira,V.W.,Alves, L.C.,DosSantos,F.A.,Silva,E.C.,2014.Ultrastructuralaspectsofmelatonin cytotoxicityonCaco-2cellsinvitro.Micron59,17–23.
Bejarano,I.,Redondo,P.C.,Espino,J.,Rosado,J.A.,Paredes,S.D.,Barriga,C.,Reiter, R.J.,Pariente,J.A.,Rodríguez,A.B.,2009.Melatonininduces
mitochondrial-mediatedapoptosisinhumanmyeloidHL-60cells.J.PinealRes. 46,392–400.
Bizzarri,M.,Proietti,S.,Cucina,A.,Reiter,R.J.,2013.Molecularmechanismsofthe pro-apoptoticactionsofmelatoninincancer:areview.ExpertOpin.Ther. Targets17,1483–1496.
Bukowska,A.,2011.Anticarcinogenicroleofmelatonin—potentialmechanisms. Med.Pr.62,425–434.
Büyükavci,M.,Ozdemir,O.,Buck,S.,Stout,M.,Ravindranath,Y.,Savas¸an,S.,2006. Melatonincytotoxicityinhumanleukemiacells:relationwithitspro-oxidant effect.Fundam.Clin.Pharmacol.20,73–79.
Codenotti,S.,Battistelli,M.,Burattini,S.,Salucci,S.,Falcieri,E.,Rezzani,R.,Faggi,F., Colombi,M.,Monti,E.,Fanzani,A.,2015.Melatonindecreasescell
proliferation:impairsmyogenicdifferentiationandtriggersapoptoticcell deathinrhabdomyosarcomacelllines.Oncol.Rep.34,279–287.
Chuffa,L.G.,Alves,M.D.,Martinez,M.,Camargo,I.,Pinheiro,P.,Domeniconi,R.,Lupi Júnior,L.A.,Martinez,F.,2015.Apoptosisistriggeredbymelatonininaninvivo modelofovariancarcinoma.Endocr.Relat.Cancer,pii:ERC-15-0463. Dantonello,T.M.,Stark,M.,Timmermann,B.,Fuchs,J.,Selle,B.,Linderkamp,C.,
Handgretinger,R.,Hagen,R.,Feuchtgruber,S.,Kube,S.,Kosztyla,D., Kazanowska,B.,Ladenstein,R.,Niggli,F.,Ljungman,G.,Bielack,S.S.,Klingebiel, T.,Koscielniak,E.,2015.Tumourvolumereductionafterneoadjuvant chemotherapyimpactsoutcomeinlocalisedembryonalrhabdomyosarcoma. Pediatr.BloodCancer62,16–23.
Faye,M.D.,Beug,S.T.,Graber,T.E.,Earl,N.,Xiang,X.,Wild,B.,Langlois,S.,Michaud, J.,Cowan,K.N.,Korneluk,R.G.,Holcik,M.,2015.IGF2BP1controlscelldeath anddrugresistanceinrhabdomyosarcomasbyregulatingtranslationofcIAP1. Oncogene34,1532–1541.
Fulda,S.,2013.Moleculartargetedtherapiesforrhabdomyosarcoma:focuson hedgehogandapoptosissignaling.Klin.Padiatr.225,115–119.
Ganie,S.A.,Dar,T.,Bhat,A.,Dar,K.,Anees,S.,Masood,A.,Zargar,M.A.,2015. Melatonin:apotentialantioxidanttherapeuticagentformitochondrial dysfunctionsandrelateddisorders.RejuvenationRes.18(June).
Gong,L.H.,Ren,D.H.,Xiong,M.,Lu,Z.Q.,Wang,X.M.,2003.Melatoninininvitro apoptosisofH22hepatocarcinomacells.ZhonghuaZhongLiuZaZhi25, 550–554.
Hong,Y.,Won,J.,Lee,Y.,Lee,S.,Park,K.,Chang,K.T.,Hong,Y.,2014.Melatonin treatmentinducesinterplayofapoptosis,autophagy,andsenescencein humancolorectalcancercells.J.PinealRes.56,264–274.
Hrushesky,W.J.,Grutsch,J.,Wood,P.,Yang,X.,Oh,E.Y.,Ansell,C.,Kidder,S., Ferrans,C.,Quiton,D.F.,Reynolds,J.,Du-Quiton,J.,Levin,R.,Lis,C.,Braun,D., 2009.Circadianclockmanipulationforcancerpreventionandcontrolandthe reliefofcancersymptoms.Integr.CancerTher.8,387–397.
Jardim-Perassi,B.V.,Arbab,A.S.,Ferreira,L.C.,Borin,T.F.,Varma,N.,Iskander,A.S., Shankar,A.,AlideCampos,M.M.,Zuccari,D.A.,2014.Effectofmelatoninon tumorgrowthandangiogenesisinxenograftmodelofbreastcancer.PLoSOne 9,e85311.
Jothi,M.,Mal,M.,Keller,C.,Mal,A.K.,2013.Smallmoleculeinhibitionof PAX3-FOXO1throughAKTactivationsuppressesmalignantphenotypesof alveolarrhabdomyosarcoma.Mol.CancerTher.12,2663–2674.
Keller,C.,Arenkiel,B.R.,Coffin,C.M.,El-Bardeesy,N.,DePinho,R.A.,Capecchi,M.R., 2004.AlveolarrhabdomyosarcomasinconditionalPax3:Fkhrmice: cooperativityofInk4a/ARFandTrp53lossoffunction.GenesDev.18, 2614–2626.
Keller,C.,Capecchi,M.R.,2005.Newgenetictacticstomodelalveolar rhabdomyosarcomainthemouse.CancerRes.65,7530–7532. Kubatka,P.,Bojková,B.,ciková-KalickáK,M.,Mníchová-Chamilová,M.,
Adámeková,E.,Ahlers,I.,Ahlersová,E.,Cermáková,M.,2001.Effectsof tamoxifenandmelatoninonmammaryglandcancerinducedby N-methyl-N-nitrosoureaandby7,12-dimethylbenz(a)anthracene respectively,infemaleSprague–Dawleyrats.FoliaBiol.(Praha)47,5–10. Lee,C.O.,2006.Complementaryandalternativemedicinespatientsaretalking
about:melatonin.Clin.J.Oncol.Nurs.10,105–107.
Leon-Blanco,M.M.,Guerrero,J.M.,Reiter,R.J.,Calvo,J.R.,Pozo,D.,2003.Melatonin inhibitstelomeraseactivityintheMCF-7tumorcelllinebothinvivoandin vitro.J.PinealRes.35,204–211.
Li,S.Q.,Cheuk,A.T.,Shern,J.F.,Song,Y.K.,Hurd,L.,Liao,H.,Wei,J.S.,Khan,J.,2013. Targetingwild-typeandmutationallyactivatedFGFR4inrhabdomyosarcoma withtheinhibitorponatinib(AP24534).PLoSOne8,e76551.
Mao,L.,Yuan,L.,Slakey,L.M.,Jones,F.E.,Burow,M.E.,Hill,S.M.,2010.Inhibitionof breastcancercellinvasionbymelatoninismediatedthroughregulationofthe p38mitogen-activatedproteinkinasesignalingpathway.BreastCancerRes. 12,R107.
Martín-Renedo,J.,Mauriz,J.L.,Jorquera,F.,Ruiz-Andrés,O.,González,P., González-Gallego,J.,2008.Melatonininducescellcyclearrestandapoptosisin hepatocarcinomaHepG2cellline.J.PinealRes.45,532–540.
Meyer,W.H.,Spunt,S.L.,2004.Softtissuesarcomasofchildhood.CancerTreat.Rev. 30,269–280.
Mills,E.,Wu,P.,Seely,D.,Guyatt,G.,2005.Melatonininthetreatmentofcancer:a systematicreviewofrandomizedcontrolledtrialsandmeta-analysis.J.Pineal Res.39,360–366.
Nowfar,S.,Teplitzky,S.R.,Melancon,K.,Kiefer,T.L.,Cheng,Q.,Dwived,P.D., Bischoff,E.D.,Moro,K.,Anderson,M.B.,Dai,J.,Lai,L.,Yuan,L.,Hill,S.M.,2002. Tumorpreventionby9-cis-retinoicacidintheN-nitroso-N-methylureamodel ofmammarycarcinogenesisispotentiatedbythepinealhormonemelatonin. BreastCancerResTreat72,33–43.
Ognjanovic,S.,Linabery,A.M.,Charbonneau,B.,Ross,J.A.,2009.Trendsin childhoodrhabdomyosarcomaincidenceandsurvivalintheUnitedStates, 1975–2005.Cancer115,4218–4226.
Perdomo,J.,Cabrera,J.,Estévez,F.,Loro,J.,Reiter,R.J.,Quintana,J.,2013.Melatonin inducesapoptosisthroughacaspase-dependentbutreactiveoxygen species-independentmechanisminhumanleukemiaMolt-3cells.J.PinealRes. 55,195–206.
S.Burattinietal./ActaHistochemica118(2016)278–285 285 Radogna,F.,Nuccitelli,S.,Mengoni,F.,Ghibelli,L.,2009.Neuroprotectionby
melatoninonastrocytomacelldeath.Ann.N.Y.Acad.Sci.1171,509–513. Rodriguez,C.,Martín,V.,Herrera,F.,García-Santos,G.,Rodriguez-Blanco,J.,
Casado-Zapico,S.,Sánchez-Sánchez,A.M.,Suárez,S.,Puente-Moncada,N., Anítua,M.J.,Antolín,I.,2013.Mechanismsinvolvedinthepro-apoptoticeffect ofmelatoninincancercells.Int.J.Mol.Sci.14,6597–6613.
Rubin,B.P.,Nishijo,K.,Chen,H.I.,Yi,X.,Schuetze,D.P.,Pal,R.,Prajapati,S.I., Abraham,J.,Arenkiel,B.R.,Chen,Q.R.,Davis,S.,McCleish,A.T.,Capecchi,M.R., Michalek,J.E.,Zarzabal,L.A.,Khan,J.,Yu,Z.,Parham,D.M.,Barr,F.G.,Meltzer, P.S.,Chen,Y.,Keller,C.,2011.Evidenceforanunanticipatedrelationship betweenundifferentiatedpleomorphicsarcomaandembryonal rhabdomyosarcoma.CancerCell19,177–191.
Sainz,R.M.,Mayo,J.C.,Rodriguez,C.,Tan,D.X.,Lopez-Burillo,S.,Reiter,R.J.,2003. Melatoninandcelldeath:differentialactionsonapoptosisinnormaland cancercells.CellMol.LifeSci.60,1407–1426.
Salucci,S.,Burattini,S.,Curzi,D.,Buontempo,F.,Martelli,A.M.,Zappia,G.,Falcieri, E.,Battistelli,M.,2014a.AntioxidantsinthepreventionofUVB-induced keratynocyteapoptosis.J.Photochem.Photobiol.B141,1–9.
Salucci,S.,Burattini,S.,Battistelli,M.,Baldassarri,V.,Curzi,D.,Valmori,A.,Falcieri, E.,2014b.Melatoninpreventschemical-inducedhaemopoieticcelldeath.Int.J. Mol.Sci.15,6625–6640.
Sánchez-Hidalgo,M.,Guerrero,J.M.,Villegas,I.,Packham,G.,delaLastra,C.A., 2012.Melatonin:anaturalprogrammedcelldeathinducerincancer.Curr. Med.Chem.19,3805–3821.
Santoro,R.,Marani,M.,Blandino,G.,Muti,P.,Strano,S.,2012.Melatonintriggers p53SerphosphorylationandpreventsDNAdamageaccumulation.Oncogene 31,2931–2942.
Soundararajan,A.,Abraham,J.,Nelon,L.D.,Prajapati,S.I.,Zarzabal,L.A.,Michalek, J.E.,McHardy,S.F.,Hawkins,D.S.,Malempati,S.,Keller,C.,2012.18F-FDG microPETimagingdetectsearlytransientresponsetoanIGF1Rinhibitorin geneticallyengineeredrhabdomyosarcomamodels.Pediatr.BloodCancer59, 485–492.
Wölfler,A.,Caluba,H.C.,Abuja,P.M.,Dohr,G.,Schauenstein,K.,Liebmann,P.M., 2001.Prooxidantactivityofmelatoninpromotesfas-inducedcelldeathin humanleukemicJurkatcells.FEBSLett.502,127–131.
Wu,S.M.,Lin,W.Y.,Shen,C.C.,Pan,H.C.,Keh-Bin,W.,Chen,Y.C.,Jan,Y.J.,Lai,D.W., Tang,S.C.,Tien,H.R.,Chiu,C.S.,Tsai,T.C.,Lai,Y.L.,Sheu,M.L.,2015.Melatonin setouttoERstresssignalingthwartsepithelialmesenchymaltransitionand peritonealdisseminationviaCalpain-mediatedC/EBPandNFBcleavage.J. PinealRes.,http://dx.doi.org/10.1111/jpi.12295.
Zanola,A.,Rossi,S.,Faggi,F.,Monti,E.,Fanzani,A.,2012.Rhabdomyosarcomas:an overviewontheexperimentalanimalmodels.J.CellMol.Med.16,1377–1391.