Cognitive impairment in patients with heart failure: an
international study
Ercole Vellone
1, Oronzo Chialà
1, Josiane Boyne
2, Leonie Klompstra
3, Lorraine S. Evangelista
4, Maria Back
5,
Tuvia Ben Gal
6,7, Jan Mårtensson
8, Anna Strömberg
5and Tiny Jaarsma
3*
1Department of Biomedicine and Prevention, University of Rome Tor Vergata, Rome, Italy;2Department of Cardiology, Maastricht University Medical Centre, Maastricht, The Netherlands;3Department of Social and Welfare Studies, Faculty of Health Science, Linköping University, Linköping, Sweden;4Sue & Bill Gross School of Nursing, University of California Irvine, Irvine, CA, USA;5Division of Physiotherapy, Department of Medical and Health Sciences, Linköping University, Linköping, Sweden;6Department of Cardiology, Rabin Medical Center (Beilinson Campus), Petah Tikva, Israel;7Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel;8Department of Nursing, School of Health and Welfare, Jönköping University, Jönköping, Sweden
Abstract
Aims Cognitive impairment (CI) in heart failure (HF) patients has mostly been studied in single countries in specific health care settings. Sociodemographic and clinical predictors of the global CI and CI dimensions are still unclear. We described CI in a diverse HF population recruited in several countries and in different health care settings and investigated sociodemographic and clinical factors associated with the global and specific CI dimensions in HF patients.
Methods and results A secondary analysis from the baseline data of the Wii-HF trial. Patients (n =605) were enrolled in Sweden, Italy, Israel, The Netherlands, Germany, and the United States. We used the Montreal Cognitive Assessment to eval-uate CI and the6 minute walk test (6MWT) to measure exercise capacity. Patients were on average 67 years old (SD, 12), and 86% were in New York Heart Association Class II and III. The mean Montreal Cognitive Assessment score was 24 (SD, 4), and 67% of patients had at least a mild CI. The item evaluating short-term memory had a considerable proportion of low scoring patients (28.1%). Worse CI was associated with patients’ older age, lower education, and lower 6MWT scores (R2=0.27). CI dimension scores were differently associated with specific clinical and demographic variables, but the 6MWT scores were as-sociated withfive out of seven CI dimension scores.
Conclusions CI is an important problem in HF patients, with specific challenges in regard to memory. Exercise capacity is a modifiable factor that could be improved in HF patients with the potential to improve cognition and other outcomes in this population.
Keywords Heart failure; Cognitive impairment; Exercise capacity
Received:9 July 2019; Revised: 14 September 2019; Accepted: 23 September 2019
*Correspondence to: Tiny Jaarsma, Department of Medical and Health Sciences, Linkoping University, Linkoping, Sweden. Email: [email protected]
Introduction
Heart failure (HF) is a prominent public health problem in the western world as a result of its rising prevalence and inci-dence.1Because of the ageing population and the improved treatment of cardiac diseases, there are about15 million peo-ple affected by HF in Europe1and more than6 million in the United States.2HF is associated with several symptoms (e.g. shortness of breath and fatigue) that impair quality of life3 and is also associated with high mortality rates, recurrent hospitalizations, and high direct and indirect costs.4–6
Cognitive impairment (CI) is a common co-morbid condi-tion in patients with HF.7 Several studies have shown that CI in HF ranges between 20% and 80%,7,8 and that CI is associated with poor HF outcomes, such as death, hospital re-admission, and poor self-care.9Despite that, the pathophysi-ology of CI in patients with HF remains unclear,10although several studies have succeeded in identifying its sociodemographic and clinical predictors. Sociodemographic predictors of CI in HF include older age,11 female gender, and lower education;12 and clinical predictors include de-creased physical activity,13 co-morbidities14 and HF severity
O R I G I N A L R E S E A R C H A R T I C L E
ESC Heart Failure2020; : 47–547
[i.e. higher New York Heart Association (NYHA) Class and lower ejection fraction].15
Although several studies have addressed CI in patients with HF,7,8the existing studies have mostly been conducted in small samples, in single countries, and specific health care settings8(e.g. only rehabilitation or outpatient settings). Also, the existing studies report inconsistencies among sociodemographic and clinical predictors of CI (i.e. gender, age, education, time after diagnosis, NYHA class, ejection fraction, comorbidities, and exercise capacity) and have not identified the sociodemographic and clinical predictors of specific cognitive dimensions in patients with HF (e.g. mem-ory). Since patients with HF receive care in a large diversity of health care settings and their cognitive dimensions might be affected by different sociodemographic and clinical vari-ables, we conducted this study to (i) describe CI in a diverse HF population recruited in several countries and in different health care settings and (ii) investigate sociodemographic and clinical factors (i.e. gender, age, education, time after di-agnosis, NYHA class, ejection fraction, comorbidities, and ex-ercise capacity) associated with global and specific CI in patients with HF.
Methods
Design
This study is a secondary analysis from the baseline data of the Wii-HF study,16 a randomized controlled trial (ClinicalTrial.gov identifier: NCT01785121) aimed at evaluat-ing the effect of exergamevaluat-ing on improvevaluat-ing exercise capacity in patients with HF enrolled in six countries.
Sample and settings
In the Wii-HF study, patients with HF were enrolled in differ-ent settings in Sweden, Italy, Israel, The Netherlands, Germany, and the United States. In Sweden, patients were enrolled in outpatient clinics offive hospitals, two university hospitals, two county hospitals, and one community hospital. In Italy, all patients were enrolled in a rehabilitation hospital; in Israel, patients were recruited from an advanced HF treat-ment centre; in The Netherlands and the United States, pa-tients were enrolled in an outpatient clinic of a university hospital; and in Germany, from a private cardiology practice. Inclusion criteria in each country were (i) diagnosis of HF with reduced or preserved ejection fraction according to the Euro-pean Society of Cardiology guidelines1 in NYHA functional Class I-IV; (ii) age older than18 years; and (iii) able to speak and understand the language of the enrolled country. The ex-clusion criteria were (i) unable to use the Wii computer be-cause of visual, hearing, and motor impairment; (ii) unable
to complete the research instruments; and, (iii) life expec-tancy shorter than 6 months. CI was not an exclusion crite-rion; however, if patients were not able to participate because of severe CI (e.g. dementia) as estimated by the study team, the patient was not eligible for the study. Two patients were excluded from the study because of their CI.
Data collection
Several instruments were used in the parent study,16but for this analysis we used the instruments described next. The Montreal cognitive assessment (MoCA),17is a 30-item valid and reliable instrument that is used worldwide (also in pa-tients with HF) to measure cognition. It evaluates seven cog-nitive domains as follows: visuospatial/executive, naming, attention, language, abstraction, delayed recall, and orienta-tion. From the MoCA it is possible to obtain a total score (ranging between0 and 30) and a dimension score with a dif-ferent range across the dimensions: a lower score means worse CI both for the total and dimension scores. As defined in prior studies,18the following categories of CI can be iden-tified according to the MoCA score: a score between 27 and 30 means ‘no to light CI’; a score between 18 and 26 means ‘mild CI’, a score between 10 and 17 means ‘moderate CI’, and a score lower than10 means ‘severe CI’.
The Charlson comorbidity index (CCI)19is a widely used in-strument that evaluates the presence of19 co-morbid condi-tions according to their gravity. Each condition could have a possible score from1 to 6, with a higher score meaning worse co-morbidity.
The 6 minute walk test (6MWT)20is a valid, reliable, and objective test of submaximal exercise capacity that measures the distance an individual is able to walk over a total of6 min on a hard,flat surface. The 6MWT score is obtained from the metres the patient walks in6 min, with a higher score mean-ing better exercise capacity. A 6MWT score >300 is associ-ated with lower event-free survival at36 months.21
Sociodemographic variables were collected for this study through self-report, including gender, age, education, and marital status. Clinical variables, including time since diagno-sis (in months), NYHA functional class and ejection fraction were abstracted from patients’ medical records.
Ethical considerations
The protocol of the study was approved by the competent In-stitutional Review Board in each centre of the six countries in which the patients were enrolled (Sweden DNR2012/247-31, The Netherlands NL48647.068.14/METC141085; Italy 0052838/272/U.V.F/1, 2014; Israel 0022-13-RMC; Germany S22(a)/2015; United States UCI IRB HS# 2016-2955). Before data collection, patients were fully informed about the study
protocol. Data collection began only after patients signed the informed consent form.
Statistical analysis
Descriptive statistics, including means, standard deviations, and frequencies were used to analyse sociodemographic and clinical variables as well as MoCA, CCI, and 6MWT scores. Comparison across MoCA score categories (no to light CI, mild CI, moderate CI, and severe CI), and patient sociodemographic and clinical variables were performed with χ2and ANOVA as appropriate. Spearman’s correlations were used to identify the significant correlations among the variables of interest. Variables significantly and indepen-dently associated with the total MoCA and dimension scores were identified with a series of regression analyses (enter procedure). In each regression, the independent variables were gender, age, education, time after diagnosis in months, NYHA class, ejection fraction, CCI, and 6MWT
scores. Data were analysed with the statistical software IBM SPSS21.
Results
The total sample included605 patients with HF (Table1). The sample mean age was 67 (SD, 12), and most were male (71%). In 68% of cases, patients were educated to primary or high school level, and 72% were married. The average CCI score was2.51 (SD, 1.71), meaning that on average pa-tients suffered from one to two diseases other than HF. The most prevalent comorbidity was diabetes (27%). Eighty-six percent of participants were in NYHA Class II and III, and the mean ejection fraction was 38.7% (11.9). The aetiology of HF was ischemic in42% of patients. The 84% of patients were treated with angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers/angiotensin recep-tor neprilysin inhibirecep-tors, 87% with B-Blocker, 48% with
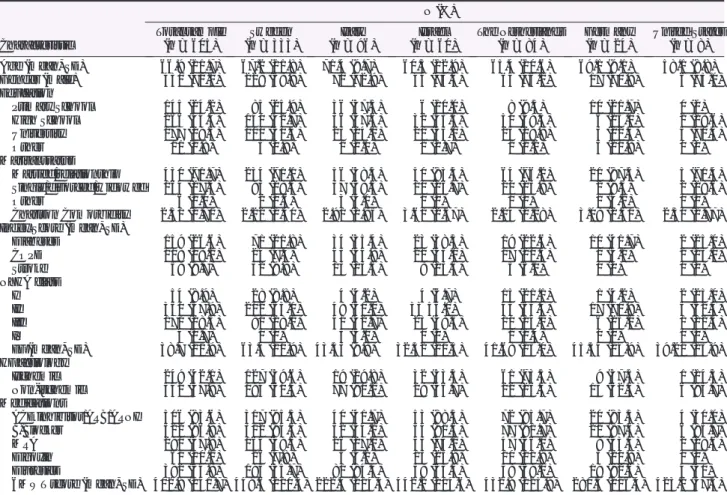
Table 1 Sociodemographic and clinical characteristics of enrolled patients (n = 605)
Characteristic N (%) Total sample (n = 605) Sweden (n = 333) Italy (n = 96) Israel (n = 60) The Netherlands (n = 84) Germany (n = 24) United States (n = 8) Age (mean, SD) 66.9 (11.7) 67.2 (11.8) 71.4 (9.7) 60.5 (12.9) 65.4 (10.6) 69.1 (9.1) 58.1 (8.8) Gender (male) 430 (71.1) 229 (68.8) 70 (72.9) 44 (73.3) 64 (76.2) 17 (70.8) 6 (75.0) Education Primary School 145 (24.2) 85 (25.9) 36 (37.5) 6 (10.0) 8 (9.5) 10 (21.7) 0 (0) High School 266 (44.4) 140 (42.7) 36 (37.5) 32 (53.3) 50 (59.5) 6 (25.0) 2 (28.6) University 177 (29.3) 100 (30.5) 23 (24.0) 21 (35.0) 25 (29.8) 3 (12.5) 5 (71.4) Other 11 (1.8) 3 (0.9) 1 (1.0) 1 (1.7) 1 (1.2) 5 (20.8) 0 (0) Marital status Married/relationship 430 (71.7) 234 (71.1) 56 (58.3) 50 (83.3) 64 (76.2) 21 (87.5) 5 (71.4) Single/divorced/widowed 164 (27.3) 93 (28.3) 37 (38.5) 10 (16.7) 20 (23.8) 2 (8.3) 2 (28.6) Other 6 (1.0) 2 (0.6) 3 (3.1) 0 (0) 0 (0) 1 (4.2) 0 (0) Charlson Comorbidity Index Score (mean, SD)
2.51 (1.71) 2.22 (1.40) 2.91 (1.85) 3.61 (2.57) 2.24 (1.18) 3.08 (2.50) 2.50 (1.77) Diabetes 159 (26.6) 71 (21.8) 34 (35.4) 23 (38.3) 19 (22.6) 10 (41.7) 2 (25.0) COPD 108 (18.2) 24 (7.4) 43 (44.8) 21 (35.0) 17 (20.5) 1 (4.2) 2 (25.0) Stroke 58 (9.7) 32 (9.9) 13 (13.5) 8 (13.3) 5 (6.0) 0 (0) 0 (0) NYHA class I 54 (8.9) 28 (8.8) 4 (4.2) 4 (6.7) 15 (21.1) 1 (4.2) 2 (25.0) II 350 (57.9) 202 (63.1) 48 (50.0) 33 55.0) 45 (63.4) 17 (70.8) 5 (62.5) III 171 (28.3) 90 (28.1) 41 (42.7) 23 (38.3) 10 (14.1) 6 (25.0) 1 (12.5) IV 4 (0.7) 0 (0) 3 (3.1) 0 (0) 1 (1.4) 0 (0) 0 (0) EF (mean, SD) 38.7 (11.9) 63.4 (10.9) 43.53 (9.8) 32.52 (10.5) 41.68 (13.0) 45.54 (14.9) 39.21 (15.9) HF aetiology Ischemic 249 (42.1) 127 (39.6) 19 (19.8) 32 (53.3) 61 (73.5) 9 (37.5) 1 (14.3) Non-ischemic 342 (57.9) 194 (60.4) 77 (80.2) 28 (46.7) 22 (26.5) 15 (62.5) 6 (85.7) Medications ACE inhibitor/ARB/ARNI 506 (83.6) 317 (95.5) 40 (41.7) 53 (88.3) 72 (85.7) 20 (83.3) 4 (50.0) B-Blocker 522 (86.9) 312 (94.3) 52 (54.2) 54 (91.5) 77 (91.7) 21 (87.5) 6 (85.7) MRA 290 (47.9) 164 (49.5) 26 (27.1) 43 (74.1) 47 (56.0) 8 (33.3) 2 (28.6) Digoxin 61 (10.1) 26 (7.8) 5 (5.2) 15 (25.9) 10 (11.9) 5 (20.8) 0 (0) Diuretics 392 (64.8) 195 (55.7) 81 (84.4) 38 (63.3) 58 (69.0) 19 (82.6) 4 (50) 6MWT score (mean, SD) 402.9 (141.7) 449.6 (110.3) 222.4 (114.3) 442.2 (114.6) 432.9 (125.9) 280.5 (116.4) 423.2 (57.5) ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor neprilysin inhibitors; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; HF, heart failure; NYHA, New York Heart Association; MRA, mineralocorticoid receptor antagonist; 6MWT, 6 minute walking test.
mineralocorticoid receptor antagonist,10% with digoxin, and 65% with digoxin.
The mean total MoCA score was24.53; 33% of the sample had no or a light CI;62 % had mild CI; 5% had moderate CI; and0.3% had severe CI (Table2). Table 3 reports the number (and percentage) of patients per each MoCA item score. Item 9, evaluating delayed recall, had a considerable proportion of patients (28.1%) who had a low score. The supporting infor-mation table reports the mean, SD, minimum, and maximum score per each MoCA item.
Table4 reports the differences among the variables in re-lation to the MoCA score categories as follows: no to light CI, mild CI, moderate CI, and severe CI. Patients in the mod-erate CI and severe CI were significantly older, with less edu-cation, with higher CCI scores, in a more advanced NYHA classification and with a lower score at the 6MWT.
Correlation and regression analysis
In the correlation analysis, lower MoCA total scores (meaning worse CI) were significantly associated with older age, lower education, higher NYHA class, lower CCI scores, and lower 6MWT scores (Table 5). When all the above variables were entered in a regression model (Table 6), lower total MoCA scores (worse CI) were independently associated with pa-tients’ older age, lower education, and lower 6MWT scores; the same variables were significantly and independently asso-ciated with lower visuospatial/executive dimension scores. Lower naming dimension scores were associated with longer time from HF diagnosis and worse6MWT scores; lower atten-tion dimension scores were associated with lower educaatten-tion and worse 6MWT scores; lower language dimension scores were associated with lower education and 6MWT scores; lower abstraction dimension scores were associated with lower ejection fraction but with a P level of0.052; lower de-layed recall dimension scores were associated only with older age;finally, lower orientation dimension scores were associ-ated with lower6MWT scores.
Discussion
To our knowledge, this is thefirst international study to de-scribe CI in a large diverse HF population recruited in six countries and in different health care settings and the first study to identify sociodemographic and clinical variables (i.e. gender, age, education, time after diagnosis, NYHA class, ejection fraction, comorbidities, and exercise capacity) inde-pendently associated with specific cognitive dimensions. These two aspects are important because prior studies have described CI in patients with HF but mostly in single countries (e.g. United States)7or in single settings (e.g. outpatient set-tings).22HF is a common health problem across the globe, Table
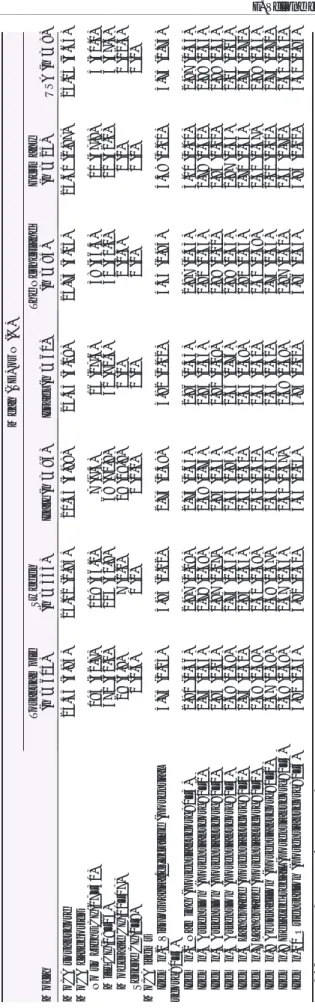
2 Cogn iti ve imp airme nt in the sample (n = 605) Mean (SD) or N (%) Moca Tot al sample (n = 605) Sweden (n= 333) Italy (n = 96) Isra el (n = 60) The Neth erland (n = 84) Germ any (n = 24) USA (n =8 ) MoCA tot al score 24.35 (3. 84) 25.0 2 (2.95) 20.5 3 (4.98) 24.4 5 (3.18) 25.7 3 (3.05) 25.61 (2. 87) 24.13 (6.64) MoCA ca tegor ies No to Light CI (27 –30) 195 (32 .7) 119 (36.1) 7 (7.3) 16 (27.6) 38 (46.3) 11 (47 .8) 4 (50.0) Mi ld CI (18 –26) 371 (62 .1) 204 (61.8) 68 (70.8) 42 (72.4) 42 (51.2) 12 (52 .2) 3 (37.5) Mo derate CI (10 –17) 29 (4. 9) 7 (2.1) 19 (19.8) 0 (0) 2 (2.4) 0 (0) 1 (12.5) Seve re CI (0 –9) 2 (0. 3) 0 (0) 2 (2.1) 0 (0) 0 (0) 0 (0) 0 (0) MoCA item s Ite m n. 1 Visuosp atial/ execut ive (p otentia l score: 0– 5) 3.75 (1. 23) 3.83 (1.12) 2.7 5 (1.38) 3.9 2 (1.02) 4.3 3 (0.93) 4.38 (1. 10) 3.75 (1.75) Ite m n .2 Namin g (potential score : 0– 3) 2.91 (0. 36) 2.97 (0.19) 2.7 5 (0.65) 2.8 6 (0.35) 2.8 7 (0.43) 3.00 (0. 00) 2.87 (0.35) Ite m n. 3 Attent ion (pot enti al score: 0– 2) 1.75 (0. 53) 1.78 (0.48) 1.4 8 (0.74) 1.8 5 (0.36) 1.8 2 (0.44) 1.79 (0. 41) 1.88 (0.35) Ite m n. 4 Attent ion (pot enti al score: 0– 1) 0.94 (0. 23) 0.97 (0.17) 0.8 4 (0.36) 0.9 2 (0.28) 0.9 9 (0.11) 0.96 (0. 20) 0.88 (0.35) Ite m n. 5 Attent ion (pot enti al score: 0– 3) 2.69 (0. 69) 2.74 (0.63) 2.3 5 (0.95) 2.6 6 (0.76) 2.8 8 (0.33) 2.87 (0. 34) 2.25 (1.16) Ite m n. 6 Langu age (pot enti al score: 0– 2) 1.76 (0. 52) 1.85 (0.44) 1.5 3 (0.60) 1.5 4 (0.68) 1.8 1 (0.45) 1.71 (0. 46) 1.75 (0.71) Ite m n. 7 Langu age (pot enti al score: 0– 1) 0.58 (0. 49) 0.61 (0.49) 0.5 0 (0.50) 0.5 3 (0.50) 0.6 1 (0.49) 0.30 (0. 47) 0.88 (0.35) Ite m n .8 Abstraction (potential score: 0– 2) 1.67 (0. 58) 1.68 (0.57) 1.5 6 (0.63) 1.5 6 (0.62) 1.7 4 (0.54) 2.00 (0. 00) 1.75 (0.71) Ite m n .9 Delaye d recall (potential score : 0– 5) 2.49 (1. 58) 2.67 (1.46) 1.4 0 (1.47) 2.6 8 (1.69) 2.8 7 (1.60) 2.65 (1. 70) 2.62 (1.60) Ite m n .1 0 Ori entation (pot enti al score: 0– 6) 5.82 (0. 55) 5.92 (0.31) 5.3 6 (1.05) 5.9 5 (0.22) 5.8 6 (0.35) 5.96 (0. 21) 5.50 (0.93) MoCA , Montrea l Cogniti ve Assessment .
and patients with HF are cared for in several health care set-tings (e.g. outpatient setset-tings, rehabilitation, and private car-diology practices). Consequently, our findings provide a better and comprehensive representation of CI in patients with HF. This might have important clinical and research implications.
Sixty-two percent (n =371) of our sample had mild CI. This percentage is similar to that identified by Harkness et al.23in a sample of44 patients with HF in an outpatient setting (70%) but higher than that identified by Gallagher et al.,24where 55% of patients with HF (n = 124) had a score <26 on the MoCA. The percentage of patients with CI falls within the
range of prior studies that have been included in systematic reviews.10Ourfinding corroborates further that CI is an im-portant problem in patients with HF.
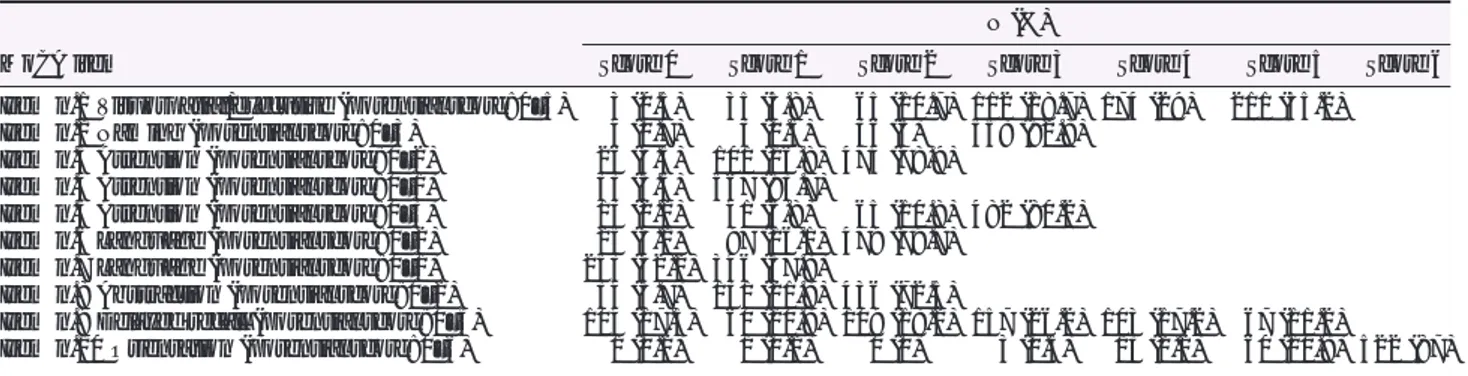
Looking at the MoCA scores across the six countries in which we enrolled the patients (Table2), we saw significant differ-ences across these subsamples. For example, Italian patients had the lowest score on the MoCA when compared with pa-tients of other countries. However, Italian papa-tients were en-rolled in a rehabilitation hospital and could have been more cognitively compromised than other patients enrolled, for ex-ample, in outpatient settings, as in Sweden. Further studies with more comparable patients should be conducted to see Table 3 Number (and percentage) of patients per each MoCA item score
MoCA item
N (%)
Score 0 Score 1 Score 2 Score 3 Score 4 Score 5 Score 6
Item n.1 Visuospatial/executive (potential score: 0–5) 3 (0.5) 35 (5.8) 65 (10.7) 112 (18.7) 174 (29) 211 (35.2)
Item n.2 Naming (potential score: 0–3) 4 (0.7) 3 (0.5) 36 (6) 558 (92.8)
Item n.3 Attention (potential score: 0–2) 26 (4.3) 101 (16.8) 474 (78.9)
Item n.4 Attention (potential score: 0–1) 33 (5.5) 567 (93.7)
Item n.5 Attention (potential score: 0–3) 13 (2.2) 41 (6.8) 65 (10.8) 482 (80.2)
Item n.6 Language (potential score: 0–2) 25 (4.2) 97 (16.1) 479 (79.7)
Item n.7 Language (potential score: 0–1) 253 (42.2) 346 (57.8)
Item n.8 Abstraction (potential score: 0–2) 34 (5.7) 131 (21.8) 436 (72.5)
Item n.9 Delayed recall (potential score: 0–5) 104 (17.3) 60 (10.8) 109 (18.2) 157 (26.2) 103 (17.2) 67 (11.2)
Item n.10 Orientation (potential score: 0–6) 1 (0.2) 1 (0.2) 0 (0) 3 (0.5) 13 (2.2) 60 (10.8) 522 (87)
MoCA, Montreal cognitive assessment.
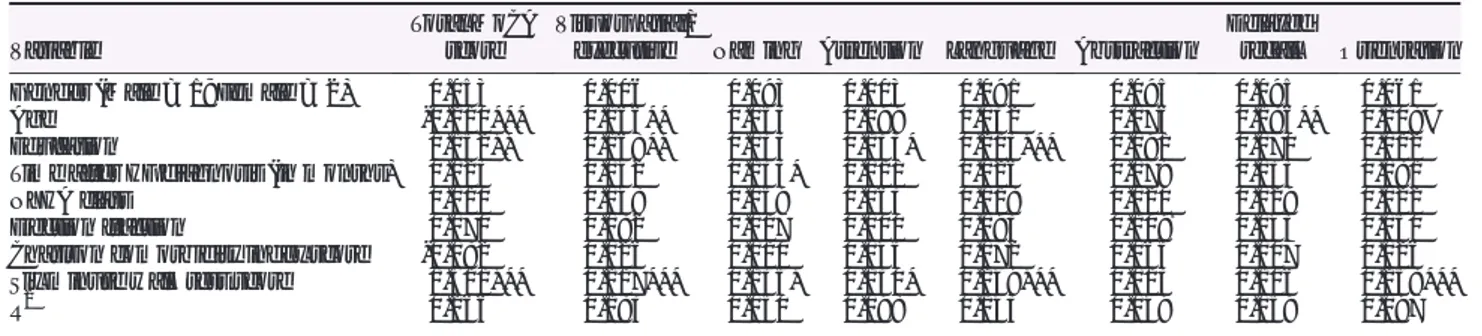
Table 4 Differences among the variables in relation to the MoCA score categories
Characteristic
MoCA score category No to light CI (Scores 27–30) M (SD) orN (%) Mild CI (Score 18–26) M (SD) orN (%) Moderate CI (Score 10–17) M (SD) orN (%) Severe CI (Score 0–9) M (SD) orN (%) P Age 62.1 (12.6) 68.6 (10.3) 77.1 (9.4) 83 (4.2) 0.000 Gender n.s. Male 132 (31.1) 273 (64.4) 18 (4.2) 1 (0.2) Female 63 (36.4) 98 (56.6) 11 (6.4) 1 (0.6) Education 0.000 Primary School 30 (20.8) 98 (68.1) 16 (11.1) 0 (0.0) High School 83 (31.7) 171 (65.3) 7 (2.7) 1 (0.4) University 78 (44.8) 92 (52.9) 4 (2.3) 0 (0.0) Marital status n.s. Married/relationship 145 (34.3) 262 (61.9) 14 (3.3) 2 (0.5) Single/divorced/widowed 48 (29.4) 102 (62.6) 13 (8.0) 0 (0.0) CCI Score 0.025 1 78 (40.6) 107 (55.7) 7 (3.6) 0 (0.0) 2 51 (30.5) 108 (64.7) 7 (4.2) 1 (0.6) 3 27 (29.3) 59 (64.1) 6 (6.5) 0 (0.0) >4 24 (19.5) 89 (72.4) 9 (7.3) 1 (0.8) NYHA class 0.000 I 24 (44.4) 28 (51.9) 2 (3.7) 0 (0.0) II 127 (37.0) 206 (60.1) 10 (2.9) 0 (0.0) III 31 (18.2) 120 (70.6) 17 (10.0) 2 (1.2) IV 0 (0.0) 4 (100) 0 (0.0) 0 (0.0) EF 38.4 (12.2) 38.7 (11.8) 40.6 (12.6) 44.0 (5.6) n.s. 6MWT score 463.3 (117.4) 385.9 (139.5) 229.3 (116.4) 163.5 (4.9) 0.000
Comparisons were performed withχ2or ANOVA as appropriate.
CCI, Charlson comorbidity index; EF, ejection fraction; NYHA, New York Heart Association; MoCA, Montreal cognitive assessment; 6MWT, 6 minute walking test.
if CI in patients with HF might be influenced by different ap-proaches in HF treatment and cultural factors across countries. Interestingly, we found that28.1 % scored 0 or 1 for delayed recall (short-term memory), meaning that our population had important memory problems, which confirms other stud-ies that found that memory and executive functions were cog-nitive areas particularly damaged in patients with HF.25This finding is important to consider when delivering patient edu-cation and when asking patients to perform self-care tasks, such as monitoring deterioration or taking medications.
In this study, we analysed sociodemographic and clinical variables independently associated with the global CI and with impairment of specific cognitive dimensions. Interestingly, we saw that older age, education, and exercise capacity were pre-dictors of the global CI, afinding that was not observed across the specific domains. For example, time after diagnosis and ex-ercise capacity were predictors of the naming dimension, and age was a predictor of delayed recall. To our knowledge, no prior studies have previously identified sociodemographic and clinical predictors of specific cognitive domains, and these newfindings may be helpful to guide future investigations and
interventions in patients with HF. For example, while patient’s age predicted the visuospatial/executive domain, it was not associated with the naming domain. Interestingly, exercise ca-pacity was associated with global CI andfive out of the seven cognitive domains. Thisfinding is important because exercise capacity could be improved in patients with HF. Prior studies have already found that physical activity was associated with better cognition in patients with HF.26
This study has strengths and limitations. A strength was the large multinational sample of patients with HF from mul-tiple settings. We have given a wide description of CI in pa-tients with HF. At the same time, our study strengths could be considered a limitation because the sample we enrolled was heterogeneous and selected with convenience proce-dures. Another limitation is that our study was a cross-sectional and secondary analysis with patients originally selected to evaluate the effectiveness of exergaming to im-prove exercise capacity in patients with HF. Even though CI was not an exclusion criterion for enrolling patients and only two patients were excluded from the study because their CI was an impediment to play with the Wii computer, we might Table 5 Correlations among the studied variables
Variable 1 2 3 4 5 6 7 8
1. Total MoCA score 1
2. Gender (Male = 1; Female = 2) 0.015 1
3. Age 0.322** 0.026 1
4. Education 0.244** 0.000 .118** 1
5. Time after HF diagnosis (in months) 0.010 0.041 0.015 0.020 1
6. NYHA class 0.261** 0.107* 0.243** 0.123** 0.021 1
7. Ejection fraction 0.036 0.105* 0.171** 0.053 0.095 0.028 1
8. Charlson comorbidity index score 0.236** 0.162** 0.221** 0.066 0.141** 0.264** 0.001 1
9. Six minute walk test score 0.396** 0.125** 0.397** 0.183** 0.052 0.458** 0.230** 0 .287**
Lower MoCA scores mean worse cognitive impairment; each number of thefirst row corresponds to the variable with the same number in thefirst column; and numbers in the cells are correlation coefficients.
HF, heart failure; NYHA, New York Heart Association; MoCA, Montreal cognitive assessment. *P < 0.05 and
**P < 0.01.
Table 6 Regression analysis predicting MoCA total score and MoCA dimension scores
Variable
Total MoCA score
Visuospatial/
executive Naming Attention Language Abstraction
Delayed
recall Orientation
Gender (Male = 1; Female = 2) 0.053 0.006 0.093 0.003 0.091 0.095 0.095 0.061
Age -0.210*** 0.166** 0.053 0.099 0.032 0.076 0.186** 0.108#
Education 0.152** 0.168** 0.033 0.133* 0.213*** 0.091 0.070 0.012
Time after HF diagnosis (in months) 0.023 0.042 0.133* 0.011 0.026 0.079 0.033 0.081
NYHA class 0.010 0.048 0.059 0.063 0.018 0.020 0.029 0.022
Ejection fraction 0.070 0.082 0.017 0.000 0.093 0.109¥ 0.043 0.050
Charlson comorbidity index score -0.082 0.026 0.010 0.045 0.072 0.065 0.007 0.026
Six minute walk test score 0.311*** 0.217*** 0.135* 0.130* 0.249*** 0.013 0.106 0.258***
R2
0.266 0.183 0.050 0.098 0.143 0.049 0.059 0.097
Lower MoCA scores mean worse cognitive impairment. Numbers in columns are standardized betas.¥P 0.052;# P 0.055
HF, heart failure; NYHA, New York Heart Association; MoCA, Montreal cognitive assessment. *P < 0.05,
**P < 0.01, ***P < 0.001.
have had a selection bias. However, the distribution of CI in our population was not different from other studies. Another limitation of our study was that we used a screening tool to evaluate CI that could be better evaluated with other tools with better sensitivity and specificity.
Our study has important practical implications because pa-tients with HF who are more cognitively compromised have worse outcomes, such as lower self-care,22higher mortality,27 and more frequent hospitalizations.28 Ourfindings showed that exercise capacity, a modifiable factor, was independently associated with CI. This means that improving physical activity has the potential to improve direct (e.g. cognition) and indirect (e.g. lower mortality rates) outcomes in patients with HF. Im-proving physical activity in HF is advocated in the international guidelines.1We have also discovered from our analysis that specific cognitive domains have different predictors that may be useful to know in clinical practice. For example, a patient’s older age was associated with worse delayed recall (memory) but not with abstraction. Practically, this means that older pa-tients with HF mayfind it difficult to memorise the medica-tions they have to take but not the fact that diuretics help to eliminate water from the body (Abstraction).
This study has several research implications. Since we ob-served different predictors for specific cognitive domains, it would be important to conduct further studies to confirm ourfindings. Another important implication is the use of lon-gitudinal designs and randomized controlled trials to examine if increased physical activity is associated with improved cog-nition. A recent pilot study29 found that a programme of combined aerobic exercise and cognitive training improved memory in patients with HF, but further studies are needed to clarify this association.
Acknowledgement
The following persons played a vital role in data collection. Norrköping: L Nestor, C Norrman, M Viklander, A Waldemar,
RM Petterson; M Wärfman; Jönköping: E Lundberg, H Sköldbäck, M Sahlin; Linköping: A Gylling, M Huss, M Jonsson, P Wodlin, L Hjelmfors; Stockholm: E Hägglund, U Lennmark; Nyköping: E Säfström; Italy: R Corsi, G Alberto Ortali; The Netherlands: HP Brunner-La Rocca, M Spanjers, A van de Voorde, G Cleuren; Israel: S Donanhirsh, Y Navon, V Yaari B. Avraham; Germany: A Hagenow, A Kuntzsch; and United States: J Ardo, J Nguyen, M Cacciata.
Con
flict of interest
None to declare
Funding
This work was supported through the Swedish National Sci-ence Council (K2013-69X-22302-01-3, 2016-01390), Swedish National Science Council/Swedish Research Council for Health, Working Life and Welfare (VR-FORTE) 2014-4100, The Swedish Heart and Lung Association E085/12, The Swed-ish Heart and Lung Foundation (20130340 and 20160439), the Vårdal Foundation (2014–0018), the Medical Research Council of Southeast Sweden (FORSS474681).
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article. Table S1. MoCA item descriptive analysis.
References
1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer
P; ESC Scientific Document Group.
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diag-nosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the
special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart
J. 2016;37: 2129–2200.
2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey de Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride P, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiol-ogy Foundation, American Heart Associ-ation Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the
management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am
Coll Cardiol 2013;62: e147–e239.
3. Heo S, Moser DK, Lennie TA, Fischer M, Kim J, Lee M, Walsh MN, Ounpraseuth S. Changes in heart failure symptoms are associated with changes in health-related quality of life over 12 months in patients with heart failure. J Cardiovasc
Nurs 2018;33: 460–466.
4. Fernández-Gassó L, Hernando-Arizaleta L, Palomar-Rodríguez JA, Abellán-Pérez
MV, Hernández-Vicente Á, Pascual-Figal DA. Population-based study offirst hos-pitalizations for heart failure and the in-teraction between readmissions and survival. Rev Española Cardiol (English
Ed.) 2018;72: 740–748.
5. Formiga F, Moreno-Gonzalez R, Chivite D, Yun S, Franco J, Ariza-Solé A, Corbella X. Sex differences in 1-year
mortality risks in older patients
experiencing a first acute heart failure hospitalization. Geriatr Gerontol Int
2019;19: 184–188.
6. Łyszczarz B. Indirect costs and public fi-nance consequences of heart failure in Poland, 2012–2015. BMC Public Health 2018;18: 1130.
7. Sterling MR, Jannat-Khah D, Bryan J, Banerjee S, McClure LA, Wadley VG, Unverzagt FW, Levitan EB, Goyal P, Peterson JC, Manly JJ. The prevalence of cognitive impairment among adults with incident heart failure: the reasons for geographic and racial differences in stroke (REGARDS) study. J Card Fail 2018;25: 130–136.
8. Cannon JA, Moffitt P, Perez-Moreno AC, Walters MR, Broomfield NM, McMurray J, Quinn TJ. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail 2017; 23: 464–475.
9. Huynh QL, Negishi K, Blizzard L, Saito M, de Pasquale CG, Hare JL, Leung D, Stanton T, Sanderson K, Venn AJ, Marwick TH. Mild cognitive impairment predicts death and readmission within 30 days of discharge for heart failure.
Int J Cardiol 2016;221: 212–217.
10. Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol 2015;178: 12–23.
11. Fendler TJ, Spertus JA, Gosch KL, Jones PG, Bruce JM, Nassif ME, Flint KM, Dunlay SM, Allen LA, Arnold SV. Inci-dence and predictors of cognitive de-cline in patients with left ventricular assist devices. Circ Cardiovasc Qual
Out-comes 2015;8: 285–291.
12. Ghanbari A, Moaddab F, Salari A, Kazemnezhad Leyli E, Sedghi Sabet M, Paryad E. The study of cognitive func-tion and related factors in patients with heart failure. Nurs midwifery Stud
2013;2: 34–38.
13. Alosco ML, Spitznagel MB, Cohen R, Sweet LH, Hayes SM, Josephson R, Hughes J, Gunstad J. Decreases in daily
physical activity predict acute decline in attention and executive function in heart failure. J Card Fail 2015; 21: 339–346.
14. Pulignano G, Del Sindaco D, De Lenarda A, Tinti MD, Tarantini L, Cioffi G, Tolone S, Pero G, Minardi G. Chronic renal dys-function and anaemia are associated with cognitive impairment in older pa-tients with heart failure. J Cardiovasc
Med 2014;15: 481–490.
15. Hanon O, Vidal JS, de Groote P, Galinier M, Isnard R, Logeart D, Komajda M. Prevalence of memory disorders in am-bulatory patients aged >/=70 years with chronic heart failure (from the EFICARE study). Am J Cardiol 2014;
113: 1205–1210.
16. Jaarsma T, Klompstra L, Ben Gal T, Boyne J, Vellone E, Bäck M, Dickstein K, Fridlund B, Hoes A, Piepoli MF, Chialà O, Mårtensson J, Strömberg A. Increas-ing exercise capacity and quality of life of patients with heart failure through Wii gaming: the rationale, design and methodology of the HF-Wii study; a multicentre randomized controlled trial.
Eur J Heart Fail 2015;17: 743–748.
17. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Mon-treal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53: 695–699.
18. Athilingam P, King KB, Burgin SW, Ackerman M, Cushman LA, Chen L.
Montreal cognitive assessment and
mini-mental status examination com-pared as cognitive screening tools in heart failure. Hear Lung 2011; 40: 521–529.
19. Charlson M, Pompei P, Ales K,
MacKenzie C. A new method of
classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383.
20. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med
Assoc J 1985; 132: 919–923. http://
www.ncbi.nlm.nih.gov/pubmed/ 3978515 (12 January 2019).
21. Rostagno C, Olivo G, Comeglio M, Boddi V, Banchelli M, Galanti G, Gensini
GF. Prognostic value of 6-minute walk corridor test in patients with mild to moderate heart failure: comparison
with other methods of functional
evaluation. Eur J Heart Fail 2003; 5: 247–252.
22. Hjelm CM, Broström A, Riegel B, Årestedt K, Strömberg A. The associa-tion between cognitive funcassocia-tion and self-care in patients with chronic heart failure. Hear Lung J Acute Crit Care 2015;44: 113–119.
23. Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal cognitive assessment tool in outpatients≥65 years of age with heart failure. Am J Cardiol 2011;107: 1203–1207.
24. Gallagher R, Sullivan A, Burke R, Hales S, Gillies G, Cameron J, Saliba B, Tofler G. Mild cognitive impairment, screening, and patient perceptions in heart failure patients. J Card Fail 2013;
19: 641–646.
25. Kim J, Shin M-S, Hwang SY, Park E, Lim YH, Shim JL, Kim SH, Kim YH, An M. Memory loss and decreased executive function are associated with limited functional capacity in patients with heart failure compared to patients with other medical conditions. Heart Lung 2018;47: 61–67.
26. Alosco ML, Brickman AM, Spitznagel MB, Sweet LH, Josephson R, Griffith EY, Narkhede A, Hughes J, Gunstad J. Daily physical activity is associated with subcortical brain volume and cognition in heart failure. J Int Neuropsychol Soc 2015;21: 851–860.
27. Lan H, Hawkins LA, Kashner M, Perez E, Firek CJ, Silvet H. Cognitive impairment predicts mortality in outpatient veterans with heart failure. Hear Lung 2018;47: 546–552.
28. Sterling MR, Safford MM, Goggins K, Nwosu SK, Schildcrout JS, Wallston KA, Mixon AS, Rothman RL, Kripalani S. Numeracy, health literacy, cognition,
and 30-day readmissions among
patients with heart failure. J Hosp Med 2018;13: 145–151.
29. Gary RA, Paul S, Corwin E, Butts B, Miller AH, Hepburn K, Williams B,
Waldrop-Valverde D. Exercise and
cognitive training as a strategy to improve neurocognitive outcomes in heart failure: a pilot study. Am J Geriatr