Dipartimento di Chimica e Chimica Industriale
Corso di Laurea Magistrale in Chimica Industriale
Tesi di Laurea Magistrale
Synthesis of Ruthenium and Osmium
Complexes with potential anticancer activity
RELATORE CONTRORELATORE
Dott. Fabio MARCHETTI Dott. ssa Tarita BIVER
CANDIDATO
Gabriele AGONIGI
1. INTRODUCTION ... 1
1.1. PECULIARITIES OF METAL-BASED DRUGS... 1
1.2. PLATINUM ANTICANCER DRUGS ... 3
1.3. RUTHENIUM ANTICANCER COMPOUNDS ... 5
1.3.1. Ruthenium (II) arene complexes ... 6
1.3.2. Ruthenium (III) complexes ... 10
1.4. OSMIUM ANTICANCER COMPOUNDS ... 13
1.5. SELECTIVITY IN CANCER TREATMENT; A MAJOR CHALLENGE ... 14
1.6. ETHACRYNIC ACID: AN INNOVATIVE SOLUTION TO ENHANCE SELECTIVITY ... 16
2. AIM OF THE WORK ... 19
3. RESULTS AND DISCUSSIONS ... 20
4. CONCLUSIONS ... 37
5. EXPERIMENTAL PART ... 38
5.1. GENERAL INFORMATION ... 38
5.2. SPECTROSCOPIC CHARACTERIZATION OF ETHACRYNIC ACID (EA-COOH,ALFA AESAR)... 38
5.3. SYNTHESIS OF ORGANIC LIGANDS ... 39
5.3.1. 4-pyridinylmethanol ... 39
5.3.2. Ethacrynic acid pyridin-4-ylmethyl ester (1) ... 39
5.3.3. Ethacrynic acid pyridin-3-yl ester (2) ... 40
5.3.4. Ethacrynic acid 2-chloro-ethyl ester (3)... 41
5.3.5. Isonicotinic acid 2-hydroxy-ethyl ester (4) ... 42
5.3.6. Nicotinic acid 2-hydroxy-ethyl ester (5) ... 42
5.3.7. Ethacrynic acid 2-hydroxy-ethyl ester (6) ... 43
5.3.8. Nicotinic acid 2-ethacrynyloxy-ethyl ester (7) ... 44
5.3.9. Ethacrynic acid 2-(3-pyridin-3-yl-propionyloxy)-ethyl ester (8) ... 45
5.3.10. 4-Diphenylphosphanyl-benzoic acid 2-ethacrynyloxy-ethyl ester (9) ... 46
5.3.11. Synthesis and reactivity of 1-(2-Hydroxy-ethyl)-3-methyl-3H-imidazol-1-ium chloride (10) 47 5.4. SYNTHESIS OF RUTHENIUM COMPLEXES ... 47
5.4.1. [Ru(η6-p-cymene)Cl2]2 (11) ... 47
5.4.2. [Ru(ethacrynic acid pyridin-4-ylmethyl ester)(η6-p-cymene)Cl2] (12) ... 48
5.4.3. [Ru(nicotinic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)Cl2] (13) ... 48
5.4.4. [(Me2SO)2H][trans-Ru(Me2SO)2Cl4] (14) ... 49
5.4.5. [(ethacrynic acid pyridin-4-yliummethyl ester)H][Ru(Me2SO)(ethacrynic acid pyridin-4-ylmethyl ester)Cl4] (15) ... 50
5.4.6. [(Ethacrynic acid pyridin-3-ylium ester)H][Ru(Me2SO)(ethacrynic acid pyridin-3-yl ester)Cl4] (16) ... 51
5.4.7. Reaction of [(Me2SO)2H][trans-Ru(Me2SO)2Cl4] (14) with nicotinic acid 2-ethacrynyloxy-ethyl ester (7)... 51
5.5. SYNTHESIS OF OSMIUM COMPLEXES ... 52
5.5.1. [Os(η6-p-cymene)Cl2]2 (17) ... 52
5.5.2. [Os(nicotinic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)Cl2] (18) ... 52
5.5.3. [Os(ethacrynic acid 2-(3-pyridin-3-yl-propionyloxy)-ethyl ester)(η6-p-cymene)Cl2] (19) ... 54
5.5.4. [Os(4-Diphenylphosphanyl-benzoic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)Cl2] (20) ... 55
5.5.5. [Os(η6-p-cymene)(η1-C-acac)(O,O’-acac)] (21) ... 56
5.5.6. [Os(η6-p-cymene)(η1-O-C(O)-EA)(O,O’-acac)] (22) ... 57
5.5.7. [Os(η6-p-cymene)(η1-O-C(O)C7H7N2O4)(O,O’-acac)] (23) ... 58
5.5.8. [Os(η6-p-cymene)Cl(O,O’-acac)] (24) ... 59
5.5.9. [Os(η6-p-cymene)(pta)Cl2] (pta = 1,3,5-triaza-7-phosphatricyclo-[3.3.1.1]decane) (25) ... 59
5.5.10. [Os(nicotinic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)(pta)Cl][Cl] (26) ... 60
5.5.11. [Os(ethacrynic acid 2-(3-pyridin-3-yl-propionyloxy)-ethyl ester)(η6-p-cymene)(pta)Cl][Cl] (27) ... 61
5.5.12. (cis)-Os{κ-S-(Me2SO)4}Cl2 (28) ... 62
5.5.14. Reaction of (cis)-Os{κ-S-(Me2SO)4}Cl2 (28) with ethacrynic acid
2-(3-pyridin-3-yl-propionyloxy)-ethyl ester (8) ... 62 5.5.15. Reaction of [Os(η6-p-cymene)(pta)Cl2] (25) with 4-Diphenylphosphanyl-benzoic acid
2-ethacrynyloxy-ethyl ester (9)... 63 5.5.16. Reaction of [Os(η6-p-cymene)(η1-C-acac)(O,O’-acac)] (21) with phenylpyridine ... 63 5.5.17. Reaction of [Os(η6-p-cymene)(O,O’-acac)Cl] (24) with ethacrynic acid ... 63
1
1. Introduction
Human body endlessly generates new cells with the aim of promoting its growth, replacing exhausted cells or regenerating marred cells after a harm.
In a healthy individual, cells born, grow and die on a regular basis and this routine is governed by a couple of genes. When some kind of alterations occurs to these genes, it may lead up to the rapid creation of abnormal cells that grow beyond their usual boundaries.
Commonly, this large group of diseases is identified as cancer and the aptitude of cancerous cells to invade adjoining parts of the body and to spread illness to other organs is referred to as metastasis.
According to World Health Organization, in 2012 14.1 million new cancer cases and 8.2 million cancer deaths were diagnosed worldwide, while 32.6 million people were living with cancer (within 5 years of diagnosis)1. Looking at these numbers, it is easy to understand the relevance
of this problem from a social point of view; furthermore economic implications are deducible and extremely touchy2,3.
1.1. Peculiarities of metal-based drugs
Cancer can be treated with several approaches including surgery, radiation therapy, immunotherapy and chemotherapy. These medical attentions could be used alone or in combination with each other, depending on the location, the characteristics of the tumour and the stage of the disease, as well the performance status (a quantification of cancer patients’ general conditions).
Without any doubt, chemotherapy plays a prominent role in the treatment of neoplastic diseases and the research of novel chemical substances capable of ceasing cancerous cells proliferation and/or damaging metastasis is an extremely active field of study.
1 World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence
Worldwide in 2012;, 2014.
2 Brooks, G.; Li, L.; Sharma, D.; Weeks, J.; Hassett, M.; Yabroff, K.; Schrag, D. J Natl Cancer Inst 2013,
105 (9), 634-642.
3 Dowling, E. C.; Chawla, N.; Forsythe, L.; de Moor, J.; McNeel, T.; Rozjabek, H.; Ekwueme, D.; Yabroff, K. Cancer 2013, 119 (18), 3393-3401.
2 With the term chemotherapy, it is usual to associate any kind of treatment involving one or more chemical substances administrated as anti-cancer drugs. Cytotoxic agents may act through several modes of action, depending on their structure and properties.
The potential activity of an anticancer agent for a particular tumour type is usually evaluated using IC50 assays with cell lines in vitro. IC50 is a value indicating the drug’s amount able to
reduce cells growth by 50%.
Unlike many other diseases, a fully successful strategy to defeat cancer has not been discovered heretofore. For this reason, research is particularly versatile and innovative towards anti-cancer drug design, and inorganic and organometallic chemistry play an important role in this field.
A variety of metal elements are essential for the human body; normally, the really indispensible component is not the element itself, but a particular compound of that element. Back-to-front several metal elements present high toxicity, but in many cases ligands can deeply influence the properties of the metal complexes thus leading to beneficial compounds (as it happens for arsenic).
The synthesis of new metal-based drugs is an extremely intriguing research field because of the high number of variables that can be considered in order to try to obtain active compounds. The change of just one parameter may provide compounds with remarkable dissimilar characteristics (see
Table 1
).Table 1 Features of metals and metal complexes to be considered in the design of therapeutic and diagnostic agents.
Features comments Coordination
number
In transition metals mainly 4-6; the range become 2-10 if also main group metals and lanthanides are considered
Geometry extremely various, from linear to trigonal bipyramidal; the most important are square planar (PtII), tetrahedral (often distorted) and
octahedral (RuIII)
Oxidation state Wide range in biological media (0-7); some metals presents couples with extremely low redox potentials
Ligand type Donors as C, N, O, halides, S, P, Se, carbenes, chelating ligands (κ2
-κ6), hapticity (e.g. η4 or η6)
Thermodynamic stability
M-L bond energies between 50 and 150 kJ mol-1
Kinetic stability M-L bond lifetimes vary from ns to years
Nuclear stability Radioactive nuclides can be used to track the metabolism of drugs (e.g.,
3
1.2. Platinum anticancer drugs
The anticancer properties of platinum compounds were serendipitously discovered by Barnett Rosenberg and co-workers in early 1960’s.
Even thought cisplatin (cis-diamminedichloroplatinum(II), Figure 1a) was described for the first time by Michel Peyrone in 1844 and the structure was successfully elucidated by Werner in 1893, medicinal purpose were not conceivable until Rosenberg observed that bacteria interrupted their mitosis processes in the presence of cisplatin4.
This breakthrough can be doubtless considered as one of the major landmarks in the history of successful anticancer drugs. Nowadays, cisplatin is one of the world’s best-selling anticancer drugs, used to treat various types of cancer, including sarcomas, some carcinomas (e.g. small cell lung cancer, and ovarian cancer), lymphomas, germ cell tumours and testicular cancer. Since the discover of the biological activity of cisplatin, thousands of platinum compounds have been prepared and screened as potential antitumor drugs5,6, in order to overcome the limitations
correlated with cisplatin. Indeed, cisplatin presents big problems regarding resistance mechanisms, range of medical use and side-effects7. Many tumours display natural resistance
to cisplatin whereas others develop a resistance after a period of time. Moreover metastasis cancers are not attacked by cisplatin8. Collateral effects compel the patients to suffer from
serious disorders or injuries.
Although the number of synthesized and tested Pt compounds is really impressive, only oxaliplatin and carboplatin (see Figure 1b and 1c) have entered clinical practice and, together with cisplatin, now they are accepted by FDA (US Food and Drug Administration) as anticancer drugs. Few other platinum drugs under investigation in Europe and USA are of current clinical use in Asia; nedaplatin9 (see Figure 1d) is employed in Japan to treat head, neck, testicular,
lung, ovarian, cervical, and non-small cell lung cancers. Heptaplatin7,10 (see Figure 1e) is used
4 Rosenberg, B.; Van Camp, L. Nature 1965, 205, 698-699.
5 Xin Zhang, C.; Lippard, S. J. Curr. Opin. Chem. Biol. 2003, 7, 481-489. 6 Wong, E.; Giandomenico, C. M. Chem. Rev. 1999, 99, 2451-2466. 7 Rabik, C.A.; Dolan, M.E. Cancer Treat. Rev. 2007, 33, 9–23. 8 Wang, D.; Lippard, S.J. Nat. Rev. Drug Discov. 2005, 4, 307–320.
9 Lee, W. S.; Lee, G. W.; Kim, H. W.; Lee, O. J.; Lee, Y. J.; Ko, G. H.; Lee, J. S.; Jang, J. S.; Ha, W. S.
Cancer. Res. Treat. 2005, 37, 208-211.
10 Ahn, J. H.; Kang, Y. K.; Kim, T. W.; Bahng, H.; Chang, H. M.; Kang, W. C.; Kim, W. K.; Lee, J. S.; Kang, J. S. Cancer. Chemother. Pharmacol. 2002, 50, 104-110.
4 against gastric cancer in South Korea. Lobaplatin11 (see Figure 1f) has been approved in China
for the treatment of chronic myelogenous leukemia, metastatic breast, and small cell lung cancer.
Figure 1. Platinum compounds approved for clinical use: cisplatin (a), carboplatin (b), oxaliplatin (c), nedaplatin (d), lobaplatin (e), heptaplatin (f).
Low efficacy, high toxicity and/or low water solubility afflict the vast majority of platinum-based compounds screened in preliminary clinical trials.
Cisplatin maintains its structure when entering into the cell (mainly exploiting copper-transporting systems), then the Cl ligands may be displaced by water molecules.
11 Voegeli, R.; Schumacher, W.; Engel, J.; Respondek, J.; Hilgard, P. J. Cancer Res. Clin. Oncol. 1990,
5
Figure 2. Cisplatin linkage ways.
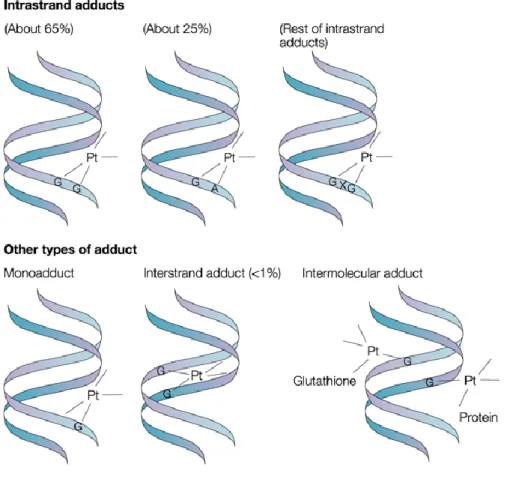
The linkage to DNA bases through two sites of the square-planar complex seems to be the key whereby cisplatin may induce cell apoptosis, however the mechanism of the cytotoxic activity has not been fully elucidated12,13. Cisplatin links in various ways DNA bases, especially guanine
or adenine nitrogens, thus determining a distortion in the DNA structure which then induces apoptosis (see Figure 2).
Cancer cells may try to repair the DNA damage, and this resistance contributes to decrease the drug activity, and consequently side-effects become heavier.
1.3. Ruthenium anticancer compounds
To overcome the limitations associated with platinum compounds, many other metal elements have been considered for drug design. In most cases, in vivo therapeutic activity has revealed to be insufficient despite favourable in vitro tests.
12 Alderden, R. A.; Hall, M. D.; Hambley, T. W. J. Chem. Ed., 2006, 83, 728-734. 13 Klein, A. V.; Hambley, T. W. Chem. Rev., 2009, 109, 4911–4920.
6 Ruthenium and osmium constitute an important frontier for the preparation of new metal-based anticancer compounds. Both Ru and Pt are relatively inert metals whose complexes exhibit comparable ligand exchange rates, within the timescale of cellular division processes.
Ruthenium presents three main oxidation states (II, III, IV); under physiological conditions only Ru(II) (d6, diamagnetic) and Ru(III) (d5, paramagnetic) are accessible. Ru complexes typically
show hexa-coordination with octahedral geometry. Likewise platinum compounds, ruthenium complexes manifest affinity towards nitrogen and sulphur ligands.
These features can explain why ruthenium is considered to be an interesting perspective; in fact, despite the analogies with Pt, Ru complexes may exhibit several geometries and up to three metal oxidation states may be exploited for useful biological actions.
Ruthenium complexes as anticancer drugs can be divided into two main groups: Organometallic Ru(II)-arene complexes;
Non organometallic Ru(III) complexes
1.3.1. Ruthenium (II) arene complexes
Ru(II)-arene bonding is highly stable under acidic, basic as well as reducing or oxidizing conditions, whereas the other ligands may be replaced. The properties of arene ruthenium complexes can be modulated by modification of the arene and/or the remaining ligands. This allows an extensive use of these complexes, e.g. for the design of catalysts, supramolecular assemblies and metal-pharmaceuticals14. As example of this versatility is given by Ru(II)
compounds used as DNA frame selectors15.
Ru(II) “piano stool” complexes (Figure 3) have been recently developed by the groups of Sadler and Dyson; many of them have revealed a really promising in vitro and in vivo activity, manifesting limited toxicity, remarkable aggressiveness towards metastasis and high activity against platinum-resistant cancers16.
14 Singh, A. K.; Pandey, D. S.; Xu, Q.; Braunstein, P. Coordin. Chem. Rev. 2014, 270, 31-56. 15 Barton, J. K.; Danishefsky, A. T.; Goldberg, J. M. J. Am. Chem. Soc.1984, 106, 2172-2176. 16 Yan, Y. K.; Melchart, M.; Habtemariam, A.; Sadler, P.J. Chem. Commun. 2005, 4764–4776.
7
Figure 3. Ru(II)-arene (“piano stool”) complexes.
Their geometry can be described either as a pseudo-tetrahedron with a six-electron donor occupying one position, or as an octahedron, assuming that the arene occupies three facial coordination positions.
Figure 4 shows two examples: [Ru(η6-arene)(XY)(Z)]0/+ (XY = bidentate ligand, Z = good leaving
group, a)
and
[Ru(η6-p-cimene)(pta)Cl2)] (known as RAPTA-C, b).
Figure 4. Piano-stool typical compounds.
Complexes of type a (see Figure 4) contain a neutral or mono-anionic N,N-, N,O-, or O,O-chelating ligand (X-Y: e.g., en, bipy, picolinate, 8-hydroxyquinolate, acetylacetonate, maltolate) while Z is typically a halide; they could present either neutral or positive charge depending on the nature of the chelating ligand (X-Y).
8 Compounds of the type [Ru(η6-arene)(en)(Cl)][PF
6] (en = ethane-1,2-diamine) have shown
promising anticancer activity both in vitro against human cancer cell lines, including some cisplatin-resistant variants, and in vivo against human ovarian cancer cells xenografts17,18.
Cytotoxicity increases on increasing the size and the hydrophobicity of the arene ligand18,19.
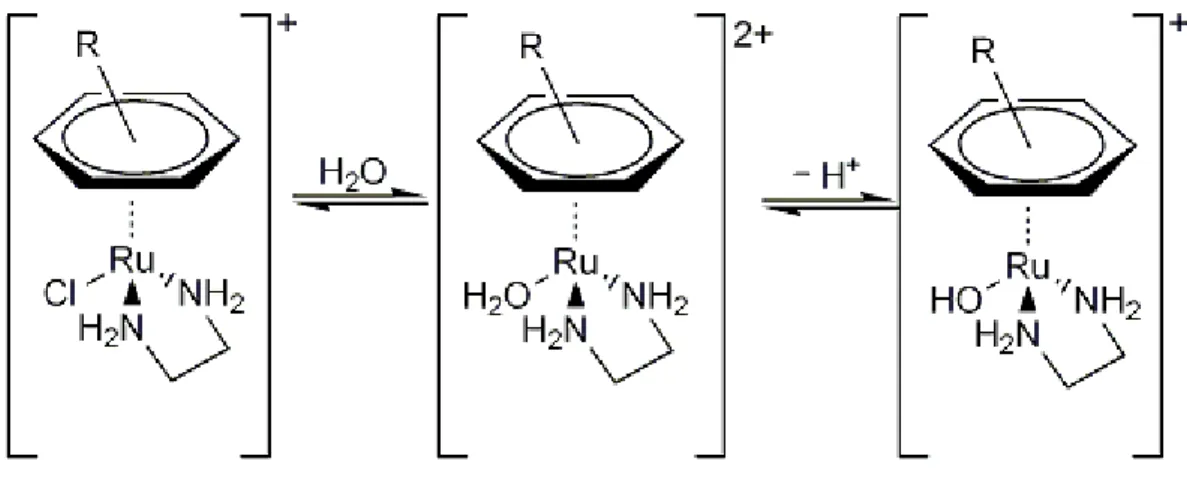
Piano-stool compounds exert their action through hydrolysis of the Ru-Cl bond, thus generating a monofunctional adduct; aquation is largely suppressed in extracellular environment (where [Cl ] is about 0.1 M), but becomes possible inside cells where [Cl ] is much lower (ca. 4-25 mM). The resulting aquo-species presents pKa values between 7 and 8; then, in physiological
conditions the [Ru-OH2] form largely prevails over the [Ru-OH] one. The activation sequence is
reported in Figure 5.
Figure 5. Speciation of Ru piano-stool compounds.
[Ru-OH2] compounds manifest their cytotoxic activity by selectively attacking guanine (G)
residues in DNA at the N7 ring position; this feature is associated with stereospecific hydrogen
bonding between one guanine-oxygen and the NH group, and with π–π stacking between the aromatic ligand and the nucleobase20. The kinetic of hydrolysis of ruthenium compounds
strongly depends on the nature of the ligands and on the net charge.
17 Peacock, A. F. A.; Sadler, P.J. Chem. Asian J. 2008, 3, 1890–1899.
18 Aird, R. E.; Cummings, J.; Ritchie A. A.; Muir, M.; Morris, R. E.; Chen, H.; Sadler, P. J.; Jodrell, D. I.
Br. J. Cancer 2002, 86, 1652 – 1657.
19 Liu, H. K.; Berners-Price, S. J.; Wang, F.; Parkinson, J. A.; Xu, J.; Bella, J.; Sadler, P.J. Angew. Chem.
Int. Ed. 2006, 45, 8153–8156.
20 Novakova, O.; Chen, H.; Vrana, O.; Rodger, A.; Sadler, P.J.; Brabec, V. Biochemistry 2003, 42, 11544– 11554.
9 Complexes containing p-cymene, biphenyl or tetrahydronaphtalene as arene ligand have provided IC50 in the range of 0.6-9 mM, comparable with platinum-based commercial drugs.
Also several chelating ligands (X-Y, see Figure 4a) have been tested, and a significant loss in cytotoxic activity has been found in those complexes were X-Y acts as π-acceptor. On the other hand, Ru-arene complexes with Z = I and a strong π-acceptor as X-Y chelating ligand (e.g. N,N-dimethylphenyl- or hydroxyphenyl-azopyridine, see Figure 6) have shown surprisingly high activity21.
Figure 6. Structure of [(η6-arene)Ru(azpy)I] [PF
6] (azpy = N,N-dimethylphenyl- or hydroxyphenyl-azopyridine).
Since the latter compounds are completely inert toward hydrolysis, they are not able to attack DNA. Instead they can enter a catalytic redox cycle that produces reactive radicals killing the cell. Thus glutathione (GSH) may be converted into its disulfide (GSSG, see Figure 7), and the consequent generation of reactive oxygen species (ROS) leads to cell death22.
21 Dougan, S.J.; Habtemariam, A.; McHale, S.E.; Parsons, S.; Sadler, P.J. PNAS, 2008 105, 11628– 11633.
22 Dougan, S.J.; Habtemariam, A.; McHale, S.E.; Parsons, S.; Sadler, P.J. PNAS, 2008 105, 11628– 11633.
10
Figure 7. Glutathione (GSH) and glutathione disulfide (GSSG) structures.
Complexes of type b (see Figure 4) present a monodentate phosphane ligand (pta = phosphatriaza-adamantane); the complex shown in Figure 4b (RAPTA-C) and the analogue containing toluene (RAPTA-T) were fully investigated both in vitro and in vivo.
These compounds exhibit low cytotoxicity on account of rapid hydrolysis and weak binding to DNA, nevertheless they present a really interesting anti-metastatic activity. Indeed RAPTA compounds avoid metastases formation via some interaction with extracellular matrix components which in turn inhibit some key steps of the metastatic process. This feature may allow to overcome one of the crucial limitations of platinum-based anticancer drugs.
In conclusion, ruthenium piano-stool compounds are really promising scaffolds for drug design.
1.3.2. Ruthenium (III) complexes
Ruthenium in the biological environment can exist as Ru(II) or Ru(III); the related redox potential is 0.249 V, thus allowing Ru(III) to Ru(II) reduction in physiological conditions. This aspect has fuelled an intense research on Ru(III) derivatives, with the aim of preparing new compounds substantially inert in the extracellular environment (so to limit side-effects) but able to become active inside cancer cells.
In fact, cancer cells present a relatively high concentration of transferrin receptors than healthy cells, in order to uptake as much iron as possible to satisfy their rapid growing23. Ru(III), due to
its similarity with Fe(III), can be easily transported in the blood by transferrin and also by albumin24.
23 Hanahan, D.; Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144 (646-674). 24 Hartinger, C. G.; Keppler, B. K. Electrophoresis 2008, 28, 3436–3446.
11 The drug is prevalently addressed to cancerous cells because of their abnormal over-expression of transferrin and albumin receptors25. Once inside the cell, after metal reduction, a
chlorine ligand is usually displaced and the complex manifests its cytotoxicity towards DNA and other cellular targets26.
Similarly, Ru(III) reduction potential is extremely adjustable, because it is strongly related to the ligand environment.
This kind of Ru(III) complexes have general formula trans-[RuA2Cl4][B], and the most widely
investigated contains: A = 1H-indazole, B = 1H-indazol-2-ium (KP1019, a), A = 1H-indazole B = sodium (KP1339, b), A = 1H-imidazole, B = 3H-imidazol-1-ium (KP418, c), A = 1H-imidazole, dimethyl sulfoxide, B = 3H-imidazol-1-ium (NAMI-A, d), see Figure 8.
Figure 8. Ruthenium III anticancer compounds.
In the sections below the principal features of the two main classes of Ru(III) anticancer compounds will be shortly described.
25 Hartinger, C. G.; Zorbas-Seifried, S.; Jakupec, M. A.; Kynast, B.; Zorbas, H.; Keppler, B. K. J. Inorg.
Biochem. 2006, 100, 891–904.
12
1.3.2.1. Keppler type complexes
An extremely large number of Ru(III) compounds have been screened as anticancer agents and those containing A = N-heterocyclic ligands were identified as the most promising ones. Compounds a, b and c reported in Figure 8 demonstrated interesting activity both against primary tumours and metastases. Biological studies over KP1019 and its sodium salt analogue KP1339 have revealed that these compounds mainly induce apoptosis via intrinsic mitochondrial pathway, and the resistance acquired by the cells appears significantly modest27,28.
KP1019 entered clinical Phase I trials in 2004 with noteworthy results in disease stabilization activity and negligible negative effects. Furthermore, KP1019 was stable enough in water solution to admit its intravenous administration. This is a key-point allowing to study pharmacokinetics29.
1.3.2.2. NAMI type complexes
In the early 1980’s, ruthenium compounds containing dimethyl sulfoxide (dmso) ligands were investigated. They appeared particularly attractive because of some distinctive features conferred by dmso: (i) dmso can bind metal centres either through the sulfur or through the oxygen, depending on both steric and electronic factors; (ii) dmso-S-Ru compounds are air-stable; (iii) dmso-S-Ru compounds present a moderately good π-acceptor behaviour and stabilize Ru(II) species, thus tolerating Ru(III) to Ru(II) reduction; (iv) dmso is a good leaving group, therefore the generation of reactive aquated derivatives takes place easily; (v) dmso confers to the complex good solubility in water and capability to penetrate non polar membranes.
Many different Ru complexes containing dmso and chlorine ligands were investigated, providing good results especially against metastases, rather than primary tumours.
In particular, NAMI-A (d, Figure 8), synthesized in order to prepare an analogue of KP418 (c, Figure 8) incorporating a dmso, was found to be highly effective towards metastases in vivo;
27 Heffeter, P.; Pongratz, M.; Steiner, E.; Chiba, P.; Jakupec, M. A.; Elbling, L.; Marian, B.; Körner, W.; Sevelda, F.; Micksche, M.; Keppler, B. K.; Berger, W. J. Pharmacol. Exp. Ther. 2005, 312, 281–289. 28 Bratsos, I.; GIanferrara T.; Alessio, E.; Hartinger, C. G.; Jakupec, M. A.; Keppler, B. J. Bioinorganic
Medicinal Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 153-160.
29 Lentz,, F.; Drescher, A.: Lindauer, A.; Henke, M.; Hilger, R. A.; Hartinger, C. G.; Scheulen, M. E.; Dittrich, C.; Keppler, B.K.; Jaehde, U. Anti-cancer Drug 2009, 20, 97–103.
13 tests on human non-small lung cancer cells xenotransplanted into nude mice revealed a significant reduction of the metastasis growth.
This achievement constitutes one of the most important one in the history of anticancer drugs: in fact metastases represent the leading cause of cancer death, because of their scarce response to chemotherapy, their multiple locations and low accessibility to surgery and/or radiotherapy.
NAMI-A successfully accomplished clinical Phase I trials showing negligible toxicity and, in 2008, it entered a therapeutic combination clinical Phase I/II trials in patients affected by non-small cell lung carcinoma.
1.4. Osmium anticancer compounds
The results obtained with ruthenium (II) and ruthenium (III) compounds prompted to investigate other similar metal-based compounds. From this viewpoint, osmium is the most interesting candidate, since it is just below ruthenium in the periodic chart and may form arene compounds that are isostructural and isoelectronic with the ruthenium congeners. Since third-row transition metals usually present high toxicity and inertness to ligand substitution, the use of osmium in therapy was initially mistrusted.
Nevertheless, many osmium compounds have been recently tested giving promising outcomes. Osmium (II) compounds with N,N or O,O chelates (e.g. the one reported in Figure 9a) were found to be scarcely cytotoxic due to slow aquation and the possible formation of inert hydroxo-bridged dimers, under physiological conditions. In spite of this fact, Os(II)-arene compounds with anionic N,O chelating ligands, such as [Os(η6-biphenyl)(pico)Cl] (pico = picolinate, see
Figure 9b), were found to be active towards both A2780 (IC50 comparable with carboplatin) and
A549 cancer cell lines30. The corresponding Ru compounds are inactive against these kinds of
cancer cells.
30 van Rijt, S. H.; Peacock, A. F. A.; Johnstone, R. D. L.; Parsons, S.; Sadler, P. J. Inorg. Chem. 2009, 48, 1753–1762.
14
Figure 9. Exemples of tested Os(II)-arene compounds.
In addition to this, some osmium complexes exhibit activity towards platinum-resistant cancer cells, thus invoking a different mechanism of action31. These facts demonstrate that the
biological activity of piano-stool compounds depends on a delicate balance of the chemical properties of all the components, including the nature of the ligands and the metal.
The recent results clearly explain why osmium arene chemistry is now attracting increasing attention in the anticancer drugs field, although it has been little investigated in comparison with the ruthenium congeners.
1.5. Selectivity in cancer treatment; a major challenge
The main troubles regarding anticancer clinical treatment are without a doubt related to side-effects. These are strictly associated with two factors: limited selectivity and limited cytotoxicity. Unfortunately a cure able to selectively attack cancerous cells without damaging healthy organs has not been developed hitherto. Otherwise chemotherapy (but generally all the clinical treatments, e.g. radiotherapy) essentially acts by killing or inducing apoptosis to cells. Another point is that metal-based drugs show intrinsic toxicity which is manifested upon drug administration32.
31 Kostrhunova, H.; Florian, J.; Novakova O.; Peacock, A. F. P.; Sadler, P. J.; Brabec, V. J. Med. Chem.
2008, 51, 3635–3643.
15 Healthy and diseased cells/tissues exhibit markedly-different chemical-physical properties. This point is crucial in order to enhance the selectivity of a designed anticancer drug. Examples are given in the following:
1) Tumour hypoxia: in solid tumours, the oxygen concentration is low (<3 µM), due to poor and irregular development of the vascular tissues and the increased level of metabolism33,34. Hypoxic cells in tumours tend to be resistant to chemotherapy due to
the over-expression of genes implicated in drug resistance. In addition, those drugs which act by generating DNA-damaging free radicals require the presence of oxygen35.
Indeed their effectiveness is reduced in hypoxic regions, where the lack of O2 allows the
DNA free radicals to return to their original form via reduction by thiol compounds36.
On account of these considerations, a really promising strategy to develop efficient drugs exploits the oxygen-depleted environment to make the drug active through reduction of the metal center. Examples regarding Ru(III)/Ru(II) have been reported above.
2) pH value: the interstitial pH in the cancer environment is significantly lower than that typical of healthy tissues, the former falling in the range 5.4–7.437. This can be attributed
to the high rate of metabolism by cancer cells, producing high amounts of CO2 which
cannot be efficiently removed due to the low development of vascular vases38.
Hence metal complexes can be conceived as potential drugs becoming cytotoxic in the acidic surroundings of cancer cells, as consequence of structural changes due to pH effects39.
33 Tredan, O.; Galmarini, C. M.; Patel, K.; Tannock, I. F. J. Natl. Cancer Inst. 2007, 99, 1441–1454. 34 Norman, j. F.; Trevor W. H. Bioinorganic Medicinal Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA,
2011, pp. 49-54.
35 Dougan, S.J.; Habtemariam, A.; McHale, S.E.; Parsons, S.; Sadler, P.J. PNAS, 2008 105, 11628– 11633.
36 Brown, J. M.; William, W. R. Nat. Rev. Cancer 2004, 4, 437–447.
37 Helmlinger, G.; Schell A.; Dellian, M.; Forbes, N. S.; Jain, R. K. Clin. Cancer Res. 2002, 8, 1284–1291. 38 Newell, K.; Franchi, A.; Pouyssegur, J.; Tannock, I. Proc. Natl. Acad. Sci. USA 1993, 90, 1127–1131. 39 Galanski, M. ; Baumgartner, C.; Meelich, K.; Arion, V. B.; Fremuth, M.; Jakupec, M. A.; Schluga, P.; Hartinger, C. G.; Von Keyserlingk, N. G.; Keppler, B. K. Inorg. Chim. Acta 2004, 357, 3237–3244.
16 3) Temperature. Due to enhanced metabolism, the temperature in cancer tissues is higher by up to 5 Celsius degrees than the temperature typical of healthy environments (ca. 37 °C). This feature can be exploited to design suitable potential ligands for appropriate metal centres, conferring increased solubility to the complex in the temperature range 37-42 °C. Ruthenium complexes, similar to RAPTA, have been successfully prepared according to this strategy40.
1.6. Ethacrynic acid: an innovative solution to enhance selectivity
A new frontier for improving anticancer activity is to combine a reactive metal moiety that has a known pharmacological activity (e.g. Pt(II), Ru(II) complexes) with an organic fragment of complementary function (multi-targeted therapeutics)41,42.
The resulting multi-functional complexes can achieve a very high selectivity, if the target of their biologically active ligands is significantly over-expressed in the diseased cells.
One of the strongest limitations involved in the clinical treatment of cancer is the progressive loss of efficacy with time. Resistance systems involve mechanisms extremely difficult to describe and to understand. Notwithstanding the research has addressed great effort aimed to develop strategies useful to overcome some of the mechanisms of resistance of the cells. One of the most successful strategies regards glutathione-S-transferase (GST), i.e. an important class of enzymes involved in cell-detoxification and apoptosis regulation. This group of enzymes catalyzes the reaction between the detoxifying agent glutathione and the substance recognized as “foreigner” by the cell, X (see Figure 10).
Figure 10. Glutathione conjugation to a generic xenobiotic (X) via GST results in the formation of a glutathione-S conjugate.
40 Clavel, C. M.; Paunescu E.; Nowak-Sliwinska P.; Dyson P. J. Chem. Sci. 2014, 5, 1097-1101. 41 Kilpin K. J.; Dyson P. J. Chem. Sci., 2013, 4, 1410-1419.
42 Nazarov, A. A.; Gardini, D.; Baquié, M.; Juillerat-Jeanneret, L.; Serkova, T. P.; Shevtsova, E. P.; Scopelliti, R.; Dyson, P. J. Dalton Trans. 2013, 42, 2347.
17 Interestingly, metal-drug resistance in some cancers, especially with reference to cisplatin, has been associated with an increased activity of the GST enzymes43.
Ethacrynic acid (see Figure 11) is a commercially available compound, commonly used as diuretic to treat high blood pressure and swelling e.g. caused by congestive heart failure, liver failure, and kidney failure. More importantly, ethacrynic acid acts as a GST inhibitor. Ethacrynic acid contains a phenoxyacetic acid derivative bearing a ketone group and a methylene group. This structure may bind cysteine and glycine residues (which are included in glutathione).
Figure 11. Ethacrynic acid (EA-COOH).
Administration of ethacrynic acid in combination with cisplatin has been demonstrated to restore the anticancer activity up to the original level.
The ethacrynic acid skeleton (EA) has been successfully incorporated in Pt(IV) (Ethacraplatin, 2005)44, Pt(II) (2011)45 and Ru(II)-arene complexes (2007, 2009)46,47 see Figure 12.
43 Mannervik B.; Danielson, U. H. Crit. Rev. Biochem. Mol., 1988, 23, 283-337.
44 Ang, W. H.; Khalaila, I.; Allardyce, C. S.; Juillerat-Jeanneret L.; Dyson, P. J.; J. Am. Chem. Soc. 2005,
127, 1382-1383.
45 Zanellato, I., Bonarrigo, I., Sardi, M.; Alessio, M.; Gabano, E.; Ravera, M.; Osella, D. ChemMedChem,
2011, 6, 2287-2293.
46 Ang, W. H.; Parker L. J.; De Luca A.; Juillerat-Jeanneret L.; Morton C. J.; Lo Bello M.; Parker M. W.; Dyson, P. J.; Angew. Chem. Int. Ed., 2009, 48, 3854-3857.
47 Ang, W. H.; De Luca C.; Chapuis-Bernasconi C.; Juillerat-Jeanneret L.; Lo Bello M.; Dyson, P. J.;
18 Figure 12. Ethacrynic acid (RCOOH) and related metal complexes: Ethacraplatin (a); cis-[Pt(NH3)2(EA)2] (b);
[Ru(EA-η6-arene)(pta)Cl
2], arene = phenylethylester (c) or benzylamide (d); [Ru(η6-p-cimene)(imidazole-EA)X2] (e).
Ethacraplatin has shown to undergo in vivo reduction to Pt(II) and release of ethacrynic acid inside the GST enzyme.Interestingly, the Ru(II)-arene derivatives, irrespective of the position of ethacrynic acid in the molecule, inhibit GST similarly to free ethacrynic acid and are considerably more cytotoxic than RAPTA-C.
The synthetic strategy involving the use of ethacrynic acid is really promising, because it potentially allows to reduce the effective dose of administrated drugs, and thus the side-effects.
19
2. Aim of the work
This work is focused on the synthesis of new Ru and Os complexes with potential anticancer activity. The design of the new compounds has been based on metal frames bearing structures with ascertained anticancer activity (ruthenium48,49 and osmium50 arenes, NAMI-like51,52); such
metal frames have been associated with newly prepared suitable ligands.
The new ligands have been designed and obtained by coupling a skeleton containing one heteroatom (N or P), able to coordinate Ru and Os centers, with a fragment derived from ethacrynic acid53. In particular, pyridines 54 and PPh
3 55 have been used to the purpose, since
both types of compounds have been reported to act as enhancers of antiproliferative activity. The synthesis of the Os complexes has been carried out at the University of Zaragoza (E), under the supervision of Prof. M. A. Esteruelas.
The anticancer activity of the prepared Ru and Os complexes will be tested at the École
Polytechnique Fédérale de Lausanne (EPFL).
48 Aird, R. E.; Cummings, J.; Ritchie, A. A.; Muir, M.; Morris, R. E.; Chen, H.; Sadler, P. J.; Jodrell, D. I.
Brit. J. Cancer, 2002, 86, 1652-1657.
49 Singh, A. K.; Pandey, D. S.; Xu, Q.; Braunstein, P. Coord. Chem. Rev., 2014, 270–271, 31–56.
50 Fu, Y.; Habtemariam, A.; Pizarro, A. M.; van Rijt, S. H.; Healey, D. J.; Cooper, P. A.; Shnyder, S. D.; Clarkson, G. J.; Sadler, P. J. J. Med. Chem. 2010, 53, 8192–8196.
51 Hartinger, C. G.; Zorbas-Seifried, S.; Jakupec, M. A.; Kynast, B.; Zorbas, H.; Keppler, B. K. J. Inorg.
Biochem., 2006, 100, 891-904.
52 Rademaker-Lakhai, J. M.; van den Bongard, D.; Pluim, D.; Beijnen, J. H.; Schellens, J. H. Clin. Cancer
Res., 2004, 10, 3717-3727.
53 Ang, W. H.; De Luca C.; Chapuis-Bernasconi C.; Juillerat-Jeanneret L.; Lo Bello M.; Dyson, P. J.;
ChemMedChem, 2007, 2, 1799-1806.
54 Grgurič-Šipka, S.; Ivanović, I.; Rakić, G. ; Todorovic, N.; Gligorijević, N.; Radulović, S.; Arion, V. B.; Keppler, B. K.; Tešic, Ž. L. Eur. J. Med. Chem. 2010, 45, 1051-1058.
55 Sàez, R.; Lorenzo, J.; Prieto, M. J.; Font-Bardia, M.; Calvet, T.; Omeñaca, N.; Vilaseca, M.; Moreno, V.
20
3. Results and discussions
Direct esterification of the alcohol functionality, bonded to a pyridine ring, with ethacrynic acid represents a simple way to obtain the desired ligands. Thus 3-hydroxypyridine and 4-pyridinemethanol were employed as starting materials (Figure 14); 3-hydroxypyridine was used as commercial compound, whereas 4-pyridinemethanol was obtained from isonicotinaldehyde (see Figure 13).
Figure 13. Synthesis of 4-pyridinemethanol.
The two alcohols were allowed to react with ethacrynic acid to obtain 1 and 2 (see Figure 14); EDCI (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide) and DMAP (4-dimethylaminopyridine) were used as coupling system. This is a well-known method to achieve esterification reactions with high selectivity and good yields56,57.
56 Dhaon, M. K.; Olsen, R. K.; Ramasamy, K. J. Org. Chem. 1982, 47, 1962-1965.
57 Sahara, H.; Hanashima, S.; Yamazaki, T.; Takahashi, S.; Sugawara, F.; Ohtani, S.; Ishikawa, M.; Mizushina, Y.; Ohta, K.; Shimozawa, K.; Gasa, S.; Jimbow, K.; Sakaguchi, K.; Sato, N.; Takahashi, N.
21
Figure 14. Synthesis of ethacrynic acid pyridin-4-ylmethyl ester (1) and ethacrynic acid pyridin-3-yl ester (2).
The reactions (Figure 14) were conducted at room temperature for 20 hours, under protection from the light. Effectively, the light sensitivity of pyridinic alcohols is well known58.
The formation of 1 and 2 was confirmed by NMR analysis (in CDCl3): the broad peak due to the
OH group in the 1H NMR spectrum of ethacrynic acid ( = 9.56 ppm, CDCl
3) disappeared,
whereas 13C NMR showed a shift of one C=O resonance from 172.8 ppm to 167.1 ppm and
166.0 ppm, in 1 and 2 respectively. Compounds 1 and 2 appeared to be light-stable, in contrast with the parent pyridine-alcohols.
An intriguing perspective would make use of naturally-available pyridine compounds. Indeed some pyridine-carboxylic acids play a biological role in human bodies, and for this reason they have found pharmacological application. In particular, nicotinic acid is an essential human nutrient (vitamin B3) and isonicotinic acid is used in tuberculosis treatment (see Figure 15).
These compounds present limited toxicity and higher stability respect to the pyridine-alcohols discussed above.
22
Figure 15. Pyridinic acids used; nicotinic acid (a), isonicotinic acid (b) and 3-(pyridin-3-yl)propionic acid (c).
Therefore the possible coupling of nicotinic acid and isonicotinic acid with ethacrynic acid was explored. Such coupling requires the preliminary conversion to alcohol of one of the two carboxylic acid functions, thus many attempts were done in this respect (see Experimental). The best strategy revealed to be that one shown in Figure 16: the reaction of ethacrynic acid with ethylene glycol in the presence of EDCI/DMAP afforded compound 6, which was purified by silica chromatography and isolated as a colourless solid in good yield.
Figure 16. Synthesis of ethacrynic acid 2-hydroxy-ethyl ester (6).
Compound 6 is stable at room temperature, and completely soluble in CHCl3, CH2Cl2 and
acetone. It was characterized by NMR spectroscopy. The 1H NMR spectrum of 6 (in CDCl3)
display two resonances at 4.27 ppm and 3.75 ppm attributed to the CH2CH2 chain, while the OH
signal has been found at 13.78 ppm. Correspondingly, the 13C spectrum contains two resonances at 66.9 ppm and 60.6 ppm due to the CH2CH2 chain.
The synthesis of 6 allows the incorporation of ethacrynic acid in suitable N- or P-donor molecules; thus 6 was combined with three compounds, i.e. two pyridines and one triphenylphosphine (Figure 17). The corresponding esterification reactions were conducted in the same conditions detailed above (EDCI/DMAP system), and led to the synthesis of the ligands 7, 8 and 9 (see Figure 17).
23 EDCI, DMAP CH2Cl2, rt, 20h O Cl Cl O O O HO + N COOH N COOH P COOH O Cl Cl O O O O N O O Cl Cl O O O O N O O Cl Cl O O O O P O 7 8 9 6
Figure 17. Synthesis of nicotinic acid 2-ethacrynyloxy-ethyl ester (7), ethacrynic acid 2-(3-pyridin-3-yl-propionyloxy)-ethyl ester (8) and 4-Diphenylphosphanyl-benzoic acid 2-ethacrynyloxy-ethyl ester (9).
Compounds 7, 8 and 9 were respectively isolated by a delicate chromatography on silica column. Partial hydrolysis of the newly formed ester functions was observed, probably due to interaction with silica. This required to separate, in every cases, a minor fraction containing impurities which were discharged, by elution with mixtures of hexane and Et2O in variable ratios.
The products were collected by using Et2O/acetone or even neat acetone as eluent, then they
were isolated in moderate to good yields (31-54%) as white solid (7), light-yellow oil (8) or colourless sticky solid (9), respectively.
Compounds 7-9 are completely soluble in CH2Cl2, CHCl3, Et2O and acetone; the
characterization was carried out through IR, 1H-NMR and 13C-NMR analysis. The data agree in
indicating the selective formation of a new ester bond (see Table 2).
Figure 18. Skeleton of synthesized ligands. NMR resonances related to C14 and C15 (highlighted in the figure) proof the formation of the new ester bond.
24
Table 2. Comparative view of significant NMR data related to 7, 8 and 9 and their parent compound.
Structure C15 - C14 δ (ppm, CDCl3) H-C14 – H-C15 δ (ppm, CDCl3) 6 O Cl Cl O O O HO 66.9, 60.6 4.27, 3.75 7 O Cl Cl O O O O N O 62.9, 62.7 4.51 (m) 8 O Cl Cl O O O O N O 63.2, 61.9 4.37, 4.30 9 O Cl Cl O O O O P O 62.4, 63.3 4.56 (m)
In summary we obtained five new functionalized molecules (1, 2, 7, 8, 9) potentially interesting in metal-based drugs synthesis, because they contain: (i) a donor atom (N for compounds 1, 2,
7 and 8, P for 9) with marked affinity towards Ru(II), Ru(III) and Os(II); (ii) the active skeleton of
ethacrynic acid, which may be released in a biological environment.
Two different ruthenium-based systems with a well-known anticancer and/or antimetastatic activity were employed to coordinate the new ligands, i.e. the Ru(II) arene dimer [Ru(η6
-p-cymene)Cl2]2 and the Ru(III) compound [(Me2SO)2H][trans-Ru(Me2SO)2Cl4] (respectively
25 Ru Cl Cl Cl Cl S S O O (MeSO)2H Ru Cl Cl 11 14
Figure 19. Ruthenium-based precursors for the new anticancer metal derivatives.
Thus [Ru(η6-p-cymene)Cl
2]2 was allowed to react in CHCl3 with 1 and 7, respectively affording
the complexes 12 and 13. The reactions were carried out at room temperature for several days.
CHCl3, rt, 5 to 9 days + O Cl Cl O O O O N O O Cl Cl O O O N Ru Cl Cl Ru Cl Cl Ru Cl Cl Cl Cl O O O O N 12 13 Ru Cl Cl O Cl Cl O O O O N O 1 7
Figure 20 Synthesis of new Ru(II) arene complexes.
Complexes 12 and 13 were purified through several washings with diethyl ether.
Red crystals of 12 suitable for X-ray analysis were obtained from a THF/hexane mixture settled aside at -30 °C for one month. The molecular structure of 12 is shown in Figure 21, while relevant bonding parameters are reported in Table 3.
26
Figure 21. ORTEP drawing of [Ru(ethacrynic acid pyridin-4-ylmethyl ester)(η6-p-cymene)Cl2)] (12); displacement ellipsoids are at the 10% probability level.
Table 3. Selected bond lengths (Å) and angles (deg) in compound 12.
Distances (Å) Angle (°)
Ru(1) Cl(1) 2.411(1) Cl(1) Ru(1) Cl(2) 89.90(4)
Ru(1) Cl(2) 2.405(1) Cl(1) Ru(1) N(1) 85.89(9)
Ru(1) C(average) 2.163(6) Cl(2) Ru(1) N(1) 86.23(9)
Ru(1) N(1) 2.134(3) Ru(1) N(1) C(1) 122.5(3)
Ru(1) C(21) 2.181(6) Ru(1) N(1) C(5) 120.5(3)
N(1) C(1) 1.338(5) C(6) O(1) C(7) 118.5(3)
O(1) C(6) 1.440(5) O(1) C(6) C(3) 107.4(4)
O(1) C(7) 1.324(5) O(1) C(7) O(2) 125.0(4)
O(2) C(7) 1.182(6) C(8) O(3) C(9) 120(1) C(3) C(6) 1.505(6) C(8) O(3) C(9) 120(1) C(7) C(8) 1.51(1) C(8) O(3) 1.43(2) O(3) C(9) 1.34(1) C(15) O(4) 1.24(1)
The structural features related to 12 were as expected. In particular, the M-arene centroid distance [Ru(1) Cy(centroid) 1.652 Å] resembles the ones reported in literature for fragments
[Ru(η6-arene)Cl
2L]59,60. Ru-Cl [average value Ru-Cl 2.442(6) Å] and Ru-C distances [average
value 2.163(6) Å] and Cl-Ru-Cl angle [89.90(4)°] resemble the respective values reported in the
59 Bagh, B.; Mc Kinty, A. M.; Lough, A. J.; Stephan, D. W. Dalton Trans. 2014,43, 12842-12850.
60 Clavel, C. M.; Paunescu, E.; Nowak-Sliwinska, P.; Griffioen, A. W.; Scopelliti, R.; Dyson, P. J. J. Med.
27 literature for similar Ru(II) arenes61,62. Moreover the bonding parameters of the ethacrynic acid
skeleton are comparable to those reported for previous relevant crystallographic characterizations63.
Compound 13 was obtained as an orange solid and characterized by 1H-NMR; the resonances due to the pyridine ring undergo significant shift compared to the situation seen in the not coordinated organic fragment (e.g. the chemical shifts due to the protons bound to the carbons close to the nitrogen shift from 9.12 ppm to 9.55 ppm and from 8.69 ppm to 9.18 ppm, respectively).
New NAMI-A type Ru(III) compounds were obtained by reaction of 14 with 1 and 2 respectively, affording the ionic compounds 15 and 16 (see Figure 22). The reactions were carried out in acetone, at room temperature for approximately 2 hours.
61 Dorcier A.; Dyson P. J.; Gossens C.; Rothlisberger U.; Scopelliti R.; Tavernelli I. Organometallics 2005, 24, 2114-2123.
62 Ang, W. H.; De Luca C.; Chapuis-Bernasconi C.; Juillerat-Jeanneret L.; Lo Bello M.; Dyson, P. J.
ChemMedChem, 2007, 2, 1799-1806.
28 Ru Cl Cl Cl Cl S S O O (MeSO)2H Ru Cl Cl Cl Cl S O 1H Ru Cl Cl Cl Cl S O 2H O Cl Cl O O O N O Cl Cl O O O N + Acetone, rt, 2h O Cl Cl O O O N O Cl Cl O O O N 15 16 14 1 2
Figure 22. Synthesis of new Ru(III) NAMI-type compounds.
Complexes 15 and 16 are soluble in DMSO, partially soluble in methanol and rather insoluble in water. Their structure was checked with IR spectroscopy (15 and 16) and mass spectrometry (15). In both cases, ESI-MS analysis indicated the presence of an anion formed from 14 by selective mono-substitution of one dmso with a pyridine ligand, associated with the corresponding pyridinium cation.
The preparation of osmium compounds containing ethacrynic acid derivatives was carried out at the University of Zaragoza, under the supervision of Prof. M. A. Esteruelas. His research group has developed a long time experience in the synthesis of osmium arene compounds.
The osmium-arene compound [Os(η6-p-cymene)Cl
2]2 (17) (see Figure 23) was employed as
precursor64; it is actually the analogue of the Ru(II) species 11 cited above (see Figure 19).
29
Figure 23. Starting material for new anticancer Os compounds.
The dissolution of 17 in pentane-2,4-dione, in the presence of a strong base, represents a straightforward route to access the intriguing “bis-acac” complex [Os(η6-p-cymene)(η1
-C-acac)(O,O’-acac)] (21) (Figure 24)65.
Figure 24. Synthesis of mononuclear Os-bis-acac complex.
Compound 21 contains two acetylacetonate ligands linked in different ways: the first unit chelates the metallic center through two O atoms, whereas the second ligand is bound through a weak η1-C interaction. As a consequence, the chemical behavior of the two acetylacetonates
is extremely different to each other ; the chelating ligand is extremely inert and thus difficult to replace, otherwise the monodentate one is very labile and can be easily substituted66.
To take advantage of the lability, compound 21 was allowed to react with ethacrynic acid. The reaction was carried out in CH2Cl2, at room temperature for approximately 24 hours and led to
the formation of the ethacrynate complex 22 (see Figure 25).
65 Michelman, R. I.; Ball, G. E.; Bergman, R. G.; Andersen, R. A. Organometallics 1994, 13, 869-881. 66 Bennett M. A.; Mitchell T. R. B.; Stevens M. R.; Willis A. C. Can. J. Chem. 2001, 79, 655-669.
30
Figure 25. Introduction of ethacrynate ligand by easy replacement of one η1-C-bound acetylacetonate in Os complex.
Complex 22 was purified through several washings with diethyl ether, finally isolated as a bright-yellow solid in good yield (54%) and fully characterized (NMR, IR, ESI-MS and elemental analysis). Yellow crystals of 22 suitable for X-ray analysis were obtained by slow diffusion of pentane into a solution of the complex in CH2Cl2. The molecular structure of 22 is shown in
Figure 26, while relevant bonding parameters are reported in Table 4.
Figure 26. ORTEP drawing of [Os(η1-O-C(O)-EA)(O,O’-acac)(η6-p-cymene)] (22); displacement ellipsoids are at the 15% probability level.
31
Table 4. Selected bond lengths (Å) and angles (deg) in compound 22.
Distances (Å) Angle (°)
Os(1) O(1) 2.081(3) O(1) Os(1) O(5) 80.60(9)
Os(1) O(5) 2.072(2) O(1) Os(1) O(6) 77.35(9)
Os(1) O(6) 2.081(2) O(5) Os(1) O(6) 88.15(9)
Os(1) C(average) 2.172(4) Os(1) O(1) C(1) 126.5(2)
O(1) C(1) 2.187(4) Os(1) O(5) C(14) 125.9(2)
O(2) C(1) 1.224(4) Os(1) O(6) C(17) 126.5(2)
O(2) C(7) 1.182(6) C(2) O(3) C(3) 117.5(3)
O(3) C(2) 1.428(4) O(1) C(1) O(2) 128.1(3)
O(3) C(3) 1.365(4) O(1) C(1) C(2) 114.0(3) O(4) C(9) 1.214(5) O(2) C(1) C(2) 117.9(3) C(1) C(2) 1.521(5) O(2) C(1) C(2) 117.9(3) C(9) C(10) 1.492(5) C(10) C(11) 1.333(5) C(10) C(12) 1.506(5)
The main structural features of 22 resemble the corresponding ones related to other osmium-acetylacetonate structures previously reported67. Moreover the geometric parameters
concerning EA are similar to those found in 12.
Complex 22 is well soluble in CH2Cl2, acetone, CH3CN, slightly soluble in diethyl ether and
almost insoluble in pentane and hexane. A solution of 22 in deuterated DMSO was analyzed by
1H-NMR, showing no substitution reactions after 6 h.
In order to incorporate EA through coordination of new ligands to the osmium centre, the successful synthetic route followed to obtain Ru(II) compounds was adopted; thus the Os(II)-arene 17 was allowed to react with ligands 7,8 and 9.
These reactions were carried out in CH2Cl2 at room temperature for 15-20 hours and led,
respectively, to the formation of the new complexes 18, 19 and 20 (see Figure 27).
32 + Os Cl Cl Os Cl Cl Os Cl Cl O Cl Cl O O O O N O Os Cl Cl O Cl Cl O O O O O P O Cl Cl O O O O N O O Cl Cl O O O O N O O Cl Cl O O O O P O Os Cl Cl O Cl Cl O O O O O N CH2Cl2, rt, 15 to 20h 19 18 20 7 8 9
Figure 27. Synthesis of new Os(II) arene complexes.
Complexes 18, 19 and 20 were washed several times with diethyl ether and with pentane to be purified, so to obtain yellow solid (18 and 19) or orange solids (20) in good yields (41-71%).
18-20 were fully characterized by NMR, IR, ESI-MS and elemental analysis. Yellow crystals of 18
suitable for X-ray analysis were collected after many attempts: the successful procedure consisted of slow diffusion of hexane into a solution of the complex in CH2Cl2 in the presence of
minor amount of hydroquinone68. Hence the molecular structure of 18 could be ascertained by
X-ray diffractometry: the structure is shown in Figure 28 while relevant bonding parameters are reported in Table 5.
33
Figure 28. ORTEP drawing of [Os(nicotinic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)Cl2] (18); displacement ellipsoids are at the 30% probability level.
Table 5. Selected bond lengths (Å) and angles (deg) in compound 18.
Distances (Å) Angle (°)
Os(1) Cl(1) 2.423(1) Cl(1) Os(1) Cl(5) 85.08(4)
Os(1) Cl(2) 2.416(1) Cl(1) Os(1) N(1) 82.2(1)
Os(1) C(average) 2.176(6) Cl(2) Os(1) N(1) 86.6(1)
Os(1) N(1) 2.119(4) Os(1) N(1) C(1) 119.9(3)
N(1) C(5) 1.343(5) Os(1) N(1) C(5) 122.2(3)
N(1) C(1) 1.356(5) C(1) N(1) C(5) 117.9(4)
O(2) C(6) 1.342(6) O(1) C(6) O(2) 124.4(4)
O(2) C(7) 1.446(5) C(4) C(6) O(1) 125.1(4)
O(3) C(8) 1.449(5) C(4) C(6) O(2) 110.5(4)
O(3) C(9) 1.332(6) C(8) O(3) C(9) 116.1(3)
O(4) C(9) 1.198(6) O(3) C(9) O(4) 125.3(4)
O(5) C(11) 1.413(7) C(10) O(5) C(11) 118.7(4) O(5) C(12) 1.368(5) C(10) O(5) C(11) 118.7(4) O(6) C(17) 1.216(6) C(4) C(6) 1.486(6) C(7) C(8) 1.495(7) C(9) C(10) 1.512(6)
34 Data obtained for compound 18, in particular, Cl [average value Cl 2.419(2) Å] and Os-Cymene distance [Os-Os-Cymenecentroid 1.644 Å] resemble the respective values reported in the
literature for similar Os(II) arenes69. Bond distances and angles for incorporated EA are rather
similar to those reported for the non coordinated compound70.
The characterization of 19 and 20 relied mainly on NMR spectra. Chemical shifts related to the cymene moiety in 17-20 are compared in Table 6
.
Table 6. Comparative view of significant NMR data related to cymene group of compounds 18, 19, and 20 and their starting compound.
Structure H-C aromatics (ppm, CD2Cl2) H-C methyl (ppm, CD2Cl2) 17 Os Cl Cl 2 6.14, 5.99 2.20 18 Os Cl Cl O Cl Cl O O O O O N 5.86, 5.64 2.03 19 Os Cl Cl O Cl Cl O O O O N O 5.80, 5.54 2.03 20 Cl Os Cl O Cl Cl O O O O O P 5.43, 5.19 1.98
Compounds 18-20 present similar solubility characteristics; more precisely, they are well soluble in CH2Cl2 and acetone, almost insoluble in diethyl ether and insoluble in pentane, hexane and
water. DMSO solutions showed to be stable for almost 4 hours (checked with 1H NMR).
69 Dorcier A.; Dyson P. J.; Gossens C.; Rothlisberger U.; Scopelliti R.; Tavernelli I. Organometallics 2005, 24, 2114-2123.
35 In order to enhance water solubility (a feature facilitating the drug use), several attempts were carried out to replace one of the chlorine ligands with pta (1,3,5-triaza-7-phosphatricyclo-[3.3.1.1]decane). First, [Os(η6-p-cymene)(pta)Cl
2] (25) was synthesized by coordination of one
pta ligand to 17 (see Figure 29): this reaction was carried out in methanol at reflux temperature for 4 hours.
Figure 29. Synthesis of [Os(η6-p-cymene)(pta)Cl2] (25).
The structure of 25, once isolated as orange solid, was confirmed through 1H NMR and 31P
NMR (in CD2Cl2); the 31P resonance in pta occurs at δ = 79.9 ppm (in CD3OD), while it resulted
shifted to 81.7 ppm in 25.
At this point, 25 was allowed to react with 7. This reaction was carried out in CH2Cl2 and was
carried on in two steps: the first one consists in the displacement of one chloride by AgBF4,
followed by in situ addition of 7 (see Experimental). This procedure led to complex 26 (see Figure 30). The same procedure was repeated with ligands 8 and 9, however these reactions showed to be scarcely selective and it was not possible to isolate any product.
Os Cl P N N N Cl 1) AgBF4 O Cl Cl O O O O N O Os Cl P N N N O Cl Cl O O O O N O 26 2) 7
Figure 30. Synthesis of [Os(nicotinic acid 2-ethacrynyloxy-ethyl ester)(η6-p-cymene)(pta)Cl][Cl] (26).
The isolation of 26 required a filtration through a celite pad, to eliminate silver salts; then the residue was washed several times with diethyl ether and precipitated with pentane in a dry ice-isopropanol bath. Complex 26 was isolated as a pale yellow solid in modest yield (28%), and then fully characterized by NMR, IR, ESI-MS and elemental analysis.
36 Figure 31 shows a comparison between the 31P-NMR spectra (in CDCl
2) of 10, 25 and 26. The
spectrum of 10 (blue line) displays a signal at 19.93 ppm, meanwhile the osmium complex “monopta” (25, green line) shows a peak at 83.04 ppm. The spectrum of 26 (red line) consists of a resonance at 77.40 ppm, coherently with the hypothesis depicted in Figure 30.
37
4. Conclusions
This work allow to obtain several new metal complexes based on Ruthenium and Osmium centers that incorporates ethacrynic acid fragment. The synthetic route followed allow to obtain ligands containing ethacrynic acid, starting from a phosphinic acid, pyridinic alcohols and pyridnic acids.
Coupling between pyridinic alcohols and ethacrynic acid were directly obtained, meanwhile to bond ethacrynic acid with carboxylic acids a general synthetic way was optimized.
Synthesized ligands were coordinated to different metal frames with recognized anticancer activity; ruthenium arene, simil NAMI and osmium arene. The presence of ethacrynic acid could be expected to increase the efficacy of these drugs through its peculiarity of inhibits enzymes involved in cells resistance mechanisms. New complexes were characterized through several analytical methods and the anticancer activity of the prepared Ru and Os complexes will be tested at the École Polytechnique Fédérale de Lausanne (EPFL).
38
5. Experimental part
5.1. General Information
Reactions involving moisture-sensitive compounds were carried out by using reaction vessels which were oven-dried at 140°C prior to use, evacuated (10–2 mmHg) and then filled with argon.
All the other operations were performed in air with common laboratory glassware. 2-propanol, methanol, acetonitrile, acetone and dimethyl sulfoxide (DMSO) were received from Sigma-Aldrich and distilled before use from appropriate drying agents. The reactants were purchased from commercial sources (Alfa Aesar or Sigma Aldrich) and used as received. Chromatography separations were carried out on columns of alumina (Fluka, Brockmann Activity I), celite (Fluka, 512 Medium) or silica (Carlo Erba, Ø = 0.05 - 0.20 mm). Infrared spectra were recorded on solid samples at 298 K, on a FT IR-Perkin Elmer spectrometer, equipped with a UATR sampling accessory. C, H, N analyses were carried out with a Perkin-Elmer 2400 CHNS/O analyzer. High-resolution electrospray mass spectra (HRMS) were recorded on samples dissolved in methanol or acetonitrile by using either a MicroTOF-Q hybrid quadrupole time-of-flight spectrometer (Bruker Daltonics, Bremen, Germany) or a Waters Micromass ZQ 4000 spectrometer. NMR spectra were recorded at 298 K on Bruker Avance 400 MHz or Bruker Avance 500 MHz spectrometer. Chemical shifts (expressed in parts per million) are referenced to residual solvent peaks. The X-ray diffractometry analyses were carried out on Bruker SMART 2000 diffractometer by Prof. Stefano Zacchini (University of Bologna, compound 12) and on a Bruker Smart APEX diffractometer by Dr. Enrique Oñate (University of Zaragoza, compound 18 and 22), respectively.
5.2. Spectroscopic characterization of Ethacrynic Acid (EA-COOH,
Alfa Aesar)
1H NMR (CDCl 3): δ = 9.56 (br, 1H, OH); 7.17 (d, 1H, 3JHH = 8.33 Hz, C10-H); 6.83 (d, 1H, 3JHH = 8.33 Hz, C11-H); 5.98 (d, 1H, 2J HH = 1.32 Hz, C4-H); 5.63 (d, 1H, 2JHH = 1.32 Hz, C4-H); 4.83 (s, 2H, C12-H); 2.48 (q, 2H, 3J HH = 7.45 Hz, C2-H); 1.16 (t, 3H, 3JHH = 7.45 Hz, C1-H) ppm.39
13C NMR (CDCl
3): δ = 196.0 (C5); 172.8 (C13); 155.1 (C9); 150.2 (C3); 134.2 (C7); 131.7 (C8);
129.0 (C4); 126.9 (C11); 123.5 (C6); 110.9 (C10); 65.7 (C12); 23.4 (C2); 12.4 (C1) ppm.
IR (solid state): ν = 2967w, 2881w, 2544w-br, 1723s (COOH), 1669m (C=O), 1660m (C=O), 1584s, 1472m, 1454w, 1423m, 1368w, 1277s, 1246vs, 1076vs, 1203m, 1122w, 1076vs, 1004m, 954m, 927m, 908s, 823w, 798s, 767m, 720m, 688m cm-1
.
5.3. Synthesis of organic ligands
5.3.1. 4-pyridinylmethanol
According to literature71, a solution of isonicotinaldehyde (4.4 mL, 0.047 mol) in THF (10 mL) was treated with a large excess of NaBH4 (8.051 g, 0.227 mol). The resulting dark-red solution
was allowed to stir at room temperature for 18 hours, then a 37% HCl solution (3.86 mL) was added dropwise. The final mixture was filtered through an alumina pad by using CH2Cl2 as
eluent. The filtrated solution was dried in vacuo. Chromatography of the dark-yellow oily residue on alumina afforded a yellow band which was separated by using Et2O as eluent. The volatile
materials were removed in vacuo, then the pale-yellow solid was dried over P2O5 and
subsequently characterized by 1H NMR spectroscopy.
5.3.2. Ethacrynic acid pyridin-4-ylmethyl ester (1)
To a solution of EA-COOH (1.00 g, 3.30 mmol) in CH2Cl2 (10 mL), protected from the light,
pyridinylmethanol (0.301 g, 2.76 mmol), EDCI (0.530 g, 2.77 mmol) and 4-dimethylaminopyridine (0.053 g, 0.43 mmol) were added in the order given. After stirring for 20
40 hours at room temperature, the mixture was filtered on a short silica pad by using acetone as eluent. Some silica was added to the filtered solution, then the volatile materials were removed under vacuum. The solid residue was charged on a silica column; the use of mixtures of hexane and Et2O (hexane/Et2O ratio progressively decreasing) as eluent allowed to isolate a colourless
fraction corresponding to 1.
The product was obtained as a yellow oily residue upon removal of the solvent (yield 0.803 g, 74%). 1H NMR (400 MHz, CDCl 3): δ = 8.54 (d, 2H, 3JHH = 4.3 Hz, C17-H); 7.46 (d, 2H, 3JHH = 4.6 Hz, -C16-H); 7.13, 6.83 (d, 2H, 3J HH = 8.3 Hz, C10-H + C11-H); 5.95, 5.57 (2m, 2H, C4-H); 5.34 (s, 2H, C12-H); 4.90 (s, 2H, C14-H); 2.45 (q, 2H, 3JHH = 7.2 Hz, C2-H); 1.16 (t, 3H, 3JHH = 6.4 Hz, C1-H) ppm. 13C NMR (400 MHz, CDCl 3): δ = 195.6 (C5); 167.1 (C13); 155.1 (C9); 150.1 (C3); 147.9 (C15); 147.6 (C17); 134.2, 131.6 (C8, C7); 128.9 (C4); 126.8, 110.9 (C11, C10); 123.4 (C6); 123.3 (C16); 66.1 (C12); 64.2 (C14); 23.4 (C2); 12.4 (C1) ppm. IR (solid state): ν= 2969w, 2935w, 2376m, 2353m, 2336w, 2315w, 2279w, 1762s, 1662s, 1633m, 1584s, 1508w, 1468m, 1436s, 1383m, 1293m, 1258m, 1185vs, 1168vs, 1122m, 1077vs, 1001m, 940w, 893w, 809s, 767m, 731w, 706w, 665w cm-1 .
5.3.3. Ethacrynic acid pyridin-3-yl ester (2)
The synthesis of 2 was carried out with a procedure analogous to that described for the 1, by allowing 3-hydroxypyridine (0.139 g, 1.46 mmol) to react with EA-COOH (0.531 g, 1.75 mmol), EDCI (0.282 g, 1.47 mmol) and 4-dimethylaminopyridine (0.030 g, 0.25 mmol) in CH2Cl2 (8 mL).
After 20 h stirring, the reaction solution was filtered on a silica pad by using acetone as eluent; the filtrated solution was added of some silica and the resulting mixture was dried under vacuum. The obtained solid was charged on a silica column; after washings with hexane/ether (variable relative amounts), the fraction corresponding to 2 was eluted with a mixture of ether


![Figure 6. Structure of [(η 6 -arene)Ru(azpy)I] [PF 6 ] (azpy = N,N-dimethylphenyl- or hydroxyphenyl-azopyridine)](https://thumb-eu.123doks.com/thumbv2/123dokorg/7942526.119935/12.893.278.674.341.622/figure-structure-arene-azpy-azpy-dimethylphenyl-hydroxyphenyl-azopyridine.webp)




