1
POLITECNICO DI MILANO
School of Industrial and Information Engineering
Master of Science in Biomedical Engineering
CHARACTERIZATION OF POLYSTYRENE
NANOPARTICLES AND QUANTITATIVE
ASSESSMENT OF THEIR
INTERACTIONS WITH HELA CELLS
Supervisor: Prof. Gabriele CANDIANI
Co-supervisor: Dr. Paolo BIGINI
Candidate:
Nora Chiara Battajni
Matr. 904898
Academic year: 2019/2020
2
Table of contents
Abstract……….4
Sommario………...8
1. Introduction………12
1.1 The relation between physico-chemical features of NPs and their biological interaction………...14
1.1.1 Geometry (size and shape).……….16
1.1.2 Surface (coating and functionalization).………..19
1.1.3 Material………....20
1.1.4 Z-potential………...21
1.2 Polymeric NPs in biology and medicine………25
1.2.1 List of the main polymers used to synthesize NPs………..27
1.2.2 Biodegradability and biocompatibility levels of the different polymers…….28
1.2.3 Nanopolymers and cell interaction………..29
1.2.4 Ps NPs, advantages and hazards………..35
1.3 NPs assessment………..36
1.3.1 Methods to characterize NPs and to visualize them in vitro and in vivo…….37
1.3.2 Methods to assess the safety of NPs in cells and in organs……….46
1.3.3 From the observation to the quantification………..49
Aim of this work……….54
2. Materials and methods………...55
2.1 NPs……….55 2.2 NPs characterization………...55 2.2.1 Fluorescence curve………..55 2.2.2 DLS and ELS………...58 2.2.3 AFM………...…….59 2.3 Cellular line………...…….59 2.4 Culture conditions………..…60 2.5 Growth curve………..…60 2.6 NPs tracking………...62 2.6.1 Time lapse………...63
3
2.6.2 Cytoskeleton stainings……….65
2.7 Fields analysis………....66
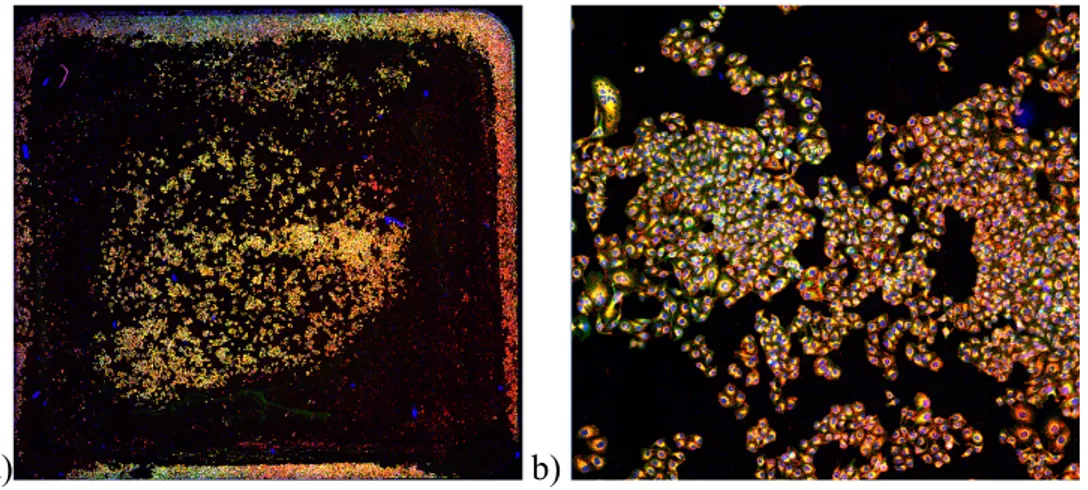
2.7.1 Overview acquisition and sampling………66
2.7.2 ImageJ analysis………68
2.8 Cell viability assays………....71
2.8.1 MTT………..…...71
2.8.2 LDH………...73
2.9 Flow cytometry………...73
2.9.1 Internalization studies………..73
2.9.2 Cell cycle and binucleation……….77
2.10 Statistical analysis………....80
3. Results and discussion………81
3.1 NPs characterization………...81 3.1.1 Fluorescence curve………..81 3.1.2 DLS and ELS………...83 3.1.3 AFM………...86 3.2 Growth curve………...87 3.3 NPs tracking………...88 3.3.1 Time lapse………...89 3.3.2 Cytoskeleton stainings……….97 3.4 Fields analysis………...101
3.4.1 Overview acquisition and sampling………..101
3.4.2 ImageJ analysis………..101
3.5 Cell viability assays………..105
3.5.1 MTT………...106
3.5.2 LDH………...108
3.6 Flow cytometry………...109
3.6.1 Internalization studies………109
3.6.2 Cell cycle and binucleation………...113
4. Conclusions and perspectives………..119
4
Abstract
Nanoparticles (NPs) are a fundamental nanomedicine tool that have been employed as drug-delivery vehicles, contrast agents and diagnostic tools. Thanks to their size, they are able to interact with cells and intracellular structures at a molecular or nanoscale level. Their effect is strongly dependent on their physico-chemical properties, especially size, shape, charge and surface functionalization.
This work focuses on the characterization of commercially available Ps latex NPs and on the assessment of their effect when they interact with a biological system, in particular with HeLa cells. The NPs were either positively charged (aminated) or negatively charged (carboxylated). Both unlabelled NPs and NPs conjugated with a red fluorophore were used. They all had a nominal diameter of 100 nm and spherical shape.
The NPs were characterized analysing their fluorescence curve, size and superficial charge. The fluorescence curve of red NPs, obtained measuring the emission curve of eight different NPs concentrations with a microplate reader, showed a good correlation between concentration and emission intensity, with an emission peak at 600 nm. The hydrodynamic diameter, measured with Dynamic Light Scattering (DLS), showed that all NPs were close to 100 nm, with slightly bigger differences for the positively charged ones. The polydispersity index (PDI) indicated good monodispersity for all batches. The Z-potential, measured with Electrophoretic Light Scattering (ELS), showed that negative NPs had a superficial charge between -20 and -25 mV. However, red positive NPs had a charge that was more than double the charge of unlabelled positive NPs (+31.4 and +14.3 mV, respectively). Atomic Force Microscopy (AFM) images also showed NPs sizes close to 100 nm and spherical shapes, with few aggregates. Analysing negative NPs with AFM proved difficult since the support for the analysis also had a negative charge.
The NPs effect on biological systems was evaluated on HeLa cells, an immortal tumoral epithelial human cell line, which is one of the most used cell lines in biomedical research. The growth curve of untreated cells was determined and their duplication time was calculated to be 20.42 h. Cells were then treated with NPs concentrations ranging from 2.5 to 25 µg/ml. Initially, only red NPs were used. Cells were observed using a confocal microscope and a SIM. Time lapse procedure was optimized, finding the best seeding concentration (10,000 cells/well using ibidi µ-slide 8-wells plates), the minimum quantity
5 of nuclear dye that guaranteed good visibility while minimizing adverse effects (2 µl/well of hoechst), and solving issues regarding temperature control and the maintenance of sterility within the microscope culture chamber. At early time points, large amounts of positive NPs were either already internalized or surrounding the cells, while there was almost no internalization of negative NPs. Internalization increased for both NPs types after 24h, when the almost totality of NPs was situated close to the nuclei, often grouped close together. Exclusively in the case of positive NPs, an unusually high number of binucleated cells was also observed. SIM observations showed additional information compared to confocal images and they allowed to better discriminate single NPs; using confocal microscopy only, they often appeared as blurred aggregates, while the higher SIM resolution gives a clearer picture of their actual position and showed they did not aggregate as much as it seemed. Projections along the xz and yz planes showed that while NPs tended to surround the nuclei, they did not enter them. Time lapse was also performed on a different line of HeLa cells, which expressed fluorescent Tubulin-eGFP and H2B-mCherry, a cytoskeleton and a histonic protein, to further investigate the presence of binucleated cells. At first, only one binucleated cell was present in the observed field, resulting from the fusion of two initially distinct cells. However, when repeating the experiment, many binucleated cells were observed. They developed as the result of an interrupted cytokinesis processes, where dividing cells had already formed two distinct nuclei and started cytokinesis but did not complete it. It appears that the main mechanism responsible for the formation of binucleated cells is an interference of the red positive NPs with the cell division.
In addition to time lapses, cytoskeleton stainings were also performed on fixed regular HeLa cells. H2B-mCherry, in fact, expresses fluorescence that overlaps with the NPs fluorescence, making it difficult to discriminate between NPs and nuclei. Both phalloidin and tubulin stainings were effective and allowed a good visualization of the structures of interest. Tubulin was preferred for its higher versatility in the choice of the fluorophore associated with the secondary antibody. Using SIM and making 3D reconstructions, it is possible to study the colocalization of NPs with other intracellular structures.
Quantitative parameters were extracted from fields acquired with the confocal microscope at a 40X magnification. Nine fields were selected for each treatment. Two types of samplings were tested: square, which selected nine adjacent fields in a grid, and random,
6 which selected nine non-adjacent fields within a certain distance from a starting point. The square one was preferred since random sampling fields that did not contain cells were more frequent and there were higher error bars. Images were then analysed using imageJ to quantify the cytoplasm area occupied by NPs and the intensity of internalized NPs at 4, 24 and 72h after incubation. The cytoskeleton staining was used to identify the region of interest and consider exclusively internalized NPs. Both positive and negative NPs were used at concentrations of 2.5, 5 and 25 µg/ml. The area occupied by negative NPs was still close to 0% after 4 and 24h, with slight increases after 72h. The area occupied by positive NPs was higher at all time points and it increased with time, with the exception of the highest concentration which decreased after 72h, possibly due to a toxic effect, since internalization was highest for this condition. Similar results were found regarding internalized NPs fluorescence.
To assess the NPs toxicity, MTT and LDH assays were performed using the same NPs concentrations. Positive NPs had a clear time and dose-dependent effect. After 72h, the highest concentration caused a strong mortality and a substantial LDH release, respectively. Results were less pronounced at lower concentrations. Negative NPs had results similar to control, only causing a slight increase in metabolic activity.
Flow cytometry was used to quantify NPs uptake and the presence of binucleated cells. After 24h cells that internalized NPs exceeded 95% for both positive and negative NPs, proving a high internalization efficacy. No significant differences were found between samples of fixated and non-fixated cells. Propidium iodide and ToPro-3 were both effective for nuclear staining, but ToPro-3 was selected for its lower overlapping with NPs fluorescence. The intensity of nuclear fluorescence was used to identify the cell cycle phase. After 24h, negative NPs induced no significant differences from control, while positive NPs caused a reduction of cells in G0/G1 and an increase of cells in S or G2 phases. Cells in G2 went from 13% to 31%, indicating an increase of binucleated cells of about 18%. To avoid any interference between NPs and nuclear dye fluorescence, the experiment was repeated using unlabelled Ps latex NPs, but no difference was found between treatments and control. Using a cell counter to compare unlabelled and red NPs toxicity, it was discovered that the unlabelled NPs dose had to be increased fivefold to observe the same toxicity as with red NPs. This difference in the response is likely due to the higher positive Z-potential of red NPs, which is capable of causing damage to the cell
7 membrane and which facilitates the NPs uptake within cells. A greater uptake of equally toxic NPs would in fact lead to a more pronounced toxicity. The manufacturer did not indicate, on the NPs datasheet, the final surface charge at the end of the synthesis. It is clear, however, that even when using NPs of the same material and size, manufactured in the same way, it is not possible to assume a constant Z-potential across different NPs types. It is fundamental to proceed to characterize each new NP type, in order to find out the specific properties of each one.
8
Sommario
Le Nanoparticelle (NPs) sono uno strumento fondamentale della nanomedicina e sono state impiegate come carrier per la somministrazione di farmaci, agenti di contrasto e strumenti diagnostici. Grazie alle loro dimensioni sono in grado di interagire con cellule e strutture intracellulari a livello molecolare o nanometrico. Il loro effetto è strettamente correlato alle loro proprietà chimco-fisiche, in particolare dimensione, morfologia, carica e funzionalizzazione superficiale.
Questo lavoro si focalizza sulla caratterizzazione di NPs commerciali in polistirene (Ps) latex e sulla valutazione del loro effetto quando interagiscono con sistemi biologici, in particolare con cellule HeLa. Le NPs avevano una carica positiva (amminate) o negativa (carbossilate). Sono state usate sia NPs non marcate sia NPs coniugate con un fluoroforo rosso. Avevano tutte un diametro nominale di 100 nm e forma sferica.
Le NPs sono state caratterizzate analizzando la loro curva di fluorescenza, la loro dimensione e la loro carica superficiale. La curva di fluorescenza delle NPs rosse, ottenuta misurando la curva di emissione di otto diverse concentrazioni di NPs con un lettore di micropiastre, mostrava una buona correlazione tra la concentrazione e l’intensità di emissione, con picco di emissione a 600 nm. Il diametro idrodinamico, misurato con dynamic light scattering (DLS), mostra che tutte le NPs erano vicine ai 100 nm, con differenze leggermente più accentuate per quelle cariche positivamente. L’indice di polidispersità (PDI) indica una buona monodispersità per tutti i campioni. Lo Z-potential, misurato con Electrophoretic Light Scattering (ELS), mostra che le NPs negative hanno una carica superficiale compresa tra -20 e -25 mV. Invece le NPs rosse hanno una carica superficiale più che doppia rispetto a quella delle NPs non marcate (+31,4 e +14,3 mV, rispettivamente). Anche le immagini acquisite con microscopia a forza atomica (AFM) mostrano che le NPs hanno dimensioni vicine ai 100 nm e forma sferica, con pochi aggregati. L’analisi delle NPs negative è stata resa difficile dal fatto che anche il supporto per l’analisi aveva carica negativa.
L’effetto delle NPs sui sistemi biologici è stato valutato su cellule HeLa, una linea di cellule immortali di tumore epiteliale umano che è tra le linee più usate nella ricerca in campo biomedico. È stata studiata la curva di crescita delle cellule non trattate e il loro tempo di duplicazione è stato calcolato essere di 20,42 ore. Le cellule sono state poi trattate
9 con concentrazioni di NPs tra i 2,5 e i 25 µg/ml. Inizialmente sono state usate solo NPs rosse. Le cellule sono state osservate con microscopio confocale e SIM. La procedura per il time lapse è stata ottimizzata, trovando la concentrazione ottimale di semina (10’000 cellule/pozzetto utilizzando piastre ibidi µ-slide 8-wells), la quantità minima di marcatore nucleare che garantisca una buona visibilità minimizzando gli effetti negativi (2 µl/well of hoechst) e risolvendo problemi connessi al controllo della temperatura e al mantenimento della sterilità all’interno della camera di coltura del microscopio. Nei primi time points, grandi quantità di NPs positive erano già state internalizzate o si trovavano disposte lungo la membrana cellulare, mentre l’internalizzazione di NPs negative era quasi nulla. Dopo 24h l’internalizzazione è aumentata per entrambi i tipi di NPs, con la quasi totalità delle NPs situate vicino ai nuclei, spesso raggruppate tra loro. Solamente per le NPs positive è stato osservato un numero insolitamente alto di cellule binucleate. Le osservazioni al SIM mostrano informazioni aggiuntive rispetto alle immagini al confocale e permettono di discriminare meglio le singole NPs; utilizzando solo il microscopio confocale, apparivano spesso come aggregati sfocati, mentre la più alta risoluzione del SIM fornisce un’immagine più chiara della loro posizione reale e mostra una minore aggregazione rispetto all’impressione iniziale. Le proiezioni lungo i piani xz e yz mostrano che anche se le NPs tendono a circondare i nuclei, non entrano al loro interno. Il time lapse è stato effettuato anche su una diversa linea di cellule HeLa, in grado di esprimere due proteine fluorescenti, una del citoscheletro (Tubulin-eGFP) e una istonica (H2B-mCherry), per investigare ulteriormente la presenza di cellule binucleate. Inizialmente, nel campo osservato era presente un’unica cellula binucleata, che era stata prodotta dalla fusione di due cellule precedentemente distinte. Tuttavia, ripetendo l’esperimento, sono state osservate molte cellule binucleate, che in questo caso si sono formate come conseguenza di un’interruzione della citodieresi. Le cellule in divisione avevano già formato due nuclei distinti e iniziato la citodieresi, ma non l’hanno portata a termine. Il meccanismo maggiormente responsabile per la formazione di cellule binucleate sembra dunque essere un’interferenza causata dalle NPs rosse positive nella divisione cellulare.
Oltre ai time lapse, sono stati fatti staining del citoscheletro su cellule HeLa non fluorescenti. La fluorescenza emessa dalla H2B-mCherry, infatti, si sovrappone con quella delle NPs, rendendo difficile la distinzione di NPs e nuclei. Sia lo staining con falloidina sia quello della tubulina sono risultati efficaci e permettono una buona visualizzazione
10 delle strutture d’interesse. La tubulina è stata preferita per la sua maggiore versatilità nella scelta del fluoroforo associato all’anticorpo secondario. Utilizzando il SIM per fare ricostruzioni 3D, è possibile studiare la colocalizzazione delle NPs con altre strutture intracellulari.
Dalle immagini al confocale con ingrandimento 40X sono stati estratti parametri quantitativi. Per ogni condizione sono stati selezionati nove campi. Sono stati testati due metodi per la selezione dei campi: campionamento quadrato, in cui venivano selezionati nove campi adiacenti e disposti in una griglia, e campionamento random, in cui venivano selezionati nove campi non adiacenti che si trovavano entro una certa distanza da un punto selezionato. Il campionamento quadrato è risultato preferibile dato che con quello random c’era un maggior numero di campi che non contenevano cellule e le barre d’errore risultavano più elevate. Le immagini sono state analizzate con ImageJ per quantificare l’area occupata dalle NPs e l’intensità di fluorescenza delle NPs dopo un’incubazione di 4, 24 e 72h. Lo staining del citoscheletro è stato utilizzato per identificare una regione d’interesse e considerare solo le NPs internalizzate. Sia le NPs negative sia quelle positive sono state usate a concentrazioni di 2,5, 5 e 25 µg/ml. L’area occupata dalle NPs negative era ancora vicina allo 0% dopo 4 e 24h, con un lieve incremento dopo 72h. L’area occupata dalle NPs positive era più elevata per tutti i time point ed è aumentata nel tempo, con l’eccezione della concentrazione più alta che diminuisce dopo 72 h, forse a causa di una tossicità delle NPs, poiché l’internalizzazione era massima in questa condizione. La fluorescenza delle NPs internalizzate ha dato risultati simili.
Per valutare la tossicità delle NPs sono stati eseguiti i saggi MTT e LDH per le precedenti concentrazioni di NPs. Le NPs positive avevano un chiaro effetto tempo e dose-dipendente. Dopo 72h la concentrazione più alta aveva causato un’elevata mortalità e un sostanzioso rilascio di LDH, rispettivamente. I risultati erano meno pronunciati per le concentrazioni più basse. Le NPs negative avevano risultati simili ai controlli, causando solamente un lieve incremento nell’attività metabolica.
La citometria a flusso è stata usata per quantificare l’internalizzazione di NPs e la presenza di cellule bionucleate. Dopo 24h le cellule che avevano internalizzato NPs superavano il 95% sia per le NPs negative sia per quelle positive, dimostrando un’elevata efficacia di internalizzazione. Non sono state trovate differenze significative tra i campioni di cellule fissate e non. Sia lo ioduro di propidio sia il ToPro-3 si sono rilevati efficaci per lo staining
11 dei nuclei, ma il ToPro-3 è stato preferito per la minore interferenza con la fluorescenza delle NPs. L’intensità di fluorescenza dei nuclei è stata usata per identificare la fase del ciclo cellulare in cui si trovava ciascuna cellula. Dopo 24h, le NPs negative non avevano indotto differenze significative rispetto al controllo, mentre le NPs positive avevano causato una riduzione delle cellule in fase G0/G1 e un aumento di cellule in fase S o G2. Le cellule in G2 erano passate dal 13% al 31%, implicando un aumento delle cellule binucleate del 18%. Per evitare qualsiasi interferenza tra la fluorescenza delle NPs e quella dello staining dei nuclei, l’esperimento è stato ripetuto utilizzando NPs non marcate, ma non è stata rilevata nessuna differenza tra il controllo e i trattamenti. Utilizzando un contacellule per confrontare la tossicità delle NPs rosse e di quelle non marcate, è stato scoperto che la dose di NPs non marcate doveva essere aumentata di cinque volte per osservare livelli di tossicità pari a quelli delle NPs rosse. Questa differenza è probabilmente dovuta alla maggiore carica positiva delle NPs rosse, che è in grado di causare danni alla membrana cellulare e che favorisce l’uptake delle NPs da parte delle cellule. Un maggiore uptake di NPs con lo stesso livello di tossicità, infatti, causerebbe una tossicità più marcata. Il produttore non aveva indicato, sulla scheda tecnica delle NPs, la carica superficiale al termine del processo di sintesi. Tuttavia, è chiaro che anche utilizzando NPs fatte con lo stesso materiale e dimensioni, prodotte nello stesso modo, non è possibile presumere uno Z-potential costante per i diversi tipi di NPs. È quindi fondamentale caratterizzare ogni nuovo tipo di NPs, in modo tale da determinare le specifiche proprietà di ciascuna.
12
1. Introduction
In recent years nanotechnology has seen an increasing development. Its applications are extremely broad, covering disciplines spanning from electronics to medicine.1
Nanomedicine is the application of nanometric sized bodies, called Nanoparticles (NPs), for therapeutics and/or diagnostics.1,2 NPs include particles from 1 to 100 nm2,3 (see Figure 1.1). However, the size range is becoming somewhat more flexible every year, also coming to include larger NPs. Common choices for NPs materials include metallic, organic, inorganic and polymeric nanostructures, including dendrimers, micelles, and liposomes.2
Figure 1.1: NPs size in relation to other objects, from molecular to macroscopic sizes. NPs are in the 1-100 µm size range. The scale is expressed in metres. Adapted from (McCarroll et al.).11
There is a restricted number of NPs approved by the FDA for use in human patients, and many more are currently undergoing clinical trials.4 Most of the approved ones are used either as imaging agents, for instance in magnetic resonance imaging (MRI), or in cancer therapy.4,5
The importance of NPs is related to their unique properties, which can be significantly different from the bulk material. In fact, due to their submicrometric size and high surface area to volume ratio, there can be strong changes in structural, biochemical, magnetic, mechanical, optical, and electronic properties.2,5
13 For nanomedical purposes drugs or contrast agents can be associated with NPs by encapsulation inside the NPs structure, by covalent linking or by adhesion to its surface.2 NPs can play a role as carriers, improving the targeted drug delivery and consequently its efficacy and safety, compared to the direct administration of the isolated drug.1,4 Unfortunately the tuning of NPs for biomedical purposes is not so trivial. Some of the major drawbacks related to the use of NPs are the following: difficult scaling up; difficult handling and storage; low drug loading capacity and efficacy; adverse cost effectiveness in their development compared to traditional drugs; not fully understood safety issues.1,6
NPs can indeed overcome some hurdles that often occur when free drugs are administered. These include: 1) limited effectiveness; 2) poor biodistribution; 3) lack of selectivity and potentially toxic accumulation in healthy tissues; 4) drug level fluctuation in plasma and; 5) temporally limited rather than sustained effect.6,7
Ideally, NPs should be able to selectively reach and accumulate in the diseased tissue. This would bring relevant advantages. The reduced accumulation in non-target cells and organs would result in a consistent reduction of systemic toxicity, while the increased percentage of the drug to successfully reach its target site would both increase the efficacy of the treatment and reduce the overall drug dose required.6 NPs may also increase the system half-life, escaping clearance for a longer time and facilitating an extended circulating time, providing a sustained therapeutic effect and a longer lasting bloodstream half-life.2,7
NPs can also improve solubility of poorly water-soluble molecules. In the case of encapsulated drugs, NPs can constitute a barrier protecting their content from degradation within the biological environment.
A specific class of NPs are the stimuli-responsive ones. These NPs are able to modify their properties, and in particular release their cargo, in response to a specific change, which can be a naturally occurring modification of the environment inside the patient’s body (i.e. pH, temperature, redox state, molarity, Ca2+ content and many others), or an externally applied stimulus (i.e. magnetic fields, ultrasounds, light, temperature).8
Another potential advantage of NPs resides in their ability to penetrate intact cells and have a therapeutic effect.9 NPs can help in this respect, facilitating cellular uptake and subsequently releasing the drug directly inside the cell. The ability to penetrate in cells exploiting mechanisms like those adopted by viruses is extremely important because it can
14 allow to generate a new type of pharmacokinetic called “intracellular pharmacokinetic”. In this context, investigations on mechanisms related to intracellular NPs internalization are crucial for future development of targeted nanomedicine. This approach could be also important to replace other techniques aimed at loading drugs inside the cells, such as electroporation, microinjection, or viral vectors. It is already known that, despite their great advantages, such techniques can induce potential cell damage. This is why, if well selected and assessed in terms of biocompatibility and biodegradation, NPs could be a promising candidate for intracellular delivery.
The safety assessment is a crucial issue because the NP properties play a crucial role in their interaction with the biological environment, influencing protein adsorption, cellular uptake, biodistribution patterns, clearance mechanisms and toxicity.5
Both physical and chemical features of NPs should be considered during their development, depending on the intended effect. It is fundamental to correctly choose and carefully characterize size, shape, surface charge, composition, surface coating and functionalization.1
Starting from the passage into biological fluids, a deep modification of NPs can occur. In particular, the NPs interaction with a high number of biomolecules, which have the possibility of attaching to the NPs surface, may lead to a strong modification of surface identity by a process universally known as protein corona formation.10
1.1 The relation between physico-chemical features of NPs and their biological interaction
It is clear from a huge number of studies that investigated bio-nano interactions that the behaviour of NPs depends on their physico-chemical properties.
It has been demonstrated that shape, size, charge and surface chemistry and functionalization can significantly alter the NPs fate.11 These properties are summarized in Figure 1.2.
15
Figure 1.2: Physicochemical factors that affect cellular uptake of NPs. a) Surface charge, b) shape, c) size and d) surface chemistry.12
Unfortunately a large number of these studies, although reliable and well explained, often lack consistency because they do not follow a common paradigm of work. Such studies are unfortunately made even more difficult by the fact that no general rule that dictates interactions seems to exist. In fact, changing for example cell type, the effect of one parameter can lead to different results.
The need of homogenous and universally accepted guidelines to evaluate the effect of each single parameter is crucial to better elucidate the ratio between the positive effect and the drawbacks in using a specific type of NP in different biological systems. When the NPs are then exposed to the biological model (cells, tissues, animals, patients), the measured biological outcomes can be univocally traced back to the parameter under investigation.13 The understanding of how the modification of NPs properties could lead to substantially different biological outcomes is fundamental for the identification and establishment of NP design rules.13
Once the effect of each parameter has been outlined, the best choices can be implemented depending on the intended target. Efficient delivery of NPs can make a significant difference in the overall success of a nanomedicine strategy.13
At the same time, NPs showing adverse effects in a biological target can be made safer simply modifying a single property.13
16 1.1.1 Geometry (size and shape)
Both size and shape of NPs can influence their ability to be internalized (uptake process) and the kinetic or the amount of this internalization process. Dimensions play an important role in circulation and biodistribution.14 They also play an indirect role in NPs toxicity.15 When working with NPs it is fundamental to characterize these properties and their uniformity among NPs belonging to the same batch and to different batches.
Size plays a crucial role in how the cellular uptake occurs and in intracellular trafficking afterwards. Cells possess different methods of internalization, and it has been demonstrated that NPs can enter cells through more than one of them. Which one they will use heavily depends on NPs size.
For instance, in a study involving carboxylated Polystyrene (Ps) NPs, with diameters of 40 nm and 150 nm, it was shown that each NP size clearly has a preferential uptake mechanism. The 40 nm PS NPs were mainly internalized through clathrin-mediated endocytosis pathway, whereas the uptake of 150 nm PS NPs relied mainly on the caveolae-mediated endocytosis.16 Another interesting result from this experiment is the fact that 150 nm NPs have a significantly higher tendency to colocalize with exosomes; this, in turn, will influence how the NPs can interact with intracellular structures and absolve their function.
Size might affect the ability of NPs to reach certain specific destinations within the body. Small sized NPs, in fact, could be retained less by filter organs thanks to their ability to escape them passing through endothelial cells.11
In most applications NPs have a spherical shape; however, many other shapes have been used too. NPs also exist in the shape of rods, wires, planes, stars, cages, multipods, and others.15
In order to tune NPs shape and morphology, it is important to choose accurately the production method and parameters. Some of the aspects that should be considered are temperature, pressure, time and pH. Knowing how they can change the characteristics of the final product can help achieving specific properties in the product.17
17 Many investigations report that NPs synthesized using the same material but with different shapes underwent a different process of internalization, with different kinetics, and even through different endocytic pathways.9
Another size related effect is the way both uptake kinetics and saturation concentrations vary according to NPs dimensions. In a study conducted with 14 nm, 50 nm and 74 nm NPs on HeLa cells, the 50 nm ones were the ones undergoing greatest uptake, suggesting the existence of an optimal size for internalization.18 However, this parameter might vary
depending not only on the type of NP, but also on the cell line, thus requiring specific new test each time a new combination is investigated.9 In the same study a shape related effect was noticed as well, with spherical NPs being internalized 5 times more than rod shape ones. This was hypothesized to depend on the fact that rods might require a longer membrane wrapping time.18
More generally, it seems that, among NPs with similar diameters and charge, the aspect ratio is a relevant feature in uptake dynamics, even more so than size. The aspect ratio is the proportion between width and height, and the most consistent uptake takes place for NPs with aspect ratio of 1 or 2.19
A study by Barua et al. compares the internalization efficacy of nanospheres, nanorods and nanodisks. The nanospheres had a diameter of 200 nm and 1 µm, while nanorods and nanodisks were obtained stretching them. NPs were used both uncoated and coated with a targeting antibody, trastuzumab. Indeed, in the case of uncoated NPs nanospheres were the ones that exhibited the highest levels of uptake, as can be seen in Figure 1.3. However, among coated NPs, the opposite effect took place, with nanorods and nanodisks being internalized more than the spheres. This could be explained as a combined effect of shape and target specificity. While spheres are the most internalized shape in nonspecific cases, in the case of coated NPs the other shapes could be responsible for an enhanced binding to the cell surface receptors.20
18
Figure 1.3: a) Scanning electron micrographs of the different NPs: microspheres, rods, and disks (scale on the left: 2 μm; scale on the right: 500 nm). b) Confocal micrographs of NP uptake in BT-474 cells. NPs on the left are uncoated, on the right they are coated with trastuzumab.20
Size affects drug release profiles as well. Smaller NPs tend to have a faster drug release, due to their larger surface area and higher number of exposed drug molecules on that surface.14
The NP size has another relevant role, in the mechanisms of NPs clearance from the body. NPs should remain in the body long enough to guarantee a prolonged drug release profile. However, it was observed that smaller NPs (<10 nm) are subjected to tissue extravasations and renal clearance whereas larger are quickly opsonized and removed from the bloodstream via the macrophages of the reticuloendothelial system.6,14 Although it has been reported that the optimal size to obtain prolonged circulation time appears to be within the range of 10-250 nm this interval of dimension is too extended and many difference can be seen comparing NP falling in this size range.14
A factor that needs to be considered when studying size related effects is the lack of colloidal stability that increases the propensity of many NPs to aggregate. This
19 phenomenon would alter both size and shape seen by the cells and change their behaviour. It is then fundamental to characterize NPs to verify the absence of aggregates, which almost always reduce the peculiar ability of NP to be internalized by cells.18
1.1.2 Surface (coating and functionalization)
Surface coating and functionalization have a primary role in the NPs fate and their interactions with macromolecules and with biological matrices. The NPs can be functionalized in many ways, to obtain a wide variety of results.
Functionalization is often used as a mean to achieve active selective targeting. It can give the following advantages: 1) make NPs stealthier and less susceptible to attack by circulatory immune system; 2) avoid the uptake from filter organs; 3) increase a receptor dependent interaction; 4) protect from degradation and aggregation in biological fluids; 5) improve the passage through biological barriers; 6) make NPs traceable; and many other functions. While passive targeting relies on the tumour property called enhanced permeability and retention (EPR), caused by the leaky vasculature of tumours, in the case of active targeting the site of interest is reached thanks to specific surface functionalization.6 For instance, it is possible to conjugate targeting ligands specific to a receptor overexpressed by tumour cells to the NPs surface. This has the double positive effect of enhancing the quantity of drugs that reach the target diseased cells and of reducing the unwanted uptake by healthy cells, where the drugs may cause adverse effects. Cell-penetrating peptides (CPPs) are also a rather common functionalization that improves cellular uptake. Their internalization mechanism relies on the addition of positively charged amino acids on the NPs surface, which interact with the negatively charged cell membrane and subsequently facilitate internalization. However, this process is not selective for a specific target, like for instance cancerous cells, so NPs may be taken up even by healthy cells.19
Other common surface modifications include the addition of natural biological molecules like oligonucleotides, peptides and glycoproteins, to improve cytocompatibility and cellular uptake.9
20 In cases when minimized NPs interactions with cells and biological components are the objective, a poly(ethylene glycol) (PEG) coating is one of the most used strategies. This neutral ligand, in fact, can mask the core NPs and is well known to somehow mitigate the protein adsorption and reduce non-specific uptake by macrophage cells.18
The non-specific adsorption of proteins on the NPs surface, called opsonization, makes NPs more easily detectable by the phagocytic cells. This could result in rapid clearance of the NPs either by phagocytosis by the mononuclear phagocyte system (MPS) in the liver or by spleen filtration.14 Not only does opsonization reduce the NPs circulation time; their
ability to reach their target is further compromised by the fact that the adsorbed proteins mask the targeting strategy on the NPs.
There are surface functionalizations that can help partially reduce this phenomenon. While PEGylation is by far the most widely employed technique to mask NPs, other strategies have also been successfully employed to address this problem. In some cases other materials with shielding properties similar to those of PEG, like for example poloxamer, polyvinyl alcohol, poly(amino acid)s and polysaccharides were used. Other approaches involve the use of CD47 peptides. Attached to the NPs surface, they are recognised as self by the phagocytic system and the drug circulation time substantially increased, as well as the accumulation in tumours. Yet another strategy uses cell membranes extracted from autologous leukocytes and red blood cells to cover the NPs. This biomimetic coating significantly prolongs circulation time as well.21
In addition to the type of surface functionalization, the way the chemical groups are arranged on the surface can also play a significant role in bio-NPs interactions.18
1.1.3 Material
Liposomes, solid lipids NPs, dendrimers, polymers, silicon or carbon materials, and magnetic NPs have all been used as drug delivery systems.6
The composition of the NPs has a great influence on how they can interact with tissues and cells and on their potential toxicity.22
21 Depending on their material, fabrication process and synthesis parameters, NPs can present varying degrees of rigidity or deformability. This parameter can change their in vivo fate and biodistribution. Rigid NPs can easily be cleared by filtering organs, such as liver and spleen, when their dimension exceeds the cut-off size limits of the fenestrated endothelia present in these organs. On the other hand, soft NPs that are more prone to deformation have shown prolonged circulation time and reduced accumulation in the spleen. In some cases, highly deformable NPs accumulated in the spleen at early time points, but their presence in the organ reduced over time, while their concentration in the blood stream increased, due to their ability to migrate through the fenestrated endothelia. Deformability is also an useful property in those situations where NPs need to pass through small capillaries.21
1.1.4 Z-potential
Superficial charge has a strong impact on how NPs are internalized by cells.
Both positively and negatively charged NPs are internalized, although to a much lesser degree in the case of negative NPs.
Positive NPs remain the ones with the greatest penetration efficacy, thanks to their ability to bind the negatively charged groups on cell membranes.9,18 There is a strong correlation between the amount of positive charge and the occurring internalization.18
However, positively charged NPs are also the ones that induce the strongest immune response.14
To minimize phagocytosis and reduce non-specific interactions, NPs with charge between -10 and +10 mv could be the most suitable.14
It was observed that, among synthetic nanomaterials, cationic NPs are capable of crossing cell membranes and be internalized much more efficiently than other NPs types.18
Superficial charge can also lead to different outcomes depending of NPs size. In a study where small positively charged NPs (2 nm) were used, the interactions with the cell
22 membrane was observed to induce a perturbation of the membrane potential, which causes Ca2+ influx into cells and the inhibition of cell proliferation.13
Currently, positively charged NPs are used for targeted chemotherapeutic drugs and gene carriers due to their high transmembrane efficiency.9
Negative NPs have a lower ability to bind to the negatively charged cell membrane.
However, internalization still occurs, possibly due to the interactions of the negative NPs with cationic lipid domains in the cell membrane.19
In some cases the desired outcome is to minimize the non-specific NPs interactions with proteins, other macromolecules and cell membranes. It appears that a neutral surface charge is the most suitable for such applications. Neutral surfaces can be obtained, for instance, by ligand functionalization, most noticeably using PEG, but also using zwitterionic ligands that provide an overall neutral charge.18
Figure 1.4 shows an example of how the variation of a single parameter, in this case size, shape and charge, respectively, can strongly vary NPs distribution among different organs, such as lungs, liver, spleen and kidneys. As for the effect of charge, positively charged NPs are more prone to sequestration by macrophages in the lungs, liver and spleen, while neutral and slightly negatively charged NPs have longer circulation lifetimes.21
23
Figure 1.4: Biodistribution of NPs with different parameters in lungs, liver, spleen and kidneys. a) Spherical NPs ranging from >2000 nm to <5 nm, divided in three size categories. b) Nanospheres, nanorods and nanodiscs, with size between 20 and 150 nm. c) Spherical NPs with negative, neutral and positive surface charge.21
The surface charge has an important effect on the formation and composition of the protein corona as well. It can modify the affinity of specific proteins to the NPs, and the proteins composing the corona will in turn influence NPs intracellular fate.
However, since the protein corona can mask the NPs superficial charge, it is possible that all those effects described as the result of NPs charge are induced by the corona composition.13
In a study conducted with six different types of Ps NPs, the effect of size and superficial charge in the formation and final composition of the protein corona has been investigated by Lundqvist et al. The NPs presented two sizes (50 nm and 100 nm) and three different surface chemistries (no modifications, carboxyl-modified and amino-modified). These surface modifications resulted in different values of z-potential: all plain and carboxyl-modified NPs had similar charges, between -40 and -42 mV, but amino-carboxyl-modified NPs acquired a z-potential of about -32 mV in the case of the 50 nm ones and +23 mV the case
24 of the 100 nm ones. It was seen that while most proteins were present across all NPs types, others were unique for each NP, proving a correlation between NPs physico-chemical properties and their interaction with the biomolecules present within the biological fluids.10 Among the proteins that differed among these three Ps NPs, many had specific key roles in various biological processes. The study also suggests that bulk material properties of the NPs are significantly less relevant than their superficial ones in terms of biological interactions. As the protein corona covers up the NPs surface, what the cells see and interact with is not the NPs material but rather the absorbed protein layer. It is then obvious that choosing carefully the NPs properties to select the protein corona that will form is fundamental for the cellular uptake of NPs and for their function, since even small properties changes could lead to significant alterations in cell-NP interactions. Further studies are still necessary to better understand the principles governing the formation of the corona.10
After internalization, NPs are often situated in endosomes, due to the uptake mechanism itself. If they remained inside them, NPs may be degraded upon the fusion of endosomes with lysosomes. For this reason, NPs ability to escape endosome vesicles is considered a desired feature. It seems that NPs superficial charge may play a relevant role in this phenomenon, with positively charged NPs being able to escape endosomes much better than other NPs. It was also observed that negatively charged Ps NPs were unable to reach the cytosolic compartment and remained confined inside endocytic vesicles.22
The surface potential also has a strong impact on other NPs properties. It can affect both the physical stability and the redispersibility (the ability to go back to an homogeneous, dispersed solution after sedimentation) of a polymer dispersion, as well as its storage stability and its propensity for aggregation.14
As for NPs charge effect on cell membranes, it seems that the binding of negatively charged NPs to a lipid bilayer causes local gelation, whereas binding of positively charged NPs induces fluidity.13
25
1.2 Polymeric NPs in biology and medicine
In the last few decades, polymeric NPs have been developed as promising tools for transmembrane delivery of low molecular weight drugs, oligonucleotides, peptides and proteins.9 They have been used in various applications: sensing, imaging and therapeutics.15
Some examples of polymeric NPs applications include their use in MRI for in vivo diagnosis. An asymmetrical cancer-targeting polymer vesicle based on R-poly(L-glutamic acid)- block-poly(ε-caprolactone), with R being either folic acid or diethylenetriami- nepentacetatic acid (DTPA) was reported as a T1 MRI contrast agent with enhanced
sensitivity, and it also served as a delivery vehicle for cancer chemotherapy and high anticancer drug loading efficiency.23,24 In another study, polymeric micelles with tertiary amines having different pKa values disassemble when they encounter a pH below their pKa values, which results in the activation of a MRI/nuclear magnetic resonance spectroscopy (NMR) signal.23,25
In fluorescence imaging a class of activatable self-assembled NPs based on the use of copolymer materials with ionizable tertiary amine groups and covalently conjugated fluorescence dyes was developed. They undergo a rapid transition when they encounter a very specific and narrow pH range, which results in the complete dissociation of the nanomicelles. Therefore, the covalently linked dyes change from a self-quenched “off” state to a highly emissive bright “on” state. An improved detection sensitivity and brighter signal is achieved since each NP contains multiple dye molecules.23,26,27
Polymeric micelles can be an advantageous solution for in vivo drug delivery. Micelles are formed by amphiphilic block copolymers, assembled to form core-shell structures with a hollow hydrophobic core that can be loaded with drugs and with a hydrophilic exterior. Encapsulation of anticancer drugs within micelles can reduce toxicity and improve circulation. For example, nephrotoxicity was significantly reduced by loading cisplatin into polymeric micelles.23,28
Polymeric NPs have been employed to obtain polymer-drug conjugates.29 Nanocarriers constituted by backbone degradable N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-drug conjugates demonstrated high efficacy in treating solid tumours. These
26 conjugates contain a degradable oligopeptide sequence in the backbone and drug in the backbone and at the termini. Thanks to their structure, the nanocarriers have a high drug load and a longer circulation time, which is extremely advantageous in increasing their accumulation into solid tumours. Their degradation products can easily be disposed of by the organism.23,30
Dendrimers are another form of polymeric NPs, characterized by a highly organized, branched structure, which makes them extremely suitable for drug delivery. Drugs can either be encapsulated or covalently bounded to the structure.14 A dendrimeric formulation
developed for anticancer therapy, called DTX- SPL8783, showed prolonged presence of the drug both in circulation and within tumours.29
Polymers are especially promising thanks to their good biocompatibility and their degradability. They also present a considerable flexibility in the tuning of their parameters. Composition, size, biodegradability, morphology, and surface functionality can be tailored according to the therapeutic indication.15
In most applications PNPs are either nanocapsules or nanospheres. While nanocapsules are composed by a polymeric shell with an empty space inside, where drugs can be stored, nanospheres have a full polymeric core structure and molecules can be attached to the external surface.17
There is the possibility to achieve the desired drug release profile either by selecting an adequate degradation kinetic or by using stimuli-responsive NPs.
Biodegradable PNPs-drug conjugates are stable in blood, non-toxic, non-thrombogenic, non-immunogenic as well as non-proinflammatory.6
Other attractive PNPs characteristics are their ease of synthesis, their versatility depending on synthesis specifics, and the fact that the surface can be easily modified and functionalized.14
27
Figure 1.5: Key features of polymeric drug delivery systems.31
1.2.1 List of the main polymers used to synthesize NPs
Polymeric NPs can be made with two kinds of polymers: natural or synthetic. Typical synthetic materials for PNPs are, among others, poly-e-caprolactone, polyacrylamide and polyacrylate. Natural polymers include albumin, DNA, chitosan, gelatine.6 They can be biodegradable, like polyesters, poly(amino esters), polyanhydrides, polyamides, chitosan, poly(L-lactide) (PLA) and polyglycolide (PGA), or non-biodegradable, like polyurethane. Biodegradable polymers are especially interesting in nanomedicine because they can
28 gradually release drugs during degradation and, if their degradation products can be disposed of by the cells, accumulation and the related toxicity can be reduced.
Furthermore, several hydrophilic polymers, such as polyethylene glycol (PEG), chitosan, and dextran, are widely used as coating agents on other NPs to enhance their aqueous dispersibility, bioavailability, and targeting efficacy.15
1.2.2 Biodegradability and biocompatibility levels of the different polymers
Natural polymer-based NPs are usually biocompatible and non-toxic, although they may suffer from stability problems when crossing biological membranes.14
Natural polymers like chitosan, alginate, gelatine and albumin are often preferred to synthetic polymers thanks to their improved biocompatibility. There are however several criticalities associated to their use, including poor batch-to-batch reproducibility and their sometimes excessive predisposition to degradation.31
The biodegradation rate can be tuned based on the preparation process to obtain the intended drug release kinetic.
Polymers have shown the ability to produce a localized, sustained release.15 It can occur in different ways.
When dealing with biodegradable NPs, the drug release is a process that takes place in concomitance with the degradation of the NP itself. As the NP carrier gradually gets degraded, the content is released into the biological environment in a time-dependent manner.15 Biodegradable NPs can be subjected to two kinds of erosions, which lead to different drug release modalities: surface and bulk erosion. The first one is preferable for controlled systems, as it gives more tunable and reproducible results. Instead, in the latter bursts or release may occur, as the phenomenon is less controllable.31
In the case of non-biodegradable NPs, drug release can still take place by diffusion through the polymer matrix.15,31 In this case, the release rate remains more stable during the entire
29 The biodegradation process is heavily dependent on the chemical composition of the core polymer. Polymers featuring a heteroatomic backbone (-C-X-) are more prone to be subjected to hydrolysis and bond cleavage and consequently degrade more quickly. On the other hand, polymers with backbones made exclusively with carbon atoms (-C-C-) show a higher stability.15
Biodegradability is often seen as an advantageous property, as it allows the disposal on NPs and reduces their accumulation, since smaller fragments are more easily eliminated. However, for NPs to effectively reach their target, i.e. tumoral cells, they still need to retain a certain level of stability. In a study comparing the suitability of polylactic acid (PLA) and polycaprolactone (PCL) NPs for the treatment of triple-negative breast cancer, it was seen that PLA NPs degrade too rapidly to reach and accumulate into tumours in a mice model. PCL, on the other hand, showed more encouraging results. Although PCL is also biodegradable it had a promising distribution profile, also due to its slower degradation rate.32
In addition, the degradation products themselves need to be biocompatible and not cause toxicity. Thus, when testing for the biocompatibility of a polymeric product, it is necessary to investigate both the degradation mechanisms and the degradation products.15
Poly lactide-co-glycolide (PLGA) has PLA and polyglycolic acid (PGA) as degradation products, which can both be metabolized by cells. This extremely favourable characteristic made it possible for PLGA to obtain FDA approval. However, even in this situation there might be local toxic effects due to the degradation products, such as local acidity and inflammation.15 Another attractive feature of PLGA is the fact that its degradation time can be regulated with relative ease by simply choosing the concentrations of PLA and PGA used for it synthesis.1
1.2.3 Nanopolymers and cell interaction
Ideally, it is desired that NPs enter only target cells, fulfil their intended function once inside, and either exit or degrade into disposable, non-toxic products afterwards. If NPs remained inside cells even after their role is completed, their accumulation might lead to cellular dysfunctions and toxicity.16 NPs have the potential of causing toxic effects at
30 molecular, cellular and tissue level, and they may even affect organ function.1 It is also important to remember that NPs do not interact exclusively with their intended target: during their transit through the patient’s body they also meet many other biological structures.19 For these reasons, it is important not only to understand the uptake mechanisms, but also the NPs intracellular fate, trafficking, and destination, including the exocytosis mechanisms. All these interactions are heavily regulated by NPs physico-chemical properties, but also depend on cell type and biological environment.16
There are different routes by which NPs can be internalized by cells. NPs physico-chemical properties contribute to determine the uptake mechanism, depending on their size, shape, charge and surface modifications. The other crucial element to take into account is the cell membrane, where properties including membrane fluidity, types of receptors, receptors density and their recycling rate should be considered to understand the initial interactions between cells and NPs.19
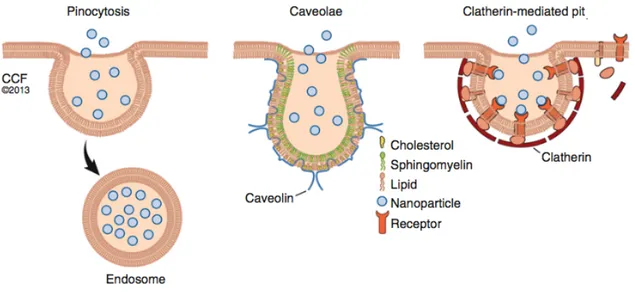
The way NPs interact with the cell membrane may in turn make a difference in intracellular fate of NPs, influencing what intracellular compartments they are sorted into, as well as the retention in the target area, and consequently the therapeutic efficacy.19 The main mechanisms of internalization are: pinocytosis, caveolae-dependent endocytosis and clathrin-mediated endocytosis. A schematic representation of these mechanisms can be seen in Figure 1.6.
31
Figure 1.6: Schematic representation of the main mechanisms of NPs internalization: pinocytosis, caveolae-dependent endocytosis and clathrin-mediated endocytosis.19
Pinocytosis is a non-selective route that consists in the internalization of the extracellular fluid, together with all its content, through an invagination of the cell membrane. While it is not necessary for NPs to interact with the cell membrane in order to be taken up through this route, it was seen that NPs that do interact with the cell membrane experience a higher rate of internalization. This pathway is the preferential one for large NPs and for microparticles.19
Clathrin-mediated endocytosis, on the contrary, is a receptor-mediated process that consists in the formation of clathrin-coated endocytic vesicles following the binding of a membrane receptor with its corresponding ligand. The vesicles have usually a diameter of approximately 100 nm. This process is exploited in active targeting strategies, where ligands corresponding to receptors of a specific cell type, i.e. tumour cells, are bound to the NPs surface. In order to induce vesicle formation, in the case of small NPs a cluster of many of them must be present.19
In caveolae-dependent endocytosis, bulb-shaped, 50-60 nm plasma membrane invaginations called caveolae are involved. These vesicles can form microdomains which can determine where the cargo is transported. This mechanism is involved in the endocytosis of anionic NPs.9,19
Another internalization mechanism is phagocytosis, which consists in the internalization of solid particles but which can only be performed by specialized cells.18
32 Different cell types may have different preferential uptake mechanisms. This, in turn, reflects in the different uptake of the same NPs by different cells. For instance, cells where pinocytosis is predominant tend to have a higher uptake of particles with diameters greater than 200 nm.19
In order to study NP-cell interactions, various membrane models have been developed. For instance, the generation of a lipid monolayer and the following analysis by Atomic Force Microscopy (AFM) is important to understand the real interaction between NPs and the lipids and its biological effect. Another existing model that can be employed is that of a lipid bilayer, the construction of which requires the use of a substrate or a support, and which allows to study its interactions with NPs or drugs using AFM, Fourier transform infrared resonance (FTIR) or X-ray photoelectron spectroscopy (XPS). Liposomes can also constitute a useful membrane model and they have been used to study the ability of NPs to cross the lipidic bilayer.19
To monitor cellular uptake and localization in actual in vitro situations, imaging and spectroscopic techniques are usually employed.19
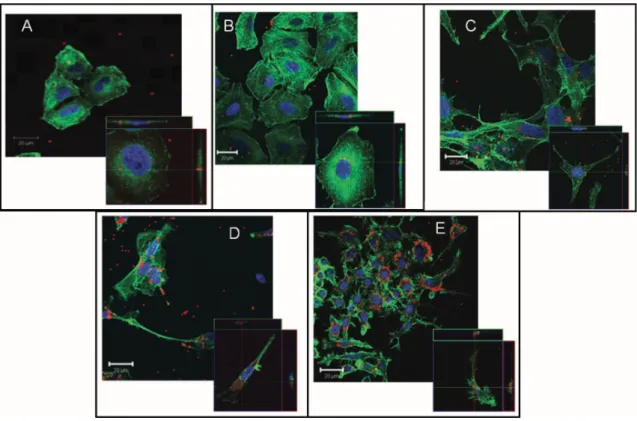
Figure 1.7 shows an example of NPs uptake in different cell lines, visualized with confocal microscopy.
33
Figure 1.7: Confocal images of a) HeLa, b) A549, c) 1321N1, d) HCMEC D3, and e) RAW 264.7 cells incubated with PS-COOH NPs. Enlarged details in the lower corners of each image represent optical sections (x-, y-axes) with projections of the x,z- and y,z-axes of a single cell, respectively. The cross points represent internalized NPs. Blue: nuclei; green: actin; red: NPs.33
Moreover, the uptake pathways can be investigated using inhibitors specific to each mechanism and suppressing one internalization route at a time.19 This strategy allows seeing the role the inhibited pathway had on the NP uptake, by observing the changes that occur when that route is no longer functioning.
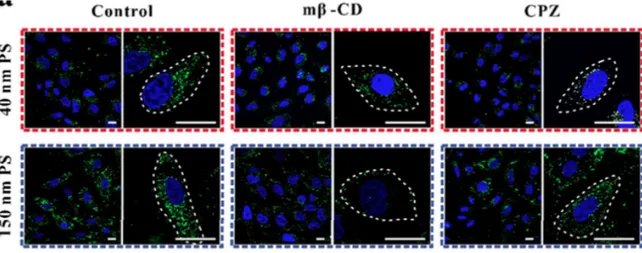
For instance, in the previously mentioned internalization study by Wang et al. aiming to identify the internalization mechanism of PS NPs with 40 and 150 nm of diameter, cells were incubated with selective pharmaceutical pathway inhibitors: Methyl-β-cyclodextrin (Mβ-CD) to block caveolae-mediated endocytosis, chlorpromazine (CPZ) to block the formation of clathrin-mediated endocytosis. The differences in the preferred internalization pathway can be seen in Figure 1.8. In the same study, small interfering RNAs (siRNA) were also used instead of pharmaceutical inhibitors to knock down the expression of key proteins of the internalization processes, producing similar results.16
34
Figure 1.8: HeLa cells treated with PS NPs with 40 (top) and 150 nm (bottom) of diameter. Left: control; centre: cells treated with a caveolae-mediated endocytosis pathway inhibitor; right: cells treated with a clathrin-mediated endocytosis pathway inhibitor.16
A more recent development is the implementation of computational models and simulations to make predictions regarding NPs behaviour and their interactions with cell membranes, and even about their potential toxicity.19 This approach can be an important tool for investigating and optimizing key NPs parameters. It addresses the current need to better understand NP behaviour in complex biological situations and to develop more effective NP therapeutics. It has the potential to facilitate and accelerate nanotechnology-based translational research, reducing the necessity for lengthy and expensive studies on cell and animal models. Numerous computational models have already been developed to study the key steps in the nanomedicine pipeline, such as drug encapsulation and release, NP targeting, delivery and uptake, and NP effects on cells and tissues.34
After internalization, NPs can undergo many different fates and localize in different cell compartments. Where they end up has important repercussions on NPs therapeutic efficiency, as well as with associated toxicity.19
Immediately after the uptake, it is common for NPs to be found inside early endosomes.16,19 Afterwards, these vesicles mature into late endosomes, which fuse with lysosomes, where the degradation of their content occurs. If NPs need to be delivered to any subcellular compartment other than lysosomes, it is necessary to find an efficient
35 strategy for endosomal escape before degradation. Some common targets inside the cell are its cytoplasm, the nucleus and mitochondria, but they are not the only ones.19
There is the possibility for NPs to be taken outside of the cells, through exocytosis. Vesicles called exosomes are transported to the plasma membrane and secreted to the extracellular space.16
Unfortunately, toxicological data in many cases has been conflicting and inconsistent.35
A concerning ongoing issue is the lack of standardized protocols to test NPs toxicity. The absence of adequate characterization in the early stages on NPs testing might significantly contribute to failure of the product in later clinical trials.1
An entirely different cause for toxicity concerns are those NPs that are not intentionally introduced in the body, but rather unknowingly assimilated. The main routes of accidental contact with NPs are inhalation, absorption through the skin, absorption through the intestinal tract. From the lungs, NPs have the potential to transfer to the blood and to systemic circulation, subsequently reaching many areas in the body.14,36
1.2.4 Ps NPs, advantages and hazards
Ps is a synthetic, aromatic polymer, composed of styrene monomers. It is one of the most widely used plastics worldwide.
In nanomedicine, Ps NPs are widely used for both cell imaging and drug delivery. They are often associated with fluorescent molecules.16
Their main advantages are their good biocompatibility, ease of synthesis and high stability.16
However, interactions of cationic Ps NPs once they are inside the cell cytoplasm have shown adverse effects such as lysosomal membrane rupture, mitochondrial damage and induction of toxic oxidative stress starting as soon as less than 1h after exposure to the NPs.37
36 Ps NPs could also represent an environmental hazard, due to their massive presence because of plastic waste accumulation. As these plastics degrade, they release micro- and nanoplastics in the environment.
Ps plastics represent about 10% of total plastic production and have been accumulation as waste for decades; thus, significative amounts of Ps NPs were produced and entered ecosystems.38,39 It has already been demonstrated that nanoplastics of waste origin can be
taken up by multicellular organisms, diffuse through membranes and enter their circulatory system.38
Nanoplastics are prone to interact with proteins, with altering effect on their secondary structure. Since proteins secondary structure plays a critical role in their function, denaturation could lead to misfunctionings in cell processes and consequent toxicity and mortality.38
In vitro genotoxicity was assessed of the human fibroblast Hs27 cell line. The
cytokinesis-block micronucleus (CBMN) assay highlighted DNA damage, with an increase in the formation of micronuclei and nuclear buds in cells exposed to 100 nm Ps NPs, as well as an increase in ROS production.40
In an experiment testing the effect of Ps NPs in Hydra attenuata, exposure to NPs led to decreased biomass, lipid peroxidation, increased polar lipid levels, viscosity, and formation of liquid crystals at the intracellular level, with NPs with 100 nm of diameter resulting significantly more toxic than 50 nm ones.39
In a study on Daphnia pulex, Ps NPs induced overproduction of ROS and enhanced antioxidant enzyme activity and gene expression. Oxidative stress may be one of the molecular mechanisms underlying the toxicological effects of nanoplastics, either directly or indirectly.41
1.3 NPs assessment
Before NPs can be used in nanomedicine, it is necessary to fully understand their behaviour in vivo and their effect and interactions with the host.
37 The starting point for these studies is the characterization of the NPs properties (size, shape, z-potential, chemical composition, surface chemistry), followed by in vitro evaluations of their behaviour. One of the first things that it is necessary to determine is how NPs interact with the cell membrane and whether they can be internalized. It is then possible to proceed to biolocalization and biokinetics studies, to better understand the fate of NPs once they are inside cells and which cellular structures they encounter.
It is essential to assess NPs toxicity as well. NPs are non-self bodies that cells might have adverse reactions to. The safety of each new NP must be carefully evaluated, initially in
vitro and then in in vivo studies.
It is important to remember that nanomaterials have peculiar properties, related to their size at the nano scale, which differentiate them from the bulk materials. Accordingly, the toxicological profile of the material at the macro scale cannot be considered reliable when investigating the effect of nanoformulations. In fact, there is evidence showing a clear tendency of increased toxicity when small NPs are used compared to larger particles with the same composition.22
It has also been hypothesized though that the toxicity mechanisms of NPs may be the same as those of larger particles, but that the effects might be magnified in NPs due to their greater surface area.22
In general, safety of nanomedicines is not fully delineated yet, and there might be still unknown hazards associated with the use of NPs.14,22
1.3.1 Methods to characterize NPs and to visualize them in vitro and in vivo
In order to properly understand how NPs interact with biological systems, keeping into account their physical and chemical properties and how they influence their behaviour, it is essential to properly characterize the NPs.
Some of the most common methods used to characterize them are:
§ Scanning Electron Microscopy (SEM) allows determining size, shape and surface morphology of NPs through their direct visualization. It is one of many different advanced microscopy techniques that allow studying size and shaping related