Contents
………..………...……... page 11. Introduction and organization of the thesis
...51.1 Biology of Annual Fish
……….………..…………...71.1.1 Historical introduction.………...…………..…....7
1.1.2 Systematics and ecology………..…………..…..………..…9
1.1.2.1 Habitat and distribution ………….……….…….………...… 9
1.1.2.2 Phylogeny ………...………..…..10
1.1.2.3 Reproductive and developmental biology ………....……...12
1.2 Nothobranchius as a laboratory animal
……….……...131.2.1 Captive care ………....………... 13
1.3 Aging phenotype of Nothobranchius
………..……... 161.4 Advances in understanding aging
……….……...…...181.5 Fish as models for aging
……….………... 211.6 Nothobranchius furzeri: an extremely short-lived
vertebrate
………..221.7 Aging-related genes in Nothobranchius
………...241.8 Biomarkers of aging
……….251.8.1 Locomotion efficiency ………25
1.8.2 Age-dependent cognitive deficit ………...………..26
1.8.3 Neuronal degeneration ………...….26
1.8.4 Lipofuscin ………..….27
1.9.1 Temperature ………...28
1.9.2 Pharmacological modulation of aging ………...28
1.9.3 Resveratrol ………...30
1.10 Nothobranchius as a genetic model for aging
…………...…….311.10.1 Effects of external mortality on evolution of aging …………...31
1.10.2 Evolution of senescence in annual fish ……….……32
1.11 Aims of the present PhD thesis
……….……….…332. Materials and Methods
………....342.1 Housing conditions
………....….342.1.1 Preparation of food pellets containing resveratrol……….…35
2.2 Methods for scoring aging and longevity
………..…...362.2.1 Survival assays ……….36
2.2.2 Histology and Immunohistochemistry ……….37
2.2.3 Behavioral assays ……….38
3. Results
………..…………..……433.1 Aging in GRZ inbred Nothobranchius furzeri strain
…………433.1.1 Life-history and growth ………...………...…43
3.1.2 Brain aging ………...………...46
3.1.3 Peripheral tissues aging ………...……....49
3.1.4 Locomotion Decline ………....51
3.1.5 Cognitive Decline ………...…………..…... 53
3.3 Effects of Resveratrol on lifespan and age-related
markers
………...………...623.4 Collection of new populations of N. furzeri from
Mozambique
………..………...…….673.5 Hybrid-line generation
………..…..763.6 Aging phenotype in Medaka
………...784.
Discussion
………..………..804.1 Short lifespan of N. furzeri is due to accelerated
life cycle
………..804.2 Brain aging, cognitive- and locomotor- decay
in N. furzeri
………...814.3 Effects of temperature on lifespan, brain aging,
cognitive and locomotor decay
………...……..834.4 Mechanisms of action of reduced temperature
………...844.5 Effects of resveratrol on mortality
………..………….864.6 Effects of resveratrol on brain aging, cognitive
and locomotor decay
………..….874.7 Resveratrol: possible mechanisms of action
………..…...884.8 Aging in different populations of N. furzeri:
effects of climatic conditions
………...… 91Appendix:
“Age-dependent remodelling of second-order
synapses in the mouse inner retina”
……… 97
1. Introduction and organization of the thesis
It is well known that the Central Nervous System undergoes plastic modifications in response to chronic and/or sub-lethal injuries as an attempt to maintain its function; it also undergoes remodelling during many physiological processes, such as development, learning, and also as a consequence of aging.
The topic of this dissertation has been to study the global effects of senescence on the Central Nervous System (CNS) by analysing structural and behavioural alterations that affect neural structures during aging in vertebrates, and by setting up experimental manipulations which can retard the aging process.
During the first year I have investigated regressive and compensatory morphological changes during physiological aging in a well known and largely investigate neural structure, the mouse retina. In neurobiology, the retina represents an ideal system of investigation: first, it is integral part of the CNS and its peripheral localization offers an easy target for many experimental procedures. Moreover, it presents a perfectly laminated structure with spatial segregation of synaptic layers performing different computations and complex of cell types whose connections and micro-circuitry is known in considerable detail. The photoreceptors are the cells of the body with the highest metabolic rate and are therefore a particularly sensitive target of aging. All this makes the retina an ideal model to characterise the effects of aging on specific neuronal circuits and synapses. Therefore, a part of this work focused on describing a form of age-dependent morphological alteration
the mouse makes it an unfavourable model for systematic life-long investigations. To experimentally manipulate aging, it is needed to use shorter-lived models.
For this reason, I choose to study a particular African annual fish,
Nothobranchius furzeri, with a life-span of only three months, as alternative
model of aging. The last two years of PhD were dedicated to the validation of this new experimental model and its application in several experimental manipulations related to the aging processes and lifespan modulation.
For the sake of a better readability of this thesis, the experiments perfomed during my first year of PhD are reported in Appendix.
The main section, that contains the bulk of the experimental data, is centred on the validation of the fish N. furzeri as new model for aging studies, and on its application to investigate environmental and pharmacological manipulations of aging, with a particular attention to the effects of age-dependent processes upon Central Nervous System functions. The text starts with an overview of the basic biology of N. furzeri. This part is necessary since no published material is present in the literature; part of this work was dedicated to characterize basic aspects of the life cycle of this fish. This is a prerequisite before any attempt of using this model in experimental manipulations.
1.1 Biology of Annual Fish
1.1 .1 Historical introduction
Annual fish are a group of Teleosts present both in South America and Africa; they successfully colonized seasonal bodies of water by producing desiccation-resistant eggs which survive the dry season (Jubb 1981). Their life expectancy in the wilderness cannot exceed the seasonal duration of their habitat. Their natural life span is therefore in the order of months, while their captive life span is likewise short even in constant presence of water. They therefore represent an excellent model for aging research.
American annual fish of the genus Cynolebias (now Austrolebias) were studied by Walford and colleagues in the 1960s and 1970s (Walford et al. 1969; Liu and Walford 1970; Liu and Walford 1975). The African annual fish
Nothobranchius guentheri was studied by Markofsky and colleagues in the
1970s (Markofsky and Perlmutter 1972; Markofsky and Perlmutter 1973; Markofsky 1976; Markofsky and Matias 1977; Markofsky and Milstoc 1979b; Markofsky and Milstoc 1979a; Inglima et al. 1981). These researchers reported that annual fish have a defined lifespan in captivity that can be modulated by water temperature; in addition, they develop age-dependent histological degenerations in a wide array of organs such as the eye, liver, kidney and thymus. These results, combined with the work of Comfort and Woodhead in the guppy (Poecilia reticulata) (Woodhead 1998), demonstrated that Teleosts are pertinent models for vertebrate aging.
The scientific community remained unimpressed by this remarkable body of evidence and these fish never gained popularity in gerontology. Only recently, riding the upsurge of interest for zebrafish genomics, some papers
on fish aging were published (Gerhard and Cheng 2002; Gerhard et al. 2002; Gerhard 2003; Kishi et al. 2003; Murtha and Keller 2003; Herrera and Jagadeeswaran 2004; Keller and Murtha 2004; Kishi 2004; Malek et al. 2004). Annual fish are not only exotic animal models: they are also among the most colourful and sought-after aquarium fish. Although the scientific community was agnostic as to their potential, a handful of dedicated hobbyists over the last 30 years systematically sampled eastern Africa looking for new species of Nothobranchius. As a consequence, detailed knowledge of their systematics, distribution and habitat is available (Wildekamp 2004). More importantly, the primary aim of these expeditions was to obtain breeding stocks and to establish captive breeding colonies. As
Nothobranchius eggs can be easily shipped by ordinary mail, after each
collection trip, the new populations were rapidly distributed to a tight-knit community of hobbyists around the world to secure their preservation in captivity. Almost a hundred different populations of Nothobranchius have been maintained without hybridization (Seegers 1997; Wildekamp 2004), and many are still extant as inbred captive strains. For this reason, not only
Nothobranchius fish represent convenient aging models due to their rapid
senescence, but they also offer an unprecedented opportunity for comparative studies.
The first aim of this PhD research has been to bring Nothobranchius fish to the attention of the aging research community, and to reveal their long overlooked potential as model organisms to study aging and age-dependent neurodegenerative diseases.
Much of the preliminary information used to design my work did not stem from the work of professional researchers, but from the hobbyist literature or personal communications from those who collect, breed, distribute, maintain, observe and enjoy these colourful fish. Noticeably, many of thes dedicated
and famous hobbyists, who are referenced here (Foersch, Huber, Jubb, Nielsen, Scheel, Seegers, Shidlovsky, Watters and Wildekamp) are/were themselves scientists, and mostly biologists, with some stature in their respective fields. Their knowledge of Nothobranchius biology is better than anyone else, having dutifully observed this fish in the wild and over many generations in captivity. This information is anecdotal and not substantiated by data collected with scientifically acceptable protocols. However, it provides an invaluable data base for future research on Nothobranchius biology.
1.1.2 Systematics and ecology
Nothobranchius is a genus of the family Cyprinodontiformes, closely related
to the pupfish species (Cyprinodon), to the mumi-chog (Fundulus
heteroclitus) of North America, and to the guppy (Poecilia reticulata)
(Wildekamp 2004; Huber 2005). The genus comprises 43 described and several undescribed species (Seegers 1997; Wildekamp 2004). These animals have a marked sexual dichromatism. Females of different species can hardly be distinguished morphologically and the classification is based mainly on male livery and size (Scheel 1968; Jubb 1981). They are relatively small fish with a median size of 5 cm (ranging from 4 to 15 cm). All these fish are adapted to survive in temporary waters and all of them produce eggs which not only resist desiccation but require a dry period to develop correctly, at least in captivity.
south (Wildekamp 2004), to Angarko, Sudan, in the north (Bellemans 2003). Zanzibar, Tanzania, is the eastern boundary of their distribution and Lake Chad the western boundary (Watters 1998). Only three species live off the African mainland, on islands (Zanzibar and Mafia Island) that were previously connected via a land bridge to Tanzania (Wildekamp 2004).
They inhabit ephemeral pools (typically on floodplains) that may, during heavy wet seasons, be temporarily connected to rivers and streams (Jubb 1981; Seegers 1997; Watters 1998; Wildekamp 2004). All known habitats have a substrate composed of vertisols developed over alluvial deposits (B. Watters, Department of Geology, University of Regina, Canada, cited in Wildekamp, 2004), which are suggested to be crucial in creating the microhabitat for long-term egg survival.
The water quality in Nothobranchius habitats is often not pristine. These pools often attract aggregations of large game which produces significant biological pollution. For example, it is reported that the location where N.
furzeri was captured was heavily contaminated by elephant faeces (Jubb. R
1972). Daily variations in temperature can be extreme, especially in the highland habitat where oscillations of more than 10°C are not uncommon.
1.1.2.2 Phylogeny
Molecular cladistic analysis indicates that the genus is monophyletic and that species diversity is due to vicariance (Murphy and Collier 1997; Murphy 1998). The data suggest that the only two species closely related to
Nothobranchius are Fundulosoma thierryi and Pronothobranchius kiyawensis. These species are morphologically similar to Nothobranchius and
show the same annual life-cycle, but are found to the west of Lake Chad (Scheel 1968). These three genera represent a distinct clade relative to other, non-annual, African Cyprinodontiformes (Murphy 1998). Annualism is
suggested to be a selective advantage that enabled these fish to exploit a unique niche and, in doing so, to escape competition with other teleost species (Seegers 1997; Murphy 1998; Hrbek 1999).
Interestingly, the only other teleost species to be found with Nothobranchius (unless the pool is invaded by fish from an adjacent river) are the lungfish
Protopterus, which are able to aestivate in a cocoon during the dry season
(Seegers 1997; Hrbek 1999). Lungfish have developed opposite adaptations to Nothobranchius: the adults and not the embryos are able to survive dormant in the dry mud. As a consequence, lungfish are very long-lived fish with captive records of more than 20 years.
Biodiversity of Nothobranchius shows marked regional variations. Tanzania is an epicentre of a relatively recent Nothobranchius radiation with no less than 16 clearly differentiated species being found within a 200 km radius of Dar es Salaam (Seegers 1997), most of them with very restricted distributions. On the other side of the spectrum, N. orthonotus and N. rachovii colonize an area of more than 2000 km including the Kruger National Park in South Africa, the coast of Mozambique up to the Zambezi River and southern Malawi (Wildekamp 2004).
Remarkable variations in regional climate and precipitation patterns are observed in Nothobranchius habitats. Some (e.g. Zanzibar) have two short dry seasons which punctuate the long wet season (Wildekamp 2004), while other localities may not dry out at all in some years (e.g. Mafia Island) (Seegers 1997). By contrast, some of the locations where Nothobranchius are found, such as Sudan or the Kruger National Park, show brief and erratic rainy seasons and may not receive sufficient rain for several years (Jubb 1981). As it will be extensively discussed in the course of my work, differences in precipitation patterns result in large-scale differences in captive
populations of the same species.
South American annual fish, by contrast, all reside in relatively humid habitats. They live longer than their African counterparts and some can reach 36 months if kept at temperatures compatible with those encountered in their natural habitat (Liu and Walford 1975).
1.1.2.3 Reproductive and developmental biology
The fish of this genus have adapted to the routine drying of their environment by evolving desiccation-resistant eggs that can lie dormant in the mud for one or perhaps more years (Watters 1996; Wildekamp 2004).
This delay in development is accomplished by the eggs going into diapause (Jubb 1981). Under laboratory conditions, diapause is characterized by three stages (Wourms 1972c; Wourms 1972b; Wourms 1972a). In DI (the first diapause) blastogenesis has completed and embryogenesis has not yet begun. At DII embryogenesis is complete and organogenesis has not begun. At DIII organogenesis is complete and the fully formed fry awaits the environmental cue to hatch.
The regulatory mechanisms of the diapauses are not well understood and are reviewed by Kadlec & Vitek (Kadlec 2003).
It is hypothesized from various researchers (Wourms 1972c) and hobbyist observations that the eggs develop in a staggered fashion over time. Bellemans (Bellemans 2003) investigated the status of eggs in mud samples collected in Sudan 3 months after the respective pan dried out and reports finding only eggs in DI. The eggs can remain in DI for a very long time, at least under captive conditions.
Eggs spawned in captivity of N. rachovii from the Kruger National Park have remained undeveloped for at least 5 years and N. orthonotus from the same locality for 4.5 years (B. Watters, personal communication). Two researchers
of the scientific group involved in this work (Alessandro Cellerino and Tyrone Genade) have inspected a sample of eggs from N. rachovii which were 14 months old and found that the large majority of the eggs were still in DI. Very long diapause is also reported for eggs of N. eggersi, N. furzeri, N.
kafuensis and N. orthonotus. These species all originate from habitats with
well-separated wet and dry seasons.
Newly hatched fry measure 5–8 mm depending on the species. As expected from animals with an accelerated life-cycle, Nothobranchius have an explosive growth. Under captive conditions, if proper feeding and water quality are provided, most species can reach sexual maturity in 6 weeks and attain adult size in 2–3 months.
Growth in the wild may possibly be faster. In the Kruger National Park almost full-grown N. orthonotus (5 cm) were collected 5 weeks after their pool received the first rain (B. Watters, personal communication).
1.2 Nothobranchius as a laboratory animal
1.2.1 Captive care
Nothobranchius males are extremely colourful and are sought-after aquarium
fish. Captive care of Nothobranchius does not require any special technical equipment: they originate from small-sized closed pools and, in captivity, are tolerant of a wide array of water parameters, resistant to poor water quality and tolerant of wide temperature ranges (Watters 1998). Although water chemistry is not a major issue, their captive care requires special attention to feeding and breeding. Culture conditions are radically different from those used for zebrafish.
Zebrafish are usually kept in recirculating systems with strong water flow. An attempt to keep N. rachovii in a recirculating zebrafish set-up resulted in massive death of the fish within less than 2 months (Herrera and Jagadeeswaran 2004). The cause of death is possibly due to exhaustion, as
Nothobranchius are not active swimmers and cannot swim against a constant
current. On the other hand, Nothobranchius can be kept in tanks without any filtration, provided that at least 50% of the water is changed every week (Markofsky and Perlmutter 1972; Valdesalici and Cellerino 2003; Herrera and Jagadeeswaran 2004). Hobbyists generally keep Nothobranchius in small (10-40L) tanks with sponge filters driven by an air supply which provides gentle filtration. The survival of N. furzeri does not change if they are kept in stagnant water or with air-driven gentle filtration (Genade et al. 2005).
The use of multiple, separate tanks is adequate when chronic pharmacological experiments have to be performed.
Another major difference with zebrafish is male aggression: Nothobranchius males often display and attack other males; they can also attack females unwilling to spawn. Aggression of the dominant male is greatly reduced if fish are kept in large groups. Ten to 20 fish of balanced sex ratio in a 40L tank is a good population for medium-sized species (N. furzeri, N. guntheri,
N. rachovii). With a turnover of 3 months, up to 80 N. furzeri can be studied
in one single 40-L tank in one year. A cupboard-sized set-up composed of 12 40-L tanks can house up to 1000 N. furzeri in one year.
A critical point in Nothobranchius care is to provide them with quantitatively and qualitatively appropriate food during their rapid development. Unlike zebrafish, they need a high-protein diet able to sustain their explosive growth. Failure to do so results in permanently stunted growth and reduced female fecundity. Fish raised by hobbyists rarely reach the size of wild-caught specimens unless large quantities of live and frozen food are provided.
Another critical point in breeding Nothobranchius is the spawning substrate. In principle, any soft substrate wherein eggs can be deposited is accepted (peat, sand, glass beads, coconut fibres, etc.). For convenience, the substrate is placed in a small flat vessel that can be easily removed. We use very fine sand, sift the eggs out of the sand, pick up the eggs and place them over damp peat-moss. If properly fed, the fish can be highly productive, with a female laying several hundreds or even thousands of eggs during her lifetime (Seegers 1997). However, the eggs are not released in one single spawn; rather, a small number of eggs (20–30) are released every day.
The third critical point is egg storage. Fresh eggs are large (about 1 mm in diameter or more, depending on the species), sturdy (can be safely picked up with blunt forceps) and can be stored in damp peat-moss for months but the humidity of the substratum is vital. Too dry or too wet a substratum will both result in egg death. A 50% egg loss is normal under these storage conditions. Within a few days after being placed on damp peat, the chorion of the eggs hardens to the point that they bounce if accidentally dropped.
The speed of egg development strongly depends on environmental factors. Many of these factors, which have been scientifically investigated, are reviewed by Kadlec & Vitek (Kadlec 2003). Peters (Peters 1963)
hypothesized that oxygen deprivation (in anoxic mud or peat) triggers DI and DII; and Watters (Watters 1996) predicted that those eggs not exposed to such conditions would develop normally without entering DI and DII.
I have observed that N. furzeri eggs not exposed to oxygen deprivation do not enter DI or DII but develop to DIII in 3 weeks, as opposed to 6 months. Similar observations are reported from the aquarium literature.
Anecdotal evidence from killifish hobbyists, our unpublished observations and experimental evidence from Markofsky & Matias (Markofsky and
completely abolish DI and DII. As a consequence, the developmental time of eggs can be manipulated to be a few weeks or several months (for some species up to years). This offers great advantages in experimental planning as well as and inexpensive methods of embryo storage.
The last critical point of Nothobranchius as laboratory animals is determining the right hatching window. If eggs are wet too soon, they do not hatch and may die. If eggs are hatched too late, the embryos are very weak and do not survive, or present abnormalities in the swim bladder and develop into ‘belly sliders’. The situation is complicated by the fact that the eggs rarely develop in synchrony. Some can be already in DIII while others are in still in DI. In summary, the husbandry and breeding of Nothobranchius requires little technical equipment but some finesse and experience to master the feeding, storage of eggs and timing of hatching. A reliable protocol or culture of N.
furzeri was developed during the course of my PhD work. However, more
work is needed for the development of artificial diets and methods for high-density, large-scale cultures which are necessary prerequisites for saturation mutagenesis, RNA interference and transgenesis.
Even though molecular genetics is not yet feasible in this spcies,
Nothobranchius are promising subjects to study the effects of environmental
manipulations and/or pharmacological treatments on longevity.
1.3 Aging phenotype of Nothobranchius
Age-dependent survival trajectories are available for N. guentheri, N. rachovii and N. furzeri. Median survival in the three species is 12 months (Markofsky and Perlmutter 1972), 6 months (Herrera and Jagadeeswaran 2004) and 9 weeks (Valdesalici and Cellerino 2003), respectively. N. rachovii shows a
gradual, almost linear decline in survival. By contrast, the survival curve of N.
furzeri shows an exponential increase in probability of death with age with a
subsequent deceleration (Valdesalici and Cellerino 2003). This temporal profile, observed in many different models as well as in humans, is considered a hallmark of age-dependent physiological deterioration and provides evidence that the short lifespan of Nothobranchius is associated with rapid aging.
Histological analysis of various organs was performed in aged N. guentheri by Markofsky and Milstoc (1979). They detected marked aging-associated degeneration of the kidney (Markofsky and Milstoc 1979b; Cooper et al. 1983) and liver (Markofsky and Milstoc 1979a), as well as increased incidence of tumours (Markofsky and Milstoc 1979b; Cooper et al. 1983). These observations were tied with a degeneration of the thymus, suggesting immune system decline with aging. Age-dependent degeneration of the pituitary was reported in N. korthausae (Ruijter 1987).
At macroscopic level, Nothobranchius fish undergo progressive and apparent age-dependent degenerations. Their stocky bodies progressively lose weight and become thinner. The females lose their rotund appearance taking on a look of emaciation and a curved spine. Calorimetric data show that emaciation is associated with a progressive loss of fat (Markofsky 1976; Balmer 1982). Spinal curvature and emaciation are associated with aging in several other fish models, e.g. guppy, Cynolebias and zebrafish (Gerhard et
al. 2002). In addition to these changes, males progressively lose their bright
colours. In all Nothobranchius species it has been observed (N. eggersi, N.
guentheri, N. korthausae, N. kunthae, N. neumanni, N. orthonotus and N. virgatus), but not in the extremely short-lived N. furzeri, the above mentioned
a progressive failure of cellular and organ maintenance, or rather the results of infectious diseases due to age-dependent degeneration of the thymus (Cooper et al. 1983).
1.4 Advances in understanding aging
Over the years, many theories have emerged to explain which changes lead to aging (Mattson et al. 2002). Most theories have old origins, but the inherent difficulties of studying human aging, such as the lack of adequate models, make experimental testing a difficult and expensive process. Moreover, interpreting the results is frequently controversial; discriminating between causes and effects of aging is often impossible. That is why, at present, no consensus exists over the causes of aging and the basis of the different aging rates of different species. Nevertheless, some theories have gathered more experimental support than others. The aging process may derive from changes occurring in parallel in different tissues due to intrinsic cellular mechanisms; or, changes in one tissue may be predominant. Some authors argue that aging is “located” within one organ, such as the brain (Mattson et al. 2002), while others defend that aging originates in all tissues (Kowald and Kirkwood 1994). Some researchers even argue that one type of cells such as bone marrow stem cells may be determinant (Geiger and Van Zant 2002; Van Zant and Liang 2003). "Big bang" simulations demonstrates how one particular system, often the endocrine system, can regulate aging (Gosden 1996). Results from C. elegans indicate that a few cellular lineages in mosaic organisms confer longevity (Apfeld and Kenyon 1998; Hsin and Kenyon 1999; Lin et al. 2001; Arantes-Oliveira et al. 2002; Patel et al. 2002), perhaps due to endocrine signals (Wolkow et al. 2000). Some results from mice also
suggest the existence of systemic factors in aging, but only to some extent (Conboy et al. 2005). Although this debate has not been settled yet, it appears that intrinsic cellular mechanisms play a role in aging, though these can be modulated by extracellular factors like hormones.
There are many types of theories of aging. For practical purposes, and also because disquisitions on gerontology are not the first target of this dissertation, one can divide this argument into “damage-based” and “programmed” theories of aging. “Damage-based theories of aging”, as the name implies, defend that aging results from a continuous process of damage accumulation originating in by-products of metabolism; in other words, a certain form of damage accumulates throughout the entire lifespan and causes most of the typical aspects of the senescent phenotype. Typically, this damage accumulation is the result of the addiction of the negative effects caused from by-product of normal cellular processes and the lost of the efficienty of the repair systems throughout the time. On the other hand, “programmed theories of aging” argue that aging is not a result of random or stochastic process but rather driven by genetically regulated processes. It is well known that aging has a strong genetic component. Even damage-based theories of aging recognize that certain genetic factors, such as defensive or protective genes, play a role in aging (Kirkwood and Austad 2000). Likewise, programmed theories of aging recognize that some forms of damage contribute to aging and that environmental factors influence the outcome of aging to some degree.
In the past few years, several molecular pathways and factors that control the lifespan in invertebrate model organisms and also in mice have been identified: the histone deacetylases of the Sir2 (sirtuin) family and their target genes (Woodhead 1998; Hwangbo et al. 2004; Wood et al. 2004), the IGF
(Migliaccio et al. 1999). In addition to the data provided by targeted gene mutations, linkage studies in humans have implicated the microsomal transport protein (mttp) in human longevity (Geesaman et al. 2003). A widely-accepted theory correlates aging with production of reactive oxygen species and oxidative damage (Beckman and Ames 1998b; Beckman and Ames 1998a; Balaban et al. 2005); these processes are also known to be key players in neurodegeneration (Siesjo 1984; Richardson et al. 1992; Bondy and LeBel 1993; Hirsch 1993; Poirier and Thiffault 1993; Gotz et al. 1994; Gutteridge 1994; Tritschler et al. 1994; Youdim et al. 1994). These genes and pathways provide molecular targets for pharmacological research to prevent and treat age-related neurological diseases. However, the lifespan of the available animal models is a bottleneck, with the result that thus far only
Drosophila or C. elegans have been adopted in systematic pharmacological
approaches to aging (Woodhead 1998; Kang et al. 2002; Wood et al. 2004; Evason et al. 2005).
Antioxydants, histone deacetylase inhibitors, sirtuin activators and anticonvulsant drugs increase the lifespan in animal models of aging (Woodhead 1998; Kang et al. 2002; Bauer et al. 2004; Wood et al. 2004; Evason et al. 2005). However, the relevance of these studies for age-related pathologies is questionable, given the completely different organization of vertebrate and invertebrate models. Moreover, C. elegans and Drosophila contain only post-mitotic cells and cannot be used to study replicative senescence, as well as tipical indicators of neurodegeneration in vertebrates, such as neurofibrillary degeneration of the brain and mild cognitive impairment (MCI). Obviously, a short-lived vertebrate with a lifespan of few months and which shows physiological expression of typical vertebrate age-related markers is highly desirable.
1.5 Fish as models for aging
The advantages of a short-lived fish as a vertebrate aging model were detailed by (Austad 2004) and will be briefly summarized here. Fish represent the most numerous group of vertebrates. Fish can be housed at high stocking density, they produce a large number of eggs which are amenable to transgenesis and gene interference and large-scale mutagenesis screening has already been performed in two different species of fish, Medaka and Zebrafish.
A vertebrate model with an extremely rapid life cycle can be used to study drugs designed to impact vertebrate-specific targets and to assess their effects not only on longevity but, more importantly, on age-related dysfunction of specific organ systems, and in particular on the efficenty of the Central Nervous System (CNS) functions.
The first studies on aging in fish were perfomed by Comfort, who showed that guppies (Poecila reticulata) have a defined lifespan and longevity can be increased by dietary restriction (Woodhead 1998); further studies in collaboration with Woodhead demonstrated age-dependent degeneration or dysfunction of several organs (Woodhead 1998) and age-related pathological changes similar to the processes described in mammals were demonstrated by Liu and Walford in the annual fish Austrolebias (Liu and Walford 1970; Liu and Walford 1972). Since then, age-dependent tissue and organ degenerations have been described in a variety of fish species (Patnaik et al. 1994; Woodhead 1998; Gerhard et al. 2002; Kishi et al. 2003) and notably in the annual fish Nothobranchius guentheri (Markofsky 1976; Markofsky and Milstoc 1979a; Markofsky and Milstoc 1979b; Balmer 1982; Cooper et al. 1983). More recently, markers of cellular senescence, protein oxidation, and
changes in heat shock proteins have been described during zebrafish aging (Kishi et al. 2003; Murtha and Keller 2003).
A further advantage of fish as aging models is that their lifespan can be easily manipulated by changing water temperature (Liu and Walford 1975; Yen et
al. 2004; Terzibasi et al. 2006; Valenzano et al. 2006a; Valenzano et al.
2006b) and low temperature retards the expression of age-related pathologies (Liu et al. 1975; Liu and Walford 1975; Yen et al. 2004; Terzibasi et al. 2006; Valenzano et al. 2006a; Valenzano et al. 2006b).
Teleost fish are also exceptional models for comparative studies. Both the smallest vertebrate (Kottelat et al. 2006), and the shortest-lived vertebrates are fish (Valdesalici and Cellerino 2003; Depczynski and Bellwood 2005), but also some of the longest-lived vertebrates are fish (205 years, the rockfish
Sebastes aleutianus (Cailliet et al. 2001)). Therefore, teleost fish might
represent interesting subjects to study comparative genomics in the future.
1.6 Nothobranchius furzeri: an extremely short-lived
vertebrate
The genus Nothobranchius compromises about hundred separated species, many still not described formally. These fish inhabit ephemeral pools (typically on flood plains) in Eastern Africa and have adapted to the routine drying of their environment by evolving desiccation resistant eggs that can remain dormant in the mud for one and maybe more years (Genade et al. 2005). This delay in development is accomplished by the eggs entering into diapause where oxygen consumption is depressed (Levels et al. 1986;
Podrabsky and Hand 1999; Podrabsky and Hand 2000; Podrabsky et al. 2000; Podrabsky et al. 2001).
N. furzeri lives in the dry lowveld of Zimbabwe, a semi-arid area, and
survives the long dry season (which can last more than ten months) as embryos encased in the dry mud. When it rains, the embryos hatch and reproduce in the few weeks before their habitat disappears. Accelerated maturation is observed in captivity as well; growth and maturation are accelerated even when compared with other, longer-living, species of the genus which originate from more humid climates with longer rain seasons (Genade et al. 2005). The laboratory strain of Nothobranchius furzeri originates from a single collection of 1968 (Jubb. R 1972) and has been maintained ever since by a handful of dedicated hobbyists. A conservative estimate of 6 months generation time leads to the conclusion that this strain underwent at least 80 generations in captivity without any outbreeding. Since hobbyists often use only one male and two females for breeding, it is reasonable to suppose that, due to repeated genetic bottlenecks, the current laboratory strain is highly homozygous.
N. furzeri develops from a larva to a sexually mature adult in 3-4 weeks,
showing a median survival of 8.5 weeks and a maximum lifespan of 13 weeks (Genade et al. 2005). This lifespan is independent on the culture condition (stagnant or with filtration) and is considerably shorter than that of other
Nothobranchius populations raised under identical conditions in our
laboratory (Genade et al. 2005) as well as the lifespan reported by other researchers for N.rachovii and N.guentheri (Markofsky and Perlmutter 1972; Herrera and Jagadeeswaran 2004).
This fish can be raised in relatively high numbers and allow to perform fine-graded age-dependent survival studies. In many organisms, death rate
observed later in life (Carey et al. 1998; Vaupel et al. 1998). Theoretical studies have modeled this late-life deceleration by applying a general theory of systems failure known as reliability theory (Gavrilov and Gavrilova 2004b; Gavrilov and Gavrilova 2004a; Gavrilova et al. 2004). Late-life deceleration of death rates can be clearly observed in the laboratory strain of
Nothobranchius furzeri (Valdesalici and Cellerino 2003).
1.7 Aging-related genes in Nothobranchius
Some genes and molecular pathways that control aging are highly conserved throughout species, e.g. the IGF signalling pathway and the Sir2 pathway. However, some genes appear to modulate aging specifically in mammals. The signalling adapter p66shc is a nuclear-encoded protein that localizes to mitochondria (Orsini et al. 2004) and has been shown to regulate the production of reactive oxygen species (Nemoto and Finkel 2002) and to modulate lifespan in mice (Migliaccio et al. 1999). The microsomal triglyceride transfer protein (mttp) was implicated in the control of human aging by linkage analysis of centenarian families (Geesaman et al. 2003). When dealing with non-model organisms, a frequently encountered problem is the paucity of genetic information about the ‘novel’ species. However, Herrera & Jagadeeswaran (Herrera and Jagadeeswaran 2004) have already shown that they could easily isolate (by PCR amplification) N. rachovii homologues of defined zebrafish ESTs that also have similarity matches in the Fugu genome. Taking advantage of the available genomic data for three teleost species (zebrafish and the two pufferfish Takifugu rubripes and
Tetraodon nigroviridis) (see, for example, http://www.ensembl.org/) and
N.furzeri. These include p66shc, mttp, sirt1 and igfr1 (Genade et al. 2005).
Genes shown to control aging provide important targets for the development of pharmaceuticals to prevent age-related pathologies in humans. Orthologs of human aging-related genes are indeed present in N. furzeri and display considerable homology with their human counterparts (Genade et al. 2005). Therefore, we suggest that N. furzeri could be used to test the effects of pharmaceuticals aimed at vertebrate-specific genes, with all the experimental advantages provided by a short-living animal model.
1.8 Biomarkers of aging
The quantification of aging rates is a key problem in biogerontology. Longevity is the most convenient endpoint and is the parameter commonly measured in pharmacological studies. However, the analysis of multiple endpoints can hardly be overemphasized. In particular, three classes of endpoints should be included in any analysis of aging: functional parameters, histological markers and fertility. It is therefore crucial to demonstrate that short lifespan in Nothobranchius furzeri is tied with accelerated expression of age-related markers and this model offers the possibility to analyze multiple endpoints. The present PhD work is concentrated upon five markers, described below.
1.8.1 Locomotion efficiency
Age-related reduction of locomotor efficiency is a marker for neuromuscular decay (Reznick et al. 2004). Kinetic parameters as indicators of swimming performance can be easily quantified by computer-assisted video tracking. This technique allows to measure swimming speed, maximum speed and time
activity and open-field exploration, a standard behavioural test used for rodents which quantifies the exploration of a new environment and shows an age-dependent decline (Furchtgott et al. 1961).
1.8.2 Age-dependent cognitive deficit
Age-dependent cognitive decay is a hallmark of mammalian aging. Learning in fish can be studied with different protocols. Particularly efficient for testing large number of subjects is a protocol of operative conditioning based on a modification of the shuttle-box. This is a protocol of active avoidance where a fish has to learn the association between the onset of a red light in one of the two compartments and a punishment. In order to avoid punishment, the fish has to move to the other compartment soon after light onset. This protocol was originally developed for goldfish (Laudien et al. 1986) and zebrafish (Pradel et al. 1999; Pradel et al. 2000), and it was conveniently adapted to N.
furzeri.
1.8.3 Neuronal degeneration
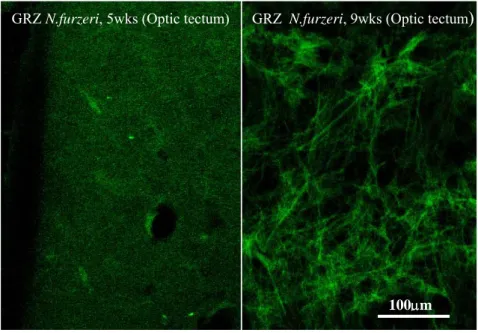
One of the hallmarks of human brain aging is the accumulation of neurofibrillary tangles. I tested the existence of age-dependent neurodegeneration in fish by using the fluorescent dye Fluoro-Jade B (Terzibasi et al. 2006; Valenzano et al. 2006a; Valenzano et al. 2006b), a specific marker of neurofibrillary degeneration. The latter is an aging-related modification of the neuronal microtubules (Schmued et al. 1997). Previous work demonstrated age-dependent neurofibrillary degeneration in the brain of post-reproductive salmon, a model of accelerated aging (Maldonado et al. 2002b; Maldonado et al. 2002a).
1.8.4 Lipofuscin
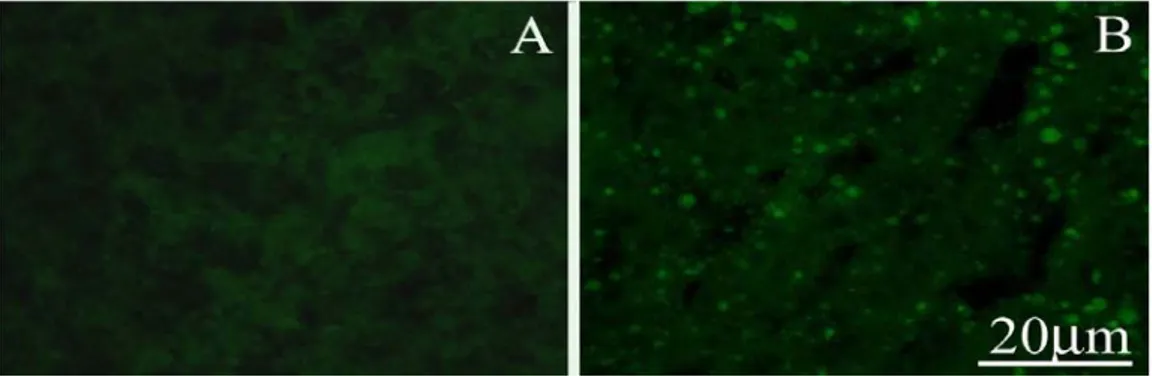
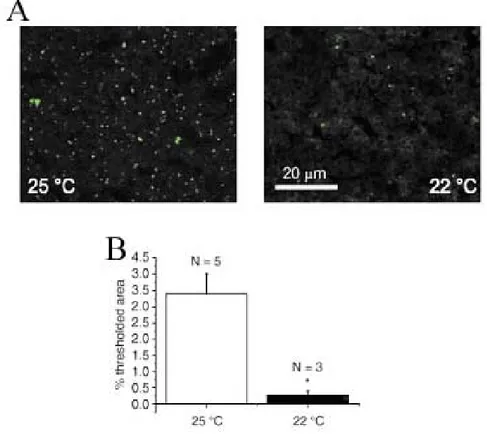
Lipofuscin (LF) is a conglomerate of lipids, metals, organic molecules, and biomolecules that commonly fluoresces at 360 to 470 nm. LF is formed within secondary lysosomes due to partially reduced oxygen species produced by mitochondria. which induce lipid peroxidation and intermolecular cross-linking (Brunk et al. 1992). LF accumulates with age in a variety of organs including brain, heart and muscle in humans. LF granules have been found in every eukaryote ever examined, and always accumulate within cells as the organism ages, hence its recognition as "the aging pigment". LF autofluorescence is a very convenient endpoint since it does not require any chemical reaction to be visualized and it can be precisely quantified by confocal microscopy (Belichenko et al. 1996; Terzibasi et al. 2006; Valenzano et al. 2006a; Valenzano et al. 2006b).
1.8.5 Senescence associated Beta-Galactosidase
Replicating cells loose their mitotic potential with aging and stop dividing. This phenomenon is named cellular senescence. Although cellular senescence is not prominent in brain, which is largely post-mitotic, it is an important causative agent of aging in peripheral organs such as the skin (Herbig et al. 2006; Jeyapalan et al. 2006). Senescence associated Beta-Galactosidase (SA-β-Gal) is a putative marker of cellular senescence (Dimri et al. 1995). Age-dependent induction of SA-Beta-Gal is observed in the dermis of humans and zebrafish (Dimri et al. 1995; Kishi et al. 2003). SA-β-Gal is a convenient marker since it does not require immunocytochemical reaction and thus possible problems of cross-species antibody recognition.
1.9 Experimental modulations of lifespan
1.9.1 Temperature
Temperature variations are known to modulate aging and life-history traits in poikilotherms as different as worms, flies and fish (Yen et al. 2004).
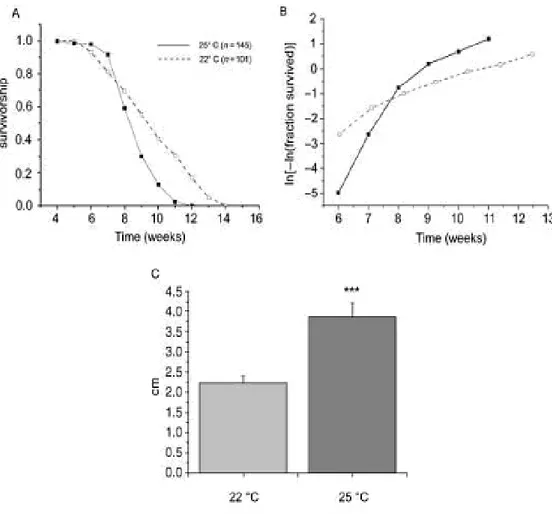
In invertebrates, temperature modulates lifespan by modulating the slope of age-dependent acceleration in death rate, which is thought to reflect the rate of age-related damage accumulation (Partridge et al. 2005). It should be remarked that life-extending effects of reduced temperature are not the result of reduced metabolism as there is no simple relationship between temperature and basal metabolism (Yen et al. 2004). Moreover, temperature variations induce very complex regulation of gene expression which include the entire mithochondrial respiration, the proteasome, lipid metabolism and transport and cromatin acetylation (Gracey 2004). At present it is unclear which of these pathways is relevant for the life-extending effects of low temperature. In the context of the present work, temperature modulation was used to demonstrate that short lifespan of N. furzeri is linked to rapid aging and it is not a consequence of some pathology.
1.9.2 Pharmacological modulation of aging
Small molecules were identified which increase lifespan of model organisms when administered with food. Some of these compounds, like ethoxyquin (Comfort et al. 1971), have toxic effects, and are able to increase lifespan of short-lived mice strains only. Others, like deprenyl (Knoll 1989; Jordens et al. 1999), have psychoactive effects. Further compouds, like 4-phenylbutyrate
(PBA), can increase flies lifespan in a dose-dependent manner, but the active dose differs between two different strains of flies (Kang et al. 2002), while higher doses are toxic. PBA (4-PhenylButyrate) induces a strong transcriptional activation and inhibits hystone deacetylases (Kang et al. 2002). The antioxidants Lipoic Acid (Bauer et al. 2004), butylated hydroxytoluene (Sharma and Wadhwa 1983), N-acetylcysteine (Brack et al. 1997), sodium solenite (Kaur et al. 1989), sodium hypophosphite (Wadhwa et
al. 1986), were all found to increase fly lifespan. Life extension was reported
using catalase mimetics in nematodes (Melov et al. 2000) (result not replicated in another experiment in nematodes (Keaney et al. 2004) and flies (Bayne and Sohal 2002)), as well as by using Ginko biloba extract EGb 761 (Wu et al. 2002) and vitamin E (Harrington and Harley 1988). The effects of antioxidant on lifespan increase are in agreement with the mitochondrial theory of aging, which underlines the importance of the mitochondrial-dependent ROS generation as the pivotal mechanism for aging initiation and progression. In fact, extension of lifespan and retardation of aging were achieved by reducing oxidative stress through the over-expression of human catalase in mice mitochondria (Schriner et al. 2005), and by mutating the mouse p66shc adaptor protein gene (Migliaccio et al. 1999), which is responsible of ROS-dependent mitochondrial apoptosis (Giorgio et al. 2005). Three anticonvulsant drugs: ethosuximide, trimethadione, and 3,3-diethyl-2-pyrrolidinone, increase nematodes lifespan and delay aging, possibly through an action directed on central and peripheral neural activity (Evason et al. 2005). This effect was however strictly dependent on culture condition: all the effects were observed at 20°C, but not at 15°C, where only 4 mg/ml of ethosuximide (a very high dosage) increased lifespan. The results also varied in relation to the food substrate on which worms were fed (Evason et al.
1.9.3 Resveratrol
Up to date, the only molecule which consistently prolongs lifespan across species is resveratrol (3,5,4’-trihydroxyl-trans-stilbene, Fig 1.1), a natural phytoalexin found in several plants where it plays its antifungal action (Langcake and Pryce 1977; Hain et al. 1990). Notably, resveratrol is present in the weed Polygonum cuspidatum and in the grape (Vitis vinifera), both very important elements for human nutrition as basic food products in China-Japan and Mediterranean populations, respectively. Resveratrol is highly concentrated in red wine (5 mg/L in average, ranging from 0.5 to 10 ppm (Celotti et al. 1996)), which is considered the principal source of this compound for humans.
Figure 1.1: Resveratrol trans-isomer
This polyphenol has been shown to have many beneficial actions, ranging from anticancer (Jang et al. 1997; Manna et al. 2000), to anti-inflammatory, estrogenic, antioxidant and chemopreventive (Granados-Soto 2003). Bioavailability of resveratrol is very low since, once assumed, it is very quickly absorbed (Bertelli et al. 1998b), and 75% of it is lost by excretion via faeces. The remaining part is mainly metabolized in glucuronide- and sulfate- resveratrol conjugates (Wenzel et al. 2005; Wenzel and Somoza 2005), which are probably the biological active forms, since no aglycone resveratrol has been found after absorption (Goldberg et al. 2003). In vitro studies show that
physiological concentrations of resveratrol modulate NF-kB (Nuclear Factor KB) dependent transcriptional activation in human endothelial cells (Pellegatta
et al. 2003). Recent studies have shown that resveratrol prolongs lifespan of
yeasts (Howitz et al. 2003) and short lived invertebrates (Bauer et al. 2004; Wood et al. 2004; Viswanathan et al. 2005). The mechanisms responsible for this reproducible effect on lifespan has been suggested to be the resveratrol-dependent activation of the NAD-resveratrol-dependent histone deacetylases belonging to the Sir2 family (Silent information regulator 2) (Howitz et al. 2003; Wood et
al. 2004).
I have tested the effects of resveratrol on longevity and on age-related markers in N. furzeri. The results of this and previous studies demonstrate that resveratrol is the first example of a molecule which shows life-extending properties in organisms as diverse as yeast, nematode, fly and fish.
1.10 Nothobranchius as a genetic model for aging
1.10.1 Effects of external mortality on evolution of aging
Extrinsic mortality is the mortality due to external causes such as predation, parasitic infection and - in the case of annual fish - the desiccation of the pool where the fish lives. All the major theories of aging (Medawar 1952; Williams 1957; Kirkwood and Holliday 1979; Hughes 2005) predict that differences in extrinsic mortality rates result in differences in growth, which eventually cause differences in senescence. In particular, high mortality rates drive evolution of rapid growth and maturation and, as a secondary consequence, of rapid senescence. These predictions were confirmed by
only limited data as to the effects of different mortality rates on the evolution of life-history traits in natural populations of vertebrates. There are examples of negative correlations between mortality rates and longevity in natural populations of opossum and salmon (Austad 1993; Hendry et al. 2004). Yet, the most complete study of natural populations of a vertebrate, performed in the guppy fish (Poecilia reticulata), does not support this theory. Guppies originating from high-predation sites (subject to high extrinsic mortality) show accelerated growth and early sexual maturation, but not reduced longevity or shortened reproductive lifespan (Reznick et al. 2004).
1.10.2 Evolution of senescence in annual fish
For annual fish, the maximum window of survival can be assumed to be directly correlated with the duration of their habitat. Owing to the huge differences in climatic conditions over their distribution area, the ease by which extrinsic mortality rates can be estimated by meteorological records, and the large number of species for which captive colonies are available,
Nothobranchius fish are an exceptionally good model system to study the
effects of extrinsic mortality rates on evolution of life-history traits.
Age-dependent survival in the laboratory has been recorded for only three species so far: N. furzeri, N. rachovii and N. guentheri. Maximal survival is 3 months (Valdesalici and Cellerino 2003), 9.5 months (Herrera and Jagadeeswaran 2004) and over 16 months (Markofsky and Perlmutter 1972), respectively. A remarkable correlation between the rainfall pattern in the biotope of origin and captive lifespan exists for these three species. Home of N.
furzeri is the low veld of Zimbabwe, which, apart from actual desert areas, is
360 mm per year(data for Breitbridge, www.worldweather.com). N. rachovii originates from the coastal city of Beira, Mozambique, with a clear separation between wet and dry season, but with a more humid climate and average annual rainfall in the order of 1600 mm per year(www.worldweather.com). N.
guntheri originates from Zanzibar, a humid mosaic forest with two wet seasons
(www.worldweather.com). The habitats of N. guentheri might not dry every year. In fact, eggs of N. guentheri, in contrast to most other Nothobranchius species, can complete their development in water without the need for a dry period (Markofsky and Perlmutter 1972).
1.11 Aims of the present PhD thesis
Aims of the present dissertations are:
1. To characterize the aging process of N. furzeri at the histological and behavioural levels to demonstrate that short lifespan is coupled to highly accelerated expression of aging characters
2. To test the effects of water temperature reduction on longevity, age-related histological damage and age-age-related behavioural deficits
3. To test the effects of resveratrol on longevity, age-related histological damage and age-related behavioural deficits
4. To characterize the longevity, related histological damage and age-related behavioural deficits of populations coming form humid and more arid habitat, by collecting wild populations of N. furzeri from Mozambique
5. To characterize longevity and age-related behavioural deficits in crosses between short-lived and long lived populations of N. furzeri
2. Materials and Methods
2.1 Housing conditions
Fish housing and care followed a specific protocol depending on the age of the fish and on the chosen experimental conditions. In standard conditions, adult fish were housed in 40L tanks at 25°C, with an “air supplied” water filtration and low water flow, since strong water flow can be lethal for this genus, as already reported for Nothobranchius rachovii. 50% of the water was changed every week; dead fish were removed from the tanks daily, providing a 10% water change for each dead fish, in order to avoid water pollution due to a raise in ammonium. As already mentioned, keeping stable water parameters is not a major issue in Nothobranchius care, as these animals live in nature in stagnant pools and can survive poor water quality and a wide range of temperatures. We chose to keep them in hard, alkaline water, as suggested by AKA guidelines (American Killifish Association). Food was supplied twice a day, and consisted of commercially available, frozen bloodworm larvae (Chironomus sp.), that are rich in proteins and fatty acids. The amount of food released in tanks equalled 50mg(food)/g(fish)/day. Fish were fed manually and, within 2 hours, uneaten food was removed from the tank to avoid water contamination. Fish were maintained in 12 hours light/dark cycles. Fish density was 20 fish per tank from the 4th week of life. Adult subjects were let spawning on a river sand substrate, which was weekly sieved and returned to the bottom of the tanks. During sieving, new eggs were removed from the sand and placed on a moist peat moss, contained in a 10cm diameter Petri dish. The peat was daily checked for dead or unfertilized
embryos which were removed to avoid fungal infections and contamination. On average, about one fourth of all the eggs become infected, appearing pale and developing a characteristic white, filamentous fungal coat. Healthy eggs appeared of amber colour . Petri dishes were sealed with parafilm to avoid excessive desiccation which can kill all the embryos. The peat used was non-chemically treated; upon use, we boiled it for 3 hours and kept it in a sterile box.
“Eyed up” embryos, i.e. embryos showing a well defined golden eye-ring, which is considered as an indicator of completed development, were put in a 2L tank, with peat extract, oxygen tablets to avoid belly sliders (fries which did not succeed in filling with air the gas bladder), peat extract and water at about 18°C, to facilitate hatching of the fries.
Fries were grown in 20L tanks at 25°C and were daily fed with nauplii of commercial fish until the fourth week of life, when they were moved to 40L tanks. Since the second week of life, fries were fed with poly-unsaturated fatty acids (PUFA) enriched Artemia salina, which provided a richer nutrient support; from the third week of life, fries started to receive also chopped bloodworms.
2.1.1 Preparation of food pellets containing resveratrol
For 120 mg/food pellet: 1.2 mg/µl resveratrol stock was prepared in 5% ethanol and stored at 4°C in the dark. Frozen Chironomus larvae were thawed, left to drip dry, and aliquoted into portions of one feeding for 10 adult fish (1g). 100 µl of the 1.2 mg/µl stock was added to each Chironomus aliquot, which was left at 4°C for 1-2 hr to soak. 5% gelatine was added to the
For feeding, frozen gelatine/Chironomus cubes were thawed in water and fed to the fish. All uneaten food was removed. Fish received 2 feedings per day. Control-fed fish were fed with the same kind of food lacking resveratrol in the stock solution.
2.2 Methods for scoring aging and longevity
2.2.1 Survival assays
Fish started to be scored for survival assays since the fourth week of life, when they are considered sexually mature and are moved to 40L tanks with a density of 20 fish per tank. Fish were counted every week, and every day, we scored dead fish. Mortality in fish younger than 4 weeks usually occurred in the very first days after hatching. For computing differences among different treatments, Graph Pad and Origin softwares were used. Since both alive fish (on a weekly basis) and dead fish (on a daily basis) were scored, we were able to assess any incongruence between these two measures as censored: i.e. it can happen that starting with n fish, after m weeks, a given number z of fish were removed from the tank for histology. In this case we have, at week m - 1, n fish and, at week m, n - z fish, without really having any death due to increased morbidity. One can remove z fish from the total number of fish or, better, consider them as censored at week n - 1. This measure allows to account for all fish, without missing those which are removed by the tank for reasons different from natural death. In other situations, the sum of all dead fish did not equal (was lower than) the initial number of fish. This can be due to unseen deterioration of dead fish bodies in the tank (which were not scored as dead), cannibalism (although very rare among same-age N. furzeri but
frequently observed in the first weeks of life in N. kuntahe), jumping out of the tank, etc. In all these cases, the missing fish were considered as censored.
2.2.2 Histology and Immunohistochemistry
Fish histology followed a standard protocol for all tissues. Chosen fish were euthanized with crushed ice for 5 min before dissection. Tissues (brain, liver, muscle+skin) were collcted from the subject, fixed by immersion in 4% paraformaldehyde/0.1M phosphate buffer (pH 7.4). Subsequently, they were infiltrated with 30% sucrose to ensure cryoprotection, embedded in Tissuetek (Reichart-Jung, Nubloch, Germany), frozen at -20°C, and serially sectioned at 18 µm of thickness on a cryostat. All assays followed the specified protocols indicated on the information sheet provided by the Company from which we purchased the required reagents.
In order to visualize presence/absence of Senescence-Associated β-Galactosidase activity in young vs old muscle tissues of the fish, the X-Gal Staining was used, following a well established protocol. Images were collected using a 20x objective and a CCD camera (Roper Scientific Photometrics) connected at a fluorescence microscope, under 488 nm excitation (Eclipse E600, Nikon).
Intracellular accumulation of Lipofuscin during aging was detected on brain and liver tissues of young and old fish in the form of light blue autofluorescent granules under UV excitation. For quantification, images were acquired using a Leica confocal microscope with a 488nm excitation laser, with fixed values of all the confocal parameters (pinhole size, photo-multiplier gain and offset, as well as , laser intensity).
Finally, FluoroJadeB staining was used to visualize neurodegeneration in brain tissues; quantification of neurofibrillary tangles on brain tissues from young and old subjects was performed on confocal images following the same procedure used for the Lipofuscin quantification.
Fluorescence analysis of images for both Lipofuscin and FluoriJadeB staining was performed with the aid of Metamorph software.
2.2.3 Behavioral assays
For scoring age-dependent behavioral decay in N. furzeri, three different behavioral assays were used: one assay designed for scoring Spontaneous Locomotion in a social condition, one assay for scoring individual fish locomotor activity in Open Field -like condition and one assy for scoring learning an Active Avoidance.
Starting at the fourth week of life, at every mesure session, 10 fish were left in their home tank with a digital video camera facing the long side of the tank. One-hour tapes with 1frame/s speed were scored in standard conditions of light and with a white background placed against the other long side of the tank in order to increase the contrast of the fish over the background. From one hour recordings, 10 starting points (a frame) were randomly chosen and, for each of these points, this procedure was adopted: a given value of image thresholding was chosen for all the experimental sets (i.e. for all visual analysis concerning one experiment, usually with 16 bit images the threshold values were 66-100); then, an image subtraction was performed between two frames 5 seconds apart, i.e. Frame10 - Frame5 and so on. In this way, a map of body displacement between two frames distant 5 seconds was obtained. A fish was considered to be significantly moving when it was
displaced from its previous position for more than half of its body-length. A fish was assigned a value of 1 when it was scored as moving and 0 when not moving. The average number of values was computed for 10 fish at every starting
point (10 starting points for every week measure). In this way, Spontaneous Locomotion was scored in age-dependent manner. This measure provides a good approximation of natural/spontaneous motility for fish of each age-class. The image analysis was performed with the software package Image J, freely available at the URL: http://rsb.info.nih.gov/ij/download.html.
Open-field like assay
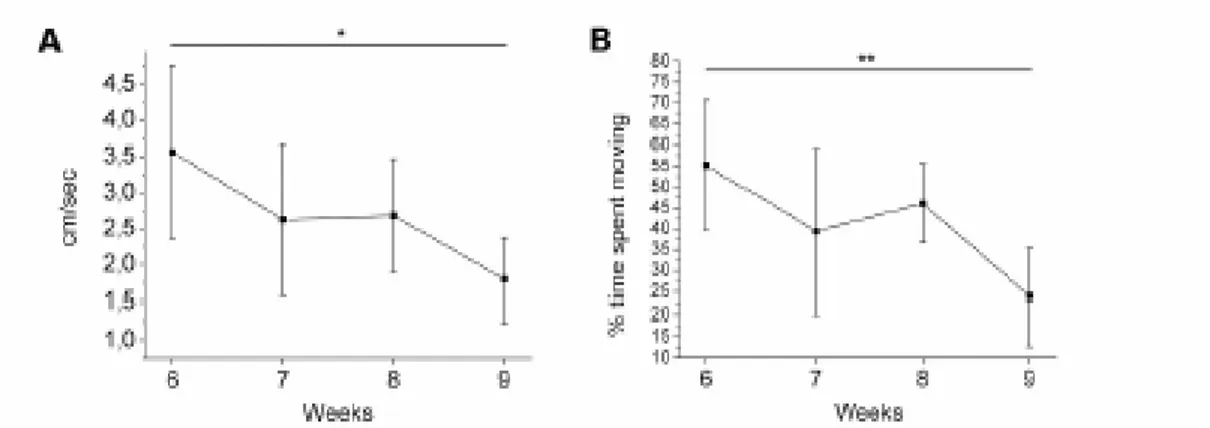
Single fish were scored for locomotor activity in a 20L test-tank having the same water temperature of the home tank. Video recordings were performed with a digital video-camera from above the tank; the water level was kept very low in order to minimize the displacement on the z axis which would not be scored by the camera while recording from above (Fig 2.1). Fish were let to habituate for 30 min within the tank before the 10 min recordings started. The image analysis were performed with Software Ethovision (NoldusTM, the Netherland) which allowed the computation of mean, maximum velocity and percentage time spent moving for every single fish belonging to any experimental group. For every groups, means and standard deviations were computed.
Figure 2.1: Snapshot from the Open Field-like test. In red is drawn the path tracked by a single fish in a sample file of 2 min. Track is drawn by the Software Ethovision (NoldusTM, the Netherland).
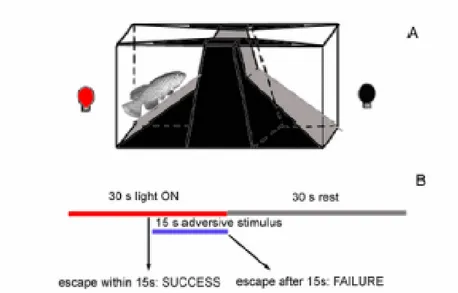
Active Avoidance Task
A researcher of our group, D.R. Valenzano, modified a learning task developed for gold fish and zebrafish, based on a shuttle-box (Fig 2.2A) consisting in a tank (38x23x18 cm), divided in two by a hurdle with a rectangular hole (3x3 cm). The two compartments were wedged-shaped to funnel the fish through the hurdle. The tank was filled with water from the housing tank and the fish was left to acclimate for 15 minutes before starting the test. Then, the conditioned stimulus (red light) was delivered in the compartment where the fish was present and it was followed by an aversive stimulus (a plastic stick whirling in the compartment). The fish always responded to the disturbance by moving to the other compartment. The aim of the test was to detect the acquisition of a strategy to escape from the aversive stimulus by crossing the hurdle upon presentation of the conditioned stimulus. The conditioned stimulus lasted for 30s. If the fish did not move to the other compartment after 15s, the adversive stimulus was delivered for 15s. The fish moved to the other compartment, resting for 30s and then the cycle was repeated. If the fish crossed the hurdle within 15s (i.e. before the onset of the red light conditioned stimulus), the trial was scored as “success”, otherwise it
was scored as “failure” (Fig 2.2B). A complete session consisted of 50 consecutive trials.
Figure 2.2: Scheme of the system used for scoring the Active Avoidance learning task. (A) Shematic representation of the shuttlebox used for training and testing. (B) Temporal structure of the test.
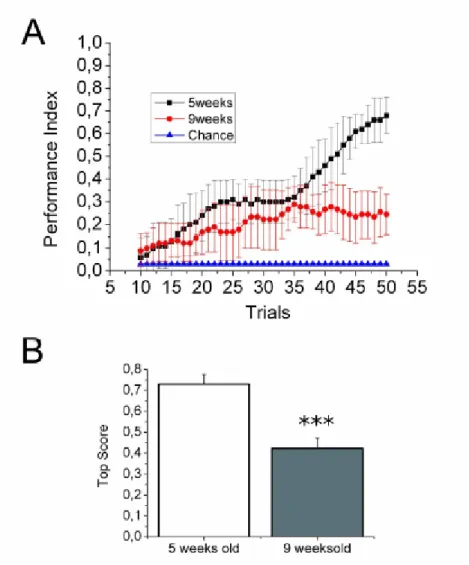
Two indexes were scored for assessing learning in each experimental group. The first measure was Performance Index, used for visualizing the evolution of the performance in function of the trial. Successful trials were scored as 1, failures with 0. For each trial, the average score was computed as follows: for example, for trial 1 in n fish, the Average Score is:
score as follows: PI(10-50) = AS1-10,AS2-11, ...,AS41-50. The chance level against which to compare the obtained measures is the average score of the first trial (i.e. before the occurrence of the conditioning) of all tested fish. This is a measure of the tendency for the fish to move to the other compartement in response to the light onset, without any conditioning. Another index used was the Top Score Index (TSI) , which measured the mean of the highest scores reached by individuals of one group during 50 trials. Compared to the Performance Index, the Top Score Index provides an absolute measure of ability to succeed in the task, independently from the trial. TSI is computed as the mean, for all the individuals in an experimental group, of:
3. Results
3.1 Aging in GRZ inbred Nothobranchius furzeri strain
3.1.1 Life-history and growth
A representative survival curve of inbred Nothobranchius furzeri GRZ strain is shown in Fig 3.1A. The graph represents the percentage of fish that survive from the fourth week of life until the death of the last animal. At four weeks, fish are sexual mature as males show typical adult coloration (Genade et al. 2005) and females start to lay fertilized eggs in the chosen substrate (see Method chapter).
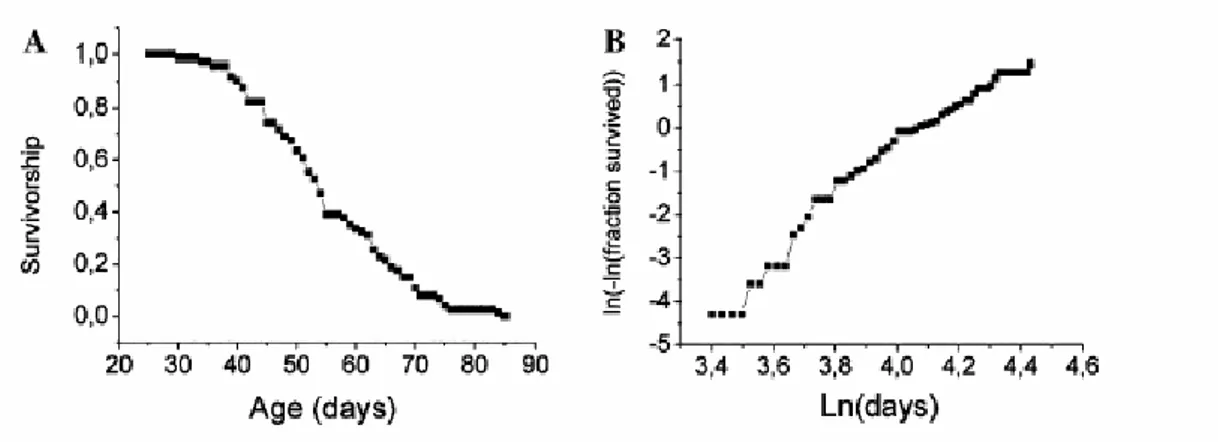
A logistic fit of mortality is shown in Fig 3.1B, to visualize the exponential increase in death rate occurring with age (Finch et al. 1990; Gavrilov and Gavrilova 2001). In demographic studies, mortality as a function of time is described as M(t) = Aeαt, where M(t) is mortality rate at time t, A is aggregate environmental danger and α is the rate constant for age-related increase in mortality. This parameter α, which is visualized as the slope of the logistic fit, is defined as aging rate of a population. One informative measure of the aging rate is the time needed for doubling mortality rate (DMR). For N.
furzeri this value is 0.483 weeks, which means that in less than half a week
the mortality rate doubles. This same index is 14.08 weeks for the mouse and 15.64 weeks for the rat (Finch et al. 1990), i.e. if we assume DMR to be a measure of aging, N. furzeri ages about 30 times faster than laboratory mice and rats.
Figure 3.1: Age-specific mortality in Nothobranchius furzeri, strain Gona Re Zhou. The survivorship curves were obtained by recording the number of dead fish every day. Total number of fish n=74. (A) Survivorship is plotted on the Y axis and age in days on the X axis. (B) Logistic fit of the mortality data.
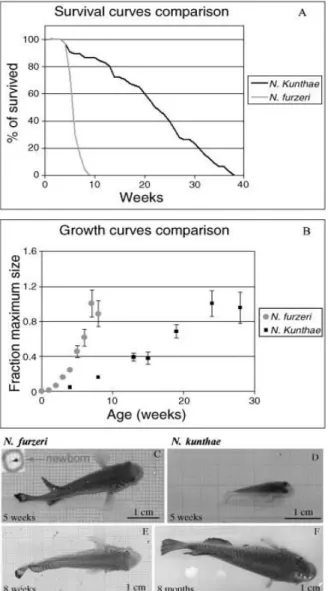
A comparison between survivorship and growth rate in two species of the genus Nothobranchius is presented in Fig 3.2 and illustrates the high variability in life expectancy present in annual killifish. Inbred GRZ N. furzeri and wild derived N. kunthae strongly differ for life expectancy in the same culture conditions, as the second one lives at least 3 times longer than the first one (Fig 3.2 A,B). Interestingly, measuring growth rates in this two species, it appears that N. furzeri reaches its plateau size significantly before N.
orthonotus var. kunthae, which grows much slower but eventually reaches a