UNIVERSITÀ DEGLI STUDI DI SASSARI CORSO DI DOTTORATO DI RICERCA
Scienze Agrarie
Curriculum in monitoraggio e controllo degli ecosistemi agrari e forestali in ambiente mediterraneo
Ciclo XXIX
Social Immunity in honeybee: behavioral, chemical and
microbiological aspects
dr. Michelina Pusceddu
Coordinatore del Corso Prof. Antonello Cannas
UNIVERSITÀ DEGLI STUDI DI SASSARI CORSO DI DOTTORATO DI RICERCA
Scienze Agrarie
Curriculum in monitoraggio e controllo degli ecosistemi agrari e forestali in ambiente mediterraneo
UNIVERSITÀ DEGLI STUDI DI SASSARI CORSO DI DOTTORATO DI RICERCA
Scienze Agrarie
Curriculum in monitoraggio e controllo degli ecosistemi agrari e forestali in ambiente mediterraneo
Ciclo XXIX
La presente tesi è stata prodotta durante la frequenza del Corso di Dottorato di ricerca in Scienze Agrarie dell’Università degli Studi di Sassari, A.A. 2013/2014 - XXIX ciclo, con il sostegno di una borsa di studio finanziata con le risorse dell’INPS – Gestione Ex INPDAP nell’ambito delle Iniziative Accademiche Homo Sapiens Sapiens.
Michelina Pusceddu presents its sincere thanks to the INPS - Gestione Ex INPDAP for the financial support of her PhD scholarship (funded by Homo Sapiens Sapiens Academic Initiatives).
This study was financially supported by the Italian ministry of education, University and Research (MIUR, Research Project PRIN 2012); “Social Immunity in honeybee: behavioral, chemical and microbiological aspects”.
THESIS INDEX
1. Introduction (Defensive strategies in honeybees) 1.1.Individual immunity system
1.2.Social immunity system
1.2.1. Preventive defenses
1.2.2. Medication or curative defenses 1.3. References
2. Agonistic interation between Apis mellifera and Vespula
germanica in a mediterranean environment
2.1. Introduction
2.2. Materials and Methods
2.2.1. Experimental apiary 2.2.2. Behavioral observations
2.2.3. Effect of predator attacks on bee foraging activity 2.2.4. Agonistic support 2.3.Ethogram 2.3.1. Wasp attack 2.3.2. Nest defense 2.4. Statistical analysis 2.5. Results 2.5.1. Wasp attack 2.5.2. Nest defense 2.5.3. Disturbance of foraging 2.5.4. Agonistic support 2.6. Discussion and Conclusions
2.7. References
2.8.Tables and Figures
3. Resin foraging dynamics in Varroa destructor infested hives. A
Pag. 1 Pag. 1 Pag. 4 Pag. 4 Pag. 5 Pag. 7 Pag. 13 Pag. 14 Pag. 15 Pag. 15 Pag. 15 Pag. 16 Pag. 17 Pag. 17 Pag. 17 Pag. 18 Pag. 19 Pag. 20 Pag. 20 Pag. 20 Pag. 21 Pag. 21 Pag. 21 Pag. 24 Pag. 29
3.2. Materials and Methods 3.2.1. Experimental apiary 3.2.2. Experiments
3.2.3. Resin foragers detection and Chemical analysis of propolis
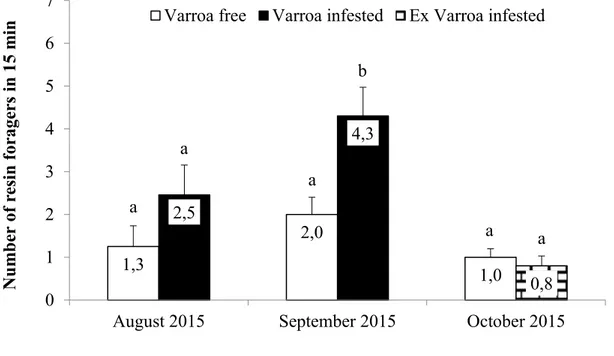
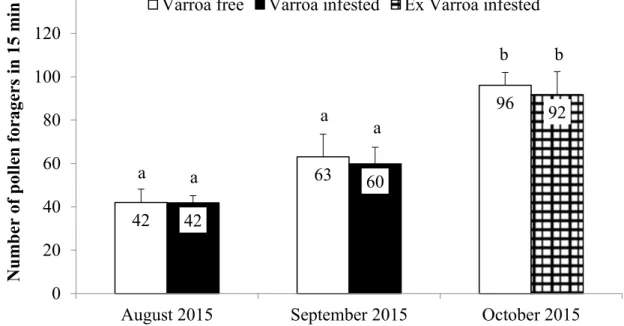
3.3. Statistical analysis 3.4. Results
3.4.1. Experiments 3.4.2. Chemical analyses 3.5. Discussion and Conclusions 3.6. References
3.7. Tables and Figures
4. Quantitative variations in the core bacterial community associated with honey bees from Varroa-infested colonies
4.1. Introduction
4.2. Materials and Methods
4.2.1. Sample collection and processing
4.2.2. RNA extraction, retro-transcription and relative quantification of immune-related genes
4.2.3. DNA extraction and relative quantification of the core bacterial community
4.3. Statistical analysis 4.4. Results
4.4.1. Varroa infestation and colony strength
4.4.2. Relative abundance of the Deformed Wing Virus (DWV) and of the overall bacterial community
4.4.3. Relative expression of immune-related genes in honeybees from infested and non infested colonies 4.4.4. Relative abundance of core bacterial community in
honeybees from infested and non-infested colonies 4.4.5. Relative expression of immune-related genes in
Pag. 36 Pag. 36 Pag. 36 Pag. 38 Pag. 39 Pag. 40 Pag. 40 Pag. 42 Pag. 42 Pag. 46 Pag. 53 Pag. 61 Pag. 62 Pag. 63 Pag. 63 Pag. 64 Pag. 65 Pag. 66 Pag. 66 Pag. 66 Pag. 67 Pag.67 Pag. 68
4.4.6. Relative abundance of core bacterial community in in emerging adults bearing sucking mites
4.5. Discussion and Conclusions 4.6. References
4.7. Tables and Figures 5. Aknowledgement Pag. 69 Pag. 69 Pag. 73 Pag. 78 Pag. 86
1. INTRODUCTION
Defensive strategies in honeybees
Colonies of social insects can be compared by a number of similarities to multicellular organisms that, due to their level of organization, have been conceptualized, since the early 1900s, as superorganisms (Wheeler, 1911).
A century later, Strassmann & Queller (2010) proposed the definition of multicellular organism for all biological entities in which components contribute in ensuring the survival of the overall group members, as also occurs in honeybee colonies (Moritz & Southwich, 1992; Thauz, 2008). In these insects, one of the main areas of cooperation among individuals is that toward the development of a social immune system of the colony. The “social immunity” is the set of collective defense mechanisms implemented by bees to combat predators, parasites and pathogens, which constantly threaten the survival of the hive (Cremer et al., 2007). In addition to the collective defense mechanisms used by Apis
mellifera, individual defense systems can also be used simultaneously, increasing thus the
resistance or tolerance against the hive intruders (Cremer & Sixt, 2009). Resistance is the ability of a system to resist infection through mechanical, chemical and physiological barriers, as well as appropriate defense responses once the infection has occurred. Conversely, tolerance against pathogens or parasites is the ability of hosts to withstand the infection and/or infestation, and is ensured by a compromise between energy costs, damages and immune response of the insect (Baracchi & Turillazzi, 2014).
1.1. Individual Immunity System
Honeybees have fewer genes involved in the immune response than several species of solitary insects, and hence this implicates less flexibility in the ability to recognize and resist pathogens (Evans et al., 2006; Weinstock et al., 2006). This finding suggests that individual defenses in social insects may be compensated by the collective defense mechanisms that emerge at the colony level (Cremer et al., 2007). Furthermore, reproduction affects the immune defenses on the level of inter- and intra-colony variability; therefore the queen bee is assumed to mate multiple times to achieve higher genetic variability, thus increasing resistance to diseases (Seeley & Tarpy, 2007; Tarpy, 2003).
The first line of individual defense in bees consists of mechanical barriers, represented by the integument (epicuticular layer, cuticle, epidermis, basal membrane), as well as the internal layers of the gut (peritrophic matrix and epithelium) that disfavor adhesion and penetration of pathogens in the organism. Another line of individual defense consists of physiological inhibitors that induce pH variations, and glandular secretions with bactericidal and/or fungicidal action such as those produced by salivary, mandibular and hypopharyngeal glands (Crailsheim & Riessberger-Galle, 2001).
Once these system defenses are breached, cellular and humoral responses are activated. The cellular immunity is the action of haemocytes to recognize, engulf and neutralize foreign bodies. Indeed, the total count of these cells allows to estimate the cellular immunocompetence of an individual (Williams, 2007; Wilson-Rich et al., 2008). On the other hand, the humoral response consists in the production of antimicrobial substances (peptides and proteins) and in retrieving an increased production of hemocyte cells, and is quantified by the number of fatty substances from which peptides and proteins with antimicrobial activity are mainly synthesized (Evans et al., 2006). At present, four antimicrobial peptides have been identified in honeybee: apidaecin, abaecin, hymenoptaecin and defensins. In particular, the defensins are presented by two isoforms, defensin 1 and 2. Defensin 1 is produced by the salivary glands and is involved in the social immune system of the honeybee. In contrast, defensin 2 is produced by the adipocytes and haemocytes, and it is therefore a component of the individual immunity (Ilyasov et al., 2012).
In addition, the production of antimicrobial peptides with a gradually higher bactericidal capacity has been crucial for the evolution of sociality in honeybees. Indeed, the antimicrobial agents of the most primitive semi-social species of Apoidea are stronger than those found in solitary species (Stow et al., 2007). This suggests that over the course of evolution and with the increase of both group size and affinity relations among individuals, there has been a tipping point in which disease control has become an imperative necessity (Stow et al., 2007). This reinforces the assumption that the presence of individual defense mechanisms in social insects are not to be considered as separate entities from social immune systems.
A further individual defensive strategy in honeybees is ensured by intestinal symbionts located in the rear intestine acquired in the first 3-5 days after emerging due to the interactions between different individuals of the colony (Anderson et al., 2016). Behaviors
such as cell cleaning, grooming, trophallaxis and oral-fecal route are essential in emerging bees to enhance the composition of the adult intestinal microbial community (Martinson et
al., 2012; Powell et al., 2014). Most of the strains isolated in honeybees belong to the genera Lactobacillus, Bifidobacterium and Bacillus (Alberoni et al., 2016). The intestinal bacterial
flora and in particular the host-symbiotic bacteria make a positive contribution to nutrition, immunity and physiology (Hamdi et al., 2011). The nutritional support to the host is due to the fact that some symbiotic species have genes that encode enzymes that are involved in the decomposition of lignin and cellulose, which are essential in a plant-based diet for energy absorption (Newton et al., 2013). Furthermore, the microbial flora produces other nutrients needed by the host as fatty acids, amino acids, vitamin B and secondary metabolites (Brodschneider & Crailsheim, 2010; Gündüz & Douglas, 2009). The protection against pathogens and/or parasites is yet another important trait frequently associated to a balanced intestinal flora. Indeed, a significant contribution to the host protection is provided by the antagonist activity of intestinal flora and its interaction with humoral and systemic immunity
(Hedges et al., 2008; Jaenike et al., 2010). More specifically, microorganisms may play a
role in the protection of their host by either stimulating the immune system of bees or inhibiting pathogens and parasites through the production of antimicrobial compounds (Alberoni et al., 2016). For example, the results of Sabaté et al. (2009) pointed out that, in honeybees, there is antagonistic action of endogenous bacteria against Paenibacillus larvae and Ascosphaera apis.
In general, the host-microbe interaction in social insects is the result of a long process of coevolution closely related to the stage of development, temporal polytheism and transmission through social interactions (Hughes et al., 2008). Several stress factors such as nutritional deficiencies, pesticides, parasites or pathogens can cause immunosuppression, leading thus to an alteration of the composition of the microbiota (Alberoni et al., 2016). The effects of the parasitic mite V. destructor on both the qualitative and quantitative composition of the microbiota of the individual bee and the entire colony will be treated in the fourth chapter of this thesis.
1.2. Social Immunity System
Social immunity systems refer to all collective defense mechanisms that bees and other colonies of social insects have evolved to combat the increased risk of disease transmission that arises from both social interactions and group living, and are the result of cooperation between each member of the colony (Cremer et al., 2007). Some of these defense systems are preventative and are intended to restrict the transmission of diseases within the nest, whereas others are activated in case of need, when pathogens and/or pests have already penetrated into the hive. High population density, frequent physical contact between nest mates as well as a reduced genetic variability are the factors that threaten the survival of social insects. Nevertheless, social wasps have exhibited a high degree of adaptation to different environments confirming thus the effectiveness of the defense strategies evolved against pathogens (Wilson, 1971). This biological success points out that social interactions, in addition to placing individuals at risk of disease, can lead to the research of new collective defense strategies as a result of coevolutionary dynamics between host and microorganisms (Baracchi & Turillazzi, 2014). This phenomenon creates indeed a balance between both entities, characterized by constant adaptations and mutual counter-adaptations.
1.2.1. Preventive Defenses
The typical organization of social insects characterized by caste divisions of various duties, temporal polyethism and spatial division of the nest represents the first preventive defense as it regulates and limits the contact among individuals, and has been defined as “organizational immunity” (Cremer et al., 2007; Naug & Smith, 2007). For instance, in ants and bumblebees, workers of the same age perform the same duties within the nest, starting their labor with nurse tasks in the center of the colony, and then drift progressively towards the periphery of the nest (Bourke & Frank, 1995; Jandt & Dornhaus, 2009). In case of a new disease transmitted by contact, this behavior, so named “centrifugal polyethism”, will clearly limit the disease spread within the colony (Bourke & Frank 1995; Jandt & Dornhaus 2009). This was also demonstrated in A. mellifera, where the interaction pattern among the individuals of a colony, at both social and spatial levels, follows the theoretical “compartment model” in which old foragers are at the outer edges, being the most exposed to external pathogens, while young bees are present in the inner area of the nest (i.e. more protected) tending the queen and brood (Baracchi & Cini, 2014).
The ability of the beekeeper to recognize diseased or parasitized individuals at the entrance of the hive prevents the entrance of pathogens and/or parasites inside the nest (Drum & Rothenbühler, 1985; Waddington & Rothenbühler, 1976). Moreover, one of the main collective defenses of social insect colonies is the use of antimicrobial secretions directly produced by insects or collected from the environment (Sadd & Schmid-Hempel, 2006). For instance, the venom gland present in ants, wasps and bees ensures the production of compounds with antimicrobial activity and represents one of the most important sources of antimicrobial compounds produced in social Hymenoptera species (Kuhn-Nentwig, 2003). The use of venom as “external immunity” has been demonstrated in ants (Tragust et al., 2013). In honeybees, the hypothesis that the venom is not only used against predators but also against pathogens and/or parasites, is supported by the antimicrobial properties of its components, in particular the melittin (Kuhn-Nentwig, 2003). Moreover, the presence of venom increases in the comb of bees nesting in cavities, which would make them more exposed than species living out of the nesting shelters (Baracchi et al., 2011). In addition, it is customary, in ants and bees, to disinfect their own nest with substances collected from the environment, which exhibit antimicrobial activity, such as fragments of solidified coniferous resin in the case of Formica paralugubris (Christe et al., 2003) or propolis in the case of A.
mellifera (Simone et al., 2009). Currently, an important scientific debate is undergoing on
the possibility that the collection of resin and use of propolis in the hive are not to be considered as exclusively a preventive defense behavior, but could be used as a self-medication behavior among individuals (Simone-Finstrom & Spivak, 2012). This topic will be the subject of extensive discussion in the third chapter of the thesis.
1.2.2. Medication or Curative Defenses
One of the main collective defense strategies in honeybees is the social fever, characterized by the increase of temperatures within the nest induced by adult bees that block the development of diseases caused by heat-sensitive pathogenic microorganisms (Starks et al., 2000). This behavior has been demonstrated as a response to Ascosphaera apis (Starks et
al., 2000). Similarly, one of the most commonly used defenses to combat ectoparasites is the
“grooming” behavior, defined as the removal of ectoparasites from the bee’s body and occurring in two types: self-grooming and allo-grooming (Pettis & Pankiw, 1998). Studies demonstrated that grooming is a frequent behavior in Apis cerana, with a good percentage
of Varroa destructor killed after removal (35%) (Buchler et al., 1992). In contrast, grooming in A. mellifera is rare, although this behavior increased in infested colonies (Buchler et al., 1992) with a percentage of 17% and 29% of Varroa killed after removal in European and Africanized bees, respectively (Invernizzi et al., 2015). In social insects, a key role is also assigned to the hygienic and the so called “undertaker” behaviors, which consist in detecting and removing diseased and parasitized brood and corpses from the nest, as well as in the maintenance of the latter from waste material (Arathi et al., 2000); Baracchi et al., 2012; Spivak & Gilliam, 1998a, b). Eventually, an extreme form of colony defense called “altruistic strategy” consists in the suicide of a diseased or parasitized group member who decides to leave the nest, preventing thus the spread of a pathogen (Rueppell et al., 2010). In ants, specifically in the genus Temnothorax, foragers cease any kind of social contact and abandon the colony when infected by entomopathogenic fungi (Heinze & Walter, 2010). Similarly, bees presenting deformations (i.e. fringed wings) are removed or leave voluntarily the nest (Rueppell et al., 2010). Moreover, in A. cerana, it was recently shown that the “altruistic suicide” defined as “social apoptosis” occurs also in immature workers besides adult bees (Page et al., 2016). The absconding behavior is another factor contributing to the reduction of the parasite load in the hive, as observed for the coleopteran species Aethina
tumida (Ellis et al., 2003). In addition, once healthy termites identify an infected colony
member, they produce vibratory alarm signals in order to isolate and surround the individual by constructing additional walls (Myles, 2002; Rosengaus et al., 1999).
The behavioral defenses that honeybees have evolved against wasps, their natural enemy, need further consideration. Indeed, as seen so far, defenses against parasites, pathogens and predators can be divided in two similar levels: preventive (warning signals) and effective (agonistic response to attack) defenses. Of warning signals, those of chemical type, namely the emission of insect-alarming pheromone, stimulate an aggressive response in the nest mates (Collins & Kubasek, 1982). Conversely, vibrational signals cause a bioacoustic effect that temporarily interrupts feeding of the colony under attack (Tan et al., 2016). Other warning signals are of visual type, such as “shimmering” in which bees would flip their abdomens upwards, producing waves once in contact with the wasp at the nest entrance (Tan
et al., 2012a). Such warning signals may induce the closest bees to the predator to change
their behavior until gasping the wasp (Seeley et al., 1982). As a matter of fact, the best-known example in collective defenses against a predator is represented by “balling”, which
consists in the formation of a winter cluster of honeybees around the predator, which is bound to succumb due to thermal increase (heat-balling) (Ono et al., 1995). This phenomenon may occur because wasps and hornets have a lower lethal thermal threshold than bees (Papachristoforou et al., 2007). In the genus Apis, this behavior was described in various species such as A. cerana, A. mellifera and Apis dorsata, but with significant differences in the heat-generating mode, duration (Tan et al., 2005), temperature reached, number of bees involved and sacrificed (Tan et al., 2012b; 2016). High temperature within the winter cluster could also not reach the lethal threshold for wasps and hornets, however they will perish due to an increase of CO2 in hemolymph (asphyxia-balling) (Papachristoforou et al., 2007; Sugahara & Sakamoto, 2009). Nevertheless, honeybees do not always show collective responses to wasp attacks, and sometimes, in case of a worker bee under attack, only one or few group companions, called “helpers”, will intervene. Such case is called “agonistic support”, which is considered a classic example of altruistic behavior in which an individual is involved in the support of another group companion in conflict, facing therefore a potential risk while the recipient reaps the benefits of the support given (Schino et al., 2007). Relative to balling, agonistic support “given by a few individuals” has been less studied in insects and will be subject of detailed study in the second chapter of the thesis.
1.3. REFERENCES
Alberoni, D., Gaggia, F., Baffoni, L., Di Gioia, D. (2016) Beneficial microorganisms for honey bees: problems and progresses. Applied Microbiology Biotechnology, 100, 9469-9482.
Anderson, K.E., Rodrigues, P.A., Mott, B.M., Maes, P., Corby-Harris, V. (2016) Ecological succession in the honey bee gut: shift in Lactobacillus strain dominance during early adult development. Microbial Ecology, 71, 1008–1019.
Arathi, H.S., Burns, I., Spivak, M. (2000). Ethology of hygienic behaviour in the honey bee
Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of hygienic bees. Ethology 106, 365–379.
Baracchi D., Francese, S., Turillazzi, S. (2011) Beyond the antipredatory defence: honey bee venom function as a component of social immunity. Toxicon, 58, 550-557.
Baracchi, D., Fadda, A., Turillazzi, S. (2012) Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. Journal Insect Physiology, 58, 1589-1596.
Baracchi, D., & Cini, A. (2014). A Socio-Spatial combined approach confirms a highly compartmentalised structure in honeybees. Ethology, 120, 1167‒1176.
Baracchi, D., & Turillazzi, S. (2014) Patologia e avversità dell’alveare. Springer-Verlag Italia.
Brodschneider, R., & Crailsheim, K. (2010) Nutrition and health in honey bees. Apidologie, 41, 278–294.
Bourke, A.F.G., & Frank, N.R. (1995) Social Evolution in ants. Monographs in behavior an ecology. Princeton University Press.
Büchler, R., Drescher, W., & Tornier, I. (1992) Grooming behaviour of Apis cerana, Apis
mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae. Experimental and applied Acarology, 16, 313-319.
Collins, A. M., & Kubasek, K. J. (1982) Field test of honey bee (Hymenoptera: Apidae) colony defensive behavior. Annals of the Entomological Society of America, 75, 383-387.
Crailsheim, K., & Riessberger-Galle, U. (2001) Honey bee age-dependent resistance against American foulbrood. Apidologie, 32, 91-103.
Cremer, S., Armitage, S.A.O., & Schmid-Hempel, P. (2007) Social immunity. Current
Biology, 17, 693-702.
Cremer, S., & Sixt, M. (2009) Analogies in the evolution of individual and social immunity.
Proceedings of the Royal Society B, 364, 129-142.
Christe, P., Oppliger, A., Bancala, F., Castella, G., and Chapuisat, M. (2003) Evidence for collective medication in ants. Ecology Letters, 6, 19–22.
Drum, N.H., & Rothenbuhler, W.C. (1985). Differences in nonstinging aggressive responses of worker honeybees to diseased and healthy bees in May and July. Journal of
Apicultural Research, 24, 184–187.
Ellis, J. D., Hepburn, R., Delaplane, K. S., & Elzen, P. J. (2003) A scientific note on small hive beetle (Aethina tumida) oviposition and behaviour during European (Apis
mellifera) honey bee clustering and absconding events. Journal of Apicultural Research, 42, 47-48.
Evans, J.D., Aronstein, K., Chen, Y. P., Hetru, C., Imler, J. L., Jiang, H., et al. (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect
Molecular Biology, 15, 645-656.
Gündüz, E.A., Douglas, A.E. (2009) Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proceeding of the Royal Society of London B: Biological Sciences, 276, 987–991.
Hamdi, C., Balloi, A., Essanaa, J., Crotti, E., Gonella, E., Raddadi, N., Ricci, I., et al. (2011) Gut microbiome dysbiosis and honeybee health. Journal of Applied Entomology, 135, 524–533.
Hedges, L.M., Brownlie, J.C., O’Neill, S.L., Johnson, K.N. (2008) Wolbachia and virus protection in insects. Science 322, 702–702.
Hughes, D.P., Pierce, N.E., Boomsma, J.J. (2008) Social insect symbionts: evolution in homeostatic fortresses. Trends in Ecology and Evolution, 23, 672–677.
Heinze, J., & Walter, B. (2010). Moribund ants leave their nests to die in social isolation. Current Biology, 20, 249-252.
Ilyasov, R.A., Gaifullina, L.R., Saltykova, E.S., Poskryakov, A.V., Nicolenko, A.G. (2012) Rewiew of the expression of antimicrobial peptide defensin in honey bees Apis
mellifera L. Journal of Apicultural Science, 56, 115-124.
Invernizzi, C., Zefferino, I., Santos, E., Sánchez, L., & Mendoza, Y. (2015) Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. Journal of Apicultural Research, 54, 321-327.
Jaenike, J., Unckless, R., Cockburn, S.N., Boelio, L.M., Perlman, S.J. (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329,212-215. Jandt, J.M., & Dornhaus, A. (2009) Spatial organizzation and division of labour in
bumblebee Bombus impatiens. Animal behaviour, 77, 641-651.
Kuhn-Nentiwig, L. (2003) Antimicrobial and cytolytic peptides of venomous arthropods.
Cellular and Molecular Life Sciences, 60, 2651-2668.
Myles, T.G. (2002) Alarm, aggregation, and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology, 40, 243-255. Martinson, V.G., Moy, J., Moran, N.A. (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Applied & Environmental Microbiology, 78, 2830–2840.
Moritz, R.F., & Southwick, E.E. (1992) Bees as superorganisms: an evolutionary reality. Springer Verlag, Berlin Heidelberg New York.
Naug, D., & Smith, B. (2007) Experimentally induced change in infectious period affects transmission dynamics in a social group. Proceedings of the Royal Society B, 274, 61-65.
Newton, I.L., Sheehan, K.B., Lee, F.J., Horton, M.A., Hicks, R.D. (2013) Invertebrate systems for hypothesis-driven microbiome research. Microbiome Science and
Medicine, 1(1), doi:10.2478/micsm-2013-0001.
Ono, M., Igarashi, T., Ohno, E., & Sasaki, M. (1995) Unusual thermal defense by a honeybee against mass attack by hornets. Nature, 377, 334-336.
Pettis, J.S., & Pankiw, T. (1998) Grooming behavior by Apis mellifera L. in the presence of
Acarapis woodi (Rennie) (Acari: Tarsonemidae). Apidologie, 29, 241-253.
Sadd, B., & Schmid-Hempel, P. (2006) Insect immunity shows specificity in protection upon secondary pathogen exposure. Current Biology, 16, 1206-1210.
Seeley, T.D., & Tarpy, D. (2007) Queen promiscuity lowers disease within honeybee colonies. Proceeding of the Royal Society B, 274, 67-72.
Simone-Finstrom, M.D., & Spivak, M. (2012) Increased resin collection after parasite challenge: a case of self-medication in honey bees? PLoS ONE, 7, e34601.
Simone, M., Evans, J.D., & Spivak, M. (2009) Resin collection and social immunity in honey bees. Evolution, 63, 3016–3022.
Starks, P.T., Blackie, C.A., & Seeley, T.D. (2000) Fever in honeybee colonies.
Naturwissenschaften, 87, 229-231.
Spivak, M., & Gilliam, M. (1998a) Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa: Part I. Hygienic behaviour and resistance to American foulbrood. Bee world, 79(3), 124-134.
Spivak, M., & Gilliam, M. (1998b) Hygienic behaviour of honey bees and its application for control of brood diseases and Varroa: Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee world, 79(4), 169-186.
Stow, A., Briscoe, D., Gillings, M., Holley, M., Smith, S., Leys, R., et al. (2007). Antimicrobial defences increase with sociality in bees. Biology Letters, 3, 422-424. Strassmann, J.E., & Queller, D.C. (2010) The social organism: congresses, parties, and
Page, P., Lin, Z., Buawangpong, N., Zheng, H., Hu, F., Neumann, P., et al. V. (2016) Social apoptosis in honey bee superorganisms. Scientific reports, 6, 1-6.
Papachristoforou, A., Rortais, A., Zafeiridou, G., Theophilidis, G., Garnery, L., Thrasyvoulou, A., et al (2007). Smothered to death: hornets asphyxiated by honeybees.
Current Biology, 17, 795-796.
Powell, J.E., Martinson, V.G., Urban-Mead, K., Moran, N.A. (2014) Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Applied and Environmental
Microbiology, 80, 7378–7387.
Rosengaus, R.B., Jordan, C., Lefebvre, M.L., and Traniello, J.F.A. (1999) Pathogen alarm behaviour in a termite: a new form of communication in social insects.
Naturwissenschaften, 86, 544–548.
Rueppell, O., Hayworth, M. K., & Ross, N. P. (2010). Altruistic self‐removal of health‐ compromised honey bee workers from their hive. Journal of evolutionary
biology, 23(7), 1538-1546.
Sabaté, D.C., Carrillo, L., Audisio, M.C. (2009) Inhibition of Paenibacillus larvae and
Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Research in Microbiology, 160, 193–199.
Schino, G., Polizzi di Sorrentino, E., & Tiddi, B. (2007) Agonistic support in juvenile japanese macaques: cognitive and functional implications. Ethology, 113, 1151-1157. Seeley, T.D., Seeley, R.H., & Akratanakul, P. (1982) Colony defense strategies of the honey
bees in Thailand. Ecological Monographs, 52, 43-63.
Sugahara, M., & Sakamoto, F. (2009) Heat and carbon dioxide generated by honeybees jointly act to kill hornets. Naturwissenschaften, 96, 1133-1136.
Tan, K., Hepburn, H.R., Radloff, S.E., Yusheng, Y., Yiqiu, L., & Danyin, Z. (2005) Heat-balling wasps by honeybees. Naturwissenschaften, 92, 492-495.
Tan, K., Wang, Z., Li, H., Yang, S., Hu, Z., Kastberger, G., & Oldroyd, B.P. (2012a) An ‘I see you’ prey–predator signal between the Asian honeybee, Apis cerana, and the hornet, Vespa velutina. Animal Behavior, 83, 879-882.
Tan, K., Yang, MX., Wang, Z.W., Li, H., Zhang, Z.Y., Radloff S.E, et al. (2012b) Cooperative wasp-killing by mixed-species colonies of honeybees, Apis cerana and
Tan, K., Dong, S., Li, X., Liu, X., Wang, C., Li, J., & Nieh, J.C. (2016) Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biology, 14, e1002423. Doi:10.1371/jounal.pbio.1002423.
Tarpy, D. (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proceeding of the Royal Society B, 270, 99-103.
Tautz, J. (2008) The buzz about bees: biology of a superorganism. Springer Verlag, Berlin Heidelberg New York.
Tragust, S., Mitteregger, B., Barone, V., Konrad, M., Ugelvig, L.V., Cremer, S. (2013) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol, 23, 76-82.
Waddington, K.D., & Rothenbuhler, W.C. (1976). Behaviour associated with hairless-black syndrome of adult honeybees. Journal of Apicultural Research, 15, 35–41.
Weinstock, G., Robinson, G., Gibbs, R., et al. (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature, 443, 931-949.
Williams, M.J., (2007) Drosophila hemopoiesis and cellular immunity. Journal
Immunology, 178, 4711-4715.
Wilson, E.O. (1971) The insect societies. Cambridge, Massachussetts: Harvard University Press.
Wilson-Rich, N., Dres, S.T., Starkd, P.T. (2008) The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). Journal of insect
Physiology, 54, 1392-1399.
CHAPTER 2
Agonistic interactions between the Italian honeybee (Apis mellifera ligustica) and the European wasp (Vespula germanica) reveal context-dependent defense strategies
Michelina Pusceddua, Ignazio Florisa, Franco Buffaa, Emanuele Salarisa, Alberto Sattaa
aDipartimento di Agraria, Sezione di Patologia Vegetale ed Entomologia, Università degli Studi di Sassari, Viale Italia 39, 07100, Sassari
2.1. INTRODUCTION
Cooperation among individuals is observed in many phylogenetically diverse taxa and has underpinned the evolution of sociality in the animal kingdom (Szathmary & Maynard Smith, 1995; Wilson, 1975). The main advantages of sociality, promoted by natural selection, include more efficient vigilance against predators, a better ability to identify food sources, and the greater survival of developing brood. However, life within a group also presents certain disadvantages, one of the most significant being the ease with which predators can detect prey. For this reason, nest protection to reduce vulnerability is another central aspect in the evolution of sociality (Hermann, 1984; Shorter & Rueppell, 2012). Eusocial insects such as the honeybee (Apis mellifera) adopt numerous general and behavioral defense mechanisms against their predators. General mechanisms include nest architecture, site and visibility, as well as species-dependent morphological adaptations such as the size of an individual (Seeley et al., 1982). In contrast, behavioral defenses are specific to particular enemies and require the prior identification of the predator based on olfactory, visual or tactile cues, recognition of movement, and information from previous encounters (Breed et
al., 2004; Tan et al., 2012a; Wood & Ratnieks, 2004). Behavioral defense can also depend
on agonistic behavior by the invader during an encounter, and in eusocial insects, on the caste to which the occupant and/or intruder belong (Breed et al., 1978). In the latter case, individuating and blocking specific predators in honeybee societies is the responsibility of guard bees. These bees adopt specialized behaviors that dissuade attacks by invertebrate predators and conspecifics from other colonies, thus preventing the loss of food and brood, and they also recruit “soldiers” to defend the nest against more aggressive predators (Breed
et al., 1990; 1992) Defense behavior in the Asiatic honeybee (Apis cerana) was recently
shown to vary not only according to the predator, but also based on the context in which the attack takes place, e.g. minimal danger caused by an attack on a single forager contrasting with the substantial threat caused by an attack at the nest entrance (Tan et al, 2016).
Wasps are major invertebrate enemies of honeybees, invading hives to steal honey, pollen, larvae and adults to provide sugar and protein for themselves and their offspring (Baracchi
et al., 2010; Matsuura & Sakagami, 1973). Current research focuses on the defense
mechanisms used by A. mellifera against the Asian predatory wasp (Vespa velutina) due to its predatory success (Tan et al., 2007) and the damage caused by its introduction into Europe
(Monceau et al., 2014). However, the study of relationships between sympatric species is also necessary even though the predators are less dangerous(Markwell et al., 1993) because such relationships underpin the evolution of defense behaviors (Arca et al, 2014; De Grandi-Hoffman et al., 1998). We therefore investigated the predator–prey relationship between two sympatric species of social Hymenoptera, namely the Italian honeybee (Apis mellifera
ligustica) and the European wasp (Vespula germanica) also known as the German wasp or
German yellowjacket, in a representative area of the European Mediterranean region (Sardinia, Italy). We evaluated the effectiveness of behavioral displays of attack and defense which have co-evolved in these two species, the defense mechanisms in various danger contexts, and the real damage and disturbance caused by this predator to the honeybee colony under attack.
2.2. MATERIALS AND METHODS
2.2.1. Experimental apiary
The experimental apiary was set up in the northwest of Sardinia inside the experimental farm (latitude 40°46'23'', longitude 8°29'34'') of the Department of Agriculture of the University of Sassari, where no specific permission was required to carry out our experiments, during March 2014. The apiary comprised 18 A. mellifera ligustica colonies maintained in new Dadan-Blatt hives containing 10 combs each. They were checked every week to confirm the presence of the queen as well as pollen and nectar provisions. We also monitored the sanitary status for evidence of microbial infections and varroosis (Pappas & Thrasyvoulou, 1988). The study did not involve endangered or protected species.
2.2.2. Behavioral observations
Agonistic events between V. germanica and A. mellifera ligustica were examined in two different contexts, one at the hive entrance where defense is thought to be initiated by the guard bees and the other on the ground close to the hive where weakened and dead bees are present. The behavioral observations were based on the “all occurrences sampling” method (Altmann, 1974) in which we recorded the frequencies of a series of behavioral events as set out in the ethogram described below.
Attacks at the nest entrance were recorded in 2014 and 2015 during September and October, when the predatory activity of wasps is more intense due to their higher nutritional requirements during reproduction and rearing of offspring (Turillazzi, 2003). Each colony was recorded for two 15-min sessions per day using a Canon LEGRIA HF R506 video camera placed ~20 cm from the opening of the hives. Recordings were taken during the hottest part of the day (between 9:30 am and 15:30 pm) when the wasps were most active. A total of 279 h of video footage was recorded (63 h in 2014 and 216 h in 2015) and all 18 colonies were observed for the same duration (15.5 h). Subsequently, two operators independently screened the video recordings using a slow motion system (VLC software, v2.2.0) and the agonistic behaviors observed were used to establish an ethogram as described below. The ethogram was supplemented with further attack and defense behaviors not observed by us but reported in the literature for similar species, or in these two species facing different antagonists. This approach allowed us to evaluate the repertoire of agonistic behavior between V. germanica and A. mellifera ligustica in a larger context following an evolutionary approach. The frequency (number of events per unit of time) was reported for all the recorded attack and defense behaviors.
Attacks at ground level (only on individuals still alive and close to the hive) were monitored in 2015 on the same colonies, concurrently with some of the observations at the nest entrance. These observations were conducted by sight, without using the video camera, for a total of 32 h. Two operators simultaneously observed the ground surface under three hives in two sessions per day, each lasting 10 min. The frequency (number of events per unit of time) was reported for all the observed attack and defense behaviors.
2.2.3. Effect of predator attacks on bee foraging activity
The 15-min video clips taken at the nest entrance in each colony were used to evaluate the disturbance caused by wasps on the foraging activity of the honeybees. We compared the frequency of pollen foragers entering the hive 5 min after wasp attack (attack context)with the frequency at random times before the attack (control context) over a fixed 2-min interval. The comparisons were carried out for 27 agonistic events observed in 2015 to account for any interference that prevented us counting the number of pollen foragers, e.g. continuation of balling, successive attacks, or other bees blocking the view of the video camera.
2.2.4. Agonistic support
To determine whether there was a correlation between the degree of agonistic support and the intensity of predator aggression, all attacks at the nest entrance were divided into two behavioral categories described as threats (attacks in which the defender did not make physical contact with the predator) and fights (where physical contact was involved) (Johnson & Hubbell, 1974; Nieh et al., 2005). For each agonistic event, we recorded the duration of the attack, the number of supporters intervening to help a nestmate under attack (in the case of individual support) and any observed cases of balling.
2.3. ETHOGRAM
2.3.1. Wasp attack
Behaviors detected in this study
Attack – The wasp swoops down to the landing board or to the ground, grasps the bee from above with its forelegs and starts biting it (usually between the head and thorax).
Fight – The predator and prey are involved in a physical encounter which may include instances of biting, aggressive gripping, and spinning on a surface or in flight.
Entering the hive – A wasp may be able to enter the nest if it is overlooked by the bees, following antennation or following a struggle.
Predation – The wasp kills the honeybee. The wasp usually goes on to dismember and consume the honeybee or to carry off parts to its offspring (see below). In some cases, the wasp may also eat the contents of the honey stomach (Baracchi et al, 2010).
Sequestration – After predation, and having divided the honeybee into three parts (head, thorax and abdomen), the wasp flies off with one of them, usually the thorax (Coelho & Hoagland, 1995; Free, 1970).
Retreat – The wasp escapes when the attack has not been successful and one or more honeybees defend themselves effectively.
Coalition attack – A coalition comprising a wasp and other conspecifics launches an attack. This behavior has been reported for the Asian giant hornet (Vespa mandarinia), which has developed a strategy of group hunting: certain individuals pillage while others defend the site against conspecifics from other colonies (Matsuura, 1991; Monceau et al., 2013).
2.3.2. Nest defense
Behaviors detected in this study
Antennation or antennal boxing – This is often the first physical contact between the occupant and invader, and most likely facilitates the recognition of intruders and conspecifics (De Wroey & Pasteels, 1978). It is defined as asymmetric when a dominant and a submissive can clearly be distinguished from the behavioral display of the two opponents or symmetric when such distinction is not possible (Denis et al., 2008).
Threat – The typical behavior of a honeybee in the presence of a conspecific intruder or other predator, consisting of open mandibles and the adoption of the so-called C posture (gaster flexion with or without extension of the sting) (Breed et al., 1978).
Agonistic support – Altruistic behavior in which an individual helps another involved in a conflict, thus facing a potential risk. It may involve a single bee or several bees (supporters) that come to the aid of their nestmates.
Balling – The formation of a ball of bees around a wasp until the latter is killed or becomes harmless. In heat-balling the wasp succumbs to the heat inside the ball because hornets and wasps have a lower thermal tolerance than bees (Ono et al., 1995; Tan et al., 2012b). In
asphyxia-balling, the heat inside the ball can be lethal to the predator but it dies due to the
increased concentration of CO2 in the hemolymph which causes asphyxiation (Papachristoforou et al., 2007; Sugahara & Sakamoto, 2009).
Killing and removal of the predator – Wasps can be killed by a single bee sting or the stings or several bees, or by balling (see above). The dead or dying wasp is then removed from the nest or landing board.
Known behaviors not detected in this study
Bee carpet – A large proportion of the colony regroups on the landing board and along the sides of the hive, forming a “bee carpet” (De Grandi-Hoffman et al., 1998). This behavior
in A. mellifera ligustica has been observed against the European hornet (Vespa crabro) (Baracchi et al., 2010).
Shimmering or shaking signal – When a wasp is seen, the guard bees simultaneously vibrate their abdomens for a few seconds from side to side, emitting a loud hissing noise (De Grandi-Hoffman et al., 1998). This behavior has been observed in the giant honeybee (Apis dorsata)
(Kasterberger et al., 2008; 2012), the dwarf honeybee (Apis florea) (Seeley et al., 1982), A.
cerana (Abrol, 2006), A. cerana nuluensis (Tan et al., 2007) and A. mellifera cypria
(Papachristoforou et al., 2011) against V. velutina and the oriental hornet (Vespa orientalis), and also in A. mellifera ligustica toward V. crabro (Baracchi et al., 2010). Shimmering is considered to be a visual signal for the predator and seems to have evolved in order to dissuade the latter from attacking, i.e. it is an honest alert signal that reduces the likelihood of predator success (Kastberger et al., 2008; Tan et al., 2012a).
Interruption of foraging – The interruption of foraging activity in a colony under attack by
V. velutina has been reported in A. cerana (Tan et al., 2005) and A. mellifera cypria
(Papachristoforou et al., 2011).
Retreat into the nest – Complete retreat into the nest during an attack has been described in
A. cerana (Tan et al., 2007)and A. mellifera cypria (Papachristoforou et al., 2011), as well
as the Cape honeybee (A. mellifera capensis), the African honeybee (A. mellifera scutellata) and the Carniolan honey bee (A. mellifera carnica) (Kastberger et al., 2009).
Attack – The switch from nest defense to attack within a larger perimeter is one of the greatest differences between European and Africanized bees (Collins et al., 1982). Moreover, the response of the African line to the same stimulus is faster, more aggressive, and involves the recruitment of more nestmates for the attack (Arca et al., 2014). Attack behavior has also been observed in A. mellifera cypria towards V. orientalis (Papachristoforou et al., 2011).
2.4. STATISTICAL ANALYSIS
The disturbance of foraging activity was measured by comparing the number of pollen foragers in the attack context to the number of pollen foragers in the control context using the Wilcoxon signed rank test (paired comparisons). A chi-squared test was used to measure
the proportional difference in support events (individual agonistic support and balling) between the threat and fight categories. To reduce the chance of a type I error, continuity correction was used for the chi-squared tests because the sample size was less than 200 (Sokal et al., 1981).
The Wilcoxon rank sum test (unpaired comparisons) was used to compare the number of supporters in the threat and fight categories (excluding balling). We also tested for correlation (non-parametric Spearman correlation) between the number of supporters and the duration of attacks. To reduce the chance of a type I error in this analysis, we used Bonferroni correction in the case of multiple testing with significance set at α = 0.05/2 = 0.025. All tests were carried out using R v3.0.2 implemented with library (exactRankTests) and library (coin).
2.5. RESULTS
2.5.1. Wasp attack
We observed 68 attacks at the hive entrance in 279 h of video footage, specifically 11 attacks in 2014 (63 h) and 57 in 2015 (216 h) representing ~0.24 attacks per hour. The most frequent outcome was wasp escape (55 events, 80.9%) and the least frequent was bee predation (1 event, 1.5%). On three occasions (4.4%) the wasp was observed entering the hive and coming out alive. On another three occasions it was not possible to confirm the fate of the wasp because observation session terminated while the wasp was still inside the hive. The average attack time was 3.5 ± 0.4 s.
We observed 465 attacks at ground level in 32 h only targeting isolated bees (~14.5 attacks per hour). In this case, the outcome was more balanced. Bee predation was observed 226 times (48.6%) and in 91 of these cases sequestration also occurred. The wasp was chased away 239 times (51.4%), which is a much lower proportion compared to hive entrance attacks. The attack behavioral display data are summarized in Table 1.
2.5.2. Nest defense
We did not observe a collective attack against any of the colonies so our data only represents defense behaviors against individual wasps. Among the 68 agonistic events observed at the hive entrance, 28 cases (41.2%) involved a single bee defending itself successfully causing
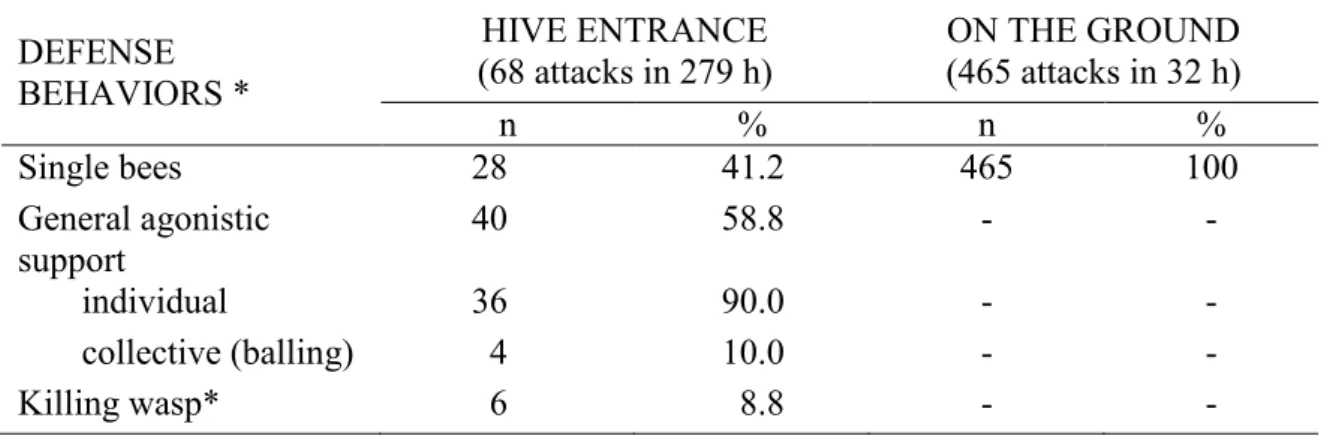
the wasp to flee. In the remaining 40 attacks (58.8%), other bees from the same nest came to the rescue. Agonistic support was exclusively individual in 90% of cases, with an average of 1.9 ± 0.2 supporters per attack, and was collective in 10% of cases, resulting in balling. In six cases (8.8%), the wasp was killed and removed from the landing board. Agonistic support of the bees under attack was never observed among the 465 ground level attacks close to the hive. The defense behavioral display data are summarized in Table 2.
2.5.3. Disturbance of foraging
We did not observe any disturbance of foraging activity when the colony was under attack. Indeed, there were no statistically significant differences between the frequency of foraging in the attack context (24.2 ± 4.1) and in the control context (23.0 ± 3.4) in 2015 (U = 154,
N1 = N2 = 27, P = 0.6378).
2.5.4. Agonistic support
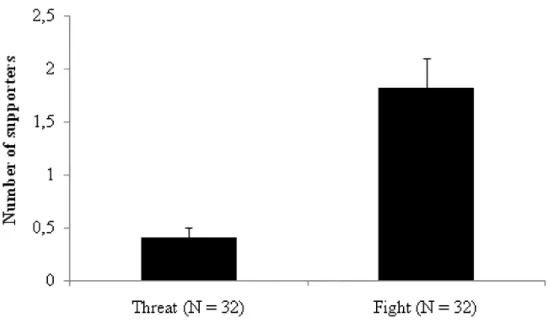
The agonistic events most commonly supported by nestmates either individually or by balling were those involving physical contact (fights) rather than warning behavior (threats). Accordingly, we observed a statistically significant difference between the number of supported threats and the number of supported fights as shown in Figure 1 (chi-squared = 13.07, df = 1, P = 0.0003). Moreover, when balling events were excluded, the average number of supporters was significantly higher in fights than threats, as shown in Figure 2 (U = 221, N1 = N2 = 32, P = 0.00001). There was also a positive correlation between the number of supporters and the duration of attack (S = 20653, P = 0.000007, rho = 0.53). Agonistic support was observed only at the hive entrance, not at the ground level.
2.6. DISCUSSION AND CONCLUSIONS
Our data revealed that V. germanica attacks A. mellifera ligustica infrequently on the landing board of the nest so there is a low risk of predation, and cases in which the predator managed to overcome the barrier of guard bees, enter the hive, pillage it and escape, were extremely rare. Instead, the predatory activity of V. germanicais clearly directed towards the bees at ground level, which are weak or isolated. This specialized form of attack, as opposed to a direct attack on the hive, achieves high predation efficiency and confirms that V. germanica
is a scavenger in the apiary ecosystem (Coelho & Hoagland, 1995). This phenomenon can be explained by the optimal foraging theory, which postulates a trade-off between energy returns and mortality due to predation (Free, 1970; Kasper et al., 2008; D’Adamo & Lozada, 2003). Our observations revealed a compromise between the reward obtained and the risk taken by the wasp, with a direct attack on the hive entrance attracting a greater risk than attacks on isolated bees. Accordingly, the isolation of foragers is essential to improve the hunting effectiveness of hornets in the vicinity of A. dorsata nests because this species is extremely effective in repelling hornets by shimmering (Kastberger et al., 2008).
Our observations also indicated that V. germanica is predominantly a solitary predator, because we found no evidence of coordinated attacks involving other conspecifics. In contrast, competition for food and pillaging among wasps were observed during predation. This probably reflects the individual and independent foraging typology of this species (D’Adamo & Lozada, 2003) hence individuals from different colonies can find themselves at the same foraging site thus explaining why each individual defends its own prey (Free, 1970). In contrast to other predators such as V. velutina and V. crabro (Monceau et al., 2013),
V. germanica has never been observed attacking forager bees in flight and returning to the
hive, only bees on the ground or on the landing board.
The relatively weak predation practiced by V. germanica is confirmed by the ability of between one and three bees to repel an attack without recourse to truly collective defense strategies such as balling. Indeed, balling by A. mellifera ligustica against V. germanica has never been reported before, and we observed this behavior only four times throughout our observation period. In contrast, balling is deployed much more frequently against Asian wasps (Arca et al., 2014; Tan et al., 2005). This is important because balling often kills some of the participating bees in addition to the predator, suggesting that A. mellifera ligustica regulates its defense behavior depending on the intensity of the threat in order to prevent unnecessary sacrifices (Helfman, 1989; Tan et al, 2012a). We also saw no evidence of alternative collective defense strategies such as a bee carpet or shimmering, which are often deployed against V. crabro (Baracchi et al., 2010). Again this suggests that A. mellifera
ligustica adjusts its defense strategy in response to different predators, and can likewise be
interpreted as a trade-off between the involvement of the colony in collective defense (with the associated risks discussed above) and the danger the predator represents.
Our study also revealed that more intense agonistic events (i.e. fights rather than threats) attract stronger support from nestmates, and showed a correlation between the degree of support and the duration of attack. This suggests that that A. mellifera ligustica can adapt its defense behavior according to the context of the danger (Tan et al., 2016). This hypothesis is further supported by the different defense responses observed at the hive entrance and on the ground. Whereas hive entrance attacks usually attracted supporters in the events we observed because such attacks are recognized as a potential danger for the entire colony, attacks against fallen individuals were never supported because these individuals were no longer recognized as nestmates.
The different nestmate-recruiting capacity observed in different behavioral danger categories probably reflects the emission of warning signals such as alarm pheromones by the bee under attack (Breed et al., 2004; Tan et al., 2016). In contrast to recent observations in colonies of A. mellifera ligustica attacked by V. velutina in Liguria, Italy (Cervo, personal communication), we never observed the interruption of foraging or complete retreat into the nest. We can therefore exclude the possibility that vibration stop signals are used to recruit supporters (Tan et al., 2016). Indeed, we found no evidence that V. germanica disrupts A.
mellifera ligustica foraging activity, providing more support for the hypothesis that the prey–
predator relationship between these two sympatric species has reached a state of balance and that V. germanica need not be considered a threat to apiculture. However, to exclude the threat to bee foraging activity completely, further observations are required in areas with a greater density of wasp colonies.
Docile characteristics are preferred when selecting genetic lines of A. mellifera and several methods have therefore been developed to evaluate the aggression of reared honeybees. It follows that an understanding of the agonistic behavioral displays in A. mellifera against natural enemies could be used to develop more effective tests to replace the current evaluation methods, which have been called into question (Zakour & Bienefeld, 2013). Indeed, the method recommended by Apimondia (International Federation of Beekeepers’ Associations) is based on subjective evaluation by the operator on a four-point scale, where 1 is most aggressive and 4 is most docile, thus establishing the protective equipment the beekeeper must use (Ruttner, 1972). This method does not account for climatic, chemical, visual, social and environmental variables that can play a role in the aggressive behavior of
a colony, and is therefore too subjective and difficult to reproduce. A much more selective method has been developed to distinguish between aggressive and docile states in A.
mellifera colonies representing the subspecies carnica, scutellata and capensis (Kastberger et al., 2009). Our data could also facilitate the selection of genetic lines of honeybees that
are less hostile to humans while maintaining aggressive behavior towards their natural enemies.
In conclusion, our study provides insight into the mechanisms of attack and defense deployed by V. germanica and A. mellifera ligustica both in terms of predator–prey coevolution (Futuyama, 1986)and in terms of potential defense strategies that can be used by native bees against alien species such as V. velutina.
2.7. REFERENCES
Abrol, D.P. (2006) Defensive behaviour of Apis cerana F. against predatory wasps. Journal
of Apicultural Science, 50, 39-46.
Altmann, J. (1974) Observational study of behaviour: sampling methods. Behaviour, 49, 227-265.
Arca, M., Papachristoforou, A., Mougel, F., Rortais, A., Monceau, K., Bonnard, O., et al. (2014) Defensive behaviour of Apis mellifera against Vespa velutina in France: Testing whether European honeybees can develop an effective collective defence against a new predator. Behavioural Processes, 106, 122-129.
Baracchi, D., Cusseau, G., Pradella, D., & Turillazzi, S. (2010) Defence reactions of Apis
mellifera ligustica against attacks from the European hornet Vespa crabro. Ethology Ecology and Evolution, 22, 1-14.
Breed, M.D., Silverman, J.M., Bell, W.J. (1978) Agonistic behavior, social interactions, and behavioral specialization in a primitively eusocial bee. Insectes Sociaux. Paris., 25, 351-364.
Breed, M.D., Robinson, G.E., & Page, R.E. (1990) Division of labor during honey bee colony defense. Behavioral Ecology and Sociobiology, 27, 395-401.
Breed, M.D., Smith, T.A., & Torres, A. (1992) Role of guard honeybees (Hymenoptera:Apidae) in nestmate discrimination and replacement of removed guards. Annals of the Entomological Society of America, 85, 633-637.
Breed, M.D., Guzman-Novoa, E., & Hunt, G.J. (2004) Defensive behavior of honey bees: Organization, genetics, and comparisons with other bees. Annual Review Entomology, 49, 271-298
Coelho, J.R., & Hoagland, J. (1995) Load-lifting capacities of three species of yellowjackets (Vespula) foraging on honey-bee corpses. Functional Ecology, 9, 171-174.
Collins, A., Rinderer, T., Harbo, J., & Bolten, A. (1982) Colony defence by Africanized and European honey bees. Science, 218, 72-74.
D’Adamo, P., & Lozada, M. (2003) The importance of location and visual cues during foraging in the German wasp (Vespula germanica F.) (Hymenoptera: Vespidae). New
Zealand Journal of Zoology, 30, 171-174.
De Grandi-Hoffman, G., Collins, A.M., Martin, J.H., Schmidt, J.O., & Spangler, H.G. (1998) Nest defense behavior in colonies from crosses between Africanized and European honey bees (Apis mellifera L.) (Hymenoptera: Apidae). Journal of Insect Behavior, 11, 37-45.
De Wroey, C., & Pasteels, J.M. (1978) Agonistic Behavior of Myrmica rubra L. Insectes
Sociaux Paris, 25, 247-265.
Denis, D., Chameron, S., Costille, L., Pocheville, A., Chaline, N., & Fresneau, D. (2008) Workers agonistic interactions in queenright and queenless nests of a polydomous ant society. Animal Behaviour, 75, 791-800.
Free, J.B. (1970) The behavior of wasp (Vespula germanica L. and V. vulgaris L.) when foraging. Insectes Sociaux. Paris, 12, 11-20.
Futuyama, D.J. (1986) The Evolution of Interactions Among Species, in: Evolutionary Biology, 2nd ed., Sinauer Associates Inc., Sunderland, Massachusetts; pp. 482–504. Gherman, B.I., Denner, A., Bobiş, O., Dezmirean, D.S., Mărghitaş, L.A., Schlüns, H., et al.
(2014) Pathogen-associated self-medication behavior in the honeybee Apis mellifera.
Behavioral Ecology and Sociobiology, 68, 1777-1784.
Helfman, G.S. (1989). Threat-sensitive predator avoidance in damselfish-trumpet fish interactions. Behavioral Ecology and Sociobiology, 24, 47-58.
Hermann, H.R. (1984) Defensive mechanisms: general considerations. In: Defensive mechanisms in Social Insects. Hermann HR. Ed., Praeger, New York; pp. 1–31. Johnson, L.K., & Hubbell, SP. (1974) Aggression and competition among stingless bees:
Kasper, M.L., Reeson, A.F., Mackay, D.A., & Austin, A.D. (2008) Environmental factors influencing daily foraging activity of Vespula germanica (Hymenoptera, Vespidae) in Mediterranean Australia. Insectes. Sociaux, 55, 288-295.
Kastberger, G., Thenius, R., Stabentheiner, A., Hepburn, R. (2009) Aggressive and docile colony defence patterns in Apis mellifera. A retreater-releaser concept. J Insect Behav., 22, 65-85.
Kastberger, G., Schmelzer, E., Kranner, I. (2008) Social waves in giant honeybees repel hornets. PLoS ONE, 3(9), e3141. Doi: 101371/journal.pone.0003141.
Kastberger, G,,Weihmann, F., Hoetzl, T., Weiss, E.S., Maurer, M., Kranner, I. (2012) How to join a wave: decision-making processes in shimmering behavior of giant honeybees
(Apis dorsata). PLoS ONE.; 7(5), e36736. Doi: 101371/journal.pone.0036736.
Markwell, T.J., Kelly, D., & Duncan, K.W. (1993) Competition between honey bees (Apis
mellifera) and wasp (Vespula spp.) in honeydew beech (Nothofagus solandri var.
solandri) forest. New Zealand Journal of Ecology, 17, 85-93.
Monceau, K., Arca, M., Leprêtre, L., Mougel, F., Bonnard, O., Silvain, F., et al. (2013) Native prey and invasive predator patterns of foraging activity: the case of the yellow-legged hornet predation at European honeybee hives. PLoS ONE, 8 (6): e66492. Doi : 101371/journal.pone.0066492.
Monceau, K., Bonnard, O., & Thiéry, D. (2014) Vespa velutina: a new invasive predator of honeybees in Europe. Journal of pest science, 87, 1-16.
Matsuura, M. & Sakagami, S.F. (1973) A bionomic sketch of the giant hornet, Vespa
mandarinia, a serious pest for Japanese apiculture. HUSCAP Journals, 19, 125-162.
Matsuura, M. (1991) The social biology of wasp. In: Ross KG, Matthews RW, editors. Ithaca: Cornell University Press; pp. 232-262.
Nieh, J.C., Kruizinga, K., Barreto, L.S., Contrera, F.A.L., & Imperatriz-Fonseca, V.L. (2005) Effect of group size on the aggression strategy of an extirpating stingless bee,
Trigona spinipe.. Insectes Sociaux, 52, 147-154.
Ono, M., Igarashi, T., Ohno, E., & Sasaki, M. (1995) Unusual thermal defense by a honeybee against mass attack by hornets. Nature, 377, 334-336.
Papachristoforou, A., Rortais, A., Zafeiridou, G., Theophilidis, G., Garnery, L., Thrasyvoulou, A., et al. (2007). Smothered to death: hornets asphyxiated by honeybees. Current Biology, 17, 795-796.
Papachristoforou, A., Rortais, A., Sueur, J., & Arnold, G. (2011) Attack or retreat: contrasted defensive tactics used by Cyprian honeybee colonies under attack from hornets.
Behavioural Processes, 86, 236-241.
Pappas, N., & Thrasyvoulou, A. (1988) Searching for an accurate method to evaluate the degree of Varroa infestation in honeybee colonies, in: Cavalloro R. (Ed.) 1988. European research on Varroatosis control, Proceedings of a meeting of the EC-Experts´Group, Bad Homburg, Germany, 15–17 October 1986, Commission of the European Communities, A.A. Balkema, Rotterdam; 1986. pp. 85–91.
Ruttner, H. (1972) Technische Empfehlungen zur Methodik der Leistungsprüfung von Bienenvöikern. In: Proceedings of Paarungskontrolle und Selektion bei der Honigbiene. Internationales Symposium. Lunz am See, Austria; pp. 112.
Seeley, T.D., Seeley, R.H., & Akratanakul, P. (1982) Colony defense strategies of the honey bees in Thailand. Ecological Monographs, 52, 43-63.
Shorter, J.R. & Rueppell, O. (2012) A review on self-destructive defense behaviours in social insects. Insect Sociaux, 59, 1-10.
Sokal, R.R., & Rohlf, F.J. (1981) Biometry, 2nd ed. W. H. freeman and Co., New York. Sugahara, M., & Sakamoto, F. (2009) Heat and carbon dioxide generated by honeybees
jointly act to kill hornets. Naturwissenschaften, 96, 1133-1136.
Szathmary, E., & Maynard Smith, J. (1995) The major evolutionary transitions. Nature, 374, 227-232.
Tan, K., Hepburn, H.R., Radloff, S.E., Yusheng, Y., Yiqiu, L., & Danyin, Z. (2005) Heat-balling wasps by honeybees. Naturwissenschaften, 92, 492-495.
Tan, K., Radloff, S.E., Li, J.J., Hepburn, H.R., Ang, M.X., Zhang, L.J, et al. (2007) Beehawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissenschaften, 94, 469-472
Tan, K., Wang, Z., Li, H., Yang, S., Hu, Z., Kastberger, G., & Oldroyd, B.P. (2012a) An ‘I see you’ prey–predator signal between the Asian honeybee, Apis cerana, and the hornet, Vespa velutina. Animal Behavior, 83, 879-882.
Tan, K., Yang, MX., Wang, Z.W., Li, H., Zhang, Z.Y., Radloff S.E, et al. (2012b) Cooperative wasp-killing by mixed-species colonies of honeybees, Apis cerana and Apis mellifera. Apidologie, 43, 195-200.
Tan, K., Dong, S., Li, X., Liu, X., Wang, C., Li, J., & Nieh, J.C. (2016) Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biology, 14 (3): e1002423. Doi:10.1371/jounal.pbio.1002423.
Turillazzi, S. (2003) Le società delle Vespe. Alberto Perdisa, Bologna. pp. 55.
Vanengelsdorp, D., Speybroeck, N., Evans, J.D., Nguyen, B.K., Mullin, C., Frazier, M., et al. (2010) Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. Journal Economic Entomology, 103, 1517-1523.
Wilson, E.O. (1975) Sociobiology: the new syntesis. Cambridge MA, twenty-fifth anniversary edition.
Wood, M.J., & Ratnieks, F.L.W. (2004) Olfactory cues and Vespula wasp recognition by honey bee guards. Apidologie; 35, 461-468.
Zakour, M.K., & Bienefeld, K. (2013) Subjective evaluation of defensive behavior in the Syrian honeybee (Apis mellifera syriaca). Journal of Apicultural Science, 57, 137-145.
2.8. TABLES AND FIGURES
Table 1. Attack behavioral display observed in colonies of A. mellifera under attack by V.
germanica.
ATTACK BEHAVIORS
HIVE ENTRANCE
(68 attacks in 279 h) (465 attacks in 32 h) ON THE GROUND
n % n %
Antennation* 11 16.2 - -
Predation* 1 1.5 226 48.6
Sequestration* - - 91 19.6
Entering the hive 6 8.8 - -
Retreat 55 80.9 239 51.4
* Behaviors which are not mutually exclusive.
Table 2. Defense behavioral display observed in colonies of A. mellifera under attack by V.
germanica when predation did not occur.
DEFENSE BEHAVIORS *
HIVE ENTRANCE
(68 attacks in 279 h) (465 attacks in 32 h) ON THE GROUND
n % n % Single bees 28 41.2 465 100 General agonistic support 40 58.8 - - individual 36 90.0 - - collective (balling) 4 10.0 - - Killing wasp* 6 8.8 - -