MicroRNA-148a reduces tumorigenesis and increases
TRAIL-induced apoptosis in NSCLC
Pooja Joshia,1, Young-Jun Jeona,1, Alessandro Laganàb, Justin Middletona, Paola Secchieroc, Michela Garofalod,2, and Carlo M. Crocea,2
aDepartment of Molecular Virology, Immunology and Medical Genetics and Comprehensive Cancer Center, Ohio State University, Columbus, OH 43210; bDepartment of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY 10029;cDepartment of Morphology and Embryology, Human Anatomy Section, University of Ferrara, 44100 Ferrara, Italy; anddTranscriptional Networks in Lung Cancer Group, Cancer Research UK Manchester Institute, University of Manchester, Manchester M20 4BX, United Kingdom
Edited by Napoleone Ferrara, University of California at San Diego, La Jolla, CA, and approved May 26, 2015 (received for review January 15, 2015)
Nonsmall cell lung cancer (NSCLC) is one of the leading causes of death worldwide. TNF-related apoptosis-inducing ligand (TRAIL) has been shown to induce apoptosis in malignant cells without inducing significant toxicity in normal cells. However, several carcinomas, including lung cancer, remain resistant to TRAIL. MicroRNAs (miRNAs) are small noncoding RNAs of ∼24 nt that block mRNA translation and/or negatively regulate its stability. They are often aberrantly expressed in cancer and have been im-plicated in increasing susceptibility or resistance to TRAIL-induced apoptosis by inhibiting key functional proteins. Here we show that miR-148a is down-regulated in cells with acquired TRAIL-resistance compared with TRAIL-sensitive cells. Enforced expression of miR-148a sensitized cells to TRAIL and reduced lung tumorigenesis in vitro and in vivo through the down-modulation of matrix metal-loproteinase 15 (MMP15) and Rho-associated kinase 1 (ROCK1). These findings suggest that miR-148a acts as a tumor suppressor and might have therapeutic application in the treatment of NSCLC. microRNA
|
chemoresistance|
lung cancerC
urrently, the largest cause of cancer-related deaths in the world is lung cancer. Nonsmall cell lung cancer (NSCLC) is the most frequent type of lung cancer, constituting more than 85% of all cases (1). It is often diagnosed at an advanced stage and has poor prognosis. The development of targeted therapies, such as promoting apoptosis, has been one of the methodologies for treatment of NSCLC (2). Tumor necrosis factor (TNF)-re-lated apoptosis inducing ligand (TRAIL) is a cytokine, and a member of the TNF family that is being tested in clinical trials. Although TRAIL has shown clinical efficacy in a subset of NSCLC patients, either alone or combined with chemotherapy, many lung tumors are resistant to TRAIL and the mechanism of this resistance is not fully understood. MicroRNAs (miRNAs), small noncoding RNAs of 19–25 nt, inhibit mRNA translation and/or negatively regulate its stability by binding to the 3′ un-translated region (3′ UTR) of target mRNAs (3). Recent evi-dence suggests that miRNAs are involved in a number of biological processes such as development, proliferation, differentiation, and apoptosis (4, 5). They are often aberrantly expressed in cancer, and their function is linked to the regulation of oncogenes and/or tumor suppressor genes involved in cell signaling pathways (6). Several miRNAs have been implicated in increasing suscepti-bility or resistance to TRAIL-induced apoptosis by inhibiting key functional proteins (7–10).The matrix metalloproteinases (MMPs) are a family of zinc proteases that play an important role in the breakdown of the extracellular matrix in normal physiological processes, such as embryonic development, tissue and bone remodeling, wound healing, and angiogenesis (11, 12). They are well characterized for their contribution to the development of cancer (13). MMP15 is a membrane-type metalloproteinase that has also been identified as an antiapoptotic marker in cancer cells, and its inhibition increases sensitivity to TRAIL-induced apoptosis (14).
Rho-associated coiled coil protein (Ser/Thr) kinase-1 (ROCK1) is an essential effector kinase downstream of Rho GTPases that has a central role in many motile responses that involve the actin cytoskeleton and/or microtubule network, from neurite extension to phagocytosis and cancer-cell invasion (15). ROCK1 has been implicated in anchorage-independent growth and metastasis in several cancers (16–19). In this study, we found that miR-148a is down-regulated in NSCLC cells with both primary and acquired TRAIL resistance compared with TRAIL-sensitive cells.MMP15 andROCK1 have been identified as miR-148a direct targets. In TRAIL-resistant cells, miR-148a induced apoptosis after TRAIL treatment by regulating MMP15 expression. miR-148a inhi-bited cell migration and invasion through the silencing of both MMP15 and ROCK1. Furthermore, the inverse relation between miR-148a and the two target genes was validated in lung tumor tissue samples.
Results
MiR-148a Targets ROCK1 and MMP15.To investigate mechanisms involved in TRAIL response, we generated TRAIL-resistant cells (H460R and H292R) by exposing the parental TRAIL sensitive H460 and H292 cells (H460S and H292S), respectively, to stepwise increases in TRAIL concentrations (1–500 ng/mL) over a period of 6 mo (20). Upon comparison of the miRNA ex-pression profile of H460R versus H460S and by quantitative real-time PCR (qRT-PCR), we found miR-148a to be markedly down-regulated in the resistant cell lines compared with their sensitive counterparts (Fig. S1A). The expression of miR-148a
Significance
Nonsmall cell lung cancer (NSCLC) is one of the deadliest cancers in the world. Although a very small subset of NSCLC patients re-spond to TNF-related apoptosis-inducing ligand (TRAIL), resistance remains a major hindrance to successful treatment. miRNAs are
small noncoding RNAs of ∼24 nt that negatively regulate gene
expression. Here, we show that miR-148a is significantly down-regulated in cells with acquired TRAIL resistance. Further-more, we have determined that miR-148a can sensitize cells to TRAIL and inhibit tumorigenesis by targeting matrix met-alloproteinase 15 and Rho-associated kinase 1 protein ex-pression. Thus, miR-148a could be a promising prognostic and therapeutic tool in NSCLC treatment.
Author contributions: P.J., Y.-J.J., M.G., and C.M.C. designed research; P.J., Y.-J.J., and J.M. performed research; A.L. and P.S. contributed new reagents/analytic tools; A.L. analyzed data; and P.J., Y.-J.J., and M.G. wrote the paper.
The authors declare no conflict of interest. This article is a PNAS Direct Submission.
1P.J. and Y.-J.J. contributed equally to this work.
2To whom correspondence should be addressed. Email: michela.garofalo@cruk.
manchester.ac.uk or [email protected].
This article contains supporting information online atwww.pnas.org/lookup/suppl/doi:10. 1073/pnas.1500886112/-/DCSupplemental.
was also found to be down-regulated in NSCLC cell lines with primary TRAIL resistance (A549, Calu-1, and H1299) compared with the more sensitive H460 and H292 cell line (Fig. 1A). To further understand the effect of TRAIL on miR-148a expression, H460-sensitive cells were treated with TRAIL (50 ng/mL) over a 24-h period. The plates were washed with PBS, and surviving cells were collected. We observed a significant decrease in the ex-pression of miR-148a by qRT-PCR in a time-dependent manner in the surviving cells (Fig. 1B). To confirm that TRAIL can affect miR-148a expression, we used siRNA against TRAIL receptors DR4 and DR5 to neutralize the effect of TRAIL treatment. H292 cells were transfected with both siRNAs together followed by 24-h TRAIL treatment (50 ng/mL). Knockdown of TRAIL receptors blocked TRAIL-induced apoptosis, and we did not see a change in miR-148a expression (Fig. S1B and C). Next, to identify miR-148a targets, we performed a bioinformatics search (TargetScan and Pictar). Among the candidates,MMP15
(nu-cleotides 4289–4295 and 4410–4416; NM_002428) and ROCK1 (nucleotides 5873–5879 and 6420–6426; NM_005406) mRNA contained regions in their 3′ UTRs that matched the seed se-quence of human miR-148a (Fig. 1C). To verify whether ROCK1 andMMP15 were direct targets of miR-148a, their 3′ UTRs were cloned into the pGL3 control vector downstream of the lucif-erase ORF. Enforced expression of these constructs in combi-nation with miR-148a in A549 cells, which express low levels of miR-148a, significantly decreased luciferase expression mea-sured as relative luciferase activity (Fig. 1D). Conversely, when we used pGL3 control vectors containingROCK1 and MMP15 3′ UTRs where miR-148a binding sites were deleted by site-directed mutagenesis, a consistent abrogation of the miR-148a inhibitory effect was observed (Fig. 1C and D). Next, we over-expressed miR-148a in A549 and Calu-1 cells, as confirmed by qRT-PCR (Fig. S1D) and checked the endogenous levels of ROCK1 and MMP15 protein and mRNA levels. Both MMP15 and ROCK1 protein and mRNA levels were reduced in cells transfected with miR-148a compared with control cells (Fig. 1E andF), suggesting that miR-148a directly regulated MMP15 and ROCK1 at the transcriptional level.
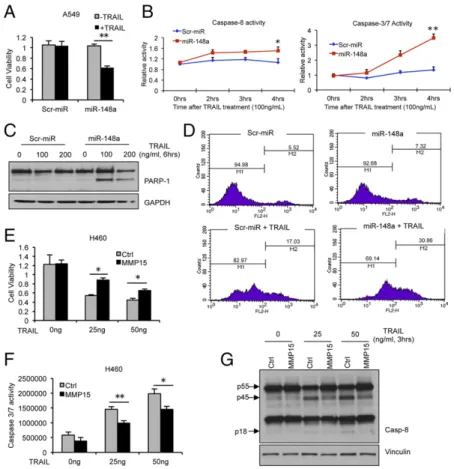
MiR-148a Overexpression Increases TRAIL Sensitivity in NSCLC.We next examined the effects of miR-148a on cell survival and TRAIL resistance in NSCLC. To test whether miR-148a over-expression in TRAIL-resistant A549 could increase the response to the drug, proliferation and apoptosis assays were performed. A549 were transfected with either a scrambled miRNA or miR-148a. After 48 h, transfected cells were exposed to TRAIL and cell viability and apoptosis were assessed by MTS, caspase-8, and caspase-3/7 activity assays, respectively. Overexpression of miR-148a in A549 cells led to reduced cell viability upon TRAIL exposure, and these cells were more sensitive to TRAIL-induced cell death as indicated by a time-dependent increase in caspase-8 and caspase-3/7 activation (Fig. 2 A and B). This effect was further confirmed in Calu-1 cells by MTS and caspase-3/7 activity assay (Fig. S2A and B). To further investigate the role of miR-148a in TRAIL-induced apoptosis, the cleavage of poly (ADP ribose) polymerase-1 (PARP-1) was tested in A549 cells after TRAIL treatment. As expected, overexpression of miR-148a in A549 cells led to increased PARP-1 cleavage (Fig. 2C). Fur-thermore, Annexin-FITC assay revealed an increase in TRAIL sensitivity after miR-148a overexpression in TRAIL-resistant Calu-1 cells (Fig. 2D). A previous study reported that MMP15 down-regulation increased the response to TRAIL-induced ap-optosis (14). To determine whether MMP15 overexpression is directly related to TRAIL resistance, we transfected H460 cells with either an expression vector containing MMP15 cDNA (Thermo Scientific) or an empty vector. After 48 h, cells were treated with TRAIL for 6 h and proliferation and caspase-3/7 activity assays were performed. H460 cells overexpressing MMP15 showed increased cell viability and resistance to TRAIL-induced apoptosis (Fig. 2E and F). Furthermore, Western blot showed a reduction in TRAIL-induced caspase-8 activation (Fig. 2G). This effect was further confirmed by using caspase-8 activity assay in H292 cells transfected with MMP15 and treated with TRAIL (25 and 50 ng/mL) (Fig. S2C). However, ROCK1 overexpression did not have any effect on TRAIL sensitivity in H460 cells (Fig. S2D and E). These results supported our hypothesis that miR-148a– mediated MMP15 down-regulation plays a crucial role in TRAIL sensitivity in NSCLC.
Demethylation of miR-148a Enhances TRAIL Sensitivity.miR-148a is located in close proximity to two CpG islands and has been observed to be hypermethylated in gastric cancer and other metastatic cancer cells (21, 22). To identify whether miR-148a was aberrantly inhibited by DNA hypermethylation, we treated A549 cells with the demethylating agent, 5-aza-dC, and then analyzed Fig. 1. MMP15 and ROCK1 are targets of miR-148a. (A) qRT-PCR showing
endogenous expression of miR-148a in NSCLC cell lines with primary TRAIL resistance (A549, Calu-1, and H1299) and TRAIL-sensitive cell lines (H460 and H292). (B) qRT-PCR showing decrease in expression of miR-148a in surviving H460 cells after TRAIL treatment. (C) MMP15 and ROCK1 3′ UTRs both contain two predicted miR-148a binding sites. The alignment of the seed regions of miR-148a with the two sites is shown. The site of target muta-genesis is indicated in red. (D) pGL3-MMP15 and pGL3-ROCK1 luciferase constructs, containing a wild-type or mutated MMP15 and ROCK1 3′ UTRs, were transfected into A549 cells. The binding sites on the MMP15 3′ UTR are close together and were cloned into the same construct, whereas for ROCK1 3′UTR each site was cloned separately. Relative repression of firefly lucifer-ase expression was standardized to a transfection control. The normalized activity of the control transfectants in each experiment was set as relative luciferase activity 1. Therefore, no error bar is shown for control transfectants. (E) MiR-148a overexpression leads to the down-regulation of endogenous levels of ROCK1 and MMP15 proteins in A549 and Calu-1 cell lines. (F) qRT-PCR in A549 cells showing a decrease in ROCK1 and MMP15 mRNAs after miR-148a enforced expression. Data are presented as± SD; *P < 0.05 and **P< 0.01 by Student’s t test.
CELL
BIO
miR-148a expression by qRT-PCR. The expression of miR-148a was significantly increased in A549 after treatment with 5-aza-dC (P < 0.05) compared with controls (Fig. 3A), suggesting that DNA methylation may be involved in the silencing of miR-148a in NSCLC. Next, we performed an apoptosis assay on 5-aza-dC– treated A549 and H1299 cells after TRAIL treatment. The cells showed an increased sensitivity to TRAIL after treatment with 5-aza-dC, as assessed by caspase-3/7 assay (Fig. 3B andFig. S3A). Also 5-aza-dC treatment followed by TRAIL led to PARP-1 cleavage and caspase-8 activation (Fig. 3C and Fig. S3B). Therefore, the use of a demethylating agent improved the re-sponse to TRAIL-induced apoptosis, at least in part by up-regulating miR-148a.
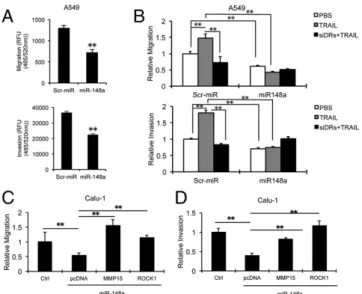
MiR-148a Inhibits Migration and Invasion in NSCLC.Next, we per-formed cell migration and invasion assays to analyze the effects of miR-148a overexpression or MMP15 and ROCK1 silencing on lung tumorigenesis. A549 and H1299 cells were seeded into the migration chambers 48 h after transient transfection with miR-148a and migration/invasion were measured after 24 h. In-terestingly, we observed a significant decrease in the migratory and invasive capabilities of miR-148a–overexpressing cells (Fig. 4A and Fig. S4A). To study the effect of TRAIL on miR-148a regulated migration and invasion, we treated A549 cells ex-pressing miR-148a with TRAIL with or without combined knockdown of TRAIL receptors (DR4 and DR5) and performed migration and invasion assays. We observed that the control cells showed increased migration and invasion on exposure to TRAIL (Fig. 4B), and this effect was suppressed when the TRAIL re-ceptors were down-regulated. However, A549 cells with miR-148a overexpression showed an inhibitory effect on cell migration and invasion regardless of the presence or absence of TRAIL. MMP15 and ROCK1 silencing also suppressed migration and invasion in A549 cells (Fig. S4B and C). Of note, when Calu-1 and A549 cells were transfected with both miR-148a and MMP15 or ROCK1
cDNA expression vectors, the decrease in cell migration and invasion was drastically reversed (Fig. 4C and D andFig. S4D and E), suggesting that miR-148a plays a major role in inhib-iting cancer cell motility and invasion via targeting MMP15 and ROCK1.
Effect of miR-148a in Lung Tumorigenicity in Vitro and in Vivo.To analyze the tumor suppressor effect of miR-148a in NSCLC, we stably infected A549 and Calu-1 cells with a GFP lentivirus construct that was either empty or contained full-length pre-cursor isoform of miR-148a. Up-regulation of miR-148a was confirmed by qRT-PCR (Fig. S5A and B). miR-148a was able to suppress colony formation in A549 and Calu-1 cells (Fig. 5A andB) but did not affect the cell cycle or proliferation rate (Fig. S5C and D). The effect of miR-148a on metastatic potential was Fig. 2. miR-148a induces TRAIL sensitivity in NSCLC by targeting MMP15. (A) Proliferation assay in A549 cells transfected with control miR or miR-148a for 48 h followed by 24-h TRAIL treatment (200 ng/mL). Cells overexpressing miR-148a were more sensitive to TRAIL-induced apoptosis. (B) Caspase-8 and caspase-3/7 activity assay in A549 cells transfected with control miR or miR-148a after TRAIL treatment (100 ng/mL) over a 4-h time period. (C) Western blot showing PARP-1 activation after 6 h TRAIL treatment (100 and 200 ng/mL) in A549 cells transfected with control miR or miR-148a. (D) Annexin-V staining showing increased sensitivity of Calu-1 cells to TRAIL-induced apoptosis 48 h after being transfected with miR-148a. Cells were treated with 1μg/mL TRAIL. M2 is percentage of apoptotic cells. (E) Proliferation assay in H460 cells transfected with control or MMP15 cDNA after TRAIL treatment (25 and 50 ng/mL). (F) Caspase-3/7 activity assay in H460 cells after MMP15 over-expression and TRAIL treatment. Cells overexpressing MMP15 showed resistance to TRAIL-induced apoptosis. (G) Western blot showing caspase-8 activation after 3-h TRAIL treatment (25 and 50 ng/mL) in H460 cells transfected with control or MMP15 cDNA. Data are presented as± SD; *P < 0.05 and **P< 0.01 by Student’s t test.
Fig. 3. Hypermethylation of miR-148a is involved in resistance to TRAIL-induced apoptosis. (A) qRT-PCR showing miR-148a expression in A549 cells after treatment with 5-aza-dC (1μM and 5 μM) for 72 h. (B) Caspase-3/7 assay after treatment of A549 cells with 5-aza-dC followed by TRAIL (100 ng/mL). (C) Western blot showing PARP-1 and caspase-8 activation after 3-h TRAIL treatment (100 ng/mL) in H1299 cells treated with 5-aza-dC for 72 h. After treatment with 5-aza-dC, NSCLC cells showed increased sensitivity to TRAIL-induced cell death as indicated by increased caspase-3/7 activity and PARP-1 and caspase-8 cleavage. Data are presented as± SD; *P < 0.05 and **P < 0.01 by Student’s t test.
analyzed by performing an anchorage-independent soft agar colony assay. A549 cells transfected with miR-148a alone or along with MMP15 or ROCK1 were plated on soft agar. After 6 d, the number of colonies in soft agar were quantified using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. miR-148a inhibited anchorage-independent growth, and this effect was reversed by MMP15 or ROCK1 overexpression (Fig. 5C). We also performed a soft agar colony assay for A549 cells expressing empty vector and miR-148a with or without knockdown of DR4 and DR5 after TRAIL treatment. In the control cells, TRAIL treatment resulted in increased anchorage-independent growth, and this effect was inhibited by knockdown of the death receptors. Interestingly, miR-148a–overexpressing cells repressed growth in the presence of both TRAIL and TRAIL neutralizing conditions (Fig. 5D). To investigate the role of miR-148a in vivo, A549-miR-148a and A549-control cells were injected s.c. into the flank of nine nude mice per group and tumor growth was measured for up to 50 d after injection. The overexpression of miR-148a resulted in a significant decrease in tumor growth (Fig. 5E) and increased tumor-free survival (Fig. 5F).
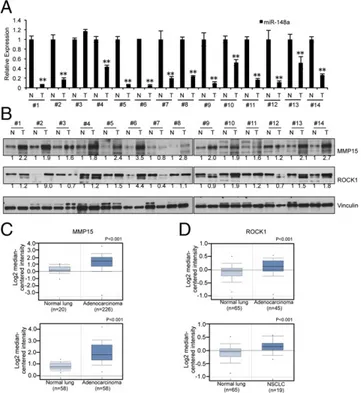
We then analyzed the expression of miR-148a in 14 sets of lung tumor samples and normal lung tissues. As seen in Fig. 6A, miR-148a expression is negligible in lung tumor samples but highly expressed in normal lung tissues. The expression of MMP15 and ROCK1 in these tumor sets was then checked by using Western blot (Fig. 6B). Interestingly, most tumor samples showed high expression of MMP15 (12 of 14) and ROCK1 (10 of 14) and low expression of miR-148a (13 of 14) (Fig. S6) compared with the corresponding normal tissue samples. Upon
examination of data available on ONCOMINE (23), we found several datasets (24–26) that indicate that both MMP15 and ROCK1 are significantly up-regulated in lung cancer compared with corresponding normal tissues (Fig. 6 C and D). These in vivo data substantiated our in vitro studies on the importance of miR-148a as a regulatory factor in lung cancer progression and chemoresistance.
Discussion
TRAIL targets cancer cells while leaving normal cells unharmed, but many human cancer cell lines show either innate or acquired resistance to TRAIL-induced apoptosis and the mechanism of such resistance is not clear. To investigate mechanisms involved in TRAIL resistance, we compared the miRNA expression profile in NSCLC cell line with acquired TRAIL resistance (H460R), previously generated in our laboratory, versus the parental TRAIL-sensitive (H460S) cells. Among the different miRNAs that were down-regulated in H460R cells, we focused on miR-148a because it exhibited the highest fold change (20). We found that miR-148a is drastically down-regulated not only in cells with acquired TRAIL resistance but is also weakly expressed in cells with primary TRAIL resistance. To further analyze the functional role of miR-148a in lung cancer, we Fig. 4. miR-148a inhibits A549 cell migration and invasion through MMP15
and ROCK1 down-regulation. (A) Transwell migration and invasion assays. A549 cells transfected with control miR or miR-148a were seeded onto transwell chambers 48 h after transfection and migration, and invasion were analyzed 24 h and 48 h later. miR-148a overexpression inhibited A549 cell migration and invasion. (B) Migration and invasion assays were conducted by using A549 cells expressing a control miR or miR-148a with or without the silencing of DR4 and DR5 and/or TRAIL (100 ng/mL) treatment. The cells were seeded onto transwell chambers, and cell migration and invasion were an-alyzed. (C) Migration assay carried out on Calu-1 cells cotransfected with miR-148a and control or MMP15 or ROCK1 cDNAs. MMP15 and ROCK1 sig-nificantly rescued inhibition of cell migration induced by miR-148a in A549 cells. (D) Calu-1 cells were cotransfected with miR-148a and control or MMP15 or ROCK1 cDNA. MMP15 and ROCK1 significantly rescued inhibition of cell invasion induced by miR-148a in Calu-1 cells. All values were measured in RFU (485/520 nm) and normalized by using values from corresponding controls. Data are presented as± SD; **P < 0.01 by Student’s t test.
Fig. 5. Effect of miR-148a in lung tumorigenesis in vivo. (A) Clonogenic assay on A549 infected with empty vector or miR-148a lentiviruses. The assay was performed three times. Representative plates are shown. (B) Clonogenic assay on A549 and Calu-1 cells infected with control or miR-148a lentiviruses. Columns indicate number of colonies derived from 2,000 cells plated after 2 wk in culture. Data are presented as± SD. (C) Anchorage-independent (soft agar) colony formation in A549 cells transfected with miR-148a alone or with MMP15 or ROCK1. miR-148a inhibited colony formation on soft agar by targeting MMP15 and ROCK1, and overexpression of MMP15 or ROCK1 re-versed this effect. Data are presented as± SD. (D) Anchorage-independent (soft agar) colony formation in A549 cells transfected with miR-148a alone or after knockdown of DR4 and DR5 and/or TRAIL treatment. TRAIL increased colony formation, but this effect was inhibited by miR-148a overexpression. Data are presented as± SD. (E) Growth curve of engrafted tumors in nude mice injected with A549 cells stably infected with empty vector or miR-148a lentiviruses. Data are presented as± SEM. (F) Tumor-free survival probability curve of nude mice injected with A549 cells stably infected with empty vector or miR-148a lentiviruses. *P< 0.05 and **P < 0.01 by Student’s t test.
CELL
BIO
investigated its potential gene targets. We identified and con-firmedMMP15 and ROCK1 as targets of miR-148a. MMP15 is an antiapoptotic protein whose down-regulation has been pre-viously shown to increase the response to TRAIL-induced apo-ptosis (13). Both MMP15 and ROCK1 have important roles in tumor cell migration and invasion. MMP15 is involved in pro-moting tumor invasiveness in gliomas (27). ROCK1 is required for anchorage-independent growth and invasion in NSCLC (28). Overexpression of miR-148a or MMP15 silencing increased the sensitivity to TRAIL-induced apoptosis. These results fur-ther corroborated our previous work showing that TIMP3, an inhibitor of MMPs activity, improves the response of NSCLC cells to TRAIL (7). It has previously been reported that MMP15-induced TRAIL resistance through the activation of MMP2 and several studies have already shown that inhibition of MMP2 can activate apoptosis-related pathways in various cancer cells lines, including A549 (29–32). As expected, knockdown of MMP15 in A549 cells reduced MMP2 activity (Fig. S7A). Furthermore, silencing of MMP2 in A549 cells increased caspase-3/7 activation in response to TRAIL (Fig. S7B). We also demonstrated that the effect of miR-148a on TRAIL response is only MMP15- and not ROCK1-dependent.
Several studies have confirmed that miR-148a acts as tumor suppressor in different tumors. miR-148a silencing resulted in stimulation of tumor cell motility through activation of targets like Wnt10B in cancer-associated fibroblasts (33). miR-148a is also known to be a potential prognostic biomarker functioning as a tumor suppressor in gastric cancer (21, 34, 35). It regulates cell survival in human pancreatic ductal adenocarcinomas by targeting
CDC25B (36), and its expression promotes apoptosis in colorectal cancer by targeting BCL2 (37). Moreover, miR-148a attenuates drug resistance in hormone-refractory drug-resistant prostate cancer cells and esophageal adenocarcinoma cells (38, 39). TRAIL signaling induces protumorigenic effects such as an increase in cell proliferation, migration, and invasion in some resistant cell lines (40, 41). We found that TRAIL induced an increase in mi-gration and invasion in resistant A549 cells, and this effect de-pended on the death receptors DR4 and DR5. Interestingly, miR-148a enforced expression decreased migration and invasion of NSCLC cells regardless of the TRAIL receptors status through the down-regulation of both ROCK1 and MMP15.
DNA methylation-associated silencing of miR-148a expression has been identified in human cancer cell lines established from lymph node metastasis of colon, melanoma, and head and neck cancer, suggesting a role in the development of metastasis (22). In this study, we reported that treatment of A549 and H1299 cells with the demethylating agent 5-aza-dC led to increased expression of miR-148a and, accordingly, to increased apoptosis and PARP-1 and caspase-8 cleavage, indicating that miR-148a methylation could increase the resistance of lung cells to TRAIL. Importantly, we found an inverse correlation between miR-148a and its targets in vitro and in vivo in a cohort of lung tumors. Finally, we further confirmed a tumor suppressor role for miR-148a in vivo by injecting A549 stably infected with miR-miR-148a into nude mice. A decrease in tumor burden in miR-148a over-expressing tumors was observed. Interestingly, seven of nine mice injected with A549 cells stably overexpressing miR-148a were tumor free for the period of the experiment. miR-148a overexpression not only decreased tumor growth but also in-creased the probability of tumor-free survival. In summary, we demonstrated an important role of miR-148a in the response of NSCLC to TRAIL-induced apoptosis and in tumorigenesis of NSCLC. Our results suggest that miR-148a could be used as prognostic and therapeutic tool in lung cancer.
Materials and Methods
Cell culture, chemicals, luciferase assay, and other standard methods are described inSI Materials and Methodsand primers used for luciferase con-structs are described inTable S1.
Target Analysis. The programs TargetScan (www.targetscan.org) and Pictar (pictar.mdc-berlin.de/) were used to perform Bioinformatics analysis. Cell Death and Cell Proliferation Assay. Cells were seeded in 96-well plates in triplicate and incubated at 37 °C in a 5% CO2incubator (ATCC). Recombinant TRAIL was used as described in figure legends. Viability of cells was exam-ined with MTT-Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega), according to the manufacturer’s protocol. Metabolically active cells were detected by adding 20μL of the reagent to each well. After 1 h of incubation, the plates were analyzed in a Multilabel Counter (Bio-Rad Laboratories). Apoptosis was assessed by using Annexin V-FITC apoptosis detection kits followed by flow cytometric analysis and using caspase-3/7 activity assay. For Annexin-FITC assay, Calu-1 cells were seeded at 1× 105 cells per 60-mm dish, grown overnight, and then transfected with precursor miR-148a or scrambled oligonucleotides for 48 h. The cells were then treated for 24 h with 1μg/mL TRAIL. Following incubation, cells were washed with cold PBS and removed from the plates by trypsinization. The resuspended cells (105) were washed with cold PBS and stained with FITC-conjugated Annexin V antibody according to the manufacturer’s instructions (Trevigen). The samples were then analyzed by using FACScan (FACScalibur; BD Bio-sciences) with the CellQuest software (BD BioBio-sciences). At least 10,000 events were analyzed and compared with control. The fraction of Calu-1 cells treated with TRAIL was taken as the apoptotic cell population. The per-centage of apoptosis indicated was corrected for background levels found in the corresponding untreated controls. For detection of caspase-8 and caspase-3/7 activity, cells were cultured in 96-well plates and treated with TRAIL and analyzed by using Caspase-Glo Assay kits (Promega) according to the manufacturer’s instructions.
Fig. 6. miR-148a expression is inversely correlated to MMP15 and ROCK1 protein expression in NSCLC tumor sets. (A) qRT-PCR showing reduced ex-pression of miR-148a in 14 pairs of tumors compared with the normal counterparts. (B) Western blot showing MMP15 and ROCK1 expression in paired sets of tumors. The band intensities were quantified by using ImageJ software, and the relative values were obtained and normalized by using values from Vinculin and their corresponding controls. (C and D) Box plots of data mined from the Oncomine datasets showing MMP15 and ROCK1 ex-pression levels respectively in normal tissues and lung cancer samples plotted on a log scale. **P< 0.01 by Student’s t test.
5-aza-2′-Deoxycytidine Treatment. A stock solution of 10mM 5-aza-2′-deoxy-cytidine (5-aza-dC) was prepared in DMSO. A549 and H1299 cells were treated for 72 h with 1 and 5μM 5-aza-dC, and total RNA was extracted for qRT-PCR. Cells treated with 5-aza-dC were plated in 96-well plates and then treated with TRAIL (100 ng/mL) for 3–6 h for caspase-3/7 activity assay and Western blot.
Migration and Invasion Assays. Assays were performed following manufac-turer’s protocol (EMD Millipore). InnoCyte cell migration and invasion assays were used. Briefly, cells were seeded in the upper chambers in serum-free media and serum-containing media was added to the lower chambers as chemoattractant. Cells that migrated/invaded to the lower chambers were dislodged and stained with a fluorescent dye. Fluorescence was measured with an excitation wavelength of∼485 nm and an emission wavelength of ∼520 nm. Generation of Stable Clones. A549 and Calu-1 cells were stably infected with Human premiRNA Expression Construct Lenti-miR expression plasmid con-taining the full-length miR-148a and the GFP gene under the control of two different promoters (System Biosciences). An empty vector was used as control. PremiR-148a and control constructs were packaged with pPACKH1 Lentivector Packaging Plasmid mix (System Biosciences) in a HEK293TN packaging cell line. Viruses were concentrated by using PEGit Virus Pre-cipitation Solution (SBI). Infected cells were selected by FACS analysis (FACSCalibur; BD Biosciences). Infection efficiency>90% was verified by fluorescent microscopy and confirmed by real-time PCR for miR expression.
Clonogenic Assay. A total of 2,000 cells each (A549-empty, A549-miR-148a, Calu-1-empty, Calu-1-miR-148a) were seeded into 100-mm plates in triplicates. Two weeks later, the cells were fixed with 4% (wt/vol) paraformaldehyde and
stained with crystal violet for 30 min. The colonies with diameters of more than 1.5 mm were counted.
Anchorage-Independent Growth Assay. A549 cells were seeded into 60-mm plates and transfected with premiR-148a alone or with MMP15 over-expression or ROCK1 overover-expression plasmid for 48 h. The cells were then trypsinized and plated onto a 96-well plate with soft agar by using Cytoselect 96-Well In Vitro Tumor Sensitivity Assay (Soft Agar Colony Formation) (Cell Biolabs). The assay was carried out according to the manufacturer’s protocol and the colony formation was detected by using MTT solution.
In Vivo Experiments. Animal studies were performed according to institutional guidelines. We used A549-empty and A549-miR-148a stably infected cells. Four- to six-week-old male athymic nu/nu mice (Harlan) were used in this study. Mice were anesthetized, and 7.5× 106cells were s.c. injected into the right flanks of the mice. Tumor size was measured every 3–5 d by a digital caliper. Tumor volumes were determined by measuring length (l) and width (w) and then calculating the volume (V= lw2/2). Thirty days after tumor initiation, the mice were euthanized. Animal experiments were conducted after approval of the Institutional Animal Care and Use Committee, Ohio State University.
Statistical Analysis. Continuous variables are expressed as mean values± SD. Student’s t test was used to determine significance. P < 0.05 was considered significant.
ACKNOWLEDGMENTS. We thank H. Alder, P. Fadda, and A. Bottoni for qRT-PCR guidance and assistance and J. Ravi for assistance with zymography.
1. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK (2014) Non-small-cell lung cancers: A heterogeneous set of diseases. Nat Rev Cancer 14(8):535–546. 2. Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev
Cancer 5(11):876–885.
3. Ambros V (2004) The functions of animal microRNAs. Nature 431(7006):350–355. 4. Bartel DP (2004) MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell
116(2):281–297.
5. Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9(10): 1274–1281.
6. Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866.
7. Garofalo M, et al. (2009) miR-221&222 regulate TRAIL resistance and enhance tu-morigenicity through PTEN and TIMP3 downregulation. Cancer Cell 16(6):498–509. 8. Razumilava N, et al. (2012) miR-25 targets TNF-related apoptosis inducing ligand
(TRAIL) death receptor-4 and promotes apoptosis resistance in cholangiocarcinoma. Hepatology 55(2):465–475.
9. Incoronato M, et al. (2010) miR-212 increases tumor necrosis factor-related apoptosis-inducing ligand sensitivity in non-small cell lung cancer by targeting the antiapoptotic protein PED. Cancer Res 70(9):3638–3646.
10. Acunzo M, et al. (2012) miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene 31(5):634–642.
11. Nagase H, Visse R, Murphy G (2006) Structure and function of matrix metal-loproteinases and TIMPs. Cardiovasc Res 69(3):562–573.
12. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: An overview. Mol Cell Biochem 253(1-2):269–285.
13. Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174.
14. Abraham R, et al. (2005) Identification of MMP-15 as an anti-apoptotic factor in cancer cells. J Biol Chem 280(40):34123–34132.
15. Riento K, Ridley AJ (2003) Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4(6):446–456.
16. Sahai E, Ishizaki T, Narumiya S, Treisman R (1999) Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol 9(3):136–145.
17. Zohrabian VM, Forzani B, Chau Z, Murali R, Jhanwar-Uniyal M (2009) Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and pro-liferation. Anticancer Res 29(1):119–123.
18. Somlyo AV, et al. (2000) Rho-kinase inhibitor retards migration and in vivo dissemi-nation of human prostate cancer cells. Biochem Biophys Res Commun 269(3):652–659. 19. Kamai T, et al. (2003) Significant association of Rho/ROCK pathway with invasion and
metastasis of bladder cancer. Clin Cancer Res 9(7):2632–2641.
20. Jeon Y-J, et al. (2015) A set of NF-κB–regulated microRNAs induces acquired TRAIL resistance in Lung cancer. Proc Natl Acad Sci USA 112(26):E3355–E3364.
21. Zhu A, et al. (2012) MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol 29(4):2701–2709.
22. Lujambio A, et al. (2008) A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA 105(36):13556–13561.
23. Rhodes DR, et al. (2004) ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia 6(1):1–6.
24. Hou J, et al. (2010) Gene expression-based classification of non-small cell lung carci-nomas and survival prediction. PLoS ONE 5(4):e10312.
25. Okayama H, et al. (2012) Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res 72(1):100–111. 26. Selamat SA, et al. (2012) Genome-scale analysis of DNA methylation in lung
adeno-carcinoma and integration with mRNA expression. Genome Res 22(7):1197–1211. 27. Zhang J, Sarkar S, Yong VW (2005) The chemokine stromal cell derived factor-1
(CXCL12) promotes glioma invasiveness through MT2-matrix metalloproteinase. Carcinogenesis 26(12):2069–2077.
28. Vigil D, et al. (2012) ROCK1 and ROCK2 are required for non-small cell lung cancer anchorage-independent growth and invasion. Cancer Res 72(20):5338–5347. 29. Morrison CJ, et al. (2001) Cellular activation of MMP-2 (gelatinase A) by MT2-MMP
occurs via a TIMP-2-independent pathway. J Biol Chem 276(50):47402–47410. 30. Chetty C, Bhoopathi P, Lakka SS, Rao JS (2007) MMP-2 siRNA induced
Fas/CD95-mediated extrinsic II apoptotic pathway in the A549 lung adenocarcinoma cell line. Oncogene 26(55):7675–7683.
31. Kesanakurti D, et al. (2011) Suppression of MMP-2 attenuates TNF-α induced NF-κB activation and leads to JNK mediated cell death in glioma. PLoS ONE 6(5):e19341. 32. Nyormoi O, Mills L, Bar-Eli M (2003) An MMP-2/MMP-9 inhibitor, 5a, enhances
apo-ptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ 10(5):558–569.
33. Aprelikova O, et al. (2013) Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene 32(27): 3246–3253.
34. Chen Y, et al. (2010) Altered expression of MiR-148a and MiR-152 in gastrointestinal cancers and its clinical significance. J Gastrointest Surg 14(7):1170–1179.
35. Guo SL, et al. (2011) miR-148a promoted cell proliferation by targeting p27 in gastric cancer cells. Int J Biol Sci 7(5):567–574.
36. Liffers ST, et al. (2011) MicroRNA-148a is down-regulated in human pancreatic ductal adenocarcinomas and regulates cell survival by targeting CDC25B. Lab Invest 91(10): 1472–1479.
37. Zhang H, et al. (2011) MiR-148a promotes apoptosis by targeting Bcl-2 in colorectal cancer. Cell Death Differ 18(11):1702–1710.
38. Fujita Y, et al. (2010) MiR-148a attenuates paclitaxel resistance of hormone-re-fractory, drug-resistant prostate cancer PC3 cells by regulating MSK1 expression. J Biol Chem 285(25):19076–19084.
39. Hummel R, et al. (2011) Mir-148a improves response to chemotherapy in sensitive and resistant oesophageal adenocarcinoma and squamous cell carcinoma cells. J Gastrointest Surg 15(3):429–438.
40. Ishimura N, Isomoto H, Bronk SF, Gores GJ (2006) Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol 290(1):G129–G136.
41. Trauzold A, et al. (2006) TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 25(56):7434–7439.
CELL
BIO