Scuola Normale Superiore
Ph.D. thesis
Dendrimers for Drug Delivery
and Molecular Imaging
Lorenzo Albertazzi
advisor Prof. Fabio Belt ram
As buds give rise by growth to fresh buds, and these, if vigorous, branch out and overtop on all sides many a feebler branch, so by generation I believe it has been with the great Tree of Life, which fills with its dead and broken branches the crust of the earth, and covers the surface with its ever-branching and beautiful ramifications.
Contents
Foreword v
Introduction 1
1 Dendrimers: Structure, Properties and Applications 5
1.1 Dendrimers: a Historical Perspective . . . 5
1.2 Dendrimers Structure and Synthesis . . . 7
1.3 PAMAM Dendrimers . . . 10
1.4 Biomedical Applications . . . 13
2 Dendrimer Internalization and Trafficking 19 2.1 Materials and Methods . . . 21
2.1.1 Materials . . . 21
2.1.2 Dendrimer Synthesis . . . 21
2.1.3 Cell Culture and Trasfection . . . 22
2.1.4 Confocal Imaging . . . 22
2.1.5 Propidium Iodide (PI) Assay . . . 22
2.1.6 Flow Cytometry Measurements . . . 23
2.1.7 Internalization Assays and Colocalization Studies . 23 2.2 Dendrimer Surface Modification and Labeling . . . 24
2.4 Time Lapse Imaging and Colocalization Assays . . . 29
2.5 TAT-Dendrimer Comparison . . . 33
2.6 Cell Line Analysis . . . 34
3 Dendrimer-based Fluorescence pH Sensors 37 3.1 Materials and Methods . . . 38
3.1.1 Materials . . . 38
3.1.2 General Procedures for Dendrimer Synthesis . . . 38
3.1.3 UV-Vis Spectrofluorimetry . . . 38
3.1.4 Cell Culture and Electroporation . . . 39
3.1.5 Confocal Imaging . . . 39
3.1.6 Kinetics of Acetylated Dendrimer Hydrolysis . . . 39
3.1.7 In Vitro Calibration . . . 40
3.1.8 Calibration and pH Imaging in HeLa Cells . . . 40
3.2 PAMAM Dendrimers-Dye Conjugates . . . 41
3.3 Electroporation and Intracellular Targeting . . . 41
3.4 Dendrimer-based pH Sensors . . . 45
3.5 In Vitro Titration . . . 47
3.6 Calibration and pH Measurements in HeLa Cells . . . 49
4 Dendrimer-PEG Copolymers: Toward the Ideal Dendritic Sensor 53 4.1 Materials and Methods . . . 54
4.1.1 Materials . . . 54
4.1.2 Synthesis and Characterization . . . 54
4.1.3 Computational Methods . . . 55
4.1.4 In vitro Fluorescence Measurements . . . 56
4.1.5 Living Cells Studies . . . 56
4.1.6 In vivo Imaging . . . 57
4.2 Synthesis and Structural Studies . . . 57
4.3 A Family of Fluorescent Sensors . . . 63
4.4 Living Cell Measurements . . . 64
4.5 In Vivo Imaging . . . 67
CONTENTS iii
5 Multifunctional Dendritic Scaffolds 73
5.1 Materials and Methods . . . 75
5.1.1 Materials and General Procedures . . . 75
5.1.2 Dendrimer Synthesis . . . 76 5.1.3 Fluorescence Spectroscopy . . . 79 5.1.4 HPLC Experiment . . . 79 5.1.5 Cell Culture . . . 80 5.1.6 Confocal Microscopy . . . 80 5.2 Dendrimer Synthesis . . . 80 5.3 In Vitro Release . . . 82
5.4 Living Cells Analysis . . . 83
5.5 Concluding Remarks . . . 87 6 Dendrimers for Drug Delivery and Imaging: Concluding
Remarks 89
List of Publications 93
Bibliography 109
Foreword
This thesis is the result of my research activity at the NEST laboratory of Scuola Normale Superiore in Pisa: I began my studies on dendrimers in 2007, prompted by the group interest in the nanomedicine sector. This research was carried out within a joint PhD program sponsored by Scuola Normale Superiore and Istituto Italiano di Tecnologia.
Introduction
Effective and selective delivery of bioactive moieties such as drugs, nu-cleic acids, contrast agents or reporters to the desired biological target is a crucial enabler for clinical diagnostics and therapy. Nanomedicine is a very promising approach in drug delivery and molecular imaging with many potential advantages with respect to present methodologies. The aim of nanomedicine is the engineering of devices at the molecular level with the purpose to achieve medical benefits. These devices often consist of a nano-sized scaffold functionalized with multiple copies of bioactive moieties such as drugs, targeting signals, contrast agents and reporters. This approach allows not only to take advantage of the biological effects of different molecules but may also exploit the scaffold properties to enhance the resulting performance in living cells and in vivo. The success of novel strategies for therapy and diagnostics relies on the development of reliable scaffolds capable of improving the efficacy of biologically active molecules. In this framework a wide range of materials were employed, yet an ideal platform is not available. In this context dendrimers are a very attractive class of macromolecules owing to their many desirable properties. They are hyperbranched nano-sized polymers that combine the advantageous features of nanoparticles (ideal size as in vivo carriers, multivalency), of polymeric materials (low cost, tunable properties, biocompatibility), and of small molecules (monodispersity and therefore precise control on their
properties). For these reasons dendrimers and dendritic polymers were recently proposed as drug-delivery vectors, transfectant agents, contrast agents and scaffolds for regenerative medicine. In this thesis I shall de-scribe the development of novel dendrimer-based nanodevices for drug delivery and fluorescence sensing. Novel dendritic materials were de-signed, synthesized and their performance evaluated in living cells and in vivo. A specific focus of this work is the understanding of the behavior of these synthetic moieties in the biological environment. This issue was addressed by means of biophysical techniques and in particular by fluo-rescence microscopy. This technique allowed us the study of the dynamics of dendrimer-based materials with high spatial and temporal resolution with minimum perturbation of the biological specimen. The resulting information was exploited to design novel dendritic structures endowed with optimized properties for specific biomedical applications.
The following sections will be organized as follows:
- In Chapter 1 I shall provide a brief review of dendrimers and their biological applications. The synthesis and properties of different dendritic architectures will be reported. Emphasis on the advantages and drawbacks in the context of drug delivery and molecular imaging will be given.
- Chapter 2 provides a detailed biophysical study of PAMAM den-drimers internalization in living cells. An array of PAMAM denden-drimers varying in size, charge and surface properties was synthesized and tracked inside cells with confocal microscopy. Flow cytometry, time lapse confocal imaging and colocalization assays were employed to unveil the endocytic properties of these dendrimers. This study yielded useful information on the molecular structure-function relationship in some crucial aspects of drug delivery, i.e. the cell membrane crossing and intracellular trafficking. - In the first part of Chapter 3 a study of the behavior of PAMAM dendrimers in the cell cytoplasm will be reported. Dendrimers were di-rectly delivered into the cell by electroporation, thus avoiding the en-dolysosomal compartment. Interestingly different dendrimers display
pe-3 culiar intracellular localization and dynamics after electroporation owing to selective interactions with intracellular biomolecules. This information is of great importance and allowed us to selectively target dendrimer-based devices to specific cell compartments such as nucleoli, lysosomes, plasma membrane and cytosol.
In the second part of the chapter the synthesis and the evaluation of dendrimer-based fluorescent pH sensors will be reported. In this frame-work dendrimers act as carriers for multiple copies of pH sensing dyes. We took advantage of dendrimer multifunctionality to obtain ratiometric targeted sensors whose properties were evaluated in vitro and in living cells. In particular information about dendrimer intracellular localization was exploited to realize organelle-targeted sensors. The enhancement of the properties of the sensing dyes showed in this chapter demonstrates how the dendritic scaffold help to overcome the intrinsic limitations of small bioactive molecules.
- Chapter 4 reports the delevopment of a ”second generation” of dendrimer-based fluorescent sensors. This novel sensor architecture shows enhanced brightness, retention of dye photophysical properties, absence of cell leakage or compartimentalization. In order to demonstrate the general applicability of this structure, three different sensors for pH, Cl
-and K+ were realized. Sensors were successfully used to measure these
biologically relevant ions in vitro, in living cells, and in vivo. In this chapter I shall demonstrate how measurements that would be impossible with standard indicators, i.e. free dyes, do became accessible thanks to the enhanced properties of the dendritic sensor.
- Widely used families of dendrimers (PAMAM, PPI, bis-MPA) can be functionalized only on the surface groups. This limitation has neg-ative consequences on the loading efficiency and monodispersity of the dendritic scaffold. In Chapter 5 I shall present the synthesis and char-acterization of a novel dendritic architecture overcoming these limitations. The presence of two orthogonal functionalities in the inner part and on the dendrimer surface allows the stoichiometric loading of two bio-active moieties. We exploited the inner dendrimer functionality to conjugate
multiple copies of payload molecules through cleavable linkers while the surface groups were decorated with chemical groups promoting membrane binding and internalization. Confocal microscopy data will demonstrate that the proposed carrier can succesfully cross the cell membrane and se-lectively release multiple cargo molecules in the cell cytoplasm in response to a specific enzymatic activity.
Chapter
1
Dendrimers: Structure, Properties
and Applications
In this chapter I shall provide a brief review of dendrimers and their appli-cations. A general description of synthesis, structure and reactivity of the most relevant dendrimer families will be given with particular emphasis on poly(amidoamine) (PAMAM) dendrimers. Since their early develop-ment in the 80s dendrimers were investigated in light of a wide range of applications. They are now routinely used for catalysis, light harvesting and various biomedical applications. A detailed review is out of the pur-pose of this chapter, however, representative examples of applications of dendrimers in life sciences will be provided.
1.1
Dendrimers: a Historical Perspective
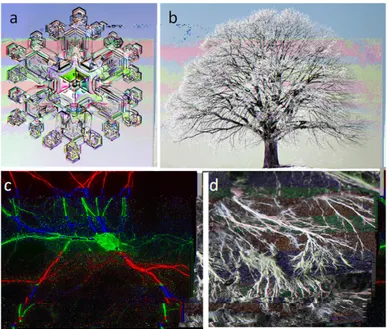
Dendrimers are polymeric molecules composed of multiple branched monomers that emanate radially from a central core in a tree-like fashion. Indeed the name dendrimer originates from the Greek word δǫνδρoν, i.e. tree. The dendritic architecture is one of the most pervasive topologies observed in nature. As shown in Fig. 1.1 many examples of branched patterns can be
found in abiotic system (e.g. snow crystals) and in biological system (e.g. in branching trees, roots, fungi, blood vessels, neurons). The reasons for the success of this topology are not fully understood but the very large interface and number of active sites per volume obtainable with a tree like structure are widely believed to play an important role. Despite this wealth of examples in nature, the synthesis of perfectly branched archi-tecture did not occur until the late 70s. The first example of repetitive growth with branching was reported in 1978 by Vogtle et al [1] at the Uni-versity of Bonn. These authors proposed an iterative procedure for the synthesis of low molecular-weight polyamines named ”cascade synthesis”. This synthetic route was plagued by low yield and isolation difficulties. It could not be used for the synthesis of large macromolecules with the unique properties now associated to the term dendrimer.
Figure 1.1: Examples of dendritic topology occurring in abiotic and living systems: a) ice crystal; b) tree; c) dendritic tree of a neuron and d) fungi hyphae
1.2 Dendrimers Structure and Synthesis 7 by Tomalia and coworkers at Dow Chemical Company. In 1985 these au-thors described the divergent synthesis of Poly(amidoamine) dendrimers (PAMAM) that overcame the problems of low yield, purity and purifi-cation encountered by Vogtle in his cascade synthesis [2]. PAMAM den-drimers of different molecular weights were synthesized in high yields and their chemical and physical properties fully characterized. In the follow-ing years other dendritic architecture based on different monomers were proposed. In particular it is worth mentioning Newkome’s synthesis of ar-borols [3] and the Frechet-type dendrons, the first example of convergent synthesis [4, 5]. These discoveries generated much interest in the field of polymer chemistry and were followed in the 90s by key contributions that opened new synthetic routes and highlighted peculiar structural features of dendrimers. In the last decade a large international effort is devoted to nanotechnology. A very relevant challenge facing nanotechnology is the development of reliable methodologies that enable the cost-effective, con-trolled assembly of nanostructures. Bottom-up synthetic strategies that produce size monodispersed, well-defined organic and inorganic nanos-tructures with dimensions ranging between 1 and 100 nm will be of cru-cial importance. In this framework dendritic architectures can play a piv-otal role thanks to their ideal properties (monodispersity, multivalency, tunable properties and low production costs) that allow the large-scale production of perfectly monodisperse nanostructure with precise control on size, shape, and surface chemistry.
1.2
Dendrimers Structure and Synthesis
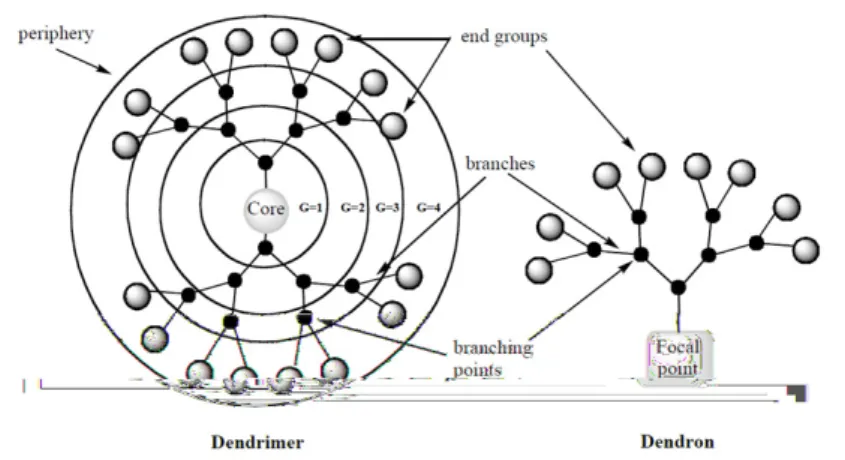
Figure 1.2 shows the schematic structure of a dendrimer. Three different regions can be indentified in the dendritic architecture: i) a central core; ii) multiple branching units and iii) periphery (or surface groups). The dendrimer core, branching units and end groups can be tailored according to requirements. The number of branching points encountered moving outwards from the core to the surface groups is called ”generation” (G1, G2, G3). Consequently increasing generations lead to larger dendrimers
with more end groups. When the dendrimer core is removed, a number of identical fragments called dendrons remain. The number of dendrons per dendrimer depends on the multiplicity of the central core.
Figure 1.2: Schematic representation of dendrimers and dendrons.
Owing to this unique topology dendrimers are endowed with many desirable properties [6]. For this reason they are particularly attractive for biological applications since they represent an ideal scaffold able to carry multiple copies of drugs, contrast agents or other bioactive moieties [7]. Figure 1.3 shows the two general approaches that were used in den-drimer synthesis: divergent synthesis and convergent synthesis. In the first approach dendrimers grow from the central core towards the periph-ery. Usually two synthetic steps (two orthogonal reactions or protection and deprotection) are required to grow one generation. These two steps are iteratively reproduced in order to obtain larger dendrimers. On the contrary in convergent synthesis dendrons of the desired generation are previously synthesized and are conjugated to a multifunctional core to obtain the dendrimer.
Both approaches show advantages and disadvantages and are widely used in dendrimer synthesis. Divergent synthesis allows the production of larger dendrimers with easy procedures. It also allows a straightforward scale up of the reactions thus facilitating production on the industrial
1.2 Dendrimers Structure and Synthesis 9
Figure 1.3: Schematic representation of the two main synthetic route for dendrimers: i) divergent synthesis (top); ii) convergent synthesis (bottom).
scale. However unwanted reactions can occur and it is very diffult to sep-arate the side products from the dendrimers. For these reasons obtaining dendrimers with high purity and monodispersity is still a challenge. On the other side convergent synthesis yields high purity, perfectly monodis-perse dendrimers. On the negative side the synthetic procedures are more complex and require the use of multiple protecting groups. More-over larger dendrimers cannot be obtained because the steric hindrance of the dendrons makes the conjugation to the core rather inefficient.
Multifunctionality is one of the most appealing features of the den-dritic architecture. It makes possible to load multiple copies of active moieties onto the dendritic scaffolds. Different strategies for dendrimer functionalization were proposed but two approaches seem particularly interesting: i) covalent conjugation of payload molecules on dendrimer surface groups; ii) non-covalent encapsulation of guest molecules inside the dendrimer structure. The ”dendritic box” reported by Meijer and coworkers represents an elegant example of these strategies [8]. Figure 1.4 shows the molecular structure of the dendritic box. The 64 surface
groups of a poly(propilenimine) dendrimer (PPI) were covalently func-tionalized with L-phenylalanine derivatives while rose bengal molecules were encapsulated in the inner part of the dendrimer. The multiple copies of the aminoacid create a dense hydrogen-bonded shell with a solid-state-like behavior, thus rose bengal molecules are entrapped and cannot escape the ”box”. These dendritic architectures represent the first example of a unimolecular compartmented structure in which guest molecules are physically locked.
Figure 1.4: Dendritic box encapsulating rose bengal (from Meijer et al [8]).
1.3
PAMAM Dendrimers
As mentioned in the previous section poly(amidoamine) dendrimers (PA-MAM) are the first synthesized and commercialized dendrimer family. They can be synthesized by means of divergent synthesis using ethylen-diamine and methyl acrylate (Fig. 1.5).
The synthesis starts from a multivalent amine and consists in the it-erative repetition of two steps: i) michael addition of a primary amine to
1.3 PAMAM Dendrimers 11
Figure 1.5: Divergent synthesis of ethylendiamine-core PAMAM den-drimers.
two molecules of methyl acrylate to produce a branched intermediate; ii) amidation of the esters by ethylenediamine to produce a dendrimer with primary amines on the surface. The synthesis does not require any pro-tection/deprotection steps and the purification can be easily achieved by monomer azeotropic distillation. This synthetic procedure can be scaled up to industrial level: this promoted the commercialization and diffu-sion of PAMAM dendrimers [9]. This synthesis method produces highly-organized and relatively-monodisperse polymers that display a controlled incremental increase in size, molecular weight, and number of surface groups with the increase in generation number. Table 1.1 shows the properties of PAMAM dendrimers as a function of the generation num-ber. Dendrimer growth reaches a critical point when the steric crowding of the branching arms limits their development into higher generations and produces defective branching architectures in a phenomenon known as de Gennes dense-packing effect.[10] This effect is observed starting with G7: the synthetic yield starts to decrease until reaching G10, when the synthesis of larger dendrimers is prohibited by steric factors.
PAMAM dendrimer structure was studied with a variety of exper-imental techniques such as small-angle X-ray scattering (SAXS) [11],
G1 G2 G3 G4 MW 1,430 3,256 6,909 14,215 Diameter (nm) 2.2 2.9 3.6 4.5 Surface Groups 8 16 32 64 G5 G6 G7 G8 MW 25,826 58,048 116,493 233,383 Diameter (nm) 5.4 6.7 8.1 9.7 Surface Groups 8 16 32 64
Table 1.1: Physical properties of PAMAM dendrimers.
chromatography [12] and optical spectroscopy [13]. These measurements provided significant information about physicochemical properties of PA-MAM dendrimers. However a detailed atomistic experimental charac-terization is extremely challenging. The problem is that these molecules possess a large number of energetically permissible conformations, and in solution, there is rapid interchange between them. Thus, diffraction techniques, NMR and IR spectroscopy yield little structural information. To overcome this limitation molecular dynamics (MD) simulations were performed by Goddard and coworkers [14]. Figure 1.6 shows a MD all-atom simulation of PAMAM dendrimers from G1 to G8. The parameters obtained by MD trajectories match the experimental data of size exclu-sion chromatography and SAXS and provide details about the structure and the dynamics of the PAMAM architecture at an atomistic level.
To date PAMAM dendrimers are the most used dendrimers and were actually employed for a wide range of applications. Representative exam-ples of PAMAM applications are reported in the next section.
1.4 Biomedical Applications 13
Figure 1.6: PAMAM dendrimers snapshots from MD simulations. From Goddard et al [14].
1.4
Biomedical Applications
Since their early development dendrimers showed a marked potential for biomedical applications such as drug delivery, gene delivery and clinical diagnostics [15]. Dendrimers are endowed with unique properties that make them particularly attractive for these applications. In particular the high control on the physicochemical properties and monodispersity yielded a very reproducible behavior in the biological environment. In this section a few representative examples of biomedical applications of dendrimers will be presented.
Selective and effective delivery of chemotherapy drugs is a major is-sue in cancer therapy. Dendritic structures were used as carriers for anti-cancer drugs and were shown to accumulate in tumor tissues and selectively release multiple cargo molecules.[16] In particular the work by Frechet and coworkers is an elegant example of a modular and ef-fective carrier design [17]. Figure 1.7 shows the schematic structure of dendritic polymer (named bow-tie dendrimer) used for doxorubicin de-livery. The carrier consist of an asymmetric dendrimer functionalized with poly(ethylenglycol) chains (PEG) on one side and doxorubicin (an anti-cancer drug) on the other.
The PEG chains increase the size of the carrier ad lead to longer body retention times. They also favor tumor accumulation because of their en-hanced permeability and retention (EPR) effect. Drugs were conjugated to the dendrimer through an acid-labile linker. These spacers are stable at physiological pH while are hydrolyzed when the pH is below 6. This
Figure 1.7: Schematic structure of bow-tie polyester dendrimers for Dox-orubicin delivery. Taken from Frechet et al [17]
allows the preferential release of drugs in tumor tissues since they are in metabolic acidosis. The combination of targeted localization and selective release results in improved anti-cancer activity with reduced side effects. An in vivo evaluation of these molecules in animal models of colon car-cinoma was reported. The doxorubicin-loaded dendrimers showed very significant advantages in comparison with the free drug in terms of effi-cacy and side-effect intensity [17].
Owing to their easy synthesis, multivalency and tunability of surface groups, dendrimers are appealing vectors also for nucleic acids delivery. The first gene transfer study using PAMAMs was reported by Haensler and Szoka in 1993 [18], and since then, several groups utilized PAMAMs and derivatives for transfection. Dendrimer-based transfection is car-ried out by low-density complexes (termed dendriplex) whose formation varies based on dendrimer-DNA charge ratio and dendrimer generation. Dendrimer-DNA complexes are formed as a result of multiple ionic in-teraction between positively-charged dendrimers and negatively-charged DNA fragments as highlighted in Fig. 1.8. Mechanistic studies show that PAMAM promotes two events that aid in cellular delivery and en-dosomal release of DNA. First dendrimers promote membrane binding
1.4 Biomedical Applications 15 and cell internalization by endocytosis [19, 20]. Following cellular up-take, endosomal chloride accumulation increased significantly and the pH of the surrounding environment increased, indicating the occurrence of endosomal swelling/lysis. Thus dendrimers are able to destabilize endo-somial vesicles facilitating DNA cytoplasmic release [21]. Recently two dendrimer-based products (Superfect and Polyfect) for in vitro gene de-livery were commercialized, both are based on G6-branched activated dendrimer formulations.
Figure 1.8: Snapshot from molecular dynamics simulation of a PAMAM dendrimer binding DNA (left). Gel electrophoresis of dendriplexes at differ-ent dendrimer/DNA ratios showing PAMAM ability to bind nucleic acids (right). From Pavan et al [22].
Another relevant clinical application of dendrimers is represented by molecular imaging devices. Many groups reported dendrimer-based agents for magnetic, optical and radionuclide imaging for diagnostic purposes. Moreover the multifunctionality of dendrimers can be exploited to de-sign multimodal imaging probes [23]. Figure 1.9 shows representative examples of in vivo imaging with dendrimer-based contrast agents. The dendritic scaffolds enhance the properties of contrast media by improving the signal, prolonging the circulation time in the body and cooperating for selective tissue localization. A complete description of dendrimer-based contrast agents is beyond the purpose of this chapter. Detailed reviews to this topic are available [24].
Figure 1.9: Examples of dendrimer-based in vivo imaging exploiting dif-ferent techniques: a) Positron Emission Tomography (PET)[25]; b) Near infrared (NIR) optical imaging[26]; c) Single photon emission computed to-mography (SPECT)[27] and d) Magnetic resonance imaging (MRI) [28].
was clear since their early development, their use as sensing devices able to image the spatiotemporal dynamics of specific bioevents is still rather un-explored. In this field the pioneering articles by Vinogradov and cowork-ers on dendrimer-based oxygen sensors deserve a careful description [29]. Oxygen levels are of crucial importance in homeostasis and tissue hypoxia is a critical parameter with respect to various tissue pathologies. Oxygen imaging in vivo can be achieved by O2-dependent quenching of porphyrin
phosphorescence combined with 2-photon excitation. Unfortunately Pt or Pd porphyrins, that are probably the most used oxygen-sensing ele-ments, have 2-photon cross sections that are too low to allow a successful imaging. Moreover porphyrins suffer from a very high affinity constant for oxygen that leads to complete quenching at in vivo concentrations. Vinogradov and coworkers described a dendrimer-based phosphorescent oxygen nanosensor overcoming these limitations. Structure and opera-tion of the sensor are reported in Fig. 1.10. The dendritic scaffold plays two main roles: i) it allows the conjugation of multiple 2-photon antenna
1.4 Biomedical Applications 17
Figure 1.10: Phosphorescent nanosensors proposed by Vinogradov and coworkers. Schematic structure of the dendritic architecture and photo-physics of the antenna-porphyrin system is reported. Taken from Brinas et al [29].
molecules that can subsequently transfer the energy to the porphyrin sens-ing molecule; ii) it encapsulates the phosphorescent sensors and controls the diffusion of oxygen thus modulating the affinity of the sensor.
This dendritic architecture limits the two main drawbacks of these widely-used oxygen optical sensors. This sensing device demonstrated its ability to measure oxygen with high spatial and temporal resolution in vivo. The sensor was successfully used to measure oxygen levels in the
Figure 1.11: Oxygen imaging in the brain. a) superimposition of maxi-mum projection of sensor signal and pseudocolor oxygen map; b) 3D ren-dering of pO2 concentrations in brain blood vessels; c) oxygen values at
brain blood vessels and in brain tissues. Figure 1.11 reports representative images of oxygen brain imaging highlighting a peculiar dependence of pO2
Chapter
2
Dendrimer Internalization and
Trafficking
The ability of a nanostructure to cross the cell membrane is of crucial importance for many biomedical applications such as drug and gene de-livery. In this chapter we shall address internalization and intracellular trafficking properties of PAMAM dendrimers in living cells. Recently a few groups reported the ability of PAMAM dendrimers to bind with high affinity the surface of cell membranes and be internalized by endocytosis [31]. The identification of the intracellular route following endocytosis, however, remains controversial. Endocytosis can take place with different mechanisms: macropinocytosis, clathrin dependent endocytosis, caveolae and clathrin- and caveolinin-independent pathways (see Fig. 2.1) [32].
In light of drug-delivery applications it is important to consider that these intracellular pathways can imply distinct chains of biochemical events. For instance in the case of macropinocytosis and clathrin depen-dent endocytosis vesicles undergo a gradual acidification while in cave-olae the pH remains neutral. Existing reports based on flow-cytometry measurements (using specific inhibitors of endocytic pathways) and im-munostaining led to different results in the elucidation of the intracellular
Figure 2.1: Pathways of entry into cells.
trafficking of PAMAM dendrimers [33, 34, 35]. Notably these techniques can provide quantitative information about dendrimer internalization but do not allow a direct visualization of endocytic processes in living cells since they usually require sample fixation.
We addressed these issues by means of fluorescence imaging and report here on the intracellular trafficking properties of PAMAM dendrimers in living HeLa cells. Exploiting the high spatial and temporal resolution of confocal fluorescence microscopy and its high biocompatibility we were able to monitor the internalization process and the subsequent intracellu-lar trafficking in living cells. We showed that dendrimers are internalized in several cell lines by macropinocytosis and clathrin dependent endocy-tosis and are eventually delivered to the lysosome. These measurements together with a direct comparison with TAT-derived peptides [36] demon-strate that PAMAM dendrimers possess similar properties to these widely used cell penetrating peptides and thanks to their chemical tunability may represent a valid alternative for drug and gene delivery.
2.1 Materials and Methods 21
2.1
Materials and Methods
2.1.1 MaterialsG2, G4, G6 and lipidated G4 PAMAM dendrimers, acetic anhydride, pro-pidium iodide, nerve growth factor (NGF), Amicon ultra 10 kDa dialysis membranes were purchased from SigmaAldrich. Alexa dyes, transferrin Alexa488, dextran-FITC, lysosensor, DAPI and lipofectamine were pur-chased from Invitrogen. TAT11-TMR conjugates were purpur-chased from Sigma Genosys. Hoechst was purchased from Gibco.
2.1.2 Dendrimer Synthesis
In order to achieve dendrimer acetylation ethylenediamine-core PAMAMs were dissolved in anhydrous methanol with triethylamine and acetic an-hydride, and the solution was stirred overnight at room temperature. The product was diluted with deionized water and extensively dialyzed against water (molecular weight cutoff 10 kDa) to afford the acetylated dendrimer. In the case of G6-Ac 100 equiv of acetic anhydride was used (0.39 equiv of dendrimer primary amines), while to obtain the fully acety-lated G4-Ac 640 equiv of Ac2O was employed (10 equiv of dendrimer
pri-mary amines). The fraction of acetylated amine groups was quantified by
1H NMR spectra in D
2O by means of a Varian Unity 300 spectrometer.
Dendrimer charge state was verified by polyacrylamide gel electrophore-sis (PAGE) and by agarose gel electrophoreelectrophore-sis. All PAMAM dendrimers were separately conjugated with Alexa647 and Alexa488 fluorophores to afford green- and infrared-labeled PAMAM dendrimers.
Conjugation was carried out via amide bond between the primary amine of the dendrimer and the N-hydroxysuccinimide activated carboxyl of the fluorophores. Dendrimers (50 nmol) were dissolved in carbonate buffer at pH = 9 and mixed with a DMSO solution of reactive dye (5 equiv for G6 and G4 or 2 equiv for G2). The solution was stirred for 4 h at room temperature and then dialyzed against water (MWCO=10 kDa) to afford dendrimer-dye conjugates. Owing to the poor solubility of
lipidated dendrimer the reaction was carried out in DMSO (4 h at room temperature) and then dialyzed against deionized water. Size exclusion chromatography was performed by means of a fast protein liquid chro-matography (FPLC) system (AKTA basic 10, GE Healthcare) equipped with a Superose 6 10/300 GL column (GE Healthcare). The isocratic mobile phase was PBS buffer pH = 7.4, and the optical density at 488 nm and 647 nm was monitored with the UV-vis detector of the FPLC system.
2.1.3 Cell Culture and Trasfection
HeLa (CCL-2), PC12 (CRL-17210), MRC5 (from normal lung tissue of a 14-week fetus, CCL-171) and HepG2 cells (HB-8065) were purchased from ATCC and cultured following manufacturer instructions. Primary astro-cytes were a kind gift of Giuseppe Bardi [37]. For live-cell microscopy cells were plated onto 35 mm glass-bottom dishes (WillCo-dish GWSt 3522) and imaged at 37◦C. PC12 cells were stimulated with 100 ng/mL of NGF
for two days to induce differentiation. Transfection of Caveolin-E1GFP
was carried out using lipofectamine reagent (Invitrogen) according to the manufacturer instruction. Cells were imaged 24 h after transfection.
2.1.4 Confocal Imaging
Cell imaging was performed on a Leica TCS SP2 inverted confocal micro-scope (Leica Microsystems) equipped with a 40x 1.25 NA oil immersion objective (Leica Microsystems). Imaging was obtained irradiating the samples with the microscope Ar and He-Ne lasers and with a 403 nm pulsed diode laser (M8903-01; Hamamatsu) at 50 MHz repetition rate. Fluorescence emission was collected with the AOBS-based built-in detec-tors of our confocal microscope (Hamamatsu R6357).
2.1.5 Propidium Iodide (PI) Assay
HeLa cells were incubated for 1 h at 37 ◦C with DMEM containing 8
2.1 Materials and Methods 23 nM, 500 nM and 1.5 µM). The medium was then discarded and cells were washed with PBS buffer containing the same concentration of propidium iodide before confocal imaging.
2.1.6 Flow Cytometry Measurements
HeLa cells were grown in a six-well plate and after treatment with Alexa488 labeled dendrimers they were detached using trypsin-EDTA, washed with PBS buffer and fixed with PFA 4%. Cells were washed with PBS until complete removal of PFA and finally resuspended in 250 µL of PBS. Flow cytometry was performed on a FACScalibur system (BD biosciences) by counting 10,000 events. Histogram plots were analyzed using WinMDI 2.9.
2.1.7 Internalization Assays and Colocalization Studies
In order to monitor dendrimer internalization cells were incubated with 100 nM Alexa647-labeled dendrimers in DMEM for 1 h at 37◦C. To
re-move unbound molecules in the medium, cells were rinsed two times with PBS. After the initial preloading and subsequent washing, cells were incu-bated again in DMEM and imaged at the indicated time point. In order to identify the endocytic vesicles involved in dendrimer internalization, we performed colocalization assays in living cells. HeLa cells were coin-cubated with dendrimers (as described above) and different dyes: with 1 mg/mL 70kDa dextran-FITC conjugate at 37 ◦C for 30 min to
la-bel macropinosomes, with 50 mM Lysosensor for 10 min to lala-bel lyso-somes, with 2 µg/mL transferrin Alexa488 conjugate to label recycling and sorting endosomes, and finally with 2 µM TAT-FITC conjugates. Images were analyzed using ImageJ software version 1.37 (NIH Image; http://rsbweb.nih.gov/ij/).
2.2
Dendrimer Surface Modification and
Label-ing
In order to analyze the internalization properties of different PAMAM dendrimers in living cells a small library of macromolecules with tuned physicochemical properties was synthesized. Particular attention was dedicated to cationic dendrimers since these show high affinity for the negatively-charged cell surface [31].
Figure 2.2 schematically shows the dendrimers investigated and their properties. Macromolecules with three different chemical surfaces were studied: (i) cationic-amine terminated (primary amines are protonated at physiological pH), (ii) neutral-acetylated amines and (iii) a cationic-lipidated dendrimer (25% of surface groups cationic-lipidated by C12 chains). Notably although fully acetylated PAMAM dendrimers display neutral acetamide groups, they retain a dynamic pH-dependent cationic behavior [38]. Additionally, a series of cationic dendrimers varying in size and surface charge were investigated in order to dissect the influence of each of these properties on cell uptake. G2, G4 and G6 dendrimers were chosen in order to analyze internalization properties on a broad range of sizes and surface charges. Moreover the highly charged G6 dendrimer was partially acetylated (100 amine groups out of 256 were acetylated) to reduce its surface charge [39]. For our endocytosis assays dendrimers were separately labeled with the Alexa488 and Alexa647 dyes to afford green- and infrared-labeled conjugates.
Labeled dendrimer properties were studied by NMR, UV-vis spec-trofluorimetry and size exclusion chromatography revealing the formation of bright and stable dendrimer-dye conjugates.
2.3
Cytotoxicity and Cell Uptake
The effect on cell viability of PAMAM dendrimers and dependence on concentration was assessed by a propidium iodide (PI) assay. This dye cannot permeate intact cell membranes while it is internalized when the
2.3 Cytotoxicity and Cell Uptake 25
Figure 2.2: Schematic diagram of dendrimers analyzed in this work. Den-drimers with different physicochemical surface properties, cationic, neutral and hydrophobic (top), and cationic dendrimers varying in size and charge (bottom) were studied. Amine terminated PAMAM dendrimers are pos-itively charged at physiological pH owing to primary amine group proto-nation (pale blue). Acetylation of primary amines (in green) reduces the surface charge density. Dendrimer hydrophobicity was modulated by adding C12 fatty acid chains (gray).
membrane is defective. With this assay we could monitor two distinct phenomena: (a) the reduction on cell viability (propidium permeates apoptotic and dead cells); (b) the reversible dendrimer-induced perme-ation of cell membranes reported by Hong et al.[39]
Figure 2.3 and Table 2.1 show our PI assay results after treatment of HeLa cells with dendrimers at 100 nM, 500 nM and 1.5 µM. Dendrimers do not display a significant reduction of cell viability up to 1.5 µM except for the highly charged G6 dendrimer. Notably the acetylation of primary amine in G6-Ac and the consequent lower surface charge strongly reduced toxicity, in line with the results by Stasko et al [40]. All experiments in the following were performed with 100 nM dendrimer concentration. At this
Figure 2.3: Propidium iodide assay on HeLa. Cells were treated with different amounts of dendrimers (100 nM, 500 nM and 1.5 µM) for 1 h, and cell viability is reported. Dendrimers display a low cytotoxicity in this concentration range except for the highly charged G6.
value none of the dendrimers displayed a relevant effect on cell viability and membrane permeability.
Cytotoxicity was also analyzed considering the large differences in molecular weight of the dendrimers studied in this work. Table 2.1 reports the molecular weight and the concentration of dendrimers administered for PI assay expressed in µg/mL in order to consider the toxicity on a mass basis.
From these data it appears that the main factor affecting cytotoxic-ity is surface charge regardless of size and mass. Since previous reports demonstrated that some cell penetrating peptides (e.g., HIV-1 TAT de-rived peptides) can induce actin reorganization causing loss of stress fibers and peripheral retraction [41], we monitored the effect of dendrimers on cytoskeleton integrity. Figure 2.4 shows phalloidin staining of actin fila-ments and indicates that no cytoskeleton perturbation occurred following dendrimer administration. In order to evaluate the cell uptake of
den-2.3 Cytotoxicity and Cell Uptake 27
MW Conc nM Conc µg/uL Viability SD
G2 3.5 100nM 0.3µg/mL 98.0 0.7 G2 3.5 500nM 1.7µg/mL 97.3 0.6 G2 3.5 1500nM 5.0µg/mL 97.2 2.0 G4 14.2 100nM 1.4µg/mL 98.0 1.0 G4 14.2 500nM 7.1µg/mL 97.2 0.6 G4 14.2 1500nM 21.3ug/mL 95.7 0.7 G6 58.0 100nM 5.8µg/mL 97.6 0.5 G6 58.0 500nM 29.0µg/mL 88.4 1.0 G6 58.0 1500nM 87.0µg/mL 67.0 5.0 G6-Ac 62.2 100nM 6.1µg/mL 87.8 0.5 G6-Ac 62.2 500nM 3.01µg/mL 95.5 0.5 G6-Ac 62.2 1500nM 92.0µg/mL 94.2 0.7 G4-C12 20.1 100nM 2.0µg/mL 97.0 2.0 G4C12 20.1 500nM 10.1µg/mL 90.1 4.0 G4-C12 20.1 1500nM 30.2µg/mL 86.9 4.0 G4-Ac 3.5 100nM 1.7µg/mL 99.0 0.6 G4-Ac 3.5 500nM 8.5µg/mL 97.2 0.5 G4-Ac 3.5 1500nM 25.3µg/mL 97.0 1.0
Table 2.1: Propidium iodide cytotoxicity assay. Concentration values are expressed both in nM and µg/mL in order to consider the toxicity both on a molecular and mass basis.
Figure 2.4: Phalloidin staining of actin filaments (green) of untreated (left) and dendrimer-incubated HeLa cells (dendrimer labeled in red). No alteration of cytoskeleton was observed. Scale bar = 10 µm.
dritic structures flow cytometry was performed: results are reported in Fig. 2.5.
The experiment was carried out incubating for 1 h HeLa cells with labeled dendrimers; as discussed in the following section in these con-ditions dendrimers enrich only cell membranes while internalization is negligible. As a consequence we can present a quantitative assay of mem-brane affinity of different PAMAM dendrimers, a crucial aspect for cel-lular uptake. Data presented in Fig. 2.5 were normalized taking into ac-count the single-dendrimer brightness values (determined by FCS). FACS analysis on HeLa cells demonstrates that membrane affinity depends on the amount of positive charge on the dendrimer surface (G6>G4>G2) and can be modulated by acetylation (G6>G6-Ac>G4-Ac). Moreover lipidated dendrimers show more affinity for cell surface than the corre-sponding cationic molecules. From the comparison of PI assay and FACS analysis we can conclude that the same groups (cationic amines and lipid chains) that promote membrane binding (and thus cell uptake) are re-sponsible for cytotoxicity. The realization of an ideal vector relies on the balance between these two aspects: according to our study G4, G4-C12
2.4 Time Lapse Imaging and Colocalization Assays 29 and acetylated G6 offer the best compromise between efficient internal-ization and low toxicity.
Figure 2.5: FACS analysis performed after 1h treatment with different dendrimers (100 nM concentration).
2.4
Time Lapse Imaging and Colocalization
As-says
In order to investigate the mechanism of dendrimer internalization in living cells we performed time-lapse imaging of HeLa cells treated with dendrimer-Alexa647 conjugates at different incubation temperatures. Fig-ure 2.6 shows dendrimer localization after 2 h when cells were incubated either at 4 or 37◦C. In both cases dendrimers bind the cell membrane, but
at 4◦C no internalization occurs. This confirms that cationic dendrimers
have a strong electrostatic affinity for the cell membrane (likely for nega-tively charged membrane proteoglycans and phospholipids) and indicates that they are internalized by an active energy-dependent process.
Figure 2.7 shows time-lapse imaging of a HeLa cell treated with the G4 dendrimer. Three main steps in dendrimer internalization can be
Figure 2.6: Dendrimer internalization at 4◦C (left) and 37 ◦C (right) of
G4. At low temperature no dendrimer signal was observed inside the cells indicating that an active cell process is necessary for internalization. Scale bar = 15 µm.
identified. First the cell is rapidly coated by fluorescent dendrimers bound to the membrane surface. In the first hour no significant internalization is observed. At a later stage vesicles moving toward the nucleus are observed. After 4 h no membrane fluorescence is detectable. Dendrimers were delivered to their intracellular fate in the perinuclear region in 12 h. Time-lapse imaging (up to 48 h) did not reveal any further dendrimer-localization changes.
Figure 2.7: Time lapse imaging of HeLa cell treated with G4 dendrimer (red). After membrane binding (1h) dendrimer is internalized and gradually delivered to the perinuclear region. Nuclei are stained with Hoechst (blue).
2.4 Time Lapse Imaging and Colocalization Assays 31 The rather long residence times on the cell membrane and correspond-ingly slow internalization kinetics are probably caused by the strong in-teractions with negatively-charged membrane proteoglycans. Dendrimer internalization in HeLa cells seems also slower than in other cell lines [33]. This may be linked to membrane composition and constitutive cel-lular endocytosis regulation. In order to identify the biological compart-ments involved in dendrimer internalization we performed colocalization assays with several biomarkers characteristic of different endocytic vesi-cles. Transferrin-Alexa488 and 70 kDa dextran-FITC were used to trace clathrin dependent pathway and macropinocytosis [42, 43], respectively. A transfection of caveolin-E1
GFP was used to label caveolae [44]. Fig-ure 2.8 shows colocalization data between dendrimers (red channel) and endocytic vesicles (green channel). Table 2.2 provides Pearsons coeffi-cients for each colocalization. Data show that all dendrimers examined strongly colocalize with transferrin and dextran (yellow vesicles) while there is no significant correlation with the caveolin-E1GFP fluorescence
signal. Regardless of size, surface functionality and total charge, all den-drimers studied appear to follow the same route. These data revealed that both clathrin-mediated endocytosis and macropinocytosis are involved in dendrimer internalization in HeLa cells, while caveolin does not play an important role. These conclusions are also supported by the colocaliza-tion with TAT peptide that is known to follow these two pathways [36, 45] and by the effective internalization observed in caveolin-lacking HepG2 cells that will be presented in the next sections. These observations are consistent with the observation of Kitchens et al [34, 46]. These au-thors reported clathrin mediated internalization for PAMAM dendrimers in Caco2 monolayer (macropinocytosis was not investigated), but dif-fer from what observed by Perumal et al [33]. This may be explained with the different behavior of the A549 cell line used in that study for which even internalization of neutral dendrimers was reported. Time-lapse imaging demonstrates that dendrimers are eventually delivered to a vesicular structure in the perinuclear region. In order to identify the latter we performed colocalization assays with the lysosome marker lysosensor.
Figure 2.8: G4 Dendrimer (red) colocalization assay with in vivo endo-cytic markers (green). High colocalization (yellow vesicles) was observed with transferrin (clathrin pathway) and dextran (macropinocytosis) while no correlation with caveolin signal was observed. Scale bar = 15µm.
Figure 2.9 shows very high colocalization with lysosensor. Thus cationic dendrimers bind the negatively-charged cell exterior and are internalized in HeLa cells via the clathrin-dependent pathway and macropinocytosis
Dextran Transferrin Caveolin G2 0.09(0.07) 0.10(0.08) 0.02(0.05) G4 0.19(0.11) 0.17(0.07) -0.04(0.08) G6 0.24(0.03) 0.17(0.06) -0.05(0.08) G6-Ac 0.30(0.09) 0.18(0.12) -0.06(0.10) G4-C12 0.26(0.10) 0.18(0.09) -0.03(0.08)
Table 2.2: Pearson’s coefficients for dendrimers colocalization with endo-cytic markers.
2.5 TAT-Dendrimer Comparison 33 and populate early endosomes and macropinosomes that are progressively acidificated until lysosome delivery. These observations are of much in-terest for drug-delivery purposes for two main reasons: a) in this acidic environment dendrimer tertiary amine protonation contributes to vesicle swelling and destabilization thus facilitating vesicle escape [21]; b) pH-triggered release based on pH cleavable linkers can be a viable strategy for the release of small molecules that can permeate destabilized acidic vesicles and diffuse in the cytoplasm [47, 17].
Figure 2.9: Colocalization of G4 dendrimers (red) with lysosome marker after 12h from dendrimer treatment reveals that dendrimers are completely delivered to the lysosomal compartment.
2.5
TAT-Dendrimer Comparison
Dendrimer endocytic properties were compared to those of a much-studied cell penetrating peptide (CPP): the arginine-rich motif derived from the HIV protein TAT. This arginine-rich peptide binds aspecifically cell mem-branes and is mainly internalized by macropinocytosis and clathrin-dependent endocytosis [36, 45]. Figure 2.10 shows HeLa cells imaged 30 min after coincubation with G4-Alexa647 and TAT-Rhodamine conjugates. As pre-viously showed G4-dendrimers at early time points are localized on the membrane while TAT displays a faster endocytosis and at about 1 h is completely localized in the perinuclear region. By incubating HeLa cells with dendrimers 2 h before TAT treatment a marked colocalization could
be observed. Notably in the cotreatment a 10-fold higher concentration of TAT was necessary owing to the larger affinity of dendrimers for cell membranes.
Figure 2.10: G4-AF647 (red) TAT-Rhodamine (green) colocalization. Co-treatment reveals faster internalization of TAT in HeLa cells (left). TAT treatment after 2h of incubation with dendrimer displays strong colocaliza-tion indicating that both vectors follow the same internalizacolocaliza-tion pathway but with different kinetics (right). Scale bar =5 µm.
These results demonstrate that PAMAM dendrimers possess similar properties to these widely used cell-penetrating peptides and thanks to their chemical tunability may represent a valid alternative for drug and gene delivery. Moreover the functionalization of dendrimers with TAT, or TAT-derived peptides with improved properties [48] could lead to an enhancement of the internalization properties of the dendrimer.
2.6
Cell Line Analysis
All experiments reported so far were carried out using HeLa cells as a model for endocytosis. We investigated dendrimer internalization also in other cell lines. In particular we considered PC-12 (neuron-like), HepG2 (human hepatocarcinoma), MRC5 (human lung fibroblast) and primary astrocytes. Figure 2.11 shows that cationic PAMAM dendrimers can cross
2.6 Cell Line Analysis 35 cell membranes in all these cell types but with some notable differences.
Figure 2.11: G4 (in red) endocytosis in different cell lines: PC-12 (upper-left), MRC5 (upper right), HepG2 (bottomleft) and Primary astrocytes cultures (bottom right). Nuclei are stained with Hoechst (blue). Scale bar = 15µm.
Dendrimers in HepG2 cells display a strong and faster endocytosis and after 4 h they reach their intracellular fate in the perinuclear re-gion. On the contrary in the case of PC-12 cells, dendrimers display high membrane affinity but very slow internalization as shown in Fig. 2.11. After 4 h the fluorescence signal is mainly localized on the membrane and few endocytic vesicles are observed inside cells, probably owing to slow membrane recyling. Finally internalization in primary cell cultures was investigated. Both astrocytes and lung fibroblasts display G4 internal-ization with a similar evolution to what observed in HeLa and analogous lysosomal delivery. This cell-line analysis reveals that cationic dendrimers are capable of crossing cell membranes of all tested cellular types but with different efficacy and dynamics that we argue are linked to the differences in membrane chemical composition and detailed recycling kinetics.
Chapter
3
Dendrimer-based Fluorescence pH
Sensors
The unique properties of dendrimers (i.e. monodispersity, chemical tun-ability and multifunctionality) were extensively exploited to realize car-riers for drug or gene delivery. These molecular features are also highly desirable for molecular sensing devices, yet just few applications of den-dritic molecules in this field were reported to date. Recent pioneering works employed dendrimers as scaffolds for the realization of colorimet-ric [49], electrochemical [50] and optical [51] sensors. The last approach seems to be particularly promising for in vivo measurements of biolog-ically relevant analytes thanks to its potentially high biocompatibility, as recently demonstrated by in vivo oxygen imaging performed by Vino-gradov and coworkers.[30] Application of these sensors to the biological environment and in particular to live cells is, however, still very limited. This likely stems from difficulties in sensor delivery and from the poor understanding of dendrimer behavior in living cells. In this chapter we describe the development of novel PAMAM dendrimer-based fluorescent pH sensors and we present a successful methodology for sensor delivery in living cells. Owing to the properties of the macromolecular scaffold
the proposed sensors allows ratiometric imaging in living cells overcom-ing many of the common drawbacks of organic sensovercom-ing dyes. Moreover organelle-targeted sensing is of much interest. Importantly, the synthetic procedure shown here can be used for other sensing dyes so that the sen-sors presented here can represent a model for a family of dendrimer-based sensors of interest for other biologically relevant ions and small molecules.
3.1
Materials and Methods
3.1.1 MaterialsG2, G4, G6 PAMAM dendrimers, acetic anhydride, Fluorescein, Amicon ultra 10 KDa dyalisis membranes were purchased from SigmaAldrich. Alexa647 dye, Oregon Green, Rhodamine Red, Lysosensor, DAPI, Syto81 and DiI were purchased from Invitrogen. NMR spectra were acquired by means of a Varian 300MHz spectrometer. IR spetra were obtained with a Perkin-Elmer FT-IR instrument.
3.1.2 General Procedures for Dendrimer Synthesis
Two sets of dendrimers were synthesized: i) neutral dendrimers labeled with Oregon Green 488 and ii) cationic dendrimers labeled with Alexa 647. Dendrimers were labeled and acetylated according to the procedures reported in Sect. 2.1.2. Materials were characterized by NMR, UV-Vis spectroscopy and Dynamic Light Scattering.
3.1.3 UV-Vis Spectrofluorimetry
Excitation and emission spectra were recorded by means of a Cary Eclipse fluorometer (Varian, Palo Alto, CA). Typically, 500 µL samples were used in quartz cuvette (Hellma, Milan, Italy). The temperature of the cell compartment was controlled by a built-in Peltier cooler (Varian). 5 nm excitation and emission band-pass filters were employed.
3.1 Materials and Methods 39
3.1.4 Cell Culture and Electroporation
HeLa and CHO cell lines were purchased from ATCC and cultured follow-ing manufacturer’s instructions. For live-cell microscopy cells were plated onto 35 mm glass-bottom dishes (WillCo-dish GWSt-3522) and imaged at 37 ◦C, 5% CO2. Electroporation was performed using Microporator
(Digital Bio Technology, Korea) according to manufacturer instructions for HeLa cultures.
Briefly, cells were resuspended in microporation buffer (Digital Bio Technology) containing dendrimers (250 nM and 2 µM for cationic and neutral PAMAM, respectively). Electroporation was performed with 10 µL volume-sized tip for neutral dendrimer and 100 µL volume-sized tip for charged dendrimers. In the case of charged dendrimers, after electro-poration cells were centrifuged for 5 min at 1200 rpm in order to remove dendrimer excess in the medium. Cells were washed and fresh medium was added before imaging, usually 12-18 h after electroporation.
3.1.5 Confocal Imaging
Cell imaging was performed with a Leica TCS SP2 inverted confocal mi-croscope (Leica Microsystems) equipped with a 40x 1.25 NA oil immersion objective (Leica Microsystems). Imaging was obtained illuminating the samples with the Ar and He-Ne lasers and with a 403 nm pulsed diode laser (M8903 01; Hamamatsu) at 50 MHz repetition rate. Fluorescence emission was collected with the microscope AOBS-based built-in detec-tors.
3.1.6 Kinetics of Acetylated Dendrimer Hydrolysis
In order to prepare cytoplasm-cell extracts U2OS cells (ATCC: HTB96) were grown in DMEM, supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 10 U/L penicillin, 10 µg/L streptomycin and 10% fetal bovine serum (Gibco). Typically 5 107 cells were used for cytoplasm extraction:
ice-cold PBS. Similarly to what already reported [52] cytoplasm was ex-tracted resuspending cells (at 4 107 cells/mL) in ice-cold 15 mM Tris-HCl
pH = 7.5, 10 mM KCl, 10 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 300
mM sucrose, 10 % glycerol and 0.1 % Triton-X100 for 7 min. This buffer was supplemented with protease inhibitors (leupeptin, aprotinin, PMSF), phosphatase inhibitors (NaF, Sodium orthovanadate), 1 mM DTT and 1mM ATP prior to use. Low-speed centrifugation (1300 g, 5 min, at 4◦C)
allowed the separation of the cytoplasm (supernatant) from intact nuclei (pellet). Lysate protein concentration was finally estimated by Brad-ford assay (Pierce). After acetylation, dialyzed dendrimer-based pH sen-sors were incubated with 50-100 g cytoplasm extract, 1:5 diluted in PBS buffer, at 37 ◦C. As a control, dendrimers were incubated, in the same
conditions, with the same volume of cytoplasm-extraction buffer. Fluo-rescence emission spectra of Oregon Green, Fluorescein, and Coumarin were collected at regular time intervals and constant T = 37◦C as
previ-ously described.
3.1.7 In Vitro Calibration
pH titration of dendrimer-based sensors fluorescence was performed on 1 µM samples dissolved in 2 mM phosphate buffer whose pH was adjusted by small addition of NaOH 0.1 M. Emission spectra of the sensing dye and Rhodamine Red were obtained for each pH.
3.1.8 Calibration and pH Imaging in HeLa Cells
HeLa cells were electroporated as described above and imaged 12 h af-ter dendrimer administration. Fluorescein and Rhodamine were imaged using the 488 nm (collection from 500 nm to 550 nm) and the 561 nm (collection from 575 nm to 700 nm) laser lines of the confocal system, respectively. To obtain a pH calibration of the sensor cells were clamped at the desired pH by using pH ionophores in a K+
3.2 PAMAM Dendrimers-Dye Conjugates 41
3.2
PAMAM Dendrimers-Dye Conjugates
The physicochemical properties of PAMAM dendrimers have been ex-tensively investigated since their early synthesis [2]. In particular amine terminated and acetylated PAMAMs were studied in much detail and their relevant properties fully characterized by experimental [38, 53] and computational techniques [17]. We exploited this information to de-sign dendrimer-based devices in which the physicochemical properties of the dendrimer periphery can modulate the interaction with biomolecules within living cells. In order to study their intracellular behavior, PA-MAM dendrimers varying in size and surface charge were labeled with bright and photostable fluorophores. Amine-terminated PAMAM den-drimers were chosen as a model for cationic denden-drimers since primary amines are protonated and bear a positive charge at physiological pH. Generations from G2 up to G6 were chosen to span a broad range of molecular-weight and surface-charge values. In order to obtain neutral structures, G4 and G6 cationic dendrimers were fully acetylated with acetic anhydride. The fine tuning of dendrimer size and surface proper-ties will be shown to yield selective dendrimer localization in living cells. Amine-terminated PAMAM dendrimers were labeled with Alexa647 while fully-acetylated neutral dendrimer were labeled with Oregon Green 488. Neutral dendrimers were labeled before acetylation. By using an excess of acetylating agent we achieved the acetylation of the free hydroxyl of the fluorophores. This will be relevant for the on/off feature of the pH sensors presented in the next sections.
3.3
Electroporation and Intracellular Targeting
In order to study the intracellular behavior of PAMAM dendrimers we developed a protocol for cell delivery based on electroporation, a tech-nique that is routinely used for the transfection of genetically-encoded GFP-based sensors and recently proposed also for quantum dots [54]. In this way we delivered fluorescent dendrimers directly into cells and could
study the dynamics of these molecules inside living HeLa cells by confo-cal microscopy. This technique offers some interesting features such as: i) fast and effective delivery; ii) direct delivery into the cytoplasm avoid-ing the complexity of endocytotic pathways [33]; iii) delivery in parallel to the whole cell population. These characteristics make electroporation quite advantageous in comparison with other delivery techniques such as microinjection, direct incubation and liposome-based transfection. We
Figure 3.1: Dendrimers varying in size (from G2 up to G6) were studied. Nuclei (in blue) were stained with Hoechst. (b) Colocalization assay for cationic dendrimers (in red) with organelle markers (in green). Scale Bar ) 15 µm.
studied cationic (G2, G4 and G6) and neutral (G4 and G6) fluorescent dendrimers and their delivery into living HeLa cells. Figure 3.1 shows the localization of different generations of cationic dendrimers in HeLa cells (Hoechst staining was also used as a nuclear marker). Images show that G2 is homogenously distributed inside cells while G4 and G6 display a peculiar patterned localizations. We performed a colocalization assay to reveal which organelles are involved in dendrimer trafficking and the results are reported in Fig. 3.2. G4 dendrimers colocalize with the syto81
3.3 Electroporation and Intracellular Targeting 43 dye, a nucleolar marker [55]. Indeed nucleolar localization was reported also for other cationic molecules able to bind negatively-charged RNA [56]. G6 dendrimers colocalize with a plasma membrane marker (DiI) and with a lysosome marker (lysotracker). Intracellular targeting to spe-cific organelles is of much interest for biosensing. It was obtained here by modulating the physicochemical properties of the dendrimer. This approach can be further extended by conjugating appropriate ligands or peptides to the scaffold in order to achieve targeting to a wider range of organelles. Figure 3.3 shows the localization of fully acetylated G4-Ac
Figure 3.2: Dendrimers (in red) were colocalized with the followings or-ganelle markers (in green): Syto81 (for nucleoli, top), lysotracker (for lyso-somes, middle) and DiI (for plasma membrane, bottom). Scale Bar = 15 µm.
and G6-Ac dendrimers. Confocal images demonstrate that neutral den-drimers show a much less structured localization with respect to their cationic analogs. The only notable aspect is the different distribution between nucleus and cytoplasm for G4 and G6 dendrimers. This can be linked to their different size (G4 14 kDa, G6 58 kDa). The larger
Figure 3.3: G4-Ac and G6-Ac dendrimers localization after electropora-tion. Nuclei (in blue) were stained with Hoechst.
G6 dendrimer cannot freely diffuse from the cytoplasm to the nucleus since its size is comparable to the nuclear-pore limit.[57] Some vesicular localization was observed for all dendrimers, probably owing to a small fraction of dendrimer being internalized by endocytosis or compartimen-talized after electroporation. Interestingly the localization of the neutral G4-Ac is pH dependent, as shown in Fig. 3.4. Indeed the acidification of the cytoplasm (induced with H+
ionophores) led to the accumulation of dendrimers in the nucleoli with a similar localization of the cationic G4 analog: at acidic pH the tertiary amines in the interior of the dendrimers are protonated and therefore the macromolecule can go from neutral to cationic [58]. This pH triggered structural change deserves further study and may be exploited for the development of stimuli-responsive materials.
Figure 3.4: Representative images of pH dependent localization of G4-Ac dendrimers. Scale Bar = 15 µm.
3.4 Dendrimer-based pH Sensors 45
3.4
Dendrimer-based pH Sensors
In this section we present synthesis and photophysical characterization of three different dendrimer-based pH sensors suitable for in vivo imaging. H+
ions are relevant in many physiological mechanisms and pH indicators were developed in order to monitor their intracellular concentration [59]. Figure 3.5 schematically shows the synthesis of the sensors. Two different
Figure 3.5: Schematic synthesis of pH sensors: (i) NHS-activated dyes, (ii) acetic anhydryde, triethylamine.
fluorophores were conjugated to the G6 dendritic scaffold: a pH sensitive dye (carboxyfluorescein in the diagram) and a pH-insensitive rhodamine dye (Rhodamine RedX). Thanks to dendrimer multifunctionality these two moieties can be conjugated to the same macromolecule and yield a ratiometric sensor. The pH sensitive fluorescein signal can be normalized to the pH-independent red signal of rhodamine, thus compensating for the signal dependence on sensor concentration. This is of crucial rele-vance for the application of the biosensors in vivo. With the same syn-thetic approach three different pH sensors with different H+ affinity were
realized. This result was achieved conjugating to the same dendritic scaf-fold (G6-Ac) different commercial sensing dyes: i) 7-Hydroxy-4-methyl-3-coumarin; ii) 5(6)-carboxyfluorescein; iii) Oregon Green 488. These three fluorophores are tuned for measurements in alkaline, neutral and acidic
environment, respectively. All conjugates were obtained from the reaction of the N-hydroxysuccinimide ester of the dye with the terminal amine of the dendrimer yielding a stable amide bond. Figure 3.6 shows the ab-sorption spectra for the three dendrimer-based sensors. All curves show the presence of the sensing dye together with the rhodamine reference. An excess of the sensing dye in comparison to the rhodamine reference (peak at 550 nm) was used. This is reflected in the relative height of the absorption peaks of the two dyes. The cationic dendrimers obtained can be acetylated in order to neutralize the surface charge of the sensor using an excess of acetic anhydride. Interestingly in these conditions the pH sensitive fluorophores are also acetylated since they all present a free hy-droxyl group that is crucial for their fluorescence and sensing properties. Indeed after acetylation the fluorescence of these molecules is completely quenched while the rhodamine fluorescence is virtually unmodified.
Figure 3.6: Absorption spectra of the three pH sensors highlight the pres-ence and the relative functionalization degree of both sensing dyes and ref-erence dyes.
We studied the hydrolysis kinetics of the acetyl esters of the three fluorophores utilized in this work by incubating them with a cytoplasmic cell lysate (i.e. a cell extract of cytoplasmic proteins) in order to mimic the intracellular conditions of hydrolysis. The recovery of the fluorescence of the pH-sensitive fluorophores induced by the cytoplasmic lysate was compared to the recovery in buffer alone as shown in Fig. 3.7. All fluo-rophores show slow fluorescence recovery in buffer that reaches a plateau
3.5 In Vitro Titration 47 within about 24 h, while the presence of cytoplasmic enzymes promotes a more rapid hydrolysis kinetics. This difference is considerable for fluo-rescein and Oregon green that display very fast kinetics in the presence of cell lysate, while it is less marked for coumarin. These large differ-ences between in vitro and in live-cell conditions can be exploited for the design of cell activated biosensors that actually turn their fluorescence on only once inside the target cells. This mechanism resembles the ace-toxymethylester (AM ester) system that was successfully applied in many in vivo measurements [60].
Figure 3.7: Hydrolysis kinetic studies for three different pH biosensors. Fluorescence recovery after acetylation was studied in PBS/lysis buffer (light-colored curves) in comparison with PBS/lysis buffer containing cy-toplasmatic protein extract (dark-colored curves).
3.5
In Vitro
Titration
Figure 3.8 reports in vitro sensor-titration data. Emission spectra of pH-sensitive carboxyfluorescein and rhodamine were recorded at differ-ent pH values (Fig. 3.8a). It can be seen that even following excitation
at 488 nm a red emission band is present as a consequence of Fluores-cence Resonance Energy Transfer (FRET): the two molecules are forced in close proximity on the dendrimer structure. Figure 3.8b reports the
Figure 3.8: (a) Fluorescence emission spectra at different pH of Fluores-cein inside G6-Ac-Fluo-RhRed sensor. (b) Green and red channel emission intensities of G6-Ac-Fluo-RhRed versus pH. (c) Fluorescence ratio at dif-ferent pH for G6-Ac-Coumarin-RhRed (blue), G6-Ac-Fluorescein-RhRed (green), and G6-Ac-Oregon Green-RhRed.
fluorescence intensities for the green and the red fluorophores upon pH titration. As expected, fluorescein emission strongly depends on pH while the red fluorophore is virtually pH insensitive in the physiological range (∆F<10%). The ratio between the sensing fluorophores and the rho-damine for the three sensors is reported in Fig. 3.8c. The three titration curves demonstrate that these sensors are suitable to measure pH in differ-ent ranges as expected from the H+
affinity values of the dyes employed. Notably some of the photophysical properties of the dyes are not fully retained after dendrimer conjugation. For example, the titration profile of Oregon green is different from the one reported by the manufacturer for the isolated fluorophore. We observed a similar behavior also with other dendrimer-fluorophore conjugates (data not shown). This does not hinder the successful applicability of the sensors presented, but we believe that a thorough understanding of the fluorescent dendrimer photophysics will allow the design of improved sensors.
3.6 Calibration and pH Measurements in HeLa Cells 49
3.6
Calibration and pH Measurements in HeLa
Cells
We applied the electroporation protocol previously described to our dendrimer-based pH sensors. We used the fluorescein-dendrimer-based sensor for living cells studies in light of its ability to measure pH around physiological val-ues; fully-acetylated G6-dendrimer sensors were therefore delivered into HeLa cells by electroporation. Figure 3.9 shows the fluorescence images of dendrimer-treated HeLa cells. Both fluorescein (green) and rhodamine
Figure 3.9: Titration of G6-Ac-Fluo-RhRed in living cells. HeLa cells were clamped at pH = 6, pH = 7, and pH = 8 with H+ionophores. A mask
was applied to remove pixels with low low signal-to-noise ratio. Scale Bar = 15 µm.
(red) fluorescence were recorded and ratiometric pH maps were calculated on a pixel-by-pixel basis. As expected the sensor retains the localization of the G6-Ac dendrimer: the cytoplasm and the nucleus of the cell are labeled albeit with a lower fluorescence signal from the latter. In order to

![Figure 1.4: Dendritic box encapsulating rose bengal (from Meijer et al [8]).](https://thumb-eu.123doks.com/thumbv2/123dokorg/4924260.51438/20.744.162.554.322.587/figure-dendritic-box-encapsulating-rose-bengal-from-meijer.webp)

![Figure 1.6: PAMAM dendrimers snapshots from MD simulations. From Goddard et al [14].](https://thumb-eu.123doks.com/thumbv2/123dokorg/4924260.51438/23.744.163.629.128.241/figure-pamam-dendrimers-snapshots-from-simulations-from-goddard.webp)


![Figure 1.9: Examples of dendrimer-based in vivo imaging exploiting dif- dif-ferent techniques: a) Positron Emission Tomography (PET)[25]; b) Near infrared (NIR) optical imaging[26]; c) Single photon emission computed to-mography (SPECT)[27] and d) Magneti](https://thumb-eu.123doks.com/thumbv2/123dokorg/4924260.51438/26.744.145.565.138.377/examples-dendrimer-exploiting-techniques-positron-emission-tomography-magneti.webp)
