CHAPTER 4: RESULTS
4.1 VECTOR GENOME ORGANIZATION
Packaging and vector constructs were derived from p∆00, a replication competent clone of FIV-Pet. The packaging construct p∆env1 (fig. 3.1) provides Gag and Pol that are expressed under the control of the CMVp, also working in non-feline cells. Construct p∆env1 lacks the untranslated 5’LTR-gag region that, together with the initial part of gag, form ψ required for viral RNA encapsidation into assembling virions. Finally, p∆env1 is also devoid of env, which is provided by a different plasmid, and the 3’ LTR that has been replaced with BGH polyA.
The SIN vector LA34 (fig. 3.2) is under control of CMVp and has few remnants of the FIV genome, namely: 1) the ψ signal (encompassing R, U5 and 120 nt of gag region) so that the construct is packaged into budding particles; 2) the RRE motif, placed downstream ψ, interacting with Rev provided by the packaging construct and necessary to export unspliced and singly spliced RNA from nucleus to cytoplasm; 3) the untranslated region between env and 3’LTR; 4) the two LTRs deleted of U3 region to avoid generation of wild type LTRs during reverse transcription. To allow transgene cloning and expression, an expression cassette made of a CMVp and MCS were inserted between RRE and 3’ LTR. Because of extensive rearrangement and deletions, LA34 is only 2.2 Kb in size.
4.2 WPRE INCREASES NON-FELINE CELLS TRANSDUCTION
Vector particles production was verified by WB on clarified supernatant of transfected cell. As expected, the protein pattern obtained by split vector particles was indistinguishable from wild type whole FIV-Pet except Env that in the vector was
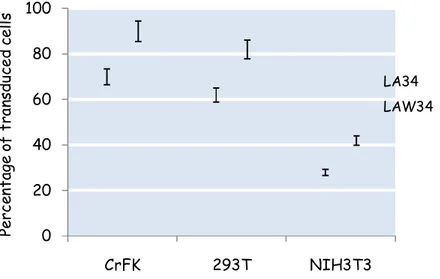
41 in the attempt to widen the breadth of tropism. We initially tried to expand the cell spectrum by adding the central polypurine tract (cPPT) element whose HIV analogous has been shown to improve nuclear translocation of pre-integration complex (PIC) and efficiency of transduction (Van Maele et al., 2003). In our system, however, the cPPT inserted upstream of expression cassette had no appreciable effects on vector performance in any cell line tested (Pistello et al., 2007). From these results we tried to increase transduction efficiency on non-feline cells by stabilizing transgene mRNAs and improving incorporation of vector RNA into pseudotyped particles, two important determinants of lentiviral cell transduction and transgene expression (Loewen et al., 2003). These features were brought about by the WPRE element that in HIV vectors has proved able to increase transduction efficiency when cloned downstream the transgene. When WPRE was inserted downstream GFP in LA34-GFP (vector LAW34-GFP), the efficiency of transduction greatly improved (fig. 4.1).
Figure 4.1: Transduction efficiency following WPRE insertion as evaluated on feline, human and murine cells using GFP as reporter gene. The WPRE element increased transduction efficiency in all cell line tested.
0 20 40 60 80 100 CrFK 293T NIH3T3 Pe rc en ta ge o f tr an sd uc e d c e ll s LA34 LAW34
In fact, compared to LA34, the efficiency of transduction doubled with NIH3T3 and reached levels around 50%.
Recent reports demonstrated that the main packaging domain in FIV gag is comprised in a 120 nt stretch (Mustafa et al., 2005). However, previous studies had suggested that the encapsidation signal was in fact encompassed with first 300 nt of gag region. From this hypothesis we constructed LA34 and LAW34 vectors with a 311 nt-long ψ (LA34L and LAW34L; fig. 3.4a and b). When compared to the respective vectors with 120 nt ψ, the longer ψ did not bring any obvious benefit as the efficiency of transduction was slightly reduced in cell lines of non-feline origin with the exception of CrFK cells for which LAW34 and LAW34L transduced at similar efficiency (Fig. 4.2). From these studies, we choose to use LAW34 for subsequent studies.
Figure 4.2: Comparison of transduction efficiency of produced vectors as evaluated in feline, human and murine cells using GFP as reporter gene. The elongation of gag slightly increased transduction on CrFK but had no appreciably influence on non-feline cells. 0 20 40 60 80 100 CrFK 293T NIH3T3 Pe rc e nt ag e o f tr an sd uc ed c el ls LA34 LA34L LAW34 LAW34L
43 Vector safety was evaluated with several approaches. Firstly, the progeny particles were checked for p∆env1 incorporation by testing generated vector and transduced cells for the gag p25 sequence derived from the packaging construct (fig 3.1). The 674 bp amplicon investigated by PCR and RT-PCR assays was readily detected in DNA of transfected cells (positive control), due to the presence of transfected p∆env1 plasmid, but was absent in vector particles (not shown) and transduced cells (Fig. 4.3). These results demonstrate that deletion of ψ in p∆env1 prevents encapsidation of the packaging construct into budding particles.
Figure 4.3: Evaluation of p∆env1 carry over from transfected to transduced cells as evaluated by gag p25 PCR with the indicated primers. Lane A: DNA extracted from transfected cells; Lane B: No template control; Lane C: DNA extracted from transduced cells; Lane D: RNA extracted from transduced cells; Lane E: DNA from mock transfected cells.
Second, we checked whether U3 deletion, located in the 3’LTR of the vector construct, was translocated to the 5’LTR of proviral DNA during reverse transcription. This was investigated by using primers that generated amplicons of different sizes from full-length (352 bp) and U3 deleted LTRs (232 bp). The PCR product obtained from DNA of transduced cells was clearly smaller compared to the one generated from the p∆00 plasmid with full-length LTRs (fig 4.4). Likewise, no amplification was observed in RNA from transfected cells thus confirming that during reverse
transcription the U3 deletion at 3' LTR is efficiently transferred to 5'LTR. The result was also validated by sequencing (data not shown).
Third, functional inactivation of the 5’ LTR was evaluated by examining transduced cells for LA34 RNA genomes, the transcription of which would have required a full-length 5’LTR. As shown by figure 4.5, while DNA of transduced cells was positive for LA34 sequences, the RNA obtained from the same cells was uniformly negative, regardless of whether it was digested or not with RNase-free DNase. Collectively, these findings demonstrated that LA34 is indeed a SIN vector, that the generated pseudotyped particles are safe, and that no vector RNA is produced from LA34 transduced cells.
Figure 4.4 Translocation of U3 deletion to 5'LTR, as checked by PCR using primers annealing to beginning U3 and within the R region of LA34 proviral DNA. Lane A: p∆00 plasmid (full-length LTR); lane B: no template control; lane C: DNA from transduced 293T cells, lane D: DNA from mock transduced cells.
45 Figure 4.5: Analysis of LTR directed transcription as tested by PCR using primers upstream the GFP promoter. Lane A: DNA of transduced 293T cells; lane B: no template control; lanes C and D: RNA from transduced 293T cells with and without DNase treatment and prior to reverse transcription; lanes E and F: RNA from mock-transduced cells treated as for C and D.
4.4 LAW34/VSV-G TRANSDUCES THE HCC1937 TUMOR CELL LINE
The HCC1937 cell line was chosen as model to test our hypothesis as it expresses an inactive form of the protein for a germ-line BRCA1 mutation (5382insC) causing a premature stop 34 amino acids before the end. This cell line was established from a 24 year-old patient diagnosed with primary breast carcinoma and is one of the few available cell line with a homozygotic inactivation of BRCA1 (Tomlinson et al., 1998). LAW34-GFP was produced as described above and pseudotyped with VSV-G or RD114/TR GPs. HCC1937 were transduced with both vectors and assessed for GFP expression 2-4 days post transduction. Human 293T cells and murine NIH3T3 were used as control.
The results showed that LAW34-GFP/VSV-G transduces about 20% of tumor cells whereas the same cells were almost refractory to transduction with LAW34-GFP/RD114/TR (fig 4.6). From these results, transduction of HCC1937 was performed only with LAW34/VSV-G and obtained GFP positive cells were maintained in colture to monitor transgene expression for up to one month.
Figure 4.6: Percentage of GFP positive cells transduced with either VSV-G or RD114/TR/TR pseudotyped vectors was evaluated 4 days post transduction. 293T cells and NIH3T3 were used as positive control. While the RD114/TR/TR was more efficient than VSV-G in NIH3T3, HCC1937 cells were transduced only when pseudotyped with VSV-G.
In particular, GFP expression was monitored every 5 days and, during the whole period of observation, the percentage of transduced cells remained almost constant in both tumor HCC1937 and control cells (fig 4.7). Moreover, the percentage fluorescent cells maintained constant following several freezing and thawing cycles (data not shown)
0 20 40 60 80 VSV-G RD114 Pe rc e nt ag e o f tr an sd uc ed c el ls 293T NIH3T3 HCC1937
47 Figure 4.7: Duration of GFP expression in cells transduced with VSV-G pseudotyped vector. Cells were monitored for one month. During this period the percentage of fluorescent cells did not change suggesting that the vector genome was integrated in host cell genome.
4.5 TRANSDUCTION PROTOCOL EFFECTS ON LAW34 EFFICIENCY
To increase transduction efficiency of HCC1937, different techniques were tested including DT and PB. PB is a small, positive charged molecule that reduces charge repulsions between vector and cell thus facilitating virion adsorbtion on cell surface. To choose the most suitable PB concentration, increasing amount of PB were added to 293T cells following transduction with LAW34-GFP. Percent of fluorescent cells, monitored 4 days post-transduction, showed that addition of 8 µg/ml led to the highest percent of GFP-positive cells (fig. 4.8); this concentration was then used for all
0 20 40 60 80 4 11 15 20 25 33 Pe rc e nt ag e o f fl uo re ce nt c el ls Days 293T NIH 3T3 HCC1937
the experiments. DT protocol, consisting in the addition of two vector preparations 4 hours apart, should increase the amount of vector per cell with a consequent increment in transduction efficiency. The two protocols were tested, separately or in combination, on 293T, NIH3T3 and HCC1937 and compared. PB almost doubled the proportion of transduced HCC1937 cells (fig. 4.9), suggesting that this protocol substantially increased virus infectivity on this cell type. Likewise, DT protocol also increased transduction of all cell types. In all, combination of DT and PB protocols was the most effective, (fig 4.8), and underlined the robustness of LAW34 under various test conditions.
Figure 4.8: Transduction efficiency on 293T cells with different PB doses. Concentration of 8 µg/mL was the most efficient concentration and was used for all experiments carried out.
0 20 40 60 80 0 3 4 5 6 8 10 Pe rc e nt ag e o f tr an sd uc ed c el ls PB (µg/mL)
49 Figure 4.9: Comparison of transduction efficiency on 293T, NIH 3T3 and HCC1937 cells using the indicated protocols. Combination of DT and PB protocols was the most effective.
4.6 BRCA1 AND GFP ARE EXPRESSED IN TRANSFECTED CELLS
The initial plan was to insert the entire BRCA1 into LAW34 at once. Unfortunately, after several unsuccessful attempts, the strategy was revised to subclone the gene in four contiguous fragments. By computer analysis we identfied 3 enzymes, namely AflII, BstZ17I and BmtI to insert three contiguous BRCA1 fragments intoLAW34 MCS to produce LAW34III. Specific oligonucleotides with the enzyme restriction site were used to amplify BRCA1 fragments and PCR product inserted in LAW34III. Every subclone was screened by PCR and sequenced. All fragments had the right sequence except the first fragment that had a G insertion at 196 nt position and had to be recloned. All amplicons presented the correct sequence and once subsequently inserted into LAW34III led to the complete BRCA1 sequence to
0 20 40 60 80 100 293T NIH 3T3 HCC1937 Pe rc en ta ge o f tr an sd uc e d ce ll s Normal PB DT DT+PB
produce LAW34-BRCA1 vector (fig. 3.5). To facilitate the functional assay we produced a bicistronic vector expressing both BRCA1 and GFP genes. GFP translation was obtained by insertion of an internal ribosome entry site (IRES) that allows co-expression of both transgenes under a unique promoter control.
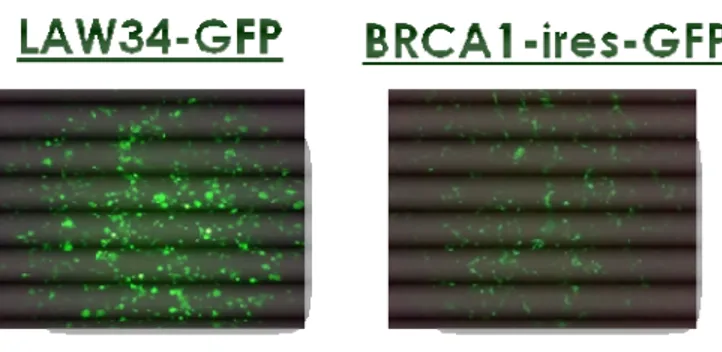
To produce the BRCA1-IRES-GFP bicistronic vector we cloned the IRES-GFP cassette (amplified from another plasmid) into LAW34-BRCA1 (fig. 3.6). BRCA1 expression of LAW34-BRCA1 and BRCA1-IRES-GFP was verified by WB of transfected 293T cells. Since BRCA1 is constitutively expressed in all cells it was not possible to discriminate between the protein naturally expressed by 293T cells and that vehiculated by the vector. However, the greater amount of BRCA1 protein in transfected compared to mock cells demonstrates the correct insertion and expression of BRCA1 (fig. 4.10). Proper GFP expression was evaluated in transfected 293T cells by fluorescent microscopy and FACS analysis. The intensity of fluorescence was compared with that of LAW34-GFP. Even if less strong than LAW34-GFP transfected cells, the bicistronic transfected cells showed a good GFP expression (fig. 4.11), which corresponded to 30% of transfected cells when investigated by FACS analysis (fig. 4.12).
Figure 4.10: BRCA1 expression in transfected and mock transfected cells. Mock transfected 293T cells are also BRCA1 positive since this gene is constitutively expressed in all cells. The stronger band of transfected cells indicate however that the exogenous protein is correctly expressed by the FIV vector.
51 Figure 4.11: GFP expression in LAW34-GFP and BRCA1-IRES-GFP transfected cells. The intensity of fluorescence in latter cells is lower most likely because GFP is encoded downstream BRCA1 in BRCA1-IRES-GFP.
Figure 4.12: Quantification of GFP expression by FACS analysis. Due to the bicistronic nature of BRCA1-IRES-GFP vector, percentage of cells transduced with this vector is lower compared to cells transduced withLAW34-GFP.
0 20 40 60 80 100 LAW34-GFP BRCA1-ires-GFP Pe rc en ta ge of t ra ns fe ct ed 2 9 3 T
4.7
LAW34-BRCA1
AND
BRCA1-IRES-GFP
ARE
EFFICIENTLY
PACKAGED
Both vector constructs produced are very long. BRCA1-IRES-GFP, in particular, exceeds FIV genome length and it has been therefore necessary to evaluate if both vectors can be packaged into virions. This was carried out by real time PCR on RNA extracted from supernatant of transfected 293T cells. To ensure that the amplification was performed on RNA, and not on plasmid remnants, the extracted RNA was treated with DNAse before reverse transcriptase reaction. To avoid possible contaminations, the real time PCR was performed by amplifying the heterologous element WPRE present only in our vector construct. The results showed that both vectors were around 1,7X107 RNA copies per ml. Moreover RNA copies were only
slightly reduced compared to shorter LAW34 and LAW34-GFP vectors that were treated in parallel (fig. 4.13). These findings demonstrate that although both vectors are longer then parental FIV genome, they are efficiently packaged into assembling virions.
Figure 4.13 RNA copies/ml quantified by real time PCR. Despite LAW34-BRCA1 and BRCA1-ires-GFP large size, they are packaged at similar efficiency compared to the
1,00E+00 1,00E+02 1,00E+04 1,00E+06 1,00E+08
LAW34 LAW34-GFP LAW34-BRCA1 BRCA1-ires-GFP
L og 10 R N A c op ie s/ m l
53 Vector particles were produced by co-transfection in 293T cells of the 3 plasmids previously described. Two days after, released vector particles were collected, titrated by real time PCR, and used to transduce HCC1937. Vector performances were assessed using bicistronic vector BRCA1-IRES-GFP and monitoring GFP expression in transduced cells 2-4 days after by FACS analysis. LAW34-GFP vector was used as control. The identification of a good percent of transduced HCC1937 fluorescent cells indicates that the bicistronic vector is working and expressing the encoded transgenes (fig 4.14).
Figure 4.14: Comparison between LAW34-GFP and BRCA1-IRES-GFP transduced cells. The intensity of fluorescence with latter vector is lower probably because GFP is encoded downstream BRCA1. .
BRCA1 expression in transduced HCC1937 was evaluated by FACS analysis, easier and faster compared to WB. Following labeling with the anti-BRCA1 mAb, we used a RPE labeled secondary Ab to monitor red fluorescence. Unfortunately since HCC1937 expresses a non-functional truncated protein shorter of 34 aa in C-terminal, there are no commercial antibodies that can discriminate the wild-type BRCA1 from
0 10 20 30 LAW34-GFP BRCA1-ires-GFP Pe rc e nt ag e o f tr an sd uc ed H C C 19 3 7
this mutated form. Therefore both mock and transduced HCC1937 showed a similar number of red cells (nearly 100%) and were indistinguishable when analyzed for percentage of fluorescence (fig. 4.15)
Figure 4.15: BRCA expression in HCC1937 A) unlabeled cells B) mock transduced cells C) transduced cells.
Although the percentage of fluorescent cells was similar between the mock and the transduced cells, the intensity of fluorescence showed a significant difference (fig. 4.16A). In fact transduced cells showed a higher intensity of fluorescence (pink line; mean fluorescence= 72.5) compared to unlabeled mock (white line; mean= 3.2), transduced (blue line; mean= 16.1) and, most importantly, labeled mock transduced (green line; mean 38.9) cells. This result demonstrates that transduced cells do have a higher number of BRCA1 copies and a higher fluorescent signal due to the presence of the wt form of BRCA1 expressed by LAW34-BRCA1. Analogous results were obtained when the same experiment was performed in BRCA1-IRES-GFP transduced HCC1937 (fig 4.16B) and in 293T cells used as control (fig 4.16C)
55 Figure 4.16: BRCA1 expression in transduced cells as determined by measuring the red fluorescence intensity. A) LAW34-BRCA1 transduced HCC1937; B) BRCA1-IRES-GFP transduced HCC1937; C) LAW34-BRCA1 transduced 293T used as positive control. The higher intensity of labeled, transduced cells indicates a higher expression level of BRCA1. mean Mock
3,2
LAW34-BRCA116,1
Mock antiBRCA138,9
LAW34-BRCA1 antiBRCA72,5
Fluorescence mean Mock3,2
BRCA1-ires-GFP9,3
Mock antiBRCA138,9
BRCA1-ires-GFP antiBRCA77,7
Fluorescence mean Mock3,5
LAW34-BRCA17,0
Mock antiBRCA124,11
LAW34-BRCA1 antiBRCA32,11
Mock Mock antiBRCA1
LAW34-BRCA1 LAW34-BRCA1 antiBRCA
B)
C)
. . . . . . . . . . . .4.9 TRANSDUCED BRCA1 RESTORES ITS ACTIVITY IN HCC1937
Once demonstrated the correct vehiculation and expression of BRCA1 in HCC1937, we investigated if the transduced transgene was functional. Since BRCA1 plays a role in maintaining genome integrity by repairing DNA DSBs arising during DNA replication or exogenous insults, we performed a RRR test to evaluate BRCA activity. This is based on the ability of a functional BRCA1 to rescue HCC1937 cells, which, as mentioned above, encode an inactive BRCA1, after irradiation with γ rays. To evaluate sensitivity to γ irradiation, HCC1937 were transduced with LAW34-GFP or BRCA1-IRES-GFP vectors, treated with ionizing radiations and analyzed by flow cytometry for one week (fig 4.17).
Figure 4.17: RRR protocol. HCC1937 cells were transduced with LAW34-GFP or BRCA1-IRES-GFP, irradiated with 2.3 Gy and monitored for 1 week by flow cytometry. RRE gag ΔU3 CMV R U5 CMV SD GFP WPRE R U5 BRCA1 ires RRE gag ΔU3 CMV R U5 CMV SD GFP WPRE R U5
57 transduced with LAW34-GFP encoding only GFP gene. Faster and more efficient rescue should also result in a higher increment of fluorescence of bicistronic transduced cells compared to control cells.
A preliminary experiment to choose the most suitable irradiation dose was carried out. Naïve HCC1937 were treated with increasing doses of irradiation (ranging from 0.8 Gy to 8 Gy) and monitored for cell viability and growth for 1 week. The results, shown in graphs 4.18 and 4.19 indicate that doses above 4.6 Gy kill the cells whereas doses lower than 1 Gy caused an only a transient delay in cell growth. A sensible reduction of cell viability and growth was observed in cell treated with 1.1 and 2.3 Gy, but since irradiation with 2.3 Gy caused a slower recovery, this dose was chosen to perform all experiments.ù
Figure 4.18: Radiation effects on HCC1937 cell viability as measured by tripan blue staining for 5 days.
Figure 4.19: Radiation effects on HCC1937 growth calculated as described in section 3.8.
HCC1937 transduced with LAW34-GFP or BRCA1-IRES-GFP were therefore irradiated with 2.3 Gy and monitored by FACS analysis for 1 week. The results showed a small increment in LAW34-GFP transduced HCC1937 followed by a slight decrement. In contrats, BRCA1-IRES-GFP transduced cells showed a 5 fold increment in fluorescence that was reached at the 5th day and remained stable thereafter (fig.
4.20). As expected, the presence of a functional BRCA1 in bicistronic transduced cells promotes more efficient DNA repair and faster cell growth as compared to naive and HCC1937 cells transduced with LAW34-GFP.
59 Figure 4.20: Increment of fluorescence of wild type BRCA1 in cells transduced with bicistronic vector as compared to LAW34-GFP transduced cells. Wild type BRCA1 repaired DNA DSBs more efficiently thus resulting in faster cell growth (increment of fluorescence).
4.10 BRCA1-IRES-GFP AND LAW34-GFP PREFERENTIALLY TRANSDUCE
PRIMARY CELLS
In view of a possible gene therapy approach, we assessed vector ability to transduce primary cells. These were collected from a breast cancer patient subjected to mastectomy. Surgical tissue was cultured and epithelial primary tumor cells partially separated from fibroblasts by tripsinization. Both cell populations were transduced with LAW34-GFP or BRCA1IRES-GFP. Percentage of fluorescent cells was then measured by FACS analysis during which a further separation between
0 1 2 3 4 5 6 0 2 4 6 8 F lu or es ce nc e in cr em en t ra te Days LAW34-GFP HCC1937 BRCA1-ires-GFP HCC1937
fibroblasts and epithelial cells was carried out thanks to different density and size of cells (fig. 4.21). FACS analysis demonstrated that both vectors could transduce epithelial cells at good levels (fig. 4.22A). Same vector preparations were less efficient on transducing primary fibroblasts suggesting that LAW34 preferentially targets epithelial cells (fig. 4.22B).
Figure 4.21: FACS separation between fibroblast and epithelial cells was based on different density and size of the two populations.. Transduction efficiency was then evaluated using the selected gate.
61 Figure 4.22: Percent transduction of: A) primary epithelial cells and B) fibroblast. Transduction efficiency was lower with latter cells suggesting that this vector preferentially transduces epithelial cells.
A)