UNIVERSITÀ DEGLI STUDI DI ROMA
"TOR VERGATA"
FACOLTA' DI MEDICINA E CHIRURGIA
DOTTORATO DI RICERCA IN PEDIATRIA MOLECOLARE
CICLO XX DEL CORSO DI DOTTORATO
Study on biological effects of β-glucuronidase in vivo and in vitro.
Silvia Mazzia
Docente Guida/Tutor: Prof. Paolo Rossi
Al mio capo Corrado Miceli †
TABLE OF CONTENTS
ABSTRACT pg 5
ABBREVIATIONS pg 6
INTRODUCTION
Allergic Disease pg 11
Dendritic cells and their role in allergic disease pg 23
Toll-like receptors pg 35
Recognition of endogenous ligands by TLRs pg 40 Regulation of TLR signaling pg 42
TLRs in allergic disease pg 46
TLR ligands as therapeutic strategies for
allergic disease pg 48
TLRs and regulatory T cells pg 50 Therapy for allergic disease
Current therapies for asthma and eczema pg 52 New therapies for asthma and eczema pg 54
Specific Immunotherapy pg 55 Subcutaneous immunotherapy pg 57 Sublingual immunotherapy pg 59 Allergoids pg 61 Ricombinant allergens pg 63 T-cell peptides pg 65 CpG oligonucleotides pg 66 Modified extracts pg 68 β-glucuronidase pg 71
AIM OF THE STUDY pg 76
RESULTS
STUDY IN VIVO
Effects of β-Glucuronidase on mouse in vivo: heterologous cutaneal passive
anaphylaxis mouse/rat pg 85
STUDY IN VITRO
Effets of β-Glucuronidase and its purified
products on dendritic cells pg 88
DISCUSSION AND CONCLUDING REMARKS pg 103
REFERENCES pg 105
ABSTRACT
The prevalence of allergic diseases like asthma, rhinitis and dermatitis, has been increasing in this last two decades from 1 to 30% and the allergic disease seems to be the more common cause of chronic diseases. Currently, the therapy commonly used is only symptom controlling.
Several works have shown the key role of dendritic cells (DC) in induction and maintenance of allergic inflammation.
Mc Ewen in 1972 treated patients allergic to grass pollen with ialuronidase and a mixture of allergens. The solution was contaminated by β -glucuronidase (BG) and he discovered that this enzyme was responsible of therapeutic benefit.
Recent works demonstrated that patients treated with BG show a decrease of symptoms accompanied with a decrease of drug intake and the analysis of cytokine production by DC showed a switch from a Th2 to a Th1 profile. We hypothesize that BG reacts with endogen agents like heparan sulphate, chondroitin sulphate or glycosaminoglycans present in the cellular matrix, and its degradation products bind the Toll-like receptor 4 (TLR4) on DC surface inducing the immune response to shift from Th2 to Th1 profile. The aim of the study is investigate the effects of BG in vivo in a mouse model of allergy and in vitro on human DC.
This study showed that BG injections in mice determines a reduced production of ovalbumin specific IgE, that BG does not modify DC differentiation from monocytes and, surprisingly, that a Low molecularweight (MW) fraction obtained by BG purification induces DC maturation and IL-12 production, promoting a Th1 response. The biochemical characterization of this fraction is going and this identification might be very important to the production of a immunotherapy that has an immediate action on symptoms and that can be used in monoallergic and poliallergic patients.
ABBREVIATIONS
AHR: airway hyperreactivity APCs: antigen presenting cells
AC: adenyl cyclase
AD: atopic dermatitis
AIC: Amb a 1 conjugated to a oligodeoxyribonucleotide ANP: atrial natriuretic peptide
AP-1: activator protein-1 BAL: bronchoalveolar lavage BCG: bacillus Calmette- Guerin BG: β-glucuronidase
BGs: Birbeck granules
BM: bone marrow
CD: cluster definition
CR: chemokine receptor
CLA: cutaneous lymphocyte-associated antigen CMV: cytomegalovirus
CpG ODNs: cytosine-guanosine dinucleotide- containing oligonucleotides CRTH2: chemoattractant receptor of TH2 cells
CS: condroitin sulfate
CSAID: cytokine suppressant anti inflammatory drugs CTLA-4: cytotoxic T-lymphocyte-associated antigen-4 DC: dendritic cell
E.P.D.: enzyme potentiated desensitisation ET-1: endothelin-1
FLAP: 5-LO-activating protein
Gs: G-protein
GATA3: GATA-binding protein-3 GlcNac: N-acetyl glucosamine
GM-CSF: granulocyte-macrophage colony stimulating factor GPI: glycosylphosphatidylinositol
GVHD: graft versus host disease HPCs: hematopoietic progenitor cells HS: heparan sulfate
Hsp: heat shock proteins HSV-1: herpes-simplex virus-1 HSV-2: herpes-simplex virus-2
ICAM-1: intercellular adhesion molecule-1 ICs: immune complexes
IDEC: inflammatory dendritic epidermal cells IFN-γ: interferon-γ
Ig: immunoglobulin IkB: inhibitor of NF-kB
IKKs: IkB kinases
iNKT: natural killer T
IL: interleukin
iNOS: NO synthase
IL-1ra : IL-1 receptor antagonist
IRAK-1: IL-1 receptor associated kinase 1 IRAK-4: IL-1 receptor associated kinase 4 IRFs: IFN regulatory factors
ISRE: interferon stimulated response element ISS: immunostimulatory sequence
JNKs: Jun N-terminal kinases
KCa: calcium-activated potassium channels LBP: LPS binding protein
LC: langerhans cells
LCMV: lymphochoriomeningitis virus LFA: leukocyte functional antigen
5-LO: 5-lipoxygenase LPS: lypopolisaccarides LTA: lipoteichoic acid MAL: MyD88-adaptor like MAP: mitogen activated protein MCh: methacholine
MCMV: murine cytomegalovirus
MCP4: macrophage chemoattractant protein-4 MDC: chemokines monocytederived chemokine MHC: major histocompatibility complex
MLCK: myosin light chain kinase MMTV: mouse mammary tumor virus MurNac: N-acetyl muramic acid
MyD88: Myeloid differentiation protein 88 MW: Molecular weight
MWCO: Molecular weight cut off NAL: nasal lavage
NDV: newcastle disease virus
NF-AT: nuclear factor of activated T-cells NF-kB: nuclear factor-kB
NIPCs: natural interferon-α producing cells
NO: nitric oxide
OD: optical density ODN: oligodeoxynucleotide OVA: ovalbumin
PAMPs: pathogen associated molecular patterns PAR2: protease-activated receptor2
PBMC: peripheral blood mononuclear cells PCA: cutaneal Passive Anaphylaxis pDC : plasmacytoid dendritic cell
PDEs: phosphodiesterases PGD2: prostaglandin D2 PGE2: prostaglandin E2
PI: phosphoinositide
PKA: protein kinase A
RIP1: receptor-interacting protein-1 RNAi: RNA interference
PRR: pattern-recognition receptor RSV: respiratory syncytial virus SCIT: subcutaneous immunotherapy sIL-4r: soluble humanized IL-4 receptor siRNA: small interfering RNA
SIT: specific immunotherapy
SLE: systemic lupus erythematosus SLIT: sublingual- swallow immunotherapy
Ss: single-stranded
TACE: TNF-α converting enzyme
TARC: thymus and activation dependent chemokine TBK1: TANK binding kinase 1
TCR: T-cell receptor Th1: T helper 1 Th2: T helper 2
TICAM1: TIR domain containing molecule 1 TGF: transforming growth factor TIRAP: TIR-associated protein TIR: toll/IL1-receptor like domain TLR: toll like receptor
TNF: tumour necrosis factor
TRAF-6: TNF receptor associated factor-6 TRAM: TRIF-related adaptor molecule
TRIF: TIR-domain containing adaptor protein inducing IFNß WNV: west nile virus
VCAM1: vascular cell-adhesion molecule-1 VIP: vasoactive intestinal peptide
VLA-4: α4 integrin very late antigen-4 VSV: vesicular stomatitis virus
INTRODUCTION
Allergic disease
Allergy can be defined as the clinically evident reaction to ubiquitous allergens. Immunological sensitization to common environmental allergens, such as house dust mites, grass and tree pollens and cat dander, can result in diseases such as allergic rhinitis and conjunctivitis, asthma and atopic dermatitis, and in the most extreme cases, in anaphylaxis and death (Kay A.B., 2001; Holgate S., 2003). Clinical symptoms vary, partly depending on how the allergen is introduced into the body.
Eczema, is a common, chronic, relapsing, inflammatory cutaneous disease, characterized by typically distributed eczematous skin lesions, dry skin and intense pruritus.
Atopic eczema is often the initial step in the so-called “atopic march”, which leads to asthma and allergic rhinitis in the majority of afflicted patients (Leung D.Y., 2004).
Allergic bronchial asthma is a complex inflammatory diseases characterized by massive infiltration with eosinophils, lymphocytes, and mast cells in the airway mucosa leading to airway hyperseisitivity, goblet cell hyperplasia and mucus overproduction.
The inflammatory process is thought to be the result of intensive T helper (Th) 2-biased immune response (Oki S., 2007).
T-helper cell type (Th)2 lymphocytes play an important role in the initiation, progression and persistence of allergic diseases, including asthma. However, little is known about immunoregulatory mechanisms that determine susceptibility to, severity of, or persistence of allergic disease. The concept of a disturbed Th1/Th2 balance, although having furthered the present understanding of immunoregulation in asthma, has recently been named a "procrustean paradigm", because of its failure to adequately explain many (pre)clinical observations.
In recent years, the general knowledge regarding the regulation of infectious, autoimmune allergic diseases, has rapidly increased (van Oosterhout A.J., 2005).
All of us come across many allergens is our life, but a person without atopy mount a low grade immunological response mainly characterized by production of allergen specific IgG1 and IgG4 antibodies, and by a modest cell proliferation and production of IFN-γ by Th1 cells. By contrast, when susceptible or atopic individuals are initially exposed or sensitized to allergens, this induces an exaggerated allergen-specific response which is characterized by CD4+ T cells producing a T helper 2 (Th2) profile of cytokines (IL-4, IL-5, IL-9 and IL-13 rather than IFN-γ and IL-2) and by the presence of allergen-specific IgE (Table 1).
In utero, T cells of the fetus are primed by common environmental allergens that cross the placenta. As a result, the immune response of virtually all newborn infants is dominated by Th2 cells (Prescott S., 1998). It has been proposed that during subsequent development, the normal (i.e., non atopic) infant’s immune system shifts in favor of a Th1-mediated response to inhaled allergens (a process termed “immune deviation”) (Holt P.Gl., 1999), whereas in the potentially atopic infant there is a further increase in Th2 cells that were primed in utero. Microbes are probably the chief stimuli of protective Th1-mediated immunity.
Macrophages that engulf microbes secrete IL-12, which induces Th1 cells and natural killer cells to produce IFN-γ, thereby shifting the immune system into an “allergy-protective” Th1-mediated response. Other factors may also influence whether Th1 or Th2 cells dominate the response, including the amount of allergen, the duration of exposure to the allergen, and the avidity of allergen-specific interactions between T cells and APC (Constant S.L., 1997; Rogers P.R., 1999). Although the picture is more complex, the immunopathological hallmark of allergic disease is the infiltration of affected tissues by Th2 cells.
Table 1. Components of Immune response to allergens in healthy and allergic
individuals (from Doctoral Dissertation of Pacciani 2007).
Specific antibody response in serum
Healthy: -No response
Detectable IgG1, IgG4 and IgA
High amounts of IgG4, relatively low amounts of IgG1 and detectable IgA and IgE
Allergic: -Relatively high amounts of IgE, together with low or high amounts of
IgG1, IgG4 and IgA
T-cell response in allergen-specific cells and in PBMC
Healthy: -No response
Th0 response in PBMC and specific T-cell clones with low frequency Tr1, particularly IL-10-dominating response with relatively high frequency
Allergic: Th2 response with varying quantities of IL-4, IL-5 and IL-13, in the
presence of detectable IL-10 and IFN-γ
Clinical outcome
Healthy: -Healthy
Skin-prick test and IgE positivity; clinical disease cannot be induced Skin-prick test and IgE positivity and healthy in normal circumstances; clinical disease can be induced by provocation tests and it is dose- dependent
Allergic: -Intermittent or persistent allergy in various clinical forms
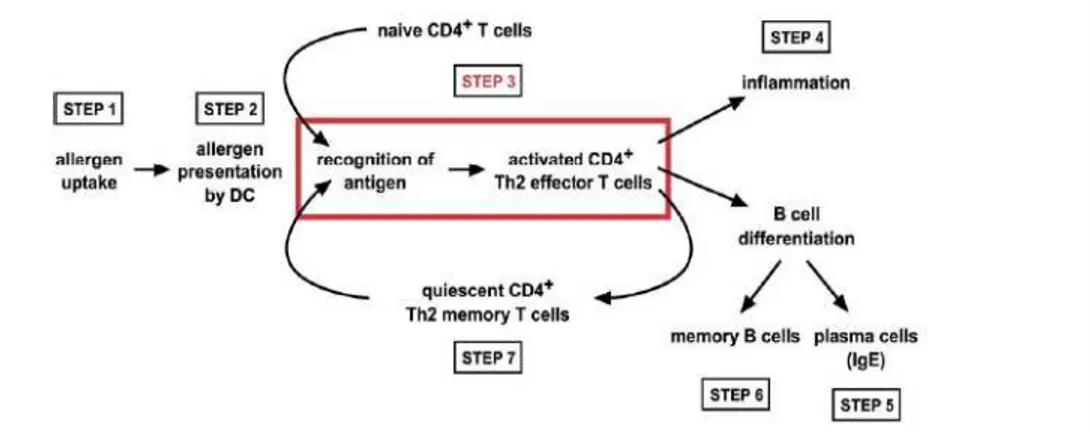
In particular, the allergen is taken up by phagocytic cells located in peripheral lung tissue and subsequently transported by DC to draining limph nodes (step 1) where is presented to T cells (step 2). Naïve T cells recognizing the allergen for the first time become activated and undergo a differentiation into Th2 effector T cells (step 3). The fully activated Th2 effector T cells initiate and sustain the local inflammatory process through secretion of soluble mediators (step 4). Concomitantly, the Th2 effector T cells instruct allergen specific B cells to differentiate to antibody-secreting plasma cells (step 5) and memory B cells (step 6). Differentiated allergen-specific T cells develop into long-lived memory T cells (step 7). Memory T cells are quiescent in the absence of antigen, but on allergen re-exposure,
they quickly become activated Th2 effector T cells again (step 3) (Ngoc L.P., 2005) (Figure 1).
Figure 1. Schematic representation of the development and function of Th2 cells in
airway disease (from Kroczek R., 2005).
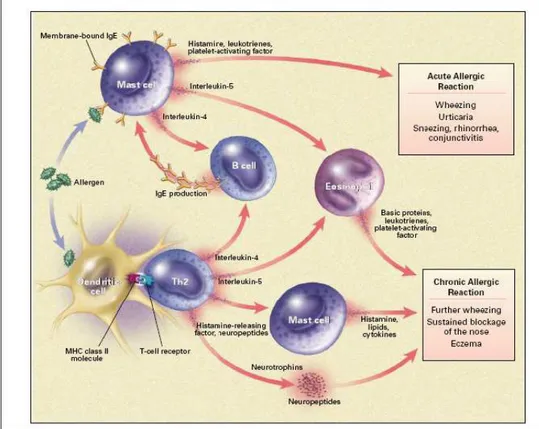
The cascade of events leading to the allergic inflammation is initiated by the process of tethering, activation, and adhesion to the endothelium followed by extravasation of inflammatory cells. This requires specific glycoprotein adhesion molecules, such as integrins and selectins, on both leukocytes and on endothelial cells, which are upregulated and show increased binding affinity in response to various inflammatory stimuli. Once the inflammatory cells have infiltrated into the tissue, they respond to chemotactic gradients established by chemoattractant cytokines and chemokines, which emanate from sites of injury or infection. More than 50 different chemokines are now recognized to be involved in the recruitment of inflammatory cells via the activation of more than 20 different surface receptors (Rossi D., 2000). These molecules play a central role in defining the nature of the inflammatory infiltrate in allergy (Kay A.B., 2001). Exposure to an allergen following allergic sensitization leads to crosslinking of allergen specific IgE bound to the surface of mast cells and basophils, degranulation of these cells and release of histamine, preformed granule-associated mediators, membrane-derived lipids, cytokines, and chemokines that cause the
symptoms associated with early or acute allergic reactions, including wheezing and conjunctivitis (Kay A.B., 2001). The release of mediators and leukotrienes causes increases in vascular permeability, smooth-muscle contraction and mucus secretion. Late-phase allergic responses are characterized by the additional recruitment and activation of eosinophils and Th2 cells at the site of allergen challenge (Figure 2).
Figure 2. Schematic drawn of immune mechanisms of allergy (from Kay A.B., 2001) Asthma prevalence in childhood, as reported in the ISAAC study, reaches in Germany 20% and in Australia 29% (Searsl M.R., 1997). In the USA asthma affects approximately 8-10% of the population and is the leading cause of hospitalization among children less than 15 years of age (Elias J.A., 2003). Eighty percent of childhood asthma is reported to be IgE mediated.
As far as eczema is concerned, globally the prevalence in children is 10-20%, and its prevalence has increased two to three-fold during the last 3 decades (Shultz–Larsen F., 2002). The frequency of the non atopic form represents about 16-45% depending on the country and the criteria for definition (ShaferT., 1999).
Although the number of people suffering of these diseases is increasing significantly there is a considerably lower prevalence of allergy in developing countries compared to Western Europe, USA and Australia, and there are also substantial differences between rural and urban areas.
According to Von Ehrenstein, in a study performed in rural Bavaria, the prevalence of a doctor's diagnosis of hay fever (1.8 vs. 4.9%) and hay fever symptoms (2.9 vs. 5.4%) was significantly lower in farmers' offspring as compared with children from a not agricultural environment. Similarly, the prevalence of a doctor's diagnosis of asthma (3.4 vs. 6.4%) and current wheeze (5.6 vs. 8.1%) was significantly lower in farmers' children, whereas no such difference was observed for eczema. The effects were stronger in children of farmers with full-time activity as compared with their peers with only part-time farming parents, suggesting a dose-response relationship (Von Ehrenstein O.S., 2000).
This and other observations, also performed on eczema (Schafer T., 2000), support the so-called hygiene hypothesis. According to this theory bacterial and viral infections in early life might direct the immune system towards a prevalent type 1 helper T (Th1) cell response which produces interleukin (IL)-2 and interferon-γ (IFN-γ), thus preventing the development of allergic diseases which are characterized by a Th2 cell response characterized by IL-4, IL-5 and IL-13 production. This theory is not easily reconciled with the increased prevalence of allergic asthma among poor blacks in the USA associated with sensitization to cockroaches and house-dust mite, and with some evidence about the increased prevalence of Th1 mediated diseases complicate the picture (Yazdanbakhsh M., 2002).
Th2 cells play a critical role in the pathogenesis of allergy and asthma. However, the immunological mechanisms that down modulate the allergic response and protect healthy individuals against the development of these disorders are poorly understood. A spectrum of CD4(+) T cells with regulatory function including Tr1, Th3 cells, CD4+CD25+ cells, Foxp3 and NKT cells play a critical role in regulating these diseases. A better understanding of the role of regulatory cells in allergic diseases may lead to the identification of novel therapeutic targets (Akbari O., 2003). In the cutaneous late-phase reaction, eosinophils and neutrophils accumulate, and then CD4+ T cells and basophils infiltrate the site (Ying S., 1999). Late-phase asthmatic (Robinson D.S., 1993) and nasal (Durham S.R., 1992) reactions have a similar pattern of cellular infiltration, although basophils are not prominent in the lower airways (Macfarlane A.J., 2000). Th2-type cytokines such as interleukin-4, 5, 9, and 13 influence a wide range of events associated with chronic allergic inflammation. IL-4 has a role in the initial derivation of the allergen-specific Th2-lineage cells, and IL-4 and IL-13 induce IgE class switching. IL-5 is a lineage-specific eosinophil differentiation and activator factor. It can be detected in the serum of mice with eosinophilia, and antibody to IL-5 blocks the development of parasite-induced eosinophilia. Although eosinophilia is often associated with high levels of IgE antibody, IL-5 appears not be involved in this response, where IL-4 appears to be main controlling factor of IgE (Sanderson C.J., 1990). IL-4 and IL-9 promote the development of mast cells; IL-9 and IL-13 help promote airway hyperresponsiveness; and IL-4, IL-9, and IL-13 promote the overproduction of mucus (Kay A.B., 2001; Robinson D.S., 2000; Romagnani S., 1994; Lloyd C.M., 2001). In the last years the important role of IL-13 in asthma has been elucidated. Originally discovered as an IL-4 like molecules, it is nowadays clear that these cytokines differ in their effector properties, IL-4 playing a more prominent role in the initiation and IL-13 in the effector phase of Th2 allergic inflammation (Zhou Y., 2001). Several studies using
overespression-transgenic animal models have provided impressive insights in the mechanism of IL-13 induced inflammation, through the chemokine receptor CCR2, and tissue fibrosis. They demonstrated, for instance, that the fibrotic response in asthma results from the ability of IL-13 to stimulate the production and activation of the fibrogenic cytokine TGF-β(Lee C.G., 2001). Airway epithelial cell-derived TGF-β1 has a potentially crucial role in the development of airway wall remodelling in asthma. Immunological mechanisms may regulate the release and accumulation of TGF-β1. Production of epithelian cell-derived TGF-β1 appear to be regulated by IL-13 (Kumar R.K., 2004). Moreover, the observation that other cytokines such us IL-9, mediate their effect in the lung through the induction of IL-13, suggests that IL-13 might be a final common pathway for Th2-mediated inflammatory response (Table 2).
Other players involved in pathogenesis of asthma have been recently studied. Among these proinflammatory factors, the main are thymic stromal lymphopoietin (TSLP), IL-25, IL-21, tumor necrosis factor α (TNF-α) and natural killer T cells (NKT cells). TSLP, which is highly expressed in Hassall’s corpuscles in the thymic medulla, appears to be important in the periphery, where it enhances the capacity of DC to induce the development of Th2 cells (Ito T., 2005).
IL-25 (also known as IL-17E) is produced by Th2 cells and mast cells and was found to induce the production of large quantities of Th2 cytokines in models of both infectious and pulmonary allergic disease (Fort M.M., 2001). it might also enhance the development of allergic inflammatory responses at mucosal sites by inducing eosinophilia, airway hyperreactivity and increased mucus production (Hurst S.D., 2002).
IL-21 is a newly described T cell-produced cytokine related to IL-2, IL-4, and IL-15 that is capable of regulating T, NK, and, especially, B cells (Parrish-Novak J., 2000; Kasaian M.T., 2002).
TNF-α is a cytokine produced by mast cells and T cells, but its role in asthma has been controversial.
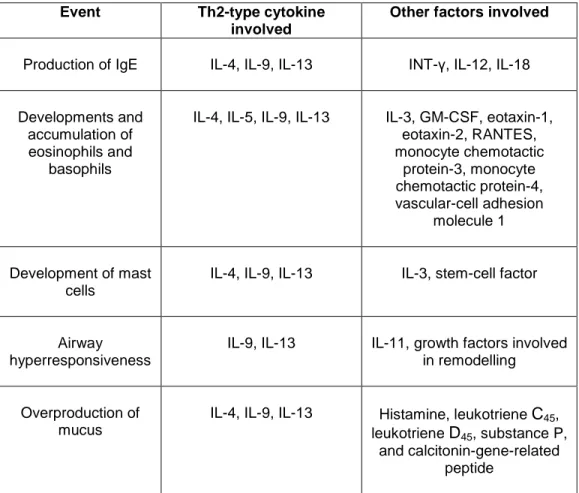
Table 2. The role of cytokines produced by Th2 cells in chronic allergic inflammation
(from Doctoral Dissertation of Pacciani 2007).
Event Th2-type cytokine
involved
Other factors involved
Production of IgE IL-4, IL-9, IL-13 INT-γ, IL-12, IL-18
Developments and accumulation of eosinophils and
basophils
IL-4, IL-5, IL-9, IL-13 IL-3, GM-CSF, eotaxin-1, eotaxin-2, RANTES, monocyte chemotactic protein-3, monocyte chemotactic protein-4, vascular-cell adhesion molecule 1 Development of mast cells
IL-4, IL-9, IL-13 IL-3, stem-cell factor
Airway hyperresponsiveness
IL-9, IL-13 IL-11, growth factors involved in remodelling
Overproduction of mucus
IL-4, IL-9, IL-13 Histamine, leukotriene C45,
leukotriene D45, substance P,
and calcitonin-gene-related peptide
However, a recent clinical study, in which patients who had refractory asthma were treated with the soluble TNF-αreceptor ‘etanercept’, suggests that the TNF-αaxis is upregulated and is proinflammatory in asthma (Berry M.A., 2006). Whether the role of TNF-αis as important in mild-to-moderate asthma as it is in refractory asthma remains to be seen.
A combination of genetic, skin barrier, immunologic factors and triggering events such as environmental, pharmacologic, psychologic factors and
infections, is considered responsible for the development and the severity of eczema. Recently the main focus of scientific attention has been the dysfunction of the immune system which could be the most relevant event for the development of eczema (Grewe M., 1998; Leung D.Y., 2004; Akdis C.A., 2003).
Several experimental results on the role of T cells and keratinocytes have led to a better understanding of eczematous inflammation and can help explain both the clinical and histological pictures of eczema. Besides activated endothelial cells and adhesion molecules, a complex interaction of numerous chemokines controls the recruitment of T cells from the blood vessels and their migration into the dermis and epidermis. Activated T cells damage the epidermis by pro-inflammatory cytokines and can induce apoptosis of individual keratinocytes through "killer molecules". Cleavage of adhesion molecules on keratinocytes leads to spongiotic changes. Keratinocytes then activate repair mechanisms, which cause acanthosis and parakeratosis in chronic eczema (Trautmann A., 2003).
Acute eczematous skin lesions present clinically as intensely pruritic, erythematous papules associated with excoriation and serous exudation. In the dermis of acute lesions, there is a marked infiltration of CD4+ activated memory T cells. When compared to normal skin or uninvolved skin of atopic eczema patients, acute skin lesions have a significantly greater number of IL-4, IL-5, and IL-13 mRNA–expressing T cells, but few IFN-γ or IL-12 mRNA–expressing T cells.
Activation and skin-selective homing of peripheral blood memory-effector T cells and effector functions in the skin represent sequential immunological events in the pathogenesis of atopic dermatitis (AD). T cells infiltrating the skin utilize the cutaneous lymphocyte-associated antigen (CLA) and other receptors to recognize and cross the vascular endothelium. In the peripheral blood of AD patients, both CD4+ and CD8 subsets of CLA+CD45RO+ T cells are in an activated state with high CD25, HLA-DR, and CD40-ligand
expression. They express upregulated Fas and Fas-ligand and undergo activation-induced apoptosis. After homing to skin these T cells form dermal infiltrates which play a key role in the pathogenesis of the disease. Skin-infiltrating T cells in AD are protected from activation-induced cell death, although they express both Fas and Fas-ligand. They are protected from apoptosis by cytokines such as IL-2, IL-4, and IL-15 and extracellular matrix components such as fibronectin and transferrin. CLA+, skin-homing T cells may play a role in peripheral blood eosinophilia and hyper IgE production by high IL-5 and IL-13 expression, respectively. These T cells secrete IFN-γ in the skin, which upregulates Fas on keratinocytes and renders them susceptible to apoptosis. Keratinocyte apoptosis is induced by Fas-ligand, either soluble or expressed on the surface of T cells, leading to eczema formation (Akdis M., 2001).
Chronic eczema skin lesions have significantly fewer IL-4 and IL-13 mRNA– expressing T cells, but greater numbers of IL-5, GM-CSF, IL-12, and IFN-γ mRNA–expressing T cells, than in acute eczema (Grewe M., 1998). Recent studies suggest that collagen deposition during chronic eczema is due to increased gene expression of the profibrotic cytokine, IL-11 (Toda M., 2003). The important role that Th1 and Th2 cytokines play in the skin inflammatory response has been demonstrated in experimental models of allergen-induced allergic skin inflammation in mice with targeted deletions or overexpression of these cytokines. In this regard, transgenic mice genetically engineered to overexpress IL-4 in their skin develop inflammatory pruritic skin lesions similar to atopic eczema, suggesting that local skin expression of Th2 cytokines plays a critical role (Chan L.S., 2001). Allergen-sensitized skin from IL-5 knockout mice has been found to have no detectable eosinophils and exhibits decreased thickening; skin from IL-4 knockout mice displays normal thickening of the skin layers, but has a reduction in eosinophils, while skin of IFN-γ knockout mice is characterized by reduced dermal thickening (Spergel J.M., 1999).
Epidermal keratinocytes from atopic eczema patients produce a unique profile of chemokines and cytokines following mechanical stimulation, e.g. scratching, or exposure to proinflammatory cytokines, including abnormally high levels of RANTES following stimulation with TNF-α and IFN-γ. They are also an important source of thymic stromal lymphopoietin, which activates DC to prime naïve T helper cells to produce IL-4 and IL-13 (Ong P.Y., 2002). DC indeed migrate to the lymph nodes and stimulate naive T cells to expand the pool of Th2 cells. The clinical importance of APC is supported by the observation that, using an experimental model of aeroallergen-induced patch test reactions on atopic skin, the presence of FcεRI+/IgE+ LC is required to provoke eczematous skin lesions (Novak N., 2003).
Dendritic cells and their role in allergy disease
When a microbe enters the body, the immune system is faced with a series of challenges.First, a decision needs to be made as to whether to respond to that specific microbe or not. Second, if a response is made, it must be tailored to fight that particular microbe. For example, in response to intracellular microbes, such as viruses and certain bacteria, CD4+ T helper (Th) cells differentiate into Th1 cells, which secrete interferon-γ (IFN-γ) and possess a specific range of functions. In contrast, extracellular pathogens such as helminths induce the development of Th2 cells, whose cytokines (interleukin-4 (IL-4), IL-5 and IL-10) direct immunoglobulin E (IgE) and eosinophil mediated destruction of the pathogens (Mosmann T.R., 1089). Generating the right kind of immune response can be a matter of life and death itself. B and T lynphocytes respond to antigens with high specifity, but they alone are not capable of making these complex decisions. These choises are made jointly by the nature of the microbe and by dendritic cells (DCs). DCs are professional antigen presenting cells (APCs) and are bone marrow-derived migratory cells that are present in most tissutes. DC were first described 30 years ago as a novel population of cells in the lymphoid organs of mice (Steinman R.M., 1973) and were subsequently shown to be highly potent accessory cells (Knight S.C., 1983) present in virtually all organs of the body. Their major role within non-lymphoid tissutes, such as epithelial and mucosal surfaces is to sample environmental antigens, and then to migrate to regional lymph nodes where they present processed antigenic peptides to T-cells and where they induce their polarizations toward a Th1 or Th2 phenotype (Lanzavecchia A., 2001).
Origin and characteristics of APCs.
Professional APCs are mainly divided into 2 systems: the DCs, including blood and tissue DCs and epidermal LCs, and the monocyte-macrophage
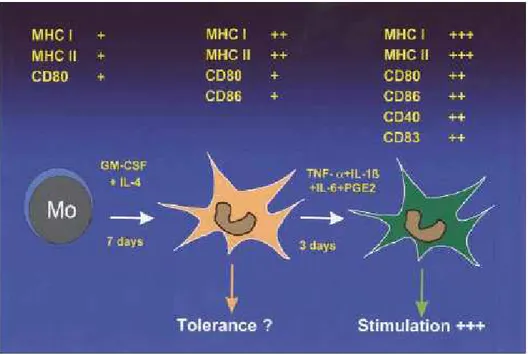
system. All these cell types originate from pluripotent bone marrow CD34+ hematopoietic progenitor cells (HPCs). During their maturation process, DCs are characterized by different phenotypes and functions. In the immature state DCs have excellent skills for the surveillance of peripheral tissues. The capacity to take up and process antigens is high, whereas these cells are weak in priming T cells. Phenotypically, they show low or absent expression of costimulatory and maturation molecules, such as CD80, CD83, or CD86, as well as low expression of major histocompatibility complex (MHC) class II. It is assumed that immature DCs are involved in peripheral self-tolerance induction because they capture apoptotic bodies from the periphery and transfer cell-associated self antigens to tolerogenic DCs in the lymph nodes. During maturation with, for example, TNF-α, they lose their capacity to take up and process antigens, and there is upregulation of costimulatory molecules and a translocation of MHC II to the cell surface. At this stage, DCs appear as cells with long dendrites. The most important changes are of functional relevance: mature DCs acquire the ability to prime T cells and are indeed the most potent inducers of primary T-cell responses (Schuler G., 1997; Banchereau J., 1998) (Figure 3).
Importantly, under certain conditions, DCs may present exogenous peptides instead of endogenous, newly synthesized peptides, together with MHC class I. This phenomenon has been termed cross-priming and allows the activation of CD8+ T cells. Cross-priming has been shown to be important in initiating MHC class I restricted responses to tumors and peripheral self, viral, and bacterial antigens. DCs may, under defined circumstances, express receptors for IgA (Geissmann F., 2001), IgG (FcγRII) (de la Salle H., 1997), and IgE (FcεRI and FcεRII (Leung D.Y., 1987; Bieber T., 1992Bieber T., 1997;) and produce various cytokines on activation (TNF-α, 6, and IL-8) (Polat G.L., 1993; Patry C., 1995).
Figure 3. Phenotypical and functional alterations of monocyte-derived DCs (from
von Bubnoff D., 2001).
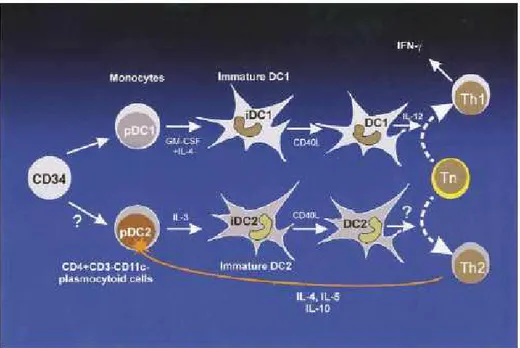
Regarding the DC system, at least 2 distinct DC precursor cells have been identified in the blood (Figure 4): the so-called DC1 subset carries the myeloid surface antigen CD11c, and the CD11c– subset forms the plasmocytoid precursors of lymphoid DCs (pDC2) (O’Doherty U., 1994; Spits H., 2000). Each subset represents only about 0.3% of mononuclear cells from the peripheral blood (Banchereau J., 2000). Plasmocytoid precursor cells (pDC2) give rise to lymphoid DCs (DC2) and are characterized by a unique surfacephenotype: CD4+ CD3– IL-3 Rα++ HLA-DR+ (Grouard G., 1997; Facchetti F., 1999). Recently, the developmental pathway from CD34+ HPCs to pDC2 was dissected by characterizing 4 distinct cell populations in vivo (Blom B., 2000). In culture, pDC2s differentiate into DC2s with IL-3 and CD40 ligand. Myeloid DCs differentiate from CD34+ HPCs or from myeloid blood precursors, such as monocytes, in vitro. Culture of CD34+ stem cells with GM-CSF and TNF-α leads to LC-like DC1s (Caux C., 1997; Caux C.,
1992). The addition of stem cell factor, FLT- 3 ligand, or both results in a higher proliferation rate of the CD1a+ LC-like DCs. These cells display surface antigens characteristic of dermal DCs (MHC class II++ CD4+ CD40+ CD54+ CD58+ CD80+ CD86+), display dendrites, and contain Birbeck granules (BGs) in 10% to 20% of cells. Peripheral blood monocyte precursors of immature DC1s have been designated pDC1s. They give rise to myeloid DC1s after culture with GM-CSF and IL-4 for 5 days (Sallusto F., 1994). After maturation with CD40 ligand or endotoxin, the cells produce large amounts of IL-12. The surface markers of DC1s mirror their origin from myeloid precursors: CD11c+ CD33+/– CD1a+ MHC class II+ CD80+ CD86+.
Figure 4. Two types of DC precursors in the blood (pDC1 and pDC2) that originate
from different cell lineages (from von Bubnoff D., 2001).
Recent reports suggest that mobilization with granulocyte colony-stimulating factor (G-CSF) containing regimens polarizes DCs into pDC2, which could potentially result with increased Th2 response and decreased
graft-versus-host disease (GVHD) in allogeneic transplantation and with decreased cytotoxic Th1 response and graft versus tumor effect, which in autologous transplantation could translate into increased relapse rate (Gazitt Y., 2006). Langerhans cells.
Langerhans cells (LC) represent one particular subpopulation of DC that are located in epithelial tissues. These cells have been most extensively characterized in the epidermis of the skin where they express a distinctive array of molecules including CD1a, Ecadherin, Langerin, and Birbeck granules (Liu Y.J., 2001). Less is known about LC in the lung, though it appears that many DC in the airway epithelium express LC-specific markers (Tazi A., 1993; Soler P., 1989). While the pathway(s) by which LC arise from precursor populations are a matter of current debate, the process of LC differentiation appears to be transforming growth factor (TGF)- β dependent. LC are absent from the epidermis in TGFb knockout mice, while DC populations in lymphoid tissues are maintained (Borkowski T.A, 1996). Moreover, TGFβ appears to be necessary for the in vitro differentiation of either CD34+ progenitors or monocytes into LC (Geissmann F., 1998). Antigen Uptake and Limph node Migration of Airway DC
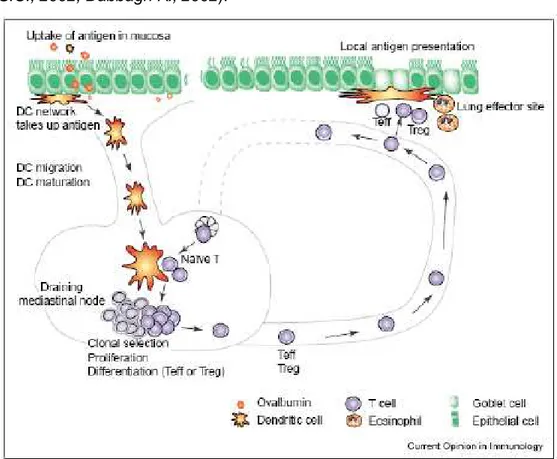
DC form a network in the upper layers of the epithelium and lamina propria of the airways. Here DC are said to be in an immature state, specialized for internalizing foreign antigens but not yet able to activate naïve T cells. With antigen uptake in the presence of a danger signal, DC undergo maturation, whereby they lose their capacity to take up antigen and acquire a phenotype of professional APC expressing all the costimulatory molecules and chemokines to attract and stimulate naïve T cells (Figure 5). A molecular basis for DC activation has been provided with the discovery of pattern-recognition receptors (PRR). PRR recognize conserved microbial structures, termed pathogen-associated molecular patterns (PAMP), and signalling via PRR leads to DC activation, defined by upregulation of MHC class II and co-stimulatory molecules. The significance of this finding for induction of
pulmonary immunity is underscored by the fact that lipopolysaccharide (LPS) is necessary for Th2 sensitization in mouse models of asthma (Eisenbarth S.C., 2002; Dabbagh K., 2002).
Figure 5. The function of DC in allergic airway disease (from Kuipers H., 2004). DC transport the antigen from the mucosa to the draining lymph nodes of the lung, after degradation of the antigen in short immunogenic peptides and loading the peptide on MHC II molecules. DC migrate to the T cell-rich area of draining lymph nodes where naïve T lymphocytes continuously pass by (Vermaelen K.Y., 2001; Lambrecht B.N., 2000). In the lymph node mature DC form an immunologic synapse with T cells in which the MHC peptide interacts with the T-cell receptor, costimulatory molecules interact with T cell–expressed coreceptors, and cytokines are released to polarize the T-cell response. The process of migration to the lymph node is driven by
chemokine signals acting on the chemokine receptor (CCR) 7 (Marsland B.J., 2005). The recognition of danger induces the surface expression of CCR7 on peripheral DC, but the responsiveness of CCR7 to CCL19 and CCL21 and the consequent lymph node migration of DC is controlled by lipid mediators, such as the leukotrienes and prostaglandins. Like skin DC, lung DC used the CCR8 receptor for the chemokine CCL-1 (also known as I-309 in human subjects and TCA-3 in mice) in concert with CCR7 for emigration of DC from the skin and lung, although the pathways governing DC migration from different tissues partially differ in molecular regulation (Jakubzick C., 2006).
How do dendritic cells induce Th1 or Th2 commitment?
The role of DC in shaping the differentiation pathway of a naïve Th cell is potentially powerful because the DC provides the precursor T-cell with its first activation signals. However, the mechanisms by which DC differentially regulate Th1 and Th2 immune responses is incompletely understood. DC express an array of costimulatory and adhesion molecules on their surface, and it has been proposed that differential expression of such surface molecules by DC influences Th1/Th2 switching (De Becker G., 1998; Lambrecht B.N., 2000). Thus, CD86 appears to be more important than CD80 for the induction of a Th2 response (Ranger A.M., 1996) and in murine models of allergic airway inflammation, blockade of CD86, but not CD80, abolishes airway eosinophilia and airway hyperresponsiveness (Haczku A., 1999). Similarly, expression of OX40 ligand (Ohshima Y., 1998) and inducible costimulatory (ICOS) ligand (Aicher A., 2000; Akbari O., 2002) on DC favours Th2 differentiation.
Numerous models of asthma have demonstrated that blocking the interaction of costimulatory molecules of the B7 superfamily (CD80, CD86, ICOS-L) or tumour necrosis factor (TNF)-R family (OX40L) can reduce features of asthma (Coyle A.J., 2000; Deurloo D.T., 2003; Harris N., 1997). However, challenge with DC derived from the BM of CD80/86 double
knockout mice in sensitized mice induced a strong airway inflammation similar to the one observed after challenge with wild-type DC. It is likely that other costimulatory molecules besides CD80/CD86 are involved in activating T cells in secondary immune responses. Several molecules on the surface of the DC, like ICOSL, OX40L, 4-1BBL, CD40 and ICAM- 1, have been reported to have costimulatory capacity, and may be responsible for the induced reaction in the absence of CD80/CD86 (Watts T.H., 1999). Strikingly, it was also observed an increase in the B7 family members PDL-1 and PDL-2, ligands of the inhibitory PD-1 receptor, on DC within eosinophilic inflammation (Van Rijt L.S., 2005). PD-1 is generally seen as an inhibitory signal but data suggest that PDL-1 might also provide a costimulatory signal to T cells (Shin T., 2003). Another costimulatory pathway able to compensate for the lack of CD80/86/B7RP-1 costimulation on DC would be OX40L. OX40 (CD134), a member of the TNFR family, is a major regulator of anti-apoptotic proteins such as Bcl-xL and Bcl-2, and strongly promotes the survival of antigen-activated primary CD4 T cells. In addition, OX40 is preferentially expressed by memory Th2 cells. Blocking of OX40–OX40L interaction impaired all features of asthma induced by adoptive transfer of OVA specific Th2 cells (Salek-Ardakani S., 2003).
Recent data suggested that OX40L expression by the initiating dendritic cell (DC) is a fundamental requirement for optimal induction of primary and memory Th2 responses in vivo (Jenkins S.J., 2007).
In allergen-challenged mice, mDC might also be a prominent source of the chemokines CCL17 and CCL22, which are involved in attracting CCR4+ Th2 cells to the airways (Kohl J., 2006). The proallergic cytokine TSLP induces the production of large amounts of CCL17 by mDC, thus contributing to the recruitment of a large number of Th2 cells to the airways (Zhou B., 2005). Mature DC produce two important polarizing cytokines: IL-12 recognized as the most powerful Th1 inducing cytokine and IL-10, a regulatory cytokine which has been shown to influence indirectly T cell polarization through its
capacity to downregulate IL-12 production by DC (Smedt T., 1997; Trinchieri G., 2003; Smits H.H,. 2004). It has been shown that retroviral overexpression of IL-12 in myeloid DC is sufficient to turn these cells into strong Th1 inducers, even in the Th2-prone milieu of the lung (Kuipers H., 2004). However, IL-12 is not necessary for Th1 development by DC, as LPS-stimulated IL-12p40-/- DC still induce Th1 development in the lung (Kuipers H., 2003). In contrast to the signals governing Th1 development, the mechanisms for DC-driven Th2 development have remained somewhat enigmatic. According to one theory, Th2 development occurs as a default in the absence of polarizing IL-12. Alternatively, some regard Th2 development as an instructive event requiring specific cytokines (such as IL-4 and IL-13) or cell surface molecules on DC.
Although the prototypic Th2 cytokine IL-4 is important for Th2 development in vivo, it is not produced by DC directly, but can be induced in other cells by DC contact. Early sources of IL-4 (and IL-13) might be naïve T cells, eosinophils or CD1d-restricted NKT cells, reacting to antigens presented by CD1d on airway DC (Voehringer D., 2004).
Recent work showed that IL-6 is an important cytokine during Th1 or Th2 development. IL-6 is a cytokine produced by DC and other cell types and has recently discovered roles in the abrogation of tolerance and in Th2 induction in several non-allergic models of disease. In addition, the production of IL-6 by pulmonary DC may favor Th2 differentiation by inhibiting Th1 responses (Dodge I.L., 2003).
IL-27 and IL-23 are produced by macrophages and DC. IL-27 and IL-23 can function as a proinflammatory cytokine because they synergize with IL-12 to induce IFN-γproduction from NK cells and to promote Th1 responses. IL-27 also Th2 properties. Addition of recombinant IL-27 to naïve T cells in culture under Th2-polarizing conditions results in decreased expression of GATA-3. GATA3 is a transcription factor important for Th2 development and by directly regulating IL-4 gene transcription through RBPJkappa sites in a 3'
enhancer (Amsen D., 2004). Concurrent with the decrease in GATA-3 was a decrease in IL-4 production. The decrease in Th2 cytokines caused by IL-27 is a result of inhibition of Th2 cell development. These results suggest that IL-27 might serve a dual role in T-cell development and the immune response by stimulating production of Th1 responses while inhibiting production of Th2 inflammatory responses (Villarino A.V., 2004). Others important players are involved in Th polarization. Among the transcription factors, T-bet and GATA-3 are the most important involved in Th1 and Th2 development, respectively. The transcription factor T-bet is necessary to induce helper T cells to differentiate into Th1 cells and to produce IFN-γ. For these reasons, T-bet is thought to be central to the feedback loops that regulate Th1 and Th2 cells, and in this way it could be important in asthma. Without any allergic sensitization of the animals, the bronchi in the T-bet -/-mice were infiltrated with eosinophils and lymphocytes and showed signs of the airway remodelling typical of allergic asthma. Moreover, these animals had airway hyperreactivity (AHR), and their BAL contained increased amounts of cytokines produced by the Th2 cells. These spontaneous changes in the T-bet-/- knockout mice were similar to those found in bronchi of wild-type mice that had been sensitized with a foreign protein and then challenged with an aerosol containing the allergen. These findings constitute strong evidence of the modulating role of IFN-γ in asthma and provide support for the hypothesis that an imbalance between Th1 and Th2 cells contributes to asthma (Finotto S., 2002; Lee J.S., 2007). Furthermore, the transcription factor T-bet was recently found to be expressed in DC in addition to T cells. T-bet is required for optimal production of IFN-γ by DC under certain conditions. T-bet-/- DC were less potent in inducing Th1 responses and produced less proinflammatory cytokines. These observations suggest that T-bet could regulate type 1 and 2 immunity by influencing genetic programs in both adaptive and innate immunity (Wang J., 2006).
Notch signaling controls cell-differentiation processes in a wide variety of tissues throughout the life of multicellular organisms, including the lineage choice between T and B lymphocytes made by hematopoietic progenitors as they become more differentiated. Mammals have four different Notch family members, Notch 1, 2, 3, and 4, which bind two conserved families of ligands, Jagged and Delta-like, encoded by two and three separate genes, respectively (Kubo M., 2007). The best-established role for Notch signaling in the hematopoietic system is the critical function of Notch1 in T cell-fate determination. Conditional loss-of-function analyses have shown that the Notch1-RBP-J signaling pathway is essential for the generation and differentiation of early T lineage progenitors in the thymus and that activation of this pathway simultaneously blocks B cell development. Outside of the thymus, Notch also contributes to many aspects of helper T cell differentiation. A recent report suggests that the Notch signal does not directly control Th2 differentiation, but rather regulates alternative mechanisms of IL-4 expression, with the initial source of IL-4 being restricted T cell subsets, such as memory type CD4+ T cells or NKT cells (Tanaka S., 2006).
A study has reported that Notch-RBP-J-mediated Th2 differentiation is regulated by antigen presenting cell (APC)-derived instructive signals. According to their model, the instructive signal comes from Jagged1 expressed on DCs. The interaction of Jagged1 with Notch during the initial stages of T cell activation controls the differentiation of naive CD4+ T cells into Th2 cells by a mechanism independent of IL-4 and STAT6 signaling (Amsen D., 2004).
The scientists have now furthered our understanding of the IL-4- and STAT6-independent Notch-RBP-J-mediated Th2 differentiation mechanism. Both groups independently find that Notch mediated binding of RBP-J to the regulatory region of exon 1a in the Gata-3 locus regulates GATA-3 expression in the absence of IL-4. GATA-3 is known to be a master regulator
controlling Th2 differentiation (Amsen D., 2007). In this regard, both reports provide a new appreciation of the important role of Notch signaling in generating Th2 immunity. Notch ligands could be one of the DC-derived instructive signals that control T cell fate during helper T cell differentiation. There is evidence that LPS-induced Jagged1 expression promotes Th2 differentiation and that Delta4 expression promotes IL-12 production by CD8-DCs and subsequently controls Th1 differentiation (Kubo M., 2007 ).
Toll-like receptors
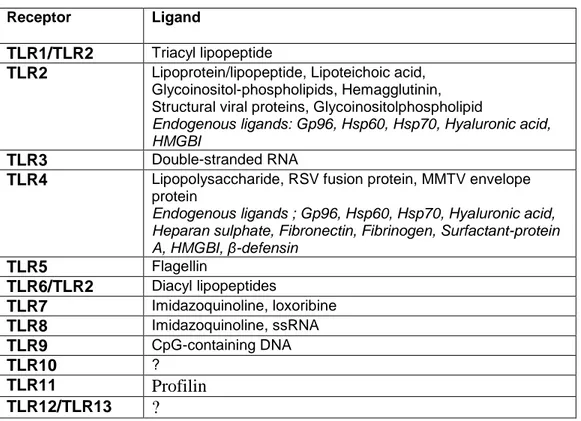
Toll receptors are type I transmembrane proteins that are evolutionarily conserved between insects and vertebrates (Rock F.L., 1998). In Drosophila, Toll was first identified as an essential molecule for dorsal-ventral patterning of the embryo and subsequently as a key molecule for the antifungal immune response in the adult animal (Anderson K.V., 1985; Lemaitre B., 1996). A homologous family of toll receptors, termed toll-like receptors (TLRs) exists in vertebrates. So far, 13 members (TLR1-13) have been reported which are fundamental in recognition of pathogen associated molecular patterns (PAMPs) (Takeda K., 2005). The family of TLRs recognizes various PAMPs from different pathogenic origins such as bacteria, viruses, fungi or protozoan parasites (Table 3) (Tabeta K., 2004). One subfamily of TLRs that consists of TLR1, 2, 4, 5, 6 and 11 is expressed on the surface of cells, recognizes a plurality of different structures and can be phagocytosed. In contrast, the other subfamily is formed by TLR3, 7, 8 and 9 that are localized inside the cell in the endoplasmic reticulum and endosomes or lysosomes where these receptors recognize nucleic acid. TLR4
The endotoxin lipopolysaccharide (LPS), a compound of the outer cell membrane of gram negative bacteria is a very potent PAMP among the cell wall components. The lipid portion of the LPS, termed Lipid A, is responsible for the immune stimulating activity (Alexander C., 2001). TLR4 is the key molecule of LPS induced signalling (Poltorak A., 1998) and utilizes several cofactors for efficient recognition. LPS associates first with LPS binding protein (LBP) and then with CD14, a glycosylphosphatidylinositol (GPI) anchored protein (Heumann D., 2003).
This complex binds to MD2 and associates with TLR4 which leads to its aggregation and subsequent signaling (Shimazu R., 1999).
Table 3. Pathogen derived ligands for TLRs (from Bauer S., 2007).
Receptor Ligand
TLR1/TLR2 Triacyl lipopeptide
TLR2 Lipoprotein/lipopeptide, Lipoteichoic acid, Glycoinositol-phospholipids, Hemagglutinin, Structural viral proteins, Glycoinositolphospholipid
Endogenous ligands: Gp96, Hsp60, Hsp70, Hyaluronic acid, HMGBI
TLR3 Double-stranded RNA
TLR4 Lipopolysaccharide, RSV fusion protein, MMTV envelope protein
Endogenous ligands ; Gp96, Hsp60, Hsp70, Hyaluronic acid, Heparan sulphate, Fibronectin, Fibrinogen, Surfactant-protein A, HMGBI, β-defensin TLR5 Flagellin TLR6/TLR2 Diacyl lipopeptides TLR7 Imidazoquinoline, loxoribine TLR8 Imidazoquinoline, ssRNA TLR9 CpG-containing DNA TLR10 ? TLR11 Profilin TLR12/TLR13 ?
Some viral-envelope proteins such as the fusion protein F from respiratory syncytial virus (RSV) and the envelope protein of mouse mammary tumor virus (MMTV) also activate TLR4 and induce cytokine production (Jude B.A., 2003). In addition, MMTV activates B cells via TLR4 and induces maturation of bone marrow-derived dendritic cells that up-regulate expression of the MMTV entry receptor (CD71) and therefore facilitate infection and may attenuate the antiviral response (Rassa J.C., 2002; Burzyn D., 2004). The IRAK-4-dependent human TLRs, except for the TLR3 and TLR4-INF-α/β
pathway, appear to play a redundant role in protective immunity to most infections, at most limited to childhood immunity to some pyogenic bacteria (Ku C.L., 2007).
TLR2, TLR1 and TLR6
Apart from LPS, other components of the cell wall found in gram positive and gram negative bacteria can stimulate innate immune cells. For example, lipoteichoic acid (LTA), an amphiphilic negatively charged glycolipid and lipoproteins are potent immune stimulators and activate TLR2 (Schwandner R., 1999). TLR2 associates with TLR1 and TLR6 and this interaction allows discrimination of differences within the lipid part of lipoproteins. Accordingly, the TLR2/TLR1 heterodimer recognizes triacylated lipopeptides, whereas the complex consisting of TLR2 and TLR6 is activated by diacylated lipopeptides (Takeuchi O., 2002). Furthermore, LPS from certain bacterial strains such as Legionella pneumophila, Leptospira interrogans and Porphyromonas gengivalis has been described to act as a ligand for TLR2 and not for TLR4 (Akamine M., 2005). However, these results must be viewed with caution since impurities in the LPS preparation could account for a LPS independent TLR2 activation (Asai Y., 2005). The recognition of peptidoglycan (PG), a large molecular structure composed of alternating N-acetyl glucosamine (GlcNac) and N-acetyl muramic acid (MurNac) sugar chains that are interlinked by peptide bridges has also been attributed to TLR2, but this observation is still controversial (Michelsen K.S., 2001). Again, the contribution of TLR2 in the recognition of PG must be viewed with caution since impurities in the biochemically purified PG such as lipoproteins or LTA could account for TLR2 activation (Travassos L.H., 2004). In addition, TLR2 is activated by viral proteins such as the hemagglutinin from measles virus and structural proteins from cytomegalovirus (CMV) and herpes-simplex virus-1 (HSV-1) (Aravalli R.N., 2005). The protozoan parasites Trypanosoma cruzi, Toxoplasma gondii, Leishmania major and Plasmodium falciparum contain further TLR2 ligands such as GPI anchors (de Veer M.J., 2003). TLR5
Many pathogens are motile and use a flagellum as the motility apparatus. The major component of the flagellum is flagellin, a potent activator of TLR5
(Hayashi F., 2001). TLR5 recognizes the constant domain D1 of flagellin that is relatively conserved among various species (Smith K.D., 2003). In epithelial cells TLR5 is expressed on the basolateral side and therefore flagellin is only recognized when flagellated pathogens have crossed the epithelium (Gewirtz A.T., 2001). A recent report demonstrates that monomeric flagellin produced by salmonella during infection of intestinal epithelial cells was not derived from polymeric cell wall-associated flagellum but instead was synthesized and secreted de novo after direct sensing of host-cell derived lysophospholipids (Subramanian N., 2006).
TLR11
Uropathogenic bacteria are recognized by TLR11 in mice (the human gene is non-functional) although the specific ligand for TLR11 has not been yet identified (Zhang D., 2004). In addition, Toxoplasma gondii contains a profilin-like molecule with unknown function that activates murine TLR11 and induces proinflammatory cytokines (Yarovinsky F., 2005).
TLR3
TLR3 recognizes dsRNA and the synthetic analog polyinosine-polycytidilic acid (polyI:C) and induces type I IFN (Alexopoulou L., 2001). dsRNA is generated as an intermediate during the replication cycle of ssRNA or DNA viruses (Matsumoto M., 2004). Since dsRNA seems to be a universal viral PAMP, TLR3 was believed to be the key receptor in an antiviral immune response. However, viral infection experiments with various viruses such as murine cytomegalovirus (MCMV), vesicular stomatitis virus (VSV), lymphochoriomeningitis virus (LCMV) and reovirus in wildtype and TLR3-/- mice revealed that TLR3 is not required for the antiviral response (Edelmann K.H., 2004). In contrast, West Nile virus (WNV), a ssRNA flavivirus, that can cause neuronal injury in man, utilizes TLR3 mediated proinflammatory cytokine production such as TNF-α. Since TNF-α leads to the disruption of the blood-brain barrier, virus induced TLR3 activation facilitates the entry into
the brain. Accordingly, TLR3-/- mice were resistant to peripheral WNV infection whereas wildtype mice succumb to infection (Wang T., 2004). TLR3 is expressed in epithelial and dendritic cells, which apparently use TLR3-independent pathways to prevent further dissemination of HSV-1 and to provide resistance to other pathogens in TLR3-deficient patients. Human TLR3 appears to be redundant in host defense to most microbes but is vital for natural immunity to HSV-1 in the central nervous system, which suggests that neurotropic viruses have contributed to the evolutionary maintenance of TLR3 (Zhang S.Y., 2007).
TLR7 and TLR8
Single-stranded (ss) guanosine and/or uridine rich RNA and ssRNA viruses such as influenza, VSV, Newcastle disease virus (NDV) are recognized by TLR7 and/or TLR8 (Kato H., 2005). Both genes are homologous to each other and are located on the X chromosome (Du X., 2000). Both receptors also recognize synthetic antiviral nucleoside analogs such as imidazoquinolines (R848 or imiquimod) or loxoribine (7-allyl-7,8-dihydro-8-oxoguanosine) (Lee J., 2003). The TLR7 mediated viral recognition of influenza, NDV and VSV is prominent in a subtype of dendritic cells, called plasmacytoid dendritic cell (pDC) or natural interferon-a producing cells (NIPCs) (Lund J.M., 2004). This cell type is characterized by their ability to secrete high amounts of IFN-a in response to viral infection (Siegal F.P., 1999). Other cell types such as conventional dendritic cells and fibroblasts also produce Type I Interferon upon infection with single-stranded RNA viruses in a TLR7 independent manner (Kato H., 2005). This observation suggests that alternative pathways do exist that sense viral infection. These TLR independent receptors in non-pDC are the RNA helicase termed RIG-I and MDA-5 that confer recognition of viral RNA and subsequent type I IFN production (Kato H., 2006).
TLR9
TLR9 recognizes bacterial genomic DNA. Studies by Tokunaga in 1984first demonstrated that bacterial DNA itself was the component of bacillus Calmette-Guerin (BCG) which promoted immunostimulatory and antitumor effects. The stimulatory effects of bacterial DNA is due to the presence of unmethylated CpG dinucleotides in a particular base context named CpG motif. Vertebrate DNA is not stimulatory due to methylation of CpG dinucleotides, their low frequency (CpG suppression) and the presence of possibly inhibitory sequences. The immunostimulatory effects of bacterial DNA can be mimicked by synthetic oligodeoxynucleotides containing a CpG-motif (CpG-ODN) (Krieg A.M., 1995). DNA viruses such MCMV, herpes-simplex virus 1 and 2 (HSV-1/HSV-2) are also recognized by TLR9 and induce production of inflammatory cytokines and type I IFN. The TLR9 mediated IFN-α response to HSV-1 and HSV-2 is limited to pDC (Krug A., 2004b). Cellular activation does not require viral infection since live, heat and UV-inactivated HSV-1/ HSV-2 produce high levels of IFN-α. In contrast, macrophages produce IFN-a upon HSV infection in a TLR9 independent manner suggesting that pDC and TLR9 independent redundant mechanisms exist that induce an effective response against DNA-viruses (Malmgaard L., 2004).
Recognition of endogenous ligands by TLRs.
The recognition of viral, bacterial, fungal and protozoan structures by TLRs supports the view that TLRs distinguish between self and foreign. Nevertheless, endogenous TLR ligands have been reported that challenge this view (Seong S.Y., 2004). Extensive work has suggested that heat shock proteins (Hsp), such as Hsp60, Hsp70 and gp96 are potent activators of the innate immune system. Hsp from bacterial and mammalian sources induce proinflammatory cytokines such as TNF-α, IL-1 and IL-6 and up-regulate costimulatory molecules on APC (Multhoff G., 2006). Similar cytokine effects
have been also reported for various molecules of mammalian origin such as fibrinogen (Smiley S.T., 2001), surfactant-protein A (Guillot L., 2002), fibronectin (Okamura Y., 2001), heparan sulphate (Johnson G.B., 2002), condhroitin sulphate, oligosaccharide of hyaluronan (Termeer C., 2002), ß-defensin (Biragyn A., 2002) and high-mobility group protein 1 (HMGB1) (Park J.S., 2004). All compounds are ligands for TLR4 and/ or TLR2, respectively. Since the cytokine effects of these endogenous ligands are similar to the cytokine pattern induced by LPS and lipoproteins, the contribution of TLR2 and TLR4 in the recognition of endogenous structures has been viewed with caution. LPS or lipoprotein contamination within biochemically purified endogenous ligands could contribute to observed effects. Furthermore, endogenous nucleic acids such as RNA and DNA can activate TLRs under certain conditions and promote or sustain autoimmune diseases such as systemic lupus erythematosus (SLE). Due to loss of tolerance to nuclear self-antigens, autoantibodies against DNA, histones, RNA and RNA-binding proteins such as Sm/RNP are produced which form immune complexes (ICs) with DNA or RNA (Migliorini P., 2005). These ICs are deposited in the kidney and lead to glomerulonephritis. The antibodies recognizing nucleic acid and/or associated proteins are produced by autoreactive B cells (Leadbetter E.A., 2003). In this system, the BCR first recognizes the Fc-region of the autoantibody and triggers the endocytosis of the ICs into endosomes where TLR9 resides. The same paradigm has been reported for RNA-containing ICs and TLR7 activation (Lau C.M., 2005). pDC activation and secretion of IFN-a is mediated through endocytosis of RNA or DNA/IC via FcgRIII (mouse) (Boule M.W., 2004) or FcgRIIa (human) (Means C.M., 2005). This uptake translocates the nucleic acid to endosomes where IFN-α is induced in a TLR7 dependent manner (Savarese E., 2006) and TLR9-dependent (Boule M.W., 2004) fashion.
Regulation of TLR signalling.
Toll-like receptors (TLRs) are involved in host defence against invading pathogens, functioning as primary sensors of microbial products and activating signalling pathways that induce the expression of immune and pro-inflammatory genes. However, TLRs have also been implicated in several immune-mediated and inflammatory diseases. As the immune system needs to constantly strike a balance between activation and inhibition to avoid detrimental and inappropriate inflammatory responses, TLR signalling must be tightly regulated (O’ Neill L.A.J., 2005).
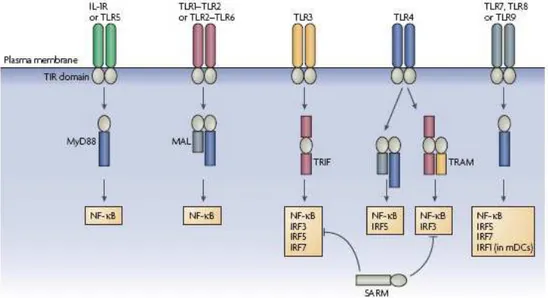
Signalling by Toll-like receptors (TLRs) involved five adaptors proteins known as MyD88, MAL, TRIF, TRAM and SARM that are characterized by the presence of a TIR domain. The TIR domain is defined by a motif of ~160 amino acids composing five β-sheets surrounded by α-helices and connected together by flexible loops. Conservation between TIR domains reflects the structural requirement of this folding pattern. However, the surface properties of TIR-domain-containing adaptors have been shown to be distinct and it has been proposed that electrostatic complementarity might explain specific interactions between adaptors and TLRs.
MyD88, Mal, TRIF and TRAM associate with the TLRs via homophilic TIR domain interactions whereas SARM utilizes its TIR domain to negatively regulate TRIF. It is well established that the differential recruitment of adaptors to TLRs provides a significant amount of specificity to the TLR-signalling pathways (Watters T.M., 2007) (Figure 6).
MyD88 was first shown to be involved in signalling by the type 1 IL-1 receptor (IL-1R1) and subsequently in signalling by various TLRs, with the crucial evidence coming from MyD88-deficient mice. These mice were shown to be profoundly unresponsive to ligands for TLR2, TLR4, TLR5, TLR7 and TLR9. Numerous studies have now been carried out on MyD88-deficient mice in the context of disease models. They are resistant to the toxic effect of LPS, and they are also immunocompromised in terms of their
ability to fight a range of pathogens, MyD88-dependent signalling is involved in transplant rejection, and it is required for the inflammation that occurs in a model of AHR involving the antigen ovalbumin. However, implicating a lack of TLR signalling in the phenotype of MyD88- deficient mice in models of infectious or inflammatory disease must be done with caution, as these mice are also impaired in their response to IL-1 and IL-18 (Adachi O., 1998). The activation of NF-ΚB, JNK (JUN N-terminal kinase) and p38 is absent in MyD88-deficient cells in response to all TLRs tested except TLR4 and TLR3. In both cases, this is because of the alternative use of the adaptor TRIF. Members of the IL-1R-associated kinase (IRAK) family are recruited immediately downstream of MyD88.
Figure 6. Overview of transcription-factor activation through TIR-domain-containing
adaptors for the TLR/IL-1R superfamily. Each adaptor is differentially used by receptor complexes to positively regulate transcription-factor activation. The exception is SARM (sterile α- and armadillo-motif-containing protein), which inhibits TRIF (Toll/IL-1R (TIR)-domain-containing adaptor protein inducing interferon-β (IFNβ))-mediated transcription-factor activation (from Luke A.J., 2007).