Italian National Agency for New Technologies, Energy and
Sustainable Economic Development
http://www.enea.it/en
http://robotica.casaccia.enea.it/index.php?lang=en
This paper is a draft. The paper is available on:
Polymer „Influence of molecular mass, thermal treatment and
nucleating agent on structrure and fracture toughness of isotactic
polypropylene“. M.Avella, R. dell’Erba , G. Ragosta and E.
Martuscelli V. 34, n.14 p.2951-60 (1993). https://doi.org/
10.1016/0032-3861(93)90620-P
I n f l u e n c e of m o l e c u l a r mass, t h e r m a l
t r e a t m e n t and n u c l e a t i n g a g e n t on
s t r u c t u r e and f r a c t u r e t o u g h n e s s of
i s o t a c t i c p o l y p r o p y l e n e
M. Avella, R. dell'Erba, E. Martuscelli* and G. Ragosta
Istituto di Ricerche su Tecnologia dei Polimeri e Reologia, CNR, Via Toiano 6, Arco Felice 80072, Napoli, Italy
(Received 24 September 1991; revised 20 November 1992)
The influence of processing, crystallization conditions and molecular characteristics on the fracture behaviour of isotactic polypropylene obtained by a new catalyst system was investigated. The sample specimens were prepared by using two extreme crystallization conditions - - quenching or slow crystallization - - in order to obtain crystals and amorphous phases with different structure. Interesting correlations between fracture parameters and some morphological parameters such as long spacing and lamellar thickness of the samples were found. The SEM fractographic analysis provides useful information on the influence of factors such as molecular weight, spherulite size and undercooling. Nucleating agents were used to tailor the dimensions of spherulites.
(Keywords: fracture toughness; polypropylene; thermal treatment)
I N T R O D U C T I O N
In a previous paper 1 we reported the results of an investigation concerning the influence of molecular weight (Mw) on the nucleation density, overall kinetics of crystallization and thermal behaviour of isotactic polypropylene (iPP) samples obtained by a new catalyst system. This catalyst process allows the production of iPP fractions with higher control of isotacticity index (up to 99%), molecular weight and molecular weight distribution z.
In this contribution the same i P P samples were used to study the effects of crystallization conditions and Mw on the fracture behaviour at high strain rate of such materials. The i P P samples were prepared by using different thermal treatments (quenching and isothermal crystallization) to obtain specimens with very different crystallinity, crystal morphological and lamellar thickness of crystals. Furthermore, a nucleating agent was used to obtain i P P spherulites with tailored dimensions.
The aim of this work was to correlate the fracture parameters with the different microstructures obtained, in order to provide useful information on the mechanism through which the various molecular and structural elements forming the spherulites control the growth and the propagation of cracks. F o r this purpose, the high-speed fracture data were analysed using the linear elastic fracture mechanics theory. The parameters of this theory, such as the critical strain energy release rate (Go) and the critical strain intensity factor (Kc), are particularly suitable for monitoring morphological and structural
* To w h o m correspondence should be addressed
changes in the material. A fractographic analysis by means of scanning electron microscopy (SEM) was also performed.
E X P E R I M E N T A L Materials
A series of iPP samples (Himont-Italia Spa) with different molar mass and molar mass distribution were used in the present work. Their molecular characteristics, code numbers and trade names are reported in Table 1. Specimen preparation
A sample of polymer was placed between two Teflon sheets and laterally contained in a 3.5 mm thick steel frame. The whole system was inserted within the plates of a hydraulic press heated at 200°C and kept for 10 min without any applied pressure, to allow complete melting. After this period a pressure of 1 0 M P a was applied for 5 min. Then the pressure was released and the samples were quenched in ice and water at about 0°C. After the compression moulding some samples (iPP1, iPP4 and iPP6) were also immersed in a thermostat-controlled bath which was set at a predetermined temperature to ensure crystallization. The crystallization temperatures were selected in order to achieve the same values of undercooling (AT) for the three different polymers defined as:
A T = T m - T c
where T~ is the crystallization temperature and Tm is the equilibrium melting temperature (the Tm of all iPP samples was determined in a previous paperl). The values of ATused are listed in Table 2. In a different procedure
0032-3861/93/142951-10
Influences on structure and fracture toughness of iPP: M. Avella et al.
Table 1 Molecular characteristics of polypropylene samples
Sample Trade name M, M, M,/M.
( x l0 s) ( x 104) iPP1 Valtec 302 1.1 1.7 6.4 iPP2 Valtec 298 1.7 1.8 9.6 iPP3 Valtec HS 013 3.1 4.5 7.0 iPP4 Valtec HS 010 3.6 5.3 6.8 iPP5 Valtec HS 005 4.5 6.6 6.7 iPP6 Valtec HL 002 7.3 9.1 8.0
Table 2 Crystallization temperatures (T~), equilibrium melting
temperatures (Tin) and undercoolings (AT) used to prepare the isothermally crystallized iPP samples
Sample T~ T m AT (°c) (oc) (oc) iPP1 127 182 55 122 182 60 117 182 65 iPP4 130 185 55 125 185 60 120 185 65 iPP6 137 192 55 132 192 60 127 192 65
the iPP1, iPP4 and iPP6 samples were premixed with 1 wt% of nucleant (sodium benzoate) by means of a Brabender-like apparatus.
Rectangular specimens were cut from the crystallized sheets for performing Charpy impact tests. Prior to testing, the samples were notched as follows: first a blunt notch was produced using a machine with a V-shaped tool and then a sharp notch 0.2 mm deep was made by a razor blade fixed to a micrometric apparatus. The final notch depth was measured after fracture, using an optical microscope.
Thermal analysis
The apparent melting temperature (T~) and the crystallinity index (Xc) for all the iPP samples, prepared both by quenching and isothermal crystallization, were determined by differential scanning calorimetry (d.s.c.) using a Mettler TA-3000 equipped with a controller and a programming unit (microprocessor TC-10). All the measurements on the samples (about 8-10mg) were tested using a scan rate of 10°C rain- 1. The values of T~ and the apparent enthalpy (AH*) were obtained from the maxima and the area of the melting peaks, respectively. The Xo values of iPP samples were calculated by the following relation:
X~ = A H * / A H °
where AH ° is the heat of fusion per g of 100% crystalline iPP, taken as 209 J g-1 (ref. 3).
S A X S measurements
Small angle X-ray scattering (SAXS) measurements were performed on quenched and isothermally crystallized iPP samples using a compact Kratky camera equipped with a Braun one-dimensional position-sensitive detector. Ni-filtered CuK~ radiation, generated from a Philips X-ray generator (PW 1730/10) operating at 40kV and 30 #A was used. The row scattering data were corrected
for parasitic scattering, absorption and slit smearing by Glatter's method 4. The desmeared intensities were corrected with the Lorentz factor 5 by multiplying by S 2 (S = 2/2 sin 0; S is the scattering vector).
Impact fracture measurements
Fracture tests were carried out on a Charpy Instrumented Pendulum machine at an impact speed of 1 m s -1. Samples with a notch depth to width ratio of 0.3 and a span length of 48 mm were fractured at room temperature. The relative curves of energy and load plotted against time or displacement were recorded.
Fracture toughness parameters
The impact data were analysed according to the linear elastic fracture mechanics approach 6. From this approach two parameters can be determined which accurately describe the conditions for the onset of crack growth in the material. One parameter is the stress intensity factor K, which determines the distribution of stress ahead of the crack tip. The fracture occurs when K achieves a critical value K¢ given by:
K¢=tr Ya 1/2 (1)
where tr is the nominal stress at the onset of crack propagation, a is the initial crack length and Y is a calibration factor dependent on the specimen geometry. For single cracked bent specimens, Yis given by Brown and Srawley 7. The other parameter is the critical strain energy release rate Go, which represents the energy necessary to initiate crack propagation. This can be expressed in terms of fracture energy by:
G¢ = U/B I4¢b (2)
where U is the fracture energy corrected for the kinetic energy contribution, B and Ware the thickness and width of the specimen, respectively, and • is a calibration factor which depends on the length of the crack and the size of the sample. The values of • were taken from Plati and Williams 8.
Fractography
Fracture surfaces of notched specimens were examined using a Philips 501 scanning electron microscope, after coating the broken surfaces with a thin layer of gold-palladium alloy.
RESULTS AND DISCUSSION
Thermal properties
Table 3 reports the apparent melting temperature (Tin) and crystallinity index (X¢) for quenched iPP samples. These values decrease with increasing molecular weight
Table 3 Apparent melting temperatures (T~) and crystallinity index
(Xc) for quenched iPP samples
Sample T~, X c (oc) (%) iPP1 169 42 iPP2 168 40 iPP3 166 39 iPP4 166 36 iPP5 166 36 iPP6 162 34
Influences on structure and fracture toughness of iPP: M. Avella et al.
of iPP, indicating that the a m o r p h o u s material increases with increasing Mw.
The thermal parameters (T~ and Xc) for i P P samples isothermally crystallized at different ATvalues, with and without nucleating agent, are reported in Table 4. As expected, for all samples examined T~ and Xc values increase with decreasing AT(increasing T~). F u r t h e r m o r e at fixed AT, the T~n points seem to decrease slightly with
Table 4 Thermal parameters obtained for isothermally crystallized iPP samples
Without nucleating agent With nucleating agent
Sample T~ AT T~, X, T~ AT T" Xo
(°c) (oc) (oc) (%) (of) (oc) (°c) (%)
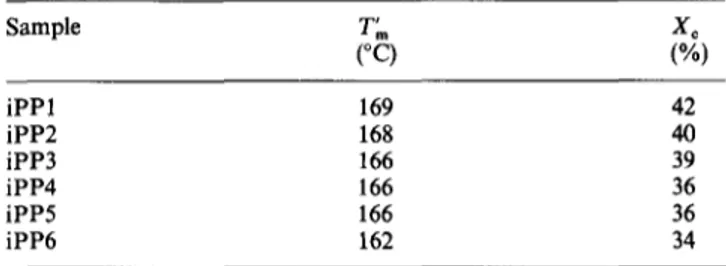
iPP1 117 65 166 46 117 65 166 47 122 60 168 48 122 60 167 50 127 55 169 52 127 55 168 50 iPP4 120 65 166 43 120 65 167 39 125 60 167 44 125 60 167 40 130 55 169 46 130 55 169 41 iPP6 127 65 164 36 127 65 163 35 132 60 165 37 132 60 165 36 137 55 166 39 137 55 165 36 48 o4 "-042 36 5 4 - a 54 I I I I I I I I 0 300 600 Mw ( * i0 -3) 900 48 o o4 ~ 4 2 36 0 Figure 1 b I I I I I I I I 5 0 0 6 0 0 900 M w ( x I0 -5)
Crystallinity index as a function of molecular weight for iPP samples (a) without nucleating agent and (b) containing sodium benzoate as nucleating agent. ATvalues: O, 65°C; /X, 60°C; [], 55°C
increase in Mw, while a strong depression of Xc values was observed (Figure la). The dependence of X c on the molecular weight of i P P can be attributed to a higher a m o u n t of chain entanglements. This is enhanced with increasing molecular weight, giving rise to a m o r e disordered crystallization process and consequently X c decreases. A similar Xc depression as a function of molecular weight, at given AT, is also seen in the case of isothermally crystallized i P P containing nucleating agent (Table 4 and Figure lb). This suggests that the nucleating agent influences only the size of spherulites and not the final crystallinity. In fact, for all the i P P samples in the A T range examined the X~ value seems to be almost constant (Table 4). This is due to the action of the nucleating agent which promotes rapid crystallization of the material at low A T a n d thus produces the same degree of crystallinity independently of the A T used.
S A X S results
A typical Lorentz-corrected desmeared pattern for an isothermally crystallized i P P sample is shown in Figure 2. The SAXS profile exhibits a well-defined maximum. By applying Bragg's law, the long period L was obtained for all samples examined, as a function of molecular weight. F o r the isothermally crystallized samples L was also calculated as a function of T c and AT.
Assuming a two-phase model for the i P P spherulite fibrillae, i.e. alternating parallel crystalline lamellae and a m o r p h o u s layers, the crystalline lamellar thickness L c was calculated from L values using the following equation9:
L c = XcL/[(pJpa) (1 - Xc) + Xc]
where X c is the d.s.c, crystallinity index, Pc and pa are the density of the crystalline and a m o r p h o u s i P P phases, respectively. The values used for the densities were3: Pc = 0.937 g c m - 3 and p, = 0.852 g c m - 3. Subtracting the L c from the L values the thickness of the a m o r p h o u s length L, was obtained. The results of SAXS analysis are summarized in Tables 5 and 6. In the case of quenched samples (Table 5) it is found that L increases with increase in Mw. This can easily be ascribed to the increase in La; in fact the Lc values seem to be almost constant.
In the case of samples isothermally crystallized (without nucleating agents) the SAXS parameters (L, Lc and L~) increase with increasing T~ (decreasing AT), as
17 15 13 II "-3 2 9 7 5 3 I 0 I I ] ] I I O0O2 0 0 0 4 0.006 S(A)
Figure 2 Typical SAXS Lorentz-corrected desmeared intensity I
versus S patterns for an isothermally crystallized iPP sample
Influences on structure and fracture toughness of iPP." M. A vella et al. Table 5 SAXS parameters calculated for quenched iPP samples
Sample L Lc
(~)
(A)
~)
iPP1 106 36 70 iPP2 113 37 76 iPP3 110 40 70 iPP4 120 40 80 iPP5 122 41 81 iPP6 124 40 84Table 6 SAXS parameters calculated for isothermally crystallized iPP
samples
Without nucleating agent With nucleating agent Sample T~ AT L L c L= T~ AT L L~ L.
(°C) (°C) (A) (~) (A) (°C) (°C) (A) (A) (A)
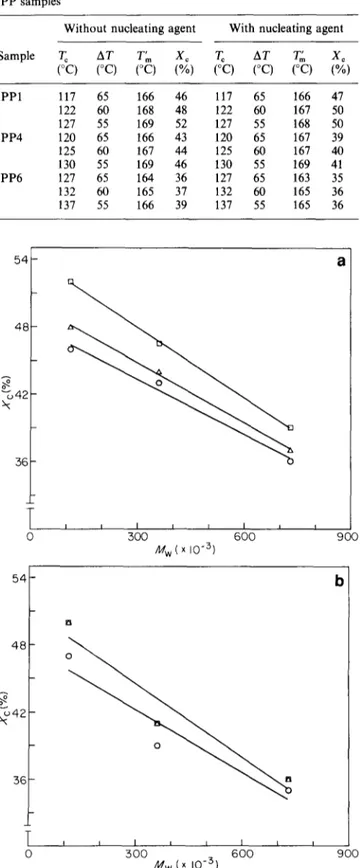
iPP1 117 65 164 71 93 117 65 171 72 99 122 60 163 74 89 122 60 178 75 103 127 55 200 90 110 127 55 187 87 103 iPP4 120 65 177 73 104 120 65 194 72 122 125 60 179 75 104 125 60 201 76 125 130 55 210 93 117 130 55 227 88 125 iPP6 127 65 224 76 148 127 65 221 73 138 132 60 228 79 148 132 60 230 79 151 137 55 261 96 165 137 55 284 97 187 a ioo 90 8c I00 I I [ I I I I I I i i i I 55 6 0 65 &T(°C) 7 0 I I I I I I I I 70 18C 16C 140 o4 % 12C
b
I 0 0 ~ ~ - " 80[--..I I I I I I t J , J I i K I , I I i I r 0 55 60 65 70 AT(°C)Figure 3 (a) Crystal lamellar thickness and (b) amorphous lamellar thickness for iPP samples as a function of undercooling. O, iPP1; A, iPP4; I , iPP6 o'~ % - . j 80 90 70 I I r J I I I I I I i i i I I I i 55 60 65 AT(°C) a I I 7O
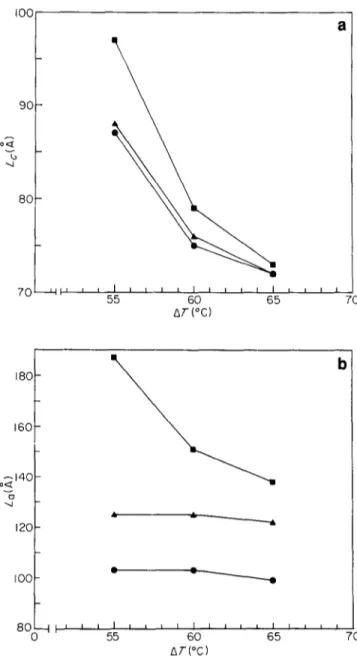
,80 f
160[- b o~14o 120 I 0 0 8 0 0 Figure 4 I I I I I I I I I [ I I I I I I I I 55 60 65 7 0 AT(°C)(a) Crystal lamellar thickness and (b) amorphous lamellar thickness for iPP samples containing sodium benzoate as nucleating agent, as a function of undercooling. O, iPP1; A , iPP4; I , iPP6
expected (see Table 6 and also Figures 3a and b where L c and La values, respectively, are plotted as a function of AT). From these figures it is evident that L= and La values, for a given AT, are affected by Mw. In fact, both Lo and L~ increase with Mw, the effects being more pronounced for L~ than Lc. Similar behaviour was also observed for samples isothermally crystallized in the presence of the nucleating agent (see Table 6 and also Figures 4a and 4b where Lo and L., respectively, are plotted against A T). Also in this case L, Lc and L~ increase with increasing T~ (decreasing AT) and seem to be dependent on the M , of iPP. However, it can be noted that the L= values obtained at the highest AT(see Figure 4a) seem to be almost constant, indicating the formation of lamellar crystals of the same thickness independently of the iPP molecular weight.
Fracture toughness and fractography
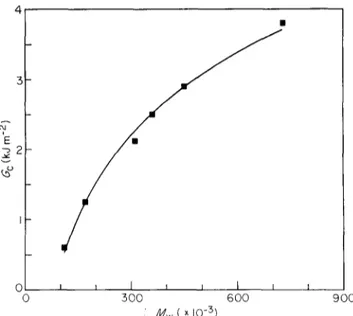
The critical strain energy release rate Gc and the critical stress factor Kc for quenched iPP samples, calculated according to equations (1) and (2), are shown as a function of Mw in Figures 5 and 6, respectively. Both G c and Kc
4 ,
u i
E v
Influences on structure and fracture toughness of iPP: M. Avella et al.
0 I i I i I I I I 0 300 600 900 : ,/k'/w(XlO-3 ) F i g u r e 5 C r i t i c a l s t r a i n e n e r g y r e l e a s e r a t e a s a f u n c t i o n o f m o l e c u l a r w e i g h t f o r q u e n c h e d i P P s a m p l e s 2 i E z 7~ v i 0 t I I i t I 0 I 0 5 0 0 6 0 0 9 0 0 M w ( × 10 -5) Figure 6 C r i t i c a l s t r e s s i n t e n s i t y f a c t o r a s a f u n c t i o n o f m o l e c u l a r w e i g h t f o r q u e n c h e d i P P s a m p l e s
increase strongly with increasing molecular weight. The parameter Go, however, seems to be more sensitive to the molecular weight change.
The key to understanding the effect of Mw on fracture toughness is the tie-molecules that join the crystalline blocks together. These molecules act as local transducers of stress among lamellae and spherulites and hence are able to control the deformation and the fracture process. Their mechanical behaviour may be compared with entanglements in amorphous thermoplastics; both are responsible for the fracture strength and without them both types of polymers are extremely weak. The concentration of tie-molecules, first reported by Keith et al. ~°, decreases markedly with decreasing Mw. Before a critical molecular weight no tie-molecules could be observed. Therefore, as the number of tie- molecules decreases, the materials become physically less interconnected and hence the applied load becomes concentrated at fewer sites at the surfaces of the crystallites, producing larger stress concentrations. Thus
a fracture at lower stress and strain levels may occur. Evidence for the role played by the tie-molecules in controlling the fracture process arises from the fractographic analysis performed on such materials.
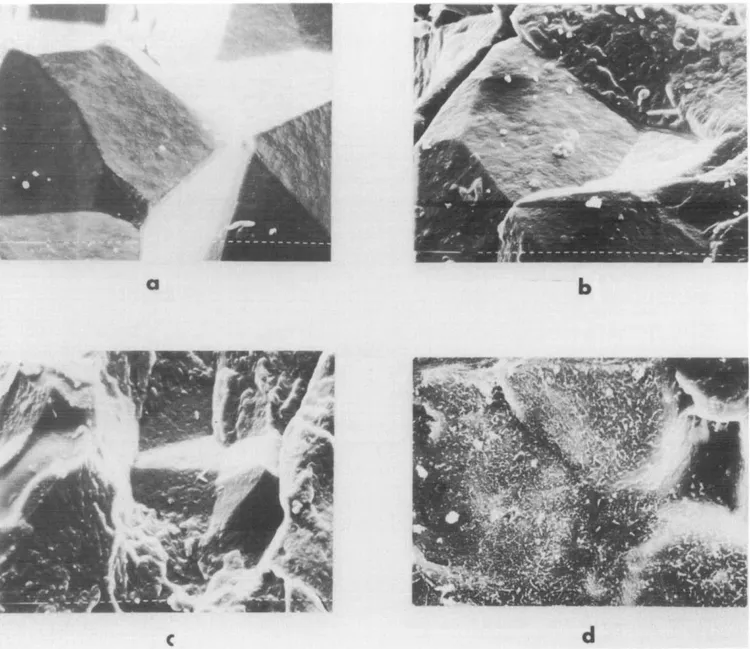
Figure 7 shows the scanning electron micrographs after impact failure of some of the quenched iPP samples. All the pictures were taken near the notch tip in the region of crack initiation. The sample with the lowest molecular weight (Figure 7a) shows a fracture morphology typical of a very brittle material with no signs of localized plastic deformation in the form of crazes or shear bands. Instead, at higher molecular weight
(Figures 7b-d), there is clear evidence of a well-defined plastic region ahead of the notch tip (the notch is situated on the left-hand side of the micrographs) which becomes markedly more extended as the molecular weight is increased. This deformed zone displays morphological features termed 'patch pattern' or 'mackerel', which are frequently reported for amorphous polymers 11-13, suggesting that the onset of crack propagation is preceded by crazing. These crazes may develop and propagate within spherulites and interspherulite regions and hence their formation is essentially determined by the degree of interconnection existing in the material, which in turn depends on the molecular weight. Previous studies on molecular weight dependence of crazing in amorphous polymers 14'15 have shown that in the case of high molecular weight polymers, the crazes were very numerous, long and straight. When the molecular weight was lowered, comparatively few, coarse-textured crazes were formed. At very low molecular weight the tendency for craze formation was greatly decreased. For iPP samples, similar results are observed as a consequence of the fact that increasing M w increases both the inter- and the intraspherulitic link density, so that the crazes formed in one of these regions have enough strength to cause further crazing in their neighbourhood before they break down. Thus, massive crazing with greater stability and resistance to crack propagation can be formed with increasing Mw. Therefore the greater toughness observed with increasing Mw arises from a larger energy dissipation due to the crazing mechanism.
A similar qualitative result may be obtained by varying undercooling, AT since the structural network (connectivity between crystallites and spherulites), where craze nucleation first takes place, is built up during the crystallization process. Figure 8 shows the effect of AT on the fracture toughness for three different iPP samples, representing low, medium and high molecular weight. The data of Figure 8 show a slight linear enhancement of Gc with increasing ATand for a constant AT, as already found, a marked increase of Gc with M w. The modest improvement in fracture toughness observed with ATis due to the limited range of T~ employed and can be attributed to an increased number of tie-chains produced at higher ATvalues. This assumption can be confirmed by electron microscopy studies conducted on the fractured surfaces.
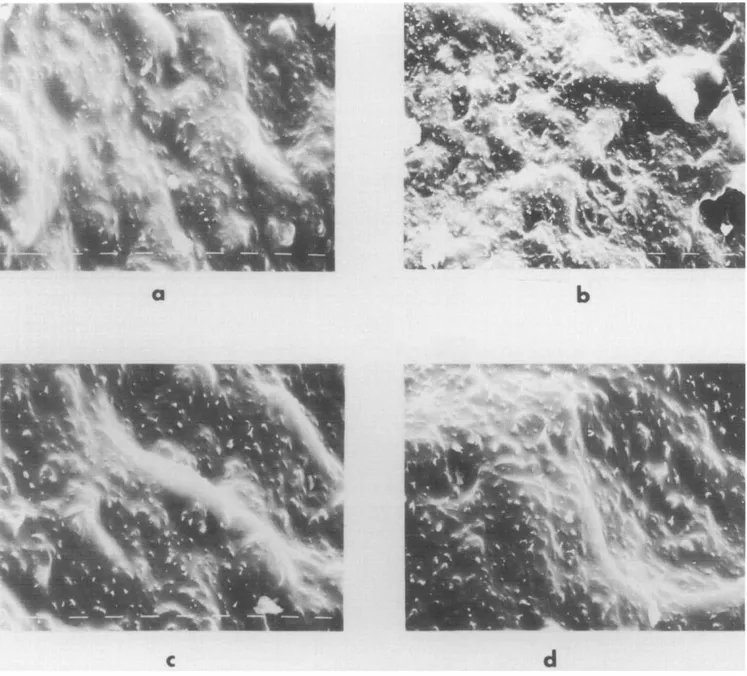
Figure 9 shows the SEM micrographs of iPP samples crystallized at ATvalues of 55 and 65°C. It can be seen from these pictures that some plastic deformations are confined to the spherulite boundaries, which are less crack resistant than the inner zones. This results in interspherulitic craze formation and subsequent boundary fracture. Also, from Figure 7d it appears that the resistance to this type of failure tends to increase with
Influences on structure and fracture toughness of iPP: M. Avella et al.
5
Figure 7 Scanning electron micrographs of fractured surfaces of quenched iPP: (a) iPP1; (b) iPP2; (c) iPP3; (d) iPP5
'E
® 2
o--- ill ~ n J I a ~ n i I I I I I I I I I I 55 60 65 70 AZ'(oc)Figure 8 Critical strain energy release rate as a function of undercooling: O, iPP1; A, iPP4; I , iPP6
increasing AT. This effect arises from the different structures formed under the different crystallization conditions and depends especially on the quality of the amorphous phase connecting the spherulites. At higher AT, the crystallization rate is higher so that poorly crystallized chains can be incorporated. Moreover, since the rate of spherulite growth is greater at large AT, small molecules can be trapped more easily between crystallites before they can diffuse away. Therefore, higher A Tshould produce the best quality boundaries enhancing the tendency for craze formation, especially among spherulite regions. This accounts for the more plastic deformation observed prior to the final fracture at increased AT.
The above results have demonstrated that, when the crystallization process is very slow, changes in AT can give only a modest increase in strength of the spherulite boundaries, which are considered to be the major factor in controlling the fracture toughness. Under such crystallization conditions, however, a more substantial improvement of the spherulite boundary properties, without large variation in other structural parameters,
Influences on structure and fracture toughness of iPP: M. Avella et al.
=
5
¢
d
Figure 9 Scanning electron micrographs of fractured surfaces of iPP samples at different undercoolings: (a) iPP1, AT-65°C; (b) iPP1, AT= 55°C; (c) iPP6, AT-65°C; (d) iPP6, AT= 55°C
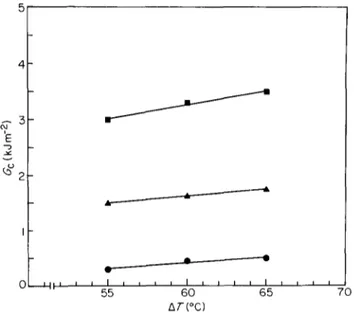
can be obtained if nucleating agents are used. Figure 10 5 shows G c versus A T for the same i P P samples as in
Figure 8 but containing sodium benzoate as nucleating
agent. As can be seen, although the general trend of Gc 4 is very similar to that of Figure 8, the values of Go,
particularly for medium (iPP4) and high (iPP6) molecular weight samples, are much higher. F o r these samples, in
fact, stress whitening was observed just ahead of the notch ~ 3 tip, indicating that extensive plastic deformation has 'E occurred. This may be readily seen from the SEM
micrographs of Figure 11 which show the presence of ~ z highly stretched material in the region of crack initiation.
Such an effect, which increases with increasing AT and
M w, is associated with the occurrence of stabilized I crazes able to accommodate very large strains before
breakdown. A possible explanation of the different fracture behaviour observed when a nucleating agent is
added can be found in the role of sodium benzoate in o reducing average spherulite size and increasing the
formation of tie-molecules. However, as previously reported, crystallinity content and lamellar thickness remain essentially invariant with sodium benzoate.
V _ . _ - - - l l t l - - - " i l l l I i I , I I i I I I I I I I I I I 55 6 0 65 70 A T ( ° C )
Figure 10 Critical strain energy release rate as a function of undercooling for iPP samples containing sodium benzoate as nucleating agent: O, i P P h A, iPP4; i , iPP6
Influences on structure and fracture toughness of iPP: M. Avella et al.
a
b
¢
d
Figure 11 Scanning electron micrographs of fractured surfaces at different undercoolings of iPP samples containing sodium benzoate as nucleating agent: (a) iPP4, AT= 65°C; (b) iPP4, AT= 55°C; (c) iPP6, AT= 65°C; (d) iPP6, AT= 55°C
Previous studies 16-1s have reported that ductility, yield strength and impact strength are improved with decreasing spherulite size. However, in most of those investigations the spherulite size was varied either by controlling the temperature of isothermal crystallization or by controlling the cooling rate. Under such conditions, variation in other structural entities (link density, crystallinity, lamellar thickness, void formation at spherulite boundaries etc.) can be obtained, hence it is not clear whether the earlier results reflect only spherulite size effects or are also caused by other structural variations. However, the trend of our results seems to indicate that the link density should have a stronger effect than spherulite size on fracture toughness, because we have found that failure is preferentially initiated at interspherulitic boundaries.
Correlation between fracture toughness and superreticular
parameters
The results presented above have shown that the parameter G¢ is very sensitive to structural changes in
bulk-crystallized P P samples. It can be taken as a measure of the structural interconnections existing in the material. On this basis we have examined the correlation of such a parameter with some structural parameters such as L, L a and Lc. This correlation for quenched i P P samples is reported in
Figures 12a-c.
In all cases, an increase of Gc is observed with increasing the superreticular parameters. This trend indicates that the crystallite level structure plays a significant role in the fracture process. However, the problem of isolating lamellar-level effects from spherulite-level effects makes it difficult to clearly establish the role of the crystallite texture.Returning to
Figures 12a
and b, the observed behaviour is due essentially to the fact that with increasing L and L a, the internal spherulite structure becomes more interconnected, enhancing the resistance to crack propagation. Since G¢ is sensitive to both intra- and interspherulitic link densities, it is reasonable to expect an increase of G¢ with increasing L and La. On the contrary, only the effects of Lo on the fracture toughness(Figure 12c)
seem to be apparent. InInfluences on structure and fracture toughness of iPP." M. A vella et al. ~ 3 E
~2
oJ E ® I I I I I I10 120LIt/
I 130 J I I I I I / 72 78 84 o Lo(A) 8 b 5 4 ~ 3 E ®2 i C i I i I ~ I 36 42 48 Zc(~-)Figure 12 Critical strain energy release rate as a function of (a) long period, (b) a m o r p h o u s lamellar thickness a n d (c) crystalline lamellar thickness, for quenched samples
fact, as pointed out previously, the quenching treatment leads to only a very small variation in the Lc values. Therefore, the contribution to the fracture toughness due to the plastic deformation on the crystallites should be
almost constant for all the iPP samples. In this way, the controlling parameter of the intraspherulitic fracture is the enhancement of the tie-chains that connect unit areas of adjacent crystallites.
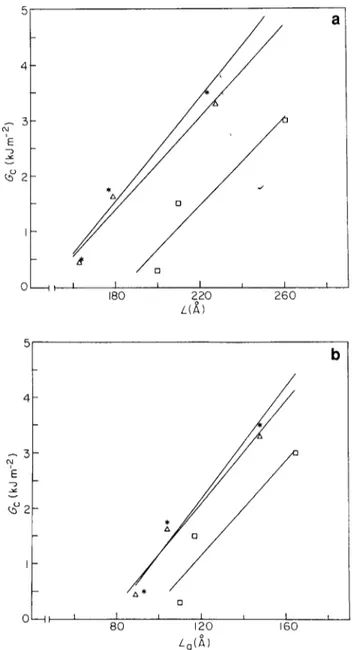
A similar correlation has been made for iPP samples isothermally crystallized with and without a nucleating agent. Figures 13a and b show the results without nucleating agent. For all the investigated undercoolings, Go appears to increase quite strongly with increasing L and L a. Such behaviour suggests a marked effect of the intraspherulitic structure on Go. This contrasts with the results of fractographic analysis, which have demonstrated that at low undercoolings the crack essentially follows the weak boundaries of the original coarse spherulites. A possible explanation of this disagreement is that the above correlation overestimates the contribution of the internal spherulitic structure. In fact, in such materials the connectivity of the spherulitic boundaries is the major factor in controlling the fracture toughness. Finally, Figures 14a and b report the correlation for isothermally crystallized samples containing a nucleating agent. As we have seen, the trend
'E 5 4 5 2 ..---I I a
//,
t I I I t I I 180 220 260 b 4 ~ 3 E¢
A I I m 0 I I t L ~ t I 80 120 160 Lo(~.)Figure 13 Critical strain energy release rate as a function of (a) long period L a n d (b) a m o r p h o u s lamellar thickness, for isothermal samples at different undercoolings: *, 65°C; A , 60°C; [~, 55°C
Influences on structure and fracture toughness of iPP: M. Avella et al. a 5 2 .~1 'E I t I I I I 180 2 2 0 2 6 0
L/~,/
~T
E ® 2 0 II i 8~0 b I ~ I I I I 0 160 o La(A)Figure 14 Critical strain energy release rate as a function of (a) long
period and (b) amorphous lamellar thickness, for isothermal samples with nucleating agent at different undercoolings: *, 65°C; A, 60°C; I-q, 55°C
is very close to that observed without a nucleating agent. However, in this case, as for quenched samples, the presence of a very fine spherulitic structure leads to competition between localized interspherulitic fracture and dispersed intraspherulitic fracture. Therefore, it is reasonable to observe a m a r k e d dependence of the fracture toughness with parameters such as L and Lo.
C O N C L U D I N G R E M A R K S
It has been shown that processing conditions and molecular characteristics strongly influence the structure
and consequently the high-speed fracture behaviour of isotactic polypropylene. In particular, from the findings presented here the following conclusions can be drawn. The a m o u n t of crystallinity is lowered by increasing undercooling and molecular weight, while the lameUar thickness increases with increasing molecular weight and decreasing undercooling. These effects can be understood on the basis of the molecular mechanism acting during the crystallization process.
The fracture toughness parameters (Go and Kc), which represent the resistance of material prior to crack propagation, increase markedly with increasing molecular weight. Moreover, the use of sodium benzoate as a nucleating agent strongly enhances the toughness of the isothermally crystallized samples. These fracture results were interpreted in terms of a fractographic analysis performed by SEM. This analysis demonstrated that the d o m i n a n t failure mechanism was localized crazing, which occurred to a greater extent as the connectivity between crystallites and spherulites was enhanced.
Finally, the correlation of the fracture toughness with some superreticular parameters has shown the i m p o r t a n t role played by the crystal-level microstructure on the failure process.
A C K N O W L E D G E M E N T
This work was partly supported by ' P r o g e t t o Finalizzato Chimica Fine 2-CNR'.
R E F E R E N C E S
1 Avella, M., dell'Erba, R. and Martuscelli, E. Mackromol. Chem.
submitted
2 Galli, P., Simonazzi, T. and Del duca, D. Acta Polym. 1988, 39, 81 3 Brandrup, J. and Immergut, E. M. (Eds) 'Polymer Handbook',
Interscience, New York, 1989
4 Glatter, 0 . J. Appl. Crystallo#r. 1974, 7, 147
5 Alexander, L. E. 'X-ray Diffraction in Polymer Science', Wiley, New York, 1969
6 Kinloch, A. J. and Young, R. J. 'Fracture Behaviour of Polymers', Applied Science, London, 1983
7 Brown, W. F. and Srawley, J. ASTM STP 410, American Society for Testing and Materials, Philadelphia, 1966, p. 13
8 Plati, E. and Williams, J. G. J. Polym. Eng. Sci. 1975, 15, 470 9 Martuscelli, E., Canetti, M. and Seves, A. Polymer 1989, 30, 304 10 Keith, H. D., Padden, F. J. Jr and Vadimsky, G. J. Polym. Sci.
A-2 1966, 4, 267
11 Greco, R. and Ragosta, G. Plast. Rubber Process. Appl. 1987, 7, 163
12 Murray, J. and Hull, D. J. Polym. Sci. A-2 1970, 8, 583 13 Van Noort, R. and Ellis, B. J. Mater. Sci. Lett. 1984, 3, 1031 14 Fellers, J. F. and Kee, B. F. J. Appl. Polym. Sci. 1974,18, 2355 15 Lainchbry, D. L. G. and Bevis, M. J. Mater. Sci. 1976,11, 2222 16 Lovinger, A. J. and Williams, M. L. J. Appl. Polym. Sci. 1980,
25, 1703
17 Way, J. L., Atkinson, J. R. and Weitting, J. J. Mater. Sci. 1974, 9, 293
18 Kleiner, L. W., Radloff, M. R., Schultz, J. M. and Chon, T. W.