Metadata of the chapter that
will be visualized online
Series Title Current Clinical Oncology
Chapter Title Renal Cancer Follow-up Counterpoint: Europe Chapter SubTitle
Copyright Year 2013
Copyright Holder Springer Science+Business Media New York
Family Name Antonelli
Particle
Given Name Alessandro
Corresponding Author
Suffix
Division Department of Urology
Organization University of Brescia, Spedali Civili Hospital Address Piazzale Spedali Civili 1, Brescia, 25123, Italy
Email [email protected]
Family Name Simeone
Particle
Given Name Claudio
Author
Suffix
Division Department of Urology
Organization University of Brescia, Spedali Civili Hospital Address Piazzale Spedali Civili 1, Brescia, 25123, Italy Email
Family Name Cunico
Particle
Given Name Sergio Cosciani
Author
Suffix
Division Department of Urology
Organization University of Brescia, Spedali Civili Hospital Address Piazzale Spedali Civili 1, Brescia, 25123, Italy Email
Abstract Despite the significant increase in early diagnoses that took place in recent years, thanks to the increase in popularity of imaging techniques (ultrasonography and CT), the kidney neoplasm is still the urologic cancer with the highest mortality rate [1] due to the significant number of cases with distant metastases for which no systemic treatment with curative potential exists. The disease has a variable and often highly unpredictable biological behavior and recurrence is possible also after radical treatment of organ-confined disease. The most common sites of relapse are lung, adrenal, liver, bone, brain, lumbar fossa, and contralateral kidney, but the literature documents that kidney carcinoma can metastasize to virtually any organ. The absence of effective systemic therapy can justify adoption of the most accurate follow-up plan available to diagnose a relapse as quickly as possible and surgically remove it. As a matter of fact, surgical metastasectomy, wherever technically feasible, can be curative and/or lead to an increase in survival duration [2].
Keywords (separated by
Despite the significant increase in early diagnoses that took place in recent years, thanks to the increase in popularity of imaging techniques (ultrasonography and CT), the kidney neoplasm is still the urologic cancer with the highest mortal-ity rate [1] due to the significant number of cases with distant metastases for which no systemic treatment with curative potential exists. The disease has a variable and often highly unpredictable biological behavior and recurrence is possible also after radical treatment of organ-confined disease. The most common sites of relapse are lung, adrenal, liver, bone, brain, lumbar fossa, and contralateral kidney, but the litera-ture documents that kidney carcinoma can metastasize to virtually any organ. The absence of effective systemic ther-apy can justify adoption of the most accurate follow-up plan available to diagnose a relapse as quickly as possible and surgically remove it. As a matter of fact, surgical metastasec-tomy, wherever technically feasible, can be curative and/or lead to an increase in survival duration [2].
Several clinical, biochemical, anatomic pathological, and molecular factors have been analyzed for their prognostic value but today anatomic pathological staging according to the TNM system remains the most important single prognos-tic factor. In order to increase its accuracy, several authors have proposed some integrated staging systems in which the TNM stage is combined with other prognostic factors [3].
In our institution, over the last two decades, we have sur-gically treated more than 1,500 patients with renal cell carci-noma. In cases where radical surgery was applied to a nonmetastatic neoplasm (pN0/Nx M0), patients are followed
with a surveillance plan independent of the disease stage. Periodic controls are done with blood tests (complete blood count, kidney and liver function tests), abdominal imaging examinations (ultrasonography or CT) and chest examina-tions (plain X-rays or CT) once each 6 months in the first 2 years after surgery and then again every year for an indefinite time. Additional examinations (brain CT and bone scintigra-phy) have been, in general, used only in the presence of specific symptoms. In light of this experience, which has allowed us to monitor these patients continuously, we have recently reviewed the results obtained and retrospectively applied an integrated staging system to assess which cases might require more intense surveillance and which cases might well be served by less intense surveillance [4].
Among the many integrated staging systems available, we have chosen the one developed at UCLA (UCLA Integrated Staging System, UISS [5]), which is based on two anatomic pathological factors (the stage according to TNM 1997 [6] and the cytonuclear grading according to Fuhrman [7]) plus a clinical factor (the performance status as defined by the ECOG score [8]) (see Table 74.1). The widespread availabil-ity of this information makes this staging system applicable in all institutions, which is one of its greatest assets. The combination of the three factors permits assignment to three risk classes, i.e., low risk (LR), intermediate risk (IR), and high risk (HR) (see Table 74.2).
We have reviewed data on 814 patients with nonmeta-static kidney cancer (pN0/Nx M0), 158 of which had under-gone nephron-sparing surgery, the remaining 656 had undergone nephrectomy. Average follow-up duration for all patients was 76 months (minimum 24 months). Relapses have occurred in 193 cases, corresponding to 24% of the total. According to UISS, 140 cases were LR, 420 IR, and 254 HR. Relapse rate in the follow-up for the three risk
Keywords
Nonmetastatic kidney cancer • Kidney • Nephrectomy • Surveillance • Papillary carcinoma
Renal Cancer Follow-up
Counterpoint: Europe
Alessandro Antonelli, Claudio Simeone, and Sergio
Cosciani Cunico
74
A. Antonelli (*) • C. Simeone • S.C. Cunico
Department of Urology, University of Brescia, Spedali Civili Hospital, Piazzale Spedali Civili 1, Brescia 25123, Italy
e-mail: [email protected]
F.E. Johnson et al. (eds.), Patient Surveillance After Cancer Treatment, Current Clinical Oncology, DOI 10.1007/978-1-60327-969-7_74, © Springer Science+Business Media New York 2013
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69
A. Antonelli et al.
classes was 10, 22, and 54%, respectively, with an average latency after surgery of 54, 36, and 30 months, respectively. The most common type of relapse was distant metastasis (73%), followed by local relapse (12%) and by the appear-ance of a new kidney neoplasm in the contralateral kidney (11%) or in the remaining kidney after nephron-sparing surgery (4%). Table 74.3 shows the distribution of relapses, with onset times, in the different UISS risk classes. It is
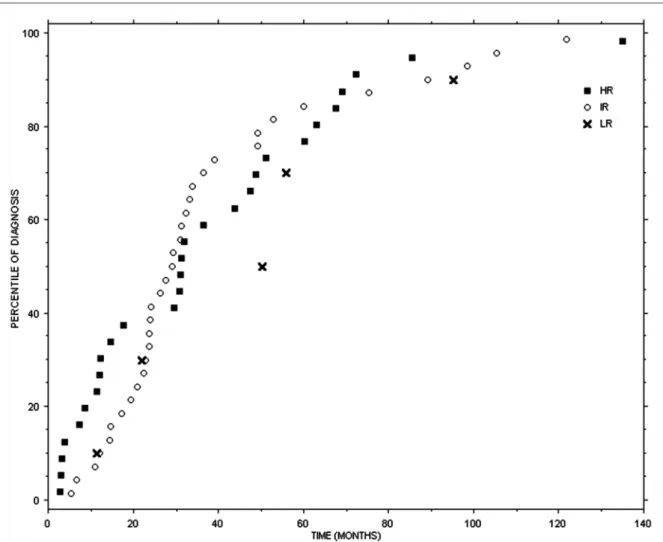
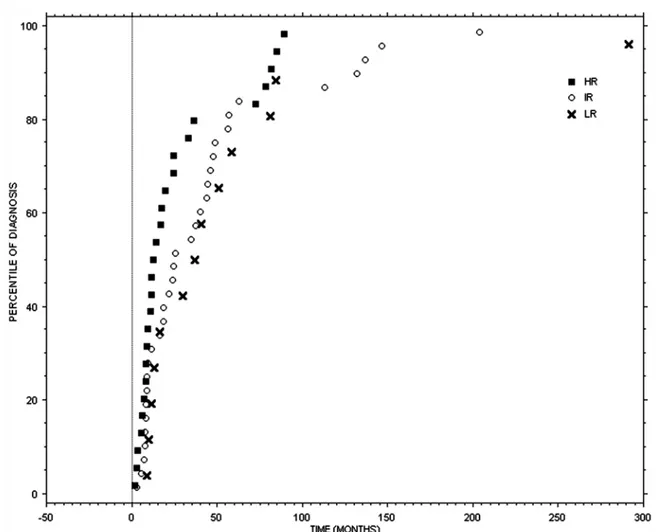
easy to note that the risk of relapse via a new renal neo-plasm decreases gradually over time among the three risk classes (LR, IR, and HR) while the chance of local relapse or distant metastasis increases from LR to IR to HR class. From a biological point of view relapses in the kidney deserve to be viewed and dealt with differently from distant metastases or local relapses. Indeed, the development of a neoplasm in the kidney undergoing nephron-sparing sur-gery may be explained by the presence of unrecognized multifocal disease or by the lack of adequate surgical mar-gins while a neoplasm in the contralateral kidney can be considered a new primary cancer. Patients with a relapse in the contralateral kidney, in the ipsilateral kidney after nephron-sparing surgery, with distant metastasis, and local recurrence have a 12-month survival rate after diagnosis of 96, 86, 70, and 44%, respectively. Figures 74.1 and 74.2 show the time distribution of the three risk classes of relapses in the chest and in the abdomen, including abdom-inal metastases, local relapses, and kidney relapses. Disease relapse in LR patients in the first 5 years of follow-up occurs chiefly at abdominal level, while the risk of lung recurrence is less serious. On the other hand, IR patients in the first 5 years after surgery have a higher risk of relapse in the lung, especially in the first 24–36 months, while risk of relapse in the abdomen is lower; the same happens, albeit at a significantly lower rate, for the subsequent 5 years. HR patients, during the earliest years of follow-up, show a high risk of relapse in the abdomen and a lower risk of lung metastasis. This risk, though still significant, decreases in the subsequent 5 years. All risk classes, after 10 years of follow-up, feature only rare relapses, chiefly in the abdomi-nal area and in the contralateral kidney. As regards the imaging methods to be used for monitoring the chest and abdomen, from a cost/benefit point of view, it is preferable
Table 74.1 Prognostic systems
TNM 1997
pT1 Tumor <= 7 cm in the greatest dimension, limited to the kidney
pT2 Tumor >7 cm in the greatest dimension, limited to the kidney
pT3 Tumor extends into major veins or directly invades the adrenal gland or perinephric fat tissues but not beyond the Gerota’s fascia
pT4 Tumor directly invades beyond Gerota’s fascia Fuhrman’s grading
G1 Tumor cells with small (~10 mm) round uniform nuclei without nucleoli
G2 Tumor cells with larger nuclei (~15 mm) with irregularities in outline nucleoli when examined under high power (400) G3 Tumor cells with even larger nuclei (~20 mm) with obvi-ously irregular outline prominent larger nucleoli even at low power (100)
G4 Tumor cells with bizarre, multilobed nuclei heavy clumps of chromatin
ECOG score
0 Fully active, able to carry on all predisease performance without restriction
1 Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work
2 Ambulatory and capable of all self care but unable to carry out any work activities. Up and about more than 50% of waking hours
3 Capable of only limited self-care, confined to bed or chair more than 50% of waking hours
4 Completely disabled. Cannot carry on any self-care. Totally confined to bed or chair
Table 74.2 UISS definitions
UISS risk class pT G ECOG
Low risk 1 1–2 0 Intermediate risk 1 1–2 >0 1 3–4 Any 2 Any Any 3 1 Any 3 >1 0 High risk 3 >1 >0 4 Any Any
Table 74.3 Site of relapse
latency (months) All patients (%) LR (%) IR (%) HR (%) Operated kidney 23.4 4.1 11.6 5.4 0 Contralateral kidney 71.8 10.9 30.1 10.1 4.0 Local recurrence 26.4 11.9 3.9 7.6 20.0 Distant metastasis 29.5 73.0 53.8 76.1 76.0 Abdomen 32.8 15.6 7.2 17.1 15.8 Chest 29.5 48.3 35.7 50.0 49.1 Bone 14.9 11.3 21.4 12.9 7.0 Others 41.4 9.9 21.4 8.6 8.8 Multiple sites 24.1 14.9 14.3 11.4 19.3 Recurrence sites and time, as a percentage of asymptomatic patients, and the distribution of different types of recurrence in UISS risk groups (LR low risk, IR intermediate risk, HR high risk; the sum of percent-ages of each site of distant metastasis is 100%)
70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 t1.1 t1.2 t1.3 t1.4 t1.5 t1.6 t1.7 t1.8 t1.9 t1.10 t1.11 t1.12 t1.13 t1.14 t1.15 t1.16 t1.17 t1.18 t1.19 t1.20 t1.21 t1.22 t1.23 t1.24 t1.25 t1.26 t1.27 t1.28 t1.29 t1.30 t1.31 t1.32 t1.33 t2.1 t2.2 t2.3 t2.4 t2.5 t2.6 t2.7 t2.8 t2.9 t2.10 t3.1 t3.2 t3.3 t3.4 t3.5 t3.6 t3.7 t3.8 t3.9 t3.10 t3.11 t3.12 t3.13 t3.14 t3.15 t3.16
Fig. 74.1 Time distribution of thoracic relapses (LR low risk, IR intermediate risk, HR high risk; marks represent the events of recurrence as
percentiles on overall recurrences in each specific site; zero point is the time of treatment of primary tumor)
to use cheaper and safer (for the patient) techniques like abdominal ultrasonography and chest radiography for lower-risk patients, using CT only for higher-risk patients. The risk of bone metastases is limited. It is higher in the time period closer to the surgery and it also pertains chiefly to HR cases. In light of these data, we think it is possible to offer different surveillance plans, depending on risk classes, as shown in Tables 74.4, 74.5, 74.6.
There is no significant difference in risk and relapse mode between patients subjected to nephrectomy and those sub-jected to nephron-sparing surgery. There is consequently no need to modify surveillance according to this factor.
One factor not included in the UISS but, in our opinion, worthy of consideration is the tumor histological subtype. Currently, according to the classification drafted in the Heidelberg and Rochester consensus conferences, there are four main histological subtype of kidney carcinoma: clear
cell (80% of cases), papillary (in turn divided in type 1 and type 2), chromophobe, and collecting duct [9]. Although the independent prognostic role of the histotype has not been clearly demonstrated, it is quite evident that patients suffer-ing with chromophobe and type 1 papillary renal cell carci-nomas usually have a highly favorable prognosis, whereas patients with collecting duct carcinoma have an extremely unfavorable prognosis and the prognosis of patients with type 2 papillary carcinoma or conventional renal cell carci-noma is somewhere in between the extremes [10, 11]. Consequently, we propose to manage follow-up of patients with favorable histotype (chromophobe and type 1 papillary renal cell carcinoma) with the plan proposed for LR class patients and to apply the HR follow-up plan for patients with the unfavorable histotype (collecting duct carcinoma). The follow-up of patients with type 2 papillary carcinoma can be decided by stratification with the UISS.
112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145
A. Antonelli et al.
References
1. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics 2004. CA Cancer J Clin. 2004;54:8–29.
2. Kuczyk MA, Anastasiadis AG, Zimmermann R, Merseburger AS, Corvin S, Stenzl A. Current aspects of the surgical management of organconfined, metastatic and recurrent renal cell cancer. BJU Int. 2005;96:721–7.
3. Furniss D, Harnden P, Ali N, et al. Prognostic factors for renal cell carcinoma. Cancer Treat Rev. 2008;34(5):407–26.
4. Antonelli A, Cozzoli A, Zani D, Zanotelli T, Nicolai M, Cosciani Cunico S, Simeone C. The follow-up management of nonmetastatic
Table 74.4 Surveillance after curative-intent treatment for renal
cancer for LR patients at Spedali Civili Hospital years posttreatment 1 2 3 4 5 5–10 >10 Chest X-ray 0 1 0 1 0 0 0 Abdomen US 1 1 1 1 1 2 1 Abdomen CT 0 0 0 0 0 0 0 Chest CT 0 0 0 0 0 0 0 Bone scan 0 0 0 0 0 0 0
The number in each cell is the number of times a particular examination is recommended in a particular posttreatment year (LR, low risk)
Fig. 74.2 Time distribution of abdominal relapses (LR low risk, IR intermediate risk, HR high risk; marks represent the events of recurrence as
percentiles on overall recurrences in each specific site; zero point is the time of treatment of primary tumor)
Table 74.5 Surveillance after curative-intent treatment for renal
cancer for IR patients at Spedali Civili Hospital years posttreatment 1 2 3 4 5 5–10 >10 Chest X-ray 1 1 2 1 1 5 0 Abdomen US 1 1 2 1 1 5 1 Abdomen CT 1 1 0 0 0 0 0 Chest CT 1 1 0 0 0 0 0 Bone scan 0 0 0 0 0 0 0
The number in each cell is the number of times a particular examination is recommended in a particular posttreatment year (IR intermediate risk)
Table 74.6 Surveillance after curative-intent treatment for renal
cancer for HR patients at Spedali Civili Hospital years posttreatment 1 2 3 4 5 5–10 >10 Chest X-ray 0 1 1 2 2 5 0 Abdomen US 0 1 1 2 2 5 1 Abdomen CT 2 1 1 0 0 0 0 Chest CT 2 1 1 0 0 0 0 Bone scan 0 1 0 0 0 0 0
The number in each cell is the number of times a particular examination is recommended in a particular posttreatment year (HR high risk)
146 147 148 149 150 151 152 153 154 155 156 t4.1 t4.2 t4.3 t4.4 t4.5 t4.6 t4.7 t4.8 t4.9 t4.10 t4.11 t5.1 t5.2 t5.3 t5.4 t5.5 t5.6 t5.7 t5.8 t5.9 t5.10 t5.11 t6.1 t6.2 t6.3 t6.4 t6.5 t6.6 t6.7 t6.8 t6.9 t6.10 t6.11
renal cell carcinoma: definition of a surveillance protocol. BJU Int. 2007;99(2):296–300.
5. Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–57.
6. Guinan P, Sobin LH, Algaba F, et al. TNM staging of renal cell carcinoma: workgroup no. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997;80:992–3.
7. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63.
8. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
9. Eble JN, World Health Organization. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon, Oxford: IARC Press & Oxford University Press; 2004.
10. Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27: 612–24.
11. Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experi-ence. J Clin Oncol. 2005;23:2763–71.
157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180