UNIVERSITY OF PISA
RESEARCH DOCTORATE
IN NEUROBIOLOGY AND CLINIC OF
AFFECTIVE DISORDERS
DEPARTMENT OF PSYCHIATRY, NEUROBIOLOGY,
PHARMACHOLOGY AND BIOTECNOLOGIES
THESIS:
MONOAMINE OXIDASE A (MAOA), CHILDHOOD
TRAUMA, ALCOHOLISM AND AGGRESSION
CANDIDATE: SUPERVISING PROFESSOR:
DR. FRANCESCA DUCCI PROF. LILIANA DELL'OSSO
HEAD OF DEPARTMENT:
SUMMARY...3
1 INTRODUCTION...5
2 METHOD ...8
2.1 Participants... 8
2.2 Psychiatric Assessment ... 8
2.3 Assessment of Childhood Sexual Abuse... 10
2.4 Genotyping... 11
2.4.1 MAOA-LPR... 11
2.4.2 SNP genotyping... 11
2.5 Statistical Analyses ... 12
3 RESULTS ...14
3.1 Main effects of MAOA and MAOB markers ... 14
3.2 Interaction between MAOA-LPR and childhood sexual abuse ... 15
3.3 Haplotype-based analyses... 16
SUMMARY
Women who have experienced childhood sexual abuse (CSA) have an increased risk of alcoholism and antisocial personality disorder (ASPD). Among males, a functional polymorphism (MAOA-LPR, monoamine oxidase A linked polymorphic region) in the promoter region of the monoamine oxidase A gene (MAOA) appears to moderate the effect of childhood maltreatment on antisocial behavior.
Our aim was to test whether MAOA-LPR influences the impact of CSA on alcoholism and ASPD in a sample of 291 women, 50% of whom have experienced CSA; we also tested whether haplotypes covering the region where both MAOA and monoamine oxidase B (MAOB) genes are located predict risk of alcoholism and ASPD better than the MAOA-LPR locus alone. Participants included 168 alcoholics (39 with ASPD [antisocial alcoholics]) and 123 controls (no Alcoholics, no ASPD). Antisocial behavior was also modeled as a continuous trait: ASPD symptoms count. The MAOA-LPR low activity allele was associated with alcoholism (p=0.005), particularly antisocial alcoholism (p=0.00009), only among sexually-abused subjects. Sexually-abused women who were homozygous for the low activity allele had higher rates of alcoholism and ASPD, and more ASPD symptoms, than abused women homozygous for the high activity allele. Heterozygous women displayed an intermediate risk pattern. In contrast, there was no relationship between alcoholism/antisocial behavior and MAOA-LPR genotype among non-abused women. The MAOA-LPR low activity allele was found on three different haplotypes. The most abundant MAOA haplotype containing the MAOA-LPR low activity allele was found in excess among alcoholics (p=0.008) and antisocial alcoholics (p=0.001). Finally, a MAOB haplotype, that we termed haplotype C, was significantly associated with alcoholism (p=0.006), and to a lesser extent with antisocial alcoholism (p=0.03). In conclusions, MAOA seems to moderate the impact of childhood trauma on adult psychopathology in females in the same way as previously shown among males. The MAOA-LPR low activity allele appears to confer increased vulnerability to the adverse psychosocial
consequences of CSA. Haplotype-based analysis of the MAOA gene appeared to strengthen the association, as compared to the MAOA-LPR locus alone. A MAOB haplotype was associated with alcoholism independently from ASPD.
1 INTRODUCTION
Thirteen percent of American women report childhood sexual abuse (CSA)1, and CSA is a critical environmental exposure for a variety of psychiatric diseases. Women exposed to CSA have an increased risk for different psychopathologies in adulthood including addictions to alcohol and other drugs 2;3;4;5; antisocial personality disorder (ASPD) 4; 5; major depression7; 8; 9, suicidal behavior4; 8; 10; 11; post traumatic stress disorder (PTSD) 4; 12; 13 and eating disorders6.
The impact of CSA on adult mental health is only one of a complex set of intercorrelated social, economic and familial disadvantages and as such the presence of CSA is an indicator of a wider and larger dysfunction within families1;
5. In addition within particular socio-cultural contexts, such as occurs in certain
American Indian tribes, rates of violence and cumulative trauma are very high, potentially amplifying within-family effects and contributing to the high vulnerability to psychiatric disorders found overall in these communities14. In the American Indian tribe which is the object of the present study, lifetime prevalences of alcohol dependence (AD) and ASPD among women are very high, 0.50 and 0.13 respectively15. By comparison, prevalences of AD and ASPD among U.S.
epidemiological samples of woman were 0.08 and 0.02 respectively16.
In this relatively isolated American Indian community4 approximately half of women were exposed to sexual abuse during childhood/early adolescence and CSA was found to predict both the development of alcoholism (OR: 2.1) and ASPD (OR: 2.9)4. In other words, despite the high prevalences of Alcoholism and ASPD
and community-pervasive risk exposures, CSA still emerged as a major risk factor. However, while CSA has profound enduring effects into adulthood, not all abused children develop psychosocial problems later in life. Interindividual differences in stress resiliency are likely to be partially moderated by genetic differences, a phenomenon known as gene by environment interaction. Recent findings 17; 18; 19; 20;
21 indicate that the Monoamine Oxidase A gene (MAOA) might be among those
genes that moderate individual response to stress.
MAOA is an X-linked gene22 encoding the mitochondrial enzyme Monoamine
Oxidase A (MAOA; EC 1.4.3.4) that metabolizes monoamines including noradrenaline (NE), dopamine (DA), and serotonin (5-HT). MAOA knockout mice have higher levels of DA, 5-HT and NE, and manifest increased aggressive behavior 23. In the humans, a common MAOA polymorphism influences MAOA transcription24. This locus, termed MAOA linked polymorphic region (MAOA-LPR), is a Variable Number Tandem Repeat (VNTR) located approximately 1.2 kb upstream to the MAOA start codon and within the gene’s transcriptional control region 24; 25. Alleles at this VNTR have different number of copies of a 30-bp repeated sequence with the 3 and 4 repeats alleles being by far the most common. Alleles with 4 repeats are transcribed more efficiently than alleles with 3 copies of the repeat and therefore are associated with higher MAOA activity 24. In a relatively recent study 17, it has been shown that the effect of adversity on vulnerability to antisocial behavior is contingent on MAOA-LPR genotype. In a longitudinal sample of males, Caspi et al (2002) have found that maltreated boys with the low activity genotype were more likely to develop antisocial problems later in life than boys with the high activity genotype. There was also a main effect of maltreatment on risk for antisocial behavior, whereas a main effect of MAOA-LPR genotype was not detected. These results were later replicated18. Overall these findings indicate that MAOA-LPR contributes to interindividual differences in stress resiliency. However, it still remains unclear whether the MAOA-LPR by environment interactive effect on antisocial behavior described for males is applicable to females. The few studies conducted on women have reported inconsistent results19; 26; 27. In a sample of 196 Caucasian women, the low activity allele was associated with adolescent but not adult antisocial behavior only among maltreated subjects27. In a sample of 119 adolescent females, the high activity genotype rather than the low activity genotype was associated with increased risk
(2004) failed to find any significant interactive effect between MAOA-LPR and maltreatment in a sample of 377 females19.
The main purpose of the present study was to evaluate whether MAOA-LPR predicts alcoholism and ASPD contingent on early (<16 years) exposure to sexual abuse in a high risk community sample of 291 adult American Indian women. A secondary aim was to test whether haplotypes covering the region of MAOA and the nearby monoamine oxidase B (MAOB) genes predict alcoholism and ASPD better than the MAOA-LPR locus alone. In this regard it is important to stress that the MAOA-LPR is located within a relatively large region of high linkage disequilibrum (LD)28;29; therefore, other functional polymorphisms might contribute to its effects on transcription. A recent study exploring 12 single nucleotide polymorphisms in addition to the MAOA-LPR suggests that this locus might not account for all the variation in MAOA transcription due to genetic factors and that other functional loci might exist28. Such functional loci might be located at the MAOA gene as well as MAOB. For this reason we genotyped nine additional SNPs encompassing the region of the two MAO genes and performed haplotype-based analyses.
2 METHOD
This protocol was approved by the Tribal Council of this Southwest Tribe,and by the Institutional Review Board of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.All subjects provided informed consent before entering the study and received $40 as compensation for their time. To maximize confidentiality, the name and exact location of the tribe are not listed.
2.1 Participants
Two hundred and ninety one women (mean age ± SD: 37.80 ± 14.58) were recruited from a Southwest American Indian tribe. In addition, 210 male subjects that were recruited were not investigated in the present report because of the low number of them who were without alcoholism or ASPD (N=22) and because of their lower level of exposure to CSA (N=21) as compared to women. Recruitment was blind to the clinical histories of subjects and their relatives. This intensively studied sample has been characterized as representative of the source population in a variety of respects including socioeconomic status, and degree of relatedness. An epidemiologic study using the CAGE has shown that the high prevalence of alcoholism in this dataset is reflective of the population30. Elder tribal members who were considered matriarchs or patriarchs and who possessed a good knowledge of family structures provided information on large multigenerational genealogies. Participants were older than21 years and eligible for tribal enrollment, having at least one quarter tribal heritage4.
2.2 Psychiatric Assessment
Psychiatric diagnoses were made according to DSM-III-R criteria using the Schedule for Affective Disorders and Schizophrenia-Lifetime version (SADS-L) by following operationally defined criteria and using the instructions of Spitzer et al.3132. ASPD was diagnosedaccording to DSM_IIIR. Validity of standard criteria for psychiatric diagnosis can be problematic within special cultural groups.
Therefore, provisions were taken to limit diagnostic errors due to culture-specific phenomena. SADS-L interviews were administered to all subjects by a psychologist with extensive experience in tribal customs and culture (R.W.R.). Because the high rate of unemployment in this population might lead to an overestimation of the prevalence of ASPD, participants were questioned in detail about the specific circumstances that may have contributedto their unemployment status. In addition, the SADS-L criterion, “does not maintain close social ties,” was added to the DSM-III-R criteria to compensate for the absence of some ASPD items on the SADS-L. Diagnoses were made from this databy two blind raters: a clinical social worker and a clinical psychologist.The rates of agreement between the two raters were acceptable; the kappa coefficient for alcohol abuse diagnosis was 0.72, for alcohol dependence 0.96 and for ASPD 0.97. Diagnostic differences between the two raters were resolved in a consensus conference that included a senior psychiatrist experienced in diagnosis in American Indian people. ASPD symptoms count was based on SADS-L items.
Among the 291 female participants, 168 had a lifetime diagnosis of an Alcohol Use Disorder (AUD) and 86% of these had alcohol dependence. Thirty-nine participants had both ASPD and AUD. All subjects with ASPD were affected by AUD. Controls (N=123) were non-ASPD and non-alcoholic. Among male participants, 89% were affected by AUD (187/210) and 39% by ASPD (82/210). Only 22 male participants were non-ASPD and non-alcoholic.
To increase our power to detect a gene effect on antisocial behavior, the latter variable was also modeled as a continuous trait: ASPD symptoms count. The mean ± SD ASPD symptom count for this sample of women was 4.18 ± 4.14. For comparison, among male participants, the mean ± SD ASPD symptom count was 7.69 ± 5.04
2.3 Assessment of Childhood Sexual Abuse
Exposure to sexual abuse during childhood was retrospectively assessed in a subset of 187 women and 126 men. The data for CSA were mainly collected during the psychiatric interview sessions and to lesser extent were based on medical records which were available in cases where the victim had received treatment at health service facilities. Therefore information on CSA was largely dependent upon recall of past events and only clearly recalled information was collected.
Childhood sexual abuse was defined as direct physical sexual contact with a perpetrator at least five years older than the victim, and in victim’s experiencing such contact prior to the age of 16 years. Peer experiences and sexual abuse not involving direct physical contact were excluded. As noted, questions about CSA were asked during SADS-L psychiatric interview sessions. Subjects had completed approximately two hours of interviewing before being asked the following seven questions:
1. Were you ever sexually abused or molested as a child before the age of 16 years? If answered yes to question 1, the subject was then asked the following questions: 2. Who first sexually abused or molested you?
3. At what age did this (first abuse or molestation) begin? 4. How often did it (the abuse or molestation) occur?
5. What did this person do to sexually abuse or molest you? Describe what s/he did. 6. Were you sexually abused or molested by an additional individual?
If answered yes to question 6, the subject was asked questions 2-6 again.
The semi-structured nature of the interview permitted opportunities for further disclosure of events by subjects including frequency, duration, and outcome of reported abuse. There is evidence that by using this format, accurate recall and reporting of childhood experiences is enhanced33. Standard measures of child sexual abuse were not used primarily because they assume a nuclear family structure, inapplicable to this Indian community, and to permit descriptions of events and experiences which may vary from existing models34.
In the current sample, 51% of interviewed women (N=95/187) admitted CSA in childhood, as previously reported 15. For comparison, prevalence of CSA in men was 16% (21/126).
2.4 Genotyping
We genotyped the MAOA-LPR locus and 9 additional SNPs spanning the 230 Kb region where both the MAOA and MAOB genes are located. These two genes are separated by approximately 20 Kb and are disposed tail-to-tail. Locations of polymorphisms within the two genes are shown in Figure 1.
2.4.1 MAOA-LPR
The MAOA gene promoter VNTR polymorphism was amplified from 25 ng genomic DNA using these primer sequences: Forward 5’ CCC AGG CTG CTC CAG AAA CAT G 3’ and Reverse 5’ GTT CGG GAC CTG GGC AGT TGT G 3’. Because of the high GC content in the VNTR region, amplification was
performed using Invitrogen’s PlatinumTaq and PCRX Enhancer System kits, according to the manufacturer’s protocol (Invitrogen, Carlsbad, California), with 5 µM of each primer and 25 mM dNTPs in a total reaction volume of 15 µl. Amplifications were performed on a Perkin Elmer 9700 thermocycler (Applied Biosystems, Foster City, CA) with one cycle at 96°C for 10 min followed by 30 cycles of 94°C for 15 sec, 60°C for 15 sec, 72°C for 30 sec, and a final 3 min extension at 72°C. The forward primer was labeled with the fluorescent dye 6-FAM, and amplicons were visualized on an ABI 3100 capillary sequencer. Allele sizes (allele 3, 263 bp; allele 3.5, 278 bp; allele 4, 293 bp; allele 5, 323 bp) were determined using Genotyper 2.5 (Applied Biosystems, Foster City, CA).
2.4.2 SNP genotyping
Nine SNPs were genotyped using the Illumina GoldenGate assay. In this method DNA samples (250 ng at 100ng/ml) are bound to paramagnetic particles and then
assay oligonucleotides are added. Three oligos are designed for each SNP including two oligos specific to each allele at the SNP site (Allele-specific Oligos, ASO) and a third oligo (Locus-specific oligo, LSO) that hybridizes several bases downstream from the SNP site. All three oligonucleotides contain a region of genomic complementarity and universal PCR primer sites. The LSO also contains a unique address sequence that targets a particular bead type. During the hybridization step, the oligonucleotides hybridize to the genomic DNA bound to paramagnetic particles. After several wash steps, extension of the appropriate ASO and ligation of the extended product to the LSO joins information about the genotype present at the SNP site to the address sequence at the LSO. These joined products are then amplified via PCR. For more detail description of the GoldenGate assay see http://www.illumina.com/products/prod_snp.ilmn.
2.5 Statistical Analyses
Genotype/allele frequencies of each marker were compared between alcoholics and controls (non AUD, non ASPD) using the 2 test. To test whether the associations between genotype and AUD were mainly driven by antisocial alcoholics, the same analyses were repeated comparing antisocial alcoholics (AUD + ASPD) to controls. Mean ASPD symptoms count were compared across genotypes using ANOVA.
To test for gene X environment interaction, both allele- and genotype-based analyses were repeated separately within the sexually abused and non-sexually abused groups. More formal gene by environment analyses were conducted using linear regression when the dependent variable was a continuous trait (ASPD symptoms count) and logistic regression when the dependent variable was categorical (AUD; AUD +ASPD). In both the logistic and linear regressions, parameters entered in the model as independent variables were: MAOA, CSA and MAOA X CSA. Of note, the linear regression was performed at the genotype level, whereas the logistic regression was performed at the allelic level due to the power
issue. In fact, allele-based analyses, which are possible only for categorical traits, have the advantage of being based on the number of chromosomes, which is twice the number of individuals and have a reduced degree of freedom (1 rather than 2) as compared to genotype-based analyses. On the other hand, certain genotypic effects can be missed, and interpretation of a positive result is more limited.
Hardy Weinberg Equilibrium (HWE) for each locus and linkage disequilibrium (LD) between each pair of markers were computed using Haploview v3.32 35. Diplotypes were assigned to each individual using PHASE 2.02 36. Haplotypes were compared between cases and controls usingthe 2 test or the Fisher’s exact test when indicated.
Although most of participants included in the present study belonged to two large multigenerational pedigrees, the degree of relatedness between any two individuals in this sample is overall low. The probability that a chromosome region from any pair of individuals is shared identical by descent (i.e. the kinship coefficient) was calculated for all possible pairs (related and unrelated) using LOKI v2.4.7 (http://loki.homeunix.net/). The mean kinship coefficient ± SE for all pairs of female subjects included in this study was only 0.0045 ± 0.0001, which is close to the average kinship of the source population and is less than the second cousin level. Therefore analyses assuming independence of individuals were undertaken and the sample was analyzed using a case/control approach.
All statistical analyses were conducted using JMP software v5.1 (SAS Institute, Cary, North Carolina). Criterion for statistical significance was set at 0.05.
3 RESULTS
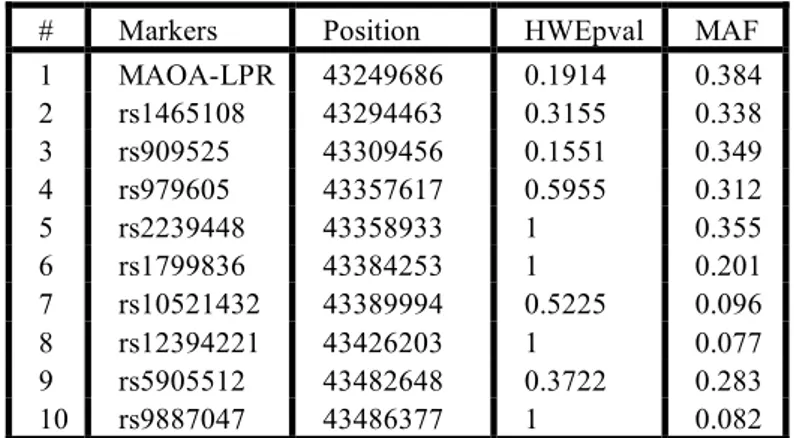
Minor allele frequency, position (according to the NCBI Human Genome Build # 35.1) and HWE of each marker are reported in table1. At the MAOA-LPR locus, similarly to what is observed in Caucasian populations, the three repeats (freq: 0.38) and the four repeats (freq: 0.62) alleles were by far the most common. The five and 3.5 repeats alleles were found only in two and one subject respectively. Since the functional effect of these rare alleles is controversial 23; 24, these individuals were excluded. Genotypes at all markers were in HWE equilibrium (See Table 1).
3.1 Main effects of MAOA and MAOB markers
Genotype and allele frequencies at each marker were compared between controls and alcoholics. To evaluate whether significant differences between controls and alcoholics were mainly driven by antisocial alcoholics, genotype/allele frequencies were also compared between controls and alcoholics with comorbid ASPD (see table two and three). Several markers across both MAOA (table 2A and B) and MAOB (Table 3) genes showed association with alcoholism.
The three repeat allele of the MAOA-LPR was significantly more common among antisocial alcoholics than in controls (df=1, χ2 =5.2, p=0.02). Only a trend toward significance was found when all alcoholics (e.g. antisocial plus non-antisocial alcoholics) were compared with controls (df=1, x2 =3.02, p=0.08).
Other markers within both genes displayed significant differences between cases and controls both at the genotypic and allelic levels (see table 2 and 3). Of note, for MAOA markers, differences in genotype/allele frequencies between controls and antisocial alcoholics were stronger than those found comparing controls with all alcoholics, indicating that signals of association with alcoholism were mainly driven by antisocial alcoholics. On the other hand, genotype/allele frequencies for
alcoholics, indicating that association between MAOB markers and alcoholism were independent from ASPD diagnosis.
3.2 Interaction between MAOA-LPR and childhood sexual abuse
As shown in table 4 and Figure 2, sexually abused women who were homozygous for the low activity MAOA-LPR allele were more likely to have alcoholism, particularly antisocial alcoholism, compared with women who were homozygous for the high activity allele. Heterozygous women displayed an intermediate risk pattern compared to homozygotes, consistent with a codominant effect of the MAOA alleles. In contrast, there was no predictive value of the MAOA-LPR genotype for antisocial alcoholism in the women who had not been sexually abused. Allelic-based analyses (see table 4) were also consistent with an interaction effect between MAOA-LPR and CSA. Among sexually abused women, the low activity allele was more common in alcoholics than in controls (df=1; χ2=7.87; p=0.005 with differences being driven by alcoholics who also had ASPD (df=1, χ2=15.42, p=0.00009). In contrast, no differences in allele frequencies were found between cases and controls among non-sexually abused women. In line with these findings, logistic regression showed that the interaction effect between CSA and MAOA-LPR was significant in predicting antisocial alcoholism (logistic regression, MAOA-LPR X CSA: df=1; χ2 =7.17; p=0.007) but not alcoholism in general (logistic regression, MAOA-LPR X CSA, df=1; χ2 =2.77; p=0.09).
Similar results emerged when antisocial behavior was modeled as a continuous trait (e.g. ASPD symptoms count) (See Figure 3). Linear regression showed a main effect of sexual abuse on antisocial behavior (β ± SE: 2.31± 0.38; p=1e-8), whereas a main effect of MAOA-LPR genotype was not found (MAOA-LPR (3/3 vs 4/4): β ± SE: 0.84 ± 0.67; p=0.22; MAOA-LPR (3/4 vs 4/4): β ± SE: 0.11± 0.46; p=0.46). The interaction effect between MAOA-LPR and CSA was significant (CSA X MAOA-LPR (3/3): β ± SE: 2.5± 0.68; p=0.0003; CSA X MAOA-LPR (3/4): β ± SE: 1.08± 0.45; p=0.02). The whole regression model explained 22% of the ASPD
symptom count variance. As shown in figure 3, within the group of sexually abused women, the mean ASPD symptoms count was the highest among women who were homozygous for the low activity allele, intermediate among heterozygous and the lowest among homozygous for the high activity allele (ANOVA: df=2; F=8.0; p=0.0006). In contrast, MAOA-LPR genotypes were not associated with antisocial behavior among non-sexually abused participants (ANOVA: df=2; F=2.07; p=0.14). Results were not driven by few outliers as shown in the supplemental material (figure 5)
MAOL-LPR has been previously reported to increase risk of exposure to maltreatment19, a phenomenon known as gene by environment correlation17. Gene by environment correlation could lead to a false significant gene by environment interactions. Therefore we compared allele frequencies at the MAO-LPR between participants exposed and not exposed to sexual abuse but we did not find a significant difference (allele based analyses: df=1; χ2=2.14, p=0.14; genotype based analyses: df=2, χ2=3.28; p=0.19) indicating that what we observed is mainly a “true” MAOA-LPR by CSA interactive effect. Nevertheless, the direction of the trend is in the same direction as Huang et al., and could tend to amplify the effect of MAOA-LPR on antisocial alcoholism.
3.3 Haplotype-based analyses
The haplotype-block structure of the region containing MAOA and MAOB genes is shown in Figure 4A. D’ values tended to be greater within each gene, therefore we divided the region into two blocks of reduced recombination: one including all five MAOA markers and one block including all MAOB markers except for SNP rs1799836 which showed very low LD with almost all other markers. However, some degree of LD was also detected between MAOA and MAOB markers. Frequencies of haplotypes encompassing each gene are shown in figure 4B. To easily identify parts shared by different haplotypes, alternative alleles at each locus are colored in red or black. Within MAOA gene, we found 5 different haplotypes
(labeled A-E) with frequencies higher than 0.01. These haplotypes accounted for 99% of all haplotype diversity in the region. Two haplotypes (A and B) were by far the most common and displayed a yin-yang configuration. The low activity allele at the MAOA-LPR locus was found on three different haplotype backgrounds. For MAOB, we found three haplotypes with frequencies higher than 0.05 that accounted for 98% of all the halotype diversity. Since frequencies of rare haplotypes computed with Phase are imprecise, carriers of rare haplotypes (freq< 1%) were excluded from the following analyses.
MAOA: within the MAOA gene, haplotype frequencies differed significantly
between all alcoholics and controls (df=4; χ2 = 11.82; p=0.02) as well as between antisocial alcoholics and controls (df= 4, χ2 =14.37, p=0.006) (see table 5). Post hoc analyses comparing each haplotype with all other haplotypes combined revealed that significance was mainly driven by the most common MAOAL-PR low activity allele-containing haplotype, namely haplotype B. This haplotype was significantly more common in alcoholics (df=1, χ2 =7.05; p=0.008) compared to controls. As previously seen for the MAOA-LPR, differences in haplotype frequencies were driven by antisocial alcoholics (df=1, χ2 = 10.41, 0.001).
Since MAOA haplotype B displayed a main effect on alcoholism which was stronger than that observed for the MAOA-LPR locus alone (see table 2), we performed interaction analysis between this haplotype and CSA.
MAOA haplotype B and CSA significantly interacted with sexual abuse (logistic regression: dependent variable = AUD: Haplotype B X CSA: df=1; χ2
= 4.47; p=0.03; dependent variable=ASPD + AUD: df=1; χ2
= 4.04;p=0.04). Similarly to what we observed with the MAOA-LPR alone (see table 6), there was a significant difference in Haplotype B between cases and controls only within women who were exposed to CSA, and but not among non-abused women.
between alcoholics and controls (AUD df=2; χ2 = 9.49; p=0.007) but only a trend was found when antisocial alcoholics were compared to controls (df=2; χ2 = 5.66; p=0.06) (see table 5). Differences were mainly driven by haplotype C, which was more common among alcoholics than controls (AUD: df=1, χ2 = 7.41; p=0.006). In contrast to what was observed for MAOA, the frequency of MAOB haplotype C was almost the same in all alcoholics and in antisocial alcoholics.
The interaction analysis between MAOB haplotype C and CSA did not reveal significant GxE (logistic regression, dependent variable= AUD: Haplotype C X CSA: df=1; χ2
= 2.11; p=0.14; dependent variable=AUDASPD: Haplotype C X CSA: df=1; χ2
4 DISCUSSION
It is well known that women exposed to CSA have an increased risk of developing a broad range of psychopathologies, including ones that are sometimes regarded as “atypical” for this sex, such as antisocial personality disorder. However, not all women exposed to CSA develop adverse psychosocial consequences. Interindividual variation in stress resiliency is at least partially mediated by genetic factors and recent findings have indicated that a functional locus within the MAOA gene (MAOA-LPR) moderates responses to childhood maltreatment 17; 18; 19; 20. However, no previous study has focused specifically on CSA and only a few studies so far have explored the interaction between MAOA and child victimization in female cohorts 19; 26; 27.
The main purpose of the present study was to test whether MAOA-LPR interacts with exposure to sexual abuse in a community sample of American Indian women with a extremely high rates of CSA4. Childhood sexual abuse in this community should be seen as an index of a more complex matrix of adverse conditions from which the abused women suffered and as part of broader familial and social dysfunction4. Consistent with previous findings derived from male samples17; 18; 19; 20, our results support the involvement of MAOA gene in moderating individual
sensitivity to childhood trauma. Sexually abused women who were homozygous for the low activity MAOA-LPR allele had higher rates of alcoholism, particularly antisocial alcoholism, and more symptoms of antisocial behavior, as compared with women who were homozygous for the high activity allele. Heterozygous women displayed an intermediate risk pattern. In contrast, there was no relationship between alcoholism/antisocial behavior and MAOA-LPR genotype in women who had not been sexually abused. This result is consistent with the gene by environment interaction model and might indicate that carriers of the low activity allele who have been exposed to sexual abuse are at higher risk of developing alcoholism with antisocial features, whereas individual with the high activity
genotype are protected from developing antisocial behavior after exposure to CSA.
The effects of CSA on adult antisocial personality disturbances were very strong (figure 3). Early life traumatic events occurring during a period of neuronal plasticity have been shown in both humans and animal models to determine long-lasting neuroendocrine changes that induce hypersensitivity of the hypothalamic-pituitary-adrenal (HPA) axis to new stressors 37, 38, 39. In humans CSA appears to cause persistent hyperreactivity of both the HPA axis and autonomic system37. Furthermore, adverse experiences early in life cause structural changes in the brain39. Rats exposed to maternal deprivation have long-lasting suppression of adult neurogenesis in the hippocampus, a brain region which is involved in the processing of emotional experience39. The effect of MAOA on the hippocampus may underlie the interaction between MAOA and childhood trauma. Carriers of the low activity variant of MAOA-LPR display hyperactivation of the hippocampus and amygdala during the retrieval of negatively valenced emotional material40. Therefore, the heightened sensitivity to adverse experiences of carriers of the low activity MAOA genotype might be due to their impaired ability to extinct adverse memories and conditioned fears
The functional effects of MAOA-LPR are more difficult to predict in females than in males. Because this gene is located on the X chromosome males are hemizygotes, whereas females have two copies of the gene. Further, among females one copy of each gene located on the X chromosome is usually inactivated through a mechanism which can be either random or selective. To date, studies exploring the inactivation status of MAOA gene on the X chromosome have reported conflicting findings 41; 42. Our results showed that heterozygous individuals displayed a risk which was intermediate between the homozygote, echoing the neuroimaging findings of Meyer-Lindenberg et al.40. Meyer-Lindenberg et al., found that the genotype conferring low MAOA activity predicted amygdala hypereactivity during emotional arousal in both males and females.
both high- and low-expressing allele was intermediate between homozygous females40. This finding is consistent with a codominant effect of the alleles not only on neurobiology but on complex behavior and might be seen as supportive of a recent study showing that MAOA is among those genes that escape X inactivation42. Alternatively, in brain the clonality of MAOA allele inactivation is such that mosaicism of allele expression of the heterozygotes is relatively fine-grained and the neurobehavioral phenotypes of heterozygotes are therefore intermediate.
MAOA-LPR is located in a relatively large region of reduced recombination. Therefore other functional loci might partially or totally account for MAOA-LPR functional effect. In line with this idea, two recent studies evaluating mRNA expression of MAOA in human brain28; 43 and MAOA activity43 suggest that not all the variance in MAOA activity/transcription due to genetic factors is explained by MAOA-LPR and other functional loci may play a role. For this reason we genotyped nine additional SNPs encompassing the region where both MAOA and MAOB genes are located. Within this 200kb region we identify two blocks of high LD which approximately correspond to the two MAO genes, although some degree of LD was also found between MAOA and MAOB markers. Consistent with previous studies, haplotype diversity within both regions was low on an overall basis29.
In the MAOA gene, the low activity MAOA-LPR allele was found in three different haplotypes one of which (haplotype B) was by far the most common. This haplotype displayed a stronger main effect on alcoholism and ASPD than MAOA-LPR alone. In contrast, other haplotypes containing the low activity allele did not show any significant associations with either alcoholism or ASPD. As previously seen for MAOA-LPR, the effect of haplotype B on alcoholism and ASPD was seen only among sexually abused subjects. Further, other polymorphisms within MAOA displayed a main effect on alcoholism which was more significant than that of the
MAOA indeed exist. However, this result should be interpreted cautiously keeping in mind that our sample size is small. This result at the haplotype level would need to be replicated in a larger sample, or a second functional locus identified, before a clear conclusion is drawn.
Haplotype-based analysis revealed an association between MAOB and alcoholism. A MAOB haplotype (termed haplotype C) was found in excess among alcoholics as compared to controls. In contrast to what we observed for MAOA, frequency of this MAOB haplotype was almost the same in all alcoholics and antisocial alcoholics, indicating that the association seen between MAOB and alcoholism in this population is independent from the ASPD diagnosis. Monoamine Oxidase B (MAOB) is located in the same chromosome region as the MAOA gene (Xp11.23-Xp11.4) and the two genes are separated by only 20Kb. These two genes have a high degree of sequence identity and most certainly have a common ancestry44. As does the MAOA enzyme, MAOB also degrades monoamines, but with a different substrate preference: MAOB has higher affinity for DA 45; 46, phenylethylamine47;
48 and benzylamine 49, whereas MAOA displays higher affinity for NA and 5-HT.
Further, both enzymes are expressed in the central nervous system (CNS) but with different distributions: MAOB is preferentially expressed on serotonergic50 and histaminergic51 neurons, while MAOA is mainly expressed in noradrenergic neurons50; 52. Both genes are expressed in glia51. Since MAOB is a regulator of monoamine tone and is expressed in the brain, it is a logical candidate for susceptibility to neuropsychiatric diseases. Nevertheless, the role of MAOB in vulnerability to psychiatric disorders has been less investigated as compared to MAOA. In contrast, there is an extensive literature relating low MAO-B enzyme activity in platelets to behavior, including alcoholism, recurrent criminality and antisocial violent behavior53; 54. However, platelet MAOB enzyme activity is also perturbed by environmental factors, including smoking, limiting the interpretability of those studies. Positive associations between MAOB polymorphisms have been reported with mood disorders55 and platelet MAO activity56. To our knowledge,
this is the first study reporting an association between genetic variation at MAOB and alcoholism.
Interestingly MAOA but not MAOB displayed a significant interaction with CSA. This result might be due to the differences in the level of expression of MAOA and MAOB in the brain during different ages. In fetal brain, MAOA activity appears earlier than MAOB and reaches a peak in the newborn period. MAOB activity progressively increases with age and peaks in late adulthood57. The MAOA/MAOB activity ratio is higher in fetal (2.43) and neonatal brain (2.39) and decreases in adulthood (0.61)58. Therefore, MAOA might be more important than MAOB in moderating the effect of traumatic events that occurs during the developmental period .
This study has strengths as well as important limitations. Strengths include the use of a female sample which is particularly suitable to explore the effect of sexual abuse given the higher prevalence of CSA in females as compared to males; the use of a specific type of childhood trauma such as CSA rather than a more heterogeneous category of adversities; the use of a relatively isolated population which is likely to be genetically and environmentally more homogenous; assessment of sexual abuse conducted through a face-to-face interview rather than a self-completed questionnaire and yielding high rates of reporting; the combination a function-based genetic association approach with haplotype-based analysis potentially accessing the effects of unknown functional loci. Finally, the high rates of sexual abuse in this high-risk population make it particularly suitable for the study of gene by stress interaction. The main limitations of the study include the relatively small sample size; and the arguably necessary retrospective rather than prospective assessment of childhood sexual abuse.
In conclusion, our results support the role of the MAOA gene in moderating the impact of childhood trauma on adult psychopathology in females, as previously
women. Both homozygotes for the low activity allele and heterozygous carriers appear to be more vulnerable to adverse psychosocial consequences of childhood sexual abuse as compared to homozygotes for the high activity allele. Haplotype-based analysis of the MAOA gene appeared to strengthen the association, as compared to the MAOA-LPR locus alone. Finally, a haplotype of the MAOB gene was associated with alcoholism independently from ASPD diagnosis, and this effect appears to be at least partially independent of MAOA. This is the first time that an association between genetic variation within MAOB and alcoholism has been reported, suggesting that closer examination of MAOB would be worthwhile, including attempts to replicate in other datasets
REFERENCES
1 Molnar, BE, SL Buka and RC Kessler. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health 2001; 91: 753-60.
2 Galaif, ER, JA Stein, MD Newcomb and DP Bernstein. Gender differences in the prediction of problem alcohol use in adulthood: exploring the influence of family factors and childhood maltreatment. J Stud Alcohol 2001; 62: 486-93.
3 Mullen, PE, JL Martin, JC Anderson, SE Romans and GP Herbison. Childhood sexual abuse and mental health in adult life. Br J Psychiatry 1993; 163: 721-32. 4 Robin, RW, B Chester, JK Rasmussen, JM Jaranson and D Goldman. Prevalence, characteristics, and impact of childhood sexual abuse in a Southwestern American Indian tribe. Child Abuse Negl 1997; 21: 769-87.
5 Horwitz, AV, CS Widom, J McLaughlin and HR White. The impact of childhood abuse and neglect on adult mental health: a prospective study. J Health Soc Behav 2001; 42: 184-201.
6 Kendler, KS, CM Bulik, J Silberg, JM Hettema, J Myers and CA Prescott. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 2000; 57: 953-9.
7 Briere, J and M Runtz. Symptomatology associated with childhood sexual victimization in a nonclinical adult sample. Child Abuse Negl 1988; 12: 51-9. 8 Nelson, EC, AC Heath, PA Madden, ML Cooper, SH Dinwiddie, KK Bucholz, et al. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry 2002; 59: 139-45.
9 Kendler, KS, JW Kuhn and CA Prescott. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 2004; 34: 1475-82. 10 Roy, A and M Janal. Gender in suicide attempt rates and childhood sexual abuse rates: is there an interaction? Suicide Life Threat Behav 2006; 36: 329-35.
attempts: A persistent and theoretically important relationship. Behav Res Ther 2006.
12 Widom, CS. Posttraumatic stress disorder in abused and neglected children grown up. Am J Psychiatry 1999; 156: 1223-9.
13 Tolin, DF and EB Foa. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull 2006; 132: 959-92.
14 Robin, RW; Chester B; Goldman, D. Cumulative trauma and PTSD in American Indian communities. In Marseala AJ, Friedman MJ; Gerrity ET and Scurfield RM (eds.), Ethnocultural aspects of posttraumatic stress disorder: Issues, research, and clinical applications. American Psychological Association: Washington DC, 1996, pp 239-253
15 Robin, RW, JC Long, JK Rasmussen, B Albaugh and D Goldman. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcohol Clin Exp Res 1998; 22: 518-23.
16 Compton, WM, KP Conway, FS Stinson, JD Colliver and BF Grant. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry 2005; 66: 677-85.
17 Caspi, A, J McClay, TE Moffitt, J Mill, J Martin, IW Craig, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851-4. 18 Foley, DL, LJ Eaves, B Wormley, JL Silberg, HH Maes, J Kuhn, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry 2004; 61: 738-44.
19 Huang, YY, SP Cate, C Battistuzzi, MA Oquendo, D Brent and JJ Mann. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology 2004; 29: 1498-505.
Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry 2006; 59: 121-7.
21 Kim-Cohen, J, A Caspi, A Taylor, B Williams, R Newcombe, IW Craig, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry 2006; 11: 903-13.
22 Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Lai E, Grimsby J, Sparkes RS, Mohandas T, Shih JC Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989, 4: 552-9.
23 Cases, O, I Seif, J Grimsby, P Gaspar, K Chen, S Pournin, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 1995; 268: 1763-6.
24 Sabol, SZ, S Hu and D Hamer. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 1998; 103: 273-9.
25 Deckert, J, M Catalano, YV Syagailo, M Bosi, O Okladnova, D Di Bella, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 1999; 8: 621-4.
26 Sjoberg, RL, KW Nilsson, HL Wargelius, J Leppert, L Lindstrom and L Oreland. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet B Neuropsychiatr Genet 2006.
27 Widom, CS and LM Brzustowicz. MAOA and the "cycle of violence:" childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry 2006; 60: 684-9.
28 Pinsonneault, JK, AC Papp and W Sadee. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: dissection of epigenetic and genetic factors. Hum Mol Genet 2006; 15: 2636-49.
29 Rosenberg, S, AR Templeton, PD Feigin, D Lancet, JS Beckmann, S Selig, et al. The association of DNA sequence variation at the MAOA genetic locus with quantitative behavioural traits in normal males. Hum Genet 2006; 120: 447-59. 30 Saremi, A, RL Hanson, DE Williams, J Roumain, RW Robin, JC Long, et al.
Alcohol 2001; 62: 294-300.
31 Spitzer, RL; Endicott, J; Robins, E. Research diagnostic criteria (RDC) for a selected group of functional disorders. Department of Research Assesment and training, New York Psychiatric Institute, New York, 1989.
32 S Spitzer, RL; Endicott, J. Schedule for affective disorders and schizophrenia lifetime version (SADS-L).Research Assesment and Training Unit. New York Psychiatric Institute. New York, 1979.
33 Fink, LA, D Bernstein, L Handelsman, J Foote and M Lovejoy. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry 1995; 152: 1329-35.
34 Conte, JR; Berliner, L . The impact of sexual abuse on children: Empirical findings. In L.K.A. Walker (ed.), handbook on sexual abuse of children: Assesment and treatment issues, New York,1998, pp 1-31.
35 Barrett, JC, B Fry, J Maller and MJ Daly. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263-5.
36 Stephens, M and P Donnelly. A comparison of bayesian methods for haplotype reconstruction frOm population genotype data. Am J Hum Genet 2003; 73: 1162-9. 37. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000, 284:592-7.
38. Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J,Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A 2004, 101:12358-63
39. Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 2004, 7:841-6.
40. Meyer-Lindenberg, A, JW Buckholtz, B Kolachana, RH A, L Pezawas, G Blasi, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A 2006; 103: 6269-74.
assessing the inactivation status of human X-linked genes. Eur J Hum Genet 2000; 8: 103-8.
42 Carrel, L and HF Willard. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005; 434: 400-4.
43 Balciuniene, J, L Emilsson, L Oreland, U Pettersson and E Jazin. Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 2002; 110: 1-7.
44 Grimsby, J, K Chen, LJ Wang, NC Lan and JC Shih. Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc Natl Acad Sci U S A 1991; 88: 3637-41.
45 Lakshmana, MK, BS Rao, NK Dhingra, R Ravikumar, Govindaiah, S Sudha, et al. Role of monoamine oxidase type A and B on the dopamine metabolism in discrete regions of the primate brain. Neurochem Res 1998; 23: 1031-7.
46 Stenstrom, A, J Hardy and L Oreland. Intra- and extra-dopamine-synaptosomal localization of monoamine oxidase in striatal homogenates from four species. Biochem Pharmacol 1987; 36: 2931-5.
47 Fowler, CJ and L Oreland. Substrate- and stereoselective inhibitor of human brain monoamine oxidase by 4-dimethylamino-alpha, 2-dimethylphenethylamine (FLA 336). J Pharm Pharmacol 1981; 33: 403-6.
48 Arai, Y, H Kinemuchi, N Hamamichi, N Satoh, T Tadano and K Kisara. Inhibition of rat brain monoamine oxidase by some analogues of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion. Neurosci Lett 1986; 66: 43-8.
49 White, HL and AT Glassman. Multiple binding sites of human brain and liver monoamine oxidase: substrate specificities, selective inhibitions, and attempts to separate enzyme forms. J Neurochem 1977; 29: 987-97.
50 Thorpe, LW, KN Westlund, LM Kochersperger, CW Abell and RM Denney. Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J Histochem Cytochem 1987; 35: 23-32.
brainstem. Neuroscience 1988; 25: 439-56.
52 Arai, R, H Kimura, I Nagatsu and T Maeda. Preferential localization of monoamine oxidase type A activity in neurons of the locus coeruleus and type B activity in neurons of the dorsal raphe nucleus of the rat: a detailed enzyme histochemical study. Brain Res 1997; 745: 352-6.
53 Devor, EJ, CW Abell, PL Hoffman, B Tabakoff and CR Cloninger. Platelet MAO activity in type I and type II alcoholism. Ann N Y Acad Sci 1994; 708: 119-28.
54 Barnholtz, JS, M de Andrade, GP Page, TM King, LE Peterson and CI Amos. Assessing linkage of monoamine oxidase B in a genome-wide scan using a univariate variance components approach. Genet Epidemiol 1999; 17 Suppl 1: S49-54.
55 Lin, S, S Jiang, X Wu, Y Qian, D Wang, G Tang, et al. Association analysis between mood disorder and monoamine oxidase gene. Am J Med Genet 2000; 96: 12-4.
56 Garpenstrand, H, J Ekblom, K Forslund, G Rylander and L Oreland. Platelet monoamine oxidase activity is related to MAOB intron 13 genotype. J Neural Transm 2000; 107: 523-30.
57. Fowler JS, Wiberg A, Oreland l, Marcusson J, Winblad. the effect of age on the activity and molecular properties of human brain monoamine oxidase. Journal of Neuroal trasmission 1980, 49: 1-20.
58. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking Int J Neuropsychopharmacol 2001, 4:33-42.
Table 1: Minor allele frequency (MAF), position and Hardy Weinberg Equilibrium (HWE) for ten MAOA/MAOB markers.
# Markers Position HWEpval MAF 1 MAOA-LPR 43249686 0.1914 0.384 2 rs1465108 43294463 0.3155 0.338 3 rs909525 43309456 0.1551 0.349 4 rs979605 43357617 0.5955 0.312 5 rs2239448 43358933 1 0.355 6 rs1799836 43384253 1 0.201 7 rs10521432 43389994 0.5225 0.096 8 rs12394221 43426203 1 0.077 9 rs5905512 43482648 0.3722 0.283 10 rs9887047 43486377 1 0.082