7. Appendix I: Rules for Determining the Priority Scale
a. For aliphatic chains the priority decreases as the number of hydrogen atoms in the
chemical group increases: C=C
>C > CH
2> CH
3> H.
b. A group containing a heteroatom has higher priority than any other functional group.
In molecules with different heteroatoms, the C=O group has the highest priority. For
the other groups with heteroatoms, the priority depends on the heteroatom type,
decreasing going to the right in the group of the periodic table (N
> O > F) and down
in the period (O
> S; F > Cl > Br > I). Once fixed the heteroatom, the priority among
the groups follows the order: (a) nitrogen N
> NH > NH
2> NO > NO
2; (b) oxygen
OH
> O; c) sulphur C=S > S > SH > S=O.
c. The edges starting from a node are ordered following the groups priority rules. If two
(or more) substituents in a node have the same priority the elements along the
substituent chains are ranked until a point of difference is reached at which a
distinction in priority is possible.
d. In a double bond the edges are numbered starting from the position cis to the root and
follow the order cis > trans > gem. When the double bond stereoisomery is cis, absent
or not specified, positions 1 and 2 are occupied according to the grouppriority rules. If
the double bond stereoisomery is trans the highest priority group occupies position 2.
8. Appendix II: References for Experimental Data
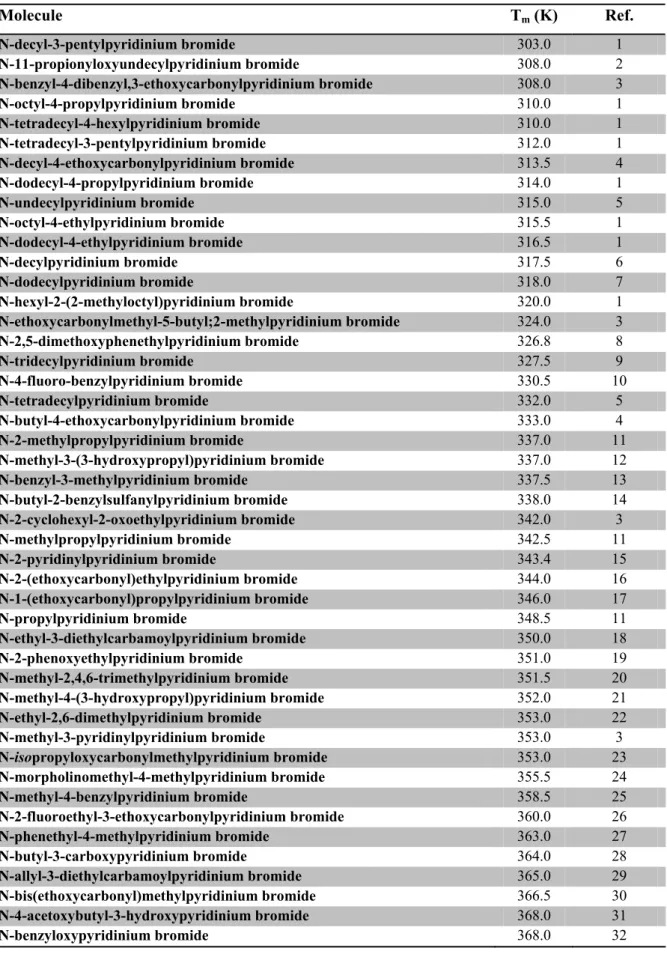
Table 8.1. Pyridinium bromides used in experiments Tm1 and Tm2.
Molecule Tm (K) Ref. N-decyl-3-pentylpyridinium bromide 303.0 1 N-11-propionyloxyundecylpyridinium bromide 308.0 2 N-benzyl-4-dibenzyl,3-ethoxycarbonylpyridinium bromide 308.0 3 N-octyl-4-propylpyridinium bromide 310.0 1 N-tetradecyl-4-hexylpyridinium bromide 310.0 1 N-tetradecyl-3-pentylpyridinium bromide 312.0 1 N-decyl-4-ethoxycarbonylpyridinium bromide 313.5 4 N-dodecyl-4-propylpyridinium bromide 314.0 1 N-undecylpyridinium bromide 315.0 5 N-octyl-4-ethylpyridinium bromide 315.5 1 N-dodecyl-4-ethylpyridinium bromide 316.5 1 N-decylpyridinium bromide 317.5 6 N-dodecylpyridinium bromide 318.0 7 N-hexyl-2-(2-methyloctyl)pyridinium bromide 320.0 1 N-ethoxycarbonylmethyl-5-butyl;2-methylpyridinium bromide 324.0 3 N-2,5-dimethoxyphenethylpyridinium bromide 326.8 8 N-tridecylpyridinium bromide 327.5 9 N-4-fluoro-benzylpyridinium bromide 330.5 10 N-tetradecylpyridinium bromide 332.0 5 N-butyl-4-ethoxycarbonylpyridinium bromide 333.0 4 N-2-methylpropylpyridinium bromide 337.0 11 N-methyl-3-(3-hydroxypropyl)pyridinium bromide 337.0 12 N-benzyl-3-methylpyridinium bromide 337.5 13 N-butyl-2-benzylsulfanylpyridinium bromide 338.0 14 N-2-cyclohexyl-2-oxoethylpyridinium bromide 342.0 3 N-methylpropylpyridinium bromide 342.5 11 N-2-pyridinylpyridinium bromide 343.4 15 N-2-(ethoxycarbonyl)ethylpyridinium bromide 344.0 16 N-1-(ethoxycarbonyl)propylpyridinium bromide 346.0 17 N-propylpyridinium bromide 348.5 11 N-ethyl-3-diethylcarbamoylpyridinium bromide 350.0 18 N-2-phenoxyethylpyridinium bromide 351.0 19 N-methyl-2,4,6-trimethylpyridinium bromide 351.5 20 N-methyl-4-(3-hydroxypropyl)pyridinium bromide 352.0 21 N-ethyl-2,6-dimethylpyridinium bromide 353.0 22 N-methyl-3-pyridinylpyridinium bromide 353.0 3 N-isopropyloxycarbonylmethylpyridinium bromide 353.0 23 N-morpholinomethyl-4-methylpyridinium bromide 355.5 24 N-methyl-4-benzylpyridinium bromide 358.5 25 N-2-fluoroethyl-3-ethoxycarbonylpyridinium bromide 360.0 26 N-phenethyl-4-methylpyridinium bromide 363.0 27 N-butyl-3-carboxypyridinium bromide 364.0 28 N-allyl-3-diethylcarbamoylpyridinium bromide 365.0 29 N-bis(ethoxycarbonyl)methylpyridinium bromide 366.5 30 N-4-acetoxybutyl-3-hydroxypyridinium bromide 368.0 31 N-benzyloxypyridinium bromide 368.0 32
Molecule Tm (K) Ref. N-allylpyridinium bromide 368.5 33 N-methyl-3-ethoxycarbonylpyridinium bromide 369.0 34 N-2-hydroxyethyl-3-methylpyridinium bromide 370.0 36 N-ethyl-2-methylpyridinium bromide 370.0 37 N-isopropylpyridinium bromide 370.0 38 N-butylpyridinium bromide 370.5 39 N-ethyl-4-(4-pyridyl)pyridinium bromide 371.0 40 N-allyl-3-hydroxypyridinium bromide 371.0 31 N-benzylpyridinium bromide 372.0 41 N-methyl-4-(2-ethoxycarbonylethyl)pyridinium bromide 372.5 42 N-ethyl-3-acetylpyridinium bromide 373.0 43 N-allyl-3-formylpyridinium bromide 373.0 44 N-acetonyl-2,6-dimethylpyridinium bromide 377.0 45 N-ethyl-3-hydroxypyridinium bromide 379.0 46 N-ethoxy-4-methoxypyridinium bromide 381.5 47 N-Propyloxycarbonylmethylpyridinium bromide 383.0 23 N-allyl-2-hydroxymethylpyridinium bromide 383.0 49 N-2-hydroxyethylpyridinium bromide 383.0 50 N-allyl-4-hydroxymethylpyridinium bromide 384.0 49 N-5-hexynylpyridinium bromide 387.0 51 N-2-cyanoethyl-3-methylpyridinium bromide 389.0 52 N-pyridinylpyridinium bromide 391.0 54 N-isopropyl-4-hydroxymethylpyridinium bromide 392.0 49 N-3-chloropropylpyridinium bromide 393.0 55 N-ethylpyridinium bromide 393.5 56 N-allyl-4-cyanopyridinium bromide 394.0 57 N-ethyl-4-methylpyridinium bromide 394.0 58 N-isopropyl-2-hydroxymethylpyridinium bromide 395.0 49 N-2-hydroxyethyl-3-hydroxypyridinium bromide 395.5 31 N-2-hydroxyethyl-3,4-dimethylpyridinium bromide 399.5 59 N-3,3-dimethylallyl-4-methylpyridinium bromide 400.5 60 N-1-methyl-2-oxopropyl-2-methylpyridinium bromide 406.0 61 N-2-cyanoethyl-3,4-dimethylpyridinium bromide 406.0 52 N-ethoxycarbonylmethylpyridinium bromide 410.0 48 N-3-bromopropyl-4-methylpyridinium bromide 412.5 63 N-(Z)-3-methylpent-2-en-4-inylpyridinium bromide 412.5 64 N-methyl-3-methoxycarbonylpyridinium bromide 414.0 35 N-2-cyanoethyl-3-aminopyridinium bromide 415.0 52 N-1-methyl-2-oxopropylpyridinium bromide 417.0 65 N-cyanomethyl-3,5-dimethylpyridinium bromide 417.0 66 N-methyl-4-methyl-3-hydroxypyridinium bromide 418.8 67 N-2-cyanoethyl-4-methylpyridinium bromide 420.0 52 N-2-cyanoethylpyridinium bromide 422.0 52 N-methylpyridinium bromide 423.0 38 N-methyl-3-hydroxypyridinium bromide 426.5 68 N-vinylpyridinium bromide 427.5 56 N-phenylpyridinium bromide 428.0 69 N-(E)-3-hydroxyprop-1-en-1-ylpyridinium bromide 429.5 70 N-2-carboxyallylpyridinium bromide 429.5 3 N-cyclohex-2-enylpyridinium bromide 432.5 71 N-2-cyanoethyl-3,5-dimethylpyridinium bromide 433.5 52
Molecule Tm (K) Ref. N-3-carboxypropylpyridinium bromide 436.0 72 N-methyl-4-methoxycarbonylpyridinium bromide 437.0 73 N-ethyl-4-cyanopyridinium bromide 438.5 74 N-cyanomethylpyridinium bromide 440.0 75 N-methyl-2-hydroxymethylpyridinium bromide 440.5 76 N-vinyl-4-methylpyridinium bromide 442.0 36 N-isopropyl-4-methoxypyridinium bromide 442.5 74 N-methyl-4-methylpyridinium bromide 446.0 77 N-methoxycarbonylmethylpyridinium bromide 447.5 23 N-methyl-3-carbamoylpyridinium bromide 448.0 78 N-ethyl-4-dimethylaminopyridinium bromide 449.0 53 N-prop-2-ynyl-4-methylpyridinium bromide 451.0 79 N-cyanomethyl-4-methylpyridinium bromide 452.0 75 N-2-fluoroethylpyridinium bromide 453.0 26 N-methyl-4-acetylpyridinium bromide 456.5 80 N-allyl-4-(hydroxyiminomethyl)pyridinium bromide 458.0 81 N-Hydrazinocarbonylmethylpyridinium bromide 458.5 82 N-2-oxopropylpyridinium bromide 460.0 62 N-ethyl-4-carbamoylpyridinium bromide 461.0 4 N-(E)-2-carboxy-1-ethenylpyridinium bromide 461.5 70 N-2-propionamidopyridinium bromide 462.5 83 N-(E)-2-carboxy-1-ethenyl-3-methylpyridinium bromide 463.5 70 N-allyl-2-(hydroxyiminomethyl)pyridinium bromide 465.5 84 N-2-oxopropyl-2-methylpyridinium bromide 469.0 85 N-2-hydroxyethyl-2-(hydroxyiminomethyl)pyridinium bromide 471.5 81 N-cyanomethyl-2,4-dimethylpyridinium bromide 472.0 86 N-carboxymethylpyridinium bromide 472.0 72 N-2-carbamoylethylpyridinium bromide 472.0 48 N-carbamoylmethylpyridinium bromide 473.0 83
References to Table 8.1
1. Harris et al., J. Am. Chem. Soc. 1951, 73, 3959.
2. Hessel, V. et al., Recl. Trav. Chim. Pays-Bas; EN 1993, 112, 339.
3. Katritzky, A. R., Jain, R., Lomaka, A., Petrukhin, R., Karelson, M., Visser, A. E., Rogers, R. D., J. Chem. Inf. Comp. Sci. 2002, 42, 71.
4. Ciusa et al., Gazz. Chim. Ital. 1958, 88, 393.
5. Breusch et al., Hoppe-Seyler's Z. Physiol. Chem. 1952, 291, 1.
6. Aksenova, V. P. et al., J.Appl.Chem.USSR (Engl.Transl.); EN 1982, 55, 1239. 7. Kolloff et al., J. Am. Pharm. Assoc., 1942, 31, 51.
8. Sugasawa et al., Chem. Ber. 1941, 74, 459.
9. Mehta et al., J. Indian chem. Soc. News 1940, 3, 137. 10. Clarke, J. Chem. Soc. 1957, 3807.
11. Katritzky, Alan R. et al., J. Am. Chem. Soc. 1990, 112, 2479. 12. Moehrle et al., Chem. Ber. 1971, 104, 1478.
13. Zhang, Xian-Man et al., J. Org. Chem. 1993, 58, 3060. 14. Renault, Ann.Chim.(Paris) 1955, 10, 135.
15. Wibaut et al., Bull. Soc. Chim. Fr. 1958, 424.
16. Lukes et al., Collect. Czech. Chem. Commun. 1956, 21, 1602.
18. Ciusa, Gazz. Chim. Ital. 1956, 86, 667.
19. Joshi, R. K. et al., Helv. Chim. Acta 1971, 54, 112.
20. Lukes et al., Collect. Czech. Chem. Commun. 1959, 24, 1868. 21. McMillan et al., J. Am. Chem. Soc. 1956, 78, 4080.
22. Ochiai et al., Yakugaku Zasshi, 1951, 71, 156; Chem.Abstr. 1951, 9542. 23. Banks, R. E. et al., J. Fluorine Chem. 1991, 53, 127.
24. Sharma,R.K. et al., J. Med. Chem. 1968, 11, 620. 25. Gray et al., J. Am. Chem. Soc. 1957, 79, 3805. 26. Saunders, J. Chem. Soc. 1949, 1279.
27. Hartwell et al., J. Am. Chem. Soc. 1946, 68, 868.
28. Gautier et al., C. R. Hebd. Seances Acad. Sci. 1948, 226, 1736. 29. Kroehnke et al., Chem. Ber. 1951, 84, 948.
30. Zaslona, A. T. et al., J. Chem. Soc. Perkin Trans. 1981, 1, 3059. 31. Shapiro et al., J. Am. Chem. Soc. 1959, 81, 5140.
32. Feely et al., J. Org. Chem. 1957, 22, 1135.
33. Macovski et al., Bul. Soc. Stiinte Cluj 1936, 8, 272. 34. Patent, Olin Mathieson Chem. Corp., US 2759942, 1952. 35. Charonnat et al., Bull. Soc. Chim. Fr. 1948, 1014. 36. Katritzky, A. R. et al., Heterocycles 1984, 22, 505. 37. Murrill, J. Am. Chem. Soc. 1899, 21, 828.
38. Barlet, R. et al., J. Chim. Phys. Phys. Chim. Biol. 1984, 81, 349. 39. Ochiai et al., Chem. Ber. 1935, 68, 2291.
40. Nuesslein, F. et al., Chem. Ber. 1989, 122, 1023. 41. Willems et al., Bull. Soc. Chim. Belg. 1957, 66, 502. 42. Patent, Warner-Lambert Pharm. Co., US 2833776, 1955. 43. Aitken, D. J. et al., J. Chem. Soc. Perkin Trans. 1 1993, 5, 597.
44. Erchak, N. P. et al., Chem.Heterocycl.Compd.(Engl.Transl.) 1993, 29, 926. 45. Jones et al., Org. Mass Spectrom., 1970, 3, 1489.
46. Patent, Lakeside Labor.Inc., US 2802007, 1954.
47. Ochiai et al., Yakugaku Zasshi 1944, 64, 210. Chem.Abstr., 1951, 5154. 48. Kondratenko et al., Pharm.Chem.J.(Engl.Transl.) 1976, 10, 201. 49. Petrova et al., Rev. Roum. Chim. 1973, 18, 1361.
50. Kataeva, O. N. et al. Bull.Acad.Sci.USSR Div.Chem.Sci.(Engl.Transl.) 1990, 39, 2371. 51. Kolotilo, N. V. et al., J.Org.Chem.USSR (Engl.Transl.) 1992, 28, 610.
52. Bunting, J. W. et al., J. Am. Chem. Soc. 1990, 112, 8878. 53. Jerchel et al., Chem. Ber. 1956, 89, 2921.
54. Patent, Chem.Fabr.v.Heyden, DE 598879, 1932.
55. Kost, A. N. et al., Chem.Heterocycl.Compd.(Engl.Transl.) 1980, 921. 56. Koizumi, T. et al., Bull. Chem. Soc. Jpn. 1986, 59, 757.
57. Biellmann et al., Bull. Soc. Chim. Fr. 1967, 397. 58. Takahashi et al., Yakugaku Zasshi 1958, 78, 467. 59. Cavallito, C. J. et al., J. Med. Chem. 1970, 13, 221. 60. Kimura et al., Chem. Pharm. Bull. 1976, 24, 515. 61. Ochiai et al., Chem. Ber. 1934, 67, 1011. 62. Henrick et al., Aust. J. Chem. 1967, 20, 2467.
63. Kharitonov, G. V. et al., J.Org.Chem.USSR (Engl.Transl.) 1980, 16, 2044. 64. Maier, W. et al., Helv. Chim. Acta 1991, 74, 1095.
65. Griesbaum et al., Chem. Ber. 1973, 106, 1041. 66. Tsuge, O. et al., Bull. Chem. Soc. Jpn. 1985, 58, 3320. 67. Ferles et al., Collect. Czech. Chem. Commun. 1972, 37, 2464. 68. Leonard et al., J. Am. Chem. Soc. 1955, 77, 2855.
69. Ismailski, Zh. Russ. Fiz.-Khim. O-va 1920, 50, 200. 70. Eicher-Lorka, O. et al., Synthesis 1999, 12, 2131. 71. Barili et al., J. Org. Chem. 1975, 40, 3331.
72. Dega-Szafran, Z. et al., Bull. Pol. Acad. Sci. Chem. 1995, 43, 295. 73. Lyle et al., J. Org. Chem. 1955, 20, 1761.
74. Claramunt, R. M. et al., J. Chim. Phys. Phys. Chim. Biol. 1981, 78, 805. 75. Wang, B. et al., J. Chem. Soc. Perkin Trans. 1 1999, 11, 1571.
76. Shapiro et al., J. Am. Chem. Soc. 1958, 80, 2743. 77. Juskowiak, B. et al., Chem. Soc. Jpn. 1999, 72, 265. 78. Ciusa et al., Gazz. Chim. Ital. 1950, 80, 604.
79. Katritzky, A. R. et al., Helv. Chim. Acta 1984, 67, 939. 80. Lukes et al., Collect. Czech. Chem. Commun. 1958, 23, 326. 81. Poziomek et al., J. Org. Chem. 1958, 23, 714.
82. Katritzky, A. R. et al., J. Prakt. Chem. 1983, 325, 177. 83. Patent, Polaroid Corp., US 4110424 1978.
84. de Jong, Leo P. A. et al., Eur. J. Med. Chem. Chim. Ther. 1981, 16, 257. 85. Tschitschibabin, Chem. Ber. 1927, 60, 1614.
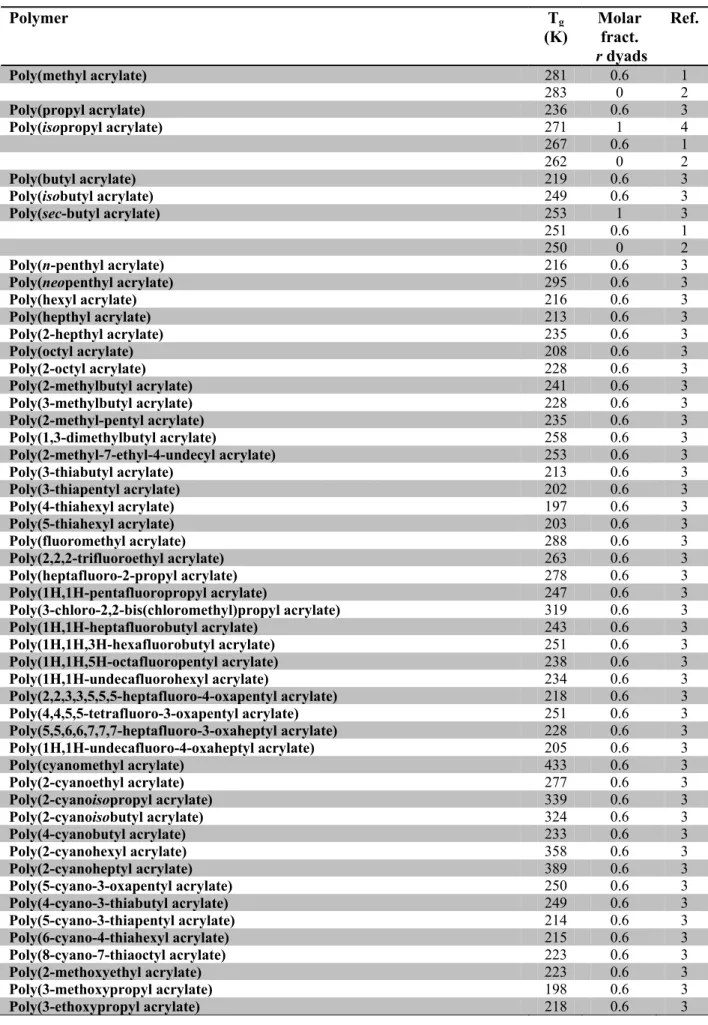
Table 8.2. Acrylic and methacrylic polymers used in experiments Tg1-Tg6.
Polymer Tg (K) Molar fract. r dyads Ref. Poly(methyl acrylate) 281 0.6 1 283 0 2 Poly(propyl acrylate) 236 0.6 3 Poly(isopropyl acrylate) 271 1 4 267 0.6 1 262 0 2 Poly(butyl acrylate) 219 0.6 3 Poly(isobutyl acrylate) 249 0.6 3 Poly(sec-butyl acrylate) 253 1 3 251 0.6 1 250 0 2 Poly(n-penthyl acrylate) 216 0.6 3 Poly(neopenthyl acrylate) 295 0.6 3 Poly(hexyl acrylate) 216 0.6 3 Poly(hepthyl acrylate) 213 0.6 3 Poly(2-hepthyl acrylate) 235 0.6 3 Poly(octyl acrylate) 208 0.6 3 Poly(2-octyl acrylate) 228 0.6 3 Poly(2-methylbutyl acrylate) 241 0.6 3 Poly(3-methylbutyl acrylate) 228 0.6 3 Poly(2-methyl-pentyl acrylate) 235 0.6 3 Poly(1,3-dimethylbutyl acrylate) 258 0.6 3 Poly(2-methyl-7-ethyl-4-undecyl acrylate) 253 0.6 3 Poly(3-thiabutyl acrylate) 213 0.6 3 Poly(3-thiapentyl acrylate) 202 0.6 3 Poly(4-thiahexyl acrylate) 197 0.6 3 Poly(5-thiahexyl acrylate) 203 0.6 3 Poly(fluoromethyl acrylate) 288 0.6 3 Poly(2,2,2-trifluoroethyl acrylate) 263 0.6 3 Poly(heptafluoro-2-propyl acrylate) 278 0.6 3 Poly(1H,1H-pentafluoropropyl acrylate) 247 0.6 3 Poly(3-chloro-2,2-bis(chloromethyl)propyl acrylate) 319 0.6 3 Poly(1H,1H-heptafluorobutyl acrylate) 243 0.6 3 Poly(1H,1H,3H-hexafluorobutyl acrylate) 251 0.6 3 Poly(1H,1H,5H-octafluoropentyl acrylate) 238 0.6 3 Poly(1H,1H-undecafluorohexyl acrylate) 234 0.6 3 Poly(2,2,3,3,5,5,5-heptafluoro-4-oxapentyl acrylate) 218 0.6 3 Poly(4,4,5,5-tetrafluoro-3-oxapentyl acrylate) 251 0.6 3 Poly(5,5,6,6,7,7,7-heptafluoro-3-oxaheptyl acrylate) 228 0.6 3 Poly(1H,1H-undecafluoro-4-oxaheptyl acrylate) 205 0.6 3 Poly(cyanomethyl acrylate) 433 0.6 3 Poly(2-cyanoethyl acrylate) 277 0.6 3 Poly(2-cyanoisopropyl acrylate) 339 0.6 3 Poly(2-cyanoisobutyl acrylate) 324 0.6 3 Poly(4-cyanobutyl acrylate) 233 0.6 3 Poly(2-cyanohexyl acrylate) 358 0.6 3 Poly(2-cyanoheptyl acrylate) 389 0.6 3 Poly(5-cyano-3-oxapentyl acrylate) 250 0.6 3 Poly(4-cyano-3-thiabutyl acrylate) 249 0.6 3 Poly(5-cyano-3-thiapentyl acrylate) 214 0.6 3 Poly(6-cyano-4-thiahexyl acrylate) 215 0.6 3 Poly(8-cyano-7-thiaoctyl acrylate) 223 0.6 3 Poly(2-methoxyethyl acrylate) 223 0.6 3 Poly(3-methoxypropyl acrylate) 198 0.6 3 Poly(3-ethoxypropyl acrylate) 218 0.6 3Polymer Tg (K) Molar fract. r dyads Ref. Poly(3-methoxybutyl acrylate) 217 0.6 3 Poly(acrylamide) 438 0.6 3 Poly(N,N-dimethylacrylamide) 362 0.6 3 Poly(N-isopropylacrylamide) 358 0.6 3 Poly(N,N-diisopropylacrylamide) 393 0.6 3 Poly(N-butylacrylamide) 319 0.6 3 Poly(N-tert-butylacrylamide) 401 0.6 3 Poly(N,N-dibutylacrylamide) 333 0.6 3 Poly(N-(1-methylbutyl)acrylamide) 380 0.6 3 Poly(N-octylacrylamide) 220 0.6 3 Poly(ethyl ethacrylate) 300 0.6 3 Poly[(1-methoxycarbonyl-1-methoxycarbonylmethylene)ethylene] 372 0.6 3 Poly(ethyl ethoxycarbonylmethacrylate) 325 0.7 3 Poly(hexyl hexyloxycarbonylmethacrylate) 269 0.7 3 Poly(methyl fluoroacrylate) 404 0.6 3 Poly(methyl fluoromethacrylate) 357 0.6 3 Poly(ethyl fluoromethacrylate) 316 0.6 3 Poly(methyl chloroacrylate) 358 0 4 380 0.35 5 409 0.52 5 411 0.59 5 419 0.71 5 424 0.75 5 450 1 4 Poly(methyl beta-chloroacrylate) 416 0.6 3 Poly(ethyl chloroacrylate) 404 1 6 377 0.8 5 367 0.71 5 356 0.54 5 325 0.27 5 320 0.16 5 308 0 6 Poly(propyl chloroacrylate) 344 0.6 3 Poly(sec-butyl chloroacrylate) 347 0.6 3 Poly(Butyl cyanoacrylate) 358 0.6 3 Poly(isopropyl chloroacrylate) 341 0 4 343 0.05 5 366 0.34 5 369 0.36 5 383 0.64 5 402 0.87 5 409 1 4 Poly(methacrylic acid) 501 0.7 3 Poly(methyl methacrylate) 328 0.01 4, 7 367 0.64 7 378 0.74 7 382 0.79 7 Poly(methyl methacrylate) 388 0.83 7 396 0.96 8 403 0.99 7, 9 Poly(propyl methacrylate) 308 0.7 3 Poly(isopropyl methacrylate) 412 1 2 359 0.75 9 307 0 9 Poly(butyl methacrylate) 293 0.7 3 Poly(butyl methacrylate) 249 0 3 Poly(sec-butyl methacrylate) 333 0.7 3
Polymer Tg (K) Molar fract. r dyads Ref. Poly(tert-butyl methacrylate) 391 0.75 10 359.5 0.55 10 350 0.1 10 Poly(pentyl methacrylate) 268 0.7 3 Poly(neopentyl methacrylate) 312 0.7 3 Poly(hexyl methacrylate) 268 0.7 3 Poly(dodecyl methacrylate) 208 0.7 3 Poly(2-ethylhexyl methacrylate) 263 0.7 3 Poly(3,3-dimethyl-2-butylbutyl methacrylate) 381 0.7 3 Poly(3,5,5-trimethylhexyl methacrylate) 274 0.7 3 Poly(dimethylaminoethyl methacrylate) 292 0.7 3 Poly(2-tert-butylaminoethyl methacrylate) 306 0.7 3 Poly(2-chloroethyl methacrylate) 365 0.7 3 Poly(2-bromoethyl methacrylate) 325 0.7 3 Poly(1,1,1-trifluoro-2-propyl methacrylate) 354 0.7 3 Poly(1H,1H-heptafluorobutyl methacrylate) 330 1 3 Poly(1H,1H,5H-octafluoropentyl methacrylate) 309 0.7 3 Poly(1H,1H,9H-hexadecafluorononyl methacrylate) 258 0.7 3 Poly(2-cyanoethyl methacrylate) 364 0.7 3 Poly(2-methoxyethyl methacrylate) 289 0.7 3 Poly(3-oxa-5-hydroxypentyl methacrylate) 278 0.7 3 Poly(2-hydroxyethyl methacrylate) 359 0.7 3 311 0.2 3 Poly(2-ethylsulfinylethyl methacrylate) 298 0.7 3 Poly(2-nitratoethyl methacrylate) 328 0.7 3 Poly(2-ethylbutyl methacrylate) 284 0.7 3 Poly(ethyl acrylate) 249 0.6 1 248 0 2 Poly(tert-butyl acrylate) 316 0.6 3 Poly(3-penthyl acrylate) 267 0.6 3 Poly(2-ethylbutyl acrylate) 223 0.6 3 Poly(4-thiapentyl acrylate) 208 0.6 3 Poly(1H,1H-nonafluoropentyl acrylate) 236 0.6 3 Poly(5,5,5-trifluoro-3-oxapentyl acrylate) 235 0.6 3 Poly(2-cyanobutyl acrylate) 384 0.6 3 Poly(6-cyano-3-thiahexyl acrylate) 215 0.6 3 Poly(2-ethoxyethyl acrylate) 223 0.6 3
Poly(butyl butoxycarbonyl methacrylate) 298 0.7 3
Poly(butyl chloroacrylate) 330 0.6 3
Poly(methyl alfa- cyanoacrylate) 433 0.6 3
Poly(ethyl methacrylate) 393 1 6, 2 359 0.85 9 338 0.7 2 281 0 1 Poly(isobutyl methacrylate) 393 1 2 326 0.7 1 Poly(isobutyl methacrylate) 281 0 1 Poly(octyl methacrylate) 253 0.7 3 Poly(3,3-dimethylbutyl methacrylate) 318 0.7 3 Poly(diethylaminoethyl methacrylate) 289 0.7 3 Poly(1H,1H,7H-dodecafluoroheptyl methacrylate) 286 0.7 3 Poly(2-hydroxypropyl methacrylate) 349 0.7 3 Poly(acrylic acid) 379 0.6 3 Poly(N-sec-butylacrylamide) 390 0.6 3 Poly(N-tert-butylmethacrylamide) 433 0.7 3 Poly(benzyl acrylate) 279 0.6 11 Poly(2-phenylethyl acrylate) 270 0.6 11
Polymer Tg (K) Molar fract. r dyads Ref. Poly(phenyl acrylate) 330 0.6 11 Poly(phenyl acrylate) 323 0.79 12 Poly(2-methylphenyl acrylate) 325 0.6 13 Poly(3-methylphenyl acrylate) 298 0.6 13 Poly(4-methylphenyl acrylate) 316 0.6 13 Poly(2-tert-butylphenyl acrylate) 345 0.6 13 Poly(4-tert-butylphenyl acrylate) 344 0.6 13 Poly(biphenyl-4-yl acrylate) 383 0.6 14 Poly(2-chlorophenyl acrylate) 326 0.6 11 Poly(2-chlorophenyl acrylate) 318 0.79 12 Poly(3-chlorophenyl acrylate) 312 0.79 12 Poly(4-chlorophenyl acrylate) 331 0.6 13 Poly(4-chlorophenyl acrylate) 330 0.79 12 Poly(2,4-dichlorophenyl acrylate) 333 0.6 11 Poly(3-dimethylaminophenyl acrylate) 320 0.6 11 Poly(4-cyanophenyl acrylate) 363 0.6 11 Poly(4-cyanobenzyl acrylate) 317 0.6 11 Poly(4-methoxyphenyl acrylate) 324 0.6 13 Poly[methyl 2-(acryloyloxy)benzoate] 319 0.6 11 Poly[methyl 3-(acryloyloxy)benzoate] 311 0.6 11 Poly[methyl 4-(acryloyloxy)benzoate] 340 0.6 11 Poly[ethyl 2-(acryloyloxy)benzoate] 303 0.6 11 Poly[ethyl 3-(acryloyloxy)benzoate] 297 0.6 11 Poly[ethyl 4-(acryloyloxy)benzoate] 310 0.6 11 Poly[butyl 4-(acryloyloxy)benzoate] 286 0.6 11 Poly(N-methyl-N-phenylacrylamide) 453 0.6 15 Poly(benzyl methacrylate) 327 0.75 11, 16 Poly(2-phenylethyl methacrylate) 299 0.75 11, 16 Poly(phenyl methacrylate) 383 0.7 17 Poly(phenyl methacrylate) 400 0.66 18 Poly(4-tert-butylphenyl methacrylate) 371 0.66 19, 20 Poly[methyl 4-(methacryloyloxy)benzoate] 379 0.7 11 Poly(4-cyanophenyl methacrylate) 428 0.7 11 Poly[4-(cyanomethyl)phenyl methacrylate] 401 0.7 11 Poly(2-methylphenyl methacrylate) 382 0.76 21, 16 Poly(3-methylphenyl methacrylate) 380 0.7 21 Poly(4-methylphenyl methacrylate) 403 0.7 21 Poly(2,3-dimethylphenyl methylacrylate) 398 0.7 21 Poly(2,4-dimethylphenyl methylacrylate) 384 0.7 21 Poly(3,4-dimethylphenyl methylacrylate) 384 0.7 21 Poly(3-methoxyphenyl methacrylate) 343 0.7 22 Poly(4-methoxyphenyl methacrylate) 379 0.7 22 Poly(biphenyl-4-yl methylacrylate) 413 0.72 23 Poly[4-(methacrylamido)benzoic acid] 473 0.7 24 Poly[methyl 4-(methacrylamido)benzoate] 453 0.7 24 Poly[ethyl 4-(methacrylamido)benzoate] 441 0.7 24 Poly[butyl 4-(methacrylamido)benzoate] 401 0.7 24 Poly(methyl atropate) 391 0.7 25 397 0.0 25 Poly(phenyl acrylate) 333 0.56 26 Poly(4-fluorophenyl acrylate) 325 0.52 26 Poly[2-((4'-methoxybiphenyl-4-yl)oxy)ethyl acrylate] 353 0.6 27 Poly[6-((4'-methoxybiphenyl-4-yl)oxy)hexyl acrylate] 338 0.6 27 Poly[6-((biphenyl-4-yl)oxy)hexyl acrylate] 321 0.6 27 Poly[3-(4-(4-nitrophenyldiazenyl)phenoxy)propyl acrylate] 319 0.6 28 Poly[4-(4-(4-nitrophenyldiazenyl)phenoxy)butyl acrylate] 310 0.6 28 Poly[6-(4-(4-nitrophenyldiazenyl)phenoxy)hexyl acrylate] 309 0.6 28
Polymer Tg (K) Molar fract. r dyads Ref. Poly[2-((4'-cyanobiphenyl-4-yl)oxy)ethyl acrylate] 323 0.6 29 Poly[5-((4'-cyanobiphenyl-4-yl)oxy)pentyl acrylate] 313 0.6 29 Poly[11-((4'-cyanobiphenyl-4-yl)oxy)undecyl acrylate] 303 0.6 29 Poly[6-((4'-cyanobiphenyl-4-yl)oxy)hexyl acrylate] 315 0.6 30 Poly[2-(2-(2-((4'-methoxybiphenyl-4-yl)oxy)ethoxy)ethoxy)ethyl acrylate] 344 0.6 31 Poly[6-(4-(4-methoxyphenyldiazenyl)phenoxy)hexyl acrylate] 319 0.6 32 Poly[6-(4-(4-cyanophenyldiazenyl)phenoxy)hexyl acrylate] 300 0.6 32 Poly[4-((4-(dimethylamino)phenyl)diazenyl)phenyl acrylate] 392 0.6 33 Poly[6-(4-((4-(dimethylamino)phenyl)diazenyl)phenoxy)hexyl acrylate] 327 0.6 33 Poly[11-(4-((4-(dimethylamino)phenyl)diazenyl)phenoxy)undecyl acrylate] 321 0.6 33 Poly(biphenyl-2-yl acrylate) 378 0.6 34 Poly[2-(4-((4-pentiloxyphenyl)diazenyl)-2-methylphenoxy)hexyl acrylate] 294 0.6 35 Poly[6-(((4'-(1-methylheptyl)oxy)biphenyl-4-yl)oxy)hexyl acrylate] 321 0.6 36 Poly[4'-((6-(acryloyloxy)hexyloxy)biphenyl-4-yl)-2-chloro-3-methylpentanoate] 301 0.6 36 Poly[8-((4'-(2-methylbutoxy)biphenyl-4-yl)oxy)octyl acrylate] 323 0.6 36 Poly[11-((4'-(2-methylbutoxy)biphenyl-4-yl)oxy)undecyl acrylate] 328 0.6 36 Poly[4'-((11-(acryloyloxy)undecyloxy)biphenyl-4-yl) 2-chloro-3-methylbutanoate] 318 0.6 36 Poly[4-(6-(acryloyloxy)hexyloxy)benzoic acid] 339 0.6 37 Poly(2-chlorophenyl methacrylate) 384 0.7 19 Poly(4-chlorophenyl methacrylate) 404 0.7 19 Poly(2,4-dichlorophenyl methacrylate) 391 0.7 19 Poly[4-(2-methoxy-2-oxoethyl)phenyl methacrylate] 354 0.7 38 Poly[4-(3-methoxy-3-oxopropyl)phenyl methacrylate] 341 0.7 38 Poly[methyl 3-(methacryloyloxy)benzoate] 345 0.7 38 Poly[methyl 2-(methacryloyloxy)benzoate] 337 0.7 38 Poly[4-(methacryloyloxy)benzoic acid] 380 0.7 38 Poly[3-(methacryloyloxy)benzoic acid] 389 0.7 38 Poly[2-(methacryloyloxy)benzoic acid] 403 0.7 38 Poly[2-((4'-cyanobiphenyl-4-yl)oxy)ethyl methacrylate] 368 0.7 29 Poly[5-((4'-cyanobiphenyl-4-yl)oxy)pentyl methacrylate] 333 0.7 29 Poly[11-((4'-cyanobiphenyl-4-yl)oxy)undecyl methacrylate] 313 0.7 29 Poly[11-(4'-cyanobiphenyl-4-yl)undecyl methacrylate] 303 0.7 29 Poly[11-((4'-cyanobiphenyl-4-yl)oxy)-11-oxoundecyl methacrylate] 318 0.7 29 Poly[4'-methoxybiphenyl-4-yl 4-(6-(methacryloyloxy)hexyloxy)benzoate] 333 0.7 39 Poly[6-(methyl(4-((4-(methylsulfonyl)phenyl)diazenyl)phenyl)amino)hexyl methacrylate] 373 0.7 40 Poly[4-(phenyldiazenyl)phenyl methacrylate] 374 0.7 41 Poly[4-((4-cyanophenyl)diazenyl)phenyl methacrylate] 363 0.7 41 Poly[2-((((4-cyanophenyl)diazenyl)phenyl)(2-methylbutyl)amino)ethyl acrylate] 384 0.7 42 Poly[2-(4-(phenyldiazenyl)phenoxy)ethyl methacrylate] 378 0.7 43 Poly[4-(4-(4-cyanophenyldiazenyl)phenoxy)butyl methacrylate] 353 0.7 44 Poly[6-(4-(4-cyanophenyldiazenyl)phenoxy)hexyl methacrylate] 333 0.7 44 Poly[8-(4-(4-cyanophenyldiazenyl)phenoxy)octyl methacrylate] 308 0.7 44 Poly[2-(2-(2-(((4-cyanophenyl)diazenyl)phenoxy)ethoxy)ethoxy)ethyl methacrylate] 310 0.7 44 Poly[2-(2-(2-(2-(((4-cyanophenyl)diazenyl)phenoxy)ethoxy) ethoxy)ethoxy)ethyl methacrylate] 293 0.7 44 Poly[6-(4-(4-methoxyphenyldiazenyl)phenoxy)hexyl methacrylate] 341 0.7 32 Poly[4-((4-(dimethylamino)phenyl)diazenyl)phenyl methacrylate] 457 0.7 33 Poly[6-(4-((4-(dimethylamino)phenyl)diazenyl)phenoxy)hexyl methacrylate] 359 0.7 33 Poly[11-(4-((4-(dimethylamino)phenyl)diazenyl)phenoxy)undecyl methacrylate] 344 0.7 33 Poly[benzyl 4-(methacryloyloxy)benzoate] 356 0.7 45 Poly[2-(4-tert-butylphenoxy)-2-oxoethyl methacrylate] 368 0.7 46 Poly(4-benzoylphenyl methacrylate) 391 0.7 47 Poly[6-(4-(4-butoxyphenyldiazenyl)phenoxy)hexyl methacrylate] 352 0.7 48 Poly[2-(2-(((4-cyanophenyl)diazenyl)phenoxy)ethoxy)ethyl methacrylate] 314 0.7 49
Polymer Tg (K) Molar fract. r dyads Ref. Poly[4-((6-((2-mehtyl-1-oxo-2-propenyl)oxy)hexyl)oxy)-benzoic acid 4'-((1-oxo-10,12-nonadecadiynyl)-oxy)-(1,1'-biphenyl)-4-yl) esther] 357 0.7 37 Poly[N-(2-methyl-4-((2-methylphenyl) diazenyl)phenyl)methacrylamide] 436 0.7 50 Poly(pentachlorophenyl acrylate) 420 11 Poly(cyclohexyl acrylate) 298 0.55 51 Poly(pentabromobenzyl acrylate) 438 0.6 52 Poly[2,2-difluoro-2-(2-heptafluorotetrahydrofuranyl)ethyl acrylate] 275 0.6 53 Poly(isobornyl acrylate) 367 0.6 1 369 1 1 363 0 1 Poly(3,5-dimethyladamantyl acrylate) 378 0.6 54 Poly(1-naphtyl acrylate) 358 0.59 13 Poly(3,3,5-trimethylcyclohexyl acrylate) 288 0.6 55 Poly(1-adamantyl acrylate) 426 0.6 54 Poly(morpholylacrylamide) 420 0.6 56 Poly(piperidylacrylamide) 381 0.6 56 Poly(4-cyclohexylphenyl acrylate) 390 0.6 57 Poly(6-(2-(4-trifluoromethylphenyl)1,3-dioxan-5-yl)hexyl acrylate 282 0.6 58 Poly(6-(2-(4-cyanophenyl)1,3-dioxan-5-yl)hexyl acrylate 298 0.6 58 Poly(6-(2-(4-(4-cyanophenoxy)carbonylphenyl)1,3-dioxan-5-yl)hexyl acrylate 315 0.6 58 Poly(6-(2-(4-(4-trifluoromethylphenoxy)carbonylphenyl)1,3-dioxan-5-yl)hexyl acrylate 310 0.6 58 Poly(6-(2-(4-(4-methoxyphenoxy)carbonylphenyl)1,3-dioxan-5-yl)hexyl acrylate 327 0.6 58 Poly(6-(2-(4-(4-cyanobenzoyloxy)phenyl)1,3-dioxan-5-yl)hexyl acrylate 298 0.6 58 Poly(6-(2-(4-(4-trifluoromethylbenzoyloxy)phenyl)1,3-dioxan-5-yl)hexyl acrylate 334 0.6 58 Poly(6-(2-(4-(4-methoxybenzoyloxy)phenyl)1,3-dioxan-5-yl)hexyl acrylate 311 0.6 58 Poly(trans-11-(4-(5-(4-methoxyphenyl)-1,3-dioxan-2-yl)- 2,6-dimethylphenoxy)undecyl acrylate 283 0.6 59 Poly(3-cyclohexyloxy-2-hydroxypropyl acrylate) 281 0.6 60 Poly(4-propanoylphenyl acrylate) 316 0.6 61 Poly[(2-ethyl-1,3-dioxan-5-yl)methyl acrylate] 307 0.6 62 Poly[(2-methyl-1,3-dioxan-5-yl)methyl acrylate] 305 0.6 62 Poly[(2-phenyl-5-ethyl-1,3-dioxan-5-yl)methyl acrylate] 330 0.6 63 Poly(1,3-dioxan-5-yl acrylate) 299 0.6 62 Poly[(5-ethyl-1,3-dioxan-5-yl)methyl acrylate] 309 0.6 64 Poly[(5-methyl-1,3-dioxan-5-yl)methyl acrylate] 314 0.6 63 Poly(cyclohexyl chloroacrylate) 387 0.73 65 Poly(cyclohexyl methacrylate) 384 0.82 66 Poly(3,5-dimethyladamantyl methacrylate) 467 0.83 54 Poly(dl-isobornyl methacrylate) 464 0.8 66 Poly(cyclobutyl methacrylate) 351 0.8 67 Poly(cyclopentyl methacrylate) 348 0.81 67 Poly(cyclooctyl methacrylate) 346 0.79 67 Poly(cyclodecyl methacrylate) 331 0.82 67 Poly(cyclododecyl methacrylate) 329 0.79 67 Poly(cyclooctylmethyl methacrylate) 326 0.7 67 Poly(2,6-dimethylphenyl methylacrylate) 449 0.7 68 Poly(3,5-dimethylphenyl methylacrylate) 382 0.7 21 Poly(2,5-dimethylphenyl methylacrylate) 379 0.7 21 Poly(2,6-diisopropylphenyl methacrylate) 455 0.7 68 Poly(3,3,5-trimethylcyclohexyl methacrylate) 398 0.7 55 Poly(2-decahydronaphthyl methacrylate) 418 0.83 66 Poly(3-tetracyclododecyl methacrylate) 477 0.81 66 Poly(1-naphthyl methacrylate) 415 0.69 18 Poly(4-tert-butylcyclohexyl methacrylate) 451 0.71 66 Poly(2-methylcyclohexyl methacrylate) 371 0.69 69
Polymer Tg (K) Molar fract. r dyads Ref. Poly(3-methylcyclohexyl methacrylate) 374 0.71 69 Poly(4-methylcyclohexyl methacrylate) 394 0.73 69 Poly(3-thienylmethyl methacrylate) 338 0.7 30 Poly(menthyl methacrylate) 402 0.7 43 Poly(glycidyl methacrylate) 347 0.7 45 Poly(tetrahydrofurfuryl methacrylate) 320 0.7 70 Poly(1,3-dioxan-5-yl methacrylate) 405 0.7 62 Poly(cyclohexylmethyl methacrylate) 351 0.7 62 Poly[(5-ethyl-1,3-dioxan-5-yl)methyl methacrylate] 396 0.7 71 Poly(trans-11-(4-(5-(4-methoxyphenyl)-1,3-dioxan-2-yl)- 2,6-dimethylphenoxy)undecyl methacrylate 294 0.7 59 Poly(2-((4-((E)-(4-(((2,6-dimethyl-pyrimidin-4-yl)amino)sulfonyl)phenyl)diazenyl)phenyl)-(methyl)amino)ethyl methacrylate) 391 0.7 72 Poly(2-(2-((4-((E)-(4-(((2,6-dimethyl-pyrimidin-4-yl)amino)sulfonyl) phenyl)diazenyl)phenyl)-(methyl)amino)ethoxy)ehtyl methacrylate) 347 0.7 72 Poly[(5-methyl-1,3-dioxan-5-yl)methyl methacrylate] 415 0.7 73 Poly[(2,2,5-trimethyl-1,3-dioxan-5-yl)methyl methacrylate] 377 0.7 73 Poly[2-(5,5-dimethyl-1,3-dioxan-2-yl)-2,2-dimethylethyl methacrylate] 384 0.7 73 Poly(cycloheptadecyl methacrylate) 329 0.82 67
All Tg data for copolymers ~ ~ 74
References to Table 8.2
1. Shetter J. A., J. Polym. Sci. B 1963, 1, 209.
2. Karasz F. E., MacKnight W. J., Macromolecules 1968, 1, 537.
3. Brandrup, J.; Immergut, E. H., Polymer Handbook, 3rd ed.; John Wiley & Sons, Inc.: New York, 1990.
4. Bicerano J., Prediction of polymer properties, Third Edition Revised and Expanded; Marcel Dekker, Inc. New York, Basel 2002.
5. Dever G. R. et al., J. Polym. Sci. A 1975, 13, 2151. 6. Wesslen B. et al., Macromolecules 1971, 4, 24. 7. Gourari A. et al., J. Polym. Sci. B 1985, 23, 889. 8. Ute K. et al., Polymer 1995, 36, 1415.
9. Walstrom A. M. et al., Polym Prepr 1986, 27, 135. 10. Gipstein E. et al., Polym Prepr 1972, 13, 1212. 11. Krause S. et al., J. Polymer Sci. A 1965, 3, 3573.
12. Diaz-Calleja R. et al., J. San Romàn, Macromolecoles 1991, 24, 1854. 13. Pizzirani G., Magagnini P. L., Chim. Ind. (Milan) 1968, 50, 1218. 14. Baccaredda M. et al., J. Polymer Sci. B 1971, 9, 303.
15. Butler K. et al., J. Polymer Sci. 1960, 48, 357.
16. Matsuzaki K. et al., Makromolekulare Chemie 1973, 174, 215. 17. Petrovich-Djakov D. M. et al., J. Thermal Analysis 1993, 40, 741. 18. Pilcher S. C., Ford W. T., J. Polym. Sci. A 2001, 39, 519.
19. Ekstrin F. A. et al., Trudy po Khimii i Khimicheskoi Tekhnologii 1970, 2, 172. 20. Niezette J., Desreux V., Makromolekulare Chemie 1971, 149, 177.
21. Velickovic J. S. et al., Polymer Bulletin 1991, 27, 331.
22. Kihira Y., Sugiyama K., J. Macromol. Sci.-Phys. 1987, B26, 227.
23. Alberda Van Ekenstein G. O. R. et al., European Polymer Journal 1989, 25, 111.
24. Chetyrkina G. M. et al., Vysokomolecul. Soedin., Vsesoyuz. Khim. Obshchestvo im. D I. Mendeleeva 1959, 1, 248 ; C. A. 1959, 53, 230591.
25. Yuki H. et al., Polymer J. 1971 (Japan), 2, 629. 26. Vasquez B. et al., Polymer 1995, 36, 3467.
27. Rottink J. B. H. et al., Polymer Bulletin 1993, 31, 221. 28. Li M. et al., Polymer Bulletin 1995, 35, 65.
29. Shibaev V. P. et al., European Polymer Journal 1982, 18, 651. 30. Yilmaz F. et al., Designed Monomers and Polymers 2005, 8, 223.
31. Pugh C., Percec V., Polymer Preprints (ACS) 1986, 27, 366. 32. Bai S., Zhao Y., Macromolecules 2002, 35, 9657.
33. Haitjema H. J. et al., European Polymer Journal 1996, 32, 1447. 34. Diaz-Calleja R. et al., J. Phys. Chem. 1992, 96, 6843.
35. Cristofolini L. et al., Molecular Crystals and Liquid Crystals Science and Technology, Section A: Molecular Crystals and Liquid Crystals 2002, 375, 689.
36. Chiellini E. et al., J. Mater. Chem. 1993, 3, 1065.
37. Chien L. -C. et al., Mol. Cryst. Liq. Cryst. 1998, 317, 273.
38. Licea-Claverie A. et al., Macromolecular Symposia 2004, 207, 193. 39. Finkelmann H. et al., Angewandte Chemie Int. Ed. Engl. 1978, 17, 935. 40. Koehler W. et al., Macromolecules 1991, 24, 4589.
41. Altomare A. et al., Macromol. Chem. Phys. 2004, 205, 1611. 42. Altomare A. et al., Gazz. Chim. Ital. 1997, 127, 143.
43. Altomare A. et al., Chirality 1991, 3, 292.
44. Altomare A. et al., Macromol. Symp. 1999, 137, 33.
45. Jone Selvamalar C. S. et al., Reactive and Functional Polymers 2003, 56, 89. 46. Soykan C., Erol I., Journal of Polymer Research 2004, 11, 53.
47. Nanjundan S. et al., Reactive and Functional Polymers 2005, 62, 11. 48. Sin S. L. et al., Macromolecules 2005, 38, 3943.
49. Han Y. K. et al., Macromolecules 2004, 37, 9355. 50. Altomare A. et al., Macromol. Mat. Eng. 2003, 288, 679. 51. Compan V. et al., Polymer 1993, 34, 2971.
52. Bromine Compounds Ltd., Israel, Patent n. IL 112326, Kind A1, Date, 20010111, Application n. IL 1995-112326, Date 19950112, Priority Application IL 1995-112326, date 19950112
53. Bovey F. A., Abere J. F., J. Polymer Sci. 1955, 15, 537. 54. Matsumoto A. et al., T. Macromolecules 1991, 24, 4017.
55. Hopfinger A. J. et al., J. Polym. Sci., Part B: Polym. Phys. 1988, 26, 2007. 56. Kovacs A. J., J. Polymer Sci. 1958, 30, 131.
57. Frosini V. et al., J. Polym. Sci.-Polymer Physics edition 1977, 15, 2239. 58. Legrand C. et al., Makromol. Chem. 1990, 191, 2971.
59. Hsu C. S., Percec V., Makromol. Chem. 1988, 189, 1141.
60. Coskun M. et al., Macrolomecular Reports 1996, A33(suppl.2), 117. 61. Sreekuttan Unnithan C. et al., J. Macromol. Sci. 2005, A42, 877. 62. Diaz-Calleja R., Riande E., Macromolecular Symposia 1999, 147, 191. 63. Diaz-Calleja R. et al., Journal of Applied Physics 1998, 84, 4436.
64. Smith Soerensen T. et al., Journal of the chemical society, Faraday transactions 1997, 93, 2399. 65. Hoff E. A. W. et al., J. Polymer Sci. 1955, 18, 161.
66. Matsumoto A. et al., J. Polym. Sci. 1993, 31, 2531. 67. Mays J. W. et al., Macromolecules 1990, 23, 3530. 68. Gargallo L. et al., Thermochimica Acta 1987, 114, 319. 69. Domìnguez-Espinosa G. et al., Polymer 2005, 46, 11351. 70. Patel M. P. et al., Biomaterials 1987, 8, 53.
71. Guzman J. et al., J. Polym. Sci. A 1997, 35, 1125. 72. Ortyl E. et al., European Polymer Journal 2002, 38, 1871. 73. Mantell G. J. et al., J. Appl. Polym. Sci. 1965, 9, 3625. 74. Penzel E. et al., Polymer 1997, 38, 325.
References for Toxicity data
Data for IGC
50of substituted phenols towards Tetrahymena pyriformis taken from:
Aptula, A. O.; Netzeva, T. I.; Valkova, I. V.; Cronin, M. T. D.; Schultz, T.W.; Kühne,
R.; Schüürmann, G. Multivariate discrimination between modes of toxic action of
phenols. Quant. Struct.-Act. Relat. 2002, 21, 12–22.
Data for LC
50of substituted benzenes towards Pimephales promelas taken from:
Hall, L. H.; Kier, L. B.; Phipps, G. Structure-activity relationship studies on the
toxicities of benzene derivatives: I. an additivity model. Environ. Toxicol. Chem.
9. Appendix III: Molecular Fragments and Vertex Labels Used
for the Experiments
All vertex labels are orthogonal to each other, except where otherwise indicated.
Experiment Tm1
Molecular fragments: CH3, CH2, C, Hsp3, Hsp2, Hsp, C≡C, C=C, Csp, C=Osp3 (bound to a
sp3 carbon), C=Osp2 (bound to a sp2 carbon), CNsp3, CNsp2, NH 2sp3,
NH2sp2, N, OHsp3, OHsp2, O, F, Cl, Br, I, C=N-OH, Phenyl,
Pyridine,
Groups with non-orthogonal labels: CH3, CH2
CH2, C Hsp3, Hsp2, Hsp C=Osp3, C=Osp2 CNsp3, CNsp2 NH2sp3, NH2sp2 OHsp3, OHsp2 F, Cl Cl, Br Br, I
Experiment Tm2
Molecular fragments: CH3, CH2sp3, CH2sp2, CHsp3, CHsp2, CHsp, Csp3, Csp2, Csp, C=O,
HC=O, CN, N, NH, NH2, OHsp3 (bound to a sp3 carbon), OHsp2
(bound to sp2 carbon), O, S, F, Cl, Br, C=N-OH, COOH, COO,
CONH2, Hexa
Groups with non-orthogonal labels: CH3, CH2sp3, CHsp3, Csp3
CH2sp2, CHsp2, Csp2 Csp, CHsp C=O, HC=O NH, NH2 OHsp3, OHsp2 O, S F, Cl Cl, Br
Experiment Tg1
Molecular fragments: H, C, CH2, CH3, CH2-CH2 (not belonging to a cyclic moiety), C≡C, F, Cl,
Br, CF2, CF3, CN, C=O, COO, O, OH, N, NH, NH2, N=N, NO2, NO3,
S, SO, SO2, Start, Stop, Phenyl
Groups with non-orthogonal labels: C, CH2, CH3
F, Cl, Br
CF2, CF3
N, NH2, NH3
SO, SO2
Experiment Tg1b
Molecular fragments: H, C, Caromatic, CH2, CH3, CH2-CH2 (not belonging to a cyclic moiety),
C≡C, F, Cl, Br, CF2, CF3, CN, C=O, COO, O, OH, N, NH, NH2,
N=N, NO2, NO3, S, SO, SO2, Start, Stop, Cut1
Groups with non-orthogonal labels: C, Caromatic, CH2, CH3
F, Cl, Br
CF2, CF3
N, NH2, NH3
SO, SO2
Experiment Tg2
Molecular fragments: H, C, Caromatic, CH2, CH3, CH2-CH2 (not belonging to a cyclic moiety),
C≡C, F, Cl, Br, CF2, CF3, CN, C=O, COO, O, OH, N, Naromatic, NH,
NH2, N=N, NO2, S, Saromatic, SO, SO2, Start, Stop, Cut1, Cut2, Cut3,
Cut4
Groups with non-orthogonal labels: C, Caromatic, CH2, CH3
F, Cl, Br
CF2, CF3
N, Naromatic, NH2, NH3
S, Saromatic
Experiment Tg2u
Molecular fragments: H, C, Caromatic, Caromatic-Caromatic, CH2, CH3, CH2-CH2 (not belonging to a
cyclic moiety), CH2-CH2 (belonging to a cyclic moiety), C≡C, F, Cl,
Br, CF2, CF3, CN, C=O, COO, O, OH, N, Naromatic, NH, NH2, N=N,
NO2, S, Saromatic, SO, SO2, StartC, Stop, Cut1, Cut2, Cut3, Cut4
Groups with non-orthogonal labels: C, Caromatic, CH2, CH3
F, Cl, Br
CF2, CF3
N, Naromatic, NH2, NH3
S, Saromatic
SO, SO2
The CH2-CH2 groups have identical labels
Experiments Tg3 and Tg4
Molecular fragments: H, C, CH2, CH3, CN, CH2-CH2, COO, Start (with one child), Start (with
two children), Stop,
Groups with non-orthogonal labels: C, CH2, CH3
The Start groups have identical labels
Experiments Tg5 and Tg6
Molecular fragments: H, C, Caromatic, Caromatic-Caromatic, CH2, CH3, CH2-CH2 (not belonging to a
cyclic moiety), CH2-CH2 (not belonging to a cyclic moiety), C≡C, F,
Cl, Br, CF2, CF3, CN, C=O, COO, O, OH, N, Naromatic, NH, NH2,
N=N, NO2, S, Saromatic, SO, SO2, Start (with one child), Start (with two
children), Stop, Cut1, Cut2, Cut3, Cut4 Groups with non-orthogonal labels: C, Caromatic, CH2, CH3
F, Cl, Br
CF2, CF3
N, Naromatic, NH2, NH3
S, Saromatic
SO, SO2
The CH2-CH2 groups have identical labels
Experiments Tox1, Tox2, Tox4 and Tox5
Molecular fragments: Haliphatic/aromatic, Haldehyde, Hacid, C, Csp2 , CH2, CH3, CH2-CH2, F, Cl,
Br, I, CN, C=O, COO, O, OH, Namine, NH2amine, NHamide, NH2amide,
Nsp2, NO, NO2, Cyclopentyl, Phenyl, Phenol
Groups with non-orthogonal labels: Namine, NH2amine
NHamide, NH2amide
NO, NO2
Experiment Tox3
Molecular fragments: Haliphatic/aromatic, Haldehyde, Hacid, C, Csp2 , CH2, CH3, CH2-CH2, F, Cl,
Br, I, CN, C=O, COO, O, OH, Namine, NH2amine, NHamide, NH2amide,
Nsp2, Cyclopentyl, Phenyl, Phenol
Groups with non-orthogonal labels: Namine, NH2amine
10. Appendix IV: Definition of the Statistical Parameters
Used for the Evaluation of Results
Mean Absolute Residual (MAR):
MAR
=
P
i exp− P
icalc i∑
n
where
P
iexp= experimental target property value for the compound i
P
icalc= calculated target property value for the compound i
(for RNN: average output over sixteen trials)
n = number of compounds in the considered data set (training or test)
Standard error of estimate/prediction (S):
S
=
P
i exp− P
icalc(
)
2 i∑
n
Squared correlation coefficient (R
2):
(
)
(
)
2 exp 2 2 exp1
calc i i i avg i iP
P
R
P
P
−
= −
−
∑
∑
where
P
avg= average value of
P
iexp