Chapter 1
Bone Tissue Engineering
1.1 Principles
Bone is a dynamic, highly vascularized tissue with the unique capacity to heal and to remodel depending on line of stress. It exhibits the unlikely combination of high compressive strength and tensile strength due to composite of calcium phosphate salts, hydroxyapatite, and collagen respectively. It is difficult to find materials to mimic such a complex system to fill bone defects. However, current research capitalizes on the dynamic properties of bone by providing a biodegradable scaffold to guide healing.
Normal bone fracture healing frequently requires temporary immobilization of area but no other intervention. The normal healing process results in reformation of organized, unscarred bone tissue.
Certain situations may arise in which bone tissue does not heal completely; such a fracture is termed a nonunion and can be due to fracture comminution or bone loss, infection, loss of blood supply, disease, or inadeguate fracture management [1]. In these situations, the patient suffers from loss of mechanical support of body’s tissues and loss of muscle attachment sites. Such skeletal defects, thus, require therapeutic intervention to restore bone to its proper form.
Reconstruction of bone defects, regardless of their origin, remains a difficult and controversial problem for orthopedic and reconstructive surgery. Many techniques to replace the structure and function of bone lost by trauma or disease have been used. The ideal reconstruction in all cases would replace bone defect with like autogenous tissue. However the supply of autograft tissue is usually limited, and the harvesting procedures can lead to creation of secondary morbid sites and potential postoperative complications. Graft tissue is also occasionally not suitable for the required reconstructions because of poor tissue quality or extreme difficulty shaping the graft. Allografts can be used in some cases, but problems associated with donor matching, integration, and tissue storage, coupled with potential complications related to blood-borne products, limit their applications and uses [2].
Alloplastic materials are another alternative, but they can show increased susceptibility or risk of infections and/or extrusion and an uncertain long-tem interaction with the host’s physiology Therapies can be categorized into essentially two groups. Permanent replacement of the bone with a foreign material is a commonly used strategy. Bone cement for injectable filling or irregularly shaped skeletal defects and prefabricated metals for hip replacement are two bone replacement materials that lend high mechanical support. However, when a very strong material is used in bone tissue replacement, it absorbs the stresses of daily activity instead of the bone; this phenomenon is called stress shielding. Since bone tissue normally maintains its strength in response to stress, the area surrounding the replacement material subsequently experiences bone loss. In addition, bone cement, which is formed by mixing prepolymerized poly(methyl methacrilate) or PMMA powder with methyl methacrilate or MMA monomer and initiator to form a moldable putty that hardens in a matter of minutes, has been associated with increased tendency for infections at the surgical site.
The second therapy involves the ultimate restoration of bone tissue at defect site. Two-well-established filler materials for this are autografts, bone harvested from cadavers. These provide a scaffold upon which the patient’s adjacent bone tissue can invade, lay down new extracellular matrix, ECM, and remodel. In fact, process of incorporation of transplanted bone is similar to normal bone regeneration after fracture or during growth [3]. Any material that provides a scaffold for bone tissue formation is termed osteoconductive.
Despite its widespread use, allograft transplantation is associated with several risks. Unprocessed allografts may carry diseases, such as hepatitis and AIDS or may be rejected by immune system. While processed allograft is widely used as an osteoconductive material, it is believed that during processing allografts loses factors that would induce bone tissue formation where and when it would not normally grow; that is, it may no longer be osteoconductive.
Problems associated with autografts are limited supply and donor site morbidity. Leaving aside these serious disadvantages, autografts satisfies three requirements necessary for bone regeneration: it is osteoconductive, osteoinductive, and contains osteogenic cells [4]. There is also no fear of rejection or transfer of disease.
Current research in bone tissue engineering uses trabecular autograft bone as so called gold standard to develop synthetic, porous, degradable scaffolds to fill defects. Since the scaffolds are degradable, bone tissue will invade the area, lay down matrix, and restore skeletal continuity while scaffold disappears as is the case with grafted bone. Unlike autograft bone, such synthetic
scaffolds do not have limited availability; there is no risk of disease transfer or rejection as is inherent in unprocessed allograft.
Considerations for engineering synthetic scaffolds involve choosing a material that is biodegradable, that allows cell attachment and proliferation, and that exhibits appropriate mechanical properties. Target properties of replacement material for human trabecular bone are minimum compressive strength of 5 MPa and minimum compressive modulus of 50 MPa. Moreover, material should be sterilizable and fairly easy to manufacture. Shelf life and availability must also be considered. In addition, potential for drug delivery and cell transplantation as well as easily manipulated are desired properties to expand bone tissue engineering applications.
Discussed are four current strategies for using synthetic scaffolds in bone regeneration, each with its own advantages and disadvantages. Common to all four is that the success of each relies on the ability of cells to attach to scaffold and eventually replace space formerly occupied by the degradable scaffold. In reality, many bone tissue engineering developments from each strategy may be contained in design of a single scaffold.
1.2 Polymers
Some skeletal defects occur adjacent to relatively healthy bone tissue, such as fracture nonunions where the defect is too large to heal on its own. One of approach to filling this type of defect is based on healthy bone tissue in neighboring region. In this strategy, a porous degradable scaffold would be used to fill the defect. Such a scaffold would have to be osteoinductive, so the osteoblasts and other cells start to colonize it. The cells would grow throughout but not beyond the scaffold: matrix would be laid down and the tissue formed and remodeled as the scaffold degrades. So, the area would result in organized bone tissue due to of its healing and remodeling potential.
The osteoconductive and osteoinductive capabilities of biomaterials are investigated in vivo using two different animal models. To investigate osteoconduction, the material is placed in the defect that would normally heal if left untreated; if normal healing occurs in the presence of biomaterial, it is considered osteoconductive but not necessary osteoinductive. To investigate osteoinduction, the material is placed in a defect that will not heal in untreated controls; such a defect may be called a nonunion or critical size defect. If the material can induce bone regeneration in this
situation, then it is osteoinductive. Clearly, osteoinduction is much more difficult criterion to satisfy in scaffold development.
It is important for tissue-engineering product developer to have several biomaterials options available, for each application calls for a unique environment for cell-cell interactions. Such applications include:
1. Support for new tissue 2. Prevention of cellular activity 3. Guided tissue response
4. Enhancement of cell attachment and subsequent cell activation 5. Inhibition of cell attachment to a vascular graft
6. Prevention of a biological response
Biodegradable polymers are applicable to those tissue-engineering products in which tissue repair or remodeling is the goal, but not where long-term materials stability is required. Biodegradable polymers must also possess:
1. Manufacturing feasibility including sufficient commercial quantities of bulk polymer 2. The capability to form polymer into the final product design
3. Mechanical properties that adequately address short-term function and do not interfere with long-term function
4. Low or negligible toxicity of degradation products in terms of both local tissue response and systemic response
5. Drug delivery compatibility in applications that call for release or attachment of active compounds
From the beginning of the material sciences, the development of highly stable materials has been major research challenge. Today, many polymers are available that are virtually indestructible in biological systems, Teflon, Kevlar, and poly(ether-ether-ketone). On the other hand, the development of degradable biomaterials is a relatively new area of research. The variety of available degradable biomaterials is still to limited to cover a wide enough range of diverse material properties. Thus, the design and synthetis of new degradable biomaterials is currently an important research challenge.
Due to the efforts of a wide range of research groups, a large number of different polymeric compositions and structures have been suggested as degradable biomaterials. However, in most cases no attempts have been made to develop these new materials for specific medical applications. Thus, detailed toxicological studies in vivo, investigations of degradation rate and mechanism, and careful evaluations of physicomechanical properties have so far been published for only a very small fractions of those polymers. This leaves tissue engineer with only a relatively limited to a review of most commonly investigated classes of biodegradable synthetic polymers. Naturally occurring hydroxyl acids, such as glycolic, lactic, and ε-caproic acids, have been utilized to synthesize an array of useful biodegradable polymers for a variety of medical products applications. As an example, bioresorbable surgical sutures made from poly(α-hydroxy acids) have been in clinical use since 1970; other implantable devices made from these versatile polymers (internal fixation devices for orthopedic repair) are becoming part of the standard surgical protocol.
The ester bond of poly(hydroxy acids) are cleaved by hydrolysis, which results in a decrease in the polymer molecular weight(but not mass) of implant. This initial degradation occurs until the molecular weight is less than 5000 Da, at which point cell degradation takes over. The final degradation and resorption of poly(hydroxy acid) implants involves inflammatory cell response such as macrophages, lymphocytes and neutrophilis. Although this late-stage inflammatory response can have a detelerious effect on some healing events, these polymers have been successfully employed as matrices for cell transplantation and tissue regeneration. The degradation rate of these polymers is determined by initial molecular weight, exposed surface area, crystallinity, and ratio of hydroxy acid monomers.
The poly(hydroxy acid) polymers have a modest range of mechanical properties and a correspondingly modest range of processing conditions. These thermoplastics can generally be formed into films, tubes, and matrices using such standard processing techniques as molding, extrusion, solvent casting and spin casting. Ordered fibers, meshes, and open-cell foams have been formed to fulfill the surface area and cellular requirements of a variety of tissue-engineering constructs. The poly(hydroxy acid) polymers have also been combined with other materials like poly(ethylene glycol), to modify the cellular response elicited by the implant and its degradation.
Poly(glycolic acid), Poly(lactic acid), and Their Copolymers
Poly(glycolic acid) (PGA), poly(lactic acid) (PLA), and their copolymers are the most widely used synthetic degradable polymers in medicine. Of this family of linear aliphatic polyesters, PGA has the simplest structure (fig 1) and consequently enjoys the largest associated literature base.
Since PGA is highly crystalline, it has a high melting point and low solubility in organic solvents. PGA was used in development of the first totally synthetic absorbable suture. The crystallinity of PGA in surgical sutures is typically in range of 46-52%. Due to its hydrophilic nature, surgical sutures made of PGA tend to lose their mechanical strength rapidly, typically over a period of two to four weeks post-implantation.
Figure 1: Poly(glycolic acid) (PGA)
In order to adapt the materials properties of PGA to a wider range of possible applications, researchers undertook an intensive investigation of copolymers of PGA with the more hydrophobic poly(lactic acid) (PLA) (fig 2).
Figure 2: Poly(lactic acid) (PLA)
Due to the presence of an extra methyl group in lactic acid, PLA (fig 2) is more hydrophobic than PGA. The hydrophobicity of high-molecular-weight PLA limits the water uptake of thin films to about 2% and results in a rate of backbone hydrolysis lower than that of PGA [5]. In addition, PLA is more soluble in organic solvents than is PGA.
It is known that there is a no linear relationship between the ratio of glycolic acid to lactic acid and the physicomechanical properties of their copolymers. Whereas PGA is highly crystalline, crystallinity is rapidly lost in PGA-PLA copolymers. These morphological changes lead to an increase in rates of hydration and hydrolysis. Thus, copolymers tend to degrade more rapidly than either PGA or PLA [6].
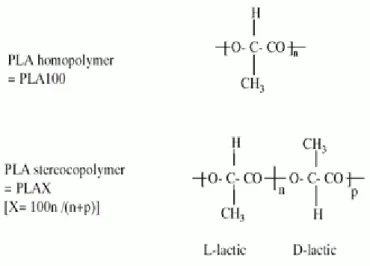
Since lactic acid is a chiral molecule, it exists in two stereoisomeric forms that give rise to four morphologically distinct polymers. D-PLA and L-PLA are the two stereoregular polymers (fig 2), D,L-PLA is the racemic polymer obtained from a mixture of D- and L-lactic acid, and meso –PLA can be obtained from D,L-lactide. The polymers derived from the optically inactive D,L-PLA is always amorphous. Generally, PLA is more frequently employed than D-PLA, since the hydrolysis of L-PLA yields L(+)-lactic acid, which is the naturally occurring stereoisomer of lactic acid.
The differences in crystallinity of D,L-PLA and L-PLA have important practical ramifications: Since D,L-PLA is an amorphous polymer, it is usually used as drug delivery system, where it is important to have a homogeneous dispersion of the active species within a monophasic matrix. On the other
hand, the semicrystalline L-PLA is preferred in applications where high mechanical strength and toughness are required (for example, sutures and orthopedic devices) [7].
Recently, PLA, PGA, and their copolymers have been combined with bioactive ceramics such as Bioglass particles and hydroxyapatite that stimulate bone regeneration while greatly improving of mechanical strength of the composite material [8].
Some controversy surrounds use of these materials for orthopedic applications. According to one review of short- and long-term response to resorbable pins made from either PGA or PGA:PLA copolymer in over 500 patients, 1.2% required reoperation due to device failure, 1.7% suffered from bacterial infection of the operative wound, and 7.9% developed a noninfectious inflammatory response that warranted operative drainage [9]. Subsequently it has become evident that the delayed inflammatory reaction represents the most serious complication of the use of the currently available degradable fixation devices.
Polydioxanone (PDS)
This poly(ether-ester) is prepared by a ring-opening polymerization of p-dioxanone. PDS has gained increasing interest in the medical field and pharmaceutical field due to its degradation to low toxicity momomers in vivo. PDS has a lower modulus than PLA or PGA; thus it became the first degradable polymer to be used to make a monofilament suture. PDS has also been introduced to the market as a suture clip as well as more recently as a bon pin marketed under the name of ORTHOSRB in United States and ETHIPIN in Europe [10].
Poly(ε-caprolactone)
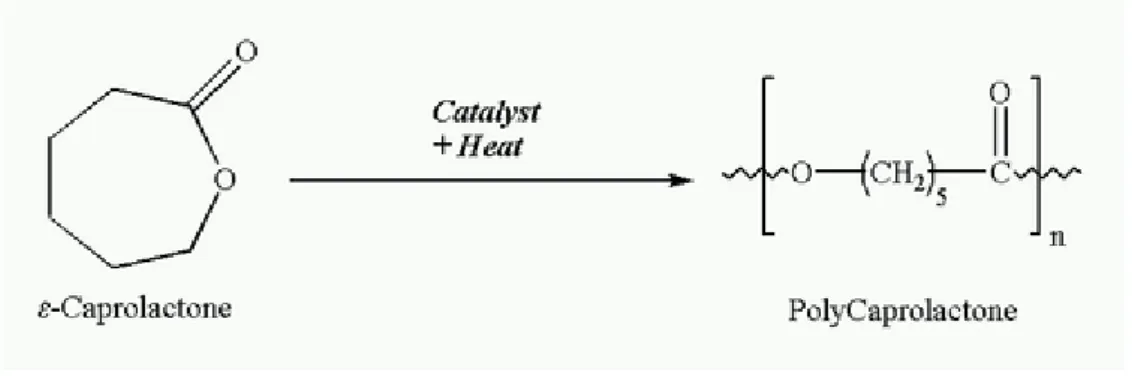
Poly(ε-caprolactone) (PCL) (fig 3) is an aliphatic polyester that has been intensively investigated as a biomaterial. The discovery that PCL can be degraded by microorganism led to evaluation of PCL as a biodegradable packaging material. Later, it was discovered that PCL can also be degraded by a hydrolytic mechanism under physiological conditions. Under certain circumstances, cross-linked PCL can be degraded enzymatically, leading to “enzymatic surface erosion”. Low-molecular weight fragments of PCL are taken up by macrophages and degraded intracellularly, with a tissue
reaction similar to that of poly(hydroxy acids). Compared with PGA or PLA, the degradation of PCL is significantly slower. PCL is therefore most suitable for the design of long-term, implantable systems such as Capronor, a one-year implantable contrapcetive device [11].
Figure 3: Poly(ε-caprolactone)
Poly(ε-caprolactone) exhibits several unusual properties not found among the other aliphatic polyesters. Most noteworthy are its exceptionally low glass transition temperature of -62˚C and its low melting temperature of 57˚C. Another unusual property of poly(ε-caprolactone) is its high thermal stability. Whereas other tested aliphatic polyesters had decomposition temperatures (Td) between 235 and 255˚C, PCL has a Td of 350˚C, which is more typical of poly(ortho esters) than of aliphatic polyesters [12]. PCL is semicristalline polymer with a low glass transition temperature of about -60˚C. Thus, PCL is always in a rubbery state at room temperature. Among the more common aliphatic polyesters, this is an unusual property, which contributes to the very high permeability of PCL for many therapeutic drugs.
Another interesting property of PCL is its propensity to form compatible blends with a wide range of other polymers. In addition, ε-caprolactone can be copolymerized with numerous other monomers. Particularly noteworthy are copolymers of ε-caprolactone and lactic acid that have been studied extensively. PCL and copolymers with PLA have been electronspun to create nonofibrous tissue-engineered scaffolds that show promise for vascular applications.
The toxicology of PCL has been extensively studied as part of the evaluation of Capronor. Based on a large numbers of tests , the monomer, ε-caprolactone, and the polymer, PCL, are currently regarded as nontoxic and tissue compatible materials. Consequently, clinical studies of the Capronor systems are currently in progress. It is interesting to note that in spite of its versability, PCL has so far been predominantly considered for controlled-release drug delivery applications. In
Europe, PCL is being used as a biodegradable staple, and it stands to reason that PCL (or blends and copolymers with PCL) will find additional medical applications in the future.
Poly(ortho esters)
Poly(ortho esters) are a family of synthetic degradable polymers that have been under development for several years. Devices made of poly(ortho esters) can be formulated in such a way that the device undergoes “surface erosion” that is, the polymeric device degrades at its surface only and will thus tend to become thinner over time rather than crumbling into pieces. Since surface-eroding, slablike device tend to release drugs embedded within the polymer at a constant rate, poly(ortho esters) appear to be particularly useful for controlled-release drug delivery.
There are two major types of poly(ortho esters). Originally, poly(ortho esters) were prepared by the condensation of 2,2-diethoxytetrahydrofuran and a dialcohol and marketed under the trade names Chronometer and Alzamer. Upon hydrolysis, these polymers release acidic by-products that autocatalyze the degradation process, resulting in degradation rates that increase with time. Later, a new type of poly(ortho ester) was synthesized based on the reaction of 3,9-bis(ethylidene 2,4,8,10-tetraoxaspiro {5,5} undecane) (DETOSU) with various dialcohols. Tese poly(ortho esters) do not release acidic by-products upon hydrolysis and thus do not exhibit autocatalytically increasing degradation rates.
Polyurethanes
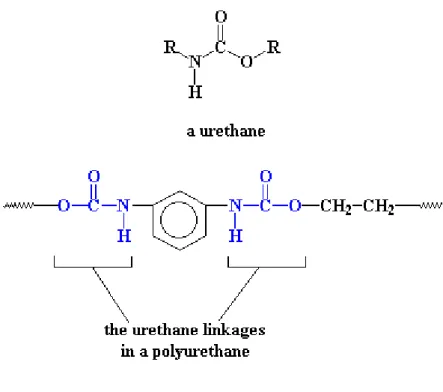
Polyurethanes, polymers in which the repeating unit contains a urethane moiety, were first produced by Bayer in 1937 (fig 4). These polymers are typically produced through the reaction of a diisocyanate with a polyol.
Conventional polyols are polyethers or polyesters. The resulting polymers are segmented block copolymers, with the polyol segment providing a low-glass-transition-temperature soft segment and the diisocyanate component, often combined with a hydrocarbon chain extender, providing
the hard segment. A wide range of physical and mechanical properties have been realized with commercial polyurethanes.
Polyurethanes have been used for nearly 50 years in biomedical applications, particularly as the blood-contacting material in cardiovascular devices. Intended as non biodegradable coatings, polyurethanes fell out of favor with the failure of pacemaker leads and breast implant coatings. Subsequent studies have elucidated much about the behavior of polyurethanes in biological systems.
Elucidation of the biodegradation mechanism and its dependence on polyurethane structure and composition have led to the development of biodegradable polyurethanes for a variety of tissue engineering applications, such as myocardial repair and vascular tissues [13].
Design of biodegradable polyurethanes has required alternative diisocyanate compounds. The traditional aromatic diisocyanates are putative carcinogenic compounds. Biodegradable polymer are made from diisocyanates, such as lysine-diisocyanate or hexamethylene diisocyanate, that release nontoxic degradation products such as lysine.
The polyol or soft-segment portion of biodegradable polyurethanes is uded to modify the degradation rate. Poly(α-hydroxy acids), including PLA, PGA and PCL, have been used as soft segments for biodegradable polyurethanes.
An interesting application of polyurethanes was the incorporation of fluoroquinolone antimicrobial drugs into the polymer as hard-segment monomers. This led to the design of drugs polymers (trade name, Epidel) that release the drug when degraded by enzymes generated by an inflammatory response. This is an example of a smart system, in that of antibacterial agents are released only while inflammation is present. Once healing occurs, the enzyme level drops and the release of drug diminishes.
Figure 4: Polyurehane
1.3 Techniques
As described before, the polymer properties and cell response to biomaterials are important selection criteria for the design of a tissue-engineered product. In addition, the ability to mold the biomaterial into the appropriate cellular-level architecture must be considered, such as the architecture must be compatible with the desired tissue response. Details such as pore size, pore structure, and oriented topography, such as aligned grooves, are often critical to form tissue with proper cell morphology, orientation, arrangement of intercellular material, and the relationship between different cell types.
In recent years, the selection of appropriate biomaterials has been aided by development of combinatorial methods and sophisticated modeling techniques that allow prediction of polymer properties and cell response to the material [14]. Such techniques promise to greatly expand the range of biodegradable polymers for tissue-engineering applications. Approaches to choosing biomaterials for various tissue-engineering applications according to type of tissue response sought are the following:
Conducting tissue response and architectures
Inducing tissue responses and architectures
Blocking tissue responses
Implicit in this consideration is that the specific material architecture (membrane, gel, matrix, tube) is critical to the tissue engineering product design and thus influences the choice of biomaterials.
Barriers: Membranes and Tubes
Design formats requiring cell activity on surface of a device while precluding transverse movement of surrounding cells onto that surface call for a barrier material. For example, peripheral nerve regeneration must allow for axonal growth and at the same time preclude fibroblast activity that could produce neural-inhibiting connective tissue. Structures such as collagen tubes can be fabricated to yield a structure dense enough to inhibit connective tissue formation along the path of repair while allowing axonal growth through lumen. Similarly, collagen membranes for periodontal repair provide an environment for periodontal ligament regrowth and attachment while preventing epithelial ingrowth into the healing site. Antiadhesion formulations using hyaluronic acid, which prevent ingrowth of connective tissue at a surgically repaired site, also work on this concept.
Gels
Gels are used to provide a hydrogel scaffold, encapsulate, or provide a specialized environment for isolated cells. For example, collagen gels for tissue engineering were first used to maintain fibroblasts, which were the basis of a living-skin equivalent. Gels have also been used for the maintenance and immunoprotection of xenografic and homograft cells, such as hepatocytes, chondrocytes, and islets of Langerhans, used for transplantation. Semipermeable gels have been created to limit cell-cell communication and interaction with surroundings tissue and to minimize movement of peptide factors and nutrients through the implant. Injectable biodegradable gels materials that form through cross-linking in situ show promise for regeneration of bone and cartilage. In general, non degradable materials are used for cell encapsulation to maximize
long-term stability of the implant. In the future, however, it may be possible to formulate novel smart gels, in which biodegradation is triggered by a specific cellular response instead of simple hydrolysis.
Matrices
It has been recognized since the mid-1970s that three-dimensional structures are an important component of engineered tissue development. Pore size, pore orientation, and fiber structure are important characteristics in the design of cell scaffolds. Several techniques have subsequently been developed to form well-defined matrices from synthetic and biologically derived polymers, and the physical characteristics of these matrices are routinely varied to maximize cellular and tissue responses. Examples of engineered matrices that have led to several resorbable templates are oriented pore structures designed for regeneration of trabecular bone.
Approaches to Bone Tissue Engineering
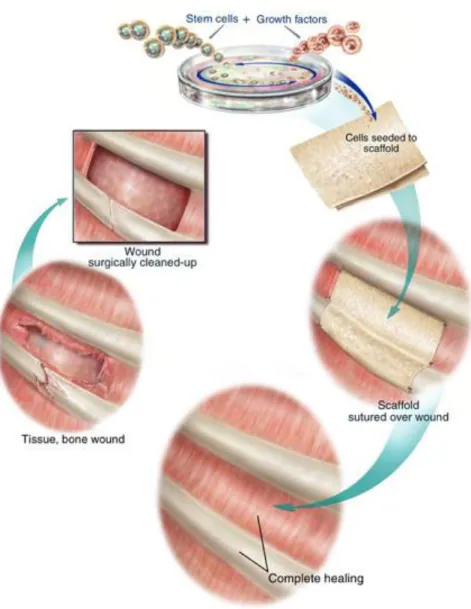
There are many approaches to bone tissue engineering, but all involve one or more of the following key ingredients: harvested cells, recombinant signaling molecules, and three-dimensional (3D) matrices. One popular approach, depicted in the figure below, involves seeding highly porous biodegradable matrices (or scaffolds), in the shape of the desired bone, with cells and signaling molecules (e.g., protein growth factors), then culturing and implanting the scaffolds into the defect to induce and direct the growth of new bone. The goal is for the cells to attach to the scaffold, multiply, differentiate (i.e., transform from a nonspecific or primitive state into cells exhibiting the bone-specific functions), and organize into normal, healthy bone as the scaffold degrades. The signaling molecules can be adhered to the scaffold or incorporated directly into the scaffold material.
Figure 5: Scaffold-guided Tissue Regeneration
In order to understand this approach, one must first understand why you can't just harvest some cells, such as osteoblasts, then culture them to create a whole bone as depicted below:
Conventional cell culturing involves growing cells in an artificial environment where they can adhere and replicate to form larger colonies of cells for applications such as diagnostic testing. These colonies, however, do not become organized into tissues or organs that could then be implanted back into patient. Cell colonies need external cues or signals to grow into functional 3D tissues or organs. In the body, cells are constantly subjected to mechanical, electrical, structural, and chemical cues that signal the cells about what they should be doing. If these signals are not properly received or processed due to disease or trauma, then the cells dedifferentiate (i.e., become nonspecific cell types), become disorganized, and eventually die.
The structural cues involve the interaction of cells with their extracellular matrix (ECM). The ECM is that part of our body which gives it form and shape. For example, bone is made up of an ECM composed of a composite fibrous network of collagen encased within a hard matrix of calcium/phosphorous. Bone cells (osteoblasts, osteoclasts, osteocytes) exist in a symbiotic relationship with ECM, first creating it, then remodeling it, and in turn being regulated by it. The physical communication between cells and ECM directly and indirectly impacts cell shape and function, and these signals are all necessary cues for normal cellular activity.
Cell actions and their responses to different environmental cues, including mechanical, electrical, structural and chemical, are mediated by protein based molecules loosely referred to as growth factors. The cellular regulatory actions of growth factors in bone include migration of cells from one site to another, morphogenesis from one cell type to another, and mitogenesis or cellular proliferation. Growth factors are produced both locally by bone cells and systemically from other sites. In mediating extracellular communications, growth factors act directly on bone cell that produced them (autocrine effect), act on neighboring cells surrounding the growth factor producing cell (paracrine effect), relay a single growth factor communication signal received by one cell to neighboring cells due to direct cell to cell interaction (juxtacrine effect), and act on cells distant from site of growth factor production by traveling through the blood stream (endocrine effect). Within the local bone environment growth factors reside in interstitial fluid, on the cell surface, and in the ECM. These growth factors are not only important for growth, development, and day-to-day maintenance of bone tissues, but are mobilized during times of bone remodeling and injury.
Tissue engineering techniques, such as depicted in Figure 5, thus involve mimicking the natural milieu by placing the cells and growth factors in synthetic scaffolds that act as temporary ECMs. However, there are numerous variations of this approach depending on:
the source of the cells; i.e., autologous (donated by the patient), allogenic (donated by another person), xenograph (from an animal)
whether or not a scaffold is even used; i.e., direct injection of cells and/or signaling molecule into the defect site may be appropriate for damaged tissue confined to a small region. Larger regions, however, will probably need the matrix as a structural cue
whether the scaffolds seeded with cells are cultured before surgery, or the cells are seeded into the matrix and immediately implanted at the time of surgery
whether or not cells are even used; i.e., just use signaling molecules
No single approach or dosage of cells and growth factors will satisfy all clinical needs; the best ‘recipe’ will depend upon particular application and the relative health status of the patient. For example in bone repair, an older diabetic patient or a smoker heals differently than a young, healthy child, so each would need a different dosing of cells and growth factors. Therapies that use a patient’s own cells are safest from an immunologic point of view, however these methods may not always be practical. For example, many surgeons and insurance carriers are not enthusiastic about performing two operations (i.e., one to harvest the cells, and another, weeks later, to implant the scaffold) because of additional costs, time, and quality control issues. Even when harvesting a patient’s own cells for immediate implantation there are two surgical sites, i.e., the implantation site and the harvesting site. In these cases, there may be donor site morbidity, including infection and chronic pain, as well as additional surgical costs. Finally, a very sick or elderly patient may not have enough virile cells, even if expanded ex vivo (outside the body), to cause the defective tissue to heal. For all these reasons, there is significant interest in having an off-the-shelf supply of donor cells. These cells would be expanded ex vivo and immortalized. Fetal or neonatal cells are extremely useful for this purpose since they are naturally non-immunogenic and are a rich source for stem cells; this approach, however, is an extremely controversial ethical issue.
Another approach will be “ex vivo gene therapy” consisting of isolation of relevant determined stem cells or committed progenitors from mature adults or from animals, expansion of them ex
vivo, transfection of them and selection of transfected cells ex vivo, and then reintroduction of the cells in vivo. Genetic engineering, however, has numerous hurdles to overcome to make this approach reliable, practical, safe, and generally accepted.
Instead of administering growth factors directly, it is also possible to use genes that encode those molecules. The genes are part of a plasmid, a circular piece of DNA constructed for this purpose. The surrounding cells take up the DNA and treat it as their own. They turn into tiny factories, churning out the factors coded for by the plasmid. Because the inserted DNA is free-floating, rather than incorporated into the cells' own DNA, it eventually degrades and the product ceases to be synthesized.
Challenges for Bone Tissue Engineering
Some of the challenges ahead for bone tissue engineering are the following:
The biggest challenge for all of tissue engineering is how to insure angiogenesis in a timely fashion within the scaffold construct; cells without a blood supply will die, and mass infection will occur.
New biomaterials are needed that cause minimal foreign body response and that degrade in a completely predictable fashion.
A basic understanding of the spatial and temporal distributions of cells and growth factors required for osteogenesis, subject to particular disease states, must still be determined; i.e., a complete bone tissue engineering knowledge base remains to be developed. To achieve this will require better experimental and analysis tools including more realistic in vitro models, better ways to non-invasively image developing tissue in vivo, suitable computational models that capture this vast, multidimensional array of information, and advanced data-mining techniques to extract salient information.
Advanced manufacturing systems are required that can fabricate complex scaffolds with spatially controlled distributions of materials, microstructures, cells and growth factors.
Design systems that encapsulate the tissue engineering knowledge-base and that understand the constraints of the manufacturing processes must be created to aid the next generation ‘tissue engineer’ in designing and manufacturing their products. Advanced CAD/CAM systems are required to create today’s complex automobiles, aircraft, and electronic products; why would anyone think that it would require anything less to design something as complex as human tissue.
References
1) Yaszemski MJ, Payne RG, Hayes WC, Langer RS, Aufdemorte TB, Mikos AG (1995): The ingrowth of new bone tissue and initial mechanical properties of a degrading polymeric composite scaffold.
2) Mankin HJ: The response of articular cartilage to mechanical injury. J Bone Joint Surg 64A: 460-66
3) Yaszemski MJ, Payne RG, Hayes WC, Langer RS, Mikos AG (1996): Evolution of bone transplantation: Molecular, cellular, and tissue strategies to engineer human bone.
Biomaterials 17: 175-185
4) Gazdag AR, Lane JM, Glaser D, Forster RA: Alternatives to autogenous bone graft: Efficacy and indications. J Am Acad Orthop Surg 3: 1-8
5) Reed, AM, Gilding: Biodegradable polymers for use in surgery-poly(glycolic)/poly(lactic acid) and copolymers. Polymer 22: 494-498
6) Gilding DK, Reed AM: Biodegradable polymers for use in surgery-poly(glycolic)/poly(lactic acid) homo and copolymers. Polymer 20: 1459-1464
7) Leenstag JW, Pennings AJ, Bos RRM, Roxema FR, Boenng G: Resorbable materials of poly-lactides. Biomaterials 8: 70-73
8) Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006): Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 4: 131-133
9) Bostman O, Pihlajamaki H (2000): Clinical biocompatibility of biodegradable orthopedic implants for internal fixation. Biomaterials 21: 2615-2621
10) Ray JA, Doddi N, Regula D, Williams JA, Melveger A: Polydioxanone (PDS), a novel monofilament synthetic absorbable suture. Surg. Gynecol. Obstet. 153: 497-507
11) Pitt CG: Poly-e-caprolactone and its copolymers. In “Biodegradable Polymers as Drug Delivery Systems”
12) Engelberg I, Kohn J: Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 12: 292-304
13) Stankus JJ, Guan J, Fujimoto K, Wagner WR (2005): Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials 27: 735-744
14) Thorstenson JB, Narasimhan B (2006): Combinatorial methods for the high-troughput characterization and screening of biodegradable polymers. In “ Handbook of Biodegradable Polymeric Materials and Their Applications”