Chapter 4
ROLE OF CYTOCHROME P450 2C9-DERIVED HYPERPOLARIZING
FACTOR IN MODULATING t-PA RELEASE IN HEALTHY SUBJECTS
AND IN HYPERTENSIVE PATIENTS
Introduction
Vascular endothelium plays a primary role in the modulation of vascular tone and structure by the production and release of nitric oxide (NO). A dysfunctional endothelium, secondary to reduced NO availability, acts to promote atherosclerosis and thus cardiovascular events 1. Besides its well documented effects on vascular function 2, NO participates in the activation of endogenous fibrinolysis 3, another crucial mechanism whereby NO may protect the vessel wall against the development of atherothrombosis. In line with experimental evidence in healthy humans, NO promotes the release of tissue-type plasminogen activator (t-PA), which is the main activator of endogenous fibrinolysis 3, 4.
Essential hypertension is a clinical condition characterized by endothelial dysfunction. A major aspect of this alteration concerns the reduced NO availability secondary to oxidative stress, which leads to both reduced endothelium-dependent vasodilation 5-7 and impaired capacity of t-PA release 4
.
Endothelial cells produce other relaxing factors, including EDHF, which cause hyperpolarization of smooth muscle cells 8. In several experimental models and clinical conditions, such as essential hypertension, EDHF induces vasodilation as a rapid compensatory mechanism for decreased NO availability 9-11. Production of EDHF involves the activation of cytochrome P450 epoxygenase (CYP 2C9) which is mainly expressed within endothelial cells 9, 12, 13. CYP 2C9 generates the metabolites of arachidonic acid epoxyeicosatrienoic acids (EETs), which either initiate the endothelial cell hyperpolarization or are released from endothelial cells to stimulate potassium on vascular smooth cells 10, 12. It has been recently reported that EETs also possess fibrinolytic properties via the modulation of t-PA release 14. Additional experimental findings suggest that physiological concentrations of EETs increase t-PA expression in endothelial cells 15, while EETs
contribute to t-PA release from HUVEC, an effect inhibited by miconazole, a selective CYP 2C9 inhibitor 16. However, so far, a possible modulating effect of EDHF on endogenous fibrinolysis has not been evaluated in humans. Therefore, the first aim of the present study was to investigate the possible role of CYP 2C9-derived EDHF in the modulation of t-PA release in forearm microcirculation of normotensive subjects. In addition, since in essential hypertension a reduced NO availability and compensatory vasodilation to EDHF production is documented 13, the possible role of cytochrome P450 epoxygenase in modulating endothelial t-PA release in essential hypertensive patients was also assessed.
Methods
Subjects
The study population included 42 healthy male volunteers and 44 age matched male patients with essential hypertension. Patients were recruited from newly diagnosed cases in the outpatient clinic. Inclusion criteria were age between 30 and 60 years, and sitting blood pressure values (after 10 minutes of rest) between 140-90 and 160-99 mmHg, confirmed on two separate occasions within one month according to European guidelines 17. Exclusion criteria were dyslipidemia, diabetes mellitus, smoking, body mass index (BMI)> 30 Kg/m2, renal or liver impairment, and established cardiovascular disease other than essential hypertension. Secondary forms of hypertension were excluded by routine diagnostic procedures. Patients either were never treated for hypertension or they did not receive any medication for ≥1 month prior to enrolment in the study. The study protocol was approved by the local ethics committee and performed according to the guidelines of our institution. All patients were aware of the nature, purpose and potential risks of the study and gave their written informed consent to it.
The perfused-forearm model used in this study has been previously described in detail 4, 7. Briefly, intravenous catheters were placed in deep antecubital veins of each arms (experimental and contra-lateral forearm) and the brachial artery was cannulated for drug infusion at systemically ineffective rates and for intra-arterial blood pressure and heart rate monitoring. Forearm blood flow (FBF) was measured in both forearms by strain gauge venous plethysmography (EC-6, D.E. Hokanson Inc., Bellevue, WA, USA). Before FBF measurement, simultaneous arterial and venous samples were obtained from the infused arm before and after each dose of study drugs. Infusions were interrupted during arterial sampling. Plasma concentrations of t-PA antigen were determined by enzyme-linked immunosorbent assay (ELISA, Tecnoclone GmbH, Vien, Austria). All samples were assayed in duplicate on the same test plate. Details concerning the methods as performed in our laboratory, including sensitivity and reproducibility, have already been published 4.
Experimental Design
Contribution of Cytochrome P450 2C9-Derived Hyperpolarizing Factor to Bradykinin-mediated t-PA release in Normotensive Subjects and in Essential Hypertensive Patients.
In 22 normotensive subjects and 20 hypertensive patients t-PA release was estimated following an intra-arterial infusion of bradykinin (0.015 µ g/100mL/min). To assess the contribution of CYP 2C9-derived EDHF on endothelial t-PA release, the infusion of bradykinin was repeated in the presence of sulfaphenazole (0.03 µg/100 ml/min), a highly selective CYP 2C9 inhibitor 13.
Finally, to exclude the possible confounding effect of flow-increase, intra-arterial sodium nitroprusside (1.0 µg/100mL/min), a direct smooth muscle cell relaxant compound, was also infused.
Contribution of NO and Cytochrome P450 2C9-Derived Hyperpolarizing Factor to Acute t-PA Release in Normotensive Subjects and Essential Hypertensive Patients.
This series was designed to assess the effect of EDHF on stimulated t-PA release in the absence and in presence of the NO inhibition. Thus, in 21 normotensive subjects and 23 hypertensive patients bradykinin was administered during infusions of saline (0.2 ml/min), sulfaphenazole (0.03 µg/100 ml/min) or the NO synthase inhibitor NG-monomethyl-L-arginine (L-NMMA) (100 µg/100mL/ min), and during L-NMMA and sulfaphenazole co-infusions. Under L-NMMA, infusions were performed according to the NO-clamp technique, which allows assessment of endothelial agonists in the presence of NO-synthase blockade without change in basal blood flow, thus avoiding any perturbation that could alter net t-PA balance. Briefly, following 10 minutes of L-NMMA infusion, sodium nitroprusside was co-infused at an adjusted dose (0.3 and 0.4 µg/100mL/min) to neutralize the L-NMMA-induced vasoconstriction and restore baseline FBF, as previously described in detail 4, 18
.
Data analysis
Forearm plasma flow was determined by FBF and hematocrit. Net release or uptake rates for t-PA were calculated by the following formula: net release=(Cv-Ca) x [FBF x (101-hematocrit)/100], where Cv and Ca are the venous and arterial concentrations, respectively. Study population characteristics, basal venous, arterial, venous-arterial concentrations and t-PA balance at baseline were compared using the Student t-test or non-parametric test, depending on the results of test for normality. Responses to intra-arterial drugs were analyzed by one-way (infusion) and two-way (group and infusion) analysis of variance (ANOVA) for repeated measures, applying the Bonferroni post-hoc analysis. Results were expressed as mean±SD. Findings were considered statistically significant at p<0.05. Computations for the power calculation and for the statistical methods were performed using SPSS 15.0® statistics software. The present study was designed to have the 80% power at the 5% level to detect a 30% modification in fibrinolytic components release after drug infusion.
Drugs
Bradykinin, L-NMMA, sulfaphenazole (Clinalfa AG) and sodium nitroprusside (Malesci SpA) were obtained from commercially available sources and diluted to the desired concentration by addition of normal saline. Sodium nitroprusside was dissolved in 5% glucose solution and protected from light by aluminum foil.
Results
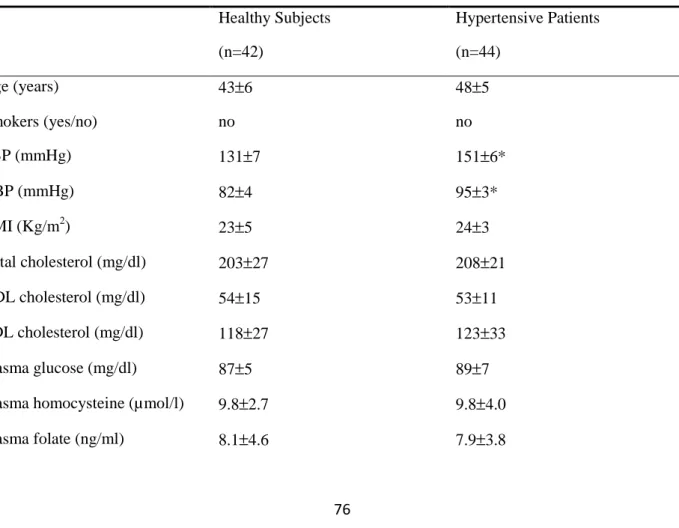
Clinical characteristics of the study population are shown in Table 1. According to exclusion criteria, groups were similar in characteristics except for systolic and diastolic blood pressure values, which were significantly higher in hypertensive group.
During intra-brachial drug infusion no change in intra-arterial blood pressure or heart rate was observed (data not shown).
Table 1. Clinical Characteristics of Study Group
Healthy Subjects (n=42) Hypertensive Patients (n=44) Age (years) 43±6 48±5 Smokers (yes/no) no no SBP (mmHg) 131±7 151±6* DBP (mmHg) 82±4 95±3* BMI (Kg/m2) 23±5 24±3 Total cholesterol (mg/dl) 203±27 208±21 HDL cholesterol (mg/dl) 54±15 53±11 LDL cholesterol (mg/dl) 118±27 123±33 Plasma glucose (mg/dl) 87±5 89±7
Plasma homocysteine (µmol/l) 9.8±2.7 9.8±4.0
Plasma vitamin B12 (pg/ml) 396.7±50.1 299.1±60.4
CRP (mg/dl) 3.1±0.9 2.8±0.8
Data are presented as mean value ± SD. SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; HDL: high density lipoprotein; LDL: low density lipoprotein; CRP: C Reactive Protein. *p<0.01 vs. healthy subjects.
Effects of Bradykinin on t-PA Release in Normotensive Subjects and Essential Hypertensive Patients
Vasodilation to bradykinin was significantly (p<0.05) higher in normotensive subjects as compared
to hypertensive patients (Figure 1).
Effect of bradykinin on forearm blood flow (FBF) in the presence of saline (open circles) or sulfaphenazole (solid circles) in normotensive subjects (A) and in essential hypertensive patients (B). Data are shown as mean ±SD. *P<0.01 vs. baseline.
At baseline, normotensive subjects showed higher arterial and venous concentration of t-PA as compared to hypertensive patients (Table 2). In normotensive group, venous concentrations of t-PA significantly increased during bradykinin infusion (Table 2). By contrast, in hypertensive patients no changes in t-PA venous concentrations were detected during bradykinin infusion (Table 2). Since arterial t-PA concentrations was not affected by drug infusion in any group, in normotensive subjects, but not in hypertensive patients, the venous concentration gradient of t-PA resulted significantly increased after bradykinin infusion (Table 2).
Table 2. Arterial (A), venous (V) and venous-arterial (V-A) concentrations gradient of t-PA at baseline and following the infusion of Bradykinin and Sodium Nitroprusside
Drug doses (µg/100ml tissue/min) Healthy Subjects (n=22) Hypertensive Patients (n=20) t-PA A t-PA V t-PA V-A t-PA A t-PA V t-PA V-A Baseline 1.31±0.35 1.36±0.35 0.05±0.06 0.72±0.12* 0.73±0.12* 0.01±0.03 BK (0.015) 1.33±0.36 1.46±0.38† 0.13±0.07† 0.73±0.11 0.79±0.13 0.06±0.02† Baseline 1.35±0.34 1.36±0.33 0.01±0.04 0.72±0.11* 0.73±0.18* 0.01±0.05 SNP (0.2) 1.34±0.35 1.35±0.32 0.01±0.06 0.73±0.11 0.74±0.11 0.01±0.04
Data are presented as the mean value ± SD. BK: Bradykinin; SNP : Sodium Nitroprusside. t-PA: tissue-type plasminogen activator. * p<0.05 vs. healthy subjects; † p<0.05 vs. baseline.
As a consequence, stimulated t-PA release was significantly (p<0.05) higher in healthy subjects (Figure 2A) compared to hypertensive patients (Figure 2B). Intra-brachial infusion of sodium nitroprusside, which induced a similar vasodilation in normotensive subjects (FBF from 2.8±0.5 to
12.1±1.8 mL/min/100 mL of forearm tissue) and in hypertensive patients (FBF from 2.9±0.7 to 12.8±2.1 mL/min/100 mL of forearm tissue) failed to induce any significant increase in both venous and venous-arterial concentration gradients of t-PA (Table 2). Therefore, no significant increase of t-PA balance was found in normotensive subjects (from 0.02±0.01 to 0.12±0.08 ng/min/100 mL of forearm tissue) and hypertensive patients (from 0.03±0.02 to 0.14±0.10 ng/min/100 mL of forearm tissue).
Effect of bradykinin on tissue-type plasminogen activator (t-PA) release in the presence of saline (open bars) or sulfaphenazole (solid bars) in normotensive subjects (A) and in essential hypertensive patients (B). Data are shown as mean ±SD. *P<0.01 vs. baseline; †P<0.01 vs. bradykinin plus saline.
Contribution of Cytochrome P450 2C9-Derived Hyperpolarizing Factor to Bradykinin-mediated t-PA release in Normotensive Subjects and in Essential Hypertensive Patients.
In both normotensive and hypertensive groups, sulfaphenazole pre-infusion did not significantly change basal FBF (normotensive group: from 2.9±0.8 to 3.0±0.8 ng/min/100 mL of forearm tissue; hypertensive group: from 3.2±1.2 to 3.3±1.4 ng/min/100 mL of forearm tissue). Sulfaphenazole infusion, while not affecting the endothelium-dependent relaxation in normotensive subjects (Figure 1A), significantly blunted the vasodilation to bradykinin in hypertensive patients (Figure 1B). Moreover, in normotensive subjects, sulfaphenazole which basally failed to affect t-PA release (from 0.10±0.02 to 0.18±0.05 ng/min/100 mL of forearm tissue), significantly reduced bradykinin-induced t-PA release (Figure 2A). A similar response was obtained in hypertensive patients, where sulfaphenazole did not significantly change basal t-PA release (from 0.13±0.3 to 0.16±0.05 ng/min/100mL forearm tissue), while it reduced the bradykinin-mediated t-PA release (Figure 2B).
Contribution of NO and Cytochrome P450 2C9-Derived Hyperpolarizing Factor to Acute t-PA Release in Normotensive Subjects and Essential Hypertensive Patients.
In this group, a higher (p<0.01) vasodilation to bradykinin in normotensive subjects as compared to
Effect of bradykinin on forearm blood flow (FBF) in the presence of saline, sulfaphenazole, L-NMMA and sulfaphenazole plus L-NMMA in normotensive subjects (A) and in essential hypertensive patients (B). Data are shown as mean ±SD. *P<0.01.
As expected, in normotensive subjects the vascular response to bradykinin was significantly reduced by L-NMMA (Figure 3A). In these subjects, sulfaphenazole administration, which did not significantly change basal FBF, was devoid of effect on response to bradykinin (Figure 3A). When L-NMMA was co-infused with sulfaphenazole, a further reduced response to bradykinin was observed (Figure 3A).
As expected, in hypertensive patients vasodilation to bradykinin was resistant to L-NMMA and significantly reduced by sulfaphenazole (Figure 3B). A similar reduced response to bradykinin was observed in the presence of L-NMMA and sulfaphenazole co-infusions (Figure 3B). The residual vasodilation to bradykinin under simultaneous L-NMMA and sulfaphenazole administrations was similar in both normotensive subjects (Figure 3A) and hypertensive patients (Figure 3B)
As already observed, in this group of normotensive subjects bradykinin-induced t-PA release was significantly (p<0.01) higher as compared to hypertensive patients (Figure 4). In this group of normotensive subjects, the presence of L-NMMA significantly decreased bradykinin-induced t-PA release (Figure 4A). Sulfaphenazole also significantly reduced the bradykinin-stimulated t-PA release (Figure 4A). When L-NMMA and sulfaphenazole were simultaneously infused, the bradykinin-induced t-PA release was almost abolished (Figure 4A). In hypertensive patients, sulfaphenazole, but not L-NMMA, significantly reduced bradykinin-induced t-PA release (Figure 4B). Finally, when simultaneously infused, L-NMMA and sulfaphenazole induced a reduction in
t-PA release similar to sulfaphenazole alone (Figure 4B).
Effect of bradykinin on tissue-type plasminogen activator (t-PA) release in the presence of saline, sulfaphenazole, L-NMMA and sulfaphenazole plus L-NMMA in normotensive subjects (A) and in essential hypertensive patients (B). Data are shown as mean ±SD. *P<0.01.
In both groups contra-lateral FBF and venous-arterial concentrations of t-PA unchanged throughout each protocol (data not shown).
Discussion
As previously reported11, 13, the present results confirm the presence of a blunted response to bradykinin in hypertensive subjects as compared to normotensive subjects. Our results also confirm that, while in healthy condition the response to bradykinin is sensitive to the NO-synthase inhibitor L-NMMA, in essential hypertension the residual vasodilation to the endothelial agonist is resistant to NO blockade but sensitive to sulfaphenazole, a specific inhibitor of CYP 2C9 13. It is of interest that the inhibitory effect of sulfaphenazole on vasodilation to bradykinin was detectable in normotensive subjects only when NO availability was abolished by concomitant L-NMMA administration. Therefore, these findings reinforce the concept that, while in healthy conditions the NO-pathway represents the main mechanism accounting for the vasodilation to bradykinin, under conditions characterized by impaired NO availability, including essential hypertension, an alternative acute compensatory pathway, possibly CYP 2C9-dependent, can be detected 13.
The major novelty of the present study is related to the mechanisms underlying bradykinin-stimulated t-PA release in humans. As previously reported 19, 20, bradykinin induces a significant increase of t-PA release in healthy subjects. This effect is specific and not flow-dependent, since it was not detected when in the same experimental conditions the infusion of the endothelium independent relaxing compound sodium nitroprusside was utilized. In addition, bradykinin-mediated t-PA release was found to be sensitive to L-NMMA, indicating a positive modulatory effect of the NO-pathway in physiological conditions, a finding in line with previous reports by applying different endothelial stimuli, such as substance P or acetylcholine 3, 4.
It is worth noting that in normotensive subjects, sulfaphenazole also significantly reduced bradykinin-induced t-PA release, indicating that a CYP 2C9 dependent pathway is physiologically
indicating that bradykinin-stimulation of CYP 2C9 is able to release EETs 10, 21, which in turn promote the induction of t-PA expression in endothelial cells15. Accordingly, in cultured human arterial endothelial cells, thrombin-induced t-PA release was found to be mediated by EETs 16. Of particular note was the finding that in the presence of simultaneous infusion of L-NMMA and sulfaphenazole, bradykinin-stimulated t-PA release was further reduced, demonstrating that the two antagonists act on different, but complimentary pathways.
In hypertensive patients bradykinin-induced t-PA release was impaired, a finding in line with a well documented reduction in endothelial fibrinolytic capacity in this clinical condition, previously demonstrated with different stimuli, including desmopressin22, substance P23 as well as acetylcholine4. It is however interesting to observe that in hypertensive patients, the residual, but still evident bradykinin-induced t-PA release, was totally resistant to L-NMMA, whereas it is blocked by sulfaphanazole. Finally, when L-NMMA was co-infused with sulfaphanazole, no further reduction in t-PA release was observed. Taken in conjunction, these findings suggest that in essential hypertension, the impairment of NO availability leads to a reduction in fibrinolytic capacity and the residual t-PA release depends on a CYP 2C9-related pathway, possible an EDHF identified with EETs 4, 22, 23. The present results demonstrate that in physiological conditions NO- and EDHFs-pathways appears to be equally involved in the modulation of stimulated t-PA release, while vascular responses seem to be almost exclusively mediated by NO. The different mechanism involved in vascular and fibrinolytic responses needs to be further explored. The possibility exists that smooth muscle cells, when stimulated by NO, are no longer sensitive to hyperpolarization while t-PA release is still sensitive to both pathways. This hypothesis is confirmed by the finding that when NO production is blocked by L-NMMA, the CYP 2C9-dependent pathway becomes detectable. Furthermore, in essential hypertension, and therefore in presence of impaired NO availability, a CYP 2C9-dependent pathway appears to operate as a “residual” mechanism responsible for endothelium-dependent vasodilation and modulation of fibrinolysis.
The present results may provide additional information concerning the pathways involved in the modulation of acute t-PA release in humans. Since, other than endothelium-dependent vasodilation 1
, also endothelial fibrinolytic capacity may predict the risk of future cardiovascular events 24, the comprehension of the pathways involved in the reduced fibrinolytic potential may add future insights for the determination of cardiovascular risk in essential hypertension
.
In addition, the restoration of endothelial fibrinolytic properties might become an adjunctive target of antihypertensive therapy. Thus, identification of the pathways that characterize impaired t-PA release can increase the knowledge on the pathophysiology of atherosclerotic disease and provide additional potential for the development of specific strategies to improve endothelial dysfunction.References
1. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363-368.
2. Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233-246.
3. Newby DE, Wright RA, Dawson P, Ludlam CA, Boon NA, Fox KA, Webb DJ. The L-arginine/nitric oxide pathway contributes to the acute release of tissue plasminogen activator in vivo in man. Cardiovasc Res. 1998;38:485-492.
4. Giannarelli C, De Negri F, Virdis A, Ghiadoni L, Cipriano A, Magagna A, Taddei S, Salvetti A. Nitric oxide modulates tissue plasminogen activator release in normotensive subjects and hypertensive patients. Hypertension. 2007;49:878-884.
5. Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468-1474.
6. Gilligan DM, Sack MN, Guetta V, Casino PR, Quyyumi AA, Rader DJ, Panza JA, Cannon RO, 3rd. Effect of antioxidant vitamins on low density lipoprotein oxidation and impaired endothelium-dependent vasodilation in patients with hypercholesterolemia. J Am Coll
Cardiol. 1994;24:1611-1617.
7. Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension.
Circulation. 1998;97:2222-2229.
8. Komori K, Vanhoutte PM. Endothelium-derived hyperpolarizing factor. Blood Vessels. 1990;27:238-245.
9. Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415-423.
10. Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666-671.
11. Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100:1400-1405. 12. Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res.
2004;49:525-533.
13. Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol. 2006;48:508-515.
14. Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433:413-420.
15. Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, Liao JK. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276:15983-15989.
16. Muldowney JA, 3rd, Painter CA, Sanders-Bush E, Brown NJ, Vaughan DE. Acute tissue-type plasminogen activator release in human microvascular endothelial cells: the roles of Galphaq, PLC-beta, IP3 and 5,6-epoxyeicosatrienoic acid. Thromb Haemost. 2007;97:263-271.
17. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053.
18. Virdis A, Ghiadoni L, Cardinal H, Favilla S, Duranti P, Birindelli R, Magagna A, Bernini G, Salvetti G, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. J Am Coll Cardiol. 2001;38:1106-1115.
19. Brown NJ, Gainer JV, Stein CM, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release in human vasculature. Hypertension. 1999;33:1431-1435.
20. Witherow FN, Dawson P, Ludlam CA, Webb DJ, Fox KA, Newby DE. Bradykinin receptor antagonism and endothelial tissue plasminogen activator release in humans. Arterioscler
Thromb Vasc Biol. 2003;23:1667-1670.
21. Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493-497.
22. Hrafnkelsdottir T, Wall U, Jern C, Jern S. Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet. 1998;352:1597-1598.
23. Ridderstrale W, Ulfhammer E, Jern S, Hrafnkelsdottir T. Impaired capacity for stimulated fibrinolysis in primary hypertension is restored by antihypertensive therapy. Hypertension. 2006;47:686-691.
24. Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler