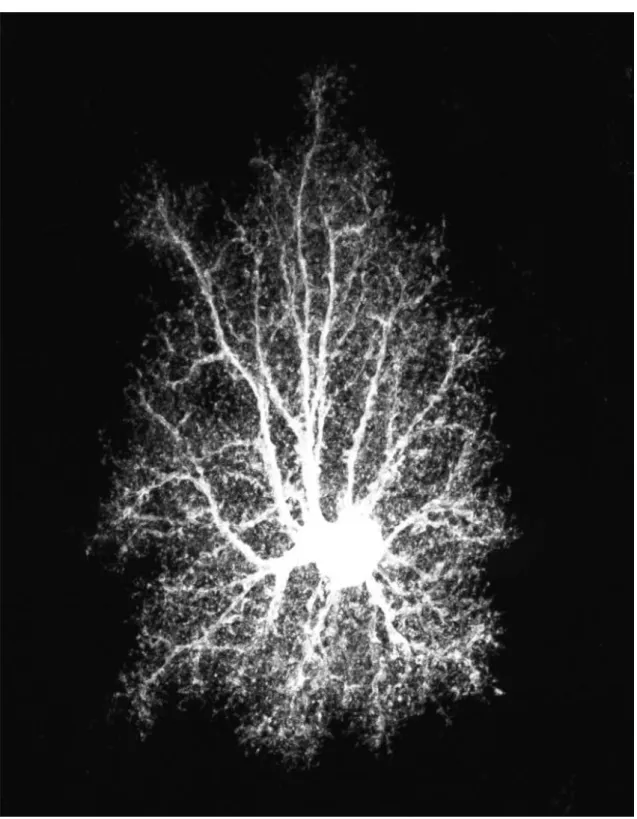
Figure 1: Stars (at last). A protoplasmic astrocyte from rat hippocampal area CA1 intracellularly injected with Lucifer Yellow and imaged by confocal microscopy. As-trocytes have been presumed for a long time to be star-shaped cells designed for the ‘brainless’ purpose of keeping neurons from bumping into one another. Experimental findings have shown instead that astrocytes could modulate synaptic transmission and neuronal excitability, hinting new starring roles for these cells in brain physiology. This possibility constitutes the premise of this study. Image courtesy of E. Bushong, The
Chapter 1
Introduction
Questioned on how the brain functions, most of us would probably think about neurons. With their long axonal projections, their rapid conduction velocity and their ability to fire action potentials, neurons have been considered for a long time the fundamental, if not the only cells playing a signalling role in the brain (Kandel et al., 2000; Ram´on y Cajal, 1906). On the contrary, glial cells (from Greek γλι α, “glue”) which in mammalian brain can be 10–50 times more numerous than neurons but do not exhibit electrical excitability, have merely been regarded as the supporting cast and scenery against which the starring neuronal roles would be played out (Kettenmann and Ransom, 2004). In the last two decades however, thanks to the development of new imaging techniques and patch-clamp recording in brain slices, this neurocentric view has substantially been debated by a large amount of seminal evidence which hinted that glial cells could be intimately involved in many of the brain’s functions, including its computational power (Haydon, 2001). A far-reaching implication of this renewed understanding of brain physiology is the notion that not only neurons and glial cells per se but also and most likely their reciprocal interactions (Ransom et al., 2003) could represent an additional tessera of the complex mosaic of the brain’s functions that provides the exquisite balance, subtlety and smoothness of operation for which nervous systems are held in awe.
Glial cell of the central nervous system (CNS) can be distinguished into four subtypes: microglia are the resident macrophages of the brain, oligodendrocytes serve a myelina-tion role, glial neuroprogenitors give rise to the neurons of the brain, and astrocytes, the focus of this study, regulate a variety of functions from homeostatic and supportive to trophic and metabolic ones, including neurotransmitter signaling and recycling, K+
homeostasis, synaptogenesis and axonal guidance and the control of blood flow (Tekk¨ok and Ransom, 2004). In addition, and probably at the origin of the current “Glia revolu-tion” (Kettenmann and Ransom, 1995), astrocytes are now regarded as active partners in synaptic transmission, both by sensing synaptic activity and reacting by elevations of their intracellular Ca2+ levels and by modulating synaptic activity itself through a
complex interplay of distinct feedback and feedforward mechanisms (Fellin et al., 2006b). As a major consequence of such a renovated role for astrocytes, the full understanding
of synaptic function is likely to be sought by coupling of astrocyte dynamics with synap-tic activity. In this regard, synapses are not bipartite but rather tripartite, as a third functional element that is the astrocyte, is part of their inherent constitution together with the presynaptic terminal and the postsynaptic neuron (Araque et al., 1999a). This aspect and his implications from the perspective of information processing in the brain, are the topic of this study.
1.1
Anatomy of neuron-glia interactions
The anatomic relationship between neurons and astrocytes dictates the opportunities for these cells to interact and sets limits on how they might do so. Although there exist some exceptions whose significance remains unknown (Alvarez-Maubecin et al., 2000; Milner et al., 1995), neurons and glial cells do not make functional synaptic or gap junctional contacts with one another. It follows that interactions between these cells must occur via the narrow separating extracellular space through movements of ions or other molecules released by both.
Astrocytes are often stellate cells with multiple fine processes, whose morphology and arrangement is probably non-casual (Chan-Ling and Stone, 1991). Processes of neigh-boring astrocytes minimally overlap (Bushong et al., 2002) and make contact through gap junctions (Giaume and McCarthy, 1996) whereas their somas distribute themselves in a non-random orderly fashion that is determined either by their morphology and by their “contact-spacing”, namely a form of astrocyte-astrocyte interaction dependent on their reciprocal positions (Chan-Ling and Stone, 1991). This arrangement is established in the early postnatal period in parallel with neuronal and vascular territories and allows each astrocyte to cover a specific territory that interfaces with the microvasculature and that might include thousands of synapses (Bushong et al., 2004, 2002).
Such a distribution of astrocytes in discrete territories although thought to be inde-pendent from the arrangement of neurons, seems to properly position them for special-ized local interactions. Synapses in the CNS in fact are often ensheathed by processes of astrocytes in the cortex (Ventura and Harris, 1999), Bergmann glia (specialized as-trocytes) in the cerebellum (Grosche et al., 1999) and M¨uller cells (astrocyte-like radial glia) in the retina (Newman, 2003b). Although these processes are highly polymorphic at first sight, volumetric reconstruction on electronic microscope data (on Bergmann glia), revealed that their geometry is probably not entirely irregular but rather has a modular constitution (Grosche et al., 2002, 1999). Such elemental modules, termed “glial microdomains”, are metabolically and electrically independent hinting a possible subcellular compartmentalization of neuron-astrocyte interactions. In addition, each mi-crodomain includes highly mobile expansions such as lamellipodia and filopodia whose morphology varies with synaptic activity and could account for highly dynamic interac-tions with the surrounding extracellular environment and therefore with the ensheathed synapses (Haber et al., 2006).
2000) and can differ both in terms of expression of ion channels or K+uptake capabilities
(Zhou and Kimelberg, 2000) and in terms of neurotransmitter receptors and transporters (Matthias et al., 2003; Zhou and Kimelberg, 2001). Such a rich diversity was suggested to account for the possible coexistence of different interactions with neuronal pathways that could take place either simultaneously and independently (Parpura, 2004).
1.2
Astrocyte calcium signalling
Although astrocytes are not electrically excitable cells, namely they cannot generate ac-tion potentials, they possess a form of chemical excitability based on variaac-tions of their intracellular calcium concentration (Cornell-Bell et al., 1990; Putney, 1993; Verkhratsky and Kettenmann, 1996). These Ca2+ variations can be transient or oscillatory and can
occur spontaneously (Hirase et al., 2004; Nett et al., 2002; Parri et al., 2001) or be in-duced by different means such as for example, by mechanical, electrical or chemical stim-ulation (Kettenmann and Schipke, 2004). In particular, astrocyte Ca2+ elevations can be
elicited in response to a variety of neurotransmitters and factors including glutamate, GABA, adrenaline, ATP, serotonin, acetylcholine, dopamine, nitric oxide (Bergmann glia) and BDNF1 (Volterra and Meldolesi, 2005). Such a response is mediated by
differ-ent receptors localized on the astrocyte plasma membrane (Porter and McCarthy, 1997) and is usually consistent with the mobilization of Ca2+ from the intracellular stores of
the endoplasmic reticulum2 (Finkbeiner, 1993).
The presence of receptors on astrocytes could be seen as the teleological evidence for neuron-to-glia signaling (Teichberg, 1991). In this regard, working on organotypic slice cultures of rat hippocampus, Dani et al. provided the first evidence that synaptically released glutamate could trigger Ca2+ elevations in astrocytes that are mediated by
mGlu receptors (Dani et al., 1992). The same phenomenon was following reported to occur in acute slices from both visual cortex and hippocampus (Pasti et al., 1997; Porter and McCarthy, 1996) and recently it was also demonstrated in vivo (Wang et al., 2006). Activation of astrocytes by neurons generally occurs through the spill-over of synaptically released neurotransmitter, however recent studies on cerebellar slices showed the existence of an alternative direct pathway of communication based on ectopic release (Matsui and Jahr, 2003). The coexistence of these two mechanisms at the same nerve terminal is likely to ensure simultaneous, but distinct communication both with the postsynaptic terminal and perisynaptic glial processes, providing additional evidence in favor of the effective existence of a tight coupling between neurons and glia (Matsui and Jahr, 2004).
The properties of Ca2+ oscillations generated in astrocytes such as their amplitude,
frequency and propagation, are governed by the intrinsic properties of both these cells
1Brain-derived neurotrophic factor.
2In the case of Bergmann glial cells, Ca2+ signalling is mediated by nitric oxide, consistent with a
Ca2+ influx from the extracellular space rather than with a release from cytoplasmic stores (Matyash
and those of neuronal inputs (Carmignoto, 2000; Zonta and Carmignoto, 2002).
Low-intensity synaptic stimulation and spontaneous astrocyte activity usually give rise to Ca2+ transients confined in small regions at the very end of distal processes
(Nett et al., 2002; Pasti et al., 1997; Porter and McCarthy, 1996) which in Bergamnn glia are also morphologically correlated with the microdomains observed in these cells (see Section 1.1 and Grosche et al., 1999). Such compartmentalization of Ca2+ signaling
supports the hypothesis that subcellular portions of astrocytes could operate as inde-pendent functional units (Perea and Araque, 2005a).
On the contrary, high-intensity synaptic activity or concurrent stimulation of several synapses, consistent with the activation of adjacent sites of the same astrocytic pro-cess, are generally associated with Ca2+ elevations that propagate along the process in
a form akin to that of regenerative Ca2+ waves (Zonta and Carmignoto, 2002). Such
waves can be either intracellular, when they remain confined within the same astrocyte, or intercellular when they propagate from one cell to neighboring astrocytes (Charles, 1998; Cornell-Bell et al., 1990; Stout et al., 2002). The mechanism of propagation of Ca2+ signalling although not yet fully understood, is likely to be due to an interplay
between diffusion of the second messenger inositol 1,4,5-trisphosphate (IP3) through gap
junctions coupling different astrocytes and release of ATP by these astrocyte themselves (Kettenmann and Schipke, 2004). In some cases, intercellular calcium waves can also travel between disconnected cells by means of purinergic (ATP) signalling (Hassinger et al., 1996; Newman, 2001).
The frequency of astrocytic Ca2+ oscillations is likely to encode the level of synaptic
activity (Carmignoto, 2000). Increases in frequency or intensity of synaptic stimulation result in fact in a corresponding increase in the frequency of Ca2+ oscillations (Pasti
et al., 1997). Notwithstanding, it is very likely that encoding of synaptic activity in the astrocyte Ca2+ signal could occur in a more complex fashion. Ca2+ oscillations indeed,
can be highly variable in amplitude (Cornell-Bell et al., 1990; Finkbeiner, 1993) and their dynamics does not simply reflect synaptic activity (Fellin et al., 2004; Perea and Araque, 2005a). In this regard recent experimental findings showed that hippocampal astrocytes in situ can discriminate among neuronal inputs of different origins, namely of different axon pathways, and can also integrate concomitant inputs (Perea and Araque, 2005b).
In spite of these large amount of evidences astrocyte Ca2+ increases evoked by
neu-ronal activity might be written off as a curious epiphenomenon of minor importance to brain function if it were not for the fact that they initiate additional astrocyte re-sponses. Perhaps the most significant of these Ca2+-dependent responses is the release
of gliotransmitters from astrocytes.
1.3
The “tripartite synapse” concept
The first evidence of Ca2+-dependent release of gliotransmitter from astrocytes is owed
as-trocytes can trigger release of glutamate (Parpura et al., 1994). This release pathway was following demonstrated not to be peculiar of the adopted cell culture system but also to exist in acute hippocampal (Bezzi et al., 1998; Pasti et al., 1997) and ventrobasal thalamic slices (Parri et al., 2001) and recently, in vivo too (Wang et al., 2006).
The mechanism by which glutamate is released remains unknown (Montana et al., 2006) however several lines of evidence indicate that it could occur through a Ca2+
-dependent exocytotic process (Bezzi et al., 1998, 2004; Zhang et al., 2004b). Such process, although similar to exocytosis of synaptic vesicles is likely to be functionally different from this latter. An accurate comparison is not yet possible but, in the case of astrocytes, glutamate vesicles seem to be fewer, their fusion significantly slower (Bezzi et al., 2004; Kreft et al., 2004) and the Ca2+ affinity of the release machinery higher than
at neuronal synapses, suggesting that the Ca2+-sensor of astrocytic exocytosis could be
different from the synaptic one (Kreft et al., 2004; Zhang et al., 2004a).
Glutamate released from astrocyte diffuses into the perisynaptic space reaching both synaptic terminals and affecting synaptic transmission and neuronal activity by means of a complex network of feedback and feedforward mechanisms.
Astrocytic glutamate can bind with presynaptic mGlu receptors which depress synap-tic release of neurotransmitter and consequently reduce the amplitude of action potential evoked postsynaptic currents (PSCs) (Araque et al., 1998a). In contrast, a fraction of this glutamate can also selectively activate presynaptic NMDA receptors (Araque et al., 1998b) which can account for a facilitation (i.e. a potentiation) of synaptic transmission either at excitatory (Jourdain et al., 2007) or inhibitory synapses (Kang et al., 1998).
At postsynaptic terminals instead, astrocytic glutamate binds with extrasynaptic NMDA receptors triggering slow inward (depolarizing) currents which can lead post-synaptic neurons to fire additional action potentials (Araque et al., 1999b; Hassinger et al., 1995). Noteworthy is the fact that such currents occur almost simultaneously in contiguous neurons belonging to the same astrocyte microdomain, thus suggesting that astrocytes could synchronize (Angulo et al., 2004; Fellin et al., 2004) the activities of neuronal circuits that are not directly connected to each others (Fellin et al., 2004; Volterra and Meldolesi, 2005).
Modulatory effects on synaptic transmission and neuronal excitability are also ob-served following the release of many other gliotransmitters (reviewed in Parpura, 2004) among which ATP and its derivative, adenosine seem to be predominant (Newman, 2003a; Pascual et al., 2005). Interestingly, purines and glutamate were found to have opposite effects on the same synaptic territory: inhibitory for purines (Zhang et al., 2003a) and stimulatory for glutamate (Fiacco and McCarthy, 2004). Astrocytes could therefore exert non-stereotyped, bimodal synaptic control through the release of these gliotransmitters. Moreover, the range of astrocyte-synapse interactions might be even more complex (Fellin et al., 2006a), given that both glutamate and purines have many receptors targets on either excitatory and inhibitory neurons, and astrocytes account for the bulk of glutamate uptake (Anderson and Swanson, 2000) at the synapse and regulate variations in the extracellular concentrations of K+ and H+ (Deitmer, 2004), .
and neurons gave birth to the concept that synapses are probably tripartite structures (Araque et al., 1999a). Therefore, synapses in the CNS are not merely constituted by a pre- and a postsynaptic terminal, but rather they include a third element, the astrocyte3,
which represents an integral modulatory component of synaptic transmission (Haydon, 2001).
The implications from a functional point of view of a revisiting of synaptic transmis-sion in terms of tripartite synapses are likely to be deep and not wholly appreciated at our current level of knowledge. Considering that a single astrocyte can cover ∼ 140 000 synapses (Bushong et al., 2002), and that each microdomain ensheathes an average of 3–5 synapses (Grosche et al., 1999), an astrocyte could be coupled with thousands of microdomains of tripartite synapses that could be independently modulated and/or syn-chronized by means of Ca2+ signalling and gliotransmitter release (Halassa et al., 2007).
Furthermore, differential synaptic stimulation could rise intercellular Ca2+ waves which
could modulate synaptic transmission over long distances by releasing gliotransmitter at synapses far from those that were initially active. This mechanism suggests an additional role for astrocytes as mediators of heterosynaptic lateral information transfer (Perea and Araque, 2005a). At last, synaptic modulation by astrocytes is probably coupled with blood flow and neural metabolism (Bernardinelli et al., 2004; Filosa et al., 2004; Zonta et al., 2003) providing a further extension for the multiple effects of neuron-astrocyte communication in the physiology of the brain.
Among all these possibilities however, we may recognize as a common denominator a novel fundamental role of astrocytes in information processing of the brain. Nonetheless it is difficult to imagine brain functions in terms of astrocytic signalling if we consider that the timescale of Ca2+ variations is slower than neuronal firing by six orders of
mag-nitude (Cornell-Bell et al., 1990) and the timescale of gliotransmitter modulatory effects at synapses ranges between hundreds of milliseconds (Fellin et al., 2004) and minutes or even tens of minutes (Araque et al., 1998a,b). Hence in order to address what roles astrocyte feedbacks on synaptic transmission could play as well as how they could influ-ence the neuronal code, we consider the case of a glutamatergic tripartite synapse only, which is currently the best characterized experimental frame in the context of neuron-glia interactions (Parpura, 2004). Accordingly, we will discuss and develop a novel biophysically-inspired mathematical model which could reproduce the essential features of neuron-astrocytes bidirectional signalling (Chapter 2). Successively we will adopt such model to explore the origin of rich astrocyte Ca2+ dynamics and how astrocytes could
encode synaptic activity (Chapter 3). Eventually we will perform a preliminary study of astrocyte feedback at the level of a single microdomain in order to quantify how it could affect the timing of action potentials fired by postsynaptic neurons (Chapters 4-5).