2
1. Introduction
Eyes are among the few identifiable organs of any vertebrate embryo during organogenesis. Not surprisingly the vertebrate eye has been the subject of developmental studies for more than a hundred years, being one of the organs that inspire Spemann’s theory of secondary induction (Martinez-Morales and Wittbrodt, 2009). Currently, the vertebrate eye is an excellent model system to study a broad range of developmental mechanisms from cell fate diversification of central nervous system to signaling cross-talk during embryogenesis.
1.1 Early development of vertebrate eye
The eye is a bilateral organ that originates from a single field positioned in the anterior neural plate. This primordium reaches its final complexity through a series of inductive and morphogenetic events coordinated by specific genetic programs which are largely conserved among different vertebrate species. There are at least three major events involved in the early development of the vertebrate eye: (1) a single eye field forms centrally within the anterior neural plate during gastrulation; it is characterized at the molecular level by the expression of “eye field transcription factors” (EFTFs); (2) the subdivision of the eye field into two optic vesicles, and the folding of the optic vesicle to form the optic cup and the optic stalks; (3) differentiation of optic cup components leads to the formation of a mature, functional eye. The morphological and molecular events underlying these processes are described in following sections.
1.1.1 Morphogenesis of eye development
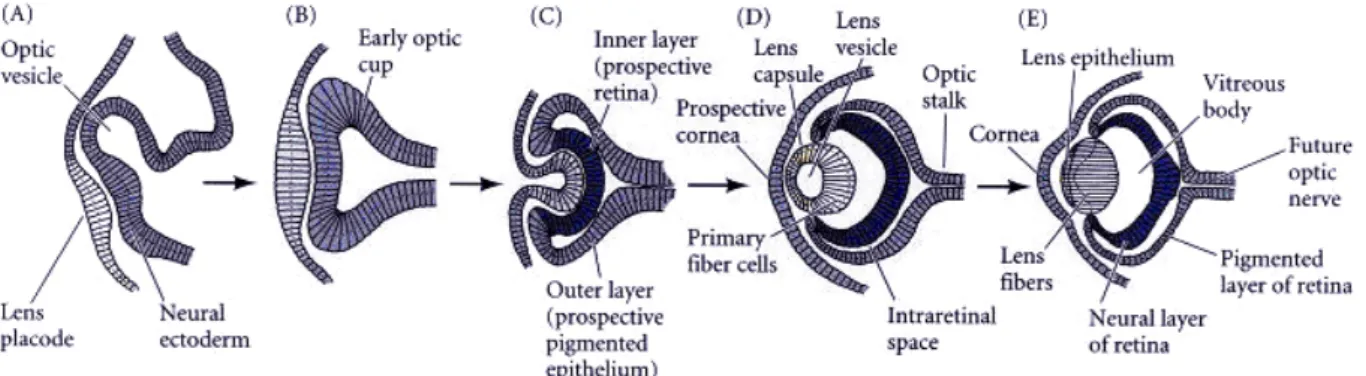
The eye is essentially a highly specialized extension of the brain. The first morphological sign of eye development in vertebrates is the bilateral evagination of the diencephalon in the early neurula (Fig.1.1A). In mammals, this is marked by the appearance of the optic pit, whereas in fish and amphibians a bulging of the optic primordia is observed. Continued evagination of the optic primordia leads to the formation of the optic vesicles. These extend towards the overlying, non-neural surface ectoderm that will ultimately give rise to the lens and cornea. Mesenchyme between the optic vesicle and the surface ectoderm (apparent in mammals and chick) is displaced as the two tissues come into close physical contact. This is a critical period in eye development during which inductive interactions between the optic vesicle and the surface ectoderm are thought to take place. At this stage, the presumptive lens also shows the first morphological signs of development. This is characterized by formation of the lens placode, a thickening of the surface ectoderm where it comes into contact with the optic vesicle (Fig.1.1A).
Coordinated invagination of the lens placode and of the optic vesicle results in the formation of the lens vesicle followed by a double-layered optic cup that provides the first indication of the final shape of the eye (Fig.1.1B,C). The cells of the inner layer proliferate rapidly and generate a variety of glia, ganglion cells, interneurons, and light-sensitive photoreceptor neurons. Collectively, these cells constitute the neural retina. The cells of the
3
outer layer produce melanin pigment and ultimately become the retinal pigment epithelium (Fig.1.1D). At the ventral extremity of the optic vesicle, the process of invagination forms a groove that runs continuously from the ventral-most region of the neural retina and along the ventral aspect of the optic stalk to the junction with the neural tube. The point at which the laterally growing edges of the optic cup fuse is known as the choroidal (or optic) fissure. This structure provides a channel for blood vessels within the eye and an exit route for projecting axons of retinal ganglion cells (Fig.1.1E).
Figure 1.1 Schematic diagram of vertebrate eye development. A: The optic vesicle evaginates from the brain and contacts the overlying ectoderm, inducing a lens placode (the prospective lens). B: The lens placode has thickened and the optic vesicle has formed an optic cup. C: Invagination of the optic vesicle results in formation of two layers, the prospective neural retina and the prospective pigmented epithelium. The central portion of the lens-forming ectoderm is internalized. D: The lens placode becomes the lens vesicle and the overall structure of the eye is established. E: The lens vesicle induces the overlying ectoderm to become the cornea, the lens consists of anterior epithelial cells and elongating posterior fiber cells. (adapted from Gilbert, 2000)
1.1.2 Specification of eye field by eye field transcription factors (EFTFs)
While the first morphological indication of eye development occurs with the bilateral evagination of the developing forebrain, the events involved in specifying eye formation begin earlier. It has been known for many years that a presumptive eye tissue (eye field) exists at the most anterior end of neural plate prior to the formation of optic vesicle. If these cells are removed from an amphibian embryo, the eyes do not form. If the eye field is transplanted to another location on the embryo or cultured in a dish, it forms eyes. When Xenopus anterior neural plate explants are isolated with underlying prechordal mesoderm at stage 12.5, two retinas form, demonstrating that the eye field is specified as early as late gastrula stages (stage 12.5) (Li et al., 1997).
But how is it that a specific region of neural ectoderm is informed that it will become the optic vesicle? Modern molecular evidence shows that the eye field is specified at the late gastrula stage when a group of transcription facotrs are expressed in the anterior neural plate, which are collectively called eye field transcription factors, EFTFs. In Xenopus, these EFTFs
4
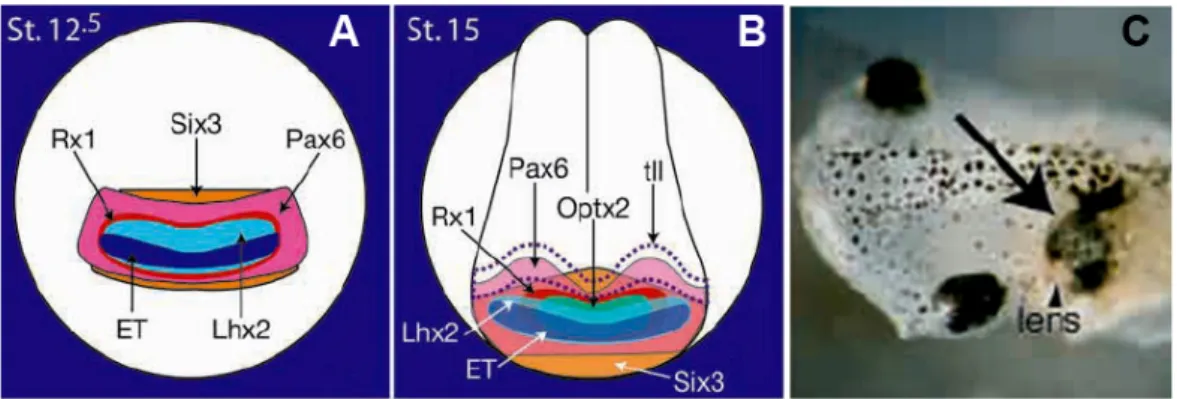
include ET, Rx1, Pax6, Six3, Lhx2, tll and Optx2 (also known as Six6) (Zuber et al., 2003). Double in situ hybridization revealed the unique, overlapping expression pattern of these EFTFs during the eye field specification and later at mid-neurula stage (Fig.1.2A,B, Zuber et al., 2003). Genetic evidence clearly demonstrates the importance of these EFTFs in vertebrate eye formation. Mutations of PAX6, SIX3 and OPTX2 in human result in malformations affecting the eyes (Wawersik and Maas, 2000). The targeted, or spontaneous mutation of Pax6, Rx, Lhx2, Tll,
Six3 and Six6 in mouse results in animals with abnormal or no eyes (Hill et al., 1991; Lagutin et
al., 2003; Li et al., 2002; Mathers et al., 1997; Porter et al., 1997; Tucker et al., 2001; Yu et al., 2000). Similar phenotypes have been observed when homologues of Six3, Pax6, tll, Rx1 and
Optx2 genes have been functionally inactivated in other vertebrate species (Carl et al., 2002;
Chow et al., 1999; Hollemann et al., 1998; Isaacs et al., 1999; Loosli et al., 2001; Zuber et al., 1999). Not only are these EFTFs necessary for eye formation, but in some contexts they are also sufficient. Overexpression of Pax6, Six3, Rx and Optx2 homologues can expand or induce eye tissues in the nervous system of vertebrates (Andreazzoli et al., 1999; Bernier et al., 2000; Chow et al., 1999; Chuang and Raymond, 2001; Loosli et al., 1999; Mathers et al., 1997; Oliver et al., 1996; Zuber et al., 1999). Overexpression of Pax6, Six3, Rx and Optx2 homologues can expand or induce eye tissues in the nervous system of vertebrates (Andreazzoli et al., 1999; Bernier et al., 2000; Chow et al., 1999; Chuang and Raymond, 2001; Loosli et al., 1999; Mathers et al., 1997; Oliver et al., 1996; Zuber et al., 1999). In Xenopus, coordinated overexpression of a cocktail of EFTFs plus Xotx2, which is a essential gene required for the establishment of presumptive forebrain, but not present in eye field (Andreazzoli et al., 1999; Kablar et al.,1996; Pannese et al., 1995), can generate a secondary eye field and ectopic eyes even outside the nervous system (Zuber et al., 2003) (Fig.1.2C). The relationship between neural induction and eye field specification will be discussed in more detail in Section 1.4.
During neurulation, one single eye field needs to separate into two retinal primordia upon the signals from underlying prechodal mesoderm, particularly, Sonic hedgehog (Shh). If the Shh gene is mutated, or if the processing of this protein is inhibited, the single median eye field will not split (Chiang et al. 1996). Consequently, the lack of Shh function results in cyclopia - a single eye in the center of the face (Roessler et al., 1996; Li et al.. 1997). It is thought that Shh protein from the prechordal plate suppresses Pax6 expression in the center of the embryo, dividing the field in two bilateral fields (Li et al. 1997; Roessler and Muenke, 2001). More recently, it was found in zebrafish that Six3 directly activates the Shh expression in the ventral midline, thereby converting the medial area of the eye field into ventral diencephalon (Geng et al., 2008).
5
Figure 1.2 Overlapping expression patterns of the eye field transcription factors at stage 12.5 (A) and stage 15 (B) are shown. (C) Embryo injected with a cocktail of mRNAs of Otx2, ET, Pax6,
Six3, Rx1, tll and Optx2, and grown to stage 45. Arrow indicates ectopic eye and arrowhead
points to lens. (adapted from Zuber et al., 2003)
1.1.3 Molecular mechanisms of vertebrate retina development 1.1.3.1 Patterning of the developing eye
The optic vesicle (OV) initially forms as an evagination of the developing forebrain, and then becomes patterned into distinct compartments. For example, the dorsal-distal region of the OV gives rise to the presumptive neural retina (NR), and the proximal region of the OV forms the optic stalk (OS) and the presumptive retinal pigmented epithelium (RPE). Thus, for proper eye development, regional specialization must occur in the optic vesicle according to the axial patterns, and the cells within the eye primordium need to acquire distinct positional cues specifying the anterior-posterior (A-P), dorsal-ventral (D-V) and medio-lateral axes. D-V specification in the early optic primodium appears to play crucial roles not only in vertebrate ocular morphogenesis, but also in establishment of the retinotectal projection. Although the precise mechanism of the determination of D-V polarity in the optic vesicle is unknown, studies in model organisms have established a network model involving transcription factors and extrinsic D-V signals(Fig.1.3).
It has been known that formation of the D-V polarity in the optic primordium is closely related to the expression of transcription factor genes, which are asymmetrically expressed and regionalize the optic vesicle into three main D-V compartments. From ventral to dorsal, these are: the OS, which expresses Pax2, Vax1 and Vax2, but not Pax6 and Tbx5; the ventral retina (VR), which expresses Vax2 and Pax6, but not Pax2, Vax1 or Tbx5; the dorsal retina (DR), which expresses Pax6 and Tbx5, but not Pax2, Vax1 and Vax2 (Lupo et al., 2005; Fig.1.3). Misexpression of these genes in early embryos or loss of function experiments in rodents results in various aberrant aspects of ocular development (Matsuo et al. 1993; Torres et al. 1996; Koshiba-Takeuchi et al. 2000; Zhang & Yang 2001). Coordinated expression patterns of the above genes along the two axes are required also for normal development of the retinotectal projection map, which is the formation of synapses between optic nerve axons and their appropriate targets in brain centers (Arvanitis and Davy, 2008).
6
transforming growth factor-β (TGF-β)/BMP4 and Wnts, whereas ventral identity is specified by Shh, and the anterior and lateral regions by FGF8 (Crossley et al. 2001; Lupo et al. 2006; Storm et al. 2006; Okada et al. 2008; Haworth et al. 2007). Retinoic acid (RA) has been implicated in regulating forebrain patterning by modulating BMP, Shh and FGF8 signaling. Deficiency of RA impairs FGF and SHH signaling (Halilagic et al. 2007; Ribes et al. 2006; Schneider et al. 2001).
These extraocularly localized signals as well as signals within the optic primordium have been shown to play an essential role also in OV development through their coordinated spatiotemporal and dose-dependent regulation, and perturbation of these signals results in aberrant polarization and subsequently failure of accurate eye formation (Lupo et al., 2005; Halilagic et al. 2007). Particularly, BMP4 is expressed in the dorsal region of the optic cup in the mouse embryo, and when mouse BMP4 is misexpressed in the ventral half of the optic cup in chick embryos, round eyes are formed with expansion of Tbx5 expression in the ventral half, and that expression of Vax and Pax2 is repressed (Koshiba-Takeuchi et al., 2000). Interaction of high levels of Shh and FGFR with low level of RA signaling repress Pax6 and induce the expression of Vax1, Pax2 and Vax2, leading to OS specification. In addition, high levels of RA repress the expression of Hh and FGFR signals, while Hh and FGFR signaling crossactivate each other at the transcriptional level (Lupo et al., 2005, see Fig. 1.3).
In a recent work in chick, Kobayashi and colleagues showed thatcoordinated regulation of BMP4 and Shh signaling specifies the polarity of the OV through FGF8 upregulation (Kobayashi et al., 2010). FGF8 expressed in the prospective NR, contributes continuously to neuronal differentiation. Shh signal induces ventral genes such as Pax2, Rx1 and Fgf8 and, at the same time, inhibits the dorsal gene Tbx5, while BMP4 maintains the expression of Tbx5,
Otx2 and Mitf. These expression patterns of genes in the outer and inner optic cup finally specify
RPE and NR domains. Another recent work in zebrafish showed that the knockdown of Zic1 in the neural midline causes increased RA signaling and results in the ventralization of the optic cup through the down-regulation of target genes of Shh and BMP (Maurus and Harris, 2009).
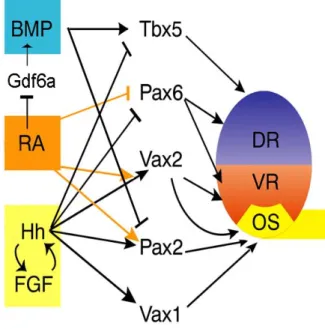
Figure 1.3 A proposed network model in the D-V patterning of the developing eye. Interaction of high levels of Hh and FGF with low level of RA signaling repress
Pax6 and Tbx5, and induce the expression
of Vax1, Pax2 and Vax2, leading to OS specification. Pax6, Tbx5 and Gdf6a expression is repressed due to elevated RA signaling. Gdf6a is a BMP ligand that functions upstream of other BMPs. DR: dorsal retina; VR: ventral retina; OS: optic stalk; RA: retinoic acid; Hh: Hedgehog. (Modified from Maurus and Harris, 2009)
7
1.1.3.2 Specification of retinal cell types
The different types of retinal neurons are organized in three laminae: the nuclei of rods and cones are in the outer nuclear layer (ONL), the nuclei of bipolar and amacrine cells (and those of another type of interneuron - horizontal cells) are in the inner nuclear layer (INL), and the innermost layer of the retina contains ganglion cells and their axons (ganglion cell layer, GCL). In addition to the neurons of the retina, there are several types of non-neuronal cells that are also critical for its function. The Müller cells are a type of glia that span the retinal epithelium and perform functions similar to astrocytes in other regions of the CNS. Adjacent to the photoreceptor layer is a layer of pigmented cells (PC), called the retinal pigmented epithelium (RPE), which are essential for the maintenance of rods and cones (Fig.1.4). During retinogenesis, these cell types are produced from a common population of retinal progenitor cells (RPCs) in an orderly fashion which is generally conserved in vertebrates (Cepko, 1999; Marquardt and Gruss, 2002; Hatakeyama and Kageyama, 2004). For instance, in the Xenopus retina, ganglion cells are always the first post-mitotic cell type, followed by horizontal cells, then cone photoreceptors, then rod photoreceptors, then amacrine cells, and bipolar cells and Müller glia are generated in the last phase of retinal neurogenesis (Wong and Rapaport, 2009). Recently, a lot of findings revealed by gene-targeting and misexpression strategies have shown that cell fate specification of RPCs is regulated by combinations of multiple transcription factors, especially basic helix-loop-helix (bHLH) and homeobox transcription factors, as summarized in Figure 1.5.
The successive production of differentiated neurons is supported by a continuous supply of RPCs. In mice, the expansion of the population of PRCs continues until after birth and proper proliferation is crucial for organization of retinal neurons in the correct numbers (Ohsawa and Kageyama, 2008). In this process, Hes1 and Hes5, the bHLH type repressors, are key regulators of the proliferation of RPCs. In Hes1-mutant embryos, precocious neurogenesis is observed in the retina as well as in other parts of the nervous system (Lee et al., 2005). Moreover, double mutations of Hes1 and Hes5 lead to more severe abnormalities in eye formation: the optic vesicles are lacking (Hatakeyama et al., 2004). Recent research showed that Hes1 and Hes5 function as targets of Notch signaling, a pathway that plays a key role in maintaining neural progenitor identity (Kageyama et al., 2005).
8
Figure 1.5 Specification of retinal cell fates by transcription factors. Three major divisions-GCL, INL, and ONL- give rise to seven retinal cell types that arise from common multipotent progenitors in a fixed order. The RGC is the first neuronal cell type and Müller glia appear last. Homeobox and bHLH transcription factors cooperate as intrinsic regulators to define the layer specificity and the neuronal cell fate. Hes1 inhibits neuronal differentiation and maintains progenitor fate. The cells that sustain Hes1/Hes5 expression during neurogenesis stages adopt the final available cell fate of Müller glial cells. The relative timing of cell appearance is for mouse development. (Adapted from Harada et al., 2007)
Generation of retinal neurons is also regulated by multiple transcription factors. Math5, is essential for the generation of retinal ganglion cells. Targeted disruption of Math5 leads to loss of more than 80% of ganglion cells (Brown et al., 2001; Wang et al., 2001). Also, introduction of Xath5, a Xenopus homologue of Math5, promotes retinal ganglion cell fate determination in
Figure 1.4 Histology of the vertebrate retina. The seven major cell types drawn on top of an actual histological section of retina. Amacrine, Müller, horizontal, and bipolar cells are located in the inner nuclear layer (INL). Rod and cone photoreceptors are located in the outer nuclear layer (ONL). The inner plexiform layer (IPL) and outer plexiform layer (OPL) form the synaptic layers. PC, pigmented cells; RPE, retinal pigmented epithelium; OS, outer segment of photoreceptors. (Adapted from Klassen et al., 2004b)
9
Xenopus retina (Kanekar et al., 1997). It has been shown that bipolar cell genesis is regulated by
two different classes of transcription factors, the bHLH genes Mash1 and Math3, and the homeobox gene Chx10 (Hatakeyama et al., 2001). Misexpression of Mash1 directs retinal progenitors towards the rod photoreceptor lineage. Although bipolar cell differentiation is delayed in Mash1-mutant retina, no apparent defect in cell fate specification is observed (Tomita et al., 1996b). However, in Mash1:Math3 compound mutants, bipolar cells are completely lost, and all cells that should normally become bipolar cells adopt a Müller glial cell fate, demonstrating that Mash1 and Math3 are required for bipolar cell genesis (Tomita et al., 2000).
Chx10 is expressed in dividing retinal progenitors, and this expression remains in differentiated
bipolar cells. Bipolar cells are completely missing in mice carrying a mutation that introduces a stop codon upstream of the Chx10 gene (Burmeister et al., 1996), indicating that Chx10 is required for bipolar cell genesis.
The bHLH transcription factor NeuroD is important for the formation of several retinal cell types and is essential for the survival of a subset of rod photoreceptors in mice (Morrow et al., 1999). Infection of a chick retina with a retrovirus overexpressing NeuroD generates a retina with three, instead of two, layers of photoreceptor cells (Yan et al., 1998). Expression of NeuroD, in turn, is regulated directly or indirectly by several other bHLH factors. For example, the bHLH protein Hes1 seems to be a negative regulator of NeuroD, and in Hes1-deficient mice retinal differentiation is accelerated, so that rods and horizontal cells appear prematurely and form abnormal rosette-like structures (Tomita et al., 1996a). It was also shown that misexpression of another bHLH gene, Hes6, in the developing retina promotes rod photoreceptor differentiation, through suppression of Hes1 function (Bae et al., 2000). Moreover, it has been shown that Math3 and NeuroD, expressed in retinal progenitors, are required for amacrine cell genesis (Hatakeyama et al., 2001; Inoue et al., 2002). Although misexpression of
Math3 or NeuroD alone fails to generate amacrine cells, misexpression of Pax6 together with Math3 or NeuroD promotes amacrine cell genesis, indicating that the combination of the bHLH
gene and the homeobox gene is critical for amacrine cell fate determination. Interestingly, while misexpression of Pax6 together with NeuroD exclusively promotes amacrine cell genesis, misexpression of Pax6 in combination with Math3 generates more horizontal cells than amacrine cells, indicating that the activity of Math3 is likely to promote horizontal cell fate over amacrine cell fate, and that the activity of Math3 and NeuroD are not exactly the same (Inoue et al., 2002).
Recent studies have further clarified the mechanism of amacrine cell genesis. The winged helix/forkhead transcription factor Foxn4 plays an essential role in generation of amacrine cells. In Foxn4-mutant retina, amacrine cells are greatly reduced in number, and horizontal cells are completely missing. Conversely, misexpression of Foxn4 into retinal explants using retroviral vectors efficiently generates amacrine cells (Li et al., 2004). It was also shown that Ptf1a is required for the generation of both amacrine cells and horizontal cells. Moreover, it has been shown that Ptf1a expression is completely lost in Foxn4- mutant retina, indicating that Ptf1a is located downstream of Foxn4 (Fujitani et al., 2006).
10
Photoreceptor cells are composed of two different populations - rod and cone photoreceptors. Recently, the molecular mechanisms underlying photoreceptor cell genesis have been at least partially clarified. The homeobox genes Crx and Otx2 are key molecules regulating photoreceptor cell development. Otx2 transactivates Crx and is required for photoreceptor cell fate determination, because deletion of Otx2 results in the conversion from photoreceptor cells to amacrine-like cells (Chen et al., 1997;Furukawa et al., 1997; Nishida et al., 2003). Likewise,
Crx-mutant retinas display defects in photoreceptor cell genesis (Furukawa et al., 1999). In Xenopus, Xotx5b, a Xenopus homologue of Crx, is expressed in both bipolar and photoreceptor
cells and selectively biases photoreceptor cell fate (Viczian et al., 2003). RaxL, a Rx gene identified in chicken, plays a role in the initiation of photoreceptor differentiation (Chen and Cepko, 2002). Rx-L, a second gene of Rx group isolated in Xenopus, functions mainly in photoreceptor cell determination (Wu et al., 2009).
Recent findings have provided an insight into molecular mechanism regulating cone and rod subtype decision. Nrl, a basic leucine zipper transcription factor, is preferentially expressed in rod photoreceptors, and it acts synergistically with Crx to regulate rhodopsin transcription (Chen et al., 1997). Targeted disruption of Nrl leads to the loss of rod cells and a transformation of rods into cone-like cells (Mears et al., 2001). In Nrl-mutant retina, expression of Nr2e3, an orphan nuclear receptor, is lost (Mears et al., 2001). Nr2e3 activates rod-specific genes but represses cone-specific genes in concert with Crx (Chen et al., 2004–2006; Peng et al., 2005). Therefore, Nrl acts as a “molecular switch” during rod development by directly activating Nr2e3.
In addition to the intrinsic transcription factors cascades outlined above, RPCs respond to extrinsic factors similar to those that stimulate proliferation of neural stem/progenitors elsewhere in the CNS, notably EGF, FGF, and Shh (see Yang, 2004 for review; see Wallace, 2008 for review of Shh). Components of the Notch pathway are also critical for the maintenance of the retinal progenitor pool (Nelson et al., 2007). More recently, a number of studies have indicated that non-protein-coding RNAs including microRNAs are prominently involved in retinal cell fate specification (Decembrini et al., 2006; Decembrini et al., 2009; see Rapicavoli and Blackshaw, 2009 for review), and increasing evidence suggests that asymmetric division and cell-cycle regulation, coupled with Notch and Shh signaling, both act to regulate cell diversification in the retina (see Cayouette et al., 2006; Andreazzoli, 2009 for review).
1.2 Xenopus laevis as a vertebrate model organism in developmental biology
Xenopus laevis, namely African clawed frogs, was in common use for human pregnancy
testing during the 1940’s and 1950’s, and was used in the very first embryological experiments.
Xenopus embryos remained the embryos of choice for experimental embryologists for many
decades. This species is currently a popularly used vertebrate organism in cell and molecular biology research.
11
As a well suited model system, the advantages of using Xenopus can be summarized as follows:
(1) Easy cultivation, external fertilization and embryonic development, large number of eggs available, and high tolerance for physical and pharmacological manipulation;
(2) Easy experimental manipulation by microinjection of RNA and antisense morpholino oligonucleotides to generate gain- and loss-of-function phenotypes;
(3) Relatively large embryo size allows microsurgical manipulations as tissue dissection/recombination from different regions of the embryos, explants heal readily after surgery and are easily cultivated in simple saline solutions, in which growth factors are easily added to evaluate inducing signals and cell competence;
(4) Accessibility of developing retina during whole retinogenesis, which enables extensive investigation of eye development from initial events to maturation. Indeed, proliferating retinal progenitors of the optic vesicle can be easily transfected by lipofection with the cDNA of interest. The effects of the lipofected gene on clonal size, cell fate and gene expression can then be analyzed at the end of retinogenesis;
(5) Extensive mapping of cell fate in early embryos, and an extensive knowledge of embryonic developmen. In our previous studies, we isolated and characterized genes involved in different aspects of retinal development, such as proliferation of precursor cells (Xrx1) (Andreazzoli et al., 2003; Casarosa et al., 2003), and differentiation of specific cell types (Xotx2, Xotx5b, Xbh1) (Viczian et al, 2003; Poggi et al. 2004). Moreover, we identified a “cocktail” of genes that is able to form an eye in non-competent ectoderm when overexpressed (Zuber et al., 2003);
(6) Tissue of the animal hemisphere (animal cap embryonic stem cells, ACES cells) of the
Xenopus blastula embryo represents a convenient system for both biochemical and
differentiation assays. Its cells are multipotent, and have been considered as stem cells. 1.2.2 Animal cap embryonic stem (ACES) cells
The blastula of amphibian embryos contains a blastocoel - a large cavity in the animal hemisphere. The lining of the blastocoelic roof is called the “animal cap” and consists of a few layers of ectodermal cells that are fated to develop into epidermis and neural tissues during normal development. However, these cells retain pluripotentiality as they express Xenopus Oct4 homologues (Morrison and Brickman, 2006), and upon exposure to specific inducers, the animal cap can differentiate into tissues of neuroectoderm (Lamb et al., 1993; Sasai et al., 1995), mesoderm (Kimelman and Kirschner, 1987; Slack et al., 1987) and endoderm (Sasai et al, 1996; Henry and Melton, 1998; Yasuo and Lemaire, 1999). In this sense, the cells of the animal cap are equivalent to mammalian embryonic stem cells, they thereby are named as animal cap embryonic stem (ACES) cells. In my project, a major advantage offered by ACES cells, compared to mouse embryonic stem cells, is the possibility to test a large number of gene combinations in a short time by a method of “animal cap assay”.
Figure 1.6 exemplifies a simple experimental method of animal cap assay, that can be used to estimate the inducing activity of soluble factors at the histological and molecular level.
12
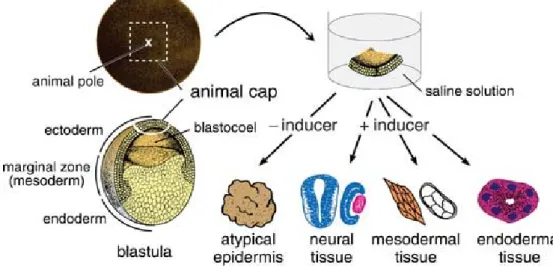
Cultured in saline for a few days, an animal cap excised from a Xenopus blastula forms atypical epidermis that shows no evident structural differentiation. If the saline contains a prospective mesodermal or neural inducer, however, respective mesodermal or neural tissue differentiation is observed in the cultured animal cap. Specific tissue differentiation can be identified by histological and molecular biological analysis. In this assay, the origin of the cells in induced tissues can be traced by the microinjection of fluorescent tracer into the animal hemisphere of the embryos at the two-cell stage. This in vitro induction systems are simple and useful for both identifying the inducibility of unknown extra- or intracellular factors and investigating the mechanisms of tissue induction in vivo (Okabayashi and Asashima, 2003).
Figure 1.6 Outline of the animal cap assay. An animal cap removed from a blastula is immersed in a saline solution that contains various concentrations of inducer. In the absence of inducer, the cap forms a cluster of epidermis, termed atypical epidermis. The differentiation of mesodermal tissues, such as the notochord and muscle, indicates the mesoderm-inducing activity of the inducer, whereas the differentiation of neural tissues, such as the brain and eyes, indicates the neural-inducing activity of the inducer. (Adapted from Ariizumi et al., 2009)
In the past decades, the animal cap of the Xenopus laevis embryo has been proven to be a versatile test tissue for a variety of molecules involved not only in animal development but also in cell regulation. Pivotally, in this project, taking advantage of the pluripotent nature of the ACES cells, we can test the ability of gene product(s) to transform animal caps into particular tissues or cell types by transplanting this tissue into the various regions of the embryo, thereby identify the factors required for tissue specification and in vitro expansion of the specific cell type which might be useful for application in cell replacement therapy.
1.3 Bone Morphogenetic Proteins (BMPs) and their antagonists
1.3.1 BMPs signaling
BMPs are a large family of secreted growth factors that belong to the transforming growth factor-β (TGF-β) superfamily. BMPs have been first isolated from bone extracts and display the ability to induce ectopic bone formation and to heal bone defects in an animal model (Reddi, 1998). However, it was later shown that BMPs function as multifunctional regulators in a
13
diverse array of developmental processes including cellular survival, proliferation, morphogenesis, lineage commitment, differentiation and apoptosis (see Botchkarev, 2003; Reddi, 2005; Miyazono et al., 2005; Walsh et al., 2010 for review). Specifically, during embryonic life, BMPs regulate neurogenesis and hematopoiesis and induce somite formation. In the limb bud, they interact with FGF4 and Shh, reducing limb bud expansion and inducing the formation of chondrocytes and osteoblasts precursors (Kishigami and Mishina, 2005). After birth, BMPs play a role in the maintenance of bone mass. They induce the differentiation of marrow stromal cells toward the osteoblastic lineage, therefore increasing the pool of mature bone forming cells, and enhance the differentiated function of osteoblasts mediated by the activation of specific BMP membrane receptors and signaling (Miyazono et al., 2005).
To date, over 20 BMP family members have been identified and characterized (Gazzerro and Canalis, 2006). BMP1 through BMP7 are expressed in skeletal tissue, and BMP2, -4 and -6 are the most readily detectable BMPs in osteoblast cultures. Studies from transgenic and knockout mice and from animals and humans with naturally occurring mutations in BMPs and related genes have shown that BMP signaling plays critical roles in heart, neural tissue and cartilage development. BMPs also play an important role in postnatal bone formation. BMP activities are regulated at different molecular levels. Preclinical and clinical studies have shown that BMP2 can be utilized in various therapeutic interventions such as bone defects, non-union fractures, spinal fusion, osteoporosis and root canal surgery (Chen et al., 2004).
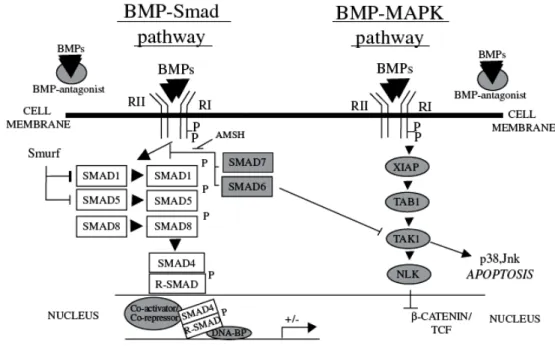
BMP signals are mediated by type I and type II serine/threonine kinase receptors. Upon ligand binding, the type II receptor forms a heterodimer with the type I receptor, and the constitutive kinase of the type II activates the type I receptor. This initiates the transmission of the intracellular signal through “canonical” and “non-canonical” BMP pathways by phosphorylating downstream cytosolic factors, which then translocate to the nucleus to activate or inhibit transcription (Miyazono et al., 2005, Fig.1.7). In canonical BMP-Smad pathway, signal transduction studies have revealed that SMAD1, 5 and 8 are the immediate downstream molecules of BMP receptors and play a central role in BMP signal transduction. There are three classes of SMADs: 1) receptor regulated SMADs (R-SMADs) that can be BMP activated, such as SMAD1, -5 and -8 or TGF-β activated, such as SMAD2 and -3; 2) common BMP and TGF-β mediator SMADs (Co-SMADs), such as SMAD4; and 3) inhibitory SMADs (I-SMADs), such as SMAD6 and -7. Following BMP receptor activation, SMAD1/5/8 are carboxy-terminally phosphorylated and translocate into the nucleus after heterodimerization with SMAD4. In the nucleus, the complex can bind to DNA sequences directly, or interact with transcription factors such as Runx-2, Menin, or Hoxc8 (Miyazono et al., 2005).
It is generally considered that the earliest requirement for BMP signaling in the developing organism is patterning of cell fates along the embryonic D-V axis in both vertebrates and invertebrates by evolutionary conserved pathway (De Robertis and Kuroda, 2004). In addition, it has been found BMPs, initially expressed throughout the ectoderm, are sufficient to repress neural fate and induce formation of epidermis. It has also been found that some neural-inducing molecules, which bind to BMPs as antagonists and prevent them from activating their receptors,
14
inhibit BMP signaling causing cells to adopt neural fates (see (Munoz-Sanjuan and Brivanlou, 2002); De Robertis and Kuroda, 2004 for review). This will be described in detail in the next section.
Figure 1.7 Schematic representation of the “canonical” BMP-Smad pathway and “non-canonical” BMP-MAPK pathway. BMP interactions with BMPR complex are regulated by diffusible BMP antagonists that prevent BMP binding to BMP receptors. Binding of the free BMP to BMP receptor complex activate either BMP-Smad or BMP-MAPK signal transduction pathways. The BMP-Smad pathway includes recruitment and phosphorylation of R-Smad followed by the formation of their complexes with Co-Smad and translocation into the nucleus to regulate gene transcription. BMP-MAPK pathway via recruitment of XIAP and TAB1 links BMP receptors with TAK1 kinase, which in turn may activate apoptosis via p38/Jnk pathway. (Adapted from Botchkarev, 2003)
1.3.2 BMP antagonists and neural induction
Neural induction constitutes the initial step in the generation of the vertebrate nervous system. In vertebrates, the primordium of neural tissue is derived from uncommitted ectoderm, which is also the source of the epidermis (skin). Neural induction was firstly discovered by the German embryologist Hans Spemann more than 90 years ago. Spemann’s experiments with newt embryos demonstrated that, when transplanted at the gastrula stage to the ventral side of a recipient embryo, a specific embryonic structure known as the dorsal blastopore lip, so-called Spemann organizer, could induce the development of a second central nervous system from the ventral ectoderm, which normally differentiates into the epidermis (Zaraisky, 2007). Since then, a large body of work mainly in frog and fish, has focused on finding out the signals that can direct prospective epidermis to adopt neural fates. Many evidences pointed to the BMP signaling pathway as a key for neural induction, but as a negative regulator, not a positive inducer. As described in the “default model” of neural induction, the activity of BMPs initially present in the embryonic ectoderm stimulates the formation of epidermis, whilst suppression of BMP signaling
15
extracellularly by their antagonists switches on the differentiation program of neural fate by default. In Xenopus, using expression screening techniques to identify novel genes that induce a neural fate in animal cap ectodermal explants, secreted antagonists of BMPs were discovered. These proteins are secreted from the organizer, such as Noggin (Lamb et al., 1993), Chordin (Sasai et al., 1994), Follistatin (Hemmati-Brivanlou et al., 1994), Cerberus (Bouwmeester et al., 1996), and XNr3 (Smith et al., 1995), and bind to BMPs in the extracellular space with high affinity and prevent activation of their receptors, causing cells to adopt neural fates. When expression of one of these secreted proteins in the organizer is blocked, BMP signaling is maintained on the dorsal side of the embryo and the neural plate does not form (Wessely et al., 2004).
Much of the knowledge of these BMP binding proteins derives from studies conducted in
Xenopus laevis, where these peptides, by opposing BMP ventralizing effects, induce
dorsalization of the embryos and neural induction. The protein sequence of the extracellular BMP antagonists is characterized by an array of evolutionary conserved cysteine-rich (CR) domains consistent with the formation of cystine knot structures. Cystine knots are functional motifs, which determine folding of the peptide and exposure of specific hydrophobic residues, facilitating diverse protein-protein interactions.
Noggin is the first neural inducer isolated from Spemann organizer and is identified as a dorsalizing factor based on rescue of dorsal development in ultraviolet-induced ventralized embryos (Smith et al., 1993). In Xenopus, injection of the putative cloned RNA into embryos resulted in large heads, hence named as Noggin (big head). Furthermore, exposure of animal cap explants to Noggin protein induces the formation of anterior neural structures in the absence of mesoderm (Lamb et al., 1993).Noggin is secreted as a glycosylated protein from the Spemann organizer and antagonizes the action of BMPs, induces neural tissue and dorsalizes ventral mesoderm. The primary structure of Noggin consists of an acidic amino-terminal region and a CR carboxy-terminal region containing a cystine knot. A central, highly basic heparin-binding segment retains Noggin at the cell surface (Gazzerro and Canalis, 2006). Noggin binds with various degrees of affinity BMP2, -4, -5, -6 and -7, GDF5, -6 and vegetally localized protein (Vg)-1, but not other members of the TGF-β family of peptides (Zimmerman et al., 1996). The crystal structure of Noggin bound to BMP7 shows that Noggin inhibits BMP signaling by blocking the molecular interfaces of the binding epitopes for both type I and type II receptors (Groppe et al., 2002). In mouse, homozygous null mutations of the noggin gene result in serious developmental abnormalities including failure of neural tube formation, dismorphogenesis of the axial skeleton and joint lesions (McMahon et al., 1998; Wijgerde et al., 2003). The embryonic lethality of noggin null mice has not allowed the definition of the noggin role in adult. The importance of the noggin gene was confirmed by human studies demonstrating that different heterozygous missense mutations of noggin coding sequence lead to proximal symphalangism and multiple synostosis syndrome (Gong et al., 1999; Marcelino et al., 2001).
During the course of investigations on Xenopus pattern formation, Chordin, another extensively studied BMP antagonist, was identified as one of neural inducers (Sasai et al., 1995).
16
Chordin has a homolog in Drosophila, short gastrulation (sog), known to bind to decapentaplegic, a BMP2/4 homolog. These BMP antagonists have thus been conserved for several million years (Reddi, 2005). Chordin is secreted as a glycosylated homodimer and is characterized by four CR domains, which are the sites of interaction with BMPs. Chordin binds specifically BMP2, -4 and -7 and does not bind other members of the TGF-β superfamily (Piccolo et al., 1996). Chordin is further regulated by delicate interactions with other secreted proteins of the extracellular matrix. The Chordin-BMP complex is a substrate for the zinc metalloprotease BMP-1/tolloid, which cleaves Chordin inactivating its biological activity and releasing free BMPs to the extracellular space (Piccolo et al., 1997). Chordin null mutations result in stillborn mice, that have normal early development and neural induction but display later defects in pharyngeal and cardiovascular organization. Double mutant noggin/chordin mice display defects in the development of the forebrain, eye and facial structures, and exhibit disrupted mesoderm development and left to right patterning (Bachiller et al., 2000). This indicates that Chordin and Noggin are required for the proper establishment of body axes in the mouse embryo.
In addition, various intracellular factors can act as antagonists of the BMP cascade in embryonic ectoderm. Among these factors, inhibitor Smads (I-Smads) play an important role. Smad6 and -7 bind to type I BMP receptors, interfering with Smad1/5/8 phosphorylation and heterodimerization with Smad 4 (Fig.1.8, Hata et al., 1998; Nakao et al., 1997). Elevated expression of the Smad7 gene induces neuralization of the embryonic ectoderm even in the absence of BMP antagonists such as Noggin and Chordin (Bhushan et al., 1998).
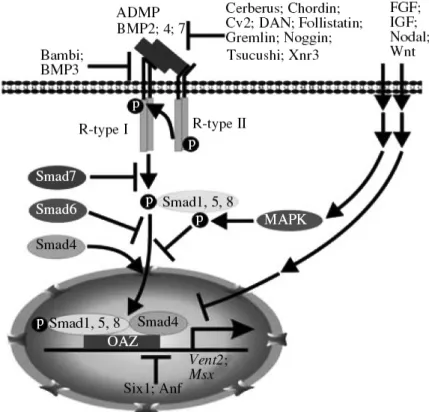
Although the interplay between BMPs and their antagonists is currently considered as being central to neural induction, more pathways and complexities are implicated in neural induction. In the last decade, one of the important achievements in understanding the mechanism of neural induction is the elucidation of the role of FGF and Wnt signaling via their interactions with the BMP cascade (Zaraisky, 2007). Now, it is clear that neural induction is regulated by integration of multiple signaling pathways at the level of Smad1 phosphorylations (Fig.1.8). It has been demonstrated that Smad1 is a target of mitogen-activated protein kinases (MAPK) activated by FGF and IGF through epidermal growth factor receptor (EGFR) ((Pera et al., 2003)), and it is also a target of glycogen synthase kinase (GSK3) activated by inhibition of Wnt (Fuentealba et al., 2007). These two kinds of phosphorylations by MAPK and GSK3 take place in the linker region of Smad1 instead of carboxy-terminus of Smad1 by BMP receptors, causing Smad1 exported from nucleus and subjected to proteasomal degradation in cytoplasm (Eivers et al., 2008). At the same time, experiments with chicken embryos showed, indirectly, that neural induction of the embryonic ectoderm can be triggered by some other mechanisms, unrelated to regulation of the BMP cascade, like FGF, casting doubt on “default model” of neural induction (Stern, 2005). However, recent studies conducted in Xenopus deeply investigated whether the requirement of FGF in neural induction is independent of BMP inhibition. Marchal and colleagues reported that BMP inhibition and FGF signaling are both important, and act sequentially. In particularly, BMP inhibition using anti-morphic form of
17
Smad5 initiate neural induction and directly activate FGF4 expression, while both normal and ectopic neural induction by the anti-morphic form of Smad5 require FGF4 activity induced by BMP inhibition. If FGF activity is blocked, neural induction is antagonized but BMP inhibition is still elevated as shown by repression of XK81 (a marker of epidermis). Therefore, consistent with default model, BMP inhibition is necessary and sufficient to initiate neural induction via activation of FGF signaling (Marchal et al., 2009).
Figure 1.8 BMP signaling cascade and mechanisms of its inhibition in neural induction. The main known proteins involved in the BMP cascade are shown. The pathways modulating the effect of the FGF, IGF, Nodal, and Wnt signaling cascades on the BMP cascade involve activation of MAP kinase with subsequent phosphorylation of Smad1 in its liker region, and direct inhibition of the genes activated by the BMP cascade. (Adapt from Zaraisky, 2007)
1.4 Neural induction and eye field specification
Since the eye is a highly specialized derivative of the central nervous system, eye development is closely associated with neural induction. Neuralized ectodermal explants of
Xenopus embryos at blastula stage give rise to anterior neural structures including eyes. Since
the potential inductive influence of mesoderm or endoderm is absent in these neuralized ectodermal explants, this result demonstrates that the molecular mechanisms directing eye specification lie downstream of neural induction and are inherent to developing anterior neural tissue (Chow and Lang, 2001). In addition, experimental evidences suggest that neural induction is initiated during gastrulation and the eye field is specified to some degree by the midgastrula stage of development (Lupo et al., 2002). Therefore, neural induction and eye development occur sequentially.
18
Before any morphological signs of an optic vesicle can be observed, eye field is formed as part of anterior neural plate and destined to give rise to the optic cup and its derivatives. Several transcription factors that pattern the anterior neural plate such as Otx2, Pax6, Rx1, Six3 and Optx2 are essential for the initiation of eye development. In Xenopus, these genes are activated almost simultaneously in a partially overlapping domain in the anterior neural plate (Lupo et al., 2000; Zuber et al., 2003).
1.4.1 Noggin and eye field transcription factors (EFTFs)
After neural induction has begun, Otx2, a homeobox gene, is strongly expressed in the the presumptive anterior neural plate (around stage 11 in Xenopus). Otx2 is required for the formation of the anterior neural region, as mice lacking Otx2 function form neither forebrain nor midbrain (Acampora et al., 1995). The role of Otx2 in the specification of retinal progenitors is not known, but Otx2 is likely to play a permissive rather than an instructive role in the formation of eye field, as its activity is suppressed in the center of the presumptive eye field, possibly by the Rx protein (Andreazzoli et al., 1999; Zuber et al., 2003). However, Otx2 expression remains in the periphery of this field. This differential inactivation of Otx2 is of functional significance, as the center of the eye field develops into the neuroretina, while the periphery of the eye field develops into the retinal pigment epithelium (Martinez-Morales et al., 2003).
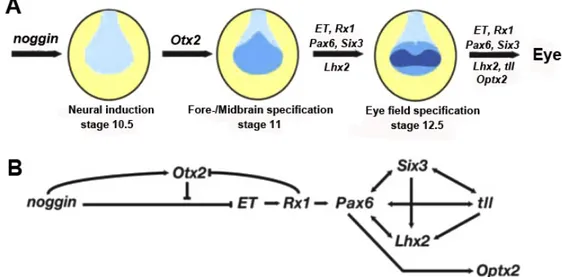
In Xenopus, at stage 12.5, Otx2 expression is significantly down-regulated in the medial region of presumptive anterior neural plate demarcating a area where expression of a suite of genes commonly called eye field transcription factors (EFTFs) begins to specify the eye field (Zuber et al., 2003). These EFTFs include ET, Rx1, Pax6, Six3, Lhx2, tll and Optx2 (Zuber et al., 2003). The EFTFs are a group of genes defined by their involvement in early eye formation. As their name implies, all are transcription factors and expressed in the eye field of the developing embryo from which the eyes form. They have been evolutionarily conserved and genetic evidence demonstrates they are required for normal eye formation (described above in Sections 1.1.2), since their mutation results in the malformation or a lack of eyes in species as diverse as flies and humans (Zuber, 2010). These EFTFs are expressed in a dynamic, overlapping pattern in the presumptive eye field and also form a functional network that is not only required, but also sufficient for eye formation.
Figure 1.9A shows a simplistic model of eye field specification in the anterior neural plate of the frog embryo. Neural induction (initiated by factors including Noggin) results in an only partially patterned neural plate. Neural patterning genes like Otx2 then specify different neural regions. The early-expressed EFTFs (ET, Rx, Pax6, Six3, and Lhx2) act coordinately to specify the eye field. Although not required for the initial specification of the eye field, evidence suggests tll and Optx2 play later roles in eye formation. Evidence for some, but not all, aspects of this predicted network are found in other species (Zuber, 2010).
Expression of an EFTF cocktail with Otx2 is sufficient to induce ectopic eyes outside the nervous system at high frequency (Zuber et al., 2003). Using both cocktail subsets and functional (inductive) analysis of individual EFTFs, a genetic network regulating vertebrate eye field specification has been revealed (Fig.1.9B). The results support a model of progressive
19
tissue specification in which neural induction then Otx2-driven neural patterning primes the anterior neural plate for eye field formation. Next, the EFTFs form a self-regulating feedback network that specifies the vertebrate eye field. Striking similarities and differences are found in the network of homologous Drosophila genes that specify the eye imaginal disc, a finding that is consistent with the idea of a partial evolutionary conservation of eye formation (Zuber, 2003).
Using animal cap assay, it has been shown that noggin ectopic expression in animal cap explants dramatically induces the expression of most of EFTFs and the expression of Otx2 (Zeber et al., 2003). In the gene regulatory network exemplified in Figure 1.9B, noggin is therefore positioned in the head of this hierarchy and positively regulates EFTFs except for ET, but this inhibition of ET by noggin overexpression can be rescued by Otx2 overexpression (Zuber et al., 2003). As a neural inducer, noggin probably provides a neutralized environment allowing the expression of EFTFs. These observations lead us to propose that noggin overexpression alone can mimic the ectopic eye field induction effect as coordinated expression of EFTFs and Otx2 in the Xenopus embryos. Thus, we started our project to investigate this hypothesis.
Figure 1.9 Neural induction and eye field specification in Xenopus. A: Relative timing of gene expression in the eye field specification in the anterior neural plate. The initial neural plate is patterned to form fate-restricted areas (noggin expression and region of neural induction are in grey). Otx2 expression is required for patterning of the early fore- and midbrain (light-blue represents the specification of fore/midbrain by Otx2 expression). Finally, the expression of EFTFs specifies the eye field (in dark-blue). B: Epigenetic interactions among the eye field transcription factors defining a genetic circuit during eye field formation. All these interactions have been studied by animal cap assay. Arrows and bars indicate the induction and repression of target gene expression, respectively. (Adapted from Zuber et al., 2003)
1.5 Retinal degenerative disease and cell replacement therapy
For humans, the visual system is the most important sense for acquiring external information. Thus, “quality of vision” is intimately tied with “quality of life” (Harada et al., 2007). However, like other regions of the nervous system, the retina is subject to many acquired
20
and inherited neuronal degenerative diseases. For example, age-related macular degeneration (AMD) is a disease of the retina characterized by the degeneration of cells in the RPE and of the photoreceptors in the central neural retina. In addition, a number of other conditions lead to degeneration of retinal neurons. Glaucoma and diabetic retinopathy also lead to neuron death in the retina; the former results in loss of ganglion cells, while in the latter the degeneration encompasses other neuronal types as well. There are also a number of inherited retinal disorders that lead to either degeneration of retinal neurons: these include Usher syndrome, Retinitis pigmentosa (RP), and Leber congenital amaurosis (Lamba et al., 2008). The loss of retinal cells results in vision impairment and potentially complete blindness. In addition, many of these degenerations begin in one cell type, only to later involve others. In AMD, for example, the pigmented epithelial cells in the RPE are the first to degenerate, but cones die soon after. In RP, it has also been observed that the mutations that cause this disorder lead to degeneration of rod photoreceptors initially, with a consequent loss in night vision, successively cone photoreceptors are affected as well, and individuals can progress to total blindness (Lamba et al., 2008).
In non-mammalian vertebrates, such as amphibians and fish, they have robust endogenous regenerative responses to injury, which can lead to the near complete restoration of the neurons lost through injury. However, in humans, there seems to be little or no recovery of lost cells (Karl and Reh, 2010). Despite extensive clinical and laboratory investigations, many diseases that impair vision lack effective treatments. The lack of an endogenous repair mechanism in the mammalian retina has thus stimulated a number of groups to explore the possibility of neuronal replacement to restore retinal function following degeneration. In 1998, Takashi et al. found that hippocampus-derived neural progenitor cells from adult rats could form a uniform layer in the adult rat eye, expressing markers of developing neurons. However, the cells did not eventually express retinal neuron markers, indicating incomplete retina formation (Takahashi et al., 1998). In 2004, Klassen and colleagues isolated retinal progenitor cells from postnatal day-1 mice and grafted these cells into degenerative retina of mature mice, and they found some improvement in light-mediated behavior of adults with retinal grafts, indicating that the neurons regenerated the retina to some extent (Klassen et al., 2004a). Two years later, MacLaren et al. showed that the committed precursors of rod photoreceptors beginning to express Nrl can be effectively incorporated in and contribute to visual function of a degenerating retina (MacLaren et al., 2006). In the same year, Lamba and colleagues showed that embryonic stem cells (ESCs) could be directed to retinal cell types, including photoreceptors expressing specific markers (Lamba et al., 2006). More recently, Lamba and colleagues showed that retinal cells derived from human ESCs will migrate into mouse retinas following intra-ocular injection, settle into the appropriate layers and express markers for differentiated cells. After transplantation of the cells into adult
Crx -/- mice (a model of Leber's Congenital Amaurosis), these human ESCs-derived retinal cells
differentiate into functional photoreceptors and restore light responses in the animals. These results demonstrate that human ESCs can, in principle, be used for photoreceptor replacement therapies.
21
pluripotent with the ability to differentiate into any cell type in the organism. Their property of indefinite self-renewal makes them an ideal source for cellular replacement therapy. The challenge is how to effectively coax them to develop as retinal progenitors or preferably postmitotic photoreceptors (Lamba et al., 2008).
In the last few years, few groups have published reports of inducing a retinal fate in mouse ESCs either using growth factors or by transfecting retinal progenitor genes (Ikeda et al., 2005; Sugie et al., 2005; Tabata et al., 2004). The most efficient induction was shown by Ikeda and coworkers using extrinsic factors normally involved in neuroretinal development including Lefty-A, Dkk1, and Activin A (Ikeda et al., 2005). They found that 25%-30% of cells expressed Pax6 and Rx, two important EFTFs. Most recently, the same group reported that combination these factors with Lectin can be more efficient to enrich photoreceptors from mouse ESCs (Mandai et al., 2010).
Human ESCs can also be directed to a retinal cell fate using a combination of a BMP inhibitor, a Wnt inhibitor, and IGF-1 (Lamba et al., 2006) or a combination of Wnt and Nodal antagonists (Osakada et al., 2008). The Lamba protocol resulted in up to 80% of all cells acquiring a retinal progenitor fate as assessed by expression of eye field transcription factors (Lamba et al., 2006). Since 2007, human ESC-like cells, known as induced pluripotent stem cells (iPSs), have been derived by overexpressing a group of key pluripotency genes in adult fibroblasts (Takahashi et al., 2007). These cells have most of the characteristics of ESCs and could help curtail some of the ethical concerns associated with the use of embryonic stem cells and also help in creating non-immunogenic cells for transplants. Thus, human ESCs could be an excellent source for retinal replacement therapy as they could provide the millions of cells needed for such procedures (Lamba et al., 2008).
The knowledge gained from these recent studies promises to improve ESCs-based cell replacement therapies for future successful treatment of degenerative retinal diseases, thereby fulfilling the hope of millions of patients suffering from impaired vision to see a bright and colorful world.
1.6 Aims of this project
Fulfilling the potential of stem cell or other cell-based replacement therapies requires a more detailed understanding of molecular mechanisms in eye development. In this project, we focused specifically on understanding the molecular mechanisms that drive ESCs toward a retinal fate, such as photoreceptors. In the past decade, our lab has been focused on the gene regulatory network involved in the development of vertebrate retina, using Xenopus leavis as a model organism. We have collected a large body of knowledge about normaleye development. Specifically, we and other labs together found that noggin overexpression can induce the transcription of most of the EFTFs in the animal cap explants. A gene regulatory network was thus established and noggin is positioned at the head of this hierarchy. To investigate whether Noggin can start the developmental program leading to retinal development, we decided to use the animal cap assay, a simple and reliable experimental protocol, to test the tissue generation potential of noggin overexpression in ACES cells, and to use the transplantation assay to test the
22
eye forming potential of noggin overepression in ACES cells, in vivo. We also characterized the
noggin-expressing ACES cells to elucidate the molecular mechanism underlying retinal
induction, thereby identifying the factors necessary and sufficient to induce the retinal cell fates together with noggin. The final goal of our project is to transfer the acquired knowledge to mammalian ESCs and provide more efficient in vitro protocols of induction and propagation of retinal neurons for future cell replacement therapy.
In this thesis, the major aims planed to achieve are:
Aim 1: To explore the tissue generation potential of noggin overexpression in ACES cells. Using animal cap assay, we investigated the tissue generation in ACES cells overexpressing different doses of noggin mRNA with molecular biological assessments.
Aim 2: To explore the the eye forming potential of noggin overepression ACES cells in
vivo.
Using transplantation assay, we analyzed the behaviors of ACES cells expressing different doses of noggin mRNA following transplantatation in sibling wild type embryos.
Aim 3: To identify the key factors necessary and sufficient to drive ACES cells towards the retinal cell fates.
Using microarray analysis, we characterized the gene expression profiles in ACES cells expressing low and high doses of noggin mRNA, and identified the key factors (genes and/or signaling molecules) acting synergistically with noggin to induce retinal fate. Our recent data coming from the microarray analysis provide us with some interesting candidate factors. These factors might be involved in the downstream events triggered by the action of Noggin in ACES cells, and might be crucial factors for retinal fate induction. We are currently attempting to verify these candidates by test their abilities to change the eye forming potential of different doses of noggin-expressing ACES cells in host embryos.