Chapter I – Introduction
Chemoreception in insects
Insects are represented by the greatest number of species (more than one million) in the animal kingdom. They have an important impact on global public health as disease vectors, such as malaria, dengue fever and other deadly pathologies. In agriculture, some species, such as honey bees, are highly beneficial as pollinators, while others, such as locusts and aphids represent major pests. The high reproductive rate and the short life cycle are the basis of their success. However their capacity to survive and reproduce depends greatly on their ability to identify and respond selectively to cues from environments.
Insects possess sensitive chemosensory systems that can detect and discriminate among a great variety of chemicals. They rely on chemical communication to identify cospecifics and enemies, locate food sources, recognise mates, oviposition sites, etc. In particular, social insects such as honey bees, ants and termites, have evolved a highly sophisticated chemical language to communicate between members of the colony.
Insect chemosensilla are the main structures involved in detection of chemical, mechanical and thermal stimuli in the enviroment. Receptor neurons, associated with these structures, convey messages from the periphery of the insect body to the central nervous sytem, where the signals are integrated and processed.
Olfactory stimuli: pheromones and general odours
The odourant compounds, that trigger the olfactory system, are usually small hydrophobic molecules transported by air. This is true for air-breathing animals, such as insects and mammals. But in aquatic animals olfaction is stimulated by compounds soluble in water, such as amino acids, glycosides and others, generally non volatile. In insects, odorants can be classified into two main groups from an ethological point of view. Chemicals with an intraspecific function are called pheromones; those with an interspecific function are called allelochemicals or general
odours. However, some chemicals can perform both functions, even in the same species (Blum, 1996).
Insect pheromones
Insect pheromones have been studied in detail and in a great number of species. Because they constitute a sophisticated communication language among individuals of the same species, they can be used to modify the behaviour of insects and control their populations. According to the types of messages they convey, we can distinguish sexual, aggregation, dispersal, alarm and trail pheromones.
Sex pheromones, which are released to attract members of the opposite sex for mating, have been extensively studied, mainly in species that represent pests in agriculture, as a way alternative to insecticides for their population control. Studies over the past three decades have demonstrated that insect sex pheromones are produced as multi-component blends in which the ratio of the individual components is precisely controlled, thus generating species-specific pheromone blends (Ando et al, 2004; Jurenka et al., 2003).
Lepidopteran pheromones were the first to be studied and represent a homogeneous class of compounds, including long chains of carbon atoms with a single functional group at the end. In other orders of insects, the pheromones belong to a great variety of chemical classes. Besides aliphatic hydrocarbons, terpenoids are very common. Mono- and sesquiterpenes are used as components of alarm pheromone in most aphids, termites, and ants. Terpenes are used by some ant species also as trail pheromones, and by bumblebees as marking compounds. Bark beetles and the cotton boll weevil, Anthonomus, use monoterpenoid compounds as attractants and aggregation pheromones. In scarab beetles there are unusual fatty acid derivates as γ-lactones and ketones, in addition to terpenoids, phenolic compounds, amino acid derivates and alkaloids (Leal et al., 1998).
Insect general odours
General odours belong to a great variety of chemical classes. Besides those produced by plants, they can also derive from active secretion and metabolism of other animal species or from organic substrates in decomposition. Specific studies have been aimed at the identification of
volatile compounds released from plants, because they are involved in the recognition of host plants by phytophagus insects. These compounds are usually hydrophobic, but relatively soluble in water, when compared to Lepidopteran pheromones. Their size may vary from two carbon atoms, as in ethanol, to 15 carbons as in cariophyllene and many other terpenoid compounds (Guerin and Visser, 1980). Some substances are present in the majority of the plants, while others are typical of few families or species. The specificity and sensitivity of insects to them are usually lower than to pheromones. For instance, the majority of phytophagus insects can perceive a complex mixture of “green” compounds, such as alcohols and aldehydes of 6-9 carbon atoms (Schweitzer et al., 1976, Van der Pers, 1981). These substances may play a relevant role in olfactory orientation, because their proportions can vary according to the host plant. Nevertheless, some cases have been reported of plant odours with olfactory thresholds as low as those of pheromones. Typical examples are trans-asaron for Psila rosae (Guerin et al., 1983), propyldisulphide for Delia antiqua and Delia brassicae (Guerin and Stadler, 1982) and β-bisabolol for Antonomus grandis (Dickens, 1984).
The strong power and efficiency of chemical stimuli on the choices of insects have suggested strategies alternative to insecticides for population control of species causing damages in agriculture or carrying serious diseases. In order to devise specific ways for interfering with the chemical communication system of insects, it is fundamental to understand their molecular language at chemical, biochemical, physiological and behavioural levels.
Insect chemosensory structures
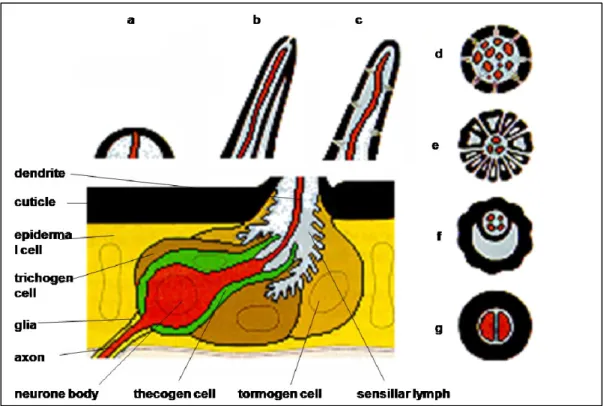
Sensory organs present a great variety of shapes and structures in different insect species, but the basic odour detection unit of the insect olfactory system is always the olfactory sensillum (Kaissling and Thorson, 1980). These can be divided into mechano-, chemo-, thermo- or hygro-sensilla. The typical insect sensillum is an oligocellular complex consisting of one or several bipolar sensory neurons of different specificity and three auxillary cells.
Insect chemosensilla come in a variety of shapes, including long and short hair-like structures and plate-like structures. All of them present an external cuticular wall, protecting the dendrites of sensory neurons. These are bathed in sensillar lymph and extend their axons to the central nervous sytems. The sensillum lymph contains very large amounts of small soluble proteins,
Fig. 1. Schematic representation of insect sensilla. a-c: Longitudinal sections. d-g: Transversal sections. a: Mechanosensitive campaniform sensillum. b, f: Gustatory sensillum; the dendrites of usually four taste neurons are exposed via a terminal pore. c-e: Olfactory sensilla; the dentrites of several neurons respond to odours that are accessible through wall pores. c-d Single-walled wall pores sensillum. e: Double-walled wall pores sensillum. g: Termo/hygrosensitive sensillum (modified from Steinbrecht, 1992a).
Odorant-binding Proteins (OBPs) and Chemosensory Proteins (CSPs), secreted and reabsorbed by specialised auxillary cells at the base of the sensillum (Vogt, 2003; Pelosi et al., 2006). The auxillary cells can vary in number, usually being three: thecogen, trichogen and tormogen (Steinbrecht 1997, 1999). Besides their ontogenetic function, they are also important elements of the cuticular apparatus. Figure 1 reports the schematic representation of some types of chemosensilla.
Olfactory receptors (ORs), seven transmembrane proteins located on the dendritic membrane, are believed to specifically interact with odorants and pheromones, thus initiating an enzymatic cascade eventually generating an electric signal (Clyne et al., 1999; Vosshall et al., 1999; Hallem et al., 2006).
The olfactory sensilla are mainly restricted to specific organs such as antennae, palps and tarsi (Steinbrecht, 1992a, 1997, 1999), but in some species they are found also in other parts of the body. They usually contain only two or three sensory neurons, but in some species this number
Fig. 2. (Left) Sensilla basiconica display a wide socket (s) and numerous wall pores as seen by scanning electron microscopy. (Right) The cross section through the shaft of a s. basiconicum reveals that the cuticular wall (cw) is perforated by many wall pores (p) connected to pore tubules (pt). The sensillum lymph cavity is almost filled with numerous dendritic branches (d). (Jin et al., 2006)
can be higher, up to 20. Olfactory sensilla exhibit electrophysiological responses to many chemical stimuli, both pheromones and general odorants. In the adult insect, each olfactory neuron sends an axon to the antennal lobes in the brain (Hildebrand, 1996; Hansson et al., 2003); in the larvae, olfactory neurons project to a location in the brain corresponding to the adult antennal lobe (Kent and Hildebrand, 1987; Python and Stocker, 2002).
There are two major subtypes of olfactory sensilla, according to the structure of the hair wall: single walled (SW) and double walled (DW) sensilla (Steinbrecht,1969). Usually SW are trichogen or basiconic sensilla, while DW are basiconic or coeloconic sensilla, although many variations of this general theme have been described (Chapman, 1998). No matter whether they are single walled or double walled, all olfactory sensilla show the presence of numerous small pores on their cuticle (Figure 2). The pores allow the entry of volatiles chemicals and their number ranges from about 5 to 50, depending on the type of sensillum.
There are two types of single-walled multiporous sensilla (Schneider and Steinbrecht, 1968): sensilla basiconica and sensilla trichodea. The former ones are short, thin-walled pegs with a high density of wall pores and many pore tubules per pore (15-20 tubules/pore), and the innervating dendrites have several branches. The sensilla trichodea are very long, the dendrites are essentially unbranched, the hair wall is thicker at the base and progressively becomes thinner
Fig. 3. Scanning electron micrographs of flagellomere 5 from Anopheles adult female antennae. This representative image shows the various types of sensilla that are found on most flagellomeres: lch – large chaetica, sch – small chaetica, st – sharp trichoid, bt – blunt trichoid, lco – large coeloconic, gp – grooved peg, E – similar to type E trichoid. (Pitts and Zwiebel, 2006)
toward the tip, and the density of pores and pore tubules is low (3-6 tubules/pore) (Figure 3). The double-walled multiporous sensilla can be distinguished in basiconic and coeloconic, although many variations of these basic structures have been described. Under the light microscope, the sensilla coeloconica appear as tiny pointed pins standing on a conical elevation (in fact the name sensillum coeloconicum means pit peg). The sensilla coeloconica are shorter than the basiconica and taper near the tip (Steinbrecht, 1987)(Figure 3).
Soluble proteins of chemoreception
In insects, odours and pheromones meet soluble proteins on their way to the membrane-bound receptors. The sensillar lymph (the area around olfactory neurons endings) is filled with a thick aqueous phase. Small soluble proteins, extremely concentrated in such regions, are able to reversibly bind odours and pheromones. Therefore, all chemical stimuli have to interact to a great extent with such proteins, that in some way modulate and modify the original chemical messages (Pelosi, 1994, 1996, 1998, 2001; Pelosi and Maida, 1995; Pelosi et al., 2006; Steinbrecht, 1998; Tegoni et al., 2004; Wanner et al., 2004).
Odorant-binding proteins of insects
In insects, two main classes of polypeptides have been identified in the lymph of chemosensilla, Odorant-binding Proteins (OBPs) and Chemosensory Proteins (CSPs). OBPs are believed to be mainly involved in olfaction, CSPs in contact chemodetection (Picimbon, 2003), but this is far from being a general rule. Both OBPs and CSPs are small, water soluble proteins, expressed in the auxillary cells of olfactory sensilla and secreted into the aqueous fluid surrounding the olfactory neurons at concentrations as high as 10 mM (Vogt et al., 1991). The secondary structure of both insect OBPs and CSPs are mainly α-helical domains, in contrast to the β-barrel motif of vertebrates’ OBPs. The three dimensional structure of insect OBPs is very compact and stabilised by three interlocked disulphide bridges connecting six cysteines that represent the main conserved amino acid of this protein family. CSPs are also mainly composed of α-helical domains and folded in a compact structure. However, CSPs present only four conserved cysteine residues establishing two small loops between adjacent residues. Figure 4 compares the main properties and the structures of OBPs and CSPs.
The six cysteines present in OBPs represent a conserved motif, that can be regarded as a signature for these proteins. Their spacing in the sequence is rather conserved, as well as their pairing into three interlocked disulfide bridges (1-3, 2-5 and 4-6) (Scaloni et al., 1999; Leal et al., 1999). This arrangement confers compactness and great stability to the protein(Sandler et
al., 2000; Tegoni et al., 2004).
The first OBP of insects was discovered at the beginning of the eighties in the giant moth
Antheraea polyphemus (Vogt and Riddiford, 1981), using the tritium-labelled specific
pheromone (E,Z)-6,11-hexadecadienyl acetate as a probe. This protein, named pheromone-binding protein (PBP), is 142 amino acids long with an isoelectric point of 4.7 (Raming et al., 1989). A great number of proteins similar in their amino acid sequences to the PBP were later identified in many Lepidopteran species, Lymantria dispar (Vogt et al., 1989), Manduca sexta (Györgyiet al., 1988), Bombyx mori (Maida et al., 1993; Krieger et al., 1996) and many others
Fig. 4. Soluble proteins of chemical communication in vertebrates and in insects. Structural comparison. In vertebrates, odorant-binding proteins (OBPs) present the typical β-barrel folding of lipocalins, the large superfamily of carrier proteins to which they belong. In insects, the two main families of polypeptides (OBPs and CSPs: chemosensory proteins) so far discovered are mainly constituted by α-helical domains in unique foldings and different arrangements of disulphide bridges.
On the basis of sequence similarity, OBPs of Lepidoptera have been classified into three main classes. PBPs (Pheromone-Binding Proteins) are preferentially expressed in pheromone-sensitive sensilla, GOBPs (General Odorant Binding Proteins) are mainly found in sensilla basiconica, that generally respond to plant odours(Steinbrecht, 1992a, Steinbrecht et al., 1995; Laue et al., 1994; Zhang et al., 2001), while ABPs (Antennal Binding Proteins) represent a third class of OBP, whose specific function is still unclear (Krieger et al.,1996).
For a while the study of OBPs was limited to Lepidopteran species. In the last few years, also thanks to the information obtained from genome sequencing, more than 400 OBPs have been identified and cloned from more than 40 insect species, belonging to 10 different orders (Pelosi et al., 2006; Zhou et al., 2010a).
Thus a greater variety of OBPs were discovered, particularly in Dipteran species, whose genomes contain 60-70 genes encoding such proteins. According to their length and the number of cysteines, insect OBPs can be grouped into: classic OBPs (having the typical six-cysteine
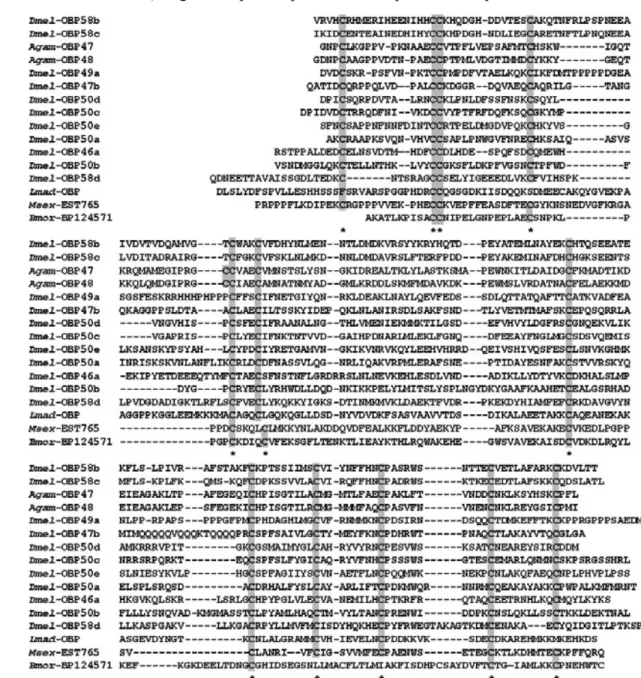
Fig. 5. Sequences of C-plus OBPs containing four to six more cysteines in addition to the six
conserved motif. Eleven sequences of this class have been annotated in the Drosophila genome and are reported as OBPs, followed by a code. The alignment includes also some representative sequences of Anopheles gambiae (Agam), one identified in the cockroach L. maderae (Lmad) and two partial sequences found in the EST database, from the two Lepidopteran species Manduca
sexta (Msex) and B. mori (Bmor). All the cysteines are highlighted.
signature and including PBPs, GOBPs, and ABPs), tandem OBPs (constituted by two classic OBPs linked by few amino acids), C-plus OBPs (containing more cysteines in addition to the six of the conserved motif), C-minus OBPs (presenting only four of the six conserved cysteines), and atypical OBPs (having a variable number of additional cysteines and generally a longer C-terminus) (Hekmat-Scafe et al., 2002; Xu et al., 2003; Riviere et al., 2003; Zhou et al., 2004a, b; Vieira et al., 2007). Figure 5 reports representative sequences of C-plus OBPs.
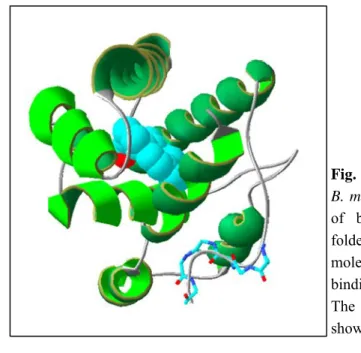
Fig. 6. Three-dimensional structure of the
B. mori PBP complexed with a molecule
of bombykol. The protein is mainly folded in α-helical domains and the molecule of bombykol sits inside the binding cavity in a bent conformation. The hydroxyl group of bombykol is shown in red.
Three dimensional structure of OBPs
To date, the structures of seven insect OBPs have been resolved. An excellent review provides most of the information available on such aspects (Tegoni et al., 2004). The first OBP structure ever determined was that of the PBP from the moth Bombyx mori (Sandler et al., 2000). Further the structure of a PBP from the cockroach Leucophaea maderae (Lartigue et al., 2003), the PBP of A. polyphemus (Mohanty et al., 2004), the pheromone binding protein LUSH of D. melanogaster (Kruse et al., 2003), the queen pheromone binding protein (ASP1) of the honeybee Apis mellifera (Lartigue et al., 2004), the OBP1 of the mosquito Anopheles gambiae (Wogulis et al., 2006) and the GOBP2 of B. mori have been published (Zhou et al., 2009). The PBP of Bombyx mori has been studied both in its crystallised form by X-ray diffraction spectroscopy (Sandler et al., 2000) and in solution, using NMR techniques (Damberger et al., 2000; Horst et al., 2001). The structure is very compact, mostly made of α-helical domains (six conserved helices). The six cysteines form three disulphide bridges C1-C3, C2-C5, C4-C6, which enforce the organization of the α-helices in the B. mori PBP structure. Helices α1, α4, α5 and α6 form a “binding pocket” and helix α3 closes one end of this pocket. The molecule of bombykol, the specific pheromone and likely its natural ligand, is bound in this cavity by numerous hydrophobic interaction, whose access requires some conformational changes.
Figure 6 shows the structure of the B. mori PBP complexed with a molecule of bombykol. The binding of the pheromone to the protein is due to specific forces and not the results of general hydrophobic interactions (Klusak et al., 2003). The structure of the protein at neutral pH without ligands has been found to be identical with that of the complex PBP/bombykol. Therefore, any conformational change allowing the ligand to enter the binding cavity would not be detectable once the complex was formed (Lee et al., 2002).
An interesting phenomenon has been observed with the B. mori PBP. A pH switch changing the conformation from an “open” form binding the pheromone at pH 6.5 to a “closed” form unable to bind ligands at pH 4.5 has been proposed. At pH 4.5 and in the absence of ligands, the C-terminus of the protein, that is not structured at neutral pH, assumes the conformation of an α-helix, which fits inside the binding cavity, taking the place occupied by bombykol at pH 6.5 (Damberger et al., 2000). It is not clear what would be the consequences of such a drastic conformational change and how this pH difference could be related to physiological conditions. However, these findings are in agreement with the hypothesis that the pH in the close proximity of the membrane could assume much lower values than in the cytoplasm. Based on such hypothesis, a model has been proposed, according to which the PBP would pick up the pheromone at pH 7, carry it to the membrane and release it in the proximity of the receptor (Horst et al., 2001). The acidity of the membrane would stabilise the C-terminal helix, that thus would push the molecule of bombykol out of the binding pocket. Figure 7 shows the structure of B. mori PBP at pH 6.5 and pH 4.5, in both cases without any ligands.
Another mechanism of action for OBPs seems to be suggested by the second structure that has been resolved for proteins of this class, the PBP of the cockroach Leucophea maderae (Lartigue et al., 2003). This protein, as other members of the same family, does not present the C-terminus common to Lepidopteran OBPs, being 19 residues shorter than BmorPBP. Therefore, the formation of a seventh α-helix interacting with the binding site could not occur in this protein. Interestingly, components of the pheromonal blend of Leucophea maderae, such as 3-hydroxy-butan-2-one are much more hydrophilic than moth pheromones and bind the PBP with good affinity (Lartigue et al., 2003). This suggests that in such case the hydrophilic pheromone could be easily released and presented to the membrane-bound receptor without need of an active mechanism. Thus, the absence of the amino acid stretch corresponding to the
Fig. 8. Three-dimensional structure of the PBP of L. maderae. This protein shows a folding similar to that of the B. mori PBP, but presents a truncated C-terminus and cannot undergo the conformational change described for B. mori PBP.
Fig. 7. Conformations of B. mori PBP at pH 7.0 (left) and pH 4.5 (right), in the absence of ligands. The C-terminus (shown in blue) is not structured at neutral pH, but assumes an α-helical conformation at pH 4.5 and enters the binding site, that otherwise would house a molecule of bombykol, the specific sex pheromone.
seventh helix together with an hydrophilic ligand suggest that an alternative mechanism of direct ligand release might be used. Figure 8 shows the three-dimensional structure of the PBP of L. maderae.
The structure of the PBP1 of the giant moth Antheraea polyphemus, has been resolved in solution, as a complex with the specific pheromone at pH 6.3 using NMR spectroscopy (Mohanty et al., 2004). A conformational change, similar to that observed for the PBP of B.
mori, also seems to occur in this protein, as reported by more recent studies (Zubkovet al., 2005;
In the structure of LUSH, an OBP of Drosophila melanogaster (Kruse et al., 2003), the C-terminus is folded back into the core of the protein at neutral pH. The authors have crystallised LUSH in the presence of small alcohols that have been found to occupy the binding cavity. However, binding assays in solution have measured good affinity only to large aromatic molecules. It has been proposed that in order to let such ligands enter the binding cavity, a conformational change involving the C-terminus of the protein should occur (Zhou et al., 2004a).
Like LUSH, the C-terminus of the honeybee Apis mellifera OBP, ASP1, which is shorter than in
B. mori PBP but longer than in L. maderae PBP, folds back into the protein core without
forming an α-helix, and partially occupies the binding cavity (Lartigue et al., 2004).
Finally, the structure of Anopheles gambiae OBP1 reveals a unique binding pocket that forms a tunnel running through two subunits of an OBP1 dimer (Wogulis et al., 2006).
Tissue and subcellular expression
In earlier studies, one of the criteria adopted for the identification of OBPs in Lepidoptera was their specific expression in the antennae. However, when investigating species of different orders of insects, proteins of the OBP family have been found also in other chemosensory organs, such as tarsi and palpi, usually covered with chemosensilla. In some species, moreover, chemosensory hairs can be present on other parts of the body, such as the wings, that may also contain OBPs. In the fly Phormia regina, an OBP, CRLBP, is expressed in palpi and tarsi, but also in wings and antennae (Ozaki et al., 1995; 2003). The presence of OBPs in taste organs suggests that these proteins could be involved also in contact chemoreception, in addition to olfaction. In D. melanogaster, An. gambiae and other Diptera, some OBPs are also expressed in non sensory organs and might be involved in functions not related to chemoreception (Zhou et
al., 2004b; Li et al., 2005; Calvo et al., 2009).
Proteins similar to OBPs have also been reported in non sensory organs, such as the pheromone glands. It is reasonable to propose that OBPs in such tissues would perform the role of carriers
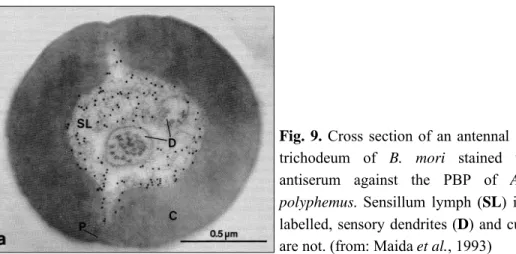
Fig. 9. Cross section of an antennal sensillum trichodeum of B. mori stained with the antiserum against the PBP of Antheraea
polyphemus. Sensillum lymph (SL) is heavily
labelled, sensory dendrites (D) and cuticle (C) are not. (from: Maida et al., 1993)
of chemical messages to the environment, rather than being involved in their perception (Calvello et al., 2003; Li et al., 2008).
In some cases, OBPs are sex-specific. Generally they are present in male antennae and absent or expressed in much lower amounts in female antennae, as in many Lepidopteran species. Based on these observations, such OBPs have been classified as PBPs. This was later supported by binding experiments with specific pheromones and their selective expression in sensilla that respond electrophysiologically to the same pheromones (Vogt et al., 1991).Interestingly, in the cockroach Leucophaea maderae, where the sex pheromone is produced by males, the PBP is specifically expressed in the antennae of females (Riviere et al., 2003).
Techniques of immunocytochemistry have been applied to evidence the sites of production and accumulation of OBPs within the chemosensilla. In all insect species, OBPs were always localised in the extracellular sensillum lymph and never on the neuron dendrites (Figure 9). The first observations indicated that, at least in Lepidoptera, each sensillum expressed a single type of OBP. Pheromone sensitive sensilla trichodea express PBPs (Steinbrecht et al., 1992a), while most sensilla basicona, that respond to general odorants, mainly express GOBPs (Laue et al., 1994). This distribution was verified in several noctuid moths (Steinbrecht et al., 1996). However, the presence of a great number of non labelled sensilla, the heavy expression of PBP also in females’ sensilla and a number of morphological sub-types of sensilla in these species make the picture more complex (Zhang et al., 2001).
In other orders of insects, the picture is much more complex, with a greater variety of sensilla, that do not have their counterparts in Lepidoptera. In Locusta migratoria manilensis, for
instance, OBPs are expressed in sensilla trichodea, basiconica and ceoloconia of the antennae (Jin et al., 2005). Moreover, two OBPs of Drosophila, OS-E and OS-F, where always found to be co-localised in the same trichoid or basiconic sensilla (Hekmat-Scafe et al., 1997). Another OBP of the same species, PBPRP2, is expressed in sensilla coeloconica. Further complication arises from the fact that some proteins classified as OBPs on the sole basis of their sequences, are actually involved in taste and expressed in contact sensilla, as in the above mentioned case of CRLBP of the fly Phormia regina (Ozaki et al., 1995, 2003).
Ligand binding studies
The widespread use of molecular biology techniques has made available relatively large amounts of insect OBPs, through expression in heterologous systems, for binding assays and structural investigation. The binding activity of OBPs to small organic compounds, such as pheromones and other semiochemicals, is the key feature of these proteins and provides essential information for understanding their physiological function.
Fluorescent assays have been adopted to measure binding of ligands to insect OBPs through displacement of fluorescent probes (Campanacci et al., 2001; Ban et al., 2003) or quenching of intrinsic fluorescence of tryptophan (Bette et al., 2002). A ‘cold’ binding protocol was recently described for OBPs, that involves separation of the complex from the free ligand by rapid ultrafiltration and evaluation of bound ligand following extraction and determination by gas chromatography-mass spectrometry (GC-MS) analysis (Leal et al., 2005a, b). Other protocols used to measure dissociation constants of ligands to insect OBPs involve calorimetry and VOBA (volatile odorant-binding assay) (Briand et al., 2001). The formation of a complex between OBPs and their ligands has also been demonstrated by X-ray crystallography, NMR spectroscopy and electrospray mass spectrometry (ESI-MS). These methods, while clearly demonstrating the presence of a ligand bound to the protein, are unable to provide any information about the relative dissociation constant.
By far the most widely used methods to study binding of ligands to OBPs and CSPs use fluorescent probes that, upon binding to the protein, modify their emission spectra. Generally a
Table 1. Structures of the fluorescent probes used with insect OBPs and CSPs and their spectral characteristics
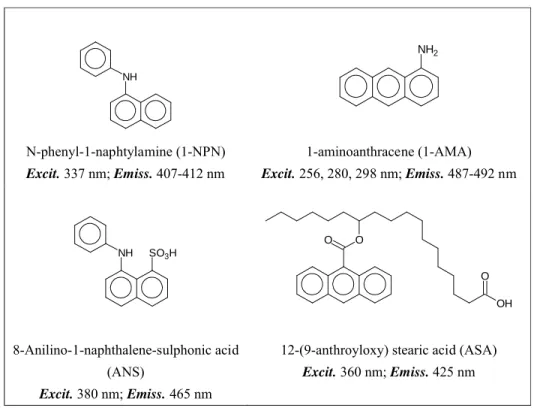
blue shift is observed, accompanied by a marked increase in intensity. The method requires that the fluorescent compound present some affinity to the protein being studied, but is simple, rapid and safe, not requiring the use of radioactive ligands. In some cases, when a suitable fluorescent probe is not available, the intrinsic fluorescence of tryptophan can be measured to monitor the presence of a ligand, provided that a tryptophan residue is present in the binding pocket and that its interaction with the ligand does affect its fluorescence properties. Typically, aromatic ligands are good quenchers, but bromo derivatives of potential ligands have also been used successfully. Table 1 reports the structures of the fluorescent ligands so far used with insect OBPs and CSPs and their spectral characteristics.
NH N-phenyl-1-naphtylamine (1-NPN) Excit. 337 nm; Emiss. 407-412 nm NH2 1-aminoanthracene (1-AMA) Excit. 256, 280, 298 nm; Emiss. 487-492 nm NH SO3H 8-Anilino-1-naphthalene-sulphonic acid (ANS) Excit. 380 nm; Emiss. 465 nm O O OH O
12-(9-anthroyloxy) stearic acid (ASA) Excit. 360 nm; Emiss. 425 nm
Reversible binding of pheromones and other odorants to insect OBPs has been demonstrated in many insect species. One important issue, related to the physiological function of OBPs, is whether these proteins can selectively bind and discriminate between the thousands of different semiochemicals present in the olfactory repertoire. There is some evidence to support their selective binding, but the full understanding of the discriminatory ability of OBPs remains elusive.
PBPs of A. polyphemus differentially bound the components of the pheromone blend (Maida et al., 2003). Also, the two PBPs of A. pernyi, AperPBP1 and AperPBP2 exhibited opposite binding affinities for two pheromone component E6,11Z-16Ac and 4E,9Z- tetradecadienyl acetate (E4,9Z-14Ac) with dissociation constants differing by about one order of magnitude (Du and Prestwich, 1995). The OBP of the paper wasp Polistes dominulus also shows a marked selective binding to oleoamide: both the trans isomer, elaidic amide, and the corresponding saturated compound, stearic amide, bind to the OBP with dissociation constants more than one order of magnitude higher than that of oleoamide (Calvello et al., 2003).
A special case, studied as part of the experimental work of this thesis, is the binding of the aphids alarm pheromone (E)-β-farnesene to the OBPs of aphids. Although each OBP of the pea aphid A. pisum binds several different componds with similar affinities, (E)-β-farnesene, as well as its structurally related farnesol and 3,7-dimethyloctyl acetate only bind to OBP3. In this case, recognition can be accomplished by the insect’s brain by analysing a complex response pattern rather than a signal generated by a specific neuron (Qiao et al., 2009).
However, the majority of OBPs do not seem to be as specific for the species’ pheromones as behavioural and electrophysiological data would suggest. For example, the PBP of M. brassicae MbraPBP1 binds all three components of the pheromone (Z)11-hexadecenol, (Z)11-hexadecenal and (Z)11-hexadecenyl acetate with similar dissociation constants (Campanacci et al., 2001). The PBP1 of the silkmoth B. mori, was found to bind very strongly to non-pheromonal compounds such as (10,12)-hexadeca-diyn-1-ol (Hooper et al., 2009).
Given the poor specificity of OBPs, as measured by direct binding, an interesting study (Bette et al., 2002; Mohl et al., 2002) has addressed the possibility that conformational changes of the protein associated with ligand binding could reveal some hidden specific interactions. Using circular dichroism measurements, the authors monitored the secondary structure of the two PBPs of A. polyphemus protein upon binding of the pheromone components. The interesting result was that although both proteins bind equally well all three components of the pheromonal blend, only one pheromone component produced a conformational change in any given protein. The conclusion of this study is that, although several compounds binds to the same protein, only one of them could induce a conformational change required for an active transmission of the signal.
The physiological function of OBPs
Since their discovery, different hypotheses and models on the function of OBPs in olfaction have been formulated (Pelosi, 1994; Rutzler, 2005; Smith, 2007). But the specific role of OBPs in the olfactory system remained elusive. In particular, the central question of whether such soluble proteins are involved in the recognition and coding of olfactory stimuli had not received until very recently a convincing answer. The extremely high concentration of OBPs around olfactory dendrites and the large number of OBPs in insect genomes indicate that these proteins are important for the insect and likely to play specific roles.
Until recently, OBPs were regarded only as passive carriers taking the semiochemicals to the membrane-bound receptors and, according to some hypotheses, also removing the same compounds from the proximity of the sensory dendrites, thus contributing to a rapid restoration of the olfactory functionality. Models have been proposed to account for this double functions (Briand et al., 2000; Klein, 1987; Kaissling, 1998, 2001, 2004). This mechanism requires that the OBP continuously switch between two conformational states, active one in the binding, the other in the releasing phase. That an OBP could exist in two conformations with different affinities for the ligand was proposed a decade ago, before any structural information on these proteins was available. Ziegelberger (1995) reported two electrophoretic bands in native conditions for the PBP of A. polyphemus and assigned them to a fully oxidized (with all six cysteines forming disulphide bridges) and a partially reduced form. Pheromone binding seemed to induce a slow partial oxidation of the reduced form, as monitored by the relative amounts of pheromone bound to the two bands. On this basis, a model was proposed where the PBP could bind the pheromone in its reduced form and deliver it to the membrane receptor, before being oxidized and act in this form as a scavenger.
In the last few years, however, OBPs have raised to a more important rank in insect olfaction, from passive carriers of hydrophobic compounds in an aqueous medium to elements responsible for the recognition and discrimination of the different chemical messages.
and Ziegelberger (1991). They managed to push the lymph out of the relatively large sensilla of the moth A. polyphemus and found that the electrophysiological response to the moth pheromone was abolished. Addition of the moth OBP in the sensillum restored the response, but, quite unexpectedly, addition of BSA also showed the same effect.
Other studies have confirmed the importance of OBPs in mediating pheromone detection, showing that the expression of different OBPs is confined to specific chemosensilla mainly in moths and in Drosophila (Laue et al., 1994; McKenna et al., 1994; Pikielny et al., 1994; Steinbrecht et al., 1992b; Steinbrecht, 1998), but also in other species.
Apparently in contrast with such observations, other studies have reported that olfactory receptors can be directly activated by odorants and pheromones, even in the absence of OBPs, as is the case when receptors are expressed in heterologous systems such as Xenopus oocytes (Wetzel et al., 2001). Following a similar approach, in a very elegant work, olfactory receptors of An. gambiae have been expressed in the antennae of D. melanogaster and shown to respond to the same odours with a specificity similar to that recorded in An. gambiae (Hallem et al., 2004), although OBPs are structurally very different between the two species.
Recent studies in vitro have provided strong evidence that OBPs increase the sensitivity and improve the specificity of olfactory receptors, when present in the fluid bathing the receptor cells (Grosse-Wilde et al., 2006).
Finally, some research performed on the Drosophila LUSH, one of the OBPs that binds with good affinity the male specific pheromone vaccenyl acetate, afforded convincing evidence that OBPs are required for the correct perception of chemical stimuli. In fact, deletion of the lush gene produces flies that do not show electrophysiological and behavioural response to the pheromone. Moreover, rescue of the gene restores the correct functioning of the olfactory system (Xu et al., 2005). These experiments clearly demonstrate that OBPs are essential for the perception of the pheromone. On the other hand, another work from the same group demonstrated that an olfactory receptor of Drosophila is also necessary for perceiving the same pheromone (Ha and Smith, 2006). The apparent contradiction reported above that odorants are also detected in the absence of OBPs is resolved in experiments performed in the presence and in the absence of OBPs. When expressing the receptor for vaccenyl acetate in the Drosophila
empty neuron model, in the absence of LUSH, a response can be recorded only with doses of about 10-2 ng of the pheromone, much higher that the 10-4 ng amounts required by the normal neuron.
How the OBP could mediate the response to the pheromone has been shown in another elegant research with LUSH. When vaccenyl acetate binds to LUSH, it induces a conformational change in the structure of the protein, which allows stimulation of the corresponding olfactory receptor. This was demonstrated by a modified LUSH protein that mimics the structure of the LUSH/vaccenyl acetate complex and is able to activate the olfactory receptor even in the absence of the pheromone (Laughlin et al., 2008).
Another important study has discovered the role of two OBPs (OBP57d and OBP57e) in the detection of two fatty acids that act as oviposition attractants in Drosophila sechellia. The same fatty acids act as repellents for D. melanogaster (and other Drosophila species). Experiments in which the genes encoding the two OBPs were exchanged between the two species of Drosophila produced, to some extent, a switch in behaviour, making the two fatty acids repellents for D. sechellia and attractants for D. simulans (Matsuo et al., 2007).
Another observation relating a specific behaviour to the presence of an OBP comes from the fire ants Solenopsis invicta (Krieger and Ross, 2002). Failure in expressing the OBP Gp-9 results in the production of abnormal with several queens colonies. Such OBP, however, was isolated not from sensory organs, but from the thorax of the queens. Therefore, an impaired perception related to the lack of this protein is difficult to explain. Alternatively, the OBP could be involved in the release of pheromones - not yet identified - from the queen, that could inhibit the maturation of other females. Thus the absence of this OBP would reduce or modify the pheromonal signal, performing a role similar to that of urinary proteins in mice (Cavaggioni et al., 1990; Bacchini et al., 1992; Robertson et al., 1993,1996; Böcskei et al., 1992; Hurst, 2002; Beynon et al., 2002).
The behavioural data reported above and the conformational changes observed with OBPs upon ligand binding strongly indicate that the functional role of OBPs goes beyond that of carriers of hydrophobic molecules. Instead, they could be directly involved, together with the membrane-bound receptors, in the recognition and discrimination of the different
semiochemicals. It remains to establish the details of how the information arriving at chemosensilla in the form of small organic molecules is transfered to the receptors by OBPs. In any case, the fact that small soluble proteins as OBPs are the elements responsible for the correct recognition of semiochemicals by the insect also provides the basis for a better and simpler analysis of their olfactory code and the possibility of interfering with their communication system. The great advantage is that OBPs are much easier to study and manipulate than olfactory receptors. In particular, their three-dimensional structures already provides clear information on how ligands can bind to OBPs and represent the basis for designing new semiochemicals to be used in insect population control.
Fig. 10. The life-cycle of winter and summer host-alternating aphids within the sub-family Aphidinae. (Birkett and Pickett, 2003)
Specific introduction on aphids
Life cycle and reproduction
Aphids (Homoptera, Aphididae) are the main insect pests in agriculture. Species of the sub-family Aphidinae, which contains the majority of pest aphids, often alternate between a winter (or primary) host, which is usually a tree or shrub, and a summer (or secondary) herbaceous host, including crops, that grow rapidly during the summer (Figure 10).
Aphids go through egg, nymph, adult and winged stages during their life cycle. Nymphs are smaller in size but closely resemble the adults. A new born nymph molts by shedding its skin three to four times before becoming an adult. This process takes 10-14 days.
Aphids reproduce both sexually and asexually. They generally reproduce sexually on the winter host, with mated females laying cold-resistant overwintering eggs (Figure 10a). The stem mother, or fundatrix, which hatches from the egg (Figure 10b), gives rise to a succession of asexual (parthenogenetic) stages on the winter host, which generate live wingless and winged females. Winged forms (Figure 10c) then migrate to the summer host, and continue to reproduce asexually. As autumn approaches, the asexually reproducing aphids on the summer host respond
Aphis fabae Megoura viciae Schizaphis graminum Cryptomyzus spp. Acyrthosiphon pisum Myzus persicae Sitobion avenae Rhopalosiphu padi Phorodon humuli 1) (4aS,7S,7aR)-nepetalactone 2) (1R, 4aS,7S,7aR)-nepetalactol 3) (1S,4aR,7S,7aS)-nepetalactol 4) (1R,4aR,7S,7aS)-nepetalactol 1 2 3 4
Fig. 11. Structures of sex pheromone components used by different aphid species. Unlike other insects, aphids use the same four components, nepetalactone (1) and three isomeric nepetalactols (2-4) in different relative proportions. This fact may be related to the very high similarity observed in OBP sequences across species.
to the reduced daylight hours by producing winged males and females (Figure 10d). Females (gynoparae) migrate to the primary host and produce wingless sexual females (oviparae), which release sex pheromones from glandular epidermal cells lying beneath scent plaques on the tibiae of the hind legs. Winged male aphids that fly separately detect the released sex pheromones via olfactory receptors situated mainly on the third segment of the six-segmented antennae (Birkett and Pickett, 2003).
Aphids pheromones
So far, sex pheromones have been identified in more than 15 aphid species (Dawson et al., 1990; Pickett et al., 1992; Boo et al., 2000; McNeil, 2004; Zhu et al., 2006). All these pheromones are comprised of either a mixture of the monoterpenoids (4aS,7S,7aR)- nepetalactone and (1R,4aS,7S,7aR)-nepetalactol or one of these two compounds alone, except the damson-hop aphid, Phorodon humili, which uses only (1S and R,4aR,7S,7aS)-nepetalactol as its pheromone (Birkett and Pickett, 2003)(Figure 11). The ratio of these two compounds in each aphid species seems to be extremely important for communication between sexes, because it is on this basis that male recognise females of their own species among those of several sympatric species (Hardie et al. 1992; Dawson et al., 1996; Boo et al., 2000; Zhu et al., 2006).
The damage brought by aphids to crops is mainly due to the vectoring of viruses that infect many plants. Aphids include a great number of species of different size, colour and shape, that occupy different niches. About 4,400 species of 10 families are known. Aphids usually grow in colonies and prefer to live on the younger parts of plants. Around 250 species are serious pests for agriculture and forest as well as an annoyance for gardeners.
As colony insects, aphids have unique systems to signal the presence of danger to other individuals. When attacked by a predator, they secrete droplets of a compound, called “alarm pheromone” from the cornicles, special tube-like structures located on the hind part of the body. In response to this pheromone, aphids abandon the site dispersing in the area. Alarm pheromones in several economically important species in the subfamily Aphididae have been isolated and identified. By far the most common component is the sesquiterpene, (E)-β-farnesene (Nishino et al. 1976). It represents the main component in 16 aphid species, alone or associated with other molecules (Pickett et al., 1992; Bowers et al., 1972; Almohamad, 2008).
Both types of pheromones unfortunately are not practical to be used in population control. The alarm pheromone (E)-β-farnesene is not persistent in the environment, being a relatively volatile hydrocarbon, and is susceptible to oxidation, owing to the presence of several double bonds in the molecule. Moreover, its chemical synthesis is complicated and expensive. However, a number of plants synthesise (E)-β-farnesene as a product of their metabolism and thus are naturally protected against these insects (Gibson and Pickett, 1983). Transgenic Arabidopsis thaliana carrying the genes for synthesising (E)-β-farnesene has been produced and shown to be protected from aphids (Beale et al., 2006). This approach could be applied to crop plants with enormous economic benefits.
The use of sex pheromones, on the other hand, is of little practical use, as these insects reproduce parthenogenetically for most of the time.
Given the great economical impact of aphids, a better understanding of their chemical communication system is highly desirable in order to devise strategies and produce chemical tools for their population control. According to the current view, odorants and pheromones interact with membrane-bound olfactory receptors (Clyne et al., 1999; Vosshall et al., 2000),
triggering olfactory neurons. However, very recently, odorant-binding proteins (OBPs), soluble polypeptides highly concentrated in the sensillar lymph bathing chemosensory neurons (Vogt and Riddiford, 1981; Pelosi et al., 2006), have been shown to be essential for pheromone and odour perception in insects (Xu et al., 2005; Matsuo et al., 2007; Laughin et al., 2008), as reported above.
In this study we have focused our attention on OBPs of aphids and their ligand-binding properties. The sequences were searched among those available in the EST database at the time when this research was performed. Later, the publication of the genome of the pea aphid, Acyrthosiphon pisum, confirmed our results, while providing a more complete pictures of the aphids OBPs. Here we describe the cloning and sequencing of four OBPs from several aphid species, their bacterial expression and purification, ligand-binding studies and immunolocalization.
Specific introduction on mosquitoes
Mosquitoes are vectors of many diseases, such as malaria, dengue and yellow fever, thus posing a major threat to human health worldwide, particularly in developing countries. The most deadly of the insect-born diseases is malaria, caused by the parasite Plasmodium falciparum, which is transmitted to humans by female anopheline mosquitoes, particularly Anopheles gambiae, during blood feeding. Malaria affects over 400 million people and kills up to 3 million people globally each year, particularly children under the age of five (Marshall, 2000).
To date, malaria control relies mainly on reducing the insect vector-human target interaction. Insecticides have been the only choice against malaria in the past and even now the control of mosquitoes mainly relies on the use of insecticides and insecticide-treated bed nets (Enserink, 2001). However, these products have severe adverse effects on public health and the general ecosystem, and also become ineffective after prolonged use due insecticide resistance in the targeted vector populations (Awolola et al., 2002; Brooke et al., 2000; Kristan et al., 2003; N'Guessan et al., 2002; Ortelli et al., 2003). Therefore, new approaches and methods for controlling the disease vector, mainly based on the use of semiochemicals, are currently being investigated (Justice et al., 2003b).
As other insects, mosquitoes locate their hosts by olfactory cues. Carbon dioxide, together with lactic acid and other volatiles produced by the host are the major attractants for mosquitoes. According to such knowledge, a strategy for controlling mosquitoes is based on the use of mosquito attractants in “bait and kill” stations. However, mosquito control devices that utilize carbon dioxide, heat, light and 1-octen-3-ol are effective on many species of mosquitoes, except for the malaria-transmitting species An. gambiae, for which no commercially available attractant exists. Consequently, the development of novel substances that could efficiently interfere with An. gambiae olfaction is one of the main goals pursued for reducing the spread of the malaria parasite worldwide.
Interestingly, field and laboratory studies have shown that female An. gambiae respond to odours emitted from humans in order to find a blood meal and have identified a variety of
chemical compounds in human sweat, that stimulate olfactory neurons and attract female mosquitoes (Cork and Park, 1996; Costantini et al., 2001; Dekker et al., 2001, 2002; Meijerink et al., 2001; Meijerink and van Loon, 1999; Qiu et al., 2006; van den Broek and den Otter, 1999). The best represented compounds include carboxylic acids (all the linear acid with 1 to 12 carbon atoms, isovaleric acid, 2-oxo-butanoic acid and lactic acid), amines (ammonia, butylamine and pentylamine) and other compounds, such as indole, o- and p-cresol, 1-octen-3-ol and 6-methyl-5-hepten-2-one.
Interestingly, human sweat also contains chemicals that have been reported to act as repellents for mosquitoes (Logan and Birkett, 2007). Synthetic mosquito repellents are commercially available, the most common being DEET and Icaridin, that however should be used in very high concentrations (10-20%) in order to be effective. In any case, their mode of action remains unclear (Justice et al., 2003a). Recently, it has been reported that they also have insecticidal action, as acetylcholine esterase inhibitors (Corbel et al., 2009). This raises some concern as to the safety of these products for humans and other animals.
A number of other volatile compounds have been reported as mosquito repellents, such as nepetalactone, cinnanic aldehyde, citronellal, isolongifolenone (Zhang et al., 2009; Gu et al., 2009; Chang et al., 2006; Cheng et al., 2004), but all of them, as DEET and Icaridin, are effective only at very high concentrations. The structural diversity of these compounds and the lack of data on their mode of action mean that there is no rationale to allow the design of chemicals with improved repellency effect. Thus the focus of the present research, is on a better understanding of the biochemical mechanisms of olfaction as a basis to devise alternative strategies to reduce the population of mosquitoes.
In order to elucidate the olfactory code of these insects, most efforts to understand mosquito olfaction have been concentrated on the two major groups of olfactory proteins, odorant binding proteins (OBPs) and odorant receptors (ORs), which are responsible for detecting and recognising host odours and pheromones. The genome of the main malaria vector Anopheles gambiae contains 79 genes encoding putative olfactory receptors and 76 encoding gustatory receptors, as well as about 60 genes for putative odorant-binding proteins (Biessmann et al., 2005; Foret and Maleszka, 2006; Vogt, 2002a; Xu et al., 2003). Of these, OBPs 1 to 29 belong to the so-called “classic OBPs”, whose members are characterised by the six conserved cysteine
motif (Pelosi and Maida, 1995; Xu et al., 2003) and a typical tertiary structure (Scaloni et al., 1999; Wojtasek and Leal, 1999; Sandler et al. 2000); OBPs 30 to 45 are called “atypical”, being longer and presenting different features, while OBPs 46 to 57 belong to the C-plus group, characterised by the presence of 12 cysteines in conserved positions (Hekmat-Scafe et al., 2002; Justice et al. 2003b; Xu et al., 2003; Zhou et al., 2004a).
Although a wide detailed screening of the specificities of 50 olfactory receptors of An. gambiae has been recently published (Allison et al., 2010; Wang et al., 2010), so far parallel information on OBPs of this mosquito is not available. Therefore, this research is focused on the characterisation of some selected OBPs of An. gambiae in terms of ligand-binding specificities towards semiochemicals and general odours. The choice of OBPs as the objects of this study is based on the recent studies, reported and discussed above (Xu et al., 2005; Laughlin et al., 2008; Matsuo et al., 2007), that have raised the role of OBPs from that of simple odorant carriers to that of active proteins responsible, together with the membrane-bound olfactory receptors, for recognition and discrimination of odorant stimuli.
More recently and specifically in mosquitoes, it has been shown that silencing the gene encoding OBP1 in An. gambiae (Biessmann et al., 2010) and in Culex quinquefasciatus (Pelletier et al., 2010) suppresses electrophysiological responses to indole, showing that in both species OBP1 is essential for the perception of indole.
Overall, results to date confirm the important role of OBPs in odour perception and discrimination and this, together with the great impact of disease vectors on human health, prompted us to study the role of OBPs in the olfaction of An. gambiae. Given the enormous amount of work that would be involved in analysing all 60 putative OBPs, we have selected, for this study, those which have been found to be most abundantly expressed in olfactory organs (Biessmann et al., 2002; Xu et al., 2003; Biessmann et al., 2005; Justice et al., 2003b; Andronopoulou et al., 2006; Iatrou and Biessmann, 2008; Dani et al., 2008). Molecular biology techniques have shown that only a small set of OBP-encoding genes are expressed in sensory organs at relatively high levels. In particular, the best represented in female antennae are the classic OBPs, 1, 3, 4, 5, 7, 9, 17 and two of the C-plus OBPs, 47 and 48. In some cases the expression of OBP genes is up-regulated or down-regulated after a blood meal, indicating that the corresponding proteins might be involved in host recognition (Iatrou and Biessmann, 2008).
It has also been reported that each of those OBPs is differentially expressed according to tissue and sex, while a substantial number of other OBPs (mainly belonging to the so-called “atypical OBPs”) are not expressed in any part of the body of either sex (Biessmann et al., 2005).
Another important aspect of the mode of action of OBPs regards their possibility of forming heterodimers, thus generating new protein species with different characteristics and enlarging the repertoire of semiochemicals that could be discriminated. Indeed interactions between OBP48 and some classic OBPs, as well as between OBP1 and OBP4 have been reported using co-immunoprecipitation methods and cross-linking studies (Andronopoulou et al., 2006).
In the current study we have expressed selected OBPs of An. gambiae in heterologous systems and investigated their binding properties towards a number of potential semiochemicals using ligand-binding assays.
Besides their role in semiochemical detection and recognition in sensory organs, OBPs can also assist the delivery of pheromones in the environment. This function is suggested by the fact that OBPs are produced also in glands that synthesise specific pheromones. This phenomenon is well documented in some mammalian species, as in the cases of urinary proteins of mouse and rat (Cavaggioni et al., 1990; Bacchini et al., 1992; Robertson et al., 1993,1996; Böcskei et al., 1992; Hurst, 2002; Beynon et al., 2002) and the salivary proteins of pig (Marchese et al., 1998; Spinelli et al., 2002). In insect, a similar situation has been documented, although the available information is still limited. In the mosquito Ae. aegypti, an OBP is produced in the male sex organ and transferred to the spermathecs of females during mating (Li et al. 2008). Besides, in some other species CSPs rather than OBPs seem to be prefered for the delivery of semiochemicals, as in the Lepidopteran Mamestra brassicae and B. mori, where these proteins have been reported to be abundantly produced in female pheromone glands (Jacquin-Joly et al., 2001; Dani et al., 2011).
Therefore, we decided to include this aspect in our research and investigate the presence of OBPs also in glands associated with pheromone synthesis and delivery. Besides, being the OBPs present in these sites directly associated with pheromones, such study could represent an alternative approach to the identification of important pheromones in mosquitoes.