Università degli Studi di Ferrara
DOTTORATO DI RICERCA IN
"FARMACOLOGIA E ONCOLOGIA MOLECOLARE"
CICLO XXVII
COORDINATORE Prof. Antonio Cuneo
REGULAR VERSUS RESCUE BUDESONIDE AND
FORMOTEROL COMBINATION FOR MODERATE ASTHMA:
A NON INFERIORITY RANDOMISED CLINICAL TRIAL
Settore Scientifico Disciplinare Med/10
Dottoranda
Dott.ssa Gnesini Giulia
Tutore
Prof. Caramori Gaetano
Co-tutore
Prof. Papi Alberto
INDEX
INTRODUCTION ... 3
Definition of Asthma ... 3
Minimum requirements for the diagnosis of asthma ... 4
Symptoms and medical history ... 4
Physical examination ... 5
Lung Function Tests ... 5
Spirometry ... 5
Peak expiratory flow ... 6
Reversibility to bronchodilators ... 6
Airway Hyperresponsiveness ... 7
Arterial Blood Gases ... 7
Allergy tests ... 7
Exhled nitric oxide ... 8
Additional tests ... 8
Imaging... 8
Assessment of Airway Inflammation ... 8
Assessing asthma symptom control ... 9
Management and treatment ... 9
Long term pharmacologic treatment ... 10
Treatment of exacerbations ... 11
STUDY ... 12
Backround and rationale ... 13
Study objectives ... 15 Primary objective ... 15 Secondary objectives ... 15 Study design ... 16 METHODS ... 18 Study Population ... 19 Inclusion criteria ... 20
Exclusion criteria ... 20
Withdrawal criteria ... 21
Treatment failure ... 21
Statistics ... 22
Role of the funding source ... 23
RESULTS ... 24
Primary efficacy outcome ... 24
Secondary efficacy outcomes ... 25
Safety ... 25
DISCUSSION ... 27
TABLES AND FIGURES ... 30
BIBLIOGRAPHY ... 43
INTRODUCTION
Definition of Asthma
Asthma is heterogeneous disease, usually characterized by chronic airway inflammation. It is defined by history of respiratory symptoms such as wheeze, shortness of breath, chest tightness, and cough that vary over time and intensity, together with variable expiratory airflow limitation (1).
Asthma is a common, chronic respiratory disease affecting 1-18% of the population in different contries; this pathology is characterized by variable simptoms and by variable expiratory airflow limitation. Both symptoms and airflow limitation characteristically vary over time and in intensity.
These variations are often triggered by factors such as exercize, allergen or irritant, change in weather, or viral respiratory infections (1).
Asthma is a heterogeneous disease, with different underlying disease processes. Recognizable clusters of demographic, clinical and/or pathophysiological characteristics are often called “asthma phenotypes”. In patients with more severe asthma, some phenotype-guided treatments are available.
More research is needed to understand the clinical utility of phenotypic classification in asthma.
Many phenotypes have been identified. Some of the most common included:
allergic asthma, non-allergic asthma, late-onset asthma, asthma with fixed airflow limitation, asthma with obesity (1).
Other characteristics of asthma are an exaggerated responsiveness of the airways to various stimuli, and in most cases, a specific type of chronic inflammation of the airways characterized by an increased number of CD4+ Th2 lymphocytes, eosinophils and methacromatic cells in the airway mucosa, and increased thickness of the reticular layer of the epithelial basement membrane, and increased volume of airway smooth muscle (1-3) (Figure 1).
Familial predisposition, atopy, and exposure to allergens and occupational sensitising agents are important risk factors for asthma, even though the causes of asthma—the factors responsible for the development of asthma rather than its exacerbations—remain largely undetermined (1).
Minimum requirements for the diagnosis of asthma
The diagnosis of asthma is based on an appropriate clinical history, together with the demonstration of variable and/or reversible airflow limitation, using lung function tests, particularly peak expiratory flow (PEF) or spirometry. Allergy tests are also often performed during the initial assessment of a patient with suspected asthma, to identify possible triggers of asthma and to guide their avoidance (1, 2).
Asthma clusters in families, and its genetic determinants appear to be linked to those of other allergic IgE-mediated diseases. Thus, a personal or family history of asthma and/or allergic rhinitis, atopic dermatitis, or eczema increases the likelihood of a diagnosis of asthma.
Symptoms and medical history
Patients with asthma seek medical attention because of respiratory symptoms. A typical feature of asthma symptoms is their variability. One or more of the following symptoms— wheezing, chest tightness, episodic shortness of breath and/or cough are reported by more than 90% of patients with asthma (1, 2). However, the presence of these symptoms is not diagnostic, because similar symptoms can be present with other respiratory or even cardiac diseases, or may be triggered by different stimuli in non-asthmatics, e.g. by acute viral infections. In some asthmatics, wheezing and chest tightness are absent, and the only symptom the patient complains of is chronic cough (“cough-variant asthma”).
Symptoms of asthma may be triggered or worsened by several factors, such as exercise, exposure to allergens, viral infections, and emotions. Recurrent exacerbations of respiratory symptoms, worsening of lung function requiring change of treatment, unscheduled requests for medical assistance, and sometimes hospitalization are also among the characteristic clinical features of asthma.
Physical activity is an important cause of symptoms for most asthma patients, particularly in children, and for some it is the only cause. Exercise-induced bronchoconstriction usually develops not during exercise, but 5 -10 minutes afterward, and it resolves spontaneously within 30-45 minutes. Prompt relief of symptoms after the use of inhaled β2 -agonist, or
their prevention by pre-treatment with an inhaled β2 -agonist before exercise, supports a
diagnosis of asthma.
Important aspects of personal history are exposure to agents known to worsen asthma in the home, such as dusty environments, forced air heating system, or exposure to allergens (e.g. pets or house dust mites, cockroaches) to which the patient is sensitized; workplace
conditions, environmental tobacco smoke, or even the general environment (e.g., diesel fumes in traffic).
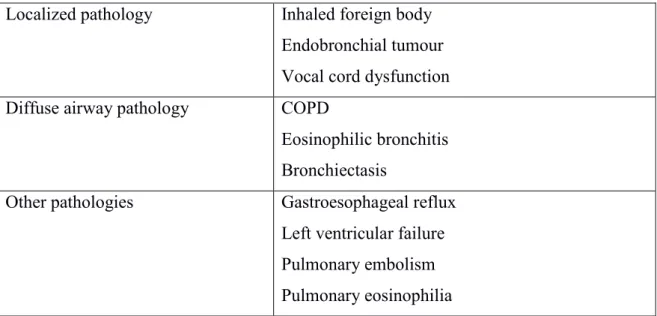
Since respiratory symptoms of asthma are non-specific, the differential diagnosis is quite extensive, and the main goal for the physician is to consider and exclude other possible diagnoses (Table 1). This is even more important if the response to a trial of therapy has been negative.
While respiratory symptoms suggest asthma, the sine qua non condition for the objective diagnosis of asthma is the presence of reversible airflow obstruction in patients who have persistent airways obstruction, or airway hyperresponsiveness or increased PEF variability in subjects presenting without airways obstruction (1,2).
Physical examination
Physical examination in patients with asthma is often normal.
The most frequent abnormality is respiratory wheezing (rhonchi) on auscultation, but it is may be absent or only heard on forced expiration (1).
Typical physical signs of asthma attacks are wheezing on auscultation, cough, expiratory rhonchi throughout the chest, and signs of acute hyperinflation (e.g., poor diaphragmatic excursion at percussion, use of accessory muscles of respiration). Some patients, particularly children, may present with a predominant non-productive cough. In some asthmatics, wheezing—which usually reflects airflow limitation—may be absent or detectable only on forced expiration, even in the presence of significant airflow limitation; this may be due to hyperinflation or to very marked airflow obstruction. In these patients, however, the severity of asthma is mostly indicated by other signs, such as cyanosis, drowsiness, difficulty in speaking, tachycardia, hyperinflated chest, use of accessory muscles, and intercostal recession.
Lung Function Tests Spirometry
Lung function tests play a crucial role in the diagnosis and follow-up of patients with asthma. Spirometric measurements, the forced expired volume in 1 second (FEV1) and
slow vital capacity (VC) or forced vital capacity (FVC), are the standard means for assessing airflow limitation. Spirometry is recommended at the time of diagnosis and for the assessment of the severity of asthma. It should be repeated to monitor the disease and when there is a need for reassessment, such as during exacerbations.
Poorly or non-reversible airflow limitation is usually defined by the absolute reduction of post-bronchodilator FEV1/FVC ratio <0.7. However, because this parameter varies with
aging, it should be confirmed by post-bronchodilator FEV1/VC values below the lower
limit of normal particularly in younger subjects. Measurements of residual volume and total lung capacity may be useful in assessing the degree of hyperinflation and/or enlargement of airspaces. Lung volumes may help in the differential diagnosis with COPD, but are not necessary for the diagnosis or for assessment of severity of asthma (1,3). In asthma, airflow limitation is usually reversible, either spontaneously or after treatment, except for moderate/severe asthma with fixed airway obstruction..
Peak expiratory flow
An important tool for the diagnosis and subsequent treatment of asthma is the PEF meter. If spirometry does not reveal airflow limitation, then home monitoring of PEF for 2-4 weeks may help to detect an increased variability of airway calibre, and assist in making the diagnosis of asthma. Daily monitoring of PEF (at least in the morning at awakening and in the evening hours, preferably after bronchodilator inhalation) is also useful to assess the severity of asthma and its response to treatment, and it can help patients to detect early signs of asthma deterioration. Diurnal variability is calculated as follows:
PEFmax – PEFmin x 100 PEFmax + PEFmin/2
A diurnal variability of PEF of more than 20% is diagnostic of asthma, and the magnitude of the variability is broadly proportional to disease severity. PEF monitoring may be of use not only in establishing a diagnosis of asthma and assessing its severity but also in uncovering an occupational cause for asthma. When used in this way, PEF should be measured more frequently than twice daily and special attention should be paid to changes occurring in and out of the workplace.
Reversibility to bronchodilators
Clinical and/or functional reversibility on repeated testing is required for the diagnosis of asthma. Thus, even a single reversibility test (defined as > 12% reversibility and/or > 200 mL in FEV1 after bronchodilator) can establish the diagnosis (1, 3). However, reversibility is often not present at the time of examination, particularly in patients on treatment, and thus the absence of reversibility does not exclude the diagnosis. Repeated testing of
reversibility of both clinical features and functional abnormalities may be useful in obtaining the best level of asthma control achievable and/or the best lung function for individual patients Achieving and maintaining lung function at the best possible level is one of the objectives of both asthma management.
Airway Hyperresponsiveness
In patients who have symptoms consistent with asthma but have normal lung function, bronchial provocation tests with methacholine, histamine or exercise are helpful in measuring airway hyperresponsiveness and thereby confirming or excluding the diagnosis of current asthma. These measurements are very sensitive, but poorly specific for a diagnosis of asthma. This means that while a negative test can be used to exclude a diagnosis of active asthma, a positive test does not always mean that a patient has asthma. While the measurement of airway hyperresponsiveness may be useful to confirm asthma in subjects with normal baseline lung function, it is not useful in presence of irreversible airflow limitation, and thus in the differential diagnosis between asthma and COPD (1, 3). Arterial Blood Gases
In severe asthma and, more important, during acute exacerbations of asthma, the measurement of arterial blood gases while the patient is breathing air and/or after oxygen administration is essential for the diagnosis of respiratory failure. This test should be performed in all patients with clinical signs of acute or chronic respiratory and/or heart failure, and in patients with an acute asthma exacerbation and PEF <50%, patients who do not respond to treatment and those with a SaO2 ≤ 92% (1, 4).
Allergy tests
The presence of allergic disorders in a patient’s family history should be investigated in all patients with symptoms of asthma. A history provides important information about the patient’s lifestyle and occupation, both of which influence exposure to allergens and the time and factors possibly involved in onset and in exacerbations of asthma. Skin tests with all relevant allergens present in the geographic area, in which the patient lives, are the primary diagnostic tool in determining allergic status. Measurement of specific IgE is not usually more informative than a skin test, and is more expensive. Measurement of total IgE in serum has no value as a diagnostic test for atopy. The main limitation of methods to assess allergic status is that a positive test does not necessarily mean that the disease is
allergic in nature or that it is causing asthma, as some individuals have specific IgE antibodies without any symptoms and it may not be causally involved. The relevant exposure and its relation to symptoms must be confirmed by patient history (1, 2).
Exhled nitric oxide
The fractional concentration of exhaled nitric oxide (FENO) can be measured in some centers. FENO is increased in eosinophilic asthma but also in non-asthma contions (eosinophilic bronchitis, atopy and allergic rhinitis) and has not been estabilished has been usuful for making a diagnosis of asthma. Feno is decreased in smokers and during bronchoconstriction, and maybe increased or decreased during viral respiratory infections in patients (mainly non-smokers) with non-specific respiratory simptoms, a finding of FENO> 50 parts of billion (PPB) was associated with a good short-term response to ICS. However, there are no long term studies examining the safety of withholding ICS in patients with low initial FENO. Consequently, FENO cannot be raccomanded at present for deciding wheter treat patients with possible asthma with ICS (1).
Additional tests
While the diagnosis and assessment of severity of asthma can be fully established on the basis of clinical history and lung function tests additional tests are sometimes helpful to better characterize individual patients.
Imaging
While chest radiography may be useful to exclude diseases that may mimic asthma, it is not required in the confirmation of the diagnosis and management of asthma. The utility of chest radiography is to exclude other conditions that may imitate or complicate asthma, particularly acute asthma. Examples include pneumonia, cardiogenic pulmonary oedema, pulmonary thromboembolism, tumours (especially those that result in airway obstruction with resulting peripheral atelectasis), and pneumothorax.
Assessment of Airway Inflammation
While airway biopsies and bronchoalveolar lavage may provide useful information in research protocols, they are considered too invasive for the diagnosis or staging of asthma. In contrast, non-invasive markers of airway inflammation have been increasingly used in research protocols, particularly to differentiate asthma from COPD and measure response
to treatment. These non-invasive measurements include induced sputum and exhaled nitric oxide (FeNO). Induced sputum is not helpful in the diagnosis of asthma, but can be very useful in the management of severe asthma. In particular, induced sputum helps identify the persistence of airway eosinophilia or airway neutrophilia in patients with difficult-to-treat asthma, which can be useful in deciding appropriate doses of inhaled corticosteroids and in reducing the risks of severe asthma exacerbations (5-7). FeNO is increased in atopic asthma, but less so in nonatopic asthma. Again it is not useful in the diagnosis, but can be helpful in monitoring adherence to inhaled corticosteroids, as it is effectively reduced by inhaled corticosteroids, but not by bronchodilators (5-7).
Assessing asthma symptom control
Directed questioning is important, as the frequency or severity of symptoms that patients regard as unacceptable or bothersome may vary for current raccomandations about the goals of asthma treatment and differs from patient to patient. To assess symptom control ask about the following in the past four weeks: frequency of asthma symtoms (days per week), any night waking due to asthma or limitation of activity, and frequency of reliever use for relief of symptoms. In general, due not include reliever taken before exercize, since this is often routine.
There are asthma symptom control tools for adults and adolescent; these can be used in primary care to quickly identify patients who needs more detailed for patients: Asthma Control Questionnaire, Asthma Control Test; when different system used for assessing asthma symptom control, the results correlate broadly with each other, but are not identical. Respiratory symptoms maybe non-specific so, when assessing changes in symptom control, it is important to clarify that symptoms are due to asthma.
The second component of assessing asthma control is to identify whether the patient is at risk of adverse asthma, particularly exacerbations, fixed airflow limitation, and side-effects of medications. Asthma symptom although an important outcome for patients, and themselves a strong predictor of future risk of exacerbations.
Management and treatment
Considering its chronic nature and life-long duration, asthma can be effectively managed only by developing a partnership between the patient and his or her doctor or health-professional, that may provide the tools for a guided self-management possibly written
plan including self-monitoring, and periodically review of treatment and level of asthma control. Education plays a major role in this partnership (1).
Long term pharmacologic treatment
The main of asthma pharmacologic treatment is to achieve and maintain current control of day-to-day symptoms, as well as preventing of the future risk of severe asthma exacerbations, while using the safest treatment algorithm (1). While the initial treatment should be started according to the degree of asthma control at the first visit, subsequently treatment should be adjusted according to the level of asthma control achieved (1, 9-11). Usually regular treatment is lowered only after a significant period of acceptable asthma control (e.g. not less than 3 months). This means that monitoring of asthma is essential to maintain asthma control and to establish the minimal treatment requirements. Step-up and step-down of treatment is not standardized, and thus should be tailored to the individual patient to achieve and maintain control with the minimum amount of medication.
Medications to treat asthma can be classified as controllers or relievers. Medications are preferably administered by inhalation, as this approach is the most effective way to treat asthma and has the fewest side effects. Controller medications (inhaled corticosteroids alone or in combination with long acting β2-agonists) are taken daily on a long-term basis
to keep asthma under clinical control. In asthma, long acting β2-agonists should be used
only in combination with inhaled corticosteroids when the latter are insufficient to achieve control, and should be discontinued only when control is maintained.
Only in patients not controlled by optimal doses of inhaled corticosteroids combined with long acting beta2-agonists, should other secondary agents may be considered. These include anti-leukotrienes, theophylline, systemic corticosteroids, or anti-IgE monoclonal antibodies in very specific cases.
Reliever medications (predominantly rapid acting β2-agonists) are medications used on an as-needed basis that act quickly to reverse bronchoconstriction and relieve asthma symptoms. Ideally, if patients are adequately controlled, they should rarely need rescue medications. The use of a combination of a inhaled rapid acting β2-agonists and corticosteroid both as controller and reliever is effective in maintaining high levels of asthma control (9-11).
Smoking asthmatics are resistant to antiasthma medications and should be primarily treated for smoking addition (12). Asthmatic smokers may develop features of COPD (1, 3, 12)..
Specific immunotherapy in asthma is limited as 1) it requires the identification of a single clinically relevant allergen, 2) can be use safely only in mild asthmatics that are usually well controlled by environmental interventions or pharmacotherapy, and 3) maybe associated to adverse events (1, 2).
Treatment of exacerbations
Shortness of breath, cough, wheezing, and/or chest tightness, may develop or worsen in subject with asthma even when they are under regular treatment (1, 4). Milder exacerbations are usually managed by the patients with an increased as needed use of rapid acting β2-agonists alone or in combination in combination with inhaled steroids. More
severe exacerbations, or exacerbations that do not respond to the increased use of rescue medications, require repetitive administration of rescue medication and systemic, preferably oral, corticosteroids, and in the very severe cases with oxygen supplementation. Severe exacerbations require medical attention or and in some instances hospital admission.
STUDY
The 2014 revision of the Global Initiative for Asthma (GINA) guidelines recommends first assessing the level of asthma control and then planning treatment accordingly.1 For patients not controlled by low-dose inhaled corticosteroid (ICS), guidelines recommend a combination of low-dose ICS and a long-acting β2-agonist (LABA) plus a rapid-acting β2
-agonist for symptom relief, or inhaled ICS/rapid-acting LABA combination both regular and for symptom relief. This approach, called same maintenance and reliever therapy (SMART), achieves similar asthma control but more effective reduction of exacerbations in moderate to severe asthmatics (13-21).
Recent studies have undermined the axiom that treatment with ICS must be regular to achieve and maintain asthma control, as equivalent control has been obtained either with prn use of an inhaled combination of a short acting beta2 agonist (SABA) and an ICS, or with a short course of 10 days high dose ICS at the start of exacerbations. In moderate-severe asthma regularly treated with inhaled ICS/LABA combination, the symptom-driven use of the same inhaled ICS/LABA combination as reliever is superior to the symptom-driven use of SABA or LABA alone. The efficacy of the symptom-symptom-driven use of ICS/bronchodilator combination on symptoms and exacerbations may be attributed to the prompt treatment (bronchodilatation and acute antiinflammatory effects) given at the time of worsening symptoms associated with both bronchoconstriction and acute inflammation. No study has been performed so far to investigate whether the symptom-driven use of an inhaled ICS/LABA combination is equivalent or inferior to the guideline recommended regular ICS/LABA combination. This study is needed! First, in Italy more than 50% of asthma medication costs is due to fixed ICS/LABA combinations. Also, there is increasing concern on the potential adverse events of the regular use both ICS and LABA. Thirdly, the compliance with regular treatment is very low, particularly for chronic diseases such asthma whose clinical manifestations are quite variable in intensity and frequency. In this study we aim to investigate whether a symptom-driven use of formoterol/budesonide is non-inferior to the regular use of formoterol/budesonide plus prn use of terbutaline in patients with mild-moderate asthma, ie in patients with FEV1>80% predicted that are not controlled by regular treatment with low dose inhaled corticosteroids, and thus require a step up to regular treatment with ICS/LABA combination.
Backround and rationale
Asthma is a problem worldwide, with an estimated 300 million affected individuals (22). There is evidence that asthma prevalence has been increasing in the last decades in some countries, including Italy (23, 24). Analyses of the cost of asthma lead to conclude that the burden of the disease depend on the extent to which exacerbations are avoided since emergency treatment is more expensive than regular treatment (25). In Italy, it has been calculated that direct costs represent 47.3% of the total economic burden of the disease, medications being the largest component of it(26). In 2001 direct medical costs for asthma were above € 800.000.000, i.e. 1% of the entire Italian health care system expenses, asthma medications being responsible for € 650.000.000 (27). Asthma is characterized by chronic inflammation of the airways with variable airflow limitation resulting in recurrent episodes of wheezing, breathlessness, chest tightness and coughing. As inhaled corticosteroids (ICS) effectively suppress airway inflammation (28), reduce symptoms and improve lung function, they are considered the most effective controller medication currently available (25).
However, recent studies in children negate that regular treatment with ICS affects the natural history of asthma (29, 30). Thus, considering the lack of treatments that may change the natural history of asthma, the main aim of the treatment is still to reach and maintain control of asthma, as defined by the absence of limitations in daily life, of troublesome diurnal and nocturnal symptoms, normal or almost normal pulmonary function, and prevention of exacerbations (25). Indeed according to the most recent update of GINA guidelines, control has become the main determinant of the assessment of severity of asthma, as the previously proposed classification based only on symptoms and lung function was useful only in untreated patients (25). In this respect, ICS are the best mantainance treatment available to achieve and particularly to mantain control (25). However, over the past decades maintenance treatment has evolved from ICS alone to combination therapy with ICS/long-acting beta2-agonist (LABA) for patients with asthma that is not controlled on low doses ICS alone (25), which in addition to improve control is associated with reduced need of higher doses of ICS (31, 32). Because of its clinical efficacy, the use of ICS/LABA combination therapy is widely diffuse in asthma management: in Italy, more than 50% of asthma medication costs iscurrently due to fixed ICS/LABA combinations (27). However, such a wide diffusion increases therisks of inappropriate (over-) treatment with ICS/LABA combination particularly because the efficacy of low doses ICS alone may not be even looked at. Nowadays, there is also
increasing concern on the potential adverse events of both ICS and LABA, which reinforces the general concept of all asthma guidelines to provide the patients with the minimum amount of medication required to achieve and maintain control (25). Indeed, a warning has been recently raised on the use of LABA in asthma since they are suspected to increase the risk of asthma exacerbations and of asthma deaths even when associated to ICS (33). Maintenance treatment generally implies a twice daily regular treatment. However, two recent different studies have shown once-daily ICS/LABA dosing to be an effective treatment for patients with moderate asthma (34, 35). Short-acting b2 agonists, alone, are currently recommended as prn medication for symptom relief at any stage of asthma severity, even though symptoms are associated not only with bronchoconstriction but also with enhanced airway inflammation (36), and inhaled corticosteroids may rapidly exert their anti-inflammatory action (16), enhance the effect of b2 agonists (37), and be as effective as systemic corticosteroids in the treatment of mild asthma exacerbations (38). While doubling the dose of ICS is ineffective in preventing exacerbations (39), short courses of high-dose ICS at the onset of asthmatic exacerbations enhance control (40). Also, the control of mild persistent asthma obtained with short courses of high-dose ICS is no different from that obtained with regular treatment with low-dose ICS (41). A recent study shows that even the simpler symptom-driven rescue use of a ICS/short-acting beta2 agonist (SABA) combination in a single inhaler in the absence of any regular treatment is no different from regular treatment with inhaled corticosteroids in controlling mild persistent asthma (42). These findings indicate that persistent suppression of airway inflammation, as obtained by regular ICS treatment (28, 43), does not imply a better clinical effect as compared to intermittent treatment (41): it may be sufficient to suppress the flares of airway inflammation associated with the development of symptoms and exacerbations (36, 44). It has also been documented in moderate-severe asthma that a symptom-driven rescue use of a ICS/LABA combination in addition to the regular use of ICS/LABA combination is superior to the guideline recommended regular use of inhaled ICS/LABA combination plus prn use of either a short- or a long- acting beta2-agonist (18). Finally, one study has shown that symptom-drived treatment of a formoterol/budesonide combination in mild intermittent asthma is more effective both clinically and as antiinflammatory than symptom drived use of formoterol alone (45). The effect of the symptom-driven rescue use of inhaled corticosteroids plus bronchodilator combination on symptoms and exacerbations can be attributed to the prompt treatment (bronchidilatation and acute antiinflammatory effects) given at the time of worsening symptoms associated
with both bronchoconstriction and acute inflammation. Considering this background, it is surprising that the requirement of the regular (i.e. daily assumption) vs intermittent treatment with inhaled ICS/LABA combination has never been challenged in moderate asthmatics. We speculated that a symptomdriven rescue use of a ICS/LABA combination, such as budesonide/formoterol, in the absence of maintenance therapy, would be as effective as regular use of budesonide/formoterol combination plus prn use of a SABA in patients that are not controlled by regular treatment with low dose inhaled corticosteroids. Study objectives
Primary objective
The aim of the study is to verify whether asthma not controlled by low doses inhaled corticosteroids, thus in need for step up therapy, can be equally controlled by guidelines recommended regular bid treatment with ICS/LABA combination or the symptom driven use of an ICS/LABA combination in the absence of maintenance therapy. The study is designed to be able to evaluate the non inferiority of regular placebo plus prn inhaled budesonide/formoterol (experimental treatment) versus regular, twice daily 160/4.5 mcg inhaled budesonide/formoterol combination plus prn inhaled terbutaline (guidelines recommended treatment).
The primary outcome for comparison between groups will be the time to treatment failure defined as the occurrence of any one of the following events: hospitalisations, unscheduled medical visit for asthma initiated by the patients or physician, use of systemic CS for asthma or open label use of ICS for asthma as determined by physician, nocturnal awakenings (2 or more on 2 consecutive days), rescue medication use (4 times or more additional puffs per day as compared with baseline last 2 weeks of run-in- on 2 consecutive days), refusal of the patient to continue because of lack of satisfaction with treatment, or judgement by a physician should stop treatment for reasons of safety (32, 43, 46). Follow-up will be continued according to protocol after the treatment failures will be noted.
Secondary objectives
Secondary outcomes will be:
1. number of treatment failures and of drop-outs, time to first treatment failure and to drop-out;
2. differences between groups of:
b) morning and evening PEF measured during the period of intense therapy– 2-wk mean values,
c) lung function parameters measured at study visits and at the end of the period of intense therapy
d) asthma QoL scores, asthma control scores (ACQ),
e) percent and mean days without rescue medication during the treatment period f) percent and mean days without asthma symptoms during the treatment period g) percent and mean days of asthma control, i.e. without asthma symptoms and
without rescue medication, throughout the study
h) asthma symptom score – 2-wk mean values, and medication use throughout the treatment; and also end study changes from baseline of secondary outcomes a to h. i) differences between treatment groups of changes (1 to 2h) from the last 2
weeks/visit of the period of intense therapy and the last 2 weeks/visit of study treatment,
j) cumulative dose of Budesonide and of Formoterol during 1 yr study treatment and k) adverse events.
Study design
This study is a 52 weeks, multicenter, national, double-blind, double-dummy, randomized, active drug-controlled, 2-arm parallel group trial in patients with asthma not controlled by low doses inhaled corticosteroids comparing, regular placebo plus prn ICS/LABA combination (experimental treatment) with the guideline recommended regular bid ICS/LABA combination plus prn terbutaline.
The study will be divided in:
a) Run-in Period (week –6 to 0) of 6 weeks duration b) Treatment period (week 0 to 52) of 52 weeks duration
c) Follow-up period/Intense Treatment (week 52 to 58) of 6 weeks duration a. Run-in period (week –6 to week 0) Visit 1 to 2
Patients meeting the inclusion criteria will enter a 6-weeks run-in period receiving open label bid inhaled 160/4.5 mcg budesonide/formoterol combination (Symbicort Turbuhaler®) plus prn Terbutaline. At the end of run-in, patients will be elegible for recruitement providing they have stable asthma (1), i.e. post-BD FEV1 > 80% and diurnal variation < than 20% in PEF, ≤ 2/week daytime symptoms, ≤ 2/week need for rescue
treatment, no nocturnal symptom/awakening, no limitation of activities and no use of oral corticosteroids during the last 14 days of the run-in.
Patients meeting the inclusion criteria will be instructed to use: 1) a peak flow meter (MiniWright®),
2) a diary to record morning and evening PEF, asthma symptoms, nocturnal awakenings, and as needed medication use.
b. Treatment period (week 0 to week 52) Visit 2 to 9
At the end of the run-in period, elegible patients will be randomized into one of the two treatment groups: (i) bid inhaled placebo plus prn inhaled 160/4.5mcg budesonide/formoterol combination (experimental treatment); or (ii) bid inhaled 160/4.5 mcg budesonide/formoterol combination plus prn 500 mcg terbutaline (guidelines treatment).
From run-in onwards, patients will be asked to withdraw any other anti-asthma medications, and to use only the study drugs. Up to 8 rescue inhalations/day will be allowed. This means a maximum of 10 puffs per day (in total) in the arm blindly treated with bid inhaled 160/4.5 mcg budesonide/formoterol combination. Up to 12 puffs a day are currently approved for this combination.
Throughout the study, patients requiring more than 8 rescue inhalations on a single day will be asked to refer to a study physician.
c. Follow-up period/Intense Treatment (week 52 to 58) Visit 9 to 10
The follow-up phase will consist of a 6-weeks period of optimal therapy (SMART Symbicort Maintenance and Reliever Therapy) (18), in this period the patients will receive the open label medication consisting of intense treatment with 160/4.5 mcg budesonide/formoterol combination 1 inalation bid plus prn 160/4.5 mcg budesonide/formoterol combination (maximum 8) (Figure 1).
METHODS
Clinic visits will take place at the beginning (visit 1) and end (visit 2) of the run-in period, and then after 4, 12, 20, 28, 36, 44, 52 (visit 9) weeks of treatment. Visit 10 will take place at the end of the 6 week treatment phase at week 58. At study visits, the investigator will review the diary cards and verify treatment compliance; physical examination will be performed, blood pressure, heart rate, and lung function measured, and asthma symptoms, asthma exacerbations, and adverse events will be reported and asthma control questionnaire (ACQ) (47) will be administered.
On clinic visits’ days, patient will be asked to abstain from the morning inhalation of the study drug. At each study visit, post-bronchodilator lung function will be measured 30 mins after inhalation of open labelled 160/4.5mcg budesonide/formoterol combination (1 inhalation).
The asthma related quality of life will be assessed by means of a questionnaire at enrolment and at the end of treatment, and end of the intense treatment period (48).
Episodes of decreased morning PEF of more than 20% below the baseline value for 2 or more consecutive days (with or without the clinical conditions of a treatment failure), will be counted separately. Also, episodes of decreased morning PEF of more than 30% below the baseline value on 2 consecutive days (with or without the clinical conditions of a treatment failure), will be counted separately (43).
Each subject will be involved in the study for 64 weeks. This period will be divided into three phases: a 6-week run-in period, a 52 week treatment period, a 6-week follow-up period.
Recruitment starting from April 2009 and continuing until May 31, 2012. At study completion, final study report will be available after full data analysis.
Organization of the study will start as soon as approval is received.
According to data from previous trials, the percentage of patients with treatment failure at 1 year in the regular use of Budesonide/Formoterol combination bid group is expected to be approximately 35%. A non-inferiority margin of 9% at 1 year, i.e., a percentage of patients with treatment failure at 1 year in the prn Budesonide/Formoterol combination group of approximately 43%, is considered to be clinically acceptable.
Under the assumptions stated above, a total number of 355 events (ie patients with at least one event) are required to test the non-inferiority (one-sided test at 0.025 significance
level) of the time to treatment failure of the prn Budesonide/Formoterol combination group vs regular use of Budesonide/Formoterol combination bid with a power of at least 80%. Planning that patients will be enrolled roughly uniformly over a period of 1 year and that the primary analysis of time to treatment failure will be performed 1 year after the last patient is accrued, there will be 860 evaluable subjects, 430 in each of the two treatment group.
Given an estimated dropout rate of 10%, approximately 960 subjects will be enrolled in the study in about 33 Italian centres.
The 33 Clinical units participating the study are expected to recruit about 32 patients/center.
Randomization procedure will be centralized in the Clinical Research Office of the University of Modena (R. D'Amico). Patients will be randomized according to a list prepared using a SAS random number generator procedure. Each investigator will be assigned the lowest available number at his site according to chronological order of entry at recruitment. The same DPI device (Turbuhaler®) will be used in all groups and treatments to ensure a double-blind design. The devices containing regular treatments will be labelled “Trattamento Regolare, 1 inalazione al mattino e 1 inalazione alla sera”, while those for prn treatment will be labelled “Da usare al bisogno”. Patients will be instructed to take 1 inhalation in the morning and 1 in the evening from the device labelled “Trattamento Regolare, 1 inalazione al mattino e 1 inalazione alla sera” and one or more inhalations when needed for symptoms relief from the labelled “Da usare al bisogno”. Patients will be instructed to measure morning and evening PEF before taking the study medication and to record daily asthma symptoms, number of nocturnal awakenings, study drug intake and number of rescue inhalations. Daytime symptoms will be evaluated in the evening on a 4-point scale ranging from 0 (no symptoms) to 4 (symptoms for most of the day which affected normal daily activities), whereas nighttime symptoms will be evaluated in the morning on a 4-point scale ranging from 0 (no symptoms during the night) to 4 (symptoms so severe that they not allow the patient to sleep at all).
Study Population
The subjects to recruit in this study protocol are asthmatic patients either not controlled by ICS (≤500 mcg BDP/d or equivalent) or controlled with the fixed combination of low doses ICS+LABA bid (step 3 GINA 2006), i.e. patients that have initiated this treatment with combination therapy in the last year because not controlled by low doses ICS.
Inclusion criteria
Male or female out-patient aged ³ 18 years and £ 65 years
Clinical diagnosis of moderate persistent asthma for at least 6 months, according to GINA revised version 2006 guidelines
Post-bronchodilator forced expiratory volume (FEV1)³ 80% of the predicted.
Either positive methacholine challenge test (PC20 FEV1< 4mg/ml or PD20 FEV1<0.8 mg) or positive response to the reversibility test in the last year
Asthma either not adequately controlled with low-dose (≤500 mcg bechlomethasone or equivalent) inhaled corticosteroids (ICS) or controlled (as defined below*) by bid inhaled combination of lowdose ICS/long acting beta-2 agonists (LABA) i.e. patients that have initiated this treatment with combination therapy in the last year because not controlled (as defined below*) by low doses ICS
A co-operative attitude and ability to be trained to correctly use the dry powder inhalator and to complete the diary cards
Written informed consent obtained in the last month ≤2 week daytime symptoms; ≤2/week need for rescue treatment, no nocturnal symptom, awakening, no limitation of activities, and no use of oral corticosteroids
Exclusion criteria
Inability to carry out pulmonary function testing
moderate severe asthma associated with reduced lung function
history of near-fatal asthma and/or admission intensive care unit because of asthma, 3 or more courses of oral corticosteroids or hospitalization for asthma during the
previous year.
Diagnosis of COPD as defined by the GOLD guidelines
Evidence of severe asthma exacerbation or symptomatic infection of the airways in the previous 8 weeks
Current smokers or recent (less than one year) ex-smokers, defined as smoking at least 10 pack/years;
History or current evidence of heart failure, coronary artery disease, myocardial infarction, severe hypertension, or cardiac arrhythmias
Percutaneous transluminal coronary angioplasty (PTCA) or coronary artery by-pass graft (CABG) during the previous six months
Abnormal ECG
Clinically significant or unstable concurrent diseases: uncontrolled hyperthyroidism, significant hepatic impairment, poorly controlled pulmonary disease (tuberculosis, active mycotic infection of the lung), gastrointestinal (e.g. active peptic ulcer), neurological or haematological autoimmune diseases
Malignancy
Any chronic diseases with prognosis < 2 years
Pregnant or lactating females or not able to esclude pregnancy during the study period History of alcohol or drug abuse
Patients treated with monoamine oxidase inhibitors, tricyclic antidepressants or beta-blockers as regular use
Allergy, sensitivity or intolerance to study drugs and/or study drug formulation ingredients
Patients unlikely to comply with the protocol or unable to understand the nature, scope and possible consequences of the study
Patients who received any investigational new drug within the last 12 weeks Patients who have been previously enrolled in this study
Withdrawal criteria
Patients have the right to withdraw from the study at any time for any reason, including personal reasons. The Investigators have also the right to withdraw patients from the study in the event of intercurrent illness that necessitates pharmacological treatment with a disallowed drug, in case of an adverse event, treatment failure, protocol violations, poor compliance, pregnancy or any other reason.
Treatment failure
Treatment failure is defined as the occurrence of any one of the following events: hospitalisations,
unscheduled medical visits for asthma initiated by the patients or physician, use of systemic CS for asthma or
nocturnal awakenings (2 or more on 2 consecutive days),
rescue medication use (4 times or more additional puffs per day as compared with baselinelast 2 weeks of run-in- on 2 consecutive days),
refusal of the patient to continue because of lack of satisfaction with treatment, or judgement by a physician should stop treatment for reasons of safety.
According to data from previous trials, the percentage of patients with treatment failure at 1 year in the regular use of Budesonide/Formoterol combination bid group is expected to be approximately 35%. A non-inferiority margin of 9% at 1 year, i.e., a percentage of patients with treatment failure at 1 year in the prn Budesonide/Formoterol combination group of approximately 43%, is considered to be clinically acceptable.
Statistics
All statistical analyses and data processing were performed using Statistical Analysis Systems (SAS®) Software (release 9.2) on a Windows 7 operating system.
The rate of patients in the regular budesonide/formoterol group with treatment failure at one year was estimated at 35%, and a non-inferiority margin of 9% at one year was considered clinically acceptable, based on an estimated effect size of 17%.7,26 A total of 355 treatment failures (in patients with at least one) were required to test the non-inferiority (one-sided test at 0·025 significance level) of the time to treatment failure of the PRN budesonide/formoterol group versus the regular combination group, with a power of at least 80%. A total of 860 evaluable patients, 430 in each group, were required to satisfy the above hypothesis.
Kaplan-Meier estimates were used to evaluate the time to treatment failure and the probability of patients with no treatment failure at 1 year. Time to treatment failure was also analysed by using a Cox proportional hazards regression model, including only treatment in the model.
Methods for the sensitivity analyses of the primary outcome, and ANCOVA models for secondary quantitative endpoints are described in the Appendix. A post-hoc analysis was performed to evaluate the effects of baseline risk factors/covariates on the probability of treatment failure by means of a logistic analysis. The primary endpoint was assessed both in the ITT and PP population, where the ITT analysis included all randomized patients who received at least one administration of the study medication and who had at least one available post-baseline efficacy evaluation and the PP population excluded from the ITT
the efficacy data collected after the start date of the major protocol deviation. All secondary endpoint were analysed in the ITT population.
Role of the funding source
The study was funded by the Italian Medicines Agency (www.agenziafarmaco.gov.it, Agenzia Italiana del Farmaco, AIFA, FARM6BWSF9) of the Italian Ministry of Health (www.salute.gov.it). All drugs were donated by AstraZeneca (AstraZeneca S.p.a. Basiglio, Milano, Italy), which had no role in the study design.
Data were collected by the clinical investigators, analysed by CROS NT (Verona, Italy), and discussed by the clinical investigators. AstraZeneca had no role in data collection and analyses, and in drafting the manuscript, nor was informed of the results of the study. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
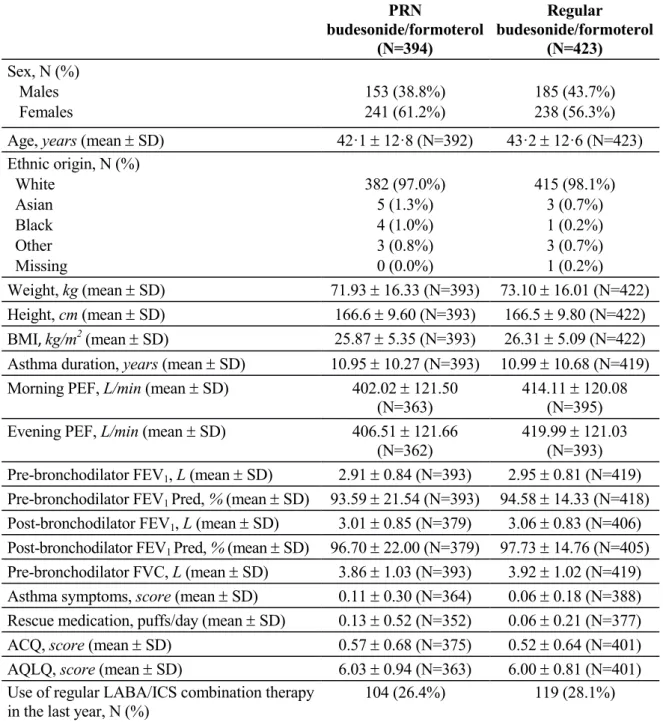
A total of 1,010 patients were screened, and 866 were randomised (424 in the PRN budesonide/formoterol group and 442 in the regular budesonide/formoterol group). Figure 1 shows the disposition of patients. The number of patients who actually received treatment (safety population) was 419 in the PRN budesonide/formoterol group and 437 in the regular budesonide/formoterol group. The intention-to-treat (ITT) population included 394 and 423 patients, respectively, and the protocol (PP) population included 393 and 422 patients, respectively.
Treatment groups were well matched in demographic and clinical characteristics at baseline (Table 3). Compliance to treatment at the end of the study period (visit 9) was of 85% (SD: 27%) and of 83% (SD: 26%) in the PRN and regular budesonide/formoterol groups, respectively (51).
Primary efficacy outcome
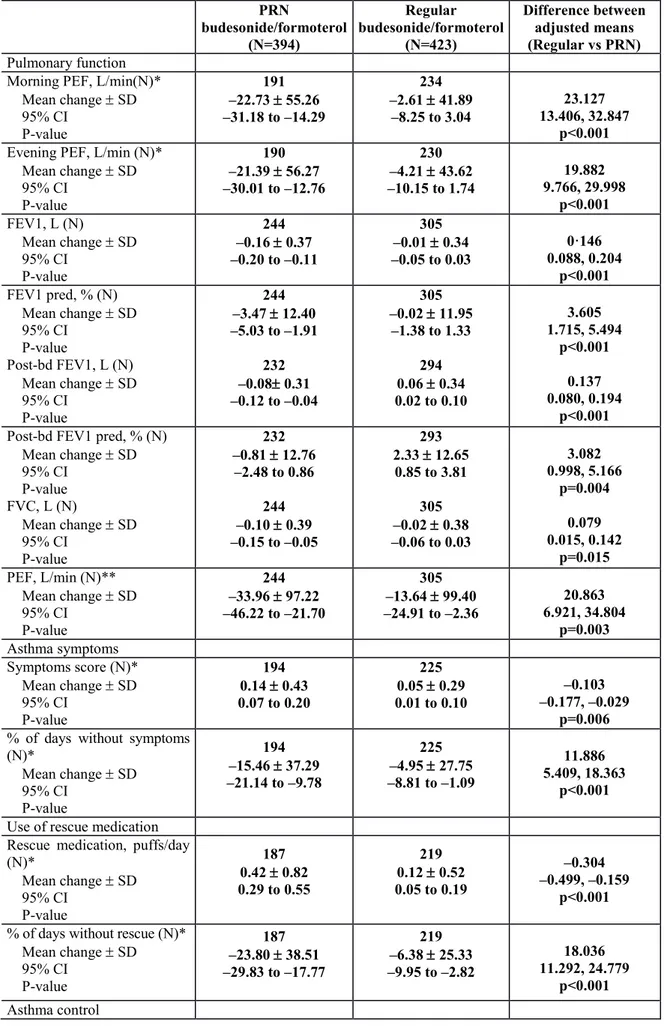
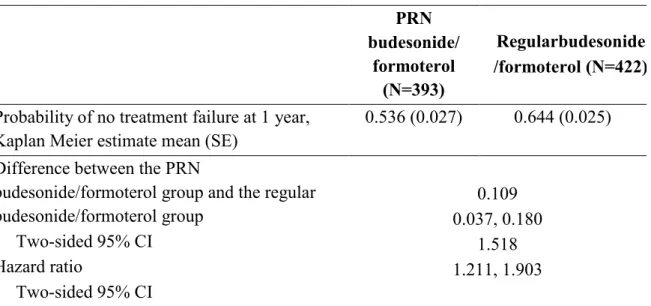
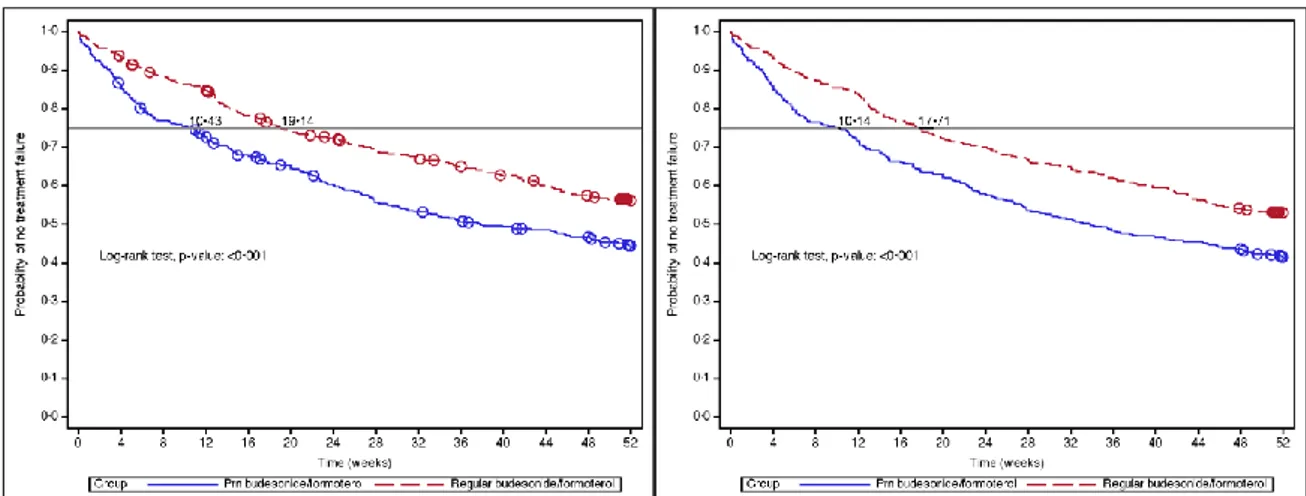
Compared to regular budesonide/formoterol therapy, patients in the PRN budesonide/formoterol group had shorter time to treatment failure (Table 4 and Figure 2) and higher probability of treatment failure (Kaplan Meier estimates, 53.6% vs 64.0%; difference: 10.3%, 95% CI: 3.2%, 17.4%, pre-defined non-inferiority limit: 9%) at one year (Table 4 and Figure 2) in the ITT population analysis. The hazard ratio between the two groups was 1.49 (95% CI, 1.19 to 1.87). The two curves for the 2 groups were resulted to be parallel and this confirmed the proportional hazards assumption. In addition we tested the correlation of the scaled Schoenfeld residuals on functions of time for both the ITT and PP population. The Pearson correlation was not statistically significant and for this reason there was not a violation of the proportional hazard assumption.
The cumulative number of patients experiencing treatment failure during the one-year study period was 170 (43.1%) in the PRN budesonide/formoterol group and 139 (32.9%) in the regular budesonide/formoterol group.
The results observed in the PP analysis were consistent with those in the ITT population (Table 4). The pre-planned sensitivity analyses, which treated drop-outs as treatment failures (Figure 2), confirmed the robustness of the results of the primary outcome. The most common reason for treatment failure was two nocturnal awakening on two consecutive days (82 patients in the PRN budesonide/formoterol group and 44 in the
regular budesonide/formoterol group). This was the only component of the composite primary outcome that differed significantly between groups (p<0.001) (Table 4a). The mean percentage of days with nocturnal awakenings was 16·17 (SD: 23.94) and 7.94 (SD: 16.07) in the PRN budesonide/formoterol and in the regular budesonide/formoterol group respectively. Female sex and smoking habit in the PRN budesonide/formoterol group, and baseline Asthma Control Questionnaire score overall and in both groups, were the factors significantly associated with higher risk of failure (Figure 3).
Secondary efficacy outcomes
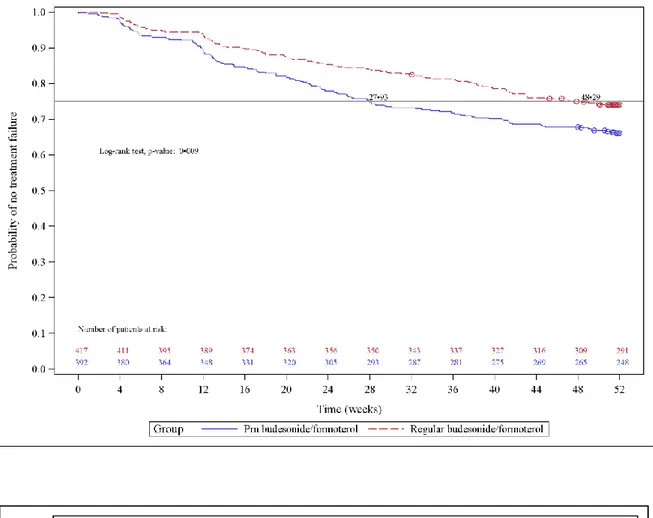
The time to drop-out was shorter in the PRN budesonide/formoterol group (28 versus 48 days, representing the time until at least 25% of the patients [first quartile] dropped out of the study; p=0.009 between groups in the log-rank test). Figure 3, shows the Kaplan-Meier plot for the time to drop-out in the ITT population. The cumulative number of patients who dropped out at the end of the randomised treatment phase was 133 (31.4%) in the PRN budesonide/formoterol group and 108 (24.4%) in the regular budesonide/formoterol group; Kaplan Meier estimates, 34.0% vs 25.9%, p=0.009).
Table 2b shows the results of the other secondary outcomes. From baseline to the end of treatment in the randomised phase of the study, there were significant differences between the two groups, in favour of regular budesonide/formoterol therapy.
After the follow-up period with open-label SMART budesonide/formoterol therapy only morning PEF (p=0.02), number of puffs of rescue medication (p=0.01), and percentage of days without use of rescue medication (p=0.004) were still significantly different in favour of regular budesonide/formoterol therapy.
Safety
Patients on PRN budesonide/formoterol combination used significantly more rescue medications, and the difference, albeit small, remained significant even at the end of the follow-up. The estimated cumulative dose of budesonide (116.8 vs 24.5 mg/year) and formoterol (3.2 mg vs 0.69 mg/year) was obviously larger in patients treated with regular budesonide/formoterol combination.
A part from the number of patients with oropharyngeal pain, the number of patients with treatment-emergent adverse events (TEAEs) was no different between the two groups (Table 4). Similarly, there were no differences in the number of patients with adverse reactions. Worsening of asthma was the most common TEAE: 48 patients (11.5%) in the
PRN budesonide/formoterol group and 40 (9.2%) in the regular budesonide/formoterol group (Table 4). From baseline to the end of the treatment period, morning serum cortisol showed no evidence of adrenal suppression in either group (data not shown).
DISCUSSION
In this one-year, randomised, double-blind, clinical trial conducted in moderate asthmatics, PRN budesonide/formoterol was inferior to regular budesonide/formoterol combination plus PRN terbutaline in preventing treatment failure. These results confirm the guideline recommended regular LABA/ICS combination treatment for patients not adequately controlled by regular ICS (1).
Nocturnal awakenings were the only component of treatment failure that was not protected by the PRN budesonide/formoterol therapy, most likely either because of lack of protection offered by the regular treatment or lack of prompt reversal of nocturnal symptoms by the PRN budesonide/formoterol combination treatment. The overall increased number of nocturnal awakening in the PRN budesonide/formoterol group was 38 episodes of nocturnal awakening in two consecutive nights in one year for the 394 patients in the ITT population, i.e. an average risk of one episode of nocturnal awakening in two consecutive night per patient in ten years, which may be considered of limited clinical relevance. The other difference between the two treatments was the higher drop-out from the study in patients on PRN budesonide/formoterol treatment (41.3% in the PRN budesonide/formoterol group and 31·2% in the regular budesonide/formoterol group). The drop-out was reported to be not related to efficacy or safety reasons, but mainly to consent withdrawn (11.5% vs 14.6%) and other logistic reasons. In a relatively young and actively working population, it is not totally surprising that the willingness (and possibility) to follow the strict rules-visit intervals- of a RCT for one entire year may be too demanding, and thus patients withdrew their consent (the main dropout reason; the same for logistic reasons), especially in a non-sponsored study, like the present one, where patients received no payment nor expense reimbursement for the participation to the study. However, the difference between the two groups (5.4% vs 8.0%) further suggest inferiority of the PRN budesonide/formoterol treatment. The results of the sensitivity analyses that took into account the study drop-outs confirmed the results of the primary analysis, excluding that they might have been affected by the different drop out.
Because of the characteristics of the population examined, i.e., patients with moderate asthma well controlled by the regular ICS/LABA combination—hence, not at high risk of exacerbations—the primary outcome of our study was rate to treatment failure. Indeed there were only 117 severe exacerbations (defined as treatment with steroids and/or
admission to the emergency room/hospital) in 817 patients during the one-year study (0.143 per patient per year): 53 (none hospitalized) in the PRN budesonide/formoterol group (0.135 per patient per year) and 64 (4 hospitalized) in the regular budesonide/formoterol group (0.151 per patient per year). Thus, both therapies were associated with a very low incidence of severe exacerbations, possibly because they were both effective in controlling exacerbations.
Poor adherence in the regular treatment might have reduced the difference in medication use between the two groups, thereby contributing the small differences in outcomes at the end of the study. In fact, Patel et al8 recently reported that adherence is lower in regular compared to SMART treatment and falls progressively over six and 12 months, suggesting that poor adherence to regular maintenance treatment might have influenced the small differences in outcomes that we observed in our study. However, the differences observed in our study likely reflect what would happen in real life by adopting the two different strategies compared in this study.
After the one-year randomised treatment, both groups of patients received a six-week SMART treatment with both maintenance and reliever budesonide/formoterol therapy to reverse uncontrolled components of asthma, if any. Both groups improved clinically and in most measurements of lung function made in the clinic (FVC, FEV1, and PEF). These
values returned to baseline, suggesting there had been no irreversible decline in lung function. However, morning PEF measured by the patient at home decreased significantly in the PRN budesonide/formoterol group and did not return to baseline after six weeks of SMART treatment. The reasons for the discrepancy between measurements of lung function made by the patient and in the clinic remain unclear (49). Although the decrease in morning PEF may suggest that the PRN budesonide/formoterol therapy may be associated with a decline in pulmonary function that was not reversible even after six weeks of SMART treatment with budesonide/formoterol, the fact that such a decline was not observed in clinically assessed PEF, FVC and FEV1 is reassuring.
The use of rescue medication and the percentage of days without the use of rescue medication remained significantly different between groups at the end of the six-week SMART follow-up therapy, suggesting that long-term PRN therapy may be associated with some persistent small reduction of control.
As expected, the number of patients with adverse reactions was low in both groups and, apart from the predictable oropharyngeal pain possibly related to the regular use of inhaled steroids, there were no other difference between groups, suggesting that safety is not an
issue in considering the two alternative therapies, at least from a one-year perspective. In particular, the use of a less intensive regimen in the PRN budesonide/formoterol group did not result in a lower risk of adverse events compared to the regular budesonide/formoterol group.
The study had some weaknesses. Two centers that initially agreed to participate in the study withdrew afterwards their willingness to participate/participation for logistic at local reasons. No patients were randomized in these centers and entered in the analysis. Also, due to the limited budget, monitoring of the centers was mainly made via teleconferences/internet and not with direct site-visits as usually performed in pharmaceutically sponsored randomized clinical trials. Moreover, paper diary card consisted a limitation for the completeness of the data related to PEF/symptoms data. This problem is well known in clinical research and the use of ePRO is more frequent to limit this aspect. The limitation of the budget was not allowing though the use of these tools. In conclusion, the results of this study show that PRN budesonide/formoterol is inferior to regular budesonide/formoterol plus PRN terbutaline in preventing treatment failure and in maintaining control. However, because the differences were small and the level of control remained above partially controlled asthma,we speculate that in recommending the regular combination treatment according to guidelines, the results of this study could be discussed with the patient, particularly reinforcing the recommendation of regular treatment with LABA/ICS combination to female patients and to patients with a significant smoking history who have a higher risk of loss of asthma control with PRN combination treatment (50),(Figure 3). Other patients could be presented with the advantages of a PRN treatment (convenience, lower cumulative dose of medications, potential long-term safety) to balance the disadvantages (lower level of control of asthma with occasional nocturnal awakening, increased use of rescue medication) (51).
TABLES AND FIGURES
Table 1: Differential diagnosis of asthma
Localized pathology Inhaled foreign body Endobronchial tumour Vocal cord dysfunction Diffuse airway pathology COPD
Eosinophilic bronchitis Bronchiectasis
Other pathologies Gastroesophageal reflux Left ventricular failure Pulmonary embolism Pulmonary eosinophilia
Table 2. Inclusion and exclusion criteria.
Inclusion criteria Exclusion criteria
1. Male or female out-patient aged 18 years and 65 years;
2. Clinical diagnosis of moderate persistent asthma for at least 6 months, according to GINA revised version 2006 guidelines;
3. Post-bronchodilator forced expiratory volume in 1 s (FEV1) 80% of the predicted;
4. Either positive methacholine challenge test (PC20
FEV1 <4 mg/ml or PD20 FEV1 <0·8 mg) or
positive response to the reversibility* test in the last year;
5. Asthma either not adequately controlled with low-dose (≤500 µg beclomethasone or equivalent) ICS or controlled (as defined below^) by b.i.d. inhaled combination of low-dose ICS/LABA, i.e., patients who had initiated this treatment with combination therapy in the last year because asthma not controlled (as defined below*) by low-dose ICS;
6. A cooperative attitude and ability to be trained to correctly use the Turbuhaler® DPI and to
complete the diary cards;
7. Written informed consent obtained.
* In the last month, ≤2 week daytime symptoms, ≤2/week need for rescue treatment, no nocturnal symptoms or awakening, no limitation of activities, and no use of oral corticosteroids.
1. Inability to carry out pulmonary function testing; 2. Moderate severe asthma associated with reduced
lung function;
3. History of near-fatal asthma and/or admission to intensive care unit because of asthma;
4. Three or more courses of oral corticosteroids or hospitalisation for asthma during the previous year;
5. Diagnosis of COPD as defined by the GOLD guidelines;
6. Evidence of severe asthma exacerbation or symptomatic infection of the airways in the previous 8 weeks;
7. Current smokers or recent (<1 year) ex-smokers, defined as smoking at least 10 pack/years; 8. History or current evidence of heart failure,
coronary artery disease, myocardial infarction, severe hypertension, or cardiac arrhythmias; 9. Diabetes mellitus;
10. Percutaneous transluminal coronary angioplasty (PTCA) or coronary artery by-pass graft (CABG) during the previous 6 months;
11. Abnormal ECG;
12. Clinically significant or unstable concurrent diseases: uncontrolled hyperthyroidism, significant hepatic impairment, poorly controlled pulmonary disease (tuberculosis, active mycotic infection of the lung), gastrointestinal (e.g., active peptic ulcer), neurological or haematological autoimmune diseases;
13. Malignancy;
14. Any chronic diseases with prognosis <2 years; 15. Pregnant or lactating females or not able to
exclude pregnancy during the study period; 16. History of alcohol or drug abuse;
17. Patients treated with monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants or beta-blockers as regular use;
18. Allergy, sensitivity or intolerance to study drugs and/or study drug formulation ingredients; 19. Patients unlikely to comply with the protocol or
unable to understand the nature, scope and possible consequences of the study;
20. Patients who received any investigational new drug within the last 12 weeks;
21. Patients who had been previously enrolled in this study.
^positive response to reversibility was considered a post-bronchodilator (400 ug salbutamol) FEV1 increase of at least 200 mL and 12% from the pre-bronchodilator values,
Table 3. Demographic and clinical characteristics of patients at baseline (ITT population) PRN budesonide/formoterol (N=394) Regular budesonide/formoterol (N=423) Sex, N (%) Males Females 153 (38.8%) 241 (61.2%) 185 (43.7%) 238 (56.3%)
Age, years (mean SD) 42·1 12·8 (N=392) 43·2 12·6 (N=423)
Ethnic origin, N (%) White Asian Black Other Missing 382 (97.0%) 5 (1.3%) 4 (1.0%) 3 (0.8%) 0 (0.0%) 415 (98.1%) 3 (0.7%) 1 (0.2%) 3 (0.7%) 1 (0.2%) Weight, kg (mean SD) 71.93 16.33 (N=393) 73.10 16.01 (N=422) Height, cm (mean SD) 166.6 9.60 (N=393) 166.5 9.80 (N=422) BMI, kg/m2 (mean SD) 25.87 5.35 (N=393) 26.31 5.09 (N=422)
Asthma duration, years (mean SD) 10.95 10.27 (N=393) 10.99 10.68 (N=419)
Morning PEF, L/min (mean SD) 402.02 121.50
(N=363)
414.11 120.08 (N=395)
Evening PEF, L/min (mean SD) 406.51 121.66
(N=362)
419.99 121.03 (N=393) Pre-bronchodilator FEV1, L (mean SD) 2.91 0.84 (N=393) 2.95 0.81 (N=419) Pre-bronchodilator FEV1 Pred, % (mean SD) 93.59 21.54 (N=393) 94.58 14.33 (N=418) Post-bronchodilator FEV1, L (mean SD) 3.01 0.85 (N=379) 3.06 0.83 (N=406) Post-bronchodilator FEV1 Pred, % (mean SD) 96.70 22.00 (N=379) 97.73 14.76 (N=405) Pre-bronchodilator FVC, L (mean SD) 3.86 1.03 (N=393) 3.92 1.02 (N=419)
Asthma symptoms, score (mean SD) 0.11 0.30 (N=364) 0.06 0.18 (N=388)
Rescue medication, puffs/day (mean SD) 0.13 0.52 (N=352) 0.06 0.21 (N=377)
ACQ, score (mean SD) 0.57 0.68 (N=375) 0.52 0.64 (N=401)
AQLQ, score (mean SD) 6.03 0.94 (N=363) 6.00 0.81 (N=401)
Use of regular LABA/ICS combination therapy in the last year, N (%)
104 (26.4%) 119 (28.1%)
N = number of patients; ACQ = Asthma Control Questionnaire; AQLQ = Asthma-Related Quality of Life Questionnaire.
Table 4. Primary and secondary outcomes
Table 4a. Primary outcome: time to treatment failure in the ITT population
PRN budesonide/ formoterol (N=394) Regular budesonide/ formoterol (N=423)
Probability of no treatment failure at 1 year, Kaplan Meier estimate (SE)
0.536 (0.026) 0.640 (0.025)
Difference between the PRN budesonide/formoterol therapy and the regular budesonide/formoterol therapy Two-sided 95% CI
Time to treatment failure during treatment period Hazard ratio Two-sided 95% CI
0.103 0.032, 0.174
1.491 1.192, 1.866
Patients who experienced at least one treatment failure
170 (43.1%) 139 (32.9%)
Reasons for first treatment failure (N)
Hospitalisation 0 3
Treatment stopped for safety reasons (physician’s judgment)
24 23
Refusal to continue because of patient
dissatisfaction with treatment 6 4
Episodes of wo nocturnal awakenings on two
consecutive days 82 44
Unscheduled medical visit for asthma worsening 6 8
Use of rescue medication 17 18
Use of systemic CS or ICS for asthma worsening 51 59
Use of systemic CS for asthma worsening 31 31