Original Paper
Ophthalmic Res 2012;47:208–213 DOI: 10.1159/000332081
Risk Factors Associated with Progression
in Exfoliative Glaucoma Patients
Gábor Holló
a
Luciano Quaranta
b
Barbara Cvenkel
d
Yuri S. Astakhov
e
Miguel A. Teus
f
Péter Kóthy
a
Stefano Miglior
c
Ivano Riva
b
Evgeny L. Akopov
e
Juan Gros
f
Jeanette A. Stewart
g
Michael S. Kristoffersen
g
Lindsay A. Nelson
g
William C. Stewart
g
a Semmelweis University, Budapest , Hungary; b University of Brescia, Brescia , and c University of Milan, Bicocca ,
Italy; d University Eye Clinic, Ljubljana , Slovenia; e Pavlov State Medical University, Saint Petersburg , Russia; f University of Alcalá, Madrid , Spain; g PRN Pharmaceutical Research Network, LLC, Cheyenne, Wyo. , USA
gressed patients (1.3, p = 0.005). A multivariate regression analysis showed higher mean, peak and variance of IOP, number of glaucoma medications at the final visit and pres-ence of a disc hemorrhage (n = 5) as independent risk factors for progression (p ^ 0.05). Conclusion: IOP reduction in XFG may be essential in reducing disease progression. The pres-ence of disc hemorrhage in XFG may suggest an increased probability of progression despite treatment to within the normal IOP range. Copyright © 2011 S. Karger AG, Basel
Introduction
Exfoliative glaucoma is the most common type of sec-ondary open-angle glaucoma [1] . Compared to primary open-angle glaucoma, it is frequently a more severe and more rapidly progressing disease [1–4] . Most authors have found that untreated intraocular pressure is higher in exfoliative glaucoma than in primary open-angle glau-coma [5–10] . Further, exfoliative glauglau-coma may be asso-ciated with greater visual field loss as well as more rapid progression of visual field and optic nerve damage than primary open-angle glaucoma [1, 2, 11] . Accordingly, treatment of exfoliative glaucoma may be more difficult
Key Words
Disc hemorrhage ⴢ Exfoliative glaucoma ⴢ Intraocular pressure ⴢ Progression ⴢ Risk factors
Abstract
Purpose: To evaluate exfoliative glaucoma (XFG) patients over 5 years, determining risk factors associated with pro-gression or non-propro-gression of glaucoma. Methods: A retro-spective, observational study. Patients were chosen from consecutive charts and data collected from each available visit included in the follow-up period. Data were abstracted for non-progressed XFG patients for 5 years and for pro-gressed patients until glaucoma worsened. Progression was determined from patient records and by disc photographs. Results: There were 71 (53%) progressed and 63 (47%) non-progressed XFG patients. Baseline parameters demonstrat-ed worse visual field damage (p = 0.014) and more prescribdemonstrat-ed medicines (p = 0.03) in progressed patients. The mean intra-ocular pressure (IOP) for progressed patients was 18.7 8 4.3 and 17.3 8 3.4 mm Hg for non-progressed patients (p = 0.047). The mean IOP that best separated the groups was 17 mm Hg with 60% staying non-progressed at or below this level and 30% above this level. At the last visit, progressed patients had more medicines prescribed (1.7) than
Received: August 4, 2011 Accepted: August 12, 2011 Published online: December 16, 2011
William C. Stewart, MD © 2011 S. Karger AG, Basel
with a higher incidence of therapeutic failure, requiring more aggressive therapy, than primary open-angle glau-coma [4, 12–14] .
Unfortunately, data on exfoliative glaucoma patients’ treatment endpoints that would help to prevent their glaucomatous progression are limited. In 2004, Konstas et al. [15] suggested that in exfoliative glaucoma patients the long-term mean intraocular pressure which best pre-vented progression was ^ 17 mm Hg. However, some pa-tients progressed despite lowering the pressure to ^ 17 mm Hg. Consequently, other risk factors for progression may exist in certain exfoliative glaucoma patients who, to date, have not been identified.
Recently, Holló et al. [16] evaluated the presence of car-diovascular disease as a potential risk factor for progres-sion of exfoliative glaucoma. Although the results were not conclusive, the study suggested that patients with a history of cardiovascular disease might require a slightly lower target intraocular pressure to prevent progression (18 mm Hg) than those without (20 mm Hg). This find-ing also suggests that additional, probably vascular risk factors of progression in exfoliative glaucoma need to be identified.
The purpose of the current study was to determine risk factors associated with progression or non-progres-sion of exfoliative glaucoma using clinical data registered during a 5-year follow-up period.
Patients and Methods
Patients
The trial was performed in five centers across Europe in Hun-gary, Italy, Slovenia, Russia and Spain. The study design was a retrospective, observational study of a single cohort. Since this was a retrospective analysis requiring no patient identifiers, an ethics committee approval was not required at any of the partici-pating research centers.
We included in this study patients with: a minimum of 5 years of records with a diagnosis of non-progressed or pro-gressed exfoliative glaucoma (based on typical anterior segment findings of exfoliation syndrome, glaucomatous optic disc and/ or nerve fiber layer changes, and/or glaucomatous visual field defect). Glaucomatous optic nerve head changes were character-ized with neuroretinal rim thinning or notching, saucerization, thin nasal rim or total cupping. Visual field changes typical for glaucoma comprised nasal step or paracentral, Seidel’s or arcu-ate scotoma. Included patients had to have at least seven visits with a documented intraocular pressure value and at least three sets of documented disc examinations (photographs, detailed disc drawings or HRT analyses) recorded during the 5-year fol-low-up period within 8 12 months of the first and final visits, respectively. Patients must have demonstrated typical anteri-or chamber findings of exfoliation syndrome including:
exfolia-tion material deposits on the lens surface, Sampaolesi’s line with irregular trabecular meshwork pigmentation by gonioscopy, moth-eaten pupillary margin and iris transillumination defects at the sphincter area [15] .
We excluded patients from this study who had progressive non-glaucomatous visual loss; refractive surgery in the study eye before or during the study period; any abnormality that prevented reliable applanation tonometry; intraocular conventional or laser surgery less than 3 months prior to the first abstracted visit; media opacity preventing reliable optic nerve head or visual field evalu-ation at the first and last abstracted visit; primary, acute or chron-ic angle closure; secondary as well as congenital glaucoma; known occludable angles by gonioscopy or presence of any other clini-cally significant angle abnormalities, or who had been enrolled in a prospective clinical trial during the follow-up period.
Procedures
Patients were chosen from consecutive charts from the prac-tices of the study investigators and reviewed alphabetically. Data collections began from the patient’s initial examination by the investigator and were recorded from each available visit included in the follow-up period. Data were abstracted for non-progressed exfoliative glaucoma patients for 5 years. In contrast, data were abstracted for progressed exfoliative glaucoma patients until the time the glaucoma worsened. Data were not abstracted after the time of progression so the information included in this study would reflect the ocular condition that worsened the glaucoma.
Data recorded from each visit included: intraocular pressure determined using Goldmann applanation tonometry, glaucoma therapeutic procedures, date of visits, dilated optic disc and vi-sual field examinations. The same investigator supervised each patient during the follow-up period. The patient’s ophthalmic and limited systemic medical history (cardiovascular, systemic hy-pertension, diabetes, and dyslipidemia), pachymetry and demo-graphics were collected at the first visit. Data on ophthalmic med-ication, cup/disc ratio, best corrected visual acuity and the visual field results were collected both at the first and last visits.
Progression was determined from the investigator’s clinical notations in the patients’ records. In each case progression was noted in the chart with the associated reason. Generally, criteria for progression were an increase in thinning of the neuroretinal rim or a reproducible worsening of glaucomatous visual field loss. In patients with total glaucomatous cupping and diffusely de-pressed visual fields, worsening of the best corrected visual acuity could also be used as the last any only measurable sign of progres-sion. Patients without ‘progression’ noted were assumed non-pro-gressed.
Statistics
PRN Pharmaceutical Research Network, LLC, analyzed the data. If both eyes of a patient met the criteria for entrance into the study, one eye was randomly chosen to be analyzed. All analyses were two sided and unpaired. A value of 0.05 was selected to de-terminate statistical significance.
An ANOVA test was used to analyze data for: age, mean and peak intraocular pressures, number of office visits, the number of medicines prescribed at baseline and at the end of the study fol-low-up period, the study term in years, baseline pachymetry, cup/ disc ratio, visual acuity, the number of laser trabeculoplasties and trabeculectomies, and systemic history [17, 18] . The F test was
used to analyze the difference in the variance (the square of the standard deviation) of the individual patient’s intraocular pres-sures measured during the follow-up period, between progressed and non-progressed patients [17] .
A 2 or Fisher’s exact test was used to analyze differences in
non-ordered scores such as: visual field diagnoses, left or right eye, gender, and the incidence of disc hemorrhage [17, 19] . The target for the intraocular pressure which best prevented glauco-matous progression was determined and described, but was not analyzed statistically. Risk factors for glaucomatous progression were also analyzed by a multivariate regression analysis.
Results
Baseline Measures
We included 134 exfoliative glaucoma patients in this study of whom 71 (53%) were progressed and 63 (47%) non-progressed. Table 1 shows the baseline characteris-tics of patients who were progressed and non-progressed over the 5-year follow-up period. At baseline, there was no statistical difference between groups for any clinical or historical parameter except for a greater number of glaucoma medicines (p = 0.03) and more visual field damage (p = 0.014) in patients who later progressed.
Table 1. B aseline patient characteristics (mean 8 SD value or number of patients)
Characteristics Detail Progressed Non-progressed p value
Patients 71 63
Age, years 69.988.2 68.088.1 0.17
Glaucoma medicines, n 1.380.9 1.080.7 0.03
Visual acuity 0.880.3 0.980.3 0.24
Pachymetry, m 535.2830.9 544.8831.0 0.10
Cup/disc ratio Vertical 0.680.3 0.680.2 0.33
Horizontal 0.680.3 0.580.3 0.09
Systemic history Cardiovascular history 9 11 0.44
Systemic hypertension 28 27 0.69
Diabetes 6 4 0.64
Dyslipidemia 2 3 0.55
Study eye Right 40 27 0.12
Left 31 36
Gender Male 31 34 0.23
Female 40 29
Visual field diagnoses Normal 19 32 0.014
Abnormal – glaucoma 42 24
Abnormal – other 4 2
T he bold line divides the characteristics of the statistical test used: above the bold line a one-way ANOVA test and below a 2 or Fisher’s exact test.
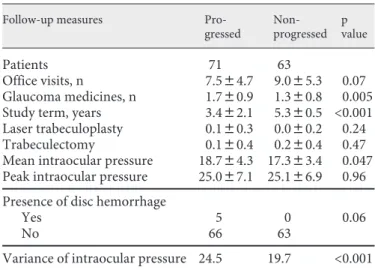
Table 2. F ollow-up measures (mean 8 SD value or number of
patients)
Follow-up measures
Pro-gressed Non-progressed p value Patients 71 63 Office visits, n 7.584.7 9.085.3 0.07 Glaucoma medicines, n 1.780.9 1.380.8 0.005 Study term, years 3.482.1 5.380.5 <0.001 Laser trabeculoplasty 0.180.3 0.080.2 0.24
Trabeculectomy 0.180.4 0.280.4 0.47
Mean intraocular pressure 18.784.3 17.383.4 0.047 Peak intraocular pressure 25.087.1 25.186.9 0.96 Presence of disc hemorrhage
Yes 5 0 0.06
No 66 63
Variance of intraocular pressure 24.5 19.7 <0.001 T he bold lines divide the characteristics by the statistical test used: the top group using one-way ANOVA test, the middle 2 or
Follow-Up Parameters
The clinical follow-up parameters are presented in ta-ble 2 . Progressed patients, as expected by the trial design of the study, had a shorter follow-up time (p ! 0.001). At the last visit, progressed patients also had more medicines prescribed (p = 0.005) as well as over the follow-up period a higher mean intraocular pressure (p = 0.047) and mean variance of the pressure (p ! 0.001).
Figure 1 shows the number of patients who progressed or were non-progressed at each mean pressure. The mean pressure which best separated non-progressed from pro-gressed patients was 17 mm Hg with 60% (45/75) staying non-progressed below this level and 40% (30/75) pro-gressed. Above this level, 30% (18/59) remained non-pro-gressed and 70% (41/59) pronon-pro-gressed.
Regression Analysis
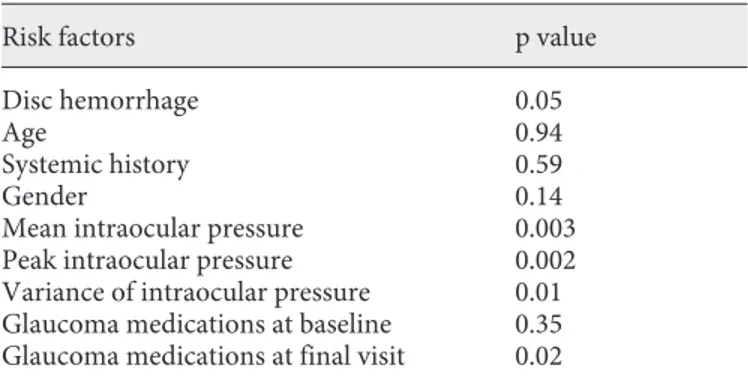
The results of the multivariate regression analysis are shown in table 3 . The mean, peak and variance of intra-ocular pressure, the number of glaucoma medications at the final visit and the presence of a disc hemorrhage were the only independent risk factors for progression (p ^ 0.05).
Discussion
The purpose of the current study was to determine risk factors associated with progression or non-progres-sion of exfoliative glaucoma using clinical data registered during a 5-year follow-up period.
Table 3. R isk factors for progression by multivariate regression
analysis
Risk factors p value
Disc hemorrhage 0.05
Age 0.94
Systemic history 0.59
Gender 0.14
Mean intraocular pressure 0.003
Peak intraocular pressure 0.002
Variance of intraocular pressure 0.01 Glaucoma medications at baseline 0.35 Glaucoma medications at final visit 0.02
0 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 Patients (n) 2 4 6 8 10 12 14 16 18 20 In traocular pr essur e (mm Hg) Non-progressed Progressed
In the current study we found that 53% of our exfolia-tive glaucoma patients under treatment progressed based on structural and/or functional findings over a 5-year follow-up period. The mean variance of the pressure was statistically higher in progressed (24.5 mm Hg) than in non-progressed patients (19.7 mm Hg). A higher variance was also previously found as a risk factor for progression in exfoliative patients by Konstas et al. [15] .
This study also found that the mean pressure was sta-tistically higher in progressed (18.7 mm Hg) than in non-progressed patients (17.3 mm Hg). The mean pressure which best separated non-progressed from progressed patients was approximately ^ 17 mm Hg. At or below this cut-off value, 60% of the exfoliative glaucoma eyes did not show progression, while at higher mean intra-ocular pressure values only 30% of the eyes remained non-progressed. This finding is consistent with the re-sults of earlier studies by Konstas et al. [15] as well as Holló et al. [16] that in exfoliative glaucoma patients the pressure level that best separated progression and non-progression over 5 years was approximately 17 mm Hg. In addition, this pressure level is similar to that found for primary open-angle glaucoma in a number, but not all studies [20–24] .
Further, in several previous investigations it has been shown that the mean intraocular pressure is an indepen-dent risk factor for progression both in exfoliative glau-coma and primary open-angle glauglau-coma by multivariate regression analysis [15, 23, 25–29] . In the current study, again the mean pressure, as well as the peak and variance of the pressure, were identified statistically as risk factors for progression. Nonetheless, despite the intraocular pressure control achieved in our current study to ^ 17 mm Hg, similarly to previously published results, 40% of the exfoliative glaucoma eyes still progressed [15] . This number is generally higher than the number reported for primary open-angle glaucoma at similar intraocular pressure levels [20–25] .
The reason that some patients progressed even with a reduced intraocular pressure has not been clarified. Since in exfoliative glaucoma cardiovascular regulation has been shown to be altered and damaged, it has been pro-posed that patients progressing despite well-controlled pressure may have resulted from a compromised cardio-vascular system [30–35] . Nonetheless, although many of our patients had a history of cardiovascular disease, the incidence was similar between progressed and non-pro-gressed patients.
However, all disc hemorrhages in our study were found in the group of progressed patients. A multivariate
regression analysis demonstrated that disc hemorrhage was a risk factor for progression of exfoliative glaucoma in our study. Bengtsson et al. [25] recently noted in a mixed primary open-angle/exfoliative glaucoma popu-lation from data obtained in the Early Manifest Glau-coma Trial, that the occurrence of a disc hemorrhage was independent of intraocular pressure achieved with treat-ment. In exfoliative glaucoma, as in primary open-angle glaucoma, a disc hemorrhage is considered to portend glaucomatous progression [34–37] . Although the num-ber of patients with disc hemorrhage in our study was small (n = 5), our results may indicate an increased risk for progressive glaucomatous damage in the presence of this finding [26, 36] .
In addition, at baseline, our progressed patients dem-onstrated more advanced glaucomatous damage and an increased need for medication at baseline, as compared to the non-progressed patients. Thus, their more ad-vanced disease may have predisposed the progressed group towards further damage during follow-up, despite effective intraocular pressure reduction.
Our results suggest that intraocular pressure reduc-tion in exfoliative glaucoma both with and without disc hemorrhage is essential to reduce disease progression. However, the presence of a disc hemorrhage in exfoliative glaucoma may indicate an increased probability of pro-gression despite treatment to within the normal intra-ocular pressure range.
The clinical importance of our data may be that exfo-liative glaucoma patients should be followed carefully be-cause of the high likelihood of progression despite an in-traocular pressure level within the normal pressure range, under treatment. Further, the presence of a disc hemorrhage may suggest an increased risk for progres-sion in exfoliative glaucoma, and may indicate a need for more intensive patient control and more aggressive intra-ocular pressure reduction. Nonetheless, prospective in-vestigations are necessary to clarify the exact role of optic nerve head hemorrhages in the progression of exfoliative glaucoma under effective intraocular pressure-lowering treatment to long-term mean intraocular pressure ! 17 mm Hg.
Disclosure Statement
References
1 Hejl A, Bengtsson B, Hyman L, Leske MC: Natural history of open-angle glaucoma. Ophthalmology 2009; 116: 2271–2276. 2 Ritch R, Schlötzer-Schrehardt U: Exfoliation
syndrome. Surv Ophthalmol 2001; 45: 265– 315.
3 Ritch R, Schlötzer-Schrehardt U, Konstas AG: Why is exfoliation syndrome associated with glaucoma? Prog Retinal Eye Res 2003; 22: 253–275.
4 Vesti E, Kivela T: Exfoliation syndrome and exfoliation glaucoma. Prog Retinal Eye Res 2001; 19: 345–368.
5 Konstas AG, Mantziris DA, Stewart WC: Di-urnal intraocular pressure in untreated exfo-liation and primary open-angle glaucoma. Arch Ophthalmol 1997; 115: 182–185. 6 Konstas AG, Stewart WC, Stroman GA, Sine
CS: Clinical presentation and initial treat-ment patterns in patients with exfoliation glaucoma versus primary open-angle glau-coma. Ophthalmic Surg Lasers 1997; 28: 111– 117.
7 Tezel G, Tezel TH: The comparative analysis of optic disc damage in exfoliative glaucoma. Acta Ophthalmol 1993; 71: 744–750. 8 Lindblom B, Thorburn W: Functional
dam-age at diagnosis of primary open angle glau-coma. Acta Ophthalmol 1984; 62: 223–229. 9 Futa R, Shimizu T, Furuyoski N, Nishiyama
M, Hagihara O: Clinical features of capsular glaucoma in comparison with primary open-angle glaucoma in Japan. Acta Oph-thalmol (Copenh) 1992; 70: 214–219. 10 Linnér E, Schwartz B, Araujo D: Optic disc
pallor and visual field defect in exfoliative and non-exfoliative, untreated ocular hyper-tension. Int Ophthalmol 1989; 13: 21–24. 11 Teus MA, Castejon MA, Calvo MA,
Pérez-Salaíces P, Marcos A: Intraocular pressure as a risk factor for visual field loss in pseudoex-foliative and in primary open-angle glauco-ma. Ophthalmology 1998; 105: 2225–2230. 12 Brooks AMV, Gillies WE: The presentation
and prognosis of glaucoma in pseudoexfolia-tion of the lens capsule. Ophthalmology 1988; 95: 271–276.
13 Blika S, Saunte E: Timolol maleate in the treatment of glaucoma simplex and glauco-ma capsulare. Acta Ophthalmol (Copenh) 1982; 60: 967–976.
14 Pohjanpelto P: Influence of exfoliation syn-drome on prognosis in ocular hypertension. Acta Ophthalmol 1986; 64: 39–44.
15 Konstas AG, Hollo G, Astakhov YS, Teus MA, Akopov EL, Jenkins JN, Stewart WC: Factors associated with long-term progres-sion or stability in exfoliation glaucoma. Arch Ophthalmol 2004; 122: 29–33.
16 Holló G, Cvenkel B, Teus MA, Irkec MT, Astakhov YS, Chiselita D, Petkova N, Lieh-neová I, Kaluzny BJ, Kóthy P, Bozkurt B, Akopov EL, Stewart JA, Kristoffersen MS, Kristoffersen CJ, Stewart WC: Is there any difference in target intraocular pressure for exfoliative glaucoma patients with cardio-vascular disease history? Eur J Ophthalmol 2010; 20: 1000–1006.
17 Book SA: Essentials of Statistics. New York, McGraw-Hill, 1978, vol 122, p 205. 18 Swinscow TD: Statistics at Square One.
Lon-don, British Medical Association, 1976, pp 54–57.
19 Moses LE, Emerson JD, Hosseini H: Statis-tics in practice. Analyzing data from ordered categories. N Engl J Med 1984; 311: 442. 20 Mao LK, Stewart WC, Shields MB:
Correla-tion between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Oph-thalmol 1991; 111: 51–55.
21 Stewart WC, Chorak RP, Hunt HH, Sethura-man G: Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Oph-thalmol 1993; 116: 176–181.
22 Stewart WC, Kolker AE, Sharpe ED, Day DG, Holmes KT, Leech JN, Johnson M, Cantrell JB: Factors associated with long-term progression or stability in primary open-angle glaucoma. Am J Ophthalmol 2000; 130: 274–279.
23 Stewart WC, Kolker AE, Sharpe ED, Day DG, Konstas AG, Hollo G, Astakhov YS, Teus MA, Stewart JA: Long-term progres-sion at individual mean intraocular pressure levels in primary open-angle and exfoliative glaucoma. Eur J Ophthalmol 2008; 18: 765– 770.
24 The AGIS Investigators: The Advanced Glaucoma Intervention Study (AGIS). 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130: 429–440.
25 Bengtsson B, Leske MC, Yang Z, Heijl A: Disc hemorrhages and treatment in the early manifest glaucoma trial. Ophthalmology 2008; 115: 2044–2048.
26 Ritch R: The Glaucomas. St Louis, Mosby-Yearbook, 1996, pp 1426–1430.
27 Seong GJ, Rho SH, Kim CS, Moon JI, Kook MS, Kim YY, Ma KT, Hong YJ, Nelson LA, Kruft B, Stewart JA, Stewart WC: Potential benefits of intraocular pressure reduction in normal-tension glaucoma in South Korea. J Ocul Pharmacol Ther 2009; 25: 91–96. 28 Nouri-Mahdavi K, Hoffman D, Coleman
AL, Liu G, Li G, Gaasterland D, Caprioli J: Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004; 111: 1627–1635.
29 Caprioli J, Coleman AL: Intraocular pres-sure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008; 115: 1123–1129. 30 Holló G, Lakatos P, Farkas K: Cold pressor
test and plasma endothelin-1 concentration in primary open-angle and capsular glauco-ma. J Glaucoma 1998; 7: 105–110.
31 Visontai Zs, Merisch B, Kollai M, Hollo G: Increase of carotid artery stiffness and de-crease of baroreflex sensitivity in exfoliation syndrome and glaucoma. Br J Ophthalmol 2006; 90: 563–567.
32 Visontai Zs, Horváth T, Kollai M, Hollo G: Decreased cardiovagal regulation in exfolia-tion syndrome. J Glaucoma 2008; 17: 133– 138.
33 Bojic L, Ermacora R, Polic S, Ivanisević M, Mandić Z, Rogosić V, Lesin M: Pseudoexfo-liation syndrome and asymptomatic myo-cardial dysfunction. Graefes Arch Clin Exp Ohthalmol 2005; 243: 446–449.
34 Prata TS, De Moraes CG, Teng CC, Tello C, Ritch R, Liebmann JM: Factors affecting rates of visual field progression in glaucoma patients with optic disc hemorrhage. Oph-thalmology 2010; 117: 24–29.
35 Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z: Predictors of long-term pro-gression in the early manifest glaucoma trial. Ophthalmology 2007; 114: 1965–1972. 36 Stewart WC: Clinical Practice of Glaucoma.
Thorofare, SLACK Inc, 1990, pp 1–40, 149– 196.
37 Sonnsjö B, Dokmo Y, Krakau T: Disc haem-orrhages, precursors of open angle glauco-ma. Prog Retin Eye Res 2002; 21: 35–56.