Alternative Recovery and Valorization of Metals from
Exhausted Catalytic Converters in a New Smart Polymetallic
Catalyst
Sebastiano Tieuli,
[a]Franco Baldi,
[a]Iztok Arčon,
[b,c]Katarina Vogel-Mikuš,
[d]Michele Gallo,
[a]Laura
Sperni,
[a]Oreste Piccolo
*,
[e]Stefano Paganelli
* [a][a] Dr. S. Tieuli, Prof. F. Baldi, M. Gallo, Dr. L. Sperni, Prof. S. Paganelli
Dipartimento di Scienze Molecolari e Nanosistemi, Università Ca’ Foscari Venezia, 30172 Venezia Mestre, Italy. E-mail: [email protected]
[b, c] Prof. Dr. I. Arčon
University of Nova Gorica, Nova Gorica 5000, Slovenia. Institut Jozef Stefan, Ljubljana 1000, Slovenia [d] Prof.Dr. K. Vogel-Mikuš
Department of Biology, Biotechnical Faculty, University of Ljubljana, Ljubljana 1000, Slovenia [e] Dr. O. Piccolo
SCSOP, via Bornò 5, 23896 Sirtori (LC), Italy. Email: [email protected]
Abstract:A new metals-polymeric composite, Metx-EPS (I), was prepared to be used as a catalyst in water or in two-phase aqueous
conditions. The metals source was an exhausted catalytic converter that was grinded and treated with an acidic solution at room temperature. After filtration, the solution was concentrated, neutralized and added to a broth of Klebsiella oxytoca DSM 29614 to produce (I) where metals are embedded in a peculiar polysaccharide structure (EPS). The composite was easily recovered from the fermentation broth and purified. The process protocol was verified many times and was shown to be reproducible satisfactorily. The % recovery of metals, originally present in the converter, was good as determined by atomic absorption. The morphology and the chemical state of main metals in (I) were investigated by X-ray absorption spectroscopy methods (XANES and EXAFS). No metallic alloy seems to be evident. The catalytic activity and a possible synergic effect due to the presence of the different metals was valuated in the hydrogenation of some substrates, valuable precursors for the production of fine chemicals.
Introduction
It is now mandatory that valuable metals have to be recovered from industrial scraps. Pyrometallurgical, hydrometallurgical or bio-hydrometallurgical methods are usually used for this purpose but each of them presents advantages and disadvantages in terms of energy consumption, necessity of special equipments or difficulty to avoid the presence of biological materials as
contaminants.[1,2] Our approach is quite specific in treating grinded exhausted catalytic converters, a very relevant metallic waste
category, as it uses a concentrated acidic solution, such as aqua regia or 80% nitric acid, at room temperature, to dissolve metals as ions, but after filtration of the solid residue, concentration and neutralization of the solution, it takes advantage of the property of the plurimetal resistant microorganism Klebsiella oxytoca DSM 29614. This microorganism, recovered from acidic waste water of an iron mine, is able to generate, in the presence of heavy metal cations, a specific capsular exopolysaccharide
(EPS) which may embed them and then be extruded from the cell so to be easily purifiable.[3,4] This is one of the resistance
mechanism of this strain to protect itself from heavy metals. So, we were able to prepare a new metals-polymeric composite,
Metx-EPS (I), which was investigated by inductively coupled plasma atomic emission spectrometry (ICP-AES), FT-IR
spectroscopy and X-ray absorption spectroscopy methods (XANES and EXAFS). To verify the catalytic activity and a possible synergic effect due to the presence of different metals embedded in EPS we decided to study, at the first, the hydrogenation of some representative molecules, precursors of valuable fine chemicals, and to compare the results with those obtained by us
using the biogenerated monometallic species Pd-EPS.[4]
Results and Discussion
Preparation and characterization of the catalyst
Metx-EPS is a new biogenerated polymetallic catalyst that contains different metals such as Al, Ce, W and the platinum-group
metals Pd, Pt and Rh. It was prepared by following a procedure tuned by us for the preparation of the mono metallic species
Pd-EPS[4] but using a complex mixture of metallic salts obtained by acid treatment of grinded exhausted catalytic converters. The
Table A. Main metals extracted from exhausted catalytic converters and present in Metx-EPSa. Analyzed Metals C (mg/L) HNO3 (n.a.) sol. (inc. ±10%)
C (mg/L) aqua regia (a.r.) sol.
(inc. ±10%) (Met
xEPS)n.a.
Metal recovery yield% b (Met xEPS)a.r.
Metal recovery yield% b
Pd 428 748 89 99 Pt 31.9 479 81 83 Rh 2.07 111 84 85 Al 1623 2413 80 >99 Ce nd 2158 nd 99 Zr nd nd nd nd W 121 174 29 10
a Experimental conditions: 20 g of exhausted catalytic converter; 400 ml of 80% HNO
3 or acqua regia; T = 20°C; t = 24 h; final
volume of the acidic solution after distillation = 80 ml.b (Amount of metal encapsulated in EPS)/(amount of metal present in the
starting acidic solution). nd = not determined
As expected, the aqua regia treatment is more efficient to extract metals from exhausted catalytic converters; however the % of recovery of metals, bonded or encapsulated in the new composite, after the biological treatment, is not significantly affected and is very high (80->99%) with the exception of tungsten (Table A). It means that using the cultural conditions for the citrate fermentation of K. oxytoca DSM 29614, the presence of chlorine anions at these working concentrations doesn’t affect the
production of its polysaccharide EPS and its ability to bind metallic species. In Figure 1 the FT-IR spectrum of (Metx-EPS),
obtained by (a.r)sol., is reported and the presence of metals bounded to capsular EPS is confirmed. It looks quite similar to the
spectrum of a previous prepared Pd-EPS[3,4] but with a very intense sharp band at 1384 cm−1.
Figure 1. FT-IR spectrum of Metx-EPS
The intense symmetric stretching of COO− vibration to 1384 cm−1, on the basis of literature, should demonstrate that carboxyl
was a contributor in the sorption of metals.[5,6] In the region between 1550-1650 cm−1 it is possible to observe two peaks, one of
them or both could be due to the asymmetric stretching of COO− vibration bounded to metals.[5] Indeed, the EPS is formed by
an eptameric structure with two D-glucuronic acids, four L-rhamnose, and one D-galactose.[7] However it is not possible to
exclude also some contribution of nitrogen species such as membrane protein amide bonds[6] or any nitrogen heterocycles such
as pyrazine or alkyl pyrazine that could be formed from ethanediol or 2,3 butanediol during the fermentation of this
microorganism.[8,9] Finally, the other intense peak at 1065 cm−1, as it was already observed for other similar EPS, is to attribute to
phosphate groups.[3,4,6] Polymetallic product (I) was also investigated by the x-ray absorption spectroscopy methods XANES and
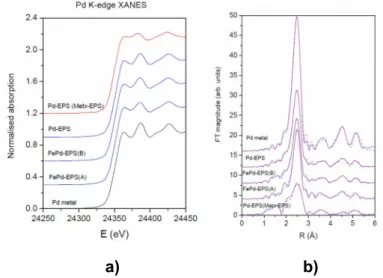
EXAFS to obtain valence and structural information of metal cations in Metx-EPS at the atomic scale. Different local environments of the cation result in different K or L absorption-edge profiles and pre-edge lines in their XANES spectra. The energy position of the absorption edge and the pre-edge features are correlated with the valence state of the absorbing atom in the sample. As known, with increasing oxidation state each absorption feature in the XANES spectrum is shifted to higher energies. Largest shifts, of the order of few eV per valence state, are observed for the energy position of the absorption edge.
[3,10] The Pd K-edge profile of Pd in Met
x-EPS are identical to the Pd XANES spectrum of the Pd metal foil with fcc crystalline
structure and also to FePd-EPS(A), FePd-EPS(B) and Pd-EPS samples, previously reported by us.[3] It clearly indicates that
palladium also in (I) is predominantly as Pd(0) (Figure 2a). For quantitative Pd EXAFS analysis, FEFF model was constructed, based on Pd metal with fcc structure with the lattice constant a = 3.8900 Å. The contributions of the first four coordination shell in the R range from 1.0 Å to 5.1 Å were analyzed (Figure 2b). The FEFF model comprised four single scattering and all multiple scattering paths from first four neighbour shell in Pd metal. In addition, one Pd-O and Pd-Pd single scattering path was included in the model, which could be expected, if part of Pd atoms is present in the form of amorphous Pd oxide or in the form of PdOx
nanoparticles. The Pd foil spectrum was used to calibrate the model. An excellent fit was obtained in the k range of 3 Å-1 to 16 Å
-1 and the R range of 1 to 3.1 Å. The complete list of best fit parameters is given in Table 1-SI in the supplementary materials.
Results of Pd K-edge EXAFS analysis (Figure 2b) confirm that Pd in (I) is in the form of Pd(0) metallic nano-particles with fcc crystalline structure, similar to that found in mono- and bi-metallic (Pd-EPSand FePd-EPS) samples from previous
investigations.[3] Pd neighbours in four coordination shells are Pd at same distances and slightly larger Debye–Waller factors as
in the Pd metal foil with fcc crystal structure, but the average coordination numbers are significantly lower than in the bulk Pd
metal or in Pd-EPS and FePd-EPS complexes.[3] This clearly indicates that smaller nano-clusters of Pd metal with fcc crystalline
structure are formed, than in the case of Pd-EPS and FePd-EPS complexes. From the observed reduction of the average number of neighbours (Table 1-SI), the size of these nano-clusters can be deduced: the average size of Pd metal nanoparticles is smaller than 1 nm. Furthermore, in EXAFS analysis of Pd neighbourhood, we found that part of Pd atoms is coordinated to oxygen atoms at about 1.97 Å which is characteristic for Pd oxides (Table 1-SI). So, it is possible to assume that about 10% of
Pd atoms is in the form of PdOx nanoparticles or that Pd atoms located on the surface of the Pd metallic nanoparticles are
oxidised. The results suggest that the Pd atoms on the surface of the Pd metallic nanoparticles may be bound to the OH or COOH groups of EPS; alternatively nano Pd oxides particles and Pd metallic nanoparticles may simply be encapsulated in the polysaccharide moiety. The results are similar, but not identical, to those on mono- and bi-metallic samples, here also reported
for a direct comparison (Figure 2b).[3]
a) b)
Figure 2. a) Pd K-edge XANES spectra of the Pd-species in Metx-EPS sample compared to Pd-EPS, EPS(A), FePd-EPS(B) samples and reference Pd metal foil with f-c.c. crystal structure; b) Pd EXAFS Fourier transform magnitude of
k3-weighted Pd EXAFS spectra of Pd-EPS, FePd-EPS(A), FePd-EPS(B), Pd-species in Metx-EPS samples and Pd metal foil,
calculated in the k range 3–16 Å-1 and R range 1–5.5 Å. Experiment: solid line; best-fit EXAFS model of the nearest coordination
shells: dashed line.
The Pt L3-edge XANES analysis was used to determine the Pt valence states in (I). If the sample contains a mixture of two or more compounds of the same cation with different local structure and valence state, the measured XANES spectrum is a linear combination of individual XANES spectra of the different cation sites. Relative amounts of the cation in each valence state in the sample can be determined by the linear combination fit (LCF) with XANES spectra of reference compounds with known valence states of the element, with similar symmetry, same type of neighbour atoms in nearest coordination shells, arranged in a similar
local structure.[10] The Pt XANES analysis would suggest that there are two forms of Pt in (I), one is metallic Pt(0) nanoparticles
(about 36 %), as expected, the other in the form of a Pt(II) complex bound to two Cl atoms and two nitrogen atoms like in PtCl2(pyridine)2 (Figure 3a). Pt L3-edge EXAFS results strongly support this finding (Figure 3b, Table 2-SI). In the local neighborhood of Pt we found in average about one N at 2.04 Å and one Cl at 2.37 Å, i.e. at distances characteristic for a square
planar PtCl2[N]2 coordination.[10] In the second coordination shell 4 to 5 carbon neighbours are detected at 2.97 Å, which might
be ascribed to the carbon atoms in two nitrogen heterocyclic rings. In addition about 7 Pt neighbours are found at the distance of 2.97 Å, characteristic for the first coordination shell of Pt in fcc crystal structure of Pt metal. The results clearly suggest that the microorganism Klebsiella oxytoca DSM 29614 encapsulates Pt species in EPS partly in the form of metallic Pt(0) nanoparticles
and partly in the form of complexes structurally similar to PtCl2(pyridine)2. It is difficult to have a clear-cut explanation for the
latter form, but perhaps PtCl2 originally added to the fermentation broth formed a complex with some nitrogen heterocycles,
11540
11560
11580
11600
0.0
0.5
1.0
1.5
Pt in Metx-EPS Pt metal (36%) N or m al is e d a b so rp tio nE (eV)
Experiment Linear combination fitPtCl2-Pyridine(64%) 0 1 2 3 4 5 6 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 Pt in Metx-EPS F T m ag ni tu de ( ar b. u ni ts ) R (Å)
a) b)
Figure 3. a) Pt L3-edge XANES spectrum measured on the Pt in Metx-EPS sample: Black solid line: experiment; magenta
dashed line: best-fit linear combination of XANES profiles of Pt fcc metal (36%) and cis-dichlorobis pyridine platinum reference compound (64%); b) Fourier transform magnitude of k2-weighted Pt L3-edge EXAFS spectra of Pt in Metx-EPS sample,
calculated in the k range 3–12 Å-1 and R range 1–3.8 Å. Experiment: solid line; best-fit EXAFS model of the nearest coordination
shells: dashed line.
The Ce L3-edge XANES analysis was used to determine the Ce valence state in the Metx-EPS sample (Figure 4). Comparison
with CeVO4 and CeO2 XANES spectra as references for Ce3+ and Ce4+, respectively, clearly shows that all Ce in the Metx-EPS
sample is in the form of three valent Ce cations, coordinated to 8 oxygen atoms, most probably in the form of Ce3+ oxide
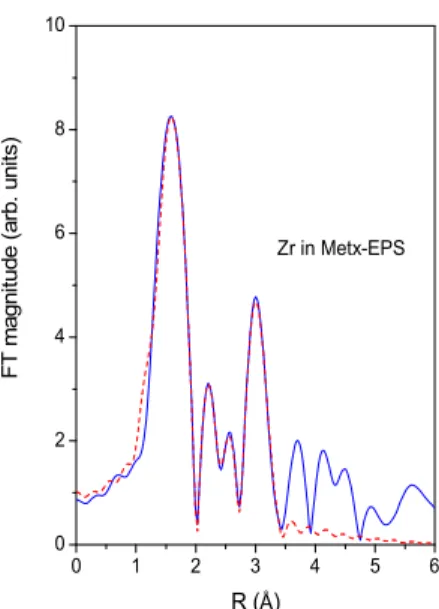
nanoparticles.[11] Finally the results of Zr and Rh EXAFS analysis of Met
x-EPS sample (Figures 5 and 6, Tables 3-SI and 4-SI)
suggest that both elements, Zr and Rh, are stored in EPS in oxidized form, as a mixture of different Zr and Rh nano-oxides.
Relatively large noise level in the measured EXAFS spectrum is due to low Rh concentration in the Metx-EPS sample. Zr is
coordinated to oxygen atoms in the nearest coordination shells and to Zr neighbours in second coordination sphere, at Zr-Zr
distances characteristic for Zr oxides.[12] Rh is coordinated to six oxygen atoms in the nearest coordination shells and to Rh
neighbours in second coordination sphere, at Rh-Rh distances characteristic for crystalline Rh2O3.[13] There is no evidences of
larger Zr or Rh oxide crystal particles. Coordination of part of Zr or Rh cations to OH and COOH groups present in EPS cannot be excluded. 5710 5720 5730 5740 5750 5760 5770 0 2 4 6 Ce in Metx-EPS CeVO4 N or m al is ed a bs or pt io n E (eV) CeO2
Figure 4. Ce L3-edge XANES spectra of the Ce species in Metx-EPS sample compared to CeVO4 and CeO2 spectra as
0 1 2 3 4 5 6 0 2 4 6 8 10 Zr in Metx-EPS F T m ag ni tu de ( ar b. u ni ts ) R (Å)
Figure 5. Fourier transform magnitude of k3-weighted Zr K-edge EXAFS spectra of Zr in Metx-EPS sample, calculated in the k
range 3–11 Å-1 and R range 1–3.4 Å. Experiment: solid line; best-fit EXAFS model of the nearest coordination shells: dashed
line. 0 1 2 3 4 5 6 0.0 0.2 0.4 0.6 0.8 1.0 Rh in Metx-EPS F T m ag ni tu de ( ar b. u ni ts ) R (Å)
Figure 6. Fourier transform magnitude of k2-weighted Rh K-edge EXAFS spectra of Rh in Metx-EPS sample, calculated in the k
range 3–11 Å-1 and R range 1–3.4 Å. Experiment: solid line; best-fit EXAFS model of the nearest coordination shells: dashed
line.
Catalyst activity
We evaluated the catalytic activity of Metx-EPS in the hydrogenation of some interesting molecules as trans-cinnamaldehyde, benzaldehyde, furfural and 5-(hydroxymethyl)furfural (HMF), key platform molecules from biomasses, (Scheme 1), and some nitroarenes as nitrobenzene, 4-nitrobenzaldehyde and 1-chloro-3-nitrobenzene (Scheme 2).
Scheme 2. Hydrogenation of nitroarenes
Hydrogenation of functionalized aldehydes
trans-Cinnamaldehyde (1) was chosen as α,β-unsaturated carbonyl model compound (Scheme 1, Table 1). The chemoselective
hydrogenation of α,β-unsaturated aldehydes to the corresponding saturated aldehydes or to the unsaturated alcohols is a very important reaction for the synthesis of valuable fine chemicals. Still now selectivity is a challenging problem and it strongly
depends on the nature of the active metal catalyst and on the hydrogenation reaction conditions.[14-17] As reported in the
literature, heterogeneous Pd catalysts preferentially reduce the carbon-carbon double bond of -unsaturated aldehydes;
however, the activity and selectivity of supported palladium catalysts can be affected by pH and by suitable promoters.[18-20] Pt
and Rh based catalysts, on the other hand, are active towards the hydrogenation of both C=C and C=O double bond.[21-24] The
first test was carried out in H2O/THF at 50°C and 0.2 MPa of H2 for 3h, with a substrate/Pd 1515/1 (mol/mol), corresponding to a
substrate/Pt 5450/1 (mol/mol). The substrate conversion was 95% and both the saturated aldehyde (2) (73%) and the corresponding alcohol (3) (21%) were formed; moreover, also a very small amount of cinnamyl alcohol (4) was observed (entry 1, Table 1). Very interestingly, the catalytic system was used in a recycle experiment without significant loss of activity (entry 2, Table 1). Noteworthy, the monometallic species Pd-EPS, previously used by us in some hydrogenation experiments, had promoted almost exclusively the hydrogenation of the carbon-carbon double bond, being the formation of alcohol (3) practically
negligible.[4] The hydrogenation of both C=C and C=O double bonds is very probably due to the synergic effect of the different
metals present in Metx-EPS. As the catalytic system is not soluble in water but only suspended in it, the same reaction was
carried out also in only THF at the above reaction conditions: conversion was 4% and 3-phenylpropanal (2) was the only reaction product (entry 3, Table 1), so confirming the importance of water for the catalytic system. This phenomenon had been already observed by using Pd-EPS; we hypothesized that water causes the swelling and the rearrangement of the polysaccharide (EPS) structure, making the active sites more available for catalysis.[4] As nanostructured metal species
incorporated in a complex organic matrix may not be completely available to the reagents, we verified if sonication could improve the catalytic performances. As a matter of fact, the sonication of a catalytic system can be very efficient by increasing
both activity and selectivity of hydrogenation reactions.[25, 26] So, Met
x-EPS was first suspended in water, sonicated for 1h, then
used in the hydrogenation of cinnamaldehyde (1) at the above reaction conditions: disappointingly, its activity was decreased, especially in the recycling experiments (data not reported in the table).
Table 1. Hydrogenation of trans-cinnamaldehyde (1) catalyzed by Metx-EPS
Reaction conditions: substrate = 1.97 mmol, substrate/Pd = 1515/1 (mol/mol), substrate/Pt = 5450/1 (mol/mol), H2O = 3 mL, THF = 3 mL, T = 50°C, t =
3h., nd = not determined. a Determined by GC. b Experiment carried out by using the catalytic phase recovered from the previous reaction. cExperiment
carried out by using only THF as solvent.
Due to the capability of Metx-EPS to catalyze also the reduction of the carbonyl group, we tested its activity in the hydrogenation
of benzaldehyde (5) to benzyl alcohol (6) (Scheme 1, Table 2). Benzyl alcohol (6) is an important commercial product, used as solvent for inks, dyes, lacquers and as precursor for the synthesis of esters employed in the cosmetics and fragrances industry.
[27] A first test was carried out at 40°C and 0.2 MPa of H
2 for 3h, with a substrate/Pt 1000/1 (mol/mol), corresponding to a
substrate/Pd 278/1 (mol/mol). Benzyl alcohol (6) was selectively obtained in quantitative yield (entry 1, Table 2), being the hydrogenation of the aromatic ring not observed. Noteworthy, the catalyst was used in two recycling experiments and its activity was maintained practically unchanged (entries 2 and 3, Table 2). A very good result was obtained using water as the sole solvent: lowering drastically the catalyst amount and increasing the reaction temperature up to 60°C, the hydrogen pressure to 1 MPa and time to 6h, benzyl alcohol (6) was obtained in 98% yield (entry 4, Table 2).
Table 2. Hydrogenation of benzaldehyde (5) catalyzed by Metx-EPS
Entry Substrate/Pt; Substrate/Pd (mol/mol) T (°C) P(H2) (MPa) t (h) Conv.(%) a 6 (%) a
1 1000; 278 40 0.2 3 >99 >99
2b 1000; 278 40 0.2 3 98 98
3b 1000; 278 40 0.2 3 97 97
4c 5000; 1390 60 1 6 98 98
Reaction conditions: substrate = 0.41 mmol, H2O = 3 mL, THF = 3 mL. a Determined by GC. b Experiment carried out by using the catalytic phase
recovered from the previous reaction.c Experiments carried out by using only H2O as solvent.
Today a great interest is devoted to the valorization of biomasses as alternative to traditional fossil resources to obtain energy,
fuels and chemicals.[28-36] Furfural (7) and 5-(hydroxymethyl)furfural (HMF) (11) are so-called platform molecules, obtained from
biomass, which can be used for the synthesis of chemicals and fuels.[37, 38] Hydrogenation is one of the most widely used
reactions for furfural valorization, yielding several products, such as furfuryl alcohol, methylfuran, methyl tetrahydrofuran, furan, tetrahydrofuran, cyclopentanone and/or olefins. Furfuryl alcohol (8), for instance, has important applications in the production of
resins, fibers or as a solvent;[39, 40] moreover, the transformation of furfural (7) into the corresponding furfuryl alcohol (8) is one of
the methods used to avoid the condensation and polymerization reactions to which furfural is subjected and that entail
considerable difficulties in its conservation and storage.[41] Copper chromite is used in the furfural industrial hydrogenation[42] but,
in order to avoid the high toxicity of chromium species, many other catalysts have been developed.[43-54] So, considering the
interest for the furfural molecule and its derivatives, we decided to test the activity and selectivity of Metx-EPS in the
hydrogenation of furfural (7) (Scheme 1, Table 3). The reaction was carried out in H2O/THF at 60°C and 1 MPa of H2 for 20h
with a substrate/Pt 500/1 (mol/mol), corresponding to a substrate/Pd 139/1 (mol/mol). Conversion was 90% and, besides
furfuryl alcohol (8), obtained in 63% yield, also 20% of tetrahydrofurfuryl alcohol (9) and 7% of tetrahydrofurfural (10) were formed (entry 1, Table 3). In this case, unlike the hydrogenation of benzaldehyde (5), the catalyst was also responsible for the reduction of the heteroaromatic ring; this fact can be attributed to the higher olefinic character of the double bonds in the heterocyclic ring. The aqueous phase containing the catalyst was then recycled at the same reaction conditions, affording 53%
Entry pH2 (Mpa) Conv.(%) a 2 (%) a 3 (%) a 4 (%) a
1 0.2 95 73 21 1
2b 0.2 92 70 20 2
of furfuryl alcohol (8), 32% of tetrahydrofurfuryl alcohol (9) and 3% of tetrahydrofurfural (10) (entry 2, Table 3). Finally, very good conversions were obtained also carrying out the reaction in water as the sole solvent in both the pristine reaction and in a recycling experiment (entries 3 and 4, Table 3).
Table 3. Hydrogenation of furfural (7) catalyzed by Metx-EPS
Reaction conditions: substrate = 0.50 mmol, H2O = 3 mL, THF = 3 mL, T = 60°C, t = 20h. a Determined by GC. bExperiment carried out by using the
catalytic phase recovered from the previous reaction. c Experiments carried out by using only H
2O as solvent.
Also HMF (11) is a very versatile molecule, from which it is possible to obtain, on a large scale, several intermediates such as 5-hydroxymethyl-furanoic acid and 2,5-dicarboxyfuranoic acid, an interesting alternative to terephthalic acid for the synthesis of
polyesters,[55] 2,5-bis(hydroxymethyl)furane (12) used, for instance, for the production of polyurethane foams,[56] and 2,5-bis
(hydroxymethyl)tetrahydrofurane (14), that can be used as a diol in the synthesis of polyesters.[57, 58] Different conventional
catalysts based on Ni, Pd, Pt, Cu and Ru have been employed for the hydrogenation of HMF ( 11) and the products distribution
depends on the reaction conditions and on the catalyst used.[29, 59-61] Therefore we considered useful to apply Met
x-EPS in the
hydrogenation of 5-hydroxymethylfurfural (11) (Scheme 1, Table 4). Due to the good solubility of 5-hydroxymethylfurfural (11) in
H2O, we carried out a hydrogenation experiment in H2O as the only solvent, at 60°C and 1MPa of H2 for 20h, with a substrate/Pt
500/1 (mol/mol), corresponding to a substrate/Pd 139/1 (mol/mol). The reaction in the homogeneous aqueous phase gave a very good conversion (87%) but selectivity was not very high, affording a mixture of 2,5-bis(hydroxymethyl)furane ( 12) (62%), tetrahydro-5-(hydroxymethyl)furan-2-carbaldehyde (13) (9%) and 2,5-bis (hydroxymethyl)tetrahydrofurane (14) (16%) (entry 1, Table 4). The catalyst was employed in a consecutive recycling experiment without significant loss of activity and the same selectivity (entry 2, Table 4). When the reaction was carried out at the same reaction conditions but with a lower amount of catalyst, conversion was only 66% without any improvement in selectivity (entry 3, Table 4).
Table 4. Hydrogenation of 5-hydroxymethylfurfural (11) catalyzed by Metx-EPS
Entry Substrate/Pt; Substrate/Pd (mol/mol) P(H2)
(MPa) Conv.(%) a 8 (%) a 9 (%) a 10 (%) a
1 500; 139 1 90 63 20 7
2b 500; 139 1 88 53 32 3
3c 1000; 278 1 84 54 23 8
Reaction conditions: substrate = 0.50 mmol, H2O = 3 mL, T = 60°C, t = 20h. a Determined by GC. b Experiment carried out by using the catalytic phase
recovered from the previous reaction.
Hydrogenation of nitroarenes
Anilino derivatives are widespread used for the industrial production of pigments, azo dyes, rubbers, amino-resins, herbicides
and other fine chemicals.[62] The hydrogenation of nitroarenes is usually the method of choice for the production of these
compounds[63] and many heterogeneous and homogeneous catalysts have been employed.[64-67] Recently, different supported
catalysts containing palladium[67-71] or platinum[67, 69, 72, 73] nanoparticles have been developed to improve reactivity, recoverability
and selectivity of this reaction. Much attention has been focused also on bi- and poly-metallic catalysts[75, 75] due to the possibility
of cooperation and synergy phenomena that can occur between the different metal centers. So Metx-EPS was tested here in the
hydrogenation of some aromatic nitrocompounds and its catalytic activity compared to that of the monometallic species Pd-EPS.
In our experiments we investigated the activity of Metx-EPS in the hydrogenation of nitrobenzene (15), chosen as model
substrate (Scheme 2, Table 5). Complete reduction of 15 to aniline (16) was obtained working with a substrate/Pd (molar ratio)
= 5000/1, corresponding to a substrate/Pt 18000/1 (mol/mol), at 30°C and 0.2 Mpa of H2 for 18h, both in the pristine reaction
and in a recycling experiment (entries 1 and 2, Table 5). The catalytic activity was strongly decreased by halving the catalyst amount: conversion was only 40% and besides 16 also 5% of nitrosobenzene was formed (entry 3, Table 5). However, even if low, the activity remained practically unchanged in a recycling experiment (entry 4, Table 5).
Table 5. Hydrogenation of nitrobenzene (15) catalyzed by Metx-EPS
Reaction conditions: substrate = 0.38 mmol, H2O = 3 mL, THF= 3 mL. a Determined by GC. b Experiment carried out by using the catalytic phase recovered from
the previous reaction. c Nitrosobenzene was found (4-5%).
The most relevant problem in the reduction of nitroarenes containing other reducible functionalities is selectivity.[63, 67] Great
efforts have been made to seek highly selective catalysts for the preferential hydrogenation of the nitro group[76-79] as
functionalized anilines are intermediates for industrially important products and fine chemicals.[65, 80] Met
x-EPS contains metals
capable, in principle, to reduce both the nitro and the carbonyl group, therefore we tested the catalytic system in the
hydrogenation of 4-nitro-benzaldehyde (17) (Scheme 2, Table 6). After 20h at 60°C and 5 Mpa of H2 substrate conversion was
quantitative and the main product was 4-aminobenzyl alcohol (18) (81%) being only 19% of 4-aminobenzaldehyde (19) formed (entry 1, Table 6). By lowering the reaction time to 6h, again conversion was complete but the selectivity was practically reverse, affording 87% of 19 and 13% of 18 (entry 2, Table 6). The results obtained show that the reduction of the nitro group is, in any case, favored with respect to the carbonyl moiety and the selectivity may be controlled by the reaction time.
Table 6. Hydrogenation of 4-nitrobenzaldehyde (17) catalyzed by Metx-EPS
Entry Substrate/Pt; Substrate/Pd (mol/mol) P(H2)
(MPa) Conv.(%) a 12 (%) a 13 (%) a 14 (%) a
1 500; 139 1 87 62 9 16
2b 500; 139 1 85 63 7 15
3 1000; 278 1 66 56 3 7
Entry Substrate/Pd; Substrate/ Pt) (mol/mol) T (°C) P(H2) (Mpa) t (h) Conv.(%) a 16 (%)a
1 5000; 18,000 30 0.2 18 >99 >99
2b 5000; 18,000 30 0.2 18 >99 >99
3c 10,000; 36,000 30 0.2 18 40 35
Entry t (h) Conv.(%)a 19 (%)a 18 (%)a
1 20 >99 19 81
2 6 >99 87 13
Reaction conditions: substrate = 0.38 mmol, substrate/Pt = 500/1, substrate/Pd = 139/1 (mol/mol), H2O = 3 mL, THF = 3 mL, T = 60°C, pH2 = 5
MPa. a Determined by GC.
Among functionalized nitroarenes, compounds containing also a halo atom on the aromatic ring are of great interest as the
corresponding aromatic haloamines are important intermediates in the synthesis of different fine chemicals.[81] These amines are
mainly produced by chemoselective hydrogenation of the corresponding halonitroarenes but hydrodehalogenation is a serious
drawback. This undesired side reaction depends on several factors: i) the type of halo atom (I> Br> Cl> F) and its position on the aromatic ring; ii) the metal component of the catalyst; iii) the reaction conditions.[81, 82] In order to improve the
chemoselectivity of this reaction, several catalysts, based on different metals, have been developed,[6, 82-84] but the selective
hydrogenation of the nitro group with no hydrogenolysis of the carbon–halo bond is indeed a challenge. The selectivity of this
reaction may be affected by the use of additives or by a pre-treatment of the catalyst[865] but also by metal dispersion, support
interactions or by the presence of a second metal.[86] Aromatic chloroanilines, obtainable by selective hydrogenation of the nitro
group of chloro nitroarenes, are valuable industrial products.[87, 88] The selectivity depends on the type of catalyst and on the
reaction conditions.[87-90] So, it was interesting to evaluate if the different metals present in Met
x-EPS could influence the
selectivity of the hydrogenation of 1-chloro-3-nitrobenzene (20) (Scheme 2, Table 7).The first test was carried out in H2O/THF at
60°C and 5 MPa of H2 for 20h. Conversion was quantitative to afford quite pure m-chloroaniline (21) (95%), being aniline (16)
formed only for (5%) (entry 1, Table 7). Also in two consecutive recycling experiments the catalyst maintained its activity, affording quantitative conversion and almost total selectivity to 21 (entries 2 and 3, Table 7).
Table 7. Hydrogenation of 1-chloro-3-nitrobenzene (20) catalyzed by Metx-EPS
Entry t (h) Conv.(%)a 16 (%)a 21 (%)a
1 20 >99 5 95
2b 20 >99 2 98
3b 20 >99 1 99
Reaction conditions: substrate = 1.34 mmol, substrate/Pd (mol/mol) = 500/1, substrate/Pt = 1700/1, H2O = 3 mL, THF = 3 mL, T = 60°C, pH2 = 5 MPa. a
Determined by GC. b Experiment carried out by using the catalytic phase recovered from the previous reaction.
Conclusions
The idea to prepare new polymetallic species embedded in a polysaccharide moiety starting from spent catalytic converters was realized, as a contribution of alternative valorisation of metallic wastes. Despite the difficulty of investigating and having detailed information on such a complex polymetallic material, atomic absorption and X-ray absorption spectroscopy methods (XANES
and EXAFS) were carried out and afforded useful data. The recovery of different metals in Metx-EPS was very high. It was
possible to establish that Pd is mainly present as nanoparticles of zerovalent metal, Pt partially as metal (0) and partially as a
complex of Pt+2 with a nitrogen ligand embedded in EPS, while other metals (Rh, Ce, Zr) are present as nanosized oxides,
which may be bound to the functional EPS groups. No metallic alloy seems to be evident. The new metals-polymeric composite Metx-EPS (I) was successfully employed, as catalyst, in the aqueous biphasic hydrogenation of different substrates, with
fine-excellent performances. Analogously to the monometallic catalytic species Pd-EPS,[4] even if not soluble in water, Met
x-EPS
showed to be active in an aqueous environment. The different metals, present in Metx-EPS, exerted a synergic effect in the
catalysis, affecting reactivity and selectivity when compared with Pd-EPS. When different functional groups were in the reacting substrate the selectivity strongly depended on the reaction conditions and on the nature of the substrate. Noteworthy, the recovered aqueous phase containing the catalyst maintained its activity in recycling experiments.
Supporting Information Summary
Supporting information PDF file contains the experimental procedure for the preparation of the new metals-polymeric composite
Metx-EPS (I) and the general procedure for the hydrogenation experiments. Moreover, parameters of the nearest coordination shells
around Pd, Pt, Zr and Rh atoms in Metx-EPS, obtained by EXAFS analysis, are reported in Tables 1-SI, 2-SI, 3-SI and 4-SI,
respectively.
Acknowledgment
The research performed at Venice university was supported by MIUR and by SCSOP. Mrs. Barbara Vicentini (Ca’ Foscari University – Venice) is acknowledged for IR spectra. The research of I. Arčon and K. Vogel-Mikus was supported by the Slovenian Research Agency (P1-0112) and by the project CALIPSOplus under the Grant Agreement 730872 from the EU Framework Programme for Research and Innovation HORIZON 2020. Access to the synchrotron radiation facilities at ALBA (BL22 (Claess), project and of ELETTRA (beamline XAFS, projects pr. 20165258 and 20160239) is acknowledged. I.A. and K.VM would like to thank Giuliana Aquilanti of ELETTRA, and Wojciech Olszewski of ALBA for support and expert advice on beamline operation.
Conflict of Interest
The authors declare no conflict of interest.
Keywords: Biogenerated polymetallic exopolysaccharide; biphasic catalysis; hydrogenation; metals-polymeric composite; new
catalyst from metallic wastes
[1] U. U. Jadhav, H. Hocheng, J. Achiev. Mat. Manufact. Eng. 2012, 54, 159-167.
[2] R. M. Izatt, S. R. Izatt, R. L. Bruening, N. E. Izatt, B. A. Moyer, Chem. Soc. Rev. 2014, 43, 2451-2475. [3] I. Arčon, S. Paganelli, O. Piccolo, M. Gallo, K. Vogel-Mikuš, F. Baldi, J. Synchrotron Rad. 2015, 22, 1215-1226. [4] S. Paganelli, O. Piccolo, F. Baldi, R.Tassini, M. Gallo, G. La Sorella, Appl. Catal. A Gen. 2013, 451, 144-152.
[5] S. K. Papageorgiou, E. P. Kouvelos, E. P. Favvas, A. A. Sapalidis, G. E. Romanos, F. K. Katsaros, Carbohydr. Res. 2010, 345, 469-473. [6] L. Tan, H. Dong, X. Liu, J. He, H. Xu , J. Xie, RSC Adv. 2017, 7, 7060-7072.
[7] S. Leone, C. De Castro, M. Parrilli, F. Baldi, R. Lanzetta. Eur. J. Org. Chem. 2007, 5183-5189. [8] W. Meng, D. Xiao, R. Wang, World J. Microbiol. Biotechnol. 2016, 32, 32-46.
[9] Y. N. Guragain, P. V. Vadlani, Process Biochem. 2017, 58, 25-34.
[10] J. Wong, F. W. Lytle, R. P. Messmer, D. H. Maylotte, Phys. Rev. B 1984, 30, 5596-5610.
[11] P. Jovanovič, N. Hodnik, F. Ruiz-Zepeda, I. Arčon, B. Jozinović, M. Zorko, M. Bele, M. Šala, V. S. Šelih, S. Hočevar, M. Gaberšček, J. Am. Chem. Soc. 2017, 139, 12837-12846.
[12] I. Arčon, A. Kodre, R. M. Abra, A. Huang, J. J. Vallner, D. D. Lasič, Colloids Surf. B Biointerfaces 2004, 33, 199-204. [13] P. Bouvier, E. Djurado, C. Ritter, A. J. Dianoux, G. Lucazeau, Int. J. Inorg. Mater. 2001, 3, 647-654.
[14] T. Dwars, G. Oehme, Adv. Synth. Catal. 2002, 344, 239-260.
[15] B. Chen, U. Dingerdissen, J. G. E. Krauter, H. G. J. Lansink Rotgerink, K. Mobus, D. J. Ostgard, P. Panster, T. H. Riermeier, S. Seebald, T. Tacke, H. Trauthwein, Appl. Catal. A Gen. 2005, 280, 17-46.
[16] F. Joò, Acc. Chem. Res. 2002, 35, 738-745.
[17] H. Gulyàs, A. C. Bényei, J. Bakos, Inorg. Chim. Acta 2004, 357, 3094-3098.
[18] G. Neri, G. Rizzo, L. De Luca, A. Donato, M. G. Mugolino and R. Pietropaolo, React. Kinet. Catal. Lett. 2008, 93, 193-202.
[19] Industrial Catalysis: A Practical Approach, (Ed. J. Hagen), Wiley VCH, Weinheim, 2006.
[21] D.D.Falcone, J. H.Hack, R. J. Davis, ChemCatChem 2016, 8, 1074-1075. [22] M. Chatterjee, Y Ikushima, F.-Y. Zhao, Catal. Lett. 2002, 82, 141-144.
[23] C. Stangel, G. Charalambidis, V. Varda, A. G. Coutsolelos, I. D. Kostas, Eur. J. Inorg. Chem. 2011, 4709-4716.
[24] T. Shimizu, M. Ota, Y. Sato, H. Inomata, Chem. Eng. Res. Des. 2015, 104, 174-179.
[25] G. Szӧllӧsi, B. Tӧrӧk, G. Szakonyi, I. Kun, M. Bartók, Appl. Catal. A Gen. 1998, 172, 225-232. [26] B. Tӧrӧk, K. Balázsik, G. Szollosi, M. Tӧrӧk, K. Felfӧldi, M. Bartók, Catal. Lett. 2002, 81, 55-62.
[27] B. Nair, Int. J. Toxicol. 2000, 20, 23-50.
[28] A. Marinas, R. A. Sheldon, Catal. Today 2011, 167, 1-2.
[29] S. Chen, R. Wojcieszak, F. Dumeignil, E. Marceau, S, Royer, Chem. Rev. 20018, 118, 11023−11117. [30] J. N. Chheda, J. A. Dumesic, Catal. Today 2007, 123, 59-70.
[31] A. Corma, O. de la Torre, M. Renz, N. Villandier, Angew. Chem. Int. Ed. 2011, 50, 2375-2378. [32] J. van Haveren, E. L. Scott, J. Sanders, Biofuels Bioprod. Bioref. 2008, 2, 41-57.
[33] P. Gallezot, Chem. Soc. Rev. 2012, 41, 1538-1558. [34] R. A. Sheldon, Green Chem. 2014, 16, 950-963.
[35] V. Montes, J.F. Miñambres, A. N. Khalilov, M. Boutonnet, J. M. Marinas, F. J. Urbano, A. M. Maharramov, A. Marinas, Catal. Today 2018, 306, 89-95. [36] R. López-Asensio, J. A. Cecilia, C. P. Jiménez-Gómez, C. García-Sancho, R. Moreno-Tost, P. Maireles-Torres, Appl. Catal. A Gen. 2018, 556, 1-9. [37] J. J. Bozell, G. R. Petersen, Green Chem. 2010, 12, 539-554.
[38] R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba, M. López Granados, Energy Environ. Sci. 2016, 9, 1144-1189. [39] M. M. Villaverde, N. M. Bertero, T. F. Garetto, A. J. Marchi, Catal. Today 2013, 213, 87-92.
[40] R. V. Sharma, U. Das, R. Sammynaiken, A. K. Dalai, Appl. Catal. A: Gen. 2013, 454, 127-136.
[41] D. E. Resasco, S. Crossley, A.I.Ch.E. J. 2009, 55, 1082-1089.
[42] D. Liu, D. Zemyanov, T. Wu, R. J. Lobo-Lapidus, J. A. Dumesic, J. T. Miller, C. L. Marshall, J. Catal. 2013, 299, 336-345 [43] S. Sitthisa, T. Sooknoi, Y. G. Ma, P. B. Balbuena, D. E. Resasco, J. Catal. 2011, 277, 1-13.
[44] C. P. Jiménez-Gómez, J. A. Cecilia, R. Moreno-Tost, P. Maireles-Torres, Topics Catal. 2017, 60, 1040-1053.
[45] C. P. Jiménez-Gómez, J. A. Cecilia, D. Durán-Martín, R. Moreno-Tost, J. Santamaría-González, J. Mérida-Robles, R. Mariscal, P. Maireles-Torres, J. Catal. 2016, 336, 107-115.
[46] C. P. Jiménez-Gómez, J. A. Cecilia, I. Márquez-Rodríguez, R. Moreno-Tost, J. Santamaría-González, J. Mérida-Robles, P. Maireles-Torres, Catal. Today 2017, 279, 327-338.
[47] S. Sitthisa, W. An, D. E. Resasco, J. Catal. 2011, 284, 90-101.
[48] T. P. Sulmonetti, S. H. Pang, M. T. Claure, S. Lee, D.A. Cullen, P. K. Agrawal, C. W. Jones, Appl. Catal. A Gen. 2016, 517, 187-195. [49] S. Srivastava, P. Mohanty, J.K. Parikh, A. K. Dalai, S. S. Amritphale, A. K. Khare, Chin. J. Catal. 2015, 36, 933-942.
[50] R. M. Mironenko, O. B. Belskaya, T. I. Gulyaeva, A. I. Nizovskii, A. V. Kalinkin, V. I. Bukhtiyarov, A. V. Lavrenov, V. A. Likholobov, Catal. Today 2015, 249, 145-152.
[51] M. Hronec, K. Fulajtárová, I. Vávra, T. Soták, E. Dobročka, M. Mičušík, Appl. Catal. B Environ. 2016, 181, 210-219. [52] J. Kijenski, P. Winiarek, T. Paryjczak, A. Lewichi, A. Mikolajska, Appl. Catal. A Gen. 2002, 233, 171-182.
[53] K. An, N. Musselwhite, G. Kennedy, V. V. Pushkarev, L. R. Baker, G. A. Somorjai, J. Colloid Interface Sci. 2013, 392, 122-128. [54] Q. Yuan, D. Zhang, L. van Haandel, F. Ye, T. Xue, E. J. M. Hensen, Y. Guan, J. Mol. Catal. A Chem. 2015, 406, 58-64. [55] E.-J. Ras, B. McKay, G. Rothenberg, Top. Catal. 2010, 53, 1202-1208.
[56] A. Corma, S. Iborra, A. Velty, Chem. Rev. 2007, 107, 2411- 2502. [57] A. Gandini, N. M. Belgacem, Polym. Int. 1998, 47, 267-276. [58] A. Gandini, M. N. Belgacem, Prog. Polym. Sci. 1997, 22, 1203-1379.
[59] R. Alamillo, M. Tucker, M. Chia, Y. Pagán-Torres, J. Dumesic, Green Chem. 2012, 14, 1413-1419. [60] Y. Nakagawa, K. Tomishige, Catal. Commun. 2010, 12, 154-156.
[61] A. Cadu, K. Sekine, J. Mormul, D. M. Ohlmann, T. Schaub, A. S. K. Hashmi, Green Chem. 2018, 20, 3386-3393. [62] Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012.
[63] J. Song, Z-F Huang, L. Pan, K. Li, X. Zhang, L. Wang, J-J. Zou, Appl. Catal. B Environ. 2018, 227, 386-408. [64] Fine chemicals through heterogeneous catalysis, (R. A. Sheldon, H. van Bekkum, Eds.), Wiley-VCH, Weinheim, 2001. [65] H. U. Blaser, H. Steiner, M. Studer, ChemCatChem 2009, 1, 210-221.
[66] M. Pietrowski, Curr. Org. Synth. 2012, 9, 470-487.
[67] M. Orlandi,D. Brenna,R. Harms,S. Jost, M. Benaglia, Org. Process Res. Dev. 2018, 22, 430-445. [68] M. L. Kantam, R. Chakravarti, U. Pal, B. Sreedhar, S. Bhargava, Adv. Synth. Catal. 2008, 350, 822-827. [69] M. Takasaki, Y. Motoyama, K. Higashi, S.-H. Yoon, I. Mochida, H. Nagashima, Org. Lett. 2008, 10, 1601-1604. [70] H. Wu, L. Zhuo, Q. He, X. Liao, B. Shi, Appl. Catal. A Gen. 2009, 366, 44-56.
[71] X. Huang, Y. Wang, X. Liao, B. Shi, Chem. Commun. 2009, 4687-4689.
[72] A. J. Kasparian, C. Savarin, A. M. Allgeier, S. D. Walker, J. Org. Chem. 2011, 76, 9841-9844. [73] V. Pandarus, R. Ciriminna, F. Béland, M. Pagliaro, Adv. Synth. Catal. 2011, 353, 1306-1316. [74] P. Buchwalter, J. Rosé, P. Braunstein, Chem. Rev. 2014, 115, 28-126.
[75] Cooperative catalysis: designing efficient catalysts for synthesis, (R. Peters Ed.). John Wiley & Sons, Stuttgart, 2015. [76] F. Cárdenas‐Lizana, S. Gómez‐Quero, A. Hugon, L. Delannoy, C. Louis, M. A. Keane, J. Catal. 2009, 262, 235-243. [77] A. Corma, C. González-Arellano, M. Iglesias, F. Sánchez, Appl. Catal. A Gen. 2009, 356, 99-102.
[78] M. R. Nabid, Y. Bide, N. Ghalavand, M. Niknezhad, Appl. Organomet. Chem. 2014, 28, 389-395.
[79] H. Wei, Y. Ren, A. Wang, X. Liu, X. Liu, L. Zhang, S. Miao, L. Li, J. Liu, J. Wang, G. Wang, D. Sua, T. Zhang, Chem. Sci. 2017, 8, 5126-5131. [80] The Chemistry of Anilines, (Z. Rappoport Ed.), John Wiley & Sons, New York, 2007.
[81] M. Liang, X. Wang, H. Liu, H. Liu, Y.Wang., J. Catal. 2008, 255, 335-342. [82] Z. Zhao, H. Yang, Y. Li, X. Guo, Green Chem. 2014, 16, 274-1281.
[83] Y. Jang, S. Kim, S. W. Jun, B. H. Kim, S. Hwang, I. K. Song, B. M. Kim, T. Hyeon, Chem. Commun. 2011, 47, 3601-3603. [84] V. D. Rathod, S. Paganelli, O. Piccolo, Catal. Commun. 2016, 84, 52-55.
[85] F. Figueras, B. Coqb, J. Mol. Catal. A: Chem. 2001, 173, 223-30.
[86] F. Cárdenas-Lizana, S. Gómez-Queroa, C. Amorimb, M. A. Keanea, Appl. Catal. A Gen. 2014, 473, 41-50. [87] F. Li, J. Liang, K. Wang, B. Cao, W. Zhu, H. Song, Reac. Kinet. Mech. Cat. 2017, 120, 651-662.
[88] C. V. Rode, R. V. Chaudhari, Ind. Eng. Chem. Res. 1994, 33, 1645-1653.
[89] J. Zhanga, Y. Wanga, H. Jia, Y. Weia, N. Wua, B. Zuob, Q. Wang, J. Catal. 2005, 229, 114-118.
[90] V. Kratkya, M. Kralika, M. Mecarovaa, M. Stolcovaa, L. Zaliberab, M. Hroneca, Appl. Catal. A Gen. 2002, 235, 225-231. [91] B. Ravel, M. Newville, J. Synchrotron Rad. 2005, 12, 537-541.
[92] J. J. Rehr, R. C.Albers, S. I. Zabinsky, Phys. Rev. Lett. 1992, 69, 3397-3400.
Table of Contents
A polymetals solution, recovered from exhausted catalytic converters, was transformed in the catalyst Metx-EPS by the use of a culture broth of Klebsiella
oxytoca DSM 29614. Indeed this microorganism produces the exopolysaccharide EPS, able to embed these metals. This new metals-polymeric composite showed a good catalytic activity and recyclability in the aqueous biphasic hydrogenation of different substrates.
Culture broth of Klebsiella oxytoca
Metx-EPS