1 Figure 1. 整理 (2010). Milk flower [Photograph].
Recovered from www.3lian.com
CHAPTER 5
Milk Production and Characteristics of the
Milk of the Jenny
Biagina Chiofalo, Paolo Polidori & Silvia Vincenzetti
1. I
NTRODUCTIONMilk is a biological fluid
designed to contain all
nutritional requirements of a specific mammalian newborn; therefore, the composition of milk differs by the needs of the neonate of different species. Although much research has
been devoted to milk
composition in the domestic horse, donkey’s milk has recently aroused scientific interest, above all among paediatric allergologists and nutritionists.
Figure 2. Lily M. (2007). A female donkey with her foal milking in Kadzidlowo [Photograph]. Recovered from www.wikipedia.org
Clinical studies have demonstrated that donkey milk may be considered a good replacer for dairy cow’s milk in feeding children with severe Ig-E mediated cow’s milk protein allergy, when human milk can not be given (Carroccio, A. et al., 2000). For these patients, donkey milk is not only useful (Monti, G. et al., 2007), but also safer compared with milk obtained by other mammalian species (Polidori, P. et al., 2009), due to the high similarity with human milk, especially considering protein fractions content (Salimei, E. et al., 2004; Vincenzetti, S. et al., 2008).
The donkey (Equus asinus) is a member of the horse family and its progenitor was the small grey donkey of northern Africa (Equus africanus) domesticated around 4000 BC on the shores of the Mediterranean Sea. It worked together with humans for centuries; the most common role was for transport. It still remains an important work animal in the poorest Regions.
Compared with ruminant’s milk, donkey milk has been studied less in the past, but in the last years research interest and capital investment in donkey milk have increased because its composition is similar to that of human milk. (see Table 1).
A donkey dairy farm may range from a few to over 600 animals. The production of donkey's milk is extremely complicated, making it a rare product. As with all mammals, lactation is triggered by the birth of a child. The animal has only two teats and no reservoir, and therefore should be treated three times a day, in order to get the average milk production of the jenny which varies between 1.5 and 2 litres maximun, taking into consideration the low volumetric capacity of the mammary gland. Milking can be done only for four to five months, since all the milk is left for the foal for the first two months. By comparison, a cow gives an average of 30 litres, year-round. Milking is done manually, and the foal needs to be present, otherwise the jenny will not give milk or even consent the milking operations. In fact, lactation is set off by the secretion of the hormone, oxytocin, caused by the foal's stimulation while nursing, so that only producing milk durng the foal's suckling period; this production stops effectively at weaning. Currently all producers work artisanally and cautiously, always respecting the animal. Of course, the jenny continues to breastfeed her foal for the entire milking period, and gradually the young donkey diversifies its diet. Donkey's milk is very white and more fluid than cow's milk, as it contains very little fat. It is also sweet. The assessment of donkey’s milk composition and its properties highlights that it could definitely be considered to be the closest natural example to woman’s milk. Its highly nourishing value led to its use as a substitute to breast milk until the beginning of the 20th
century, a fact that would remain existing, with a lesser importance, in the interwar years according to the testimony of Dr. Charles Porcher
(1872-1933) of the Lyon National Veterinary Institution in 1928. The
reason why the use of ass milk had been considered when raising children in their early infancy, even more in cases of delicate health or better said, why donkey’s milk had not been quite totally abandoned, may be that 25 or 30 years ago, a few well looked-after asses could easily be found in the city to provide milk nourishing young babies, unfortunately that is no longer the case today (www.donkeysandco.com).
2
Table 1. Comparison of chemical composition and physical properties of donkey’s,
mare’s, human’s and cow’s milk (Guo, H.Y et al., 2007).
Composition Donkey Mare Human Cow
pH 7.0 – 7.2 7.18 7.0 – 7.5 6.6 – 6.8 Protein g/100g 1.5 – 1.8 1.5 – 2.8 0.9 – 1.7 3.1 – 3.8 Fat g/100g 0.3 – 1.8 0.5 – 2.0 3.5 – 4.0 3.5 – 3.9 Lactose g/100g 5.8 – 7.4 5.8 – 7.0 6.3 – 7.0 4.4 – 4.9 Total Solids (TS) g/100 g 8.8-11.7 9.3-11.6 11.7-12.9 12.5-13.0 Casein Nitrogen (CN) g/100 g 0.64-1.03 0.94-1.2 0.32-0.42 2.46-2.80 Whey protein g/100 g 0.49-0.80 0.74-0.91 0.68-0.83 0.55-0.70 NPN g/100 g 0.18-0.41 0.17-0.35 0.26-0.32 0.1-0.19 Casein Nitrogen (CN) % 47.28 50 26.06 77.23 Whey protein % 36.96 38.79 53.52 17.54 NPN % 15.76 11.21 20.42 5.23
Donkey, mare and human milk present a relatively similar poor protein and fat percentage but a rich lactose one. The casein to whey protein ratio is intermediate between human milk and cow milk. Gross composition of whole milk differs by the mother's lactation stage. Ash and protein content shows a decreasing trend, while pH, percentage of whey protein, and amino acid content remain the same.
Figure 3. Donkeys & Co. (1877). Jennies farm at Saint Vincent de Paul Hospital, [Etching].
Recovered from www.donkeysandco.com
In the 19th and 20th centuries, donkey’s milk replaced milk in orphanages. In 1877, the “Saint Vincent de Paul Hospital” decided to set up a stable next to the dormitory for direct feeding of children. At that time, this beverage was also distributed in some large cities of France (Toulouse and Paris).
Figures 4, 5, 6, 7 & 8. (Figures 6, 7 & 8) Biagina Chiofalo, Paolo Polidori
&Silvia Vincenzetti (2013). Milking methods: (Figure 4) Hand-milking, /Figure 5)
Milk coming out of the udder, (Figure 6) Milking Machine/Milker, (Figure 7) Mechanical milking, (Figure 8) Teat cups, vaccum tubes, claw & milk hose
[Photographs]. Figure 4 Recovered from www.asineriedupaysdescollines.be,
Figure 5 Recovered from www.donkeysandco.com. The results of studies recently carried out on the characteristics of
the milk production of the jennie, both in the cosmetic and the food environment have revealed that it would be totally feasible to have a donkey dairy industry.
Dairy jennies quickly adapt to experimental conditions and the average productive level increase significantly the second year of the study and during the day, while studies concerning nutritional characterization has highlighted the slightly reduced content in fractions of allergenic proteins and an interesting acid pattern of the lipids. Donkey's milk has proved to be hypolipidic; however improving breeding techniques, standardising the milking routine, developing nutritional strategies as well as genetic selection will be able to help raise the lipid level of Donkey's milk and thus its nutritive value.
On the other hand the hygienic and sanitary aspect of the milk produced has proved to be good; the high level of lysozyme observed could not only contribute to the good microbiological quality of the Donkey's milk but would also represent a possible explanation regarding the use, acknowledged since the beginning of time, of Donkey's milk in dermatology.
In terms of reintroducing antique traditions and food and safeguarding alternative species for breeding, realising an innovative jennies’ milk operating system appears particularly interesting in central and Mediterranean Europe where indigenous species that are extremely hardy and today fewer in number, could represent a zootechnic resource of noteworthy interest for hilly regions, regions at the foot of mountains and for our islands, especially if associated with such initiatives as rural tourism or hippotherapy for example. The current objective would be therefore to set up a cooperative project between different European breeders capable of respecting a quality charter and providing excellent quality milk so it can be handled and packaged to then be correctly marketed (www.eurolactis.com)
3
2. Cow’s milk protein allergy
Adverse reactions to food are currently classified into toxic and non-toxic reactions. Non-non-toxic adverse reactions to milk are primarily caused by either lactose intolerance or milk allergy. Milk intolerance is due to the inherited lack of the specific enzyme, β-galactosidase that is required to hydrolyze lactose. For lactose intolerance, the most common therapeutic approach excludes lactose-containing milk from the diet.
Cow’s milk protein allergy (CMPA) is defined as an immunological reaction to one or more milk proteins (Hill, D.J et al., 1986). A variety of symptoms can be suggestive for CMPA. CMPA is suspected clinically in 5-15% of infants (Høst, A., 2002), while most estimates of prevalence of CMPA vary from only 2 to 5 %. Confusion regarding CMPA prevalence is often due to differences in study population, and a lack of defined diagnostic criteria for CMPA. The importance of defined diagnostic criteria needs to be emphasised. It precludes infants from an unnecessary diet (Vandenplas, Y. et al., 2007) and avoids delay in diagnosis, which can lead to malnutrition (Viera, M. et al., 2010).
Cow’s milk is a member of the “Big-8” food allergens that include egg, soy, wheat, peanuts, tree nuts, fish and shellfish in terms of prevalence (Crittenden, R.G. and Bennett, L.E., 2005). The incidence of CMPA varies with age. CMPA is prevalent in early childhood with reported incidences between 2 and 6% (Garcia-Ara, M.C. et al., 2004) and decreases into adulthood to an incidence of 0.1–0.5% (Woods, R.K et al., 2002).
It has been suggested that infants have milk allergies because milk is usually the first source of foreign antigens that they ingest in large quantities, and the infant intestinal system is insufficiently developed to digest and immunologically react to milk proteins. When milk is eliminated, the inflammation response is controlled. After several years, oral tolerance is developed, and milk can again be tolerated (Høst, A.,
1999).
For human beings cow’s milk represents the most common feeding during the infant weaning, but also the first allergen in life. In many countries cow’s milk is the most important food allergen in babies and children (Hill,
D.J. and Hosking, C.S, 1996). Adverse reactions to cow’s milk were found in
2% of babies during the first year of life: 30% of cases at the first month, 60% before the third and 96% within the twelfth (Stintzing, G. and
Zetterstrom, R., 1979). Symptoms can even appear during the breast-feeding
because newborn reacts against a small amount of cow milk proteins present in maternal milk (Høst, A. et al., 1988). Children followed for the first 3 years of life, 56% of cases had recovered from cow’s milk allergy at 1-year age, 77% at 2 years and 87% at 3 years age (Høst, A. and Halken, S.
1990). However allergy can persist for all life. Considering the possible use
of alternative milk sources for human in cases of cow’s milk allergy, the use of goat’s milk should be avoided because of the high risk of cross-reactivity , while mare’s and donkey’s milks, used in popular practice for allergic children, are valid alternative protein sources when appropriately evaluated from the hygienic point of view (Restani, P. et al., 2002).
The discussion on the use of soy-based infant formula is difficult, since scientific societies have different recommendations. There is a broad consensus on the following statements: the incidence of soy allergy in soy fed infants is comparable to that of CMPA in cows’ milk formula-fed babies (De Greef, E. et al, 2012). Cross reactivity to soy has been reported in 10 to 35% of infants with CMPA, regardless whether they were positive or negative for specific IgE for CMPA. In particular, infants with multiple food allergies and eosinophilic enterocolitis also react to soy protein; Its healing virtues had been known since Antiquity so that
doctors formerly recommended and used it in medicine to cure diverse affections. Hippocrates (460 – 370 BC), the father of medicine, prescribed ass milk for numerous purposes, such as liver troubles, infectious diseases, fevers, oedema, nose bleeds, poisonings, and wounds.
In his encyclopedic work Naturalis Historia, volume 28, dealing with remedies derived from animals, Pliny the Elder (23 – 79 AD) proposed it to fight poisonings, fever, fatigue, eye stains, weakened teeth, face wrinkles, ulcerations, asthma and certain gynecological troubles:
<<Asses' milk, in cases where gypsum, white-lead, sulphur, or quick-silver, have been taken internally. This last is good too for constipation attendant upon fever, and is remarkably useful as a gargle for ulcerations of the throat. It is taken, also, internally, by patients suffering from atrophy, for the purpose of recruiting their exhausted strength; as also in cases of fever unattended with headache. The ancients held it as one of their grand secrets, to administer to children, before taking food, a semisextarius of asses' milk, or for want of that, goats' milk; a similar dose, too, was given to children troubled with chafing of the rectum at stool. In case where persons have swallowed quicksilver, bacon is the proper remedy to be employed. Poisons are neutralized by taking asses' milk ; henbane more particularly, mistletoe, hem- lock, the flesh of the sea-hare, opocarpathon, pharicon, and dorycnium: the same, too, where coagulated milk has been productive of bad effects, for the biestings,' JO or first curdled milk, should be reckoned as nothing short of a poison. We shall have to mention many other uses to which asses' milk is applied ; but it should be remembered that in all cases it must be used fresh, or, if not, as new as possible, and warmed, for there is nothing that more speedily loses its virtue.
When the teeth have been loosened by a blow, they are strengthened by using asses' milk or else ashes of the burnt teeth of that animal, or a horse's lichen, reduced to powder, and injected into the ear with oil.
An ass’s hoof, reduced to ashes and applied with asses' milk, is used for the removal of marks in the eyes and indurations of the crystalline humours.
Ulcerations of the stomach are effectually treated with asses' milk or cows' milk. Asses' milk boiled with bulbs, the whey being the part used, with the addition of nasturtium steeped in water and tempered with honey, in the proportion of one cyathus of nasturtium to three semi-sextarii of whey. The liver or lights of a fox, taken in red wine, or bear's gall in water, facilitate the respiration.
The disease called tenesmus, or in other words, a frequent and ineffectual desire to go to stool, is removed by drinking asses' milk or cows' milk.
If pains are felt in the breasts, they will be alleviated by drinking asses' milk; and the same milk, taken with honey, has considerable efficacy as an emmenagogue.>> Similarly, Georges-Louis Leclerc, Comte de Buffon, (1707–1788) mentions the benefits of ass milk in his Histoire naturelle: <<Ass’s
milk, on the contrary, is a well-tried remedy specific to certain illnesses, and the use of this remedy has been retained from the Greeks until us. >>
In the South Indian state of Tamil Nadu, a popular folk belief states that donkey milk can aid infants' immune systems and voice development. However doctors have warned nursing mothers against the practice, citing the potential risk of infection.
4 therefore, different specialist groups have different standpoints on the
use of Soy formula for CMPA, but is generally not recommended before the age of 6 months (De Greef, E. et al, 2012).
Figure 9. Renato Contado (2006). Donkey & cow grazing, [Photograph]. Recovered
from www.flickr.com
3. Donkey milk protein fractions
In a study performed in order to determine the different protein fractions in donkey milk (Vincenzetti, S. et al, 2008) it was possible to separate 9 peaks that were identified as β-caseins (sequence: REKEELNVSS) and αS1-caseins (sequence: RPKLPHRQPE), having
different molecular weights.
Reversed-phase chromatography on HPLC (RP-HPLC) followed by 15% SDS-PAGE and N-terminal analysis was performed on the skimmed donkey’s milk giving as a result three main peaks identified as lysozyme (sequence, KVFSKXELA), α-lactalbumin, (sequence, KQFTKXELSQVLXSM), and β-lactoglobulin (sequence TNIPQTMQ), respectively (Table 2).
This study revealed the presence of -caseins (sequence: REKEELNVSS) and s1-caseins (sequence: RPKLPHRQPE), which presented marked
homology with s1- and -caseins from mare’s milk (Egito, A.S. et al.
2002), while the presence of other types of caseins, such as s2-, - and k-
were not determined in donkey milk. This result show another high similarity between donkey and human milk: in fact, the presence of s2
-caseins in human milk has not been demonstrated (Egito, A.S. et al. 2002).
Table 2. Donkey’s milk protein fractions (Vincenzetti, S. et al, 2008).
Protein kDa N-terminal sequence
Lysozyme 14.60 KVFSKXELA
α-lactalbumin 14.12 KQFTKXELSQVLXSM β-lactoglobulin 22.40 TNIPQTMQ αs1-casein 33.30 RPKLPHQPE
β-casein 37.50 REKEELNVS
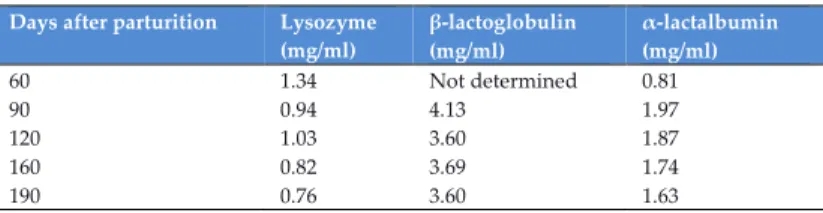
Thanks to RP-HPLC analysis, it was possible also to calculate the lysozyme, β-lactoglubulin and α-lactalbumin concentrations (in mg/ml) at different stages of lactation (60, 90, 120, 160 and 190 days after parturition), the results are shown in Table 3.
Table 3. Quantitative determination of lysozyme, -lactoglobulin, -lactalbumin in different stages of lactation (Vincenzetti, S. et al, 2008).
Days after parturition Lysozyme (mg/ml) β-lactoglobulin (mg/ml) α-lactalbumin (mg/ml) 60 1.34 Not determined 0.81 90 0.94 4.13 1.97 120 1.03 3.60 1.87 160 0.82 3.69 1.74 190 0.76 3.60 1.63
The amount of lysozyme in donkey’s milk varied considerably during the different stages of lactation, with a mean value of 1.0 mg/ml, and proved to be higher with respect tothat in bovine (traces), human (0.12 mg/ml) and goat’smilk (traces), whereas, it was very close to mare’s milk (0.79 mg/ml), as demonstrated by Miranda, G. et al. (2004).
The mean β-lactoglobulin content in donkey’s milk (3.75 mg/ml) was very close to that of bovine milk (3.3 mg/ml) and pony mare’s milk (3.0 mg/ml), whereas in human milk the β-lactoglobulin is absent (Egito, A.S. et al. 2002). The α-lactalbumin content increased in the three months after parturition till the value of 1.8 mg/ml, close to the α-lactalbumin content in human milk (1.6 mg/ml) but lowest compared to the pony mare’s -lactalbumin content (3.3 mg/ml) (Uniacke-Lowe, T. et al., 2010).
4. Donkey milk: A nutrilipidomic approach for
human health
Donkey milk lipids have been suggested as “nutraceuticals” for human nutrition since they contain a number of nutrient substances, fatty acids and triglycerides, interesting not only in the diet of the infants but also in patients with osteogenesis processes, premature senescence, coronary diseases or in the atherosclerosis therapy and hypocholesterolemic diets (Chiofalo B., Salimei E., 2001; D’Alessandro A.G. et al., 2010).
Moreover, donkey milk lipids are characterized by particular prebiotic and probiotic properties because capable to conditioning indirectly or directly the intestinal environment and immunity (Amati L., et al., 2010), taking part in the prevention and cures of some pathologies (Chiofalo B. et al., 2003;
Tafaro A. et al., 2007).
In fact, recent studies (Laiho K. et al., 2002) on the immunomodulatory properties of fatty acids and antioxidant, including alfa-tocopherol, of certain nutrients, have shown that food not only is a source of dietary antigens causing sensitization but also may contain protective factors, that through the regulation of the immune function, may counteract with the oxidative stress and may diminish the inflammatory response in allergic disease.
Approximately 2.5 percent of children younger than three years of age are allergic to milk. Nearly all infants who develop an allergy to milk do so in their first year of life. Most children eventually outgrow a milk allergy. The allergy is most likely to persist in children who have high levels of cow’s antibodies in their blood (www.foodallergy.org/allergens/milk-allergy)
5 Figure 11. Support the udder of the
jenny (Frandson, 1986 & Navas, F.J., 2013).
Figure 10. Sub-division of the udder of
the jenny into left and right (Quinn, 1990 & Navas, F.J., 2013).
Figure 14. H.M. Farrell Jr. et al (2006)
A typical lactating cell indicating active secretion of protein and lipid by distinct mechanisms.
Figure 13. H.M. Farrell Jr. et al (2006). A single alveolus consisting of
lactating epithelial cells (SEC) surrounding the lumen.
Figure 15. Peta A. Jones (2005). Jennies’
pregnancy recognition according to the udder state.
Figure 12. Components of udder showing blood supply (Frandson, 1986).
5. Donkey milk lipid fractions
Lipids, in addition to being an important source of energy, play several important biological roles (Kaila M. et al., 1999). The knowledge of the composition of milk lipids, in terms of molecular species of triacylglycerols and fatty acids, is fundamental in order to explain elucidate the physical properties, the sensorial characteristics, the nutritional value of milk and the biosynthetic pathways of milk fat (Morera Pons S. et al., 1998) as well as it appears to be a particularly challenging and important task, also considering the pivotal role of fat-derived inflammatory substances. Moreover, most of the studies present in literature demonstrate that lipid structure is an important parameter affecting lipid absorption because of lipid digestion is dependant on the milk fatty acid composition and on the fatty acid position on the glycerol bone of the milk triglycerides. Human milk presents palmitic acid preferentially on the sn-2 position in the triglyceride molecule, and it is assumed to be absorbed as sn-2 monoacylglycerol rather than as free fatty acid (Bemback S. et al., 1990; Lopez-Lopez A. et al., 2001).
On the contrary, infant formula, mainly composed by vegetable oil and cow-milk fat, although characterised by palmitic acid as the major components in analogy with human milk composition, presents this fatty acid predominately esterified in the sn-1,3 position of the triglyceride (Small D.M., 1991; Collomb M. et al., 2002). Therefore, during the lipid digestion in the new born that occurs in the stomach, for the specific activity of the pancreatic lipase towards the primary ethereal bonds, the digestion of the human’s milk permits a greater absorption of the palmitic acid like 2-monoacylglyceride (Hamosh M., 1995), while, during the digestion of the cow’s milk, the palmitic acid, frees from the glycerol, binds itself to the calcium present in the intestinal lumen, precipitate as calcium soaps which are not soluble at body temperature, therefore it is excreted through the faeces with consequent a twofold nutritional damage for the infants: the loss of palmitic acid and of calcium from the body (Chiofalo B. et al., 2006a). Therefore, it is important to study the positional isomery of triglycerides that occurs in human and donkey milk, for a more detailed nutritional evaluation.
Data about the human milk can be found from the literature (Kallio H., Rua P., 1994), but few data on the quali-quantitative triacylglycerol composition in lactating donkeys (Chiofalo et al., 2006b) can be found and, in authors knowledge, no data about triglyceride positional isomery in donkey milk can be found. In this direction, our studies (Dugo P. et al.,
2005; Dugo P. et al., 2006) carried out by using an innovative analytical
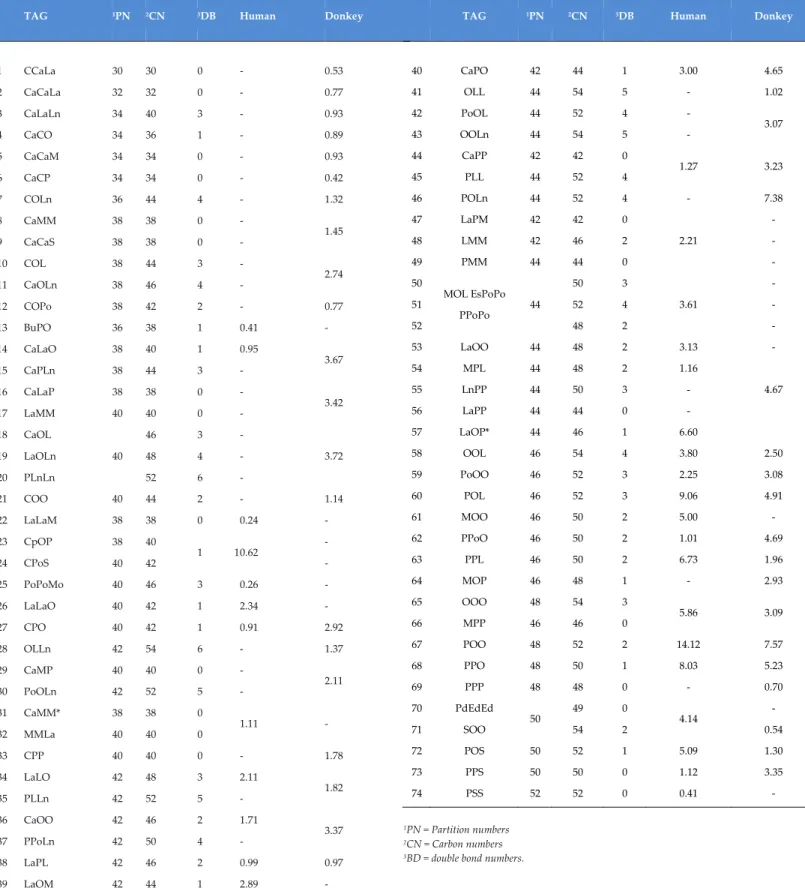
techniques, the RP-HPLC-APCI-MS, show the quali-quantitative similarities in the fatty acid typology that form the triglycerides of human milk in comparison with those of human milk (Table 4).
As reported in Table 4, the mostly represented triglycerides in human milk are: Palmitic-Oleic-Oleic (14.1%), Palmitic-Oleic-Linoleic (9.1%), Palmitic-Palmitic-Oleic (8.0%), Lauric-Oleic-Palmitic (6.6%), Oleic-Oleic-Oleic (5.9%), Myristic-Oleic-Oleic-Oleic-Oleic-Oleic-Oleic (5.0%) and in donkey milk are: Oleic-Linolenic (7.4%), Oleic-Oleic (7.6%), Palmitic-Palmitic-Oleic (5.23%), Palmitic-Oleic-Linoleic (4.9%), Palmitic-Palmitoleic-Oleic (4.69%), Capric-Palmitic-Oleic (4.65%) (Chiofalo et al.,
2011). Therefore, the high presence in both milk of the triglycerides:
Palmitic-Oleic-Oleic, Palmitic-Palmitic-Oleic and Palmitic-Oleic-Linoleic, confirm that the Palmitic and Oleic acids are the most abundant fatty acids and that, the palmitic acid is approximately for the 80% on the sn-2 position. This means that the composition of donkey milk lipids, in terms of molecular species of triacylglycerols greatly resembles that of human milk.
6
Table 4. Average composition of TAGs determined in the human and donkey milk (expressed as area %, average of 3 different human and 3 donkey milk samples) analysed by
RP-HPLC-APCI-MS.
TAG 1PN 2CN 3DB Human Donkey
1 CCaLa 30 30 0 - 0.53 2 CaCaLa 32 32 0 - 0.77 3 CaLaLn 34 40 3 - 0.93 4 CaCO 34 36 1 - 0.89 5 CaCaM 34 34 0 - 0.93 6 CaCP 34 34 0 - 0.42 7 COLn 36 44 4 - 1.32 8 9 CaMM CaCaS 38 38 38 38 0 0 - - 1.45 10 11 COL CaOLn 38 38 44 46 3 4 - - 2.74 12 COPo 38 42 2 - 0.77 13 BuPO 36 38 1 0.41 - 14 15 CaLaO CaPLn 38 38 40 44 1 3 0.95 - 3.67 16 17 CaLaP LaMM 38 40 38 40 0 0 - - 3.42 18 19 20 CaOL LaOLn PLnLn 40 46 48 52 3 4 6 - - - 3.72 21 COO 40 44 2 - 1.14 22 LaLaM 38 38 0 0.24 - 23 24 CpOP CPoS 38 40 40 42 1 1 0.62 - - 25 PoPoMo 40 46 3 0.26 - 26 LaLaO 40 42 1 2.34 - 27 CPO 40 42 1 0.91 2.92 28 OLLn 42 54 6 - 1.37 29 30 CaMP PoOLn 40 42 40 52 0 5 - - 2.11 31 32 CaMM* MMLa 38 40 38 40 0 0 1.11 - 33 CPP 40 40 0 - 1.78 34 35 LaLO PLLn 42 42 48 52 3 5 2.11 - 1.82 36 37 CaOO PPoLn 42 42 46 50 2 4 1.71 - 3.37 38 LaPL 42 46 2 0.99 0.97 39 LaOM 42 44 1 2.89 -
TAG 1PN 2CN 3DB Human Donkey
40 CaPO 42 44 1 3.00 4.65 41 OLL 44 54 5 - 1.02 42 43 PoOL OOLn 44 44 52 54 4 5 - - 3.07 44 45 CaPP PLL 42 44 42 52 0 4 1.27 3.23 46 POLn 44 52 4 - 7.38 47 48 49 LaPM LMM PMM 42 42 44 42 46 44 0 2 0 2.21 - - - 50 51 52 MOL EsPoPo PPoPo 44 50 52 48 3 4 2 3.61 - - - 53 LaOO 44 48 2 3.13 - 54 55 56 57 MPL LnPP LaPP LaOP* 44 44 44 44 48 50 44 46 2 3 0 1 1.16 - - 6.60 4.67 58 OOL 46 54 4 3.80 2.50 59 PoOO 46 52 3 2.25 3.08 60 POL 46 52 3 9.06 4.91 61 MOO 46 50 2 5.00 - 62 PPoO 46 50 2 1.01 4.69 63 64 PPL MOP 46 46 50 48 2 1 6.73 - 1.96 2.93 65 66 OOO MPP 48 46 54 46 3 0 5.86 3.09 67 POO 48 52 2 14.12 7.57 68 PPO 48 50 1 8.03 5.23 69 PPP 48 48 0 - 0.70 70 71 PdEdEd SOO 50 49 54 0 2 4.14 - 0.54 72 POS 50 52 1 5.09 1.30 73 PPS 50 50 0 1.12 3.35 74 PSS 52 52 0 0.41 - 1PN = Partition numbers 2CN = Carbon numbers
7 On the contrary, triglycerides in human and donkey milk are very
dissimilar from the those of cow milk; in the latter, the main components are Palmitic-Oleic, Palmitic-Plamitic and Butyric-Myristic-Palmitic whereas, triglycerides containing polyunsaturated fatty acids (Linoleic and Linolenic acids), are not present (Blasi F. et al., 2008). This confirms that the digestibility of donkey milk is very similar to that of human milk and higher than that of cow milk.
Indeed, the presence, among the main components of the triacylglycerol fraction of donkey milk, of Palmitic-Oleic-Linolenic, Palmitic-Oleic-Oleic, Palmitic-Palmitic-Oleic, Capric-Palmitic-Oleic, Palmitic-OleicLinoleic and Palmitic-Palmitoleic-Oleic, in comparison with triglycerides of cow milk, testify the absence in the small intestine of donkey of isomerization and hydrogenation processes of the fatty acids prior to absorption and esterification in blood (Chiofalo B. et al., 2006b), and confirms the nutritional value of donkey milk fat composition in relation to human health.
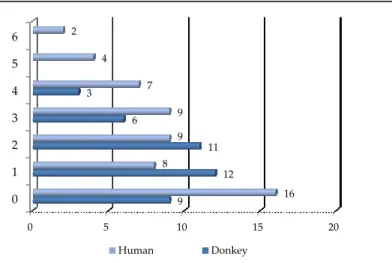
To better compare the composition of triacylglycerol fraction of human and donkey milk kinds, Figure 1 reports the graph relative to the triglyceride groups based on double bond numbers for each milk kind. As it is possible to see, donkey milk is characterised by numerous triglycerides with high unsaturation degree. Human milk presents a distribution similar to donkey milk for triglycerides content with double bond numbers=3; other triglycerides showed quantitative differences.
Figure 16. Qualitative distribution of TAGs in function of DB, determined in human and
donkey milk. The numbers above the bars represent the number of TAGs of each group
[Graphic].
Human milk triglyceride fraction is characterised by the presence of 9 triglycerides with double bond numbers=0 representing about 12%, 12 triglycerides with double bond numbers=1 that count about 12%, 11 triglycerides with double bond numbers=2 that represent about 10%, 6 triglycerides with double bond numbers=3 and 3 with double bond numbers=4 that represent 7% and 2% respectively.
The triacylglycerol fraction of donkey milk lipid (Figure 1) is characterised by the presence of 16 saturated (double bond numbers=0) triglycerides that count for less than 14%, 8 triglycerides with double bond numbers=1 that represent 8%, 9 triglycerides with double bond numbers=2 that represent about 10%, 9 triglycerides with double bond numbers=3 that represent about 9%, 7 triglycerides with double bond numbers=4 representing about 7%, 4 triglycerides with double bond
numbers=5 and 2 triglycerides with double bond numbers=6 that represent 4% and 2% respectively.
Results underline some quali-quantitative similarities in the fatty acid typology that form the donkey’s milk triglycerides in comparison with those of human’s milk and they underline some differences in comparison with thoseof cow milk (Gresti J. et al., 1993, Blasi F., et al., 2008).
As regards the trend of the triglycerides in donkey milk during the lactation, previous studies (Chiofalo B. et al., 2006b, Morera S., 2003), report a certain degree of similarity with that of triglyceride profile in human milk; in fact, during the lactation some triglyceride species such as Caprylic-Oleic-Linolenic, Caprylic-Palmitic-Oleic, Oleic-Linoleic-Linolenic and Capric-Palmitic-Oleic, show in human and donkey milk relatively constant levels (Chiofalo B. et al., 2006b) and could be therefore considered as markers of the triglyceride profile of these milk kinds.
Figure 2 shows the fatty acid composition of human and donkey milk. Comparing the fatty acid content of human and donkey milk, the most abundant fatty acids in both milks are: among the saturated fatty acids, palmitic acid (24% in human milk and 26% in donkey milk), among the monounsaturated fatty acids, oleic acid (38% in human milk and 32% in donkey milk) and among the polyunsaturated fatty acids, linoleic acid (10% in human milk and 8% in donkey milk). The main differences among the fatty acids are given by the presence of higher amounts of short chain fatty acids, C6-C10, and of a considerable higher level of linolenic acid in donkey milk (8.1%) than the human milk (0.7%).
Donkey milk in fact is characterised by large amounts of polyunsaturated fatty acids. This composition renders donkey’s milk a good source of essential fatty acids (n-6 and n-3) which counteracting with the oxidative stress and, therefore, diminishing the inflammatory response in allergic disease, may regulate the immune function (Salimei E, and Chiofalo B., 2006). Moreover, the low presence of short chained fatty acids renders it adequate (better tolerance) for infant diet (Chiofalo B. et al., 2003, Chiofalo B.
et al., 2005a, Salimei E. et al., 2004). The data obtained are in agreement with
Collomb et al. (2002).
The considerable presence of unsaturated fatty acids together with the low atherogenic (0,80) and thrombogenic (0,32) indices (Chiofalo B. et al., 2005b) observed in donkey milk make it very interesting in the prevention of the cardiovascular, auto-immune and inflammatory diseases (Kinsella J. et al.,
1990). In particular, the higher levels of n-3 polyunsaturated fatty acids
content of donkey milk, characteristic constituents of the fish oils, can counteract the above-mentioned pathologies through the synthesis of antiinflammatory, antiaggregant and non-immunosuppressant substances, like lipid mediators (eicosanoids), prostaglandins (PGE3) and leukotrienes
(LTB5), and protein mediators (cytokines), interleukins (IL4, IL10, IL13, IL1ra),
tumor necrosis factor, etc. (Williams C.M., 2000).
Moreover, several studies report that the addition of linolenic acid in the human diet improves the degree of eczematous involvement of the skin in some atopic dermatitis patients (Chiofalo B. et al, 2011; Horrobin D.F., 2000). Chiofalo et al. (2006a), evaluating the fatty acid composition of donkey milk, with a view to its potential use as a good linoleic and linolenic source, suggest that donkey milk can also be considered in the future a potential nutritional source for patients suffering from atopic dermatitis.
0 5 10 15 20 0 1 2 3 4 5 6 9 12 11 6 3 16 8 9 9 7 4 2 Human Donkey
8 Figure 17. Fatty Acid distribution in human and donkey milk, determined by GC-FID [Graphic].
9
6. Conclusions
In conclusion, donkey milk has been indicated as a valuable and safe source for the nutrition of cow-milk intolerant infants as well as an interesting nutraceutical food for older people, therefore as a brand new functional food. Moreover, the results here discussed confirm that it represents a natural source of lipids with high similarity to human milk and therefore high nutritional value in the regulation of the immune-inflammatory system. Nevertheless, the use of donkey milk for therapeutic purposes is hampered by several factors: difficulties in finding food, ensuring continuity of supply, medical legal risk associated with the administration of non-conventional diet therapy. In this context the valorisation of “donkey milk” as food, considering that the Regulation (EC) No 853/2004 lays down specific hygienic rules for food on animal origin including donkey raw milk, could represent an incentive for the breeding of donkeys and, therefore, an indispensable factor to preserve the animal biodiversity. Therefore, the implementation of the good practices throughout the global “dairy donkey” food chain is a crucial topic to guarantee the safety and quality of donkey milk for Public health.
7. Bibliography & References
Amati, L, Marzulli G., Martelli, M., Tafaro, A., Jirillo, F., Pugliese, V., Martemucci, G., D'Alessandro, A.G. and Jirillo, E. [2010]: Donkey and goat milk intake and modulation of the human aged immune response. Current Pharmaceutical Design, 16(7), pp. 864-69.
Bemback, S., Blackberg, L. and Hernell, O. [1990]: The complete digestion of human milk triacylglycerol in vitro requires gastric lipase, pancreatic colipase-dependent lipase, and bile salt-stimulated lipase.
Journal of Clinical Investigation, 85(4), pp. 1221-26.
Blasi, F., Montesano, D., De Angelis, M., Maurizi, A., Ventura, F., Cossignani, L., Simonetti, M.S. and Damiani, P. [2008]: Results of stereospecific analysis of triacylglycerol fraction from donkey, cow, ewe, goat and buffalo milk. Journal of Food Composition and Analysis, 21, pp. 1-7.
Carroccio, A., Cavataio, F., Montalto, G., D’Amico, D., Alabrese, L. and Iacono, G. [2000]: Intolerance to hydrolysed cow’s milk proteins in infants: clinical characteristics and dietary treatment. Clinical &
Experimental Allergy, 30, pp. 1597-603.
Chiofalo, B. and Salimei, E. [2001]: Ass's milk: Exploitation of an alimentary resource. Folium, 3, pp. 235-41.
Chiofalo, B., Azzara, V., Piccolo, D., Liotta, L., Chiofalo, L. [2005a]: Il latte di asina al traguardo della ricerca. Gli acidi grassi nel corso della lattazione. Large Animal Review, 11(1), pp. 39-44.
Chiofalo, B., Drogoul, C. and Salimei, E. [2006a]: Other utilisation of mare’s and ass’s milk. In: Miraglia N, Martin-Rosset W (eds) Nutrition
and feeding of the broodmare. Part B: Lactation. The Netherlands:
Wageningen Academic Publishers: EAAP publication No. 120, pp. 133-47.
Chiofalo, B., Dugo, P., Bonaccorsi, I. and Mondello, L. [2011]: Comparison of major lipid components in Human and Donkey Milk. New perspectives for a hypoallergenic diet in humans.
Immunopharmacology and Immunotoxicology, 33, pp. 633- 44.
Chiofalo, B., Polidori, M., Costa, R. and Salimei, E. [2005b]: Fresh forage in dairy ass's ration: effect on milk fatty acids and flavours.
Italian Journal of Animal Science, 4(Suppl. 2), pp. 433-35.
Chiofalo, B., Salimei, E. and Chiofalo, L. [2003]: Acidi grassi del latte d’asina: proprietà bio-nutrizionali ed extranutrizionali. Large Animal
Review, 9(5), pp. 1-6.
Chiofalo, B., Salimei, E., Dugo, P., Kumm, T., Piccolo, D. and Mondello, L. [2006b]: Evaluation of triglycerides in donkey milk during lactation. In: Proc 2nd European Equine Health & Nutrition Congress, Ghent, Belgium, 17-18 March, pp. 102-4.
Collomb, M., Butikofer, U., Sieber, R., Jeangros, B. and Bosset, J.O. [2002]: Composition of fatty acids in cow's milk fat produced in the lowlands, mountains and highlands of Switzerland using high-resolution gas chromatography. International Dairy Journal, 12, pp. 649-59.
Crittenden, R.G. and Bennett, LE. [2005]: Cow's milk allergy: a complex disorder. Journal of the American College of Nutrition, 24(6 Suppl), pp. 582S-91S.
D’Alessandro, A.G., Martemucci, G., Jirillo, E. and De Leo, V. [2011]: Major whey proteins in donkey’s milk: effect of season and lactation stage. Implications for potential dietary interventions in human diseases.
Immunopharmacology and Immunotoxicology, 33(2), pp. 259-65.
De Greef, E., Veereman-Wauters, G., Devreker, T., Hauser, B. and Vandenplas, Y. [2012]: “Diagnosis and Management of Cows’ Milk Protein Allergy in Infants”, in C. Pereira (ed.) Allergic Diseases - Highlights
in the Clinic, Mechanisms and Treatment, Rijeka, Croatia: Intech Publisher,
pp. 279-88.
Dugo, P., Kumm, T., Lo Presti, M., Chiofalo, B., Salimei, E., Cotroneo, A. and Mondello, L. [2005]: Determination of triacylglycerols in donkey milk by using high performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. Journal of
Separation Science, 28, pp. 1023-30.
Dugo, P., Kuum, T., Chiofalo, B., Cotroneo, A. and Mondello, L. [2006]: Separation of triacylglycerols in a complex lipidic matrix by using comprehensive two dimensional high performance liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometric detection. Journal of Separation Science, 29, pp. 1146-54. Egito, A.S., Miclo, L., Lopez, C., Adam, A., Girardet, J.M. and Gaillard, J.L. [2002]: Separation and characterization of mare’s milk s1-, -, k-caseins, -casein-like and proteose peptone component 5-like peptides. Journal of Dairy Science, 85, pp. 697-706.
Frandson, F.R., 1986. Anatomy And Physiology Of Farm Animals. Lea & Febiger. Philadelphia
Garcia-Ara, M.C., Boyano-Martinez, M.T., Diaz-Pena, J.M., Martin-Munoz, M.F. and Martin-Esteban, M. [2004]: Cow’s milk-specific immuno-globulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clinical & Experimental Allergy, 34, pp. 866-70.
Gresti, J., Bugaut, M., Maniongui, C. and Bezard, J. [1993]: Composition of molecular species of triacylglycerols in bovine milk fat.
Journal of Dairy Science, 76, pp. 1850-69.
Guo, H.Y., Pang, K., Zhang, X.Y., Zhao, L., Chen, S.W., Dong, M.L. and Ren, F.Z. [2007]: Composition, Physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. Journal of
Dairy Science, 90, pp. 1635-43.
H.M. Farrell Jr., E.L. Malin, E.M. Brown, P.X. Qi, Casein micelle structure: What can be learned from milk synthesis and structural biology?, Current Opinion in Colloid & Interface Science, Volume 11, Issues
10 2–3, June 2006, pp. 135-147.
Hamosh, M. [1995]: Lipid metabolism in paediatric nutrition.
Pediatric Clinics of North America, 12, pp. 839-59.
Hill, D.J. and Hosking, C.S. [1996]: Cow milk allergy in infancy and early childhood. Clinical Experimental Allergy, 26, pp. 254-61.
Hill, D.J., Firer, M.A., Shelton, M.J. and Hosking, C.S. [1986]: Manifestations of milk allergy in infancy: clinical and immunologic findings. The Journal of Pediatrics, 109, pp. 270-6.
Horrobin, D.F. [2000]: Essential fatty acid metabolism and its modification in atopic eczema American Journal of Clinical Nutrition, 71, pp. 367S-72S.
Høst, A and Halken, S. [1990]: A prospective study of cow’s milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy, 45, pp. 587-96.
Høst, A. [1999]: Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on HypoallergenicFormulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Archives of Disease in Childhood, 81, pp. 80-4.
Høst, A. [2002]: Frequency of cow's milk allergy in childhood.
Annals of Allergy, Asthma & Immunology, 89, pp. 33-7.
Høst, A., Husby, S. and Osterballe, O. [1988]: A prospective study of cow’s milk allergy in exclusively breast-fed infants. Incidence, pathogenic role of early exposure to cow’s milk formula, and characterization of bovine milk protein in human milk. Acta Paediatric
Scandinavian, 77, pp. 663-70.
Kaila, M., Salo, M.K. and Isolauri, E. [1999]: Fatty acids in substitute formulas for cow's milk allergy. Allergy, 54, pp. 74-7.
Kallio, H. and Rua, P. [1994]: Distribution of the major fatty acids of human milk between sn-2 and sn-1,3 positions of triacylglycerols.
Journal of the American Oil Chemists’ Society, 71, pp. 985-92.
Keeanan, T.W., Morre, J.T. & Huang, C.M., 1974. Membranes Of The
Mammary Gland. In: Lactation. Vol. II. Eds. B.L. Larson & V.R. Smith.
Academic Press. New York.
Kinsella, J.E., Shane Broughton, K. and Whelan, J. W. [1990]: Dietary unsaturated fatty acids: interactions and possible needs in relation to eicosanoid synthesis. Journal of Nutritional Biochemestry, 1, pp. 123-41.
Laiho, K., Ouwehand, A., Salminen, S. and Isolauri, I. [2002]: Inventing probiotic functional foods for patients with allergic disease.
Annals of Allergy, Asthma & Immunology, 89(Suppl.), pp. 75-82.
Lopez-Lopez, A., Castellote-Bargallo, A.I., Campoy-Folgoso, C., Rivero-Urgel, M., Tormo-Carnice, R., Infante-Pina, D. and Lopez-Sabater, M.C. [2001]: The influence of dietary palmitic acid triacylglyceride position on the fatty acid, calcium and magnesium contents of at term newborn faeces.
Early Human Development, 65s, pp. 83-94.
Miranda, G., Mahé, M.F., Leroux. C. and Martin, P. [2004]: Proteomic tools to characterize the protein fractions of Equidae milk.
Proteomics, 4, pp. 2496–509.
Monti, G., Bertino, E., Muratore, M.C., Coscia, A., Cresi, F., Silvestro, L., Fabris, C., Fortunato, D., Giuffrida, M.G. and Conti, A.
[2007]: Efficacy of don key’s milk in treating highly problematic cow’s milk allergic children: an in vivo and in vitro study. Pediatric Allergy
Immunology, 18, pp. 258-64.
Morera Pons, S., Castellote Bargalló, A.I. and López Sabater, M.C. [1998]: Evaluation by high-performance liquid chromatography of the hydrolysis of human milk triacylglycerides during storage at low temperatures. Journal of Chromatography A, 823, pp. 467-74.
Morera Pons, S., Castellote Bargalló, A.I., Jauregui, O., Casals, I. and López Sabater, M.C. [2003]: Triacylglycerol markers of mature human milk. European Journal of Clinical Nutrition, 57, pp. 1621-26.
Mu, H. and Porsgaard, T. . [2005]: The metabolism of structured triacylglycerols. Progress in Lipid Research, 44(6), pp. 430-48.
Polidori, P., Beghelli. D., Mariani, P. and Vincenzetti, S. [2009]: Donkey milk production: state of the art. Italian Journal of Animal Science, 8(Suppl. 2), pp. 677-83.
Quinn, T., 1980. Dairy Farm Management. Van Nostrand Reinhold. New York.
REGULATION (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Official Journal of the European Union, L. 139/55,
Restani, P., Beretta, B., Fiocchi, A., Ballabio, C. and Galli, C.L. [2002]: Cross-reactivity between mammalian proteins. Annals Allergy Asthma
Immunology, 89(Suppl.), pp. 11-15.
Salimei, E. and Chiofalo B. [2006]: Asses: milk yield and composition. In: Miraglia N, Martin-Rosset W (eds) Nutrition and feeding of the
broodmare. Part B: Lactation. The Netherlands: Wageningen Academic
Publishers: EAAP publication No. 120, pp. 117-31.
Salimei, E., Fantuz, F., Coppola, R., Chiofalo, B., Polidori, P. and Varisco, G. [2004]: Composition and characteristics of ass’s milk. Animal
Research, 53, pp. 67-78.
Small, D.M. [1991]: The effects of glyceride structure on absorption and metabolism. Annual Review of Nutrition, 11, pp. 413-34.
Stintzing, G. and Zetterstrom, R. [1979]: Cow’s milk allergy, incidence and pathogenetic role of early exposure to cow’s milk formula.
Acta Paediatric Scandinavian, 68, pp. 383-7.
Tafaro, A., Magrone, T., Jirillo, F., Martemucci, G., D'Alessandro, A.G., Amati, L. and Jirillo, E. [2007]: Immunological properties of donkey's milk: its potential use in the prevention of atherosclerosis.
Current Pharmaceutical Design, 13, pp. 3711-17.
Uniacke-Lowe, T., Huppertz, T. and Fox, P.F. [2010]: Equine milk proteins: chemistry, structure and nutritional significance. International
Dairy Journal, 20, pp. 609-29.
Vandenplas, Y., Koletzko, S., Isolauri, E., Hill, D., Oranje, A.P., Brueton, M., Staiano, A. and Dupont, C. [2007]: Guidelines for the diagnosis and management of cow's milk protein allergy in infants.
Archives of disease in childhood, 92, pp. 902-8.
Viera, M., Spolidoro, J.V.N., Toporovski, M.S., Cardoso, A.L., Traujo, G.T.B., Nudelman, V. and Fonseca, M.C.M. [2010]: A survey on clinical presentation and nutritional status of infants with suspected cow’milk allergy. BMC Pediatrics, 10, pp. 25.
Vincenzetti, S., Polidori, P., Mariani, P., Cammertoni, N., Fantuz, F. and Vita, A. [2008]: Donkey’s milk protein fractions characterization. Food
11
Chemistry, 106, pp. 640-49.
Whittemore, C.T., 1980. Lactation Of The Dairy Cow. Longman. New York.
Williams, C.M. [2000]: Dietary fatty acids and human health.
Annales de Zootechnie, 49, pp. 165-80.
Woods R.K., Thien, F., Raven, J., Walters, E.H. and Abramson, M.A. [2002]: Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Annals of Allergy,
![Figure 2. Lily M. (2007). A female donkey with her foal milking in Kadzidlowo [Photograph]](https://thumb-eu.123doks.com/thumbv2/123dokorg/5388322.56880/1.931.74.475.220.789/figure-lily-female-donkey-foal-milking-kadzidlowo-photograph.webp)