SUMMARY
SUMMARY ... 1 ABSTRACT ... 3 CHAPTER 1: INTRODUCTION ... 4 A) ATOPIC DERMATITIS ... 5 Introduction ... 5Genetic and environmental factors ... 5
Pathogenesis of canine atopic dermatitis ... 8
Clinical aspects of canine atopic dermatitis ... 14
Diagnosis of atopic dermatitis ... 16
Therapy of atopic dermatitis... 19
B) MAST CELLS IN ATOPIC DERMATITIS ... 26
C) ENDOCANNABINOID SYSTEM ... 31
Introduction ... 31
Cannabinoid receptors ... 32
The endocannabinoid system in skin ... 33
CHAPTER 2: EXPERIMENTAL PART ... 37
A) AIM OF THE STUDY ... 38
B) MATERIAL AND METHODS ... 40
Animals... 40
Skin samples ... 43
Mast cell’s proliferating index ... 45
Lipids, PEA and FAAs extraction from the skin ... 47
Cannabinoid receptors distribution ... 47
C) RESULTS ... 50
Mast cells’s morphometry ... 50
Mast cells’s proliferating index ... 52
Lipids, PEA and FAAs extraction from the skin ... 55
Cannabinoid receptors distribution ... 56
D) DISCUSSION AND CONCLUSION ... 63
ABSTRACT
Atopic dermatitis (AD) is one of the most frequent skin disease of dogs. Although its pathogenesis is excedingly complex, mast cells (MCs) have been shown to have a key role in their determination. The endocannabinoid system (ECS) have been previously shown to play a protective role in inflammatory processes including AD and MCs are intimately correlated to this system. Studies on humans have already given evidence for the presence of Cannabinoid Receptors 1 (CB1) and 2 (CB2) in the skin. The aim of this study is to determine the density of dermal MCs in skin of healty and atopic dogs, to measure the MC’s proliferating index (PI) in dogs with AD, to determine the skin total amount of lipids and the levels of palmytoylethanolamide (PEA) and finally to determine the distribution of CB1 and CB2 in skin of healty and atopic dogs. Skin from 5 healthy dogs and skin from 15 dogs with AD were studied. Frozen and formalin fixed skin samples were collected to perform respectively lipids extraction and histologycal/histochemical/immunohistochemical procedures. The results of this study showed a statistically significant increase of MC’s density in dogs with AD in respect to healty subjects, a MC’s PI in dogs with AD of 7,73% and a statistically significant reduced total amount of skin lipids with an increase of PEA levels (30-times higher) in lesional skin in respect to normal skin. Finally our results showed the presence of CB1 and CB2 in various types of cells in the epidermis, follicle, glands and in dermal cells including perivascular cells with MC morphology, fibroblasts, and endotheliocytes of both, healty and atopic dogs. This finding suggests that the role of MCs during AD is also numerical and not only functional and support the hypothesis of their in-situ proliferation. Furthermore, for the first time, we provide evidence for the presence of CB1 and CB2 in the canine skin and contribute to knowledge of the ECS of dogs suggesting that it may be a target for treatment of AD in dogs.
A) ATOPIC DERMATITIS
Introduction
The atopic dermatitis (AD) is “a genetically predisposed inflammatory and
pruritic allergic skin disease with characteristic clinical features associated with IgE antibodies most commonly directed against environmental allergens”
(Halliwell, 2006) and shares many features with human atopic dermatitis, such as a familial or hereditary predisposition, similar histopathology and pruritus as the predominant clinical sign (Rhodes et al, 1987).
There are many report of the incidence of AD in canine population. The incidence has been estimated to be around 10-15% of the general population (Chamberlain, 1974; Reedy et al., 1997; Scott et al., 2001) and 8% of referrals to a teaching hospital, which obviously represent a selected and biased population (Scott and Paradis, 1990). Unfortunately none of these figures are based on reliable epidemiological data and the true prevalence and incidence of AD in the general dog population thus remains unknown; nevertheless its possible say that wich is one of the most frequent cause of itching togheter with flea allergy dermatitis (Hillier and Griffin, 2001).
Genetic and environmental factors
Under the original definition of an atopic disease, a genetic trait is a prerequisite, and familial tendencies have long been known. Hence breed predisposition are inevitabile and are well-known. The predisposed breed can vary from one part of the world to another. In a study conducted at the University of California the
Labrador retriever, Golden retriever, West Highland white terrier, Chinese shar-pei, Bull terrier, Bichon frisé, Tibetan terrier and English springer spaniel were all significantly over-represented, whereas mixed breeds were resistant (Zur, et al, 2002). In a study conducted on all Switzerland canine population, the West Highland white terrier, Boxer, French bulldog, Bull terrier, Dalmatian, Vizsla and Basset hound resulted predisposed (Picco et al, 2008). In a study conducted in the United Kingdom, the heritability of AD amongst Labrador and Golden retrievers was found to be high, implying a strong genetic trait (Shaw et al, 2004). Reports of sex predisposition in AD dogs are inconsistent. Some study reported predisposition for male, female or for neither sex (Griffin and DeBoer, 2001). In another study conducted on a large population of 843 AD dogs no sex-predilection was detected (Favrot et al, 2010).
In the scientific literature many reports document differences in gene frequency from healthy subjects and subjects with atopic dermatitis; these genes are particularly important for the efficency of skin barrier and immune system (Coleman et al., 1997; Schultz Larsen, 1993; Schultz Larsen and Holm, 1985, Wood et al., 2009, Cookson, 2004, Morar et al., 2006, Merryman-Simpson et al., 2008). This disease is a polygenetic disorder influenced by hereditability and not depending on a single gene defect (Bu et al., 2006; Nodvedt et al., 2007).
The genetic predisposition is necessary but not enough to develop the disease. Environmental factors clearly influence whether a subject with genetic background of AD will become clinically atopic (Tarpataki, 2006). In humans the lower prevalence of atopic dermatitis in rural as compared with urban areas
suggests a link to the “hygiene hypothesis,” which postulates that the absence of early childhood exposure to infectious agents increases susceptibility to allergic diseases (Strachan, 1989). The children early infections in fact, would play a pivotal role for the proper development of the cell-mediate immunity and of the immunity tollerance (Bieber, 2008); particularly these conditions tends to promote a Th1 bias to the immune system and a lower prevalence of Th2-biased hypersensitivity disease (DeBoer, 2010). Also dietary factors can be considered the cause of many forms of sensitization and can therefore lead to clinical manifestations of AD. Some authors suggested that a restricted diet, without foods held responsible of sensitization as eggs, fish and milk, in breast-feeding women can prevent atopic dermatitis in their offspring (Businco et al., 1983; Lovegrove et al., 1994). Other authors reported that breast-feeding reduce the risk of development of AD (Laubereau et al., 2004; Schoetzau, et al., 2002; Kull et al., 2005) and also the juvenile intake of pre and pro-biotics substances (Lee et al., 2008; Moro et al., 2006) or vitamins (Hoppu et al., 2005; Kawai et al., 2007) can play a protective role.
To date, in dogs, the influence of environmental factors in development of AD is largely unknown; nevertheless in Sweden, several factors were found to increase the risk of AD, including living in a city or in central or southern Sweden and being born in the autumn (Nodvedt et al., 2006); furthermore the feeding of bitch during lactation with a diet including noncommercial products had a protective effect on the development of AD in her offspring (Nodvedt et al., 2007). The
indication of home-made diets as protective against development of canine AD can be seen as supportive of the hygiene hypothesis also in this species.
Pathogenesis of canine atopic dermatitis
Atopic dermatitis is a hypersensitivity phenomenon directed against various environmental but not only allergens. These include dust and storage mite antigens, house dust, pollens from grasses, trees and weeds, mould spores, epidermal antigens, insect antigens, miscellaneous antigens such as kapok (Hill and De Boer, 2001) and food allergens (Olivry et al., 2007).
The allergens are protein or glycoprotein of small size (20.000-40.000 daltons), therefore able to reach the deep respiratory tract by inhalation and to penetrate the skin barrier, and the mucous membranes of the digestive system when ingested (Hill and DeBoer, 2001; Olivry and Hill, 2001).
The definition of canine atopic dermatitis implies a pivotal role for IgE in their pathogenesis; however this is exceedingly complex, and can be categorized under three headings: defects in innate immunity, defects in barrier function and defects in acquired immunity (Halliwell, 2009).
Defects in innate immunity
The innate immune system is phylogenetically the oldest, and provides the first line of defence against invading microorganisms. In man, 80-100% of non-lesional skin of AD patients is colonized with Staphilococcus aureus as compared with 5-30% of healty subjects (De Benedetto et al, 2009). This and many other abnormalities that accompany AD are attributable to defects in the
innate immune system. In veterinary medicine there are a few studies about this topic, but the similarities in the disease between man and dog are such that similar abnormalities are likely.
Following are reported some of the abnormalities of the innate immune system reported for human AD and hypothized for canine AD. Toll-like receptors 2 (TLR2) have been shown to be deficient in some AD subjects thus rendering the skin more susceptible to infection (De Benedetto et al, 2009).
These receptors, also known as “pattern recognition receptors” recognize pathogens in the context of broad molecular pattern termed “pathogen-associated molecular patterns” (PAMPs). They are expressed by antigen presenting cells, mast cells, neutrophils and keratinocytes. A similar mechanism could be implicates in canine atopic dermatitis as well. In the skin of both humans and dogs with AD a few neutrophils are usually detected by histopathology, even in the face of significant infection (Ginn, et al., 2007) In man it has been shown that the leucocyte adhesion molecule CD11b is markedly decreased in AD patients as compared to healty subjects (De Benedetto et al, 2009).
Another important elements of the innate immune system are the antimicrobial peptides (AMPs). These come from many sources, including kerainocytes, neutrophils, sebocytes and the cells of sweat gland ducts. They are generally present at low or undetectable levels but are markedly increased in the face of injury. They have broad antimicrobial activity and have been shown to be
deficient in human’s AD patients (De Benedetto, et al, 2009). In dogs, studies on AMPs, however, have so far shown equivocal results (Van Damme et al, 2009).
Defects in barrier function
The integrity of skin barrier is very important to prevent AD; in fact in dogs, the epicutaneous route of allergens contact is the prevalent (Olivry and Hill, 2001).
The barrier function is abnormal in both human and canine AD. Barrier function is at two levels: at the level of the stratum corneum, composed of terminally differentiated corneocytes surrounded by a matrix of specialized lipids, and the tight junctions of the stratum granulosum (Halliwell, 2009). For some authors, genetic defects in the skin barrier function should be recognized as major risk factors for the development of atopic dermatitis (Tarpataki, 2006; Vickery, 2007). Recently it has been suggested that inflammation in AD is the early result of inherited and acquired insults that only after converge to alter epidermal structure and function, followed by immune system activation, which in turn has negative consequences for skin-barrier homeostasis. This cycle comprises an “outside–inside–outside” model of AD pathogenesis (Elias and Steinhoff, 2008). In human, a mutation of filaggrin has been shown to be highly associated with AD, but is not present in all cases, and so other abnormalities must contribute (Barker et al, 2007). In the dog, studies regarding filaggrin are ongoing, but it has been shown that the skin of dogs with AD contain lipids in globules, rather than dispersed to fill in all the intercellular spaces (Inman et al, 2001; Marsella and Girolomoni, 2009).
A noninvasive method to assess barrier function is the measurement of transepidermal water loss (TEWL).(Wilson and Maibach, 1989; Pinnagoda et al, 1990) TEWL is the rate at which evaporated water is lost from the skin. Thus, it is an assessment of skin permeability and epidermal barrier function. TEWL is low in the skin of normal individuals when compared with AD patients.(Loden et al, 1992; Lee et al, 2006) Furthermore it is known that the stratum corneum of individuals with AD is on average thinner than the stratum corneum of normal individuals (White et al, 1987) and that the thickness of the stratum corneum varies between different body regions and was thinner in the sites predisposed to show symptoms of AD (Barker, 1951; Lee and Hwang, 2002; Southwood, 1955). In one recent study, TEWL was evaluated in a colony of atopic beagles. TEWL was found to be higher in atopic beagles when compared with normal controls, matched for age and breed, additionally, within the atopic group it was found that sites predisposed to AD lesions had higher TEWL than other sites, and this change was particularly evident in young dogs. Again, these changes were noted in the absence of clinical evidence of AD (Hightower et al, 2008; Cornegliani et al, 2011).
Defects in acquired immunity
Study of the histopathology and immunohistochemistry of infiltrating cells in AD gives valuable insights into disease pathogenesis. Biopsies of canine AD show evidence of epidermal Langerhans’ cell hyperplasia, and these often appear in cluster (Olivry et al, 1996) and this aggregation seems to facilitate their linkage with a higher number of IgE antibodies (Marsella et al., 2006). Armed with IgE
antibodies, these play a pivotal role in allergen capture and processing. Also noted are an increased number of dermal dendritic cells that have similar functions (Olivry et al 1996; Olivry et al, 1997). Mast cells hyperplasia is frequently noted on histopathology reports, but a really increased density in the dermis of atopic dogs in respect of healty subjects are to date in debate (Olivry et al, 1997; Nimmo Wilkie et al, 1990; Welle et al, 1999). Lymphocytes are frequent in the cellular infiltrate, with a prevalence of T cells, with only a few B cells (Olivry et al , 1997). Both CD4+ and CD8+ cells are found in increased numbers, with a prevalence of CD8+ cells in the epidermis. Also neutrophils and eosinophils are frequently seen in biopsies of affected skin, but in neither cases are they represent a dominant feature. It is well estabilished in both humans and dogs that T cell responses fall in to one of the following two patterns: a Th1 response associated with IL-2, IL-12, γ-IFN and IL-18, wich is expressed as cell-mediated immunity, and a Th2 response associated with 4, 5, 6 and IL-13 that facilitate the antibody production, including IgE. Many studies undertaken in dogs suggested a Th2 response associated with the acute phase of AD, whereas a Th1 response is associated with the chronic phase, were secondary infections is superimposed (Olivry et al, 1999; Nuttal et al, 2002; ; Marsella et al, 2006). The cytokines derived respectively from a Th2 and Th1 response promote that response via a positive feedback mechanism. Conversely they are mutually inhibitory, for example γ-IFN inhibits the Th2 response and this has led to the use of γ-IFN as a treatment for canine AD (Iwasaki and Hasegawa, 2006). Similarly, successful immunotherapy in canine AD are
accompanied by a shift from Th2 to Th1 (Shida et al, 2004). In canine atopic dermatitis, also the preformed MCs derived mediators, are clearly significant. These include histamine, proteases and serotonin, although there is a little evidence that the latter is contributory. The little efficacy of antihistamines as a sole treatment implies that other mediators are likely to have more pronounced pruritogenic and inflammatory effects. The membrane-derived mediators, especially the leukotrienes, and particularly LTB4 are implicated in a range of inflammatory dermatosis included AD and are promising targets for pharmacologic intervention (Kietzman, 1990). In addition the inflammatory cell mileu that characterizes AD offers the potential for the involvement of many other inflammatory mediators that are derived from keratinocytes and other epidermal and dermal sources. Secondary bacterial infection are common features of canine AD; a major reason for this is the enhanced ability of canine staphylococcal species to adhere to corneocytes of atopic dogs. This has been demonstrated by both in vitro and in vivo studies (McEwan et al, 2005; Simou et al, 2005). The fact that IgE antibodies to antigens of these organisms can develop makes this an important factor in the disease process (Morales et al 1994). Similarly, also malassezia overgrowth is well documented (Nardoni et al, 2007) and as in the case of bacterial infection, an IgE response can compound the disease process (Nuttal and Halliwell, 2001). The immunopathogenesis of AD is very complex, but a schematic representation of the chain of events involved can is helpful to simplified it:
Initially an impaired barrier function facilitates the percutaneous absorption of allergen.
The allergen is capturated by Langerhans’ cells armed with IgE antibodies.
In the resultant immune-response to the allergen, the genetic features of the atopic trait favour the development of an IgE response Th2-mediated wich is largely elaborated in the local lymph node.
The exposure of MCs armed with IgE antibodies initiates release of preformed and newly generated mediators, wich aids the influx of inflammatory cells.
These latter, release other pro-inflammatory mediators.
Particularly in the chronic phase, a concomitant Th1 response occurs with γ-IFN prominent.
Secondary infection compounds the problem, leading to further Th1 responses.
Finally a failure of the immune regulation allows the continuation of the immune responses and resultant inflammation.
Clinical aspects of canine atopic dermatitis
Information regarding the history of the affected dogs are very important for the corrected diagnosis and should be recorded carefully. Some important questions
have already been mentioned (age at onset, breed, familial predisposition) but some others such as seasonality, presence of pruritus without skin changes at onset, efficacy of previous treatment, should be asked before any clinical examination. The typical age at onset of canine AD is reported to be between 6 months and 3 years (Griffin and DeBoer, 2001). Furthermore, it has been recently shown that about 78% of AD dogs present with clinical signs before three years of age (Favrot, et al, 2010). Clinical signs of canine AD may be seasonal or not, but seasonality is often present at onset (42-75%) (Griffin and DeBoer, 2001). Approximately 80% of dogs with seasonal signs are symptomatic in spring or summer, while the others exhibit signs in winter or autumn (Williams, 1996). It should be mentioned that some non seasonal atopic dogs exhibit worsening of clinical signs during one specific season. Pruritus must be present and its absence rules out the diagnosis of AD; furthermore in a study, 61% of dogs with AD exhibit initially pruritus without skin changes (Favrot, et al, 2010). As well 43% of AD dogs presented first with an episode of otitis externa while the associated conjunctivitis/blepharitis are rarely seen (Favrot, et al, 2010). The atopic dogs are often treated with glucocoricoids and 78% of dogs with AD respond adeguately to such treatment, prevently in the first stage of disease, while in chronic phase, the development of secondary bacterial or yeast infections usually corresponds with a poorer response to such treatment (Favrot et al, 2010). It should be mentioned that 82% of atopic dogs spend most of their time indoor and this suggest that prolonged exposure to house dust mites may trigger or worsen CAD clinical signs (Favrot et al 2010). Erytema and pruritus
are virtually always present and often represent the first clinical signs; however mild pruritus may remain unrecognized by the owner and the veterinarian may rely on indirect proofs of pruritus such as the presence of excoriations or saliva-coloured hairs (Favrot et al, 2010). Most of the signs are due to self-trauma and/or secondary infections. In fact, small erythematous papules, which are considered the primary lesion of CAD, are rarely observed in AD dogs (Griffin and DeBoer, 2001). Usually consequences of inflammation and pruritus such as excoriations and self-induced alopecia and/or the signs of the secondary bacterial (papules, pustules, crusts, erosions) or yeast (epidermal hyperplasia, hyperpigmentation, lichenification) infections are observed. In a study, bacterial infection was observed in 66% of dogs with AD, while malassezia dermatitis and otitis externa were present in 33% and 50% of all these affected dogs, respectively (Favrot et al, 2010). Also the distribution of the lesions is helpful in diagnosing AD. In fact, the most often affected areas are the pinnae (58%), the axillae (62%), the abdomen (66%), the front (79%) and hind feet (75%), the lips (42%) and the perineal area (43%) (Favrot et al., 2010).
Diagnosis of atopic dermatitis
Several set of criteria have been proposed for make diagnosis of CAD (Willemse , 1986; Prealud et al, 1998; Williams, 1996; Favrot et al 2010). Until few years ago, the most used criteria to make diagnosis of AD were that suggested by Willemse in 1986 (Table 1). Unfortunately these criteria were not evaluate with regard to sensitivity, specificity and accuracy for diagnosis of canine AD. More recently, a list of five major criteria for canine AD was proposed by Prelaud et al,
(1998) using a survey of seven veterinarians examining 96 canine patients. The presence of 3 out of five of these criteria in a patient resulted in diagnostic sensitivity and specificity of approximately 80% (Table 1). The last set of criteria was proposed by Favrot et al in 2010. In this study, performed on 1800 pruritic dogs, the measure of 5 out of 8 criteria has been associate with a sensitivity and specificity of about 80% (Table 2).
On the base of Favrot’s criteria, the diagnosis of CAD can be made on the history (age at onset, seasonality, pruritus without skin changes at onset, familial or breed predisposition, previous response to glucocorticoids), the progress of the disease and the lesional pattern (Favrot et al 2010). Neverthless a diagnosis of CAD should never been made before other skin diseases such as fleas, ectoparasites and primary skin infections have been rule out. In the same way, inorder to identify food-induced atopic dermatitis, a 6-8 week elimination diet and a subsequent challenge with the previous food should be carried out in all dogs with clinical signs of CAD. Also, allergy testing (serological evaluation of allergen-specific IgE and intradermal skin testing) are not regarded as criteria for the diagnosing of CAD. This is because numerous healty dogs are sensitized to environmental allergens and are consequently positive. These test should consequently used only to identify the offending allergens in order to use them for allergen-specific immune-terapy desensitization (Favrot et al, 2010). The histological aspect of allergic skin is usually not specific and consequently not enough to make a diagnosis. However it may be indicated to perform a skin
biopsy to rule out some differential diagnosis such as for example skin infections or cutaneous lymphoma.
Table 1: Criteria for diagnosing of CAD proposed by Willemse (1986) and Prélaud et al (1998).
Willemse, 1986 Prélaud et al, 1998
Patients must have at least 3 of the following
major features:
Pruritus
Typical morphology and distribution: facial and/or digital involvement, lichenification of the flexor surface of the tarsal joint and/or the extensor surface of the carpal joint
Chronic or chronic relapsing dermatitis
Individual or family history of atopy and or breed predisposition
Patients must have at least 3 of the following
minor features:
Onset of symptoms before 3 years
Facial erythema and cheilitis
Bacterial conjunctivitis
Superficial staphylococcal pyoderma
Hyperhidrosis
Immediate positive intraadermal test to inhalants
Elevated serum allergen-specific IgE
Elevated serum allergen-specific IgGd
Patients must have at least 3 out of the following 5 major features:
Corticosteroid-sensitive pruritus
Erythema of pinnae
Bilateral cranial erythematous pododermatitis
Cheilitis
Apparence of first signs between the age of 6 months and 3 years
Table 2: Criteria for the diagnosing of CAD and associated sensitivity and specificity (Favrot et al., 2009).
1. Age at onset<3 years
2. Mostly indoor
3. Corticosteroid-responsive pruritus
4. Chronic or recurrent yeast infections
5. Affected front feet
6. Affected ear pinnae
7. Non-affected ear margins
8. Non-affected dorso-lumbar area
Sensitivity when 5 criteria are fulfilled: 85%
Specificity when 5 criteria are fulfilled: 79%
Sensitivity when 6 criteria are fulfilled: 58%
Specificity when 6 criteria are fulfilled: 88%
Therapy of atopic dermatitis
The treatment of CAD is multifaceted and consists of a combination of actions that include the use of allergen avoidance, anti-inflammatory agents, allergen-specific immunotherapy, and antimicrobial drugs. Unfurtunately a permanent curative theraphy presently is unavailable. Treatment of the disease includes first the treatment of dermatosis wich are related or secondary to AD such as: microbial infection (bacteria and Malassezia), flea allergy dermatitis, food reactions, keratoseborrhoeic skin disease, otitis externa and pyotraumatic dermatitis. Specific therapy includes allergen removal and allergen specific
immunotherapy wich is a major approach for the long term management of the disease. Finally symptomatic therapy with various systemic and topicals drugs can be use alone or in association of specific immunotherapy.
Treatment of microbial infections
An adeguate antibacterial treatment regimen of secondary pyoderma, based upon systemic antibiotics and appropriate antibacterial topicals, may return the animal to a normal state (Mueller and Bettenay, 1996). The same reasoning is applicable to cases of Malassezia dermatitis. Systemic ketoconazole and topical therapy are required with a carefull follow-up (Guillot and Bond, 1999).
Treatment of flea allergy (FAD)
A rigorous flea control regimen can eliminate the FAD and consequently, in certain cases can enable the animal to fall under its pruritic threshold (Carlotti and Jacobs, 2000). In the casese in wich clinical signs persist after the flea control regimen, AD should be treated while maintaining absolute antiparasitic treatment (Carlotti and Jacobs, 2000).
Management of food reaction
Food intolerance may resemble CAD or may trigger flares of atopic dermatitis (Olivry et al, 2007). An elimination diet should be performed in all cases of CAD and followed by sequential reintroductions in case of good response. If an offending food is identified, this should be definitely withdrawn from the patient’s diet.
Treatment of keratoseborrhoeic skin disease
A keratoseborrhoeic disorder can occur in CAD, particularly in ancient cases. Treatment is mainly topical with shampoos and moisturizing, moreover systemic Essential Fatty Acids may have good effects on seborrhea (Carlotti and Bensignor, 2002).
Treatment of otitis externa
Otitis externa is a major feature of CAD wich causes inflammation of the external ear canal and ear pinnae. Secondary infections occur and perpetuating factors such as hyperplasia of epidermis and of both sebaceous and apocrine glands lead to chronicity. It is typically erythemato-ceruminous at the beginning of the disease and it becomes eventually suppurative (Carlotti, 2009). Numerous commercial otic preparations are available which are usually easy to use and effective (Carlotti, 2009). They contain active substance such as antibiotic, antifungal and corticosteroid agents. Corticosteroids reduce pruritus, pain, proliferative reactions and cerumen secretion (Carlotti, 2009). Systemic antibiotic therapy can be useful in otitis externa due to CAD, particularly if it is suppurative (Carlotti, 2009).
Treatment of pyotraumatic dermatitis
Lesion of pyotraumatic dermatitis are common in CAD; clipping and cleansing with antiseptic shampoos can be followed by the application of creams containing antibiotics and corticosteroids. If pruritus or pain are important, a short systemic glucocorticoid treatment is useful (Holm et al, 2004).
Specific treatment
The allergic eviction is “theoretically” the ideal treatment for all cases of allergic dermatitis. Not all allergens can be destroyed and for example pollens cannot be avoided. Nevertheless house dust mites can be destroyed with the use of acaricide sprays and foggers. The elimination of house dust mites showed good efficacy in a study conducted on dogs (Swinnen and Vroom, 2004). The allergen specific immunotherapy (ASIT) acting with the stimulation of production of blocking antibodies (IgG), which bind with allergens before that they combine with the IgE (Griffin and Hilier, 2001; Loewenstein and Mueller, 2009; Hou et al, 2008). In particular, acting on Th2-Th1 shift will lead to a reduction in the IL-4 production and an increase in the interferon-gamma production (Shida et al, 2004). The choice of allergens mainly depends on the in vivo (skin test) and/or in
vitro (ELISA = Enzime-Linked Immuno-Sorbent Assay) test results. The results
are to be interpreted in an anamnesis and clinical view in each of the cases. A positive test means only that the animal has developed specific IgE towards allergens and does not mean that the clinical signs are linked to the sensitization. In addition, in case of positive reactions, allergens should be included in an immunotherapy protocol only if they are present in the environment (Bollinger et al, 1996). The results of ASIT are more or less difficult to evaluate in dogs. In fact they depend on the animals, evaluation criteria, follow-up duration and recognition or not of “loss of follow-up” as setbacks. Presently is considered that 50 to 100% of animals respond to immunotherapy in open studies (Griffin and Hilier, 2001; Loewenstein an Mueller, 2009).
Symptomatic treatment
Symptomatic treatment is useful at the beginning of immunotherapy (within the first year in successful cases) or on a long-term basis in failed cases, or even in cases where immunotherapy is not required (aged animal, owner’s hesitation or even, clinically slightly worrying cases a part from a few signs) (Olivry and Mueller, 2003; Olivry et al, 2009).
Glucocorticoids are the most effective medications to treat allergic dermatitis. They have inflammatory and pruritic properties as well as anti-proliferative and immunosuppressive effects (Carlotti, 2009). They act in almost all inflammation and immunologic response stages but unfortunately, their activity, varies tremendously and the effect is reduced over time with consequently increasing of the doses required (Carlotti, 2009). Glucocorticoids are used topically or systemically. The topical glucocorticoids ointments, creams and gels are useful for localized lesions and in the most of the traditional veterinary formulations are combined with antimicrobials and contain less potent agents. However, their overuse can lead to tachyphylaxis, atrophy and microbial infections. Systemic glucocorticoid therapy should be limited to the administration of prednisolone or methyl-prednisolone by oral route (0,5 to 1 mg/kg/day during 5 to 7 days followed by 1 mg/kg every other day, as shortly as possible) (Carlotti, 2009). They have significant side effects including polyuria-polydipsia, polyphagia, hepatomegaly, inhibition of the hypothalamo-hypophyso-adrenal axis, dryness of the skin and haircoat and even iatrogenic Cushing’s syndrome with alopecia; secondary infection can occur (Carlotti, 2009).
Regarding their long term side effects, glucocorticoids should be used as little as possible, at the lowest possible dose and only if alternative anti-pruritic medications are not effective enough (Carlotti, 2009).
Non steroidal topical one sprays, rinses or shampoos with anti-pruritic, rehydrating and sometimes antimicrobial effects. One of these, containing Hamamelis extracts and menthol has showed to kill Malassezia (Carlotti and Reme, 2004). Also shampoos containing fatty acids can help in allergic skin disorders (Löflath, et al 2007).
Anti-H1 anti-histamines, wich block H1 receptor, may be useful (whereas antiH2 are inefficient); but in dogs, their efficacy for treatment of CAD is only of 15 to 25% (close to a placebo effect) (DeBoer and Griffin, 2001).
Many clinical studies have been done in dogs on the use of Essential fatty acids (EFA) for the treatment of CAD. These exhibit potential efficacy in treatment of allergic dermatitis through the modulation of prostaglandin and leukotriene production, the inhibition of cellular activation and cytokine secretion as well as the alteration of the composition and function of the epidermal lipid barrier (Olivry et al, 2001). They are polyunsatured, administerd by oral route, particularly omega-3 series eicosapentanoic acid (EPA) and omega-6 series gamma linolenic acid (GLA). These EFA anti-inflammatory and pro-inflammatory activities reinforce the defective cutaneous barrier of atopic dogs (Carlotti, 2009). There are still doubts, however, on the real efficacy of EFA in
CAD; furthermore the reaction of atopic dogs to these substances varies and there is no a supplement or diet that is appropriate to all (Carlotti, 2009).
Ciclosporin, an orally administered calcineurin inhibitor, is an effective drug for the treatment of CAD (Steffan et al, 2006). This molecule has become, despite its high cost, a very effective symptomatic treatment of CAD, particularly in severe forms. Its efficacy is dose-dependent but the assay of its blood level has no value since it cannot predict the clinical response. Secondary effects are limited and are mainly gastro-intestinal, with more rarely gingival hyperplasia, verrucous lesions or hypertrichosis. The long-term secondary effects known in man (renal insufficiency and hypertension) have not been reported in dogs. Last but not least ciclosporin does not enhance the risk of secondary infections (Carlotti, 2009). Finally the recombinant canine gamma interferon injections showed a good –to-excellent efficacy to control skin lesions or pruritus in about 80% of dogs with CAD and in a recent study, it has been showed a good efficacy of the recombinant omega interferon, comparable with ciclosporin (Carlotti, 2009).
In conclusion, long-term management of CAD is difficult; therapy of dermatosis wich are related or secondary is essential. The treatment shall act on the pruritic threshold, by specific immunotherapy and symptomatic treatments and can also include, when it is possible, also the allergen eviction.
B) MAST CELLS IN ATOPIC DERMATITIS
Mast cells play a pivotal role in immediate hypersensitivity and chronic allergic reactions that can contribute to skin allergic disease such as AD (Okayama and Kawakami, 2006). This function is mediated to the linkage of IgE antibodies at high-affinity IgE receptor (FcεRI) present on their membrane. Cross-linking of the FcεRI on MCs activates multiple signaling pathways that lead to degranulation, de novo synthesis of arachidonic acid metabolites, and production of various cytokines and chemokines including histamine, triptase, chimase, leucotrien, the tumor necrosis factor (TNF-α), IL-4, IL-13 and IL-8 (Metcalfe et al., 1997; Marsella and Olivry, 2001). Beyond this classical role of mast cell activation in allergic reactions, some studies have expanded our understanding of the involvement of mast cells in the defense against bacteria (Galli et al., 1999) and parasites (Metcalfe et al., 1997; Galli et al., 1999).
On the basis of their granule content, three different MCs populations have been demonstrated in dogs: MCs with both triptase and chimase (TC-MCs), MCs with only triptase (T-MCs) and finally MCs with only chimase (C-MCs) (Noli et al., 2003; Welle et al., 1999).
Mast cells are normal residents of connective tissue and are found in the highest number in areas of the body that interface with the environment, such as skin, lung and gastrointestinal tract (Foreman, 1993). In contrast to other cells of the hematopoietic stem cell lineage, wich differentiate in the bone marrow before being released into the circulation, MCs do not circulate as mature cells. Morphologically unidentifiable precursor migrate in the blood and invade
connective or mucosal tissues where they proliferate and differentiate into mature MCs (Kitamara et al., 1979; Zucker-Franklin et al., 1981; Rodewald et al., 1996). The differentiation and proliferation of mature MCs from these progenitor cells is regulated by fibroblast/keratinocyte derived stem cell factor (SCF) and T cells-derived interleukin (IL)-3, IL-4, IL-9 and IL-10 (Nocka et al., 1990; Zsebo et al., 1990; Huang et al., 1990; Wiliams et al., 1990; Tsai et al., 1991; Galli et al., 1993; Haig et al., 1994). Stem cell factor is also chemotactic agent for MCs (Meininger et al., 1992), influences the adhesion of MCs to the dermis (Kinashi and Springer, 1994), regulates the synthesis of MCs mediators (Ziegler et al., 1993) and modulates their secretory function (Coleman et al., 1993). The IL-3, IL-4, IL-9 and IL-10 instead are resulted more important for the proliferations of MCs seen in gastrointestinal tract nematode infections (Abe et al., 1988) but is also likely to be involved in the proliferation of skin MCs (Hill and Olivry, 2001).
An immunohistochemistry study revealed that there was a difference in the detectable presence of SCF between areas of skin sites without lesions from atopic dogs and same-site skin from normal dogs (Hammerberg et al., 2001). Stem cell factor has been shown to enhance human (Frenz et al., 1997), as well as canine (Brazis et al., 2000), mast cell degranulation response to cross-linking of high-affinity receptors for IgE (FceRI). Naturally occurring ‘hyperexcitability’ by blood leukocytes releasing a greater percentage of their histamine in response to anti-IgE cross-linking of FceRI has been shown to distinguish atopic dogs from non-atopic dogs (Jackson et al., 1996). Mast cells from the skin of atopic
dogs released a greater percentage of histamine than cells from the skin of normal dogs in response to concanavalin A (ConA) (de Mora et al., 1996). Stem cell factor performs its activity on MCs by the binding of its receptor Kit (or CD117) that is present on the membrane of MCs. The linkage of SCF to Kit, induces the dimerization or oligomerization of Kit leading to the activation of its intrinsic kinase activity (Okayama and Kawakami, 2006).
In humans, it has been suggested that the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP) may initiate atopic dermatitis through a dendritic cell–mediated Th2 response (Allakhverdi et al., 2007; Hirasawa et al., 2009). Thymic stromal lymphopoietin might initiate and aggravate allergic inflammation in the absence of T lymphocytes and immunoglobulin E antibodies via the innate immune system; in fact, TSLP, synergistically with interleukin 1 and tumor necrosis factor, stimulates the production of high levels of Th2 cytokines by human MCs (Allakhverdi et al., 2007). Thymic stromal lymphopoietin is released by epithelial cells in response to certain microbial products, physical injury, or inflammatory cytokines. Direct epithelial cell– mediated, TSLP-dependent activation of MCs may play a central role in “intrinsic” forms of atopic diseases and explain the aggravating role of infection and scratching in these diseases (Allakhverdi et al., 2007; Hirasawa et al., 2009).
Furthermore the MCs degranulation during an inflammatory process such as AD, is down-regulated by endogen fatty acid amides (FAAs) through a negative feed-back due to the so called ”Autacoid, Local, Injury, Antagonism” (ALIA) mechanism (Aloe et al., 1993; Levi Montalcini et al., 1996).
The number of mast cells in inflamed tissue can be variable based on their proliferation, migration, and survival characteristics. The number of tissue mast cells in healthy individuals is stable, but this homeostasis is disturbed by a number of pathophysiologic conditions: their numbers increase in inflamed tissues in allergic diseases, such as allergic rhinitis (Viegas et al., 1987) and allergic asthma (Gibson et al., 1993).
In literature there are disagreement about a possible increase of MCs number in the skin of atopic dogs (Nimmo Wilkie et al., 1990; Olivry et al., 1997; Olivry et al., 2001; Welle et al., 1999). The increase of MCs number in skin of atopic dogs can be due only to an increased migration from vascular system or at least in part, to an in-situ proliferation of these cells. There are few studies in literature studying this issue of MC’s biology; Sugiura and Uehara (1993) have observed, although in small number, mitoses of cutaneous MCs of atopic humans in 6 out of 55 subjects examined, suggesting that in-situ proliferation can contribute to MCs increase (Sugiura and Uehara, 1993). Differently in a study conducted marking skin biopsies of 3 atopic and 3 healty dogs with bromodeoxyuridine (BrdU) and proliferating cell nuclear antigen (PCNA), MC’s proliferation was not observed (Ordeix et al., 2001). In humans, in the acute stage of AD the dermal MCs were not increased in number but more degranulated; instead in chronic phases of disease there are a significant increasing in MC’s number, expecially in the areas infiltrated by many lymphocytes (Irani et al., 1989; Soter, 1989).
Furthermore MCs in healty subjects, are not evenly distributed in all regions of body but appear to be imore numerous in the ear pinnae and in the interdigital spaces; that is the areas in wich the lesions of AD first appear (Hill and Olivry, 2001). Nevertheless a significant correlation between MC’s density of some body’s regions and the tendency to develop itching in these areas is not still documented and then, the involvment of MCs in AD would seem to be mainly functional and not numerical (Welle et al., 1999; Olivry et al., 1997; Auxilla and Hill, 2000).
C) ENDOCANNABINOID SYSTEM
Introduction
Historically, cannabis/marijuana has been recognized as an anti-inflammatory drug with therapeutic effects for rheumatism in the third millenium BC, by the chinese emperor Shen-nung and it was then recommended by British physicians in the 19° century not only as an appetite stimulant and analgesic but also in treating of various infections, chronic cough, stomach pain and convulsion (Tomida et al., 2004). The physiological basis for these therapeutic effects was not understood until the discovery of the endocannabninoid system consisting of receptors and ligands in various organs and tissues in the body. It is now clear that this system is operating in both brain and peripheral tissues such as joint, bone, Immune system, gastro intestinal tract and skin (Richardson, 2007; Onaivi, 2006; Wright et al., 2008; Sylvaine et al., 1995; Stander et al., 2005).
The main psychoactive substance in Cannabis extracts was isolated and synthesized in 1964 and shown to be the Δ9-tetrahidrocannabinol (THC) (Gaoni and Mechoulam, 1964). The defined physiological and pharmacological effects of marijuana cannabinoid suggested the possible existence of an endogenous ligand with similar activity and the isolation of the first of these was reported in 1992 (Devane et al., 1992). This endogenous cannabinoids, so-called “endocannabinoid” was demonstrated to be the arachidonic acid derivative N-arachidonoyl ethanolamide (AEA), and since its discovery, several other similar compounds have been isolated and extensively studied including N-palmitoylethanolamide (PEA) and 2-arachidonoylglycerol (2-AG) (Aloe et al.,
1993; Mechoulam et al., 1995). The exogenous cannabinoids with therapeutic potential and endogenous ligands acts via a direct or indirect interaction with one or both of the two known cannabinoid receptors CB1 and CB2.
Cannabinoid receptors
The first cannabinoid receptor CB1 was cloned from a rat brain c-DNA library and the predicted amino acid sequence identified it as a seven –transmembrane G protein-coupled receptor (Matsuda et al., 1990). The second receptor CB2 was cloned in 1993 from the leukocyte cell line, HL60, and from rat spleen (Munro et al., 1993). Both receptors are coupled through Gi and G0 and inhibit adenyl cyclase as well as a variety of other second messenger and signaling components found in neural and immune tissues (Klein et al., 2003; Howlett et al., 2004). CB1 receptor ortholog have been demonstrated in many species from invertebrate sea squirt to humans and is well conserved among these organisms (Anday and Mercier, 2005). On the contrary, the structure of CB2 is less conserved among species; however, critical similarities persist because many different cannabinoid ligands activate both receptors with similar affinities (Anday and Mercier, 2005). The CB1 receptor was expressed extensively in the central and peripheral nervous system and it is particularly enriched in cortex, hyppocampus, amigdala, basal ganglia outflow tracts and cerebellum (Mackie K, 2005). Furthermore CB1 was expressed in lower levels also in numerous other organs and tissues including heart, vessels, testis, immune system and skin (Klein et al., 2007). The CB2 receptor on the contrary, shows only little expression in nervous tissues and is primarly restricted to expression in immune system (Klein
et al., 2007); also CB2 was expressed in numerous other organs and tissues including skin (Stander et al., 2005).
Besides CB1 and CB2, other receptors are stimulated by cannabinoid ligands; in fact, recent trials on endocannabinoids indicate the possible presence of a CB3 receptor, wich still has to be cloned and characterized (Breivogel et al., 2001). Furthermore cannabinoids could activate also the receptors belonging to peroxisome-proliferators-activated receptor (PPAR) family as well as a transient receptor potential vanilloid-1 (TRPV-1) (Elphick et al., 2001; Elmes et al., 2004; Breivogel et al., 2001; Brown, 2007). Remarkably, some authors even observed that endocannabinoids can simultaneously activate various receptors on the same cell and only the interaction with all these receptors produced the full action of endocannabinoids (Costa et al., 2008).
The endocannabinoid system in skin
The distribution of CB1 and CB2 and the phisiological functions of endocannabinoids system in skin were studied prevently in humans (Stander et al., 2005). The CB1 was predominantly observed to be espressed on cutaneous nerves, keratinocytes of the stratum spinosum and granulosum and on epithelial cells of infundibulum and the inner root sheet in hair follicle (Stander et al., 2005). Cannabinoid receptor 1 was also be found on a portion of CD68-positive macrophages and on all dermal mast cells (Stander et al., 2005). Cannabinoid receptor 2 has been found in the skin on large myelinated nerve fibre bundles of the superficial and deep reticular dermis, small unmyelinated nerves of the papillary dermis and occasionally on nerves of the epidermis (Stander et al.,
2005). In the epidermis, immunoreactivity for CB2 has been mainly noted in the basal layer. In contrast to CB1, CB2 expression was detected in cells of the infundibulum, in the outer root sheet and in the bulb of hair follicles, suggesting that both receptors play different role during differentiation of follicular keratinocytes. Finally CB2 expression was noted also in mast cells and CD68-positive macrophages (Stander et al., 2005). In dog skin, the distribution and expression of CB1 and CB2 receptor are almost totally unknown and to date only the presence of CB1 in the inner root sheet of hair follicle Has been documented (Mercati et al., 2011).
The cutaneous endocannabinoid system is functional and has been implicated in numerous physiological mechanisms that described below. In epidermis, both phytocannabinoids and synthetic CB agonists inhibited proliferation of human epidermal keratinocyte with a CB1/CB2-independent mechanism (Wilkinson and Williamson, 2007). In contrast the CB1 and CB2 antagonists can prevented the growth-inhibitory actions of synthetic CB agonists. (Casanova et al., 2003) Furthermore it has been recently shown that AEA markedly inhibits cellular growth and induce dose- and CB1-dependent apoptosis in human keratinocytes (Birò et al., 2006). It is also noteworthy that endocannabinoid system regulates human epidermal differentiation, probably via the CB1-dependent mechanism. (Maccarone et al., 2003) Involvement of CB1 in the regulation of epidermal differentiation is also suggested by the differential in situ expression of CB1 in human epidermis, being higher in the more differentiated (spinosum and granulosum) layers (Stander et al., 2005; Casanova et al., 2003). The
endocannabinoid system might have a regulatory role also in human pilosebaceous unit. The pilosebaceous unit can be regarded as the “brain” of the skin because it controls a wide-array of the biological functions of this organ (Roosterman et al., 2006; Paus et al., 2006) Both human organ-culture hair follicles and sebocytes have been reported to produce AEA and 2-AG (Telek et al., 2007; Dobrosi et al., 2008).
Cannabinoids dose-dependently inhibeted hair shaft elongation and the proliferation of hair matrix keratinocytes and intraepithelial apoptosis and premature hair follicle’s regression (catagen transformation) (Birò, Dajnoki et al., 2009; Mercati et al., 2011). A recent study demonstrated that CB1 receptor antagonists induced hair growth in mice (Srivastava et al., 2009). These data support the idea that human hair follicles exploit a CB1-mediated endocannabinoid signaling system that might act as an autocrine-paracrine negative regulator of human hair growth. Interestingly, differential CB2-dependent regulation by endocannabinoids has been observed in human sebocytes (Dobrosi et al., 2008). In accordance with these findings sebocyes predominantely express CB2, suggesting that CB2 is largerly expressed in undifferentiated epithelial cells of human sebaceous gland (Stander et al., 2005; Dobrosi et al., 2008). In sebaceous glands cannabinoids, via a CB2-dependent mechanism, enhanced lipid production and induced cell death, hallmarks of sebocyte differentiation and hence a model of holocrine sebum production (Dobrosi et al., 2008).
The endocannabinoids system has also immunomodulatory and anti-inflammatory effects (suppression of production of various cytokines, chemokines, arachidonic acid-derived proinflammatory metabolites and nitric oxide) and exert a protective functions in a large number of acute and chronic inflammatory diseases (Pacher et al., 2006; Klein et al., 2005, Cerrato et al., 2010).
Collectively, it seems that the main physiological function of the cutaneous endocannabinoid system is to constitutively control the proper and well-balanced proliferation, differentiation and survival as well as immune competence and/or tolerance, of skin cells. Pathological alterations in the activity of this system might promote or lead to the development of certain skin diseases. Therefore, it is envisaged that the targeted manipulation of the endocannabinoid system, with the aim of to normalize the skin cell growth, sebum production and skin inflammation, might be beneficial in a multitude of skin diseases.
A) AIM OF THE STUDY
Atopic dermatitis (AD) is one of the most frequent cause of itching in dogs, as well as flea allergy dermatitis (Hillier and Griffin, 2001). The pathogenesis of this disease is complex and not yet fully known.
Mast cells play a key role in the pathogenesis of AD (Okayama and Kawakamy 2006) and their function is in part linked to the degranulation phenomenon (Metcalfe et al., 1997; Marsella and Olivry, 2001). Whether the number of MCs in normal and/or lesional skin of dogs with AD is increased, is still in debated (Olivry et al., 1997; Welle et al., 1999). A further question arises, namely whether the increased number of MCs, as proposed by some authors (Sugiura and Uehara, 1993), derives from their in-situ proliferation or only from their migration through the vascular system.
The cannabimimetic compound PEA have previously shown to play a protective role in AD and this ability should be due at least in part by its documented inhibitory effect on the degranulation of MCs (Cerrato et al., 2010). The endocannabinoid system and their cannabimimetic compounds such as PEA thus represent a potential target for therapeutic intervention. The distribution of cannabinoid receptors in tissues is well known in humans and laboratory animals (Stander et al., 2005; Mackie, 2005) while in dogs, to date, it is largely unknown and only been reported in the salivary glands and hair follicles (Dall’Aglio et al., 2010; Mercati et al., 2011).
Since canine AD is a major issue in veterinary dermatology, a better knowledge of MC involvment in this disease and information about the endocannabinoid system in this species, might be of interest also for potential therapeutic applications. To this end, the aims of this study were:
1. To investigate the number of MCs in biopsies of dogs affected by AD and normal skin of healthy dogs.
2. To investigate the proliferation index of MCs in atopic dogs.
3. To analyse the lipid’s content and the levels of the aliamides PEA and other FAAs in skin biopsies of dogs affected by AD and in normal skin of healthy dogs.
4. To investigate the CB1 and CB2 distribution in canine skin and appendages in both normal dogs and dogs affected with AD.
B) MATERIAL AND METHODS
Animals
For the aims of the study three groups of animals were used. The investigation of the number of MCs, the analysis of skin lipid’s content, of skin levels of the aliamides PEA and other FAAs and the investigation of CBs distribution have been performed on a prospective study composed of 5 dogs with AD (Group 1) and 5 healty subjects (Group 2). In a second phase, a retrospective study including 9 skin biopsies of dogs with AD (Group 3) was performed in order to increase the number of cases (group 1) for the investigation on the proliferation index of MCs.
Group 1 (prospective study) included five dogs with AD selected from patients
of the Clinica Veterinaria “L’Arca” in Naples (Italy). Signalment of these dogs is reported in table 1. The dogs were selected among those with a diagnosis of AD based on the presence of at least three of the major and three of the minor features proposed by Willemse (1986). Clinical lesions were mainly represented by erythema, alopecia, pruritus and mild crusting. The severity of erythema, lichenification and escoriation has been graded as follows: (0) absent; (1) minimal; (2) mild to moderate; (3) severe. The score result is shown in table 1. In particular, the dogs were enrolled in the study if they showed skin lesions in selected areas, the ventral neck and axilla, in order to avoid bias in histological differences due to selection of anatomically different sites. All dogs underwent flea control and were fed an 8-week hypoallergenic restriction diet to rule out flea allergy and adverse food reactions, respectively. When necessary they were
treated for yeasts and/or bacterial secondary infections. At the moment they were enrolled into the study nor bacteria neither fungi were detected by cytological examination and specific treatments had been withdrown for at least 2/3 weeks prior to skin sampling. The diagnosis of atopic dermatitis was confirmed by the positivity to the intradermal-reaction test performed in patients under general anesthesia on an area of 10X10 cm on the thorax after having clipped the mantle.
Group 2 (control group of the prospective study) consisted of five dogs, patients of the same clinic, that did not show any lesion on the skin and which were recovered for surgery (sterilization). The signalment of these dogs is also reported in table 1.
Table 1: signalment and clinical lesion score of subjects belonging to group 1 and 2.
Group Case
No. Breed Age Gender Diagnosis
Clinical lesions score
Erythema Escoriation Lichenification
1
1 Mixed
breed 8 years FS
Atopic
dermatitis 2 1 1
2 Pitbull 5 years FS Atopic
dermatitis 2 1 1 3 Dogo Argentino 4 months M Atopic dermatitis 1 1 0
4 Pitbull 11 years M Atopic
dermatitis 2 2 0 5 Mixed breed 11 years F Atopic dermatitis 2 0 2 2
1 Yorkshire 13 years FS Healty 0 0 0
2 Cane
corso 4 years F Healty 0 0 0
3 Cane
corso 4 years F Healty 0 0 0
4 Mixed
breed 13 years FS Healty 0 0 0
5 Golden
Group 3 (retrospective study) included 9 dogs with a clinical diagnosis of atopic
dermatitis whose biopsies were collected and archived in the Diagnostic Dermatological Database of the Department of Animal Pathology of the University of Pisa. The signalment of these dogs is reported in table 2. The clinical diagnosis of AD in these cases was based on the presence of at least five of the criteria delineated by Favrot et al., (2010) as reported in table 2. When necessary the dogs had been treated for yeasts and/or bacterial secondary infections. To be enrolled in the study skin biopsies were scrutined among a larger series of dogs affected by AD in order to obtain uniformity in the histopathological pattern of the lesions. The selected cases were characterized by hyperplastic epidermal changes without secondary complication (malassezia, bacteria, pustules and crusts), mixed perivascular to interstitial dermatitis mainly composed of mast cells, lymphocytes, plasmacells and histiocytes and hyperplastic adnexal changes (mainly sebaceous gland hyperplasia).
Table 2: signalment and clinical features of subjects of group 3.
Case
No. Breed Age Gender Diagnosis Favrot's features
1 German
Shepherd 3 years M
Atopic
dermatitis A3, CRP, CYI, AFF, NAEM, NADL 2 Fox terrier 5 years F Atopic
dermatitis A3, MI, CRP, AEP, NADL 3 Springer
spaniel 8 years F
Atopic
dermatitis CRP, CYI, AFF, NAEM, NADL 4 English
Setter
11
years M
Atopic
dermatitis MI, CRP, AFF, NAEM, NADL 5 Dalmatian 4 years F Atopic
dermatitis A3, MI, CRP, AFF, AEP, NADL
6 Mixed
breed
11
months M
Atopic
dermatitis A3, MI, AFF, NAEM, NADL 7 German
Shepherd 5 years M
Atopic
dermatitis A3, CRP, CYI, AFF, NAEM
8 Shitzu 8 years F Atopic
dermatitis MI, CRP, CYI, NAEM, NADL
9 Mixed
breed
13
years M
Atopic
dermatitis MI, CRP, CYI, AFF, NAEM 10 Alaskan
Malamute 8 years M
Atopic
dermatitis A3, CRP, CYI, NAEM, NADL
Abbreviations
A3 = Age at onset < 3 years; MI = Mostly indoor; CRP = Corticosteroid-responsive pruritus; CYI = Chronic or recurrent yeast infection; AFF = Affected front feet; AEP = Affected ear pinnae; NAEM = Non-affected ear margins; NADL = Non-affected dorso-lumbar area.
Skin samples
From dogs of group 1 and 2 eight millimeter punch biopsy samples were collected, in duplicate from adjacent sites, from the skin lesions (group 1) and normal skin (group 2) under general anesthesia. One sample was fixed in a 10% buffered formalin solution and paraffin embedded for hematoxylin-eosin (H&E) and toluidine blu (TB) stainings; the adjacent biopsy was snap frozen in liquid nitrogen for lipid extraction. Skin sampling from both groups of dogs was
performed after the experimental procedures were approved and certified by written consensus by the owner.
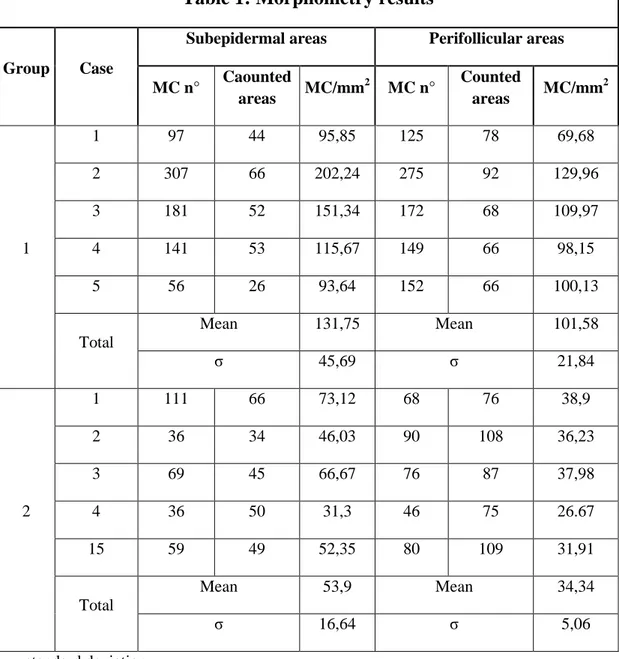
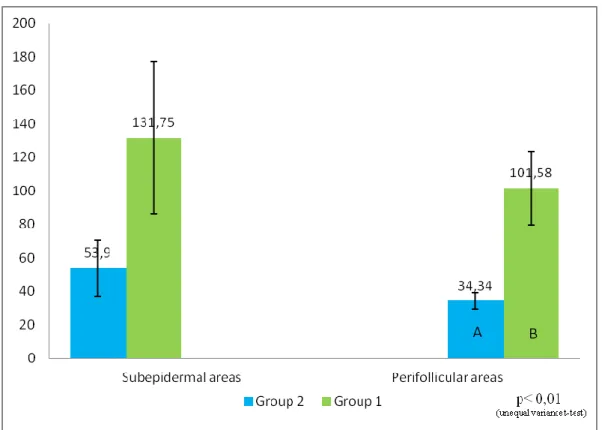
Mast cell’s morphometry
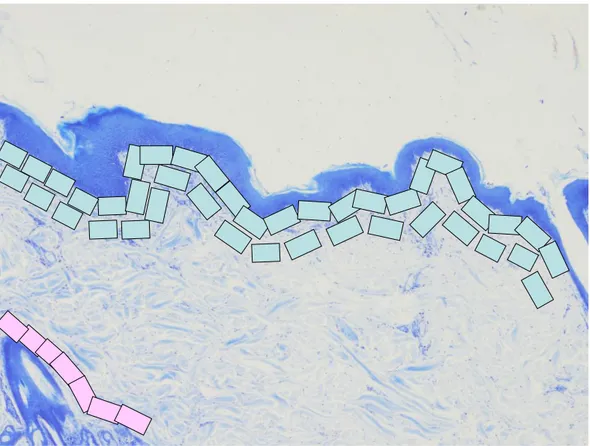
The number of MCs was measured on samples of group 1 and 2 by using the Lucia Measurements computerized analyser system (Nikon, Tokyo, Japan) on sections of 5µm thickness stained with TB following a standard procedure. The number of MCs was assessed at high power field (magnification x400). Images were captured on the monitor and MCs counted in consecutive fields from the subepidermal and perifollicular dermis (Figure 1). The number of MCs was expressed as cells/mm2. Descriptive statistic was calculated. The non-parametric Mann-Whitney U test was carried out and p values were considered significant when < 0.05.
Figure 1: Mast cells morphometry; a rappresentation of investigation (pink rectangle= perifollicular areas; bright blue rectangle= subepidermal areas. Section of skin stained with BT (magnification x 40).
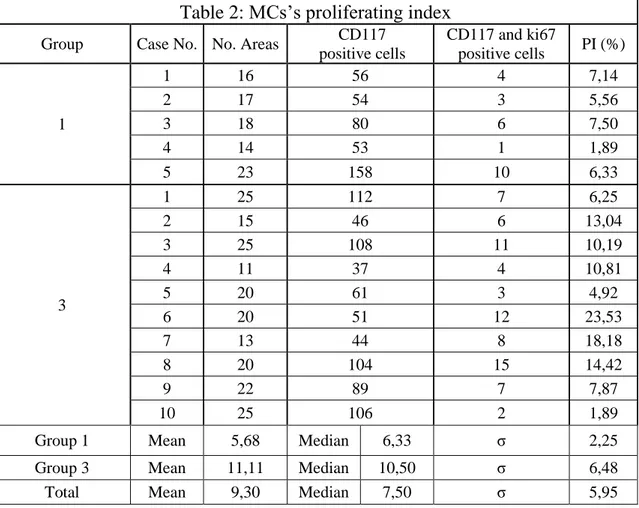
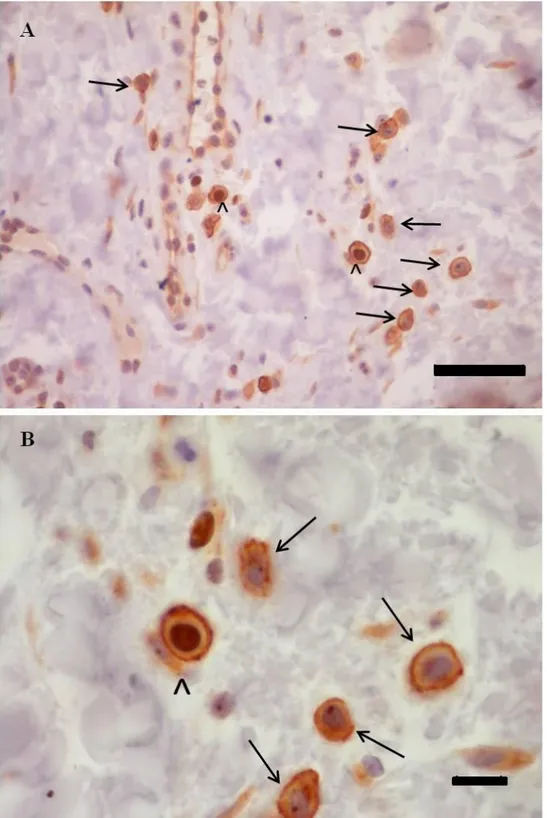
Mast cell’s proliferating index
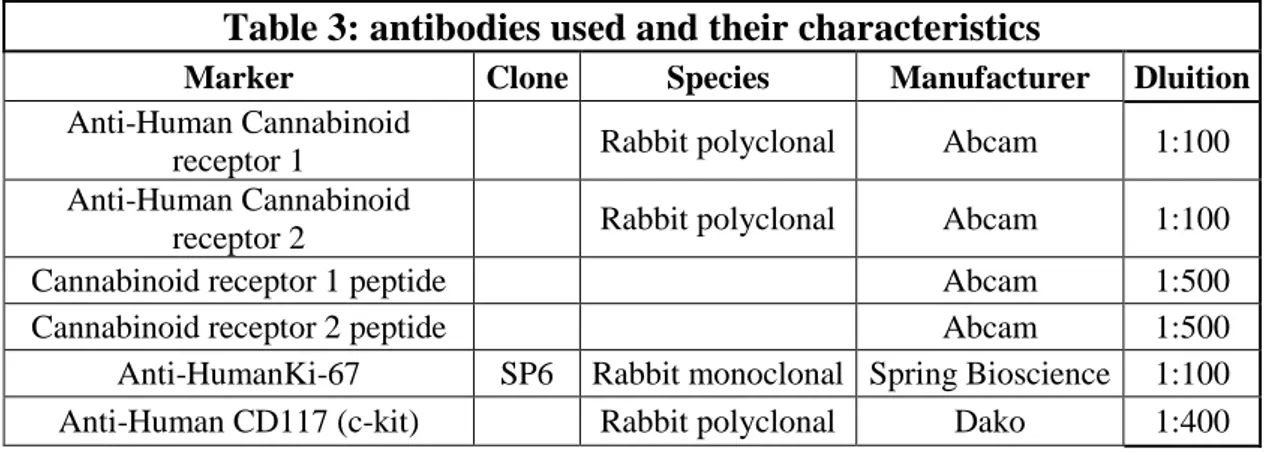
The MCs’s proliferating index (PI) was calculated on skin samples of dogs belonging to group 1 and 3; on these cases a double immunostaining was performed using CD117 (code A4502, Dako, Carpinteria, CA, USA ) and Ki67 (cat No. M3060, Spring Bioscience, Pleasanton, CA, USA ). Details of antibodies used in this study are reported in table 3. Five micrometer thick paraffin sections were prepared, mounted on SuperfrostPlus slides (Fisher Scientific, Pittsburgh, PA, USA), deparaffinized and then rehydrated. Quenching of endogenous peroxidases was carried out with 3% H2O2 in distilled water, for 30 min at room temperature. Subsequently sections were placed in citrate buffer
pH 6 (UCS Diagnostic S.r.l., Morlupo, Rome, Italy) and boiled in microwave for 15 minutes. Non-specific binding was prevented by incubation of slides with a serum-free protein block (Super Block, Scy Tek Laboratories, Logan, UT, USA) for 10 minutes. Slides were then incubated with the first primary antibody (Ki67) diluited 1:100 in 0.1M phosphate buffered saline solution + Tween (PBST, UCS Diagnostic S.r.l., Morlupo, Rome, Italy), overnight at 4°C, and subsequently, after 4 washes with PBST between each step, the slides were incubated for 10 minutes with the secondary antibody (Ultra Tek Anti-Polyvalent, Scy Tek Laboratories, Logan, UT, USA) and with a peroxidase-conjugated Streptavidin (Ultra Tek HRP, Scy Tek Laboratories, Logan, UT, USA). Immunoreactivity was visualised by diaminobenzidine incubation for 10 minutes (DAB Chromogen, Scy Tek Laboratories, Logan, UT, USA). Section were then placed in PBST for 10 minutes and incubated with a serum-free protein block for 10 minutes. After this, the sections were incubated with the second primary antibody (CD117) diluited 1:400 in PBST for 1 hour at room temperature and subsequently, after 4 washes with PBST between each step, the slides were incubated for 10 minutes with the secondary antibody and with a peroxidase-conjugated Streptavidin. Immunoreactivity was visualised by a chromogen peroxidase-substrate kit (Vector® NovaRED, Vector Laboratories, Burlingame, CA, USA). Sections were then counterstained with Haematoxylin, dehydrated and mounted with a permanent mounting medium.
The PI of MCs was assessed at high power field (magnification x400). Images were captured on the monitor and the number of cells positive for CD117 but
negative for Ki67 (non proliferating MCs) or positive for both CD117 and KI67 (proliferating MCs) were counted. The proliferating index was expressed as a percentage of proliferating MCs on total MCs as assessed by their CD117 positivity (proliferating MCs x 100/ total MCs).
Table 3: antibodies used and their characteristics
Marker Clone Species Manufacturer Dluition
Anti-Human Cannabinoid
receptor 1 Rabbit polyclonal Abcam 1:100
Anti-Human Cannabinoid
receptor 2 Rabbit polyclonal Abcam 1:100
Cannabinoid receptor 1 peptide Abcam 1:500
Cannabinoid receptor 2 peptide Abcam 1:500
Anti-HumanKi-67 SP6 Rabbit monoclonal Spring Bioscience 1:100
Anti-Human CD117 (c-kit) Rabbit polyclonal Dako 1:400
Lipids, PEA and FAAs extraction from the skin
Frozen tissue samples collected from group 1 and 2 were homogenized and lipid-containing organic phase was dried down, weighed and pre-purified by open-bed chromatography on silica gel. The amounts of PEA and other FAAs were determined by isotope diluition liquid chromatography-atmospheric pressure chemical ionization mass spectrometry (LC-APCI-MS). The amounts of lipid extracts and FAAs of dogs with AD and healty subjects were compared by the Wilcoxon signed-rank test.
Cannabinoid receptors distribution
The cannabinoid receptor immunolocalization was performed on skin samples from dogs belonging to group 1 and 2. Sections of 5 normal healthy brains (hippocampus) and 5 normal lymph nodes, collected from dogs without clinical