Contents lists available atScienceDirect
Interdisciplinary Neurosurgery
journal homepage:www.elsevier.com/locate/inatTechnical Notes & Surgical Techniques
Cyberknife stereotactic treatment of pituitary adenomas: A single center
experience using di
fferent irradiation schemes and modalities
Giovanni Ra
ffa
a,c,d,⁎,1, Oana Ruxandra Cotta
a,1, Francesco Ferraù
b, Alfredo Conti
c,d,
Antonio Pontoriero
c,f, Maria Luisa Torre
b, Marta Ragonese
e, Erika Messina
a, Federica Spagnolo
a,
Adriana Albani
a, Felice Esposito
c,d, Filippo Flavio Angileri
c,d, Antonino Germanò
c,d,
Stefano Pergolizzi
c,f, Salvatore Cannavò
b,eaDepartment of Clinical and Experimental Medicine, University of Messina, Italy bDepartment of Human Pathology, University of Messina, Italy
cDepartment of Biomedical Sciences, Dentistry and Biomorphological and Functional Imaging, University of Messina, Italy dUnit of Neurosurgery, University Hospital of Messina, Italy
eUnit of Endocrinology, University Hospital of Messina, Italy fUnit of Radiotherapy, University Hospital of Messina, Italy
A R T I C L E I N F O Keywords: Cyberknife Pituitary adenomas Radiosurgery A B S T R A C T
Background: Stereotactic irradiation is proposed for treatment of pituitary adenomas (PAs) in patients with tumor progression/recurrence after surgery and in cases not eligible for other therapies. In the literature several papers have been published on the role of the Gamma-Knife stereotactic irradiation for management of PAs, but data on the role of stereotactic Cyberknife (CK) are still limited. We describe a single-center experience using CK stereotactic radiosurgery (CK-SRS) and hypofractionated radiotherapy (CK-SRT) for PAs treatment, analyzing its efficacy/safety in the light of current literature.
Methods: We retrospectively collected clinical data from PAs patients treated using CK system at the University Hospital of Messina, Italy, between 2008 and 2018. The efficacy was evaluated by analyzing the tumor growth/ biochemical disease control rates, and the safety by evaluating post-treatment pituitary and/or visual deficits. Results: Twenty-four PAs patients were included in the study. The mean follow-up was 42.21 ± 32.67 months. The overall tumor growth control rate was 91.6%, but it was higher using the single-session scheme (100%) than using hypofractionated sessions (80%). The biochemical disease control rate was 60%, but increased to 80% in GH-secreting PAs. Post-treatment hypopituitarism occurred in 41.66% of cases, being 35.71% using a hypo-fractionated scheme, and 50% using a single-session treatment. No cases of post-treatment visual deterioration were observed. Overall and progression-free survival were respectively 84.6% and 83.6% at three years. Conclusions: Cyberknife irradiation is an effective and safe option for PAs treatment, being associated to an excellent tumor growth/biochemical control of the disease, and to a low rate of post-treatment complications. The efficacy seems to be higher when using a single-session scheme even if this could be associated to a higher incidence of post-treatment hypopituitarism.
https://doi.org/10.1016/j.inat.2018.12.004
Received 3 December 2018; Accepted 9 December 2018
Abbreviations: ACTH, adrenocorticotropic hormone; BED, biological equivalent dose; CK, Cyberknife; Dmax, maximal dose; FSH, follicle-stimulating hormone; FT4, free thyroxine; GH, growth hormone; GHD, growth hormone deficiency; GHRH, GH-releasing hormone; GK, Gamma-Knife; Gy, Grey; IGF-1, insulin-like growth factor-1; LH, luteinizing hormone; MRI, magnetic resonance imaging; NFPAs, non-functioning pituitary adenomas; OC, optic chiasm; ON, optic nerve; OS, overall survival; PAs, pituitary adenomas; PFS, progression free survival; PRL, prolactin; SRS, stereotactic radiosurgery; SRT, stereotactic radiotherapy; TE, echo time; TR, repetition time; TSH, thyroid-stimulating hormone; UFC, free urinary cortisol
⁎Corresponding author at: Department of Clinical and Experimental Medicine, Department of Biomedical Sciences, Dentistry and Biomorphological and Functional
Imaging, and Unit of Neurosurgery, University of Messina, A.O.U. Policlinico“G. Martino”, Via Consolare Valeria 1, 98125, Messina, Italy. E-mail address:giovanni.raff[email protected](G. Raffa).
1These authors contributed equally to the study.
2214-7519/ © 2018 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
1. Introduction
Pituitary adenomas (PAs) account for about 15% of all intracranial tumors [1]. Surgery is considered the best treatment strategy for both non-functioning [2] and functioning PA [3,4], except for prolacti-nomas, which can be usually successfully managed through medical treatment [5]. Nevertheless, stereotactic irradiation has been increas-ingly used as treatment option in cases of PAs showing a volumetric progression and/or hormonal hypersecretion resistant to other thera-pies, or in patients not eligible to other therapies including surgery (i.e. high anaesthesiological risk, refusing surgical treatment) [6–14]. Dif-ferent options for stereotactic irradiation are available today, including different systems such as the frame-based Gamma-Knife (GK) (Elekta AB, Stockholm, Sweden) and the frame-less Cyberknife (CK) (Accuray, Sunnyvale, California, USA). Moreover, different therapeutic schemes have been proposed, ranging from the single-session stereotactic radiosurgery (SRS) to the new hypofractioned schemes of stereotactic radiotherapy (SRT) for tumors compressing the optic apparatus [15], with the latter being preferred to reduce the radiation dose delivered to the vulnerable optic chiasm/nerves and pituitary stalk [16].
Despite the frame-less CK system could easily deliver the radiation dose using hypofractionated schemes [8], to date only few papers evaluated the efficacy and safety of the CK stereotactic irradiation for PAs [6–10,12,17–22], usually on small patient series.
According to this premise, the aim of the present study is to con-tribute to the actual available literature in the field by reporting our single-center experience on the treatment of PA using the CK stereo-tactic irradiation. We analyzed its clinical efficacy in terms of the tumor-growth/biochemical disease control rates, and its safety by as-sessing the incidence of post-treatment visual/endocrinological deficits. Findings were compared with the actual available data reported in the literature, and, for thefirst time, also according to the different treat-ment modalities and schemes available in the clinical practice. 2. Materials and methods
2.1. Patients
We retrospectively reviewed clinical and neuro-radiological data of all patients admitted at the University Hospital of Messina, Italy, be-tween 2008 and 2018 who were treated by CK stereotactic irradiation for primary, residual or recurrent PAs.
First line CK treatment was performed in inoperable patients for a high anaesthesiological risk or because they refused surgery. Adjuvant CK irradiation was performed after surgery in cases of residual tumor that showed a progressive increase in size or hormone hypersecretion not controlled by other treatments, and in case of recurrence during the neuro-radiological follow-up that could not benefit from a further sur-gical debulking.
We evaluated biochemical data collected before CK treatment and during the follow-up. Pituitary function was evaluated by the random measurement of FT4, prolactin, and IGF-1 levels, testosterone levels in men and FSH levels in amenorrhoic women, by stimulation test with GHRH + arginine (0.5 g/kg arginine, iv, from 0 to +30 min, up to a maximum of 30 g + GHRH 1μg/kg iv bolus at 0 min) for the assessment of GH reserve, and with low-doses ACTH (1μg im of synthetic ACTH from 0 to 90 min) for the assessment of hypothalamic-pituitary-adrenal axis. Hormone hypersecretion status was evaluated accordingly with most recent guidelines [3–5].
Hormones assays measurements were performed in the centralized laboratory of the University Hospital of Messina, by using commercial kits (TSH, FT4, PRL, GH, IGF-1, ACTH, FSH and LH by chemilumines-cent assays. Cortisol and testosterone by radioimmunoassay assays).
A brain contrast-enhanced MRI scan was performed before CK treatment and at 3, 6, and 12 months after treatment during thefirst year, and every year thereafter. Pre- and post-treatment volumetric
analysis was performed on a T1-weighted (TR/repetition time = 8.1, TE/echo time = 3.7, slice thickness 1 mm) contrast-enhanced sequence (Achieve 1.5 T, Philips Medical Systems, The Netherlands) by an ex-perienced neuroradiologists who was blind to the clinical outcome, using the OsiriX Imaging Software© (Pixmeo SARL, Bernex, Switzerland) as previously reported [23–25]. According to the Guide-lines of the Committee of the Brain Tumor Registry of Japan, changes of tumor size were considered significant if a variation (increase/de-crease) > 25% of the contrast-enhanced tumor was recorded [20,26,27].
Visual field evaluation was performed before CK treatment and during follow-up by the Humphrey Field Analyzer (HFA II, Carl Zeiss AG, Oberkochen, Germany).
In patients submitted to adjuvant CK irradiation the diagnosis was previously confirmed by histological examination, while in the other cases diagnosis was exclusively based on endocrinological and neuro-radiologicalfindings.
The study was conducted in accordance with the Declaration of Helsinki and its later amendments. All patients signed an informed consent for the collection and scientific use of their data.
2.2. CK stereotactic irradiation technique
An inverse planning algorithm using a non-isocentric technique with dose constraints was used to plan the best irradiation strategy to maximize doses to the tumor and minimize irradiation of the optic apparatus.
A single-session scheme (CK-SRS) was chosen for PAs located at ≥3 mm from the optic apparatus [10,18]. A marginal dose of maximum 20 Gy was planned, maintaining the dose to optic nerves/chiasm lower than 8–10 Gy (Fig. 1) to reduce the risk of radiation-induced optic neuropathy (RION) [10,15,28–32]. Indeed, it has been demonstrated that, for single-session treatment, delivering a single-dose of 10 Gy is associated to 1% of probability to develop the RION [30].
A hypofractioned scheme consisting of 3 to 5 sessions (CK-SRT) was used for PAs close to the optic apparatus (≤2 mm) [18]. The planned dose to the target volume was 21 Gy in 3 fractions or 25 Gy in 5 frac-tions. As well, the maximum doses allowed for the optic apparatus were 20 Gy in 3 fractions or 25 Gy in 5 fractions. These doses constraints were defined according to the literature to reduce the risk of RION [30]. All irradiations were given once a day, 3–5 days a week.
Dose prescription and constraints for critical structures were the same for functioning and non-functioning PAs.
2.3. Data collection and analysis
Pre- and post-treatment data and imaging were collected from the clinical charts, PACS system, and out-patients clinic records, and ana-lyzed to assess 1) the tumor growth control rate; 2) the biochemical disease control rate; and 3) post-treatment acute complications, and long-term occurrence of hypopituitarism and/or visual deterioration; data were compared according to the treatment modality (first line/ adjuvant) and scheme (single-session or CK-SRS/hypofractionated or CK-SRT). We also investigated an eventual correlation between the post-CK tumor volume and the biochemical disease control rate/oc-currence of hypopituitarism.
2.4. Statistical analysis
Pre- and post-CK treatment quantitative data were compared by using the paired Student t-test, whereas qualitative data were compared by using the Chi-square and Fisher tests. The Pearson test was used to investigate an eventual correlation between the post-CK tumor volume and the biochemical disease control rate/occurrence of hypopitui-tarism. Logistic regression was used for multivariate analysis of the correlation between radiation doses and the occurrence of
post-treatment hypopituitarism. The Kaplan-Meier method was used to de-fine the overall and progression-free survival (respectively OS, and PFS). Statistical significance was defined as a p value < 0.05. Data analysis was performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, California, USA, www. graphpad.com.
3. Results 3.1. Population
Twenty-four patients (13 males, 11 females, median age 56.5 years old, range 15–79) were included in the study (Table 1). The mean follow-up was 43.96 ± 32.66 months (median 32.5, range 6–122). Only two patients had a 6-months follow-up, that is the minimum value reported also in other studies [17,22].
Fourteen patients were affected by non-functioning PAs (NFPAs). Among the remaining 10 PAs, 5 were GH-secreting (including one case of GH/TSH co-secretion), 4 ACTH secreting (including 1 case pre-senting as Nelson's syndrome) and 1 PRL-secreting tumors. Two of 4 ACTH secreting PAs turned out to be pituitary carcinomas because of the appearance of systemic metastases during the follow up. At baseline 14 (58.33%) patients presented pituitary function impairment: there were 6 (25%) cases of isolated pituitary deficiency, 7 (29.16%) cases of multiple pituitary deficiency and 1 (4.17%) case of panhypopituitarism. Growth hormone deficiency (GHD) was present in 9 (37.5%) cases, central hypothyroidism in 8 (33.33%), hypogonadotropic hypogo-nadism in 10 (41.66%) and central hypoadrenalism was demonstrated in 2 (8.33%) cases.
Four patients underwent CK irradiation asfirst line therapy because refused surgical treatment, in three cases, or showed rapid volumetric progression of ACTH-secreting giant adenoma, in one case. In that case a surgical debulking was not possible due to serious comorbidities. The remaining 20 patients received adjuvant CK treatment after endoscopic endonasal trans-sphenoidal surgery.
Fourteen patients were submitted to a hypofractioned scheme (3–5 sessions), while 10 patients received a single-session scheme. The mean follow-up of patients receiving a single-session treatment was 44 ± 33.66 months (median 40, range 6–87). For patients treated by multi-sessions CK, the mean follow-up was 43.93 ± 33.20 (median 32.5, range 6–122). The details of the delivered doses are reported on Table 2.
An overview of the pre- and post-treatment results is reported in
Table 3.
3.2. Tumor growth control
The mean postoperative tumor size was significantly reduced (7.13 ± 9.88 cm3vs. 8.33 ± 10.03 cm3, p = 0.03). CK treatment de-termined a volumetric decrease in 13 cases (54.16%) (Figs. 2 and 3), and in 3 of these the PA was no longer visible during the MRI follow-up. In 9 cases (37.5%) the tumor size was stable, whereas a volumetric increase was evident in 2 out of the 24 cases (8.3%).
The overall tumor growth control rate was 91.6%. It was 100% for NFPAs, but decreased to 80% in the group of functioning tumors. In particular, it was 100% (4 of 4) for ACTH-secreting, 80% (4 of 5) for GH-secreting and 0 for the unique case of PRL-secreting PA. No sig-nificant differences were observed for the tumor growth control rate when distinguishing between the treatment scheme and modality (Table 3).
The two PAs in progression were invasive and aggressive: one was a GH-secreting tumor with invasion of the cavernous sinus and an evident suprasellar extension; the other one was a PRL-secreting PA invading the sphenoidal, ethmoidal and the cavernous sinuses, and the nasal cavities. Both patients received a dose of 25 Gy using a 5-sessions scheme, after transphenoidal surgery. There was no dose under cov-erage in both cases. Probably the reason of CK treatment failure was the very large size of the PA before treatment: in particular the GH-se-creting one had a volume of 27.61 cm3, while the PRL-secreting one had a volume of 31.8 cm3. Despite an initial volumetric control, the
pro-gression-free survival was 30 months for the GH-secreting PA, and 36 for the PRL-secreting PA. The patient with the GH-secreting PA died after 33 months from CK treatment.
3.3. Biochemical disease control
Among 10 functioning PAs, a hormonal control was achieved in 6 patients (60%) at last visit. The mean follow-up was 50.9 ± 36.54 months (median 32.5, range 10–122 months). In 5 out of 6 cases medical therapy was continued because necessary to maintain the hormonal level normalization. The median time to biochemical control was 12 months (range 6–30).
Normalization of the IGF-1 levels was demonstrated in 4 (80%) out of 5 cases of acromegaly, and medical therapy was withdrawn in one case after 18 months from CK treatment.
Free urinary cortisol (UFC) levels definitively normalized in 2 out of Fig. 1. Example of CK planning for treatment of a case of PA, showing the planned doses. Please, note the constrained Dmax planned to be delivered to the optic chiasm (8.6 Gy).
4 (50%) cases of Cushing's disease. The other cases experienced a transient UFC levels normalization for 6 and 9 months, respectively.
In the patient with PRL-secreting aggressive PA, CK irradiation progressively reduced (−80%), but did not normalize, PRL levels during the first 36 months. Nevertheless, hormonal values increased again thereafter.
No significant differences in biochemical disease control rates were observed using different CK irradiation modalities or schemes (Table 3). 3.4. Safety of the CK treatment
3.4.1. Endocrinological outcome
At last visit, a worsening of the pituitary function was recorded in 10 out of 24 patients (41.66%). Among 10 patients with a normal pi-tuitary function before treatment, 5 (50%) developed some degree of pituitary function impairment (3 developed isolated GHD and 2 mul-tiple pituitary deficits). Conversely, a further pituitary function wor-sening was demonstrated in 5 out of the 14 already hypopituitary
patients (35.71%).
Among patients who received a single session treatment (CK-SRS), a worsening of the pituitary function was observed in 5 out of 10 (50%). It consisted in panhypopituitarism in 3 patients, a single deficit in 1 (GH) and a multiple deficiency in the remaining one (TSH-GH-LH/ FSH). Five out of 14 (35.71%) patients who underwent hypo-fractionated treatment (CK-SRT) showed an endocrinological wor-sening. In particular, 2 cases consisted in panhypopituitarism, 2 cases in a single deficit (GH), and the remaining 1 in a multiple deficiency (GH-LH/FSH). The length of the follow-up was not different between the two groups (44 ± 33.66 vs. 43.93 ± 33.20 months,). Differences ac-cording to the treatment modality and scheme did not reach the sta-tistical significance (Table 3).
Logistic regression applied to a model including 5 variables (iso-dose, marginal (iso-dose, number of fractions, biological equivalent dose or BED, and dose max) predicted the post-treatment pituitary function impairment with an accuracy of 75% (p = 0.04). The BED was the only independent predictor of new pituitary deficiency, having a lower BED Table 1
Clinical characteristics of patients.
Patient Sex Age Adenoma type
CK modality CK scheme Post-CK PA size (time to progression) Biochemical disease control (time to response)
Pre-CK pituitary deficits Post-CK new hormonal deficits Post-CK visual deterioration Post-CK death
1 M 56 NF First line 4 Reduced N/A No GH No No
2 F 46 NF Adjuvant 1 Reduced N/A TSH-GH-LH/FSH Panhypopituitarism No No
3 F 42 NF Adjuvant 1 Reduced N/A TSH-GH Panhypopituitarism No No
4 F 79 NF Adjuvant 3 Stable N/A TSH-GH-LH/FSH Panhypopituitarism No No 5 M 57 NF Adjuvant 1 Reduced N/A TSH-GH-LH/FSH Panhypopituitarism No No
6 F 43 NF Adjuvant 5 Reduced N/A No GH No No
7 M 69 NF Adjuvant 5 Reduced N/A GH No No No
8 M 75 NF First Line 1 Stable N/A No GH No No
9 F 51 NF Adjuvant 1 Reduced N/A No TSH-GH-LH/FSH No No
10 F 54 NF Adjuvant 1 Reduced N/A No No No No
11 F 75 NF Adjuvant 5 Stable N/A No GH-LH/FSH No No
12 M 69 NF Adjuvant 4 Reduced N/A LH/FSH No No No
13 F 61 NF Adjuvant 1 Stable N/A No No No No
14 M 57 NF Adjuvant 5 Stable N/A Panhypopituitarism No No No
15 M 36 GH Adjuvant 1 Stable Yes (6 months) LH/FSH No No No
16 F 59 GH Adjuvant 1 Stable Yes (30 months) No No No No
17 M 65 GH (aggressive)
Adjuvant 5 Increased (30 m)
No TSH-LH/FSH-ACTH No No Yes
18 F 75 GH Adjuvant 3 Reduced Yes (6 months) No No No No
19 M 65 GH/TSH First Line 1 Stable Yes (18 months) No No No No
20 M 49 ACTH (carcinoma)
Adjuvant 5 Stable No TSH-GH-LH/FSH No No Yes
21 M 35 ACTH (carcinoma)
Adjuvant 5 Reduced No GH-LH/FSH No No Yes
22 F 37 ACTH First Line 5 Reduced Yes (12 months) TSH-LH/FSH No No No 23 M 15 ACTH
(Nelson's syndrome)
Adjuvant 5 Reduced Yes (12 months) GH No No No
24 M 49 PRL (aggressive)
Adjuvant 5 Increased (36 m)
No GH Panhypopituitarism No No
Abbreviations: ACTH = adrenocorticotropic hormone, CK = Cyberknife; F = female, FSH = follicle-stimulating hormone, GH = growth hormone, LH = luteinizing hormone, M = male, m = months, N/A = not available, NF = non-functioning, PA = pituitary adenoma, PRL = prolactin, TSH = thyroid-stimulating hormone.
Table 2
Characteristics of the CK prescribed doses.
Characteristics of the CK treatment Values
Number of fractions 1 in 10 patients; 3–5 in 14 patients
Marginal dose Mean 21 ± 4.52 Gy (median 21, range 15–30) Maximum dose Mean 27.76 ± 6.51 Gy (median 25.32, range 20–40) Prescribed isodose Mean 74.25 ± 8.35% (median 75, range 49–87) Biological equivalent dose (BED) 115.5 ± 30.77 Gy (median107.5, range 62.33–199.3) Optic nerves/chiasm maximum dose 13.45 ± 6.41 Gy (median 10.65, range 6.2–30.3)
Single session 8.71 ± 2.17 Gy (median 8.6, range 6.2–13.5) Hypofractionated 16.82 ± 6.32 (median 17.29, range 8.94–30.31)
a slightly protective effect against the occurrence of post-treatment hypopituitarism (p = 0.03; OR 0.87).
3.4.2. Visual outcome
All patients showed a stable visual condition and no cases of visual deterioration were recorded.
3.4.3. Acute complications
No cases of acute complications, including adrenal crisis, visual impairment or cranial nerves palsy were observed in the short term (4 weeks) after CK irradiation.
3.5. Correlation between the post-CK tumor volume and the biochemical disease control rate/occurrence of hypopituitarism
We observed a significant inverse correlation between the post-CK tumor volume and the biochemical control of the disease: a larger tumor was associated to a lower biochemical control of the disease (r =−0.88, p = 0.006). No correlation was found between the post-CK tumor volume and the worsening of the pituitary function.
3.6. Progression-free and overall survival rates
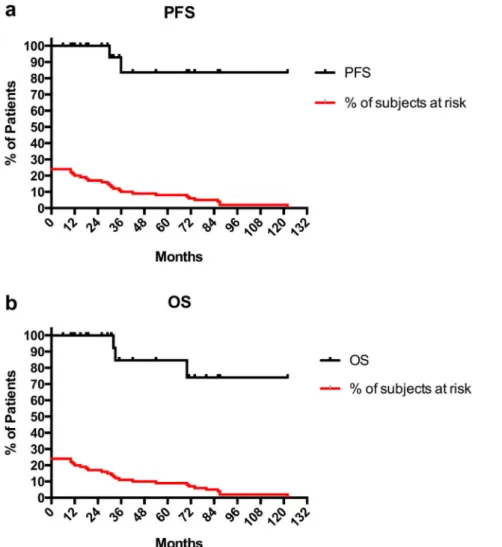
In the two cases with PA volumetric increase, the progression was noted after 30 (GH-secreting) and 36 (PRL-secreting) months from treatment. The PFS rate was 100% at two years, but decreased to 83.6% at three years (Fig. 4a).
The OS was 100% at two years, 84.6% at three years, and decreased to 74.4% after the 6th year from treatment (Fig. 4b). Three patients died for disease progression: two had an ACTH-secreting pituitary carcinoma and one a giant aggressive GH-secreting PA, all having re-ceived adjuvant 5-sessions CK-SRT.
4. Discussion
The frame-based Gamma-knife has historically been thefirst used SRS system for treatment of PAs, and its efficacy using a single-session scheme has been demonstrated by several studies with long-term follow-up [7,11,18,33].
Conversely, the frameless CK system easily allows for hypo-fractionated schemes, thus reducing the administered dose during each session, and the risk of damage to the surrounding critical structures (i.e. the optic nerves/chiasm, pituitary stalk). CK actually represents the most commonly reported system for the hypofractionated SRT of dif-ferent intracranial perioptic lesions, but in the literature only few pa-pers have analyzed its safety and efficacy for PAs treatment [7–10,12,17–22,26]. Several studies (9 of 12) are focused on small series, ranging from 7 to 26 patients, and are heterogeneous and not easily comparable: some included only NFPAs, others only specific hormone-secreting PAs, some others mixed series of NFPAs and func-tioning PAs (Table 4).
4.1. Efficacy of CK treatment
In our series of mixed PAs, we achieved a tumor growth control rate of 91.6%. This result is concordant with the current literature that re-ports an excellent and quite homogenous tumor growth control, ran-ging from 92.3% to 100% for CK treatment [7–10,12,17–22,26], and from 83.3% to 100% for GK-SRS [6]. It is note of worthy that the two patients showing tumor progression harbored aggressive PAs, bigger than the other ones and extensively invasive. On the contrary, the two pituitary carcinomas were controlled by CK irradiation.
The overall biochemical disease control rate was 60% that is com-prised in the ranges of 17–80% reported by the CK literature (Table 4). Nevertheless, the efficacy of the stereotactic irradiation can be different according to the biology of the PAs. Kobayashi et al. reported that NFPAs showed a better response than functioning PAs to GK-SRS. In-deed, higher doses were necessary for tumor growth and biochemical control especially in GH- and PRL-secreting tumors [11]. This is what exactly happened in our series with one GH- and one PRL-secreting aggressive PAs that progressed over time. Therefore, a different re-sponse to CK irradiation between functioning PAs and NFPA could be explained on the basis of a different biological behavior.
4.2. Safety
An overall worsening of the pituitary function was observed in 41.66% of cases. Thesefindings seem worse compared to CK literature Table 3
Pre- and post-treatment results.
Characteristics Values Differences (p value)
Age Median 56.5 years old (range 15–79) / Sex 13 m, 11 f / Number of patients 24 NFPA 14 GH 5 (1 GH/TSH) / ACTH 4 (2 carcinomas) PRL 1 CK irradiation modality First line 4 / Adjuvant 20 CK irradiation scheme Single Session (CK-SRS) 10 / Hypofractionated (CK-SRT) 14 (3–5 sessions) Follow-up 43.96 ± 32.66 months (Median 32.5, range 6–122) /
Pre-treatment tumor size Mean 8.33 ± 10.03 cm3 p = 0.03
Post-treatment tumor size Mean 7.13 ± 9.88 cm3
Tumor Growth Control Rate (overall) 91.6% (22 of 24) (volumetric reduction in 13, stability in 9, progression in 2) / NFPAs 100% (14 of 14) ns Functioning 80% (8 of 10) Single Session (CK-SRS) 100% (10 of 10) ns Hypofractionated (CK-SRT) 85.71% (12 of 14) First-line 100% (4 of 4) ns Adjuvant 90% (18 of 20) Biochemical Disease Control Rate (overall)
60% (6 of 10) / Single Session (CK-SRS) 100% (3 of 3) ns Hypofractionated (CK-SRT) 42.85% (3 of 7) First-line 100% (2 of 2) ns Adjuvant 50% (4 of 8) GH 80% (4 of 5) ns ACTH 50% (2 of 4) PRL 0% (1 case)
Time to biochemical control Median 12 months (range 6–30) / Post-treatment hypopituitarism (overall) 41.66% (10 of 24) / Single Session (CK-SRS) 50% (5 of 10) ns Hypofractionated (CK-SRT) 35.71% (5 of 14) First-line 50% (2 of 4) ns Adjuvant 40% (8 of 20)
Correlation with BED OR 0.87 p = 0.03 Post-treatment visual defect 0 / Overall survival rate 84.6%(at 3 years) / Progression-free survival 83.6% (at 3 years) /
Abbreviations: ACTH = adrenocorticotropic hormone; BED = biological e ffec-tive dose; CK-SRS = Cyberknife Stereotactic Radiosurgery; CK-SRT = Cyberknife Stereotactic Radiotherapy; GH = growth hormone; NFPAs = non-functioning pituitary adenomas, PRL = Prolactin; TSH = thyroid-stimulating hormone.
reporting rates ranging from 0 to 33% [7–9,12,17–21,26], with only one series having demonstrated a cumulative incidence of 50% [22] (Table 4). Nevertheless, our data are in line with GK results, which range between 0 and 47% [33]. This could suggest that the single-session SRS treatment (frequently used by GK, and also used in 10 patients of our series) could have a heavier impact on the pituitary function. However, the real occurrence of hypopituitarism after SRS is difficult to establish because the reported data is seldom heterogeneous with different patients, doses and follow-up periods being considered. Moreover, the likelihood to develop a pituitary deficiency after
stereotactic irradiation increases in a time- and dose-dependent manner [34,35]. The rate of hypopituitarism occurrence in our series could therefore be explained by a longer follow-up period (mean 43.96 ± 32.66 months) than many previous CK studies, and high percentage of a single-session scheme treatment (Table 4).
Interestingly, we recorded no visual deterioration cases, which we consider an excellent result as it has been reported ranging from 0 to 7.69% after CK treatment (Table 4). Probably, this was the result of different scheme choices after careful patients' stratification according to the distance from the optic nerves/chiasm [10,15].
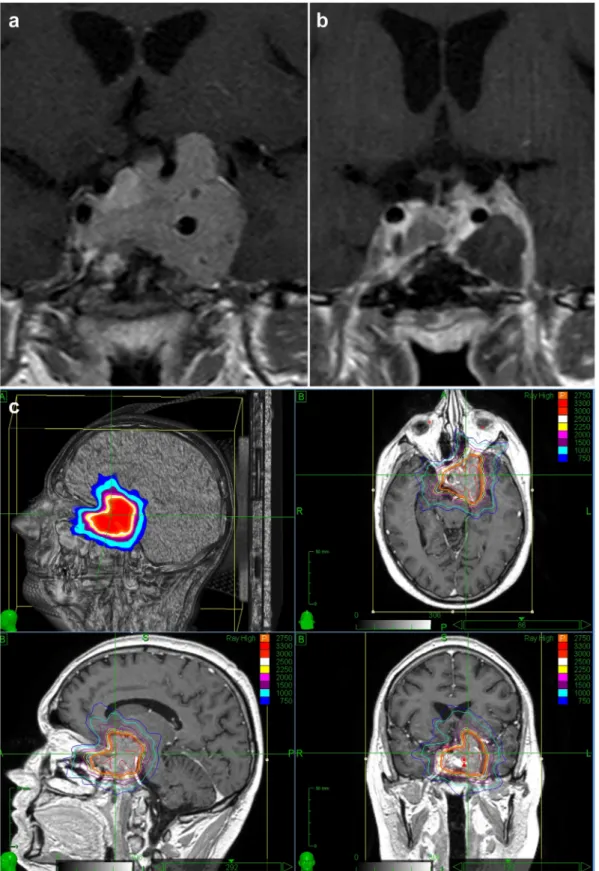
Fig. 2. Case-example of a PA case that experienced shrinkage afterfirst line CK irradiation. The PA before irradiation (a) underwent complete shrinkage after treatment (b). Isodose lines are indicated by colors and percentages (c).
4.3. NFPAs
The tumor growth control was higher for NFPAs (100%) vs. func-tioning PAs (80%), supporting the hypothesized better response of these lesions to stereotactic irradiation [11]. Unfortunately, 64.28% of cases developed hypopituitarism. This could be explained considering
that 50% received a single-session scheme that could have an increased treatment efficacy, but also a higher dose delivered to the pituitary stalk. Thesefindings suggest that the single-session CK-SRS could be more effective for tumor growth control despite it could increase the risk of hypopituitarism.
Fig. 3. Case-example of a PA case that responded to the adjuvant hypofractionated CK-SRT. The PA size before treatment (a) significantly reduced after irradiation (b). Isodose lines are indicated by colors and percentages (c).
4.4. ACTH-secreting PAs
A 5-sessions CK-SRT was effective in our series: tumor growth and biochemical control rates were 100% and 50% respectively, with no post-treatment complications. Interestingly, this is actually the second largest study on ACTH-secreting PAs. Thefirst one included 7 patients reporting a tumor growth control rate of 100%, a biochemical remission rate of 57.1%, the absence of visual deterioration, and the occurrence of hypopituitarism in 14.28% of cases [20] using both single-session and hypofractionated schemes. Conversely, GK-SRS studies reported a bio-chemical remission rate ranging from 27.9% to 67%, but a higher in-cidence of post-treatment hypopituitarism (0 to 50%), and visual de-terioration (0 to 5.6%) using a single-session scheme [6,20]. Our results are therefore concordant with the literature for tumor growth and biochemical control rates, but they seem better in terms of visual or pituitary complications. It could be argued that a hypofractionated CK-SRT scheme could reduce complications, ensuring a good local tumor growth and/or biochemical control, even in carcinoma cases. However, thesefindings are based on few cases and larger studies are therefore strongly advocated.
4.5. GH-secreting PAs
We observed a tumor growth/biochemical control in 80% of pa-tients, without any pituitary function worsening. The biochemical control seems higher as compared to previous CK studies reporting a
range between 17% and 59% (Table 4), but more similar to many GK studies reporting a range of 17–82% [6,36]. This could be explained by the use of the single-session scheme (typical of GK) in 60% of patients that probably is more effective in controlling the endocrine disease than the hypofractionated scheme (usually used by CK).
4.6. Irradiation modality:first line vs. adjuvant CK treatment
CK treatment is usually considered a second-line therapy in cases of PAs showing a volumetric progression and/or hormonal hypersecretion resistant to other therapies, or in patients not eligible to other therapies including surgery (i.e. high anaesthesiological risk, refusing surgical treatment) [6–14]. In our series, the CK treatment was reserved asfirst line therapy in four patients: three refused surgery and one was affected by a giant aggressive adenoma and serious comorbidities that contra-indicated surgical resection. The tumor/biochemical control rates and the occurrence of hypopituitarism were higher using CK irradiation as first line treatment (Table 3), even if the difference was not significant. This suggests a higher efficacy of this modality, at the expenses of a slightly increased risk of hypopituitarism. On the other hand, a lower BED could have a significant protective effect on pituitary function regardless of the treatment modality/scheme. Nevertheless, as reported in two series of acromegalic patients, the BED was higher in cured patients than in those with a persistent disease [17,22]. Therefore, the choice of the BED must be carefully balanced with the need to achieve biochemical control.
Fig. 4. Survival Kaplan-Meier curves describing the PFS and OS in our series. The PFS rate was 100% at two years, but decreased to 83.6% at three years. Only two PA cases were not controlled by CK irradiation. The progression was noted after 30 (1 GH-secreting) and 36 (1 PRL-secreting) months from treatment (a). The OS rate was 100% at two years, 84.6% at three years, but decreased to 74.4% after the 6th year from treatment (b).
Table 4 Detailed comparison between data from current series and previous CK studies. Studies Pham et al. 2004 [12 ] Kajiwara et al. 2005 [ 26 ] Roberts et al. 2007 [ 17 ] Adler et al. 2008 [ 10 ] Cho et al. 2009 [ 19 ] Killory et al. 2009 [ 18 ] Iwata et al. 2011 [ 8 ] Chen et al. 2013 [ 21 ] Puataweepong et al. 2015 [ 7 ] Iwata et al. 2016 [ 9 ] Sala et al. 2018 [ 22 ] Moore et al. 2018 [ 20 ] Current series Patients number 14 21 9 19 26 20 100 22 40 52 22 7 24 Adenoma type 7 NFPAs; 3 GH; 3 PRL; 1 ACTH 14 NFPAs; 3 ACTH; 3 PRL; 1G H GH NA 17 NFPAs; 6 GH; 3 PRL 14 NFPAs; 4 GH; 1 PRL; 1 TSH NFPAs 17 NFPAs; 4 PRL; 1 GH 27 NFPAs; 7 GH; 5 PRL; 1 ACTH GH GH ACTH 14 NFPAs; 5 GH; 4 ACTH; 1 PRL Treatment modality First line 0 0 0 NA 4 0 6 1 2 1 1 0 4 Adjuvant 14 21 9 NA 22 20 94 21 38 51 21 7 20 Treatment scheme Single session (CK-SRS) 0 1 pts 5 0 5 0 0 0 0 0 14 4 10 Hypofractionated (CK-SRT) 14 (2 –5 sessions) 20 pts. (2 –5 sessions) 4( 2– 3 sessions) 19 (2 –5 sessions) 21 (3 sessions) 20 (5 sessions) 100 (3/5 sessions) 22 (5 sessions) 40 (3 –5 sessions) 52(3/5 sessions) 8( 2– 5 sessions) 3 (3/5 sessions) 14 (3 –5 sessions) Treatment parameters (Gy) Mean BED NA NA 137 ± 47 (72 –216) NA NA NA NA NA NA NA 132.6 ± 51 (median 131.2) 131 (median 143) 115.5 ± 30.7 (median 107.5; 62.33 –199.3) Mean marginal dose 20.0 (15 –30) a 14.3 ± 4.5 21 (18 –24) 20.3 (15 –30) a 19.23 (NF PAs); 19.11 (functioning PAs) NA 21.0 (17.0 –25.0) NA 25 (20 –28) 21.0 (17.4 –26.8) for 3 sessions/ 25.0 (20.0 –32.0) for 5 sessions 24 (18 –30) 25 (21 –35.5) 21 ± 4.52 (median 21; 15 –30) Mean Dmax to the optic nerves/ chiasm ≤ 5 a NA NA NA NA 4.5 ± 3.5 (3.6 –5) per fraction 19.9 (1.4 –25.0) ONs; 20.3 (2.6 –25.0) OC 2.4 ± 0.31 (1.5 –3.0) per fraction 20 (7.2 –31.95) OC; 17.9 (2.4 –30.8) right ONs; 14.8 (5.2 –23.32) left ON 15.6 (1.2 –21.0)/ 23.3 (16.1 –25.0) for OC; 16.8 (1.0 –21.0)/ 22.2 (8.6 –25.0) for ONs 7.88 Gy (1.5 –22.7 Gy) for OC; 7.35 (0 –24.6) for left ON; 7.65 (0 –18.7) for right ON 6.1 (0–28.5) for OC 13.45 ± 6.41 (6.2 –30.3); 8.71 ± 2.17 (6.2 –13.5) for single session; 16.82 ± 6.32 (8.94 –30.31) for hypofractionated Follow-up in months (mean/median; range) Mean 29 (15 –62) a Mean 35.3 ± 10.7 (18 –59) Mean 25.4 ± 14.0 (6 –53) Mean 46 (13 − 100) a Mean 30 ± 12.7 (7 –47) Mean 25.2 ± 7.3 (16.2 –37.7) Median 33 (12 –118.5) Mean 33 (NA) Median 38.5 m (12 –71) Median 60 (27 –137) Median 43.2 (6 –153) Median 55.4 (9–159) 43.96 ± 32.66 months (6 –122) Tumor growth control rate 94.1% a 95.2% 100% 94.7% 92.3% 100% 95% 95.6% 97.5% 100% at 5 years; then 98.1% 100% 100% 91.6% (100% NFPAs; 80% functioning PAs) Biochemical disease control rate NA 28.57% 55% NA 44% 40% NA 80% 54% (57% for GH-secreting; 33% for PRL-secreting) 17% 59% 57.1% 60% Post-treatment hypopituitarism NA 9.5% 33 NA 0 12.5% 3% 0 0 1.9% 50% 14.2% 41.66% Post-treatment visual deterioration 7.14% 4.76% 0 5.26% 7.69% 0 1% 0 0 0 0 0 0 Survival rate NA NA NA 90% a NA NA 98% (at 3 years) NA 100% 100% (at 5 years) NA NA 84.6 (at 3 years) (continued on next page )
4.7. Irradiation scheme: single session SRS and hypofractionated CK-SRT
To date, this study is the second largest PAs series treated with single-session CK-SRS, after that published by Sala et al. [22] (Table 4). We observed a high tumor growth/biochemical control rates but also a higher occurrence of hypopituitarism in the single-session cases (Table 3). This suggest that a single-session scheme is therefore possible even using CK if higher tumor growth/biochemical control is desired, considering the increased risk of hypopituitarism. Nevertheless, the selection of the CK irradiation scheme should be based on the distance from the optic apparatus to avoid visual deterioration [10,15].
4.8. Survival
To date only two papers reported the analysis of the OS and PFS of PAs patients after CK treatment. The OS and the PFS were respectively 98% and 96% after three years in a series of NFPAs [8], and 100% and 96% after 5 years in a series of GH-secreting PAs [9]. We report a lower PFS (83.6%) and OS (84.6%) after three years. Nevertheless, we in-cluded two carcinomas and two aggressive PAs, and this probably makes our results worse than previous studies. Interestingly, all the three recorded deaths consisted of functioning PAs that received ad-juvant 5-sessions CK-SRT. This could suggest a different response of functioning PAs to the hypofractionated scheme as compared to NFPAs. Therefore, further studies are strongly needed to investigate if specific CK treatment modalities and schemes or the PAs biology could also influence these important variables.
4.9. Limitations
The retrospective nature of the present study represents its main limitation, since this type of studies is associated to the well-known possibility of selection and expertise biases. Moreover, the small number of the included patients limited the statistical significance of our comparisons and results, as well as their generalizability. Nevertheless, among the currently available literature on the (CK) treatment of PAs that collectively accounts to 12 studies, only three studies reported significantly larger series [7–9] than ours, one reported 26 cases [19], and the remaining 8 studies reported a smaller number of patients than our series (Table 4). Therefore, this study must be in-tended simply as a further contribution to current limited literature on CK treatment for PAs. Finally, it must be considered that CK effects and complications are time-dependent: nonetheless, among the currently available literature, the follow-up in our study is longer than at least 6 studies [12,17–19,21,26], and similar to other two [8,10] (Table 4).
5. Conclusions
Our experience is in accordance with current literature, suggesting Cyberknife stereotactic irradiation represents a valid treatment option of PAs. It provides a tumor growth control in almost all cases, and a biochemical remission in more than half of patients. Nevertheless, the safety profile of CK still needs to be better defined, since the incidence of post-treatment visual and/or pituitary deficits critically depends on the dose delivered and the distance from the vulnerable optic appa-ratus. In our experience the hypofractionated CK-SRT scheme actually seems to be safer but less effective on tumor growth and biochemical control, especially in cases of aggressive PAs, than the single session CK-SRS. In addition, our study suggests that CK irradiation could be con-sidered an effective first line treatment in selected cases, not eligible for other treatments. Larger prospective studies to define the optimal treatment protocol in order to increase CK efficacy and safety are warranted. Table 4 (continued ) Studies Pham et al. 2004 [12 ] Kajiwara et al. 2005 [ 26 ] Roberts et al. 2007 [ 17 ] Adler et al. 2008 [ 10 ] Cho et al. 2009 [ 19 ] Killory et al. 2009 [ 18 ] Iwata et al. 2011 [ 8 ] Chen et al. 2013 [ 21 ] Puataweepong et al. 2015 [ 7 ] Iwata et al. 2016 [ 9 ] Sala et al. 2018 [ 22 ] Moore et al. 2018 [ 20 ] Current series Progression-free survival rate 91% (at 2 years) a NA NA NA NA NA 96% (at 3 years) NA NA 96% (at 5 years) NA NA 83.6 (at 3 years) Abbreviations: ACTH = adrenocorticotropic hormone; BED = biological eff ective dose; CK-SRS = Cyberknife Stereotactic Radiosurgery; CK-SRT = Cyberknife Stereotactic Radiotherapy; Dmax = maximal dose; m = months; NA = not available; NFPAs = non-functioning pituitary adenomas; OC = optic chiasm; ON = optic nerve, F = female, FSH = Follicle-stimulating h ormone, GH = growth hormone, LH = Luteinizing hormone, M = male, NFPA = non-functioning pituitary adenomas, PRL = prolactin, TSH = thyroid-stimulating hormone. a Reported data refers to a larger series of perioptic lesions, including meningiomas. Data from PAs only are not available.
Compliance with ethical standards
Funding: This study has been funded by the Department of University and Scientific Research of the Italian Government, Rome, Italy (PRIN 2015 2015ZHKFTA. Recipient: Prof. Salvatore Cannavò), and by the Italian Society of Neurosurgery - SINch, Naples, Italy (Premio Bartolazzi 2017. Recipient: Dott. Giovanni Raffa).
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: Prot. 69/18 Comitato Etico Messina.
Informed consent: Informed consent was obtained from all in-dividual participants included in the study.
References
[1] S. Ezzat, S.L. Asa, W.T. Couldwell, et al., The prevalence of pituitary adenomas: a systematic review, Cancer 101 (3) (2004) 613–619,https://doi.org/10.1002/cncr.
20412.
[2] J.W. Lucas, M.E. Bodach, L.M. Tumialan, et al., Congress of neurological surgeons systematic review and evidence-based guideline on primary management of pa-tients with nonfunctioning pituitary adenomas, Neurosurgery 79 (4) (2016) E533–E535,https://doi.org/10.1227/NEU.0000000000001389.
[3] L. Katznelson, E.R. Laws Jr., S. Melmed, et al., Acromegaly: an endocrine society clinical practice guideline, J. Clin. Endocrinol. Metab. 99 (11) (2014) 3933–3951,
https://doi.org/10.1210/jc.2014-2700.
[4] L.K. Nieman, B.M. Biller, J.W. Findling, et al., Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab. 100 (8) (2015) 2807–2831,https://doi.org/10.1210/jc.2015-1818.
[5] F.F. Casanueva, M.E. Molitch, J.A. Schlechte, et al., Guidelines of the pituitary society for the diagnosis and management of prolactinomas, Clin. Endocrinol. 65 (2) (2006) 265–273,https://doi.org/10.1111/j.1365-2265.2006.02562.x. [6] K. Kajiwara, K. Saito, K. Yoshikawa, et al., Stereotactic radiosurgery/radiotherapy
for pituitary adenomas: a review of recent literature, Neurol. Med. Chir. 50 (9) (2010) 749–755https://www.ncbi.nlm.nih.gov/pubmed/20885109.
[7] P. Puataweepong, M. Dhanachai, A. Hansasuta, et al., The clinical outcome of hy-pofractionated stereotactic radiotherapy with CyberKnife robotic radiosurgery for perioptic pituitary adenoma, Technol. Cancer Res. Treat. 15 (6) (2016) NP10–NP15,https://doi.org/10.1177/1533034615607113.
[8] H. Iwata, K. Sato, K. Tatewaki, et al., Hypofractionated stereotactic radiotherapy
with CyberKnife for nonfunctioning pituitary adenoma: high local control with low
toxicity, Neuro-Oncology 13 (8) (2011) 916–922.
[9] H. Iwata, K. Sato, R. Nomura, et al., Long-term results of hypofractionated
stereo-tactic radiotherapy with CyberKnife for growth hormone-secreting pituitary ade-noma: evaluation by the Cortina consensus, J. Neuro-Oncol. 128 (2) (2016)
267–275.
[10] J.R. Adler Jr., I.C. Gibbs, P. Puataweepong, S.D. Chang, Visualfield preservation
after multisession cyberknife radiosurgery for perioptic lesions, Neurosurgery 62
(Suppl. 2) (2008) 733–743.
[11] T. Kobayashi, Long-term results of stereotactic gamma knife radiosurgery for
pi-tuitary adenomas. Specific strategies for different types of adenoma, Prog. Neurol.
Surg. 22 (2009) 77–95.
[12] C.J. Pham, S.D. Chang, I.C. Gibbs, P. Jones, M.P. Heilbrun, J.R. Adler Jr., Preliminary visualfield preservation after staged CyberKnife radiosurgery for perioptic lesions, Neurosurgery 54 (4) (2004) 792–799https://www.ncbi.nlm.nih.
gov/pubmed/15046645.
[13] S.M. Priola, F. Esposito, S. Cannavo, et al., Aggressive pituitary adenomas: the dark side of the moon, World Neurosurg. 97 (2017) 140–155,https://doi.org/10.1016/j.
wneu.2016.09.092.
[14] M. Losa, F. Bogazzi, S. Cannavo, et al., Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas, J. Neuro-Oncol. 126 (3) (2016) 519–525,https://doi.org/10.1007/s11060-015-1991-y.
[15] A. Conti, A. Pontoriero, F. Midili, et al., CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic me-ningiomas: intermediate-term results and radiobiological considerations, Springerplus 4 (2015) 37,https://doi.org/10.1186/s40064-015-0804-2.
[16] C. Mayo, M.K. Martel, L.B. Marks, J. Flickinger, J. Nam, J. Kirkpatrick, Radiation
dose-volume effects of optic nerves and chiasm, Int. J. Radiat. Oncol. Biol. Phys. 76
(3 Suppl) (2010) S28–S35.
[17] B.K. Roberts, D.L. Ouyang, S.P. Lad, et al., Efficacy and safety of CyberKnife
radiosurgery for acromegaly, Pituitary 10 (1) (2007) 17.
[18] B.D. Killory, J.J. Kresl, S.D. Wait, F.A. Ponce, R. Porter, W.L. White,
Hypofractionated CyberKnife radiosurgery for perichiasmatic pituitary adenomas:
early results, Neurosurgery 64 (2 Suppl) (2009) A19–A25.
[19] C.B. Cho, H.K. Park, Joo W. Il, C.K. Chough, K.J. Lee, H.K. Rha, Stereotactic
radiosurgery with the CyberKnife for pituitary adenomas, J. Korean Neurosurg. Soc.
45 (3) (2009) 157–163.
[20] J.M. Moore, E. Sala, A. Amorin, et al., CyberKnife radiosurgery in the multimodal
management of patients with Cushing disease, World Neurosurg. 112 (2018)
e425–e430.
[21] Y.-H. Chen, S.D. Chang, H.-I. Ma, et al., Multisession CyberKnife radiosurgery for
post-surgical residual and recurrent pituitary adenoma: preliminary result from one
center, J. Radiosurg. SBRT 2 (2) (2013) 105–117.
[22] E. Sala, J.M. Moore, A. Amorin, et al., CyberKnife robotic radiosurgery in the
multimodal management of acromegaly patients with invasive macroadenoma: a
single center's experience, J. Neuro-Oncol. 138 (2) (2018) 291–298.
[23] G. Raffa, A. Conti, A. Scibilia, et al., The impact of diffusion tensor imaging fiber tracking of the corticospinal tract based on navigated transcranial magnetic sti-mulation on surgery of motor-eloquent brain lesions, Neurosurgery 83 (4) (2018) 768–782,https://doi.org/10.1093/neuros/nyx554.
[24] G. Raffa, I. Bährend, H. Schneider, et al., A novel technique for region and linguistic specific nTMS-based DTI fiber tracking of language pathways in brain tumor pa-tients, Front. Neurosci. 10 (552) (2016),https://doi.org/10.3389/fnins.2016.
00552.
[25] G. Raffa, A. Conti, A. Scibilia, et al., Functional reconstruction of motor and lan-guage pathways based on navigated transcranial magnetic stimulation and DTIfiber tracking for the preoperative planning of low grade glioma surgery: a new tool for preservation and restoration of eloquent network, Trends Reconstr. Neurosurg. Neurorehabil. Restor. Reconstr. 124 (2017) 251–261,https://doi.org/10.1007/
978-3-319-39546-3_37.
[26] K. Kajiwara, K. Saito, K. Yoshikawa, et al., Image-guided stereotactic radiosurgery
with the CyberKnife for pituitary adenomas, Minim. Invasive Neurosurg. 48 (2)
(2005) 91–96.
[27] K. Takakura, General rules for clinical and pathological studies on brain tumors,
Diagnosis of Brain Tumor— The Committee of Brain Tumor Registry of Japan (Ed),
Kanehara Publishing Co., Tokyo, Japan, 2002, pp. 62–63.
[28] R.B. Tishler, J.S. Loeffler, L.D. Lunsford, et al., Tolerance of cranial nerves of the cavernous sinus to radiosurgery, Int. J. Radiat. Oncol. Biol. Phys. 27 (2) (1993) 215–221,https://doi.org/10.1016/0360-3016(93)90230-S.
[29] M.T. Milano, K.Y. Usuki, K.A. Walter, D. Clark, M.C. Schell, Stereotactic radio-surgery and hypofractionated stereotactic radiotherapy: normal tissue dose con-straints of the central nervous system, Cancer Treat. Rev. 37 (7) (2011) 567–578,
https://doi.org/10.1016/j.ctrv.2011.04.004.
[30] M.T. Milano, J. Grimm, S.G. Soltys, et al., Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways, Int. J. Radiat. Oncol. Biol. Phys. (2018),https://doi.org/10.1016/j.ijrobp.2018.01.053(pii: S0360-3016(18) 30125-1).
[31] B.E. Pollock, M.J. Link, J.A. Leavitt, S.L. Stafford, Dose-volume analysis of radia-tion-induced optic neuropathy after single-fraction stereotactic radiosurgery, Neurosurgery 75 (4) (2014) 456–460,https://doi.org/10.1227/NEU.
0000000000000457.
[32] J.A. Leavitt, S.L. Stafford, M.J. Link, B.E. Pollock, Long-term evaluation of radia-tion-induced optic neuropathy after single-fraction stereotactic radiosurgery, Int. J. Radiat. Oncol. Biol. Phys. 87 (3) (2013) 524–527,https://doi.org/10.1016/j.ijrobp.
2013.06.2047.
[33] C.J. Stapleton, C.Y. Liu, M.H. Weiss, The role of stereotactic radiosurgery in the multimodal management of growth hormone-secreting pituitary adenomas, Neurosurg. Focus. 29 (4) (2010) E11,https://doi.org/10.3171/2010.7.
FOCUS10159.
[34] C.S. Graffeo, M.J. Link, P.D. Brown, W.F. Young Jr., B.E. Pollock, Hypopituitarism
after single-fraction pituitary adenoma radiosurgery: dosimetric analysis based on patients treated using contemporary techniques, Int. J. Radiat. Oncol. Biol. Phys.
101 (3) (2018) 618–623.
[35] G. Minniti, M.F. Osti, M. Niyazi, Target delineation and optimal radiosurgical dose
for pituitary tumors, Radiat. Oncol. 11 (1) (2016) 135.
[36] N.C. Rowland, M.K. Aghi, Radiation treatment strategies for acromegaly,