UNIVERSITA’ DEGLI STUDI DI SIENA
Dipartimento Biotecnologie, Chimica e Farmacia
DOTTORATO DI RICERCA IN
Biochemistry and Molecular Biology - Bibim 2.0
CICLO XXXIII
COORDINATORE: Prof.ssa Lorenza Trabalzini
The nickel hyperaccumulating plants of genus Odontarrhena (Brassicaceae):
novel insights from molecular, physiological and biochemical analyses
SETTORE SCIENTIFICO-DISCIPLINARE: BIO/10
.
Dottoranda:
Isabella Bettarini
1
stTutor: Prof.ssa Luigia Pazzagli - 2
ndTutor: Prof.ssa Cristina Gonnelli
ANNO ACCADEMICO: 2019-2020
Firmato digitalmen te da BETTARINI ISABELLA C=IT1
INDEX
Summary 2
Abbreviations 4
Chapter 1. General introduction 6
1.1 Metallophytes and serpentine soils 7
1.2 Nickel and Ni-hyperaccumulating plants 9
1.2.1 Nickel: sources, characteristics, functions and toxicity
1.2.2 Nickel hyperaccumulators
1.2.3 Nickel hyperaccumulation mechanisms
1.2.4 Nickel hyperaccumulator plants: biotechnological applications
1.3 The target plant genus 15
1.4 General methodological approach 18
1.4.1 Field work
1.4.2 Experiments in controlled conditions
1.5 Outline of the thesis 20
Chapter 2. Population genetics of Odontarrhena (Brassicaceae) from Albania: the effects of
anthropic habitat disturbance, soil and altitude on a Ni-hyperaccumulator plant group from a major serpentine hotspot.29
Chapter 3. Unravelling soil and plant metal relationships in Albanian Ni-hyperaccumulators of genus
Odontarrhena (syn. Alyssum sect. Odontarrhena, Brassicaceae) 52
Chapter 4. Contrasting patterns of Ni-accumulation ability in Odontarrhena (Brassicaceae):
new evidence from CE Mediterranean taxa and populations of serpentine and non-serpentine soils 70
Chapter 5. Inability to accumulate Ni in a genus of hyperaccumulators: the paradox of Odontarrhena
sibirica (Brassicaceae) 86
Chapter 6. Photosynthesizing while hyperaccumulating nickel: insights into the genus Odontarrhena 103
Chapter 7. The activity of urease in Ni-hyperaccumulating plants: new insights from genus
Odontarrhena (Brassicaceae) 124
Chapter 8. Final remarks 133
Literature cited 139
Appendix. Supplementary tables and figures to chapters 2, 3, 5, 6 160
2
SUMMARY
Due to the high nickel concentrations, serpentine soils provide a very restrictive and selective environment for plant life. Some plants, termed “Ni-hyperaccumulators”, are adapted to live on these heavy-metal-enriched soils without toxicity symptoms. Ni-hyperaccumulators are increasingly important for research on metal tolerance, homeostasis and biotechnological applications.
This project aims to investigate nickel accumulation in taxa and populations of Odontarrhena, a genus of tribe Alysseae (Brassicaceae) that includes over 85 species many of which are Ni-hyperaccumulators.
Based on a previous systematic study conducted on poorly-known populations of Odontarrhena native to Albania we performed a molecular study to characterize taxa and populations of this genus. To this purpose we used DNA sequencing and the AFLP-fingerprint technique to reconstruct the species phylogenetic relationships and the population differentiation patterns in relation to their distribution, ploidy level, intensity of anthropic site disturbance, altitude, soil type and metal concentration population (Ni, Cr, Co, Ca, Mg). We found significant population differentiation, dominance of within-population variation, no isolation by geographic distance and existence of six genetic groups variously represented across the six taxa possibly due to hybridization especially in disturbed sites.
Next, we compared metal concentrations in native Odontarrhena populations from Albania in relation to their soil of origin. We determined the concentration of the most important trace metals (Ni, Co, Cr, Mg, Ca, K, Fe and Mn) in soil, plant roots and shoots of five taxa from 20 different outcrops. We found large differences in mineral element concentrations in soils and also between the plants; shoot Ni concentrations in Albanian Odontarrhena taxa depend on soil Ni concentrations but not on species identity. For O. chalcidica, the most widely distributed species, this “environmental fingerprint” was found not only for Ni, but also for Ca and Mg. After these investigations on native populations from the natural environment, we designed an experimental study in controlled conditions. Plant seedlings of seven taxa and 11 populations of Odontarrhena from serpentine and non-serpentine sites of the Balkan peninsula and Italy were cultivated in hydroponics with increasing NiSO4 concentrations to determine plant growth and Ni accumulation. These plantlets were
analyzed to test inter- and intra-specific differences in nickel tolerance and accumulation, in relation to Ni levels in the soils and in wild plants. We found a metal stimulatory effect on growth that was present in the low-dose zone and significantly fitted the Brain-Cousens hormetic model. Taxa showed broad variation in tolerance, with the most tolerant plants requiring the highest Ni concentration for optimal growth. Our data suggested that tolerance is associated with hyperaccumulation ability.
Among the obligate and facultative serpentinophytic species of Odontarrhena that have been investigated we found a notable exception, O. sibirica, a facultative serpentinophyte in which accumulation ability was enigmatic from previous studies. We addressed this issue using observational and experimental methods as in our previous researches. We found that Ni-concentrations in the native populations sampled on serpentine soils in Greece were always much lower than the hyperaccumulation threshold. When cultivated together with other Ni-accumulating Odontarrhena species on the same natural ultramafic soil, O. sibirica was the only one unable to accumulate the metal. When grown in hydroponics at different NiSO4 levels Ni-accumulation occurred only
3 at higher concentrations which, however, had a toxic effect. This peculiar combination of Ni-response traits could be the result of a partial evolutionary loss of ability with respect to all other Ni-accumulating congeneric species. For its unique characteristics, O. sibirica could therefore represent a unique model system for further studies on the evolutionary dynamics, physiological mechanisms and genetic control of metal accumulation and homeostasis.
In a parallel study, we investigated photosynthesis responses of the same plants using an experimental approach. In non-hyperaccumulator plants, toxicity symptoms to above 10 µg g-1 DW nickel concentrations in
soils can include inhibition of photosynthesis, impaired nitrogen assimilation and disturbed enzyme activity. However, there is a complete lack of information about how Ni-hyperaccumulators reconcile that extraordinary amount of metal in their shoots with an efficient photosynthetic activity, or at least on which photosynthetic parameters the excess of Ni impacts less in these plants. We measured Ni effects on growth, root and shoot metal accumulation and several photosynthetic parameters, such as gas exchange, chlorophyll fluorescence analyses and pigments content in three Odontarrhena taxa (two hyperaccumulators, one not) grown in hydroponics and exposed to three NiSO4 treatments. We found that Ni-hyperaccumulators species are
photosynthetically more efficient under Ni excess in respect to the non-accumulating species. In fact, Ni treatment in O. chalcidica increased not only the photochemical efficiency of PSII and the CO2 assimilation
rate, but also the stomatal conductance.
Finally, this project focused on the determination of the activity of the enzyme urease, the only Ni-metalloenzyme known so far in plants, in selected Odontarrhena taxa. The hypothesis to test was whether the high basal requirement for this micronutrient in these plants could be linked to a depletion of the Ni cytosolic pool at low external metal concentration, due to hyperaccumulation mechanism and impairing urease activity. To this purpose, enzyme activity and Ni shoot concentration were determined in plants of accumulating and non-accumulating taxa of Odontarrhena cultivated on Ni-rich serpentine soil and on garden soil, as well as in samples of O. bertolonii cultivated in hydroponics at increasing Ni concentrations. Odontarrhena hyperaccumulators showed similar urease activity when grown on both kinds of soils, with no relation between the enzyme activity and the leaf Ni accumulation. Contrarily, clear indications came from the experiment in controlled conditions, where the presence of Ni determined a progressive stimulation, in respect to control samples, of the activity of the enzyme, associated with an increase in shoot metal concentration. A significant relationship was found between the levels of urease activity and the amount of Ni accumulated in the leaves. Therefore, the already known Ni-stimulated growth of O. bertolonii at increasing metal concentrations in the low-dose zone could be explained by a Ni-induced activity of urease, associable to an enhanced nitrogen metabolism, unless other still unknown physiological functions of Ni in hyperaccumulating plants.
4
ABBREVIATIONS
A: steady-state photosynthetic CO2 assimilation rate
Amax: light saturated rate of photosynthesis at growth CO2 concentration
AFLPs: Amplified Fragment Length Polymorphisms AMOVA: analysis of molecular variance
ANOVA: analysis of variance
AQY: maximum apparent quantum efficiency (moles of CO2 fixed per incident photons)
Car: carotenoids
Cc: CO2 concentration in chloroplasts
Chl a: chlorophyll a Chl b: chlorophyll b
Ci: intercellular CO2 concentration
Cm: carbon per leaf mass
Cys: cysteine amino acid DAT: days after treatment df: degree of freedom DW or dw: dry weight E: transpiration rate
ETR: electron transport rate
Fm: maximum fluorescence yield with all PSII reaction centres in the reduced state
Fo: minimal fluorescence yield emitted by the leaves in the dark-adapted state.
Fs: fluorescence at the actual state of PSII reaction centres during actinic illumination
Fv/Fm: potential efficiency of PSII photochemistry
FST: mean genetic distance between populations within taxa FW or f.w.: fresh weight
GLY: glyoxalases Gly: glycine amino acid Glu: glutamic amino acid gm: mesophyll conductance
gs: stomatal conductance
GSH: reduced glutathione He: population heterozygosity
Hs: mean species heterozygosity
ITS: internal transcribed spacer IGS: intergenic spacer
Jmax: maximal light-driven electron transport rate
5 Me: metal
MFA: multiple factor analysis MG: methylglyoxal
Na: Nitrogen per leaf area
Nm: nitrogen per leaf mass
NPQ: Stern-Volmer non-photochemical quenching OUTL: mean number of outlier loci per population PB: proportion of leaf N invested in bioenergetic pools
PL: proportion of leaf N invested in light-harvesting components
PNUE: photosynthetic N-use efficiency PPFD: photosynthetic photon flux density PR: proportion of leaf N invested in Rubisco
PS: photosystem PSII: photosystem II Rl: respiration in the light
Rubisco: ribulose-1,5-bisphosphate carboxylase-oxygenase RuBP: ribulose biphosphate
Vcmax: maximal carboxylation rate
Γ*: CO2 compensation point between photosynthesis and photorespiration
PSII: actual photon yield of PSII photochemistry6
CHAPTER 1
GENERAL INTRODUCTION
7
1.1 Metallophytes and serpentine soils
Plants that are adapted to live on heavy-metal-enriched soils and able to survive and reproduce without suffering from toxicity are termed ‘metallophytes’ (Baker et al., 2010; Wójcik et al., 2017). From a chemical point of view, the term heavy metal is used strictly for transition metals with atomic mass over 20 and specific gravity above 5. In biology, “heavy” refers to a series of metals and also metalloids that can be toxic to both plants and animals even at very low concentrations (Rascio and Navarri-Izzo, 2011; Reeves et al., 2017). Some of these heavy metals, such as As, Cd, Hg, Pb or Se, do not play any known physiological function in plants. Others, such as Co, Cu, Fe, Mn, Mo, Ni and Zn, are required for normal growth and metabolism of plants. These latter elements can easily lead to poisoning when their concentration exceeds optimal values (Rascio and Navarri-Izzo, 2011).
High metal concentrations also occur naturally in areas with particular rock types, such the so-called ultramafics. The most typical and common soils derived from these rocks are termed “serpentine soils” and are characterized by high levels of Ni, Co and Cr, low levels of nutrients and a high Mg/Ca ratio (Brooks, 1987; Gonnelli and Renella, 2012). Outcrops of serpentine soils are actually considered ‘ecological islands’ in the “sea of normal soils” (Lefèbvre and Vernet, 1990), uninhabitable by most plant species and favorable for the evolution of endemic taxa (Kruckeberg, 1954; Kruckeberg and Kruckeberg, 1990). With a documentation dating back to the 16th century, serpentine areas have been the first, among the various types of metal-rich environments on Earth, to be reported possessing a characteristic flora (Vergnano-Gambi, 1992).
Several categories can be distinguished based on whether these plants are restricted to metal-rich soils (obligate) or not (facultative), or the type of metal they are able to cope with (Fig.1). Facultative metallophytes are species occurring both on metalliferous and on “normal” (nonmetalliferous) soil. In facultative metallophytes, metal tolerance is expressed only by the populations growing on metalliferous soils (Macnair, 1993) whereas the non metallicolous populations can contain tolerant mutants in low frequencies (Wu et al., 1975).
Due to their adaptive nature, obligate metallophytes require relatively high levels of metals for optimal growth and reproduction because they are poor competitors with other plants on "normal" soils.
Plant growing on metalliferous soils have developed three main strategies to cope with heavy metals (Fig. 1). The first and most common strategy is to exclude metals from the tissues. These plants, called “excluders” are able to restrict either the entry of metals into the roots or their transport to the shoot whose cells remain sensitive to the phytotoxic effects over a wide range of soil metal concentration (Rascio and Navarri-Izzo, 2011). The second strategy is found in the so called “indicator” plants, which allow the metals to enter their tissue (Wójcik et al., 2017). In these plants shoot metal concentration reflect the soil metal concentration (shoot:soil metal concentration ratio close to unity), therefore these have been proposed as biomonitoring organisms for environmental pollution. Finally, the third and most remarkable strategy is that of so called “metal accumulators”, e.g. those plants which are characterized by an efficient metal uptake and translocation to the shoots without showing any toxicity symptom. In metal accumulators the shoot:root and the shoot:soil metal concentration ratio are typically higher than unit (Fig. 1).
9 The hyperaccumulator species are distributed in a wide range of phylogenetically unrelated families, showing that this ability has evolved independently multiple times during plant evolution, under the pressure of selective ecological factors. Hence, hyperaccumulators are exceptional models for fundamental science to understand metal regulation including the physiology of metal uptake, transport and sequestration, as well as evolution and adaptation in extreme environments (Reeves et al., 2017).
As of March 2020, in consideration of recent discoveries and new accumulation thresholds, the Global Hyperaccumulator Database (http://hyperaccumulators.smi.uq.edu.au/collection/, Reeves et al., 2017) includes 759 species, some of which can store more than one element (Manara et al., 2020). A majority of hyperaccumulators are obligate metallophytes, however some of them also occur on normal non metalliferous soils (facultative metallophytes) (Fig.1). The majority of hyperaccumulator species are Ni hyperaccumulators (more than 75%, in 532 different species), which is updated as of March 2020 by browsing the Global Hyperaccumulator Database (http://hyperaccumulators.smi.uq.edu. au/collection/), which are found in a large number of naturally Ni enriched serpentine soils worldwide (Reeves, 2003; Van der Ent et al., 2013, Manara et al., 2020).
The global centers of distribution for Ni-hyperaccumulator plants include the Mediterranean Region, mainly with species of family Brassicaceae. The most diverse genus of Ni-hyperaccumulator plants is Odontarrhena, until recently considered a section of Alyssum (Reeves et al., 2017).
1.2 Nickel and Ni-hyperaccumulating plants
1.2.1 Nickel: sources, characteristics, functions and toxicity
Nickel, the 28th element of the periodic table, is the 22ndmost abundant element in the Earth’s crust where it
occurs in igneous rocks as a free metal or together with Fe (Sunderman and Oskarsson, 1991). It is a silver-white metal found in several oxidation states (from -1 to +4), though the Ni2+ form is stable over a wide range
of pH values and redox conditions prevalent in soils (Yusuf et al., 2011). This oxidation state is the most common one also in biological systems (Kenkhaus and Salnikow, 2002).
Nickel is the element that has been recognized in more recent times to be very important for plant mineral nutrition (Brown et al. 1987). In fact, it was identified as a fundamental component of the enzyme urease only in the middle of 1970s (Dixon et al., 1975; Fishbein et al., 1976). This enzyme (Fig. 2) contains a bi-nickel center (Balasubramanian and Ponnuraj, 2010) and a second nickel-dependent protein, the glyoxalase I, that was only recently discovered (Mustafiz et al., 2014, Fabiano et al., 2015). Glyoxalases I and II participate in the degradation pathway of methylglyoxal (MG), a toxic alpha-ketoaldehyde that may be lethal to cell functions. This degradation is initiated by a spontaneous reaction between MG and reduced glutathione (GSH) that forms hemithioacetal, which is then converted in S-Dlactoylglutathione in a reaction catalized by GLY-I. GSH is an essential tripeptide (γ-Glu–Cys–Gly) that plays a fundamental dual role in maintaining the oxidative status in plants exposed to metal stress: first, as an antioxidant to mitigate the redox imbalance caused by toxic metal accumulation and second, as a precursor of the ligand peptides phytochelatins responsible for free metal
10 ion chelation and compartmentation in the vacuole (Freeman et al., 2004; Wójcik and Tukiendorf, 2011). The fact that Ni is an activator of GLY-I suggests that it may play an important role in antioxidant metabolism (Fabiano et al., 2015).
The major role of urease is to prevent the accumulation of urea which is generated during the various metabolic processes, as this it is toxic to plants when in high concentrations. Role and functioning of urease in plants were extensively investigated in soybean (Fig. 2), which has two enzyme isoformes, a highly expressed embryo-specific urease and an ubiquitous urease found in all tissues (Meyerbothling et al., 1987).
Higher plants typically contain nickel concentrations in the range of 0.5–10 mg kg-1 DW (Chen et al., 2009),
while concentrations over 10–50 mg kg-1 DW (depending on the plant species concerned) are associated with
nickel toxicity effects. Toxicity symptoms can include inhibition of photosynthesis, impaired nitrogen assimilation, disturbed enzyme activity, genotoxic effects and the generation of reactive oxygen species (Yusuf et al., 2011). Nickel affects various physiological processes in plants starting from several enzyme
activities and it is not easy to discern direct and indirect effects of the metal on enzyme activities themselves (Seregin and Kozhevnikova, 2006).
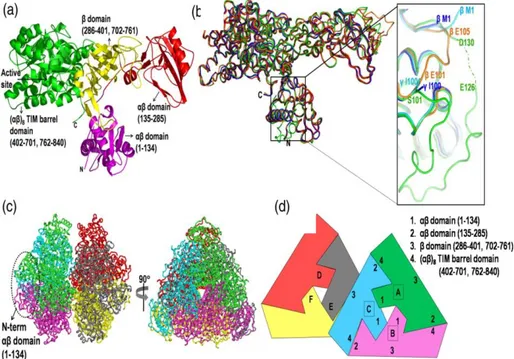
Figure 2. Overall structure of JBU monomer and its oligomeric assembly. (a) A hammer or T-shaped JBU monomer consists of four domains: the N-terminal αβ domain (magenta, handle of the hammer), another αβ domain (red, one end of the hammer head), a β domain (yellow, the middle region of the hammer head), and C-terminal (αβ)8 TIM barrel domain (green, other end of the hammer head). (c) The backbone representation of JBU hexamer. Three monomers are associated in a triangular fashion generating a planer surface on face of the triangle, whereas the other face has a small protrusion formed by the N-terminal αβ domain (1–134). The two trimers pack against one another through their planer surface and form a functional hexamer. View of the hexamer down the crystallographic 3-fold axis is shown in right. (b). (from Balasubramanian and Ponnuraj, 2010)
11 1.2.2 Nickel hyperaccumulators
In 1977, Brooks et al. (1977) quantified for the first time the accumulation threshold for nickel at shoot concentrations > 1000 µg g−1 (0.1%) on dry weight (DW). This is an exceptionally high concentration threshold, considering that Ni toxicity takes place at 10 to 15 µg g−1 in vegetative organs of most plants (Yusuf et al., 2011).
Among the various metals that can be hyperaccumulated by plants, Nickel is the most frequent across Angiosperm taxa. In fact, over 75% of the metal-hyperaccumulator plants are specialized for Ni, while only 5 species are known, for example, to have the same ability for Cd, one of the most toxic heavy metals (Reeves et al., 2017). Nickel is also the metal that has been shown to reach the highest possible concentrations in plant tissues. This was discovered in Sebertia acuminata (Sapotaceae), a tree endemic to the serpentine soil from New Caledonia, which accumulates up to 26% Ni (dry mass) in its latex (Sagner et al., 1998).
Accumulation of nickel was first discovered in the late 1940s in the Tosco-Ligurian endemic Odontarrhena bertolonii (Desv.) Jord. & Fourr. (syn. Alyssum bertolonii Desv.; Minguzzi and Vergnano, 1948), a species of the most diverse and widespread group of hyperaccumulators in Europe and west Asia, the genus Odontarrhena C.A.Mey. ex Ledeb. [= Alyssum L. subgen. Odontarrhena (C.A.Mey. ex Ledeb.) W.D.G.Koch; Brooks et al. 1979; Reeves et al. 1983]. Indeed, O. bertolonii is only one of the many members of this genus that grow on the ultramafics of the Euro-Mediterranean region and Western Asia and is able to accumulate this metal well over 1000 µg g−1 d.w.in its shoots(Galardi et al., 2007).
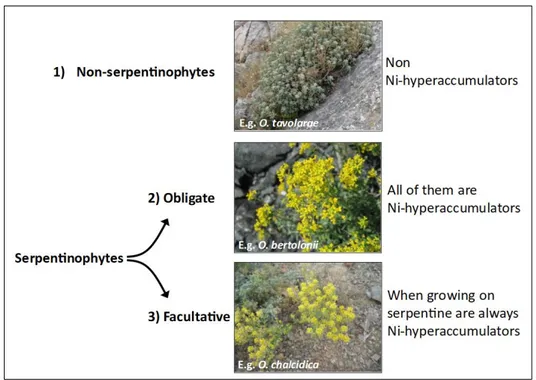
Based on their soil preferences, the ca. 90 species of Odontarrhena (Španiel et al. 2015; AlyBase, http://www.alysseae.sav.sk/) can be grouped into three major categories (Fig.3):
1) facultative serpentinophytes, including populations from ultramafic and non-ultramafic soils, 2) obligate serpentinophytes, often endemic to outcrops of more or less restricted geographic areas,
3) non-serpentinophytes, occurring only on non-ultramafic soils, though often rich in Mg, as for example dolomite.
Based on present-day knowledge, all taxa in the genus belonging to the second category are “obligate hyperaccumulators” (sensu Pollard et al. 2002; Brooks et al. 1979), while the first category is that of “facultative hyperaccumulators” (sensu Pollard et al. 2014; Reeves et al. 2015). In these species, only the populations from ultramafic soils are able to hyperaccumulate nickel in their leaves, while those from other soil types with low nickel concentration are not (Fig.3).
Several hypotheses have been offered to explain the adaptive function of metal hyperaccumulation in plants. According to the hypothesis of elementary allelopathy, shedding plant organs with high heavy metal content, such as leaves, would enrich soil surface with this metal and inhibit the growth of competing neighbour species that are not tolerant to high nickel concentration in soil (Brooks 1998). To date the “elemental defence” hypothesis is the most widely accepted. This hypothesis, formulated by Boyd and Martens (1998), proposes that the elevated concentrations of sequestered metal ions protect hyperaccumulators against attack by
12 herbivores and pathogens. The defence hypothesis was supported by several experimental studies as reported by van der Pas and Ingle (2019).
1.2.3 Nickel hyperaccumulation mechanisms
Nickel-hyperaccumulating plants display enhanced uptake, root to shoot translocation, and ability to detoxify and sequester nickel compared to non-accumulator species (Rascio and Navari-Izzo, 2011). However, despite their relative wide distribution on Earth, information about the physiological and molecular bases of Ni-hyperaccumulation is coming to light slowly if compared to Zn and Cd Ni-hyperaccumulation. In two Zn/Cd two hyperaccumulators expression profiling experiments have shown that these have constitutively elevated expression of genes involved in the uptake, chelation, and xylem loading of Zn/Cd in comparison to related non-accumulators (Beker et al. 2004, Weber et al. 2004)]. While it has been assumed that the same must hold true in Ni hyperaccumulators, the lack of genomic resources for these species has made this difficult to determine. This situation has changed recently with the advent of RNA-Seq technology which allows the de novo assembly of transcriptomes and quantification of transcript abundance in the absence of any prior sequence information. To date only four such studies have been published on Ni hyperaccumulators as reported by van der Pas and Ingle (2019) in his review on what is currently known about the molecular basis of the physiological processes underlying Ni hyperaccumulation in plants.
Figure 3 – The three main edaphic categories found in genus Odontarrhena, and corresponding Ni-behavior
Indirect evidence has suggested that hyperaccumulating plants absorb nickel mainly as Ni2+. For example the
13 is presumably due to a reduction of the free Ni2+ concentration in the soil solution. Few studies have been
conducted to investigate the Ni2+ uptake kinetics. Nickel uptake has been suggested to be a kinetic process,
with Michaelis-Menten constant values (Km) ranging from 0.51 to 379 μM (Deng et al, 2018). The relatively high Km values indicate that Ni2+ is mainly absorbed via low-affinity transport systems, and nickel uptake by
roots appears to be mediated by poorly selective cation transporters, notably members of the ZRT/IRT-like (ZIP) family (Nishida et al. 2015). So far, no high-affinity Ni-specific transporter in plants of this cation from soil have been identified in hyperaccumulating plants (van der Pas and Ingle, 2019).
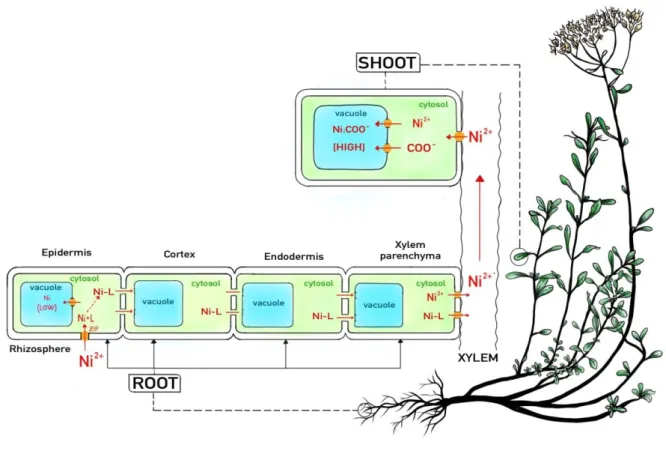
Once reached the root symplast, metal ions are rapidly complexed by organic ligands to alleviate toxicity (Haydon and Cobbett 2007). Formation of a nickel-ligand complex ([Ni-L] in the Figure 4) may also impede the vacuolar sequestration of nickel in root tissues by tonoplast localized iron-regulated/ferroportin (IREG/FPN) transporters. Histidine concentrations are found to be constitutively high in roots of the nickel hyperaccumulator Alyssum lesbiacum [= Odontarrhena lesbiaca] in comparison to the non-hyperaccumulator Brassica juncea (Kerkeb and Krämer 2003), which results from the overexpression of the histidine biosynthetic pathway in this species (Ingle et al. 2005). Histidine acts as a nickel transport facilitator in the cytoplasmic matrix of root cells. Due to the weak sequestration capacity of root vacuoles, most of the nickel is then transferred radially from epidermis to pericycle (Fig. 4).
When reaching the xylem by radial transport (from epidermis trough cortex, endodermis and xylem parenchima) nickel is then efficiently exported from xylem parenchyma and loaded into xylem vessels. Nickel then reaches the shoots in the xylem sap. The identity of the transport protein(s) responsible for the xylem loading of nickel remains elusive. Some studies seem to indicate that nickel hyperaccumulators may possess a transport protein, lacking in other plant species, that loads the Ni-His complex into the xylem, though no such transporter has been identified to date (van der Pas and Ingle, 2019). Nickel moves up to shoots following the xylem flow, most of which find their final destination in mature leaves due to the strong transpiration in these tissues, while young leaves receive relatively small proportion of nickel in xylem (Fig. 4).
Nickel is translocated from roots to shoots in several forms with citrate and malate complex, however it is also clear that the majority of nickel in xylem sap remains as a hydrated cation [Ni(H2O)6]2+ as in the Odontarrhena
(= Alyssum) nickel hyperaccumulators (Kramer et al, 1996; Centofanti et al. 2013). This is possible because Ni2+ is quite stable in the acidic xylem sap (pH 5–6), and xylem vessels are non-living cells which should not
be affected by high concentrations of Ni2+ (Fig. 4).
On reaching leaves, the main storage organ, nickel is then transferred across the whole apoplastic space via leaf veins. The transporters responsible for the unloading of nickel (and Fe) from xylem into shoot cells are unknown but may include members of the ZIP family. Here, nickel remains in the apoplast or is either absorbed by leaf cells (symplast), at subcellular level the vacuole is the primary site of nickel storage in shoot cell (Krämer et al. 2000; Robinson et al. 2003). Compartmentalization of nickel in the vacuole is a key mechanism to remove excess metal ions from the cytosol and seems to be crucial for metal tolerance. A number of tonoplast transporters are responsible for metal transport into the vacuole, both in the form of cations and in complexes with ligands (Wòicik et al. 2017). In the vacuole nickel is complexed by carboxylic acids (COO-), with Ni-citrate or Ni-malate the predominant complexes identified to date (Van der Pas et al., 2019). nickel is
14 accumulated primarily in the shoot epidermis in most species. Secondly, leaf apoplast also acts as an important sink for redundant nickel, in particular when large amounts of Ni2+ are transported to leaves from the xylem.
For example, cell walls, cuticles and epidermal trichomes can store high concentrations of nickel (Fig. 4).
Figure 4 - A general model for nickel uptake, transport and accumulation in plants (modified from Deng et al. 2018).
1.2.4 Nickel hyperaccumulator plants: biotechnological applications
Next to fundamental research on the genetic bases and molecular mechanisms of metal homeostasis, evolution and adaptation in extreme environments, Nickel accumulating plants are currently attracting considerable interest for biotechnological applications as well (Wójcik et al., 2017; Reeves et al., 2018; van der Ent et al., 2018). Hyperaccumulator plants have potential for "phytomining", which utilizes hyperaccumulators as ‘metal crops’ to sequester soil Ni in harvestable biomass that can then be used to produce fine Ni chemicals or eco-catalysts (see the review of Deng et al., 2017). Phytomining of Ni is especially promising, due to the high market price of Ni, the large variety of hyperaccumulating species adapted to a wide climatic range, and numerous tracts of suitable application areas, e.g. natural ultramafic soils (van der Ent et al. 2015). The applicability and economic value of phytomining in given social and environmental contexts have been demonstrated at field scale in various countries with extensive serpentine outrops that are not economically exploitable in other ways, such as Albania, for example (Bani et al. 2007; Bani et al. 2015; Figure 5). The optimization of phytomining/phytoextraction technologies requires in-depth knowledge of the metal uptake
15 and transport mechanisms in hyperaccumulator plants. For example, unravelling the pathways associated with Ni uptake will allow for a better application of specific soil amendments to increase the Ni extraction yield in metal crops (Deng et al. 2017), Furthermore, insights into the physiological mechanisms of metal hyperaccumulation has important implications for advancing the understanding of the uptake and transport of trace elements in ‘normal plants’ such as food crops (Bani et al. 2007; White and Broadley 2011).
Figure 5 – Massive cultivation of Odontarrhena chalcidica for phytomining on serpentine-soil fields around Lake Ohrid, Eastern Albania.
1.3 The target plant genus
The present thesis is focused on species of Odontarrhena, a genus of tribe Alysseae (Brassicaceae) that includes over 85 species many of which are Ni-hyperaccumulators. Until recently, these species were referred to genus Alyssum sect. Odontarrhena due to a superficial resemblance to species of Alyssum sect. Alyssum. However, recent molecular phylogenetic studies have shown that Alyssum and Odontarrhena are two monophyletic clades representing well distinct genera without close affinity to each other (Warwick et al., 2008; Cecchi et. al. 2010; Resetnik 2013). While metal accumulation is totally lacking in Alyssum, this ability is widespread in Odontarrhena. In fact, some species of this genus are among the most important model systems to investigate the physiological mechanisms, molecular pathways and genetic control of metal accumulation in plants (Reeves and Baker, 2000).
16 Morphologically, species of Odontarrhena are small shrublets or long-lived perennial herbs with robust taproot and numerous sterile shoots from the base. Most aerial parts of the plants are usually covered with dense stellate hairs, which often gives to the plants a silvery-greyish color. Inflorescence is branched in a compound corymbus, only rarely it is reduced to a simple raceme. The flowers consist of five ovate sepals, and five cuneate-spathulate petals of bright yellow color. Stamens filaments typically bear denticulate appendages, while the fruits are silicles with two seeds, one for each loculus.
The genus is distributed from western Asia to the western Mediterranea basin. Several species complexes are still taxonomically poorly resolved and many taxa are not clearly defined, especially those from the Middle East and the Balkan countries, both of which represent important centres of diversity for this genus. In the framework of the European Project AGRONICKEL it was thus necessary to elucidate the systematics of some of these critical species groups before performing analyses of plant metal content and metal accumulation capacity. A robust taxonomy of metallophytes is in fact crucial for any other type of scientific study or practical use of these plants. To this purpose, our systematic investigation focused the taxa native to Albania, a major European hotspot of metallophytes and Odontarrhena thanks to the vast occurrence of ultramafic outcrops. Based on plant material collected during several field trips across Albania and relevant herbarium material, morphological, karyological, ecological data were obtained and analysed, allowing to circumscribe seven taxa: O. albiflora, O. chalcidica, O. moravensis, O. rigida, O. decipiens, O. smolikana subsp. glabra and O. sibirica (Fig. 6). In the taxonomic paper by Cecchi, Bettarini et al. (2018) a formal and complete morphological description is given for each of these taxa, as well as all available information about nomenclatural issues, chromosome numbers, distribution, and phenology. Moreover, all taxa were illustrated by the original iconographies that are provided at the end of this chapter.
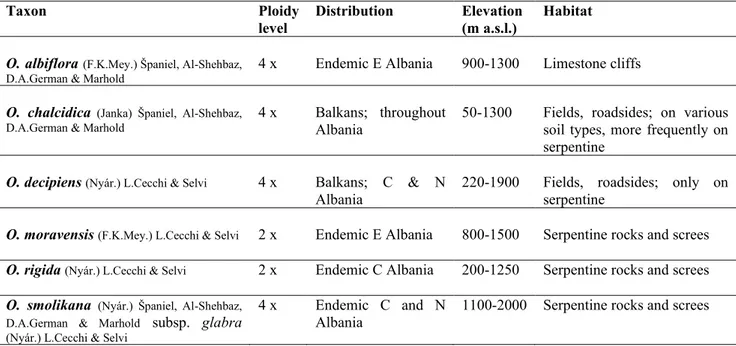
In summary, O. albiflora is a tetraploid endemic known from a single site in Eastern Albania, on calcareous cliffs at 900–1300 m a.s.l.; O. smolikana subsp. glabra is a tetraploid obligate serpentine endemic, occurring in rocky subalpine slopes at 1200–2200 m a.s.l.; O. rigida is a diploid serpentine endemic of Central Albania at 250–1200 m a.s.l., in mostly undisturbed sites; O. moravensis is also diploid and restricted to undisturbed serpentine sites of the E Albanian mountains, at 800–1500 m a.s.l.; O. chalcidica is a tetraploid species widely distributed especially in the northern, central, and eastern parts, on serpentine and other soil types (schist, flysch), at 50–1300 m a.s.l., usually in anthropogenic habitats; O. decipiens is a putative allotetraploid hybrid between O. smolikana and O. chalcidica, found at 220–1900 m a.s.l. mostly in disturbed sites of Northern and Central Albania (and Northern Greece). Odontarrhena sibirica, a facultative serpentine species, was collected only once at the border with Greece but no recent confirmation is available for Albania and its presence in this country remains uncertain.
Flowering and fruiting time is also widely variable between species (Fig.7), often independently from altitude or habitat, providing an useful taxonomic character.
Besides the characters above, Nickel concentrations in all taxa and accessions sampled were also analysed, revealing in all cases values well above the hyperaccumulation threshold of 1000 μg · g−1 leaf dw (Table 1).
17 Figure 6. The Albanian species of Odontarrhena in their natural habitat: A) O. albiflora (Mt. Tathë); B) O. chalcidica (Pishkash); C) O. decipiens (Bulqizë); D) O. moravensis (Voskopojë); E) O. rigida (Mt. Shpat); F) O. smolikana subsp.
glabra (Mt. Shebenik) [From Cecchi, Bettarini et al., 2018].
Figure 7. Phenological differences between species of Odontarrhena in Albania. Bars in solid grey indicate the time interval between the decade in which at least 50% of the population is in flower and the decade when full flowering (100%) of the population occurs; black bars indicate the same interval for the fruiting process (50%-100% of the population with ripe fruits and seeds). [From Cecchi, Bettarini et al., 2018]
18 TABLE 1. Nickel concentration in leaf samples (µg · g-1 dw) of six out of seven taxa of Odontarrhena from Albania; minimum and maximum values, determined in different populations/sites (except for O. albiflora, only one population), are given as means ± standard deviation of five plants (three replicates per plant); details of the voucher specimens are given in Appendix 1.
Species Ni concentration Origin Vouchers
min max
O. albiflora — 2718 ± 96 Mt. Thatë FI050840
O. chalcidica 4585 ± 501 23267 ± 982 Barmash, Pogradec FI050417, FI050416
O. decipiens 7916 ± 526 17296 ± 1301 Fierzë, Shtamë FI050444, FI050442
O. moravensis 5510 ± 133 14276 ± 1277 Mt. Morave, Voskopoje FI050828, FI050441
O. rigida 7517 ± 470 17055 ± 732 Mt. Shpat, Mt. Shebenik FI050434, FI050437
O. smolikana
subsp. glabra 7705 ± 409 14050 ± 1926 Shtamë, Krastë FI050431, FI050831
1.4 General methodological approach
The work is based first on an observational and comparative approach by which we could first elucidate the poorly-known taxonomy of the Albanian taxa and then characterize their genetic relationships using molecular tools. This step was necessary before investigating the levels of metal concentration in soils and populations of these species (also including other taxa from Greece) and the possible influencing factors. To test the hypotheses based on evidence from the observational studies above, we then adopted an experimental approach to evaluate the accumulation and tolerance to nickel and other metals in controlled conditions. We also included the investigation of the still unknown relationships between nickel accumulation and photosynthetic efficiency in three taxa of this genus with different soil preferences. Using an ad-hoc experimental design, we finally explored the role of Ni in relation to the activity of the enzyme urease in Ni-hyperaccumulators. In particular, we tested the hypothesis that nickel concentration in these plants may be associated with an enhanced requirement of the metal to assure an adequate activity of this nickel-dependent enzyme which catalyzes the hydrolysis of urea to ammonia and bicarbonate (Dixon et al., 1975; Eskew et al., 1984; Polacco et al., 2013).
1.4.1 Field work
Field sampling and plant collection of native populations were performed during field trips across Albania (2016, 2017, 2018), Greece (2018, 2019) and Romania (2018). During these trips we sampled several populations at different sites and in different seasons to collect ripe seeds, plants (shoots and roots) and bulk
19 soil samples for all subsequent analyses. The full list of taxa and populations surveyed during these trips and used for the present thesis is given in Table 2
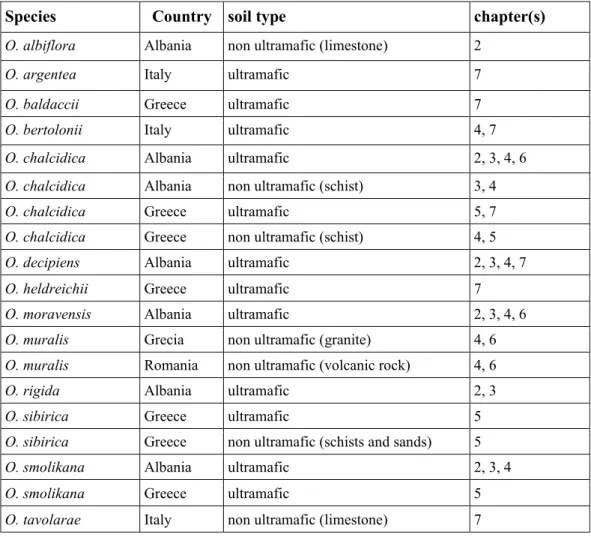
Table 2 - List of investigated species of Odontarrhena, with country, soil type of origin and chapters of this thesis where they have been used for the experimental work (more details about populations are given in each chapter)
Species Country soil type chapter(s)
O. albiflora Albania non ultramafic (limestone) 2
O. argentea Italy ultramafic 7
O. baldaccii Greece ultramafic 7
O. bertolonii Italy ultramafic 4, 7
O. chalcidica Albania ultramafic 2, 3, 4, 6
O. chalcidica Albania non ultramafic (schist) 3, 4
O. chalcidica Greece ultramafic 5, 7
O. chalcidica Greece non ultramafic (schist) 4, 5
O. decipiens Albania ultramafic 2, 3, 4, 7
O. heldreichii Greece ultramafic 7
O. moravensis Albania ultramafic 2, 3, 4, 6
O. muralis Grecia non ultramafic (granite) 4, 6
O. muralis Romania non ultramafic (volcanic rock) 4, 6
O. rigida Albania ultramafic 2, 3
O. sibirica Greece ultramafic 5
O. sibirica Greece non ultramafic (schists and sands) 5
O. smolikana Albania ultramafic 2, 3, 4
O. smolikana Greece ultramafic 5
O. tavolarae Italy non ultramafic (limestone) 7
1.4.2 Experiments in controlled conditions
Seeds collected during field trips were stored at about 4°C for at least four weeks before sowing in peat soil.
Six-week-old seedlings
were then transferred to hydroponic culture in 1-L polyethylene containers with Hoagland’s solution (Fig. 8). Nutrient solutions were changed weekly and plants were grown in a growth chamber (24/16°C day/night; light intensity 100 µmol-2 s-1, 16-h (day) photoperiod; relative humidity 60-65%).Then plantlets were exposed to a series of increasing NiSO4 concentrations, in a background solution of the
same composition as the pre-culture solution, with no addition of NiSO4 (Ni concentration < 150 nM). More
20 Figure 8 - 1-L polyethylene pots with Odontarrhena seedlings in growth chambers
1.5 Outline of the thesis
Chapter 2 provides the results of our investigation on the phylogenetic relationships and population genetic structure of Odontarrhena in Albania, performed on 32 populations of the six taxa from all major ultramafic outcrops in the country. This was necessary before performing subsequent analyses of metal levels and Ni-accumulation capacity. In this work, we estimated species-level phylogenetic relationships using molecular markers from the nuclear and plastid genomes (ITS-5.8S and trnL-trnF), and analyzed population genetic structure using the DNA-fingerprinting approach based on the Amplified Fragment Length Polymorphism (AFLPs). Phylogenetic relationships and patterns of population genetic differentiation were then interpreted in relation to distribution and ploidy level of the taxa, intensity of anthropic site disturbance, altitude, soil type and metal concentration at each population site.
Chapter 3 presents the study of metal concentrations in the same populations and taxa. Here we analyzed the levels of Ni, Co, Cr, Mg, Ca, K, Fe and Mn in roots and shoots of all specimens sampled, in relation to those in the relative soil samples of origin.
In chapter 4 we reported our experimental investigation about tolerance and accumulation ability in several populations of different Odontarrhena species. Plantlets from seeds were grown in hydroponic cultivation in growth chambers at increasing Ni-concentrations. Measurements of growth, root and shoot metal accumulation were made to fit an adequate model of the nickel dose/response curve. This model was applied to obtain reliable tolerance descriptors for testing the effects that the metal can have on the growth of these plants.
In Chapter 5 we investigated the particular case of O. sibirica, a facultative serpentinophyte in which Ni-accumulation ability was still enigmatic from previous studies. To address this question we used an observational and experimental approach based on natural populations, herbarium specimens and plants in controlled conditions. Nickel concentration level was measured in plants and soil samples from native populations collected on ultramafic and non-ultramafic soils in Greece. The same analyses were conducted on
21 plant material from 33 specimens obtained from five major European Herbarium collections. Plantlets from seeds of O. sibirica were grown in controlled conditions in hydroponic cultivation and exposed to different levels of nickel concentrations to test tolerance and accumulation ability. In parallel, we performed a common garden experiment with O. sibirica and three other Odontarrhena species for comparison, which were grown on natural ultramafic soils.
In chapter 6 we investigated the effects of nickel on photosynthesis in Ni-hyperaccumulating plants. To date there is a complete lack of information about how Ni-hyperaccumulators reconcile that extraordinary amount of metal in their shoots with an efficient photosynthetic activity, or at least on which photosynthetic parameters the excess of nickel impacts less in plants which such an ability compared to normal plants. In this chapter measurements of growth, root and shoot metal accumulation and several photosynthesis-related parameters, such as gas exchange, chlorophyll fluorescence and photosynthetic pigments, were determined in three species of Odontarrhena grown in hydroponics and exposed to two different levels of NiSO4.
Chapter 7 presents the experimental set up, methodology and preliminary results of our study on the enzyme urease. This is the only enzymatic Ni metal-protein in plants, catalyzing the hydrolysis of urea to ammonia and bicarbonate. Whether the high nickel concentration in the shoots of wild Ni-hyperacumulator species is associated with an increased expression or activity of urease has not been investigated so far. Urease activity was measured on eight taxa of Odontarrhena with different edaphism (Fig. 3). These plants were collected during field trips in Albania, Greece and Italy, and then grown in the Botanical Garden of Firenze University. The urease assays were performed on fresh, green, fully expanded leaves. Additional assays were also performed on plantlets from seeds of O. bertolonii, an obligate hyperaccumulator, grown in controlled conditions in hydroponic cultivation and exposed to different nickel levels.
22
Odontarrhena albiflora
A) habit (specimens at different fruiting stages);
B) flowering (left) and sterile (right) shoots cauline leaves, showing their upper (left) and lower surface (right); C) flower from above and in lateral view, and single petal;
D) lateral fruiting raceme;
E) silicles of different size in lateral, inner and outer view.
23
Odontarrhena chalcidica
A, F) habit (flowering and fruiting specimens of different stature);
B) cauline leaves of flowering (left) and sterile (right) shoots, showing their upper and lower surface on the left and on the right, respectively;
C) lateral fruiting raceme;
D) silicle in lateral, inner and outer view.
24
Odontarrhena moravensis A) habit (fruiting specimens);
B) leaf of sterile shoot, showing its upper (left) and lower surface (right); C) lateral fruiting raceme;
E) silicle in lateral, inner and outer view.
25
Odontarrhena rigida
A) habit (fruiting specimen);
B) leaf of sterile shoot, showing its upper (left) and lower surface (right); C) lateral fruiting raceme;
D) silicle in lateral, inner and outer view.
26
Odontarrhena decipiens
A) habit (flowering specimen and fruiting shoot);
B) leaf of sterile shoot, showing its upper (on the left) and lower surface (right); C) lateral fruiting racemes;
E) closed and open silicles of different size and shape.
27
Odontarrhena smolikana subsp. glabra A) habit (fruiting specimen);
B) leaf of sterile shoot, showing its upper (on the left) and lower surface (right); C) lateral fruiting raceme;
D) flower in lateral view, with isolated sepal and petal; E) silicle in lateral, inner and outer view.
28
Odontarrhena sibirica A) habit (fruiting specimens);
B) leaves of sterile shoot from above (left) and below (right); C) lateral fruiting raceme;
E) silicle in lateral, inner and outer view
Original drawing by L. Cecchi (based on the isotype of Alyssum suffrutescens var. epirotum BM000750156) [from Cecchi, Bettarini et al, 2018]
29
CHAPTER 2
ABSTRACT
Albanian taxa and populations of the genus Odontarrhena are most promising candidates for research on metal tolerance and Ni-agromining, but their genetic structure remains unknown. We investigated phylogenetic relationships and genetic differentiation in relation to distribution and ploidy of the taxa, anthropic site disturbance, elevation, soil type, and trace metals at each population site. After performing DNA sequencing of selected accessions, we applied DNA-fingerprinting to analyze the genetic structure of 32 populations from ultramafic and non-ultramafic outcrops across Albania. Low sequence divergence resulted in poorly resolved phylograms, but supported affinity between the two diploid serpentine endemics O. moravensis and O. rigida. Analysis of molecular variance (AMOVA) revealed significant population differentiation, but no isolation by distance. Among-population variation was higher in polyploids than in diploids, in which genetic distances were lower. Genetic admixing at population and individual level occurred especially in the polyploids O. chalcidica, O. decipiens, and O. smolikana. Admixing increased with site disturbance. Outlier loci were higher in serpentine populations but decreased along altitude with lower drought and heat stress. Genetic variability gained by gene flow and hybridization at contact zones with “resident” species of primary ultramafic habitats promoted expansion of the tetraploid O. chalcidica across anthropogenic sites.
30
2.1 INTRODUCTION
Nickel accumulating plants are currently attracting considerable interest for both biotechnological applications and fundamental research on the genetic bases and molecular mechanisms of metal homeostasis, evolution and adaptation in extreme environments (Wójcik et al., 2017; Reeves et al., 2018; van der Ent et al., 2018). Because of the phylogenetic constraints involved in metal accumulation ability (Broadley et al., 2001), research in this field requires the use of appropriate model systems formed by closely related accumulating and non-accumulating taxa and/or species with populations from sites with high and low levels of Ni in the soil (Reeves & Baker, 1984; Krämer, 2010). In Europe, broad opportunities for research and phytoextraction technologies are offered by the most diverse groups of Ni-accumulators in the continent, e.g. the Brassicaceae genera Bornmuellera and Odontarrhena (syn. Alyssum sect. Odontarrhena; Reeves et al., 1983; Reeves, 1988; Peer et al., 2006; Nkrumah et al., 2016; Kidd et al., 2018). The latter genus consists of nearly 90 species ranging from the Iberian peninsula to Iran and adjacent regions (Španiel et al., 2015), of which about 60 are able to accumulate Ni well above 1000 μg g-1 dw (Brooks et al., 1979; Global
Hyperaccumulator Database; http://hyperaccumulators.smi.uq.edu.au/collection/; Reeves et al., 2018). Such plants are often obligate endemics of Ni-rich ultramafic outcrops (mostly “serpentine” soils) in given regions, while others grow either on or outside these outcrops and include both accumulator and non-accumulator populations (Reeves 1988; Brooks et al., 1981). Previous studies showed that accumulator and non-accumulator species do not form separate clades (Mengoni et al., 2003a; Cecchi et al., 2010, 2013), and that no genetic differentiation between serpentine and non-serpentine accessions of facultative species (Sobczyk et al., 2017). This suggested pre-adaptive capacity to accumulate Ni when growing on ultramafics, and repeated micro-evolutionary events of local adaptation on distinct outcrops in different regions and species complexes. However, most molecular studies on Ni-accumulating plants of this genus have focused on single obligate serpentine endemics, such as O. bertolonii (Mengoni et al., 2003b) or O. lesbiaca (Adamidis et al., 2014a), whilst none have examined the population genetic structure of entire species complexes including obligate and facultative serpentinophytes from ultramafic and non-ultramafic soils patchily distributed in geologically variable regions. Notwithstanding, such complexes provide a unique opportunity to examine the patterns of population differentiation between conspecific and heterospecific populations, and to shed light on the evolutionary dynamics of edaphic specialization, ecotypic variation and speciation.
The present study focuses on this general topic using the whole Odontarrhena group from Albania as a model system. Ultramafic areas cover 11% of this rugged country and extend from 100 to 2400 m a.s.l. (Estrade et al., 2015), which makes it a major center of diversity of metal-accumulating plants (and serpentine flora in general) and a most promising land for Ni-agromining (Kidd et al., 2018). In a recent study (Cecchi et al, 2018) we could elucidate its complicated systematics and ascertain the presence of six taxa that were already recognized by previous students of the genus and of the Albanian flora (Nyárády 1949; Meyer and Beiträge, 2011). Most of these taxa are allopatric and restricted to undisturbed serpentine or limestone sites in separate mountain massifs, river basins or altitudinal belts, which might involve high inter-population genetic differentiation and isolation by distance. Previous studies on single Ni-accumulator
31 Odontarrhena endemics from non-insular areas (Mengoni et al., 2003b; Sobczyk et al., 2017) and on other serpentine species (Wolf et al., 2000; Mengoni et al., 2006; Coppi et al., 2014) pointed to strong genetic differentiation between populations in relation to the patchy distribution of the ultramafic outcrops. In Albania, however, such a pattern may be blurred by O. chalcidica and O. decipiens, since these polyploid species are largely sympatric and overlapping with the others over a wide altitudinal range, on both serpentine and non-serpentine soils (Cecchi et al., 2018). Their wide distribution and clear preference for anthropogenic habitats suggest that historical land-use activities (i.e. agriculture, sheep farming, mining, industrialization, urbanization) may have promoted hybridization and range expansion, in line with the disturbance hypothesis of Anderson & Stebbins (Anderson & Stebbins, 1954). Moreover, recent evidence shows that species range expansion into novel habitats, including anthropic ones, is often fostered by enhanced genetic variation gained through hybridization and introgression with resident species (Pierce et al., 2017). Thus, Albanian taxa of Odontarrhena provide a good opportunity to examine this topic, and to investigate the levels and drivers of genetic divergence between taxa and populations from a vast ultramafic ‘archipelago’ along altitudinal and site disturbance gradients.
Using molecular markers from the nuclear and plastid genomes, we first estimated the level of DNA sequence divergence and phylogenetic relationships of all Albanian taxa, within a wide group of mainly Balkan representatives. Then, we used the more variable dominant nuclear markers Amplified Fragment Length Polymorphisms (AFLPs): 1) compare the levels of genetic diversity and divergence within and between species and populations, 2) assess the possible effects of site conditions such as elevation, soil type and metal concentration (Ni, Cr, Co, Ca, Mg), and 3) test the hypothesis that human disturbance could have contributed to the shaping of genetic structure in the group by promoting hybridization. AFLPs were used also to identify deviant loci potentially involved in adaptation to serpentine soil, trace metal concentration and elevation.
Elucidating the genetic structure in this group also pointed to the potential risk of using it for agromining applications on ultramafic outcrops outside its native range and inhabited by native Odontarrhena species.
2.2. RESULTS
2.2.1. DNA sequence divergence and phylogeny
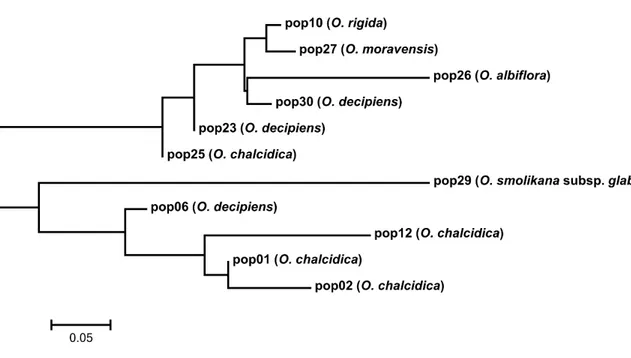
The nuclear ribosomal DNA (rDNA) internal transcribed spacer region (ITS) alignment consisted of 629 bp (including 22 coded gaps 608-629), of which 479 conserved, 145 variable but phylogenetically non-informative and 99 (15.7%) non-informative; considering only the ingroup (i.e. only Odontarrhena accessions), only 50 positions (7.9%) were phylogenetically informative. The mean genetic distance among ingroup accessions was 0.015. The Bayesian consensus phylogram (Figure 1) retrieved O. fallacina from Crete as sister to the rest of the ingroup that remained substantially unresolved.
32 Figure 1. Bayesian consensus phylogram from ITS-5.8S sequences showing relationships of taxa and accessions of Odontarrhena from Albania (in blue). Posterior probability values > 80% are shown at the corresponding nodes.
33 All Albanian taxa and accessions were included in a large unresolved group. However, the two diploids O. moravensis and O. rigida formed a well-supported clade (1.00), as well as the three accessions of typical O. muralis from outside Albania. Small terminal clades with PP > 0.90 were formed by other Balkan species but these did not include Albanian accessions.
The chloroplast DNA trnL-trnF IGS (trnL-F) alignment was 733 bp long, (gaps in pos. 712-733). By excluding the Alyssum representatives (outgroup) 565 sites were conserved, 16 were variable but non-informative and only 5 were (0.68%) phylogenetically informative. Mean genetic distance in the ingroup was 0.003. The IGS sequences therefore provided even less phylogenetic signal than did ITS. The resulting Bayesian tree was completely unresolved and is therefore not shown.
2.2.2. Genetic structure
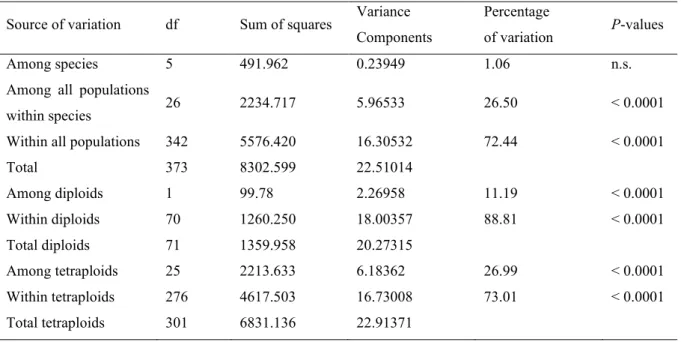
AFLP-fingerprinting was successfully performed on 374 individual samples and produced a total of 137 loci. The fragment length range was 41-511 bp. AMOVA showed significant differentiation between populations (FST = 0.273; p < 0.001) and a genetic structure dominated by within-population variation (72.44%; Table 1).
Table 1. Partitioning of genetic variance. AMOVA was performed at three hierarchical levels to test the differentiation between 374 individual samples from 32 populations and six species. The table shows: degrees of freedom (df), sum of squared deviations, variance component estimates, percentages of total variance contributed by each component, and the probability of obtaining a more extreme component estimate by chance alone (p). P-values were estimated with 1023 permutations.
Source of variation df Sum of squares Variance Components
Percentage
of variation P-values
Among species 5 491.962 0.23949 1.06 n.s.
Among all populations
within species 26 2234.717 5.96533 26.50 < 0.0001
Within all populations 342 5576.420 16.30532 72.44 < 0.0001
Total 373 8302.599 22.51014 Among diploids 1 99.78 2.26958 11.19 < 0.0001 Within diploids 70 1260.250 18.00357 88.81 < 0.0001 Total diploids 71 1359.958 20.27315 Among tetraploids 25 2213.633 6.18362 26.99 < 0.0001 Within tetraploids 276 4617.503 16.73008 73.01 < 0.0001 Total tetraploids 301 6831.136 22.91371
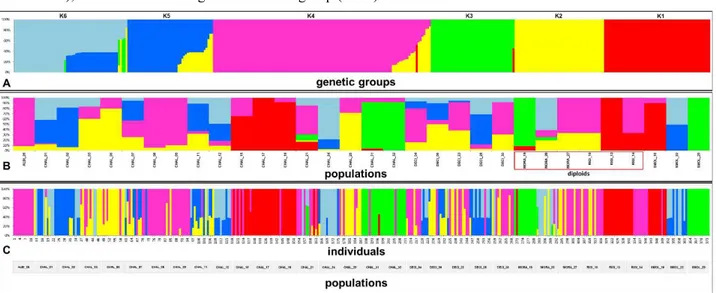
34 Figure 2. Bayesian clustering of AFLP-data from STRUCTURE, showing: a) Relative proportion of each of the six genetic groups (1-6) across the 32 populations (width of the colored band); b) proportion of population membership to each of the six genetic groups; populations including more than one group (color) are admixed; diploid populations are indicated (red box); c) proportion of individual membership to the six genetic groups across the 32 populations (374 individuals); individuals including more than one group (color) are admixed.
Figure 3. a) Principal Components analysis biplot showing relationships between the six genetic groups (K; numbers as in Figure 2a) and the six Albanian taxa of Odontarrhena (dots are diploid taxa, squares are tetraploids); b) Scattergram from Multifactorial Analysis showing direction of variation of number genetic groups per population (KPOP), genetically admixed individuals (MIND), number of outlier loci (OUTL) and heterozygosity HE in relation to anthropic site disturbance (ASD), altitude (ALTILOG), Ni and Cr soil concentration. The cos2 values, represented in the colour scale barplot, show the contribution of the variables on both dimensions (Dim 1 and Dim 2).
Grouping populations by species identity yielded non-significant results, whereas differences between populations within species accounted for a significant fraction of variation in the sample (26.50%; overall among-population + among-species variation = 27.56%). Among-population variation was considerably
35 higher in tetraploid than in diploid accessions (27.0% vs. 11.2%, respectively; Table 1), and population differentiation was, therefore, stronger (FST = 0.270 vs. 0.112 in diploids).
STRUCTURE Harvester detected six genetic groups (K = 6; Supplementary Figure S1 and Supplementary Table 4) and the presence of significant admixing zones (Figure 2a).
Groups were differently represented across the 32 populations (Figure 2b) and correspondence with species identity was weak. Principal component analysis (Figure 3a) explained 88.2% of the total variation and showed that O. rigida and O. albiflora are dominated by K1 and K4 , while the tetraploids O. chalcidica, O. decipiens, and O. smolikana include variable proportions of four to six groups.
This was the case of also O. moravensis, in which, however, only one of three populations (no. 20) was deviating in having four genetic groups instead of two as in pops. 19 and 27 (Figure 2b, Table 2).
Genetically admixed populations (with at least two groups both of which with ≥ 10% posterior probability of belonging into that genetic group) were the large majority (29 = 91%); only three populations consisted of only one group (Figure 2b, Table 2). However, diploid populations (excluding pop. 20 of O. moravensis mentioned above) showed a significantly lower admixing than tetraploid populations (p = 0.00443).
At the individual level, 75 samples (20%) included more than one genetic group (Figure 2c, Table 2); O. albiflora and O. rigida included no admixed individuals, whereas the great majority of the O. chalcidica accessions (14 out of 17) and all those of its hybrid O. decipiens included a variable proportion of admixed individuals. The two O. moravensis accessions no. 19 and no. 27 were formed by non-admixed individuals, while population no. 20 included five admixed individuals. When excluding the latter, the diploid profiles showed a significantly lower admixing than the tetraploids (p = 0.00338).
Based on multifactorial analysis (Figure 3b) the number of both genetic groups and admixed individuals were positively related to site disturbance severity. Populations from severely disturbed sites were also more admixed and consisted of more numerous genetic groups, regardless of species identity (Kruskal–Wallis test p = 0.0147).
Neighbor-Net analysis (Supplementary Figure 2) confirmed no clustering of individuals by species identity or population geographic origin, except for population 26 of O. albiflora and population 14 of O. rigida. Most individuals of O. chalcidica and O. decipiens were mixed with those of other species.
36 Table 2. Main parameters of genetic diversity in Albanian populations and taxa of Odontarrhena: number of genetic groups (reaching a proportion of at least 10%) in each population (KPop) and number of genetically admixed individuals (Mind), number (no. poly) and percentage (%poly) of polymorphic loci; population heterozygosity (He), mean species heterozygosity (Hs), mean number of outlier loci per population (Out) and mean genetic distance between populations within taxa (FST).
Taxa/pop. KPop Mind no. poly %poly He Hs Out FST
O. albiflora 26 0.113 — 2 0 49 35.766 0.113 2.08 O. chalcidica 0.247 0.267 1 4 3 113 82.48 0.323 — 6.25 — 2 3 4 114 83.21 0.338 — 6.92 — 3 3 3 81 59.12 0.217 — 4.83 — 5 2 2 74 54.02 0.196 — 3.25 — 7 4 6 101 73.72 0.261 — 4.83 — 8 2 1 47 34.31 0.126 — 0.83 — 9 2 1 61 44.53 0.147 — 0.82 — 11 4 10 101 73.72 0.275 — 5.25 — 12 4 7 120 87.59 0.335 — 5.82 — 15 2 1 68 49.64 0.189 — 1.80 — 17 1 0 81 59.12 0.201 — 1.67 — 18 2 0 86 62.77 0.215 — 1.83 — 21 5 4 92 67.15 0.251 — 2.92 — 24 3 2 103 75.18 0.296 — 5.73 — 25 2 1 72 52.56 0.191 — 3.83 — 31 3 1 113 82.48 0.317 — 3.08 — 32 2 0 114 83.21 0.322 — 4.92 — O. decipiens 0.231 0.224 4 4 3 81 59.12 0.185 — 2.50 — 6 4 6 96 70.07 0.252 — 5.75 — 23 4 2 78 56.93 0.199 — 2.67 — 28 4 7 120 87.59 0.348 — 5.83 — 30 3 2 69 50.37 0.171 — 2.83 — O. moravensis 0.239 0.279 19 2 0 103 75.18 0.273 — 3.17 — 20 4 5 110 80.29 0.314 — 3.67 — 27 2 0 45 32.84 0.130 — 2.08 — O. rigida 0.185 0.230 10 2 0 69 50.36 0.171 — 2.08 — 13 1 0 80 58.39 0.232 — 3.58 — 14 2 0 69 50.36 0.153 — 1.08 — O. smolikana 0.293 0.302 16 2 0 95 69.34 0.267 — 2.70 — 22 2 4 113 82.48 0.352 — 6.45 — 29 1 0 94 68.61 0.259 — 3.50 —
37 Figure 4. a) Distribution of the 137 loci (black dots) detected by BayeScan analysis of 374 individual samples of Albanian Odontarrhena; dots that fall over the red threshold line [Log (Posterior Odds, PO) = 0.5]) are identified as outlier loci. b) variation of the mean number of outliers per population with altitude (log); colours and species names abbreviations follow Figures 1 and 6.
38 Figure 5. a) Box-Whiskers plot of outlier variation across the six Albanian taxa (ALBI: O. albiflora; CHAL: O.
chalcidica; DECI: O. decipiens; MORA: O. moravensis; RIGI: O. rigida; SMOL: O. smolikana subsp. glabra);
asterisks indicate the serpentine obligate taxa, and letters indicate statistically different groups at p < 0.05; b) variation in serpentine (S) vs. non-serpentine (NS) accessions
2.2.3. Genetic diversity
All loci resulted polymorphic, showing that the primer combination was effective in distinguishing all individuals of the six taxa as unique genotypes. The lowest polymorphism (32.8%) was in the population of O. moravensis from the type locality (no. 27), whilst the highest (87.6%) was in those of O. decipiens and O. chalcidica (Table 2). The latter taxon showed the broadest inter-population variation, due to low polymorphism in accessions 8 and 9. At the species level, polymorphism was highest in O. smolikana (73.5%) and lowest in O. albiflora (35.8%), though no significant differences existed between taxa. Diploid and tetraploid populations/taxa also did not differ significantly, but diploids had on average a lower polymorphism than tetraploids (57.9% vs. 66.8%). Similarly, polymorphism was higher in serpentine vs. non-serpentine populations (66.2% vs. 52.8%), though differences were not significant because of large