ORIGINAL CONTRIBUTION
Starch-enriched diet modulates the glucidic profile in the rat
colonic mucosa
Maria Gabriella Gabrielli1 · Daniele Tomassoni1
Received: 1 August 2016 / Accepted: 2 February 2017 © Springer-Verlag Berlin Heidelberg 2017
Conclusions Although the mechanisms leading to such a
modulation are at present unknown, either an altered intes-tinal microbiota or a dysregulation of glycosylation pat-terns might be responsible for the types and distribution of changes in the glucidic profile here observed.
Keywords Lectin histochemistry · Colon · Sialic acid ·
Starch-rich diet · Glycosylation
Introduction
The gastrointestinal tract is a complex and dynamic ecosys-tem resulting from the interaction of distinct cell types, sur-face and secretory glycoconjugates, resident microbiota, and luminal contents. Growing research interest is focused on studying the multiple exogenous and endogenous factors that modulate the critical equilibrium of these different compo-nents as well as their effects on the intestinal mucosa and its secretory products. These consist mainly in glycoconjugates which form, as glycoproteins and glycolipids, the epithelial glycocalyx associated with the brush border membrane or are secreted as mucins, mainly by goblet cells, thus largely con-tributing to the first line of protection between the secreting epithelium and the luminal contents. The carbohydrate com-ponents of this epithelial mucous coat are essential to main-tain a homeostatic relationship with the microbiota: they act not only as a site for bacteria adhesion but also as metabolic substrates for their survival and colonization [1, 2]. Thus, changes in glucidic composition of the intestinal mucous layer may alter the composition and the equilibrium of the intestinal microbiota, which in turn, can affect the nature and production rates of the colonic mucus [3, 4] resulting in
Abstract
Purpose The protective function of the intestinal mucosa
largely depends on carbohydrate moieties that as a part of glycoproteins and glycolipids form the epithelial glycoca-lyx or are secreted as mucins. Modifications of their expres-sion can be induced by an altered intestinal microenviron-ment and have been associated with inflammatory disorders and colorectal cancer. Given the influence of dietary factors on the gut ecosystem, here we have investigated whether a long term feeding on a starch-rich diet can modulate the glucidic profile in the colonic mucosa of rats.
Methods Animals were divided into two groups and
main-tained for 9 months at different diets: one group was fed a standard diet, the second was fed a starch-enriched diet. Samples of colonic mucosa, divided in proximal and dis-tal portions, were processed for microscopic analysis. Con-ventional stainings and lectin histochemistry were applied to identify acidic glycoconjugates and specific sugar resi-dues in oligosaccharide chains, respectively. Some lectins were applied on adjacent sections after sialidase/fucosi-dase digestion, deacetylation, and oxidation to characterize either terminal dimers or sialic acid acetylation.
Results An increase in sulfomucins was found to be
asso-ciated with the starch-enriched diet that affected also the expression of several sugar residues as well as fucosylated and sialylated sequences in both proximal and distal colon.
* Maria Gabriella Gabrielli [email protected]
1 School of Biosciences and Veterinary Medicine, University
of Camerino, Via Gentile III da Varano, I, Camerino, 62032 Macerata, Italy
reduced viability of the defensive barrier and susceptibility to gastrointestinal diseases [5, 6]. Altered glycosylation pat-terns of the intestinal mucosa, in particular the expression of certain sialylated sugar chains as well as changes of sialic acid O-acetylation, have been associated with inflammatory intestinal disorders and colorectal adenomas and carcinomas [7–11]. The mechanisms for these glycosylation changes are probably complex. Evidences for the involvement of specific sialyltransferase and fucosyltransferase in colorectal cancer have been reported [12]. Other glycosylation changes tend not to correlate so well with glycosyltransferase activity and could be a consequence of bacterial metabolism, or a result of the ongoing inflammatory response.
Even the contribution of dietary factors to this critical intestinal environment still requires to be fully clarified, also to identify appropriate modifications of the diet that may protect the intestinal mucosa from various diseases. In sev-eral species, experimental data pointed out the potential of dietary factors in affecting morphological features and crypt-villus architecture of the large intestinal epithelium, cell pro-liferation, and the thickness and chemical characteristics of the intestinal mucus layer [13–17]. In particular, the type of the carbohydrate in the diet, whether it is a simple or a com-plex sugar, seems to differently influence the colonic mucosa. Indeed, dietary sucrose has been suggested to represent a risk factor of colon cancer, while complex carbohydrates, like starch, have been widely associated with a protective effect against several diseases [18–22]. Both sucrose and starch are generally well digested in the small intestine although a certain portion of starch, resistant starch, can reach the large intestine for fermentation.
In a previous study [23], a diet rich in starch has been proven to modify the microbiota composition and the faecal short-chain fatty acid (SCFA) contents in rats. To provide additional data on the effects of starch on the intestinal micro-environment, we aimed here to verify whether a long-term feeding on a high starch diet can affect the glucidic profile of the colonic mucosa of rats. The study has been performed by application of a series of lectins that allow the distribu-tional patterns of terminal and internal sugar residues and sequences to be visualized. In addition, chemical and enzy-matic pretreatments, combined with appropriate lectins, have been addressed to both localize sialylated glycan sequences and clarify the degree and position of acetylated substituents in sialic acid residues [24].
The finding of distinct modifications in the intestinal gly-coconjugates in response to a particular dietary pattern, such as starch, might provide new insights into the suggested rela-tion between diet, intestinal microenvironment, and colonic dysfunction.
Materials and methods Animals
Eleven–twelve-week-old female Sprague–Dawley rats (Morini, Reggio Emilia, Italy) were kept in the Univer-sity’s Animal Care Facility; they were housed in plas-tic cages with wire tops and bottoms and maintained at constant environmental temperature (20–22 °C) on a 12 h light–dark cycle, according to internationally accepted guidelines. The animals and animal use proto-cols were approved by Institution’s Animal Care and Use Committee.
Dietary treatment
A starch-enriched diet (corn starch: 64%) was prepared, based on the AIN-76 diet (American Institute of Nutri-tion, 1977) slightly modified and containing a lower casein content (16%) and a slightly higher fat content (both corn and olive oil). Dietary components were from Piccioni Laboratories (Gessate, Italy).
The animals were divided in two groups (eight animals per group): one group were fed on the starch-enriched diet (STD); in the second group (control animals), the sugar composition was based on the AIN-76 diet (Table 1). Food and water were provided ad libitum for 9 months.
Tissue processing
The anaesthetized animals were killed by cervical dis-location. The entire colon was removed immediately and washed thoroughly in 0.1 M phosphate buffered saline solution (PBS) to remove luminal contents. Two cm segments from each colon were taken 1 cm distal to
Table 1 Composition of the experimental diets
Ingredient Control (%) STD (%)
Casein purified high nitrogen 16.0 16.0
DL-methionine 0.3 0.3
Sucrose 50.0 –
Corn starch 14.0 64.0
Corn oil 4.0 4.0
Olive oil 8.0 8.0
Alphacel, non-nutritive bulk 2.5 2.5
Choline bitartrate 0.2 0.2
Mineral mix AIN 4.0 4.0
the cecum in the proximal colon and 2 cm from the anus in the distal colon. All manipulations were performed according to the authorized investigators. The segments were then placed for 24 h at room temperature in Car-noy’s fluid and postfixed in a 2% calcium acetate–4% paraformaldehyde solution (1:1) for 3 h. Following dehydration through a graded series of ethanols, speci-mens were embedded in paraffin wax. Sections were serially cut at 5 µm.
Histochemical stainings
Sections were processed for carbohydrate histochemistry using the Alcian blue 8GX staining at either pH 2.5 (AB 2.5) or pH 1.0 (AB 1.0) according to routine methods. The AB 2.5 stains carboxylated and sulfated types of acidic mucins, and AB 1.0 stains sulfomucins. To dis-criminate between weakly acidic and strongly acidic gly-coconjugates, the Low Iron Diamine (LID) and the High Iron Diamine (HID) reactions were also applied [25].
Lectin histochemistry
A panel of eight horseradish peroxidase (HRP)-conju-gated lectins (Sigma Chemicals, St. Louis, MO, USA) were used for detection of specific sugar residues, as indicated in Table 2. Histochemical staining was per-formed as previously detailed [26]; briefly, after endog-enous peroxidase inactivation with 0.3% H2O2 in
meth-anol for 30 min, sections were incubated for 30 min at room temperature with the lectin diluted in PBS (Table 2). The peroxidase binding sites were visualized with a solution of 3,3′ diaminobenzidine (DAB kit, Vec-tor LaboraVec-tories, Burlingame, Calif., USA).
Chemical and enzymatic treatments
Adjacent, serially cut sections were subjected to PNA and GSA II lectins with and without digestion with 1 U/ ml bovine epididymis α-fucosidase (Sigma Chemicals) in 0.1 M sodium citrate buffer, pH 6.0, containing 25 mM EDTA, for 16 h at 37 °C.
Other adjacent sections were subjected to PNA and DBA lectins binding with and without the following pretreatments:
• Digestion with 0.5 U/ml sialidase from Clostridium
perfringens (Type V, Sigma) for 16 h at 37 °C in 0.1 M
acetate buffer, pH 5.5, containing 10 mM CaCl2;
• deacetylation with 0.5% potassium hydroxide in 70% ethanol solution for 30 min at room temperature. This treatment, by detaching acetyl substituents, renders sialic acid residues susceptible to sialidase digestion; • mild and strong periodate oxidation (PO) with a 1 mM
and 44 mM aqueous solution of periodic acid, respec-tively, for 15 min at room temperature. Mild oxidation abolishes the lectin binding with sialidase or KOH/ sialidase when sialic acids do not contain C7- and/or
C8- and/or C9-O-acetyl groups in the side chain. Strong
oxidation blocks the subsequent staining with sialidase/ lectin or KOH/sialidase/lectin except for C9 acetylated sialic acids linked via a α2–3 bond to the penultimate ß-galactose.
The histochemical experiments specific for demonstra-tion of sialic acid derivatives were formulated according to previous reports [26].
Controls
Controls to verify the lectin specificity included substitution of the conjugated lectins for the respective unconjugated
Table 2 Lectins used in the study, working concentration, specificity, inhibitory sugars
Fuc Fucose, Gal galactose, GalNAc N-acetylgalactosamine, Glc glucose, GlcNAc N-acetylglucosamine, Man mannose
Lectins
Concentra-tion μg/ml Nominal specificity Inhibitory sugar WGA Triticum vulgaris 5 (β-D-GlcNAc)2>>sialic acid D-GlcNAc
GSA II Griffonia simplicifolia 50 D-GlcNAc D-GlcNAc
LTA Lotus tetragonolobus 100 α-L-Fuc L-Fuc
PNA Arachis hypogaea 60 β-D-Gal(1,3)-D-GalNAc D-Gal
Con A Canavalia ensiformis 100 α-D-Man > α-D-Glc D-Man
GSA I-B4 Griffonia simplicifolia 20 α-D-Gal D-Gal
PSA Pisum sativum 100 α-Man D-Man
lectins as well as pre-incubation of lectins with the corre-sponding hapten sugars at concentration of 0.1–0.4 M.
Controls for fucosidase and sialidase digestion aimed to determine the influence of the enzyme-free buffers and to investigate the efficacy and the specificity of treatments.
Results
Histochemical stainings of carbohydrates
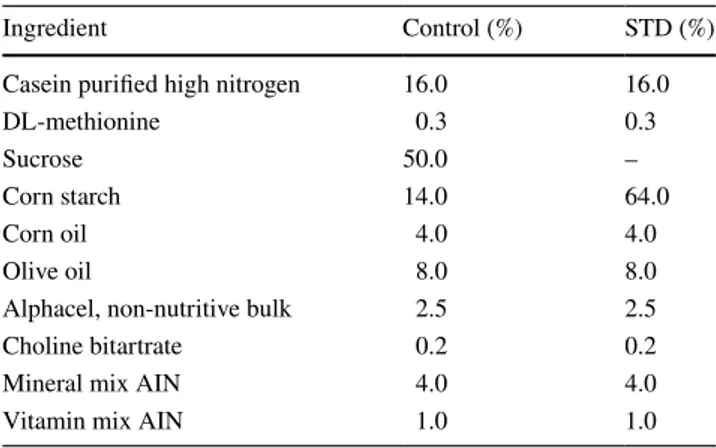
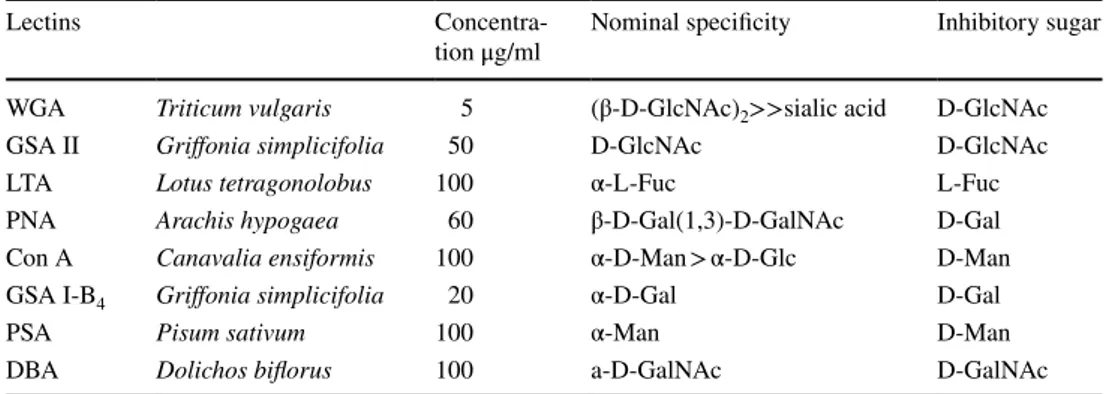
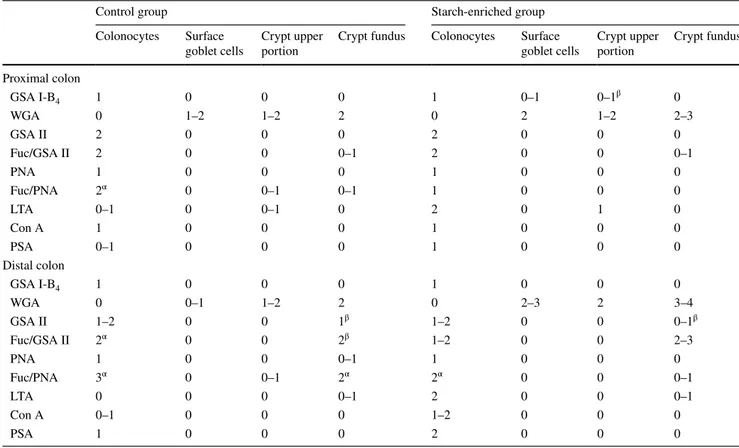
All the conventional reactions employed for the visualiza-tion of acidic glycoconjugates stained the goblet cells as well as the secretory mucous products adherent to the lumi-nal surface of colonocytes or discharged into the lumen. The histochemical patterns produced by the AB (pH 2.5) and LID reactions indicated an overall slight reduction of the staining intensity, indicative of acidic mucins, in the animals fed on the starch-enriched diet compared with the control group (Figs. 1, 2a, b). This finding was more appreciable in the distal than in the proximal colon (Fig. 1). Both HID and AB (pH 1) reactions resulted in a lower dis-tribution of staining, compared with LID and AB (pH 2.5) reactivity, respectively. Such a decrease appeared to be less marked in the colonic epithelium of the STD rats than in the control group (Fig. 2).
Lectin histochemistry
The lectin binding patterns produced in the proximal and distal portions of the rat colonic mucosa by GSA I-B4,
WGA, LTA, Con A, PSA, GSA II, and PNA, the last two with and without fucosidase predigestion, are reported in Table 3. The comparative analysis of the lectin binding patterns pointed out some differences in the experimental
conditions assayed. In particular, GSAI-B4, which is selec-tive for terminal α-D-Gal residues, weakly labelled goblet cells and upper crypts only in the proximal colon upon the starch-rich diet. WGA, which specifically recognizes termi-nal and intertermi-nal β-d-GlcNAc residues and sialic acid
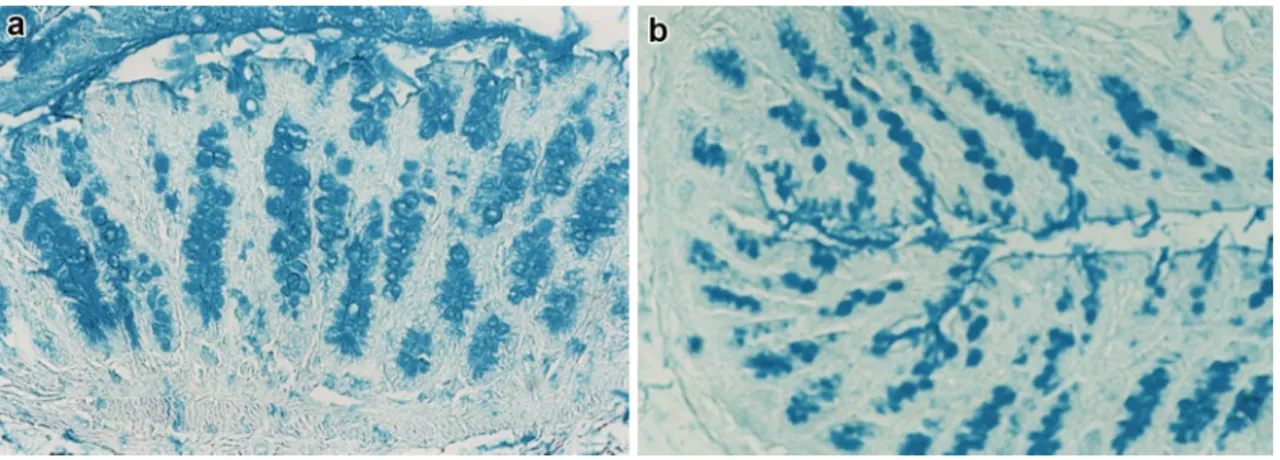
resi-dues, showed a higher reactivity in the surface goblet cells and crypt fundus of the colonic mucosa of rats fed on the starch-enriched diet, compared with the control group. This binding pattern exhibited the most intense reactivity in the distal colon (Table 3). In both groups, GSAII lectin, which binds with high selectivity terminal α- and β-D-GlcNAc residues, produced a moderate staining in the colonic epi-thelium. Only in the distal colon, the GSAII labelling was increased by predigestion with fucosidase that induced a new staining in the colonocyte supranuclear cytoplasm in the control group, not in STD rats, and increased the reac-tivity at the crypt fundus in both groups, at a higher degree in the STD animals (Table 3). When fucosidase digestion was performed prior to PNA, new reactive sites, suggestive of Fuc-d-Gal-β(1–3)-d-GalNAc terminal sequences, were
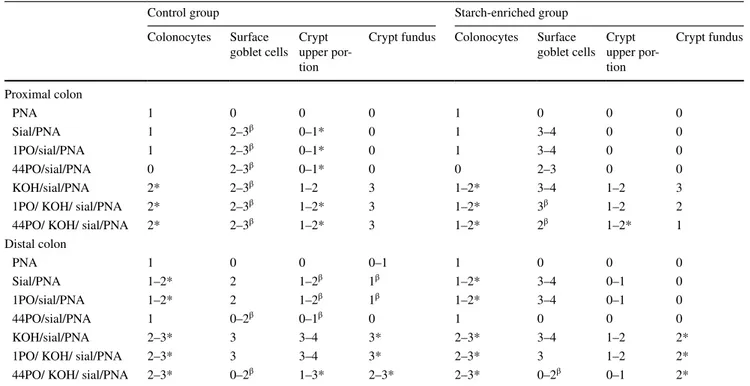
clearly identified in colonocytes and crypts of the control group. In the STD rats, a weak PNA staining was induced by fucosidase treatment only in the distal colon (Fig. 3a, b). An opposite situation was observed for fucose-specific LTA lectin which produced a more market reactivity in the colonocytes of the STD group than in the controls (Table 3; Fig. 3c, d).
The starch-enriched diet proved to affect also the expres-sion of sialylated components as well as the structural fea-tures of sialic acid residues, as deduced by the comparative evaluation of the effects of deacetylation and differential oxidation on sialidase/PNA and sialidase/DBA binding patterns (Tables 4, 5). In particular, a lowered expression of sialic acid-D-Gal-β(1,3)-d-GalNAc sequences can be
detected at the luminal cell border in the colonocytes of
Fig. 1 a, b Histological appearance of the distal colon of rats in AB pH 2.5. a Control rats show strong staining in the goblet cells of the surface epithelium and along the crypts. b STD rats show more reduced staining. Original magnification ×20
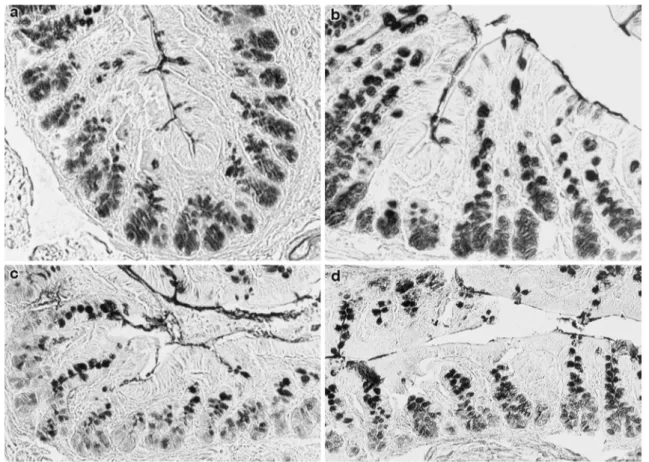
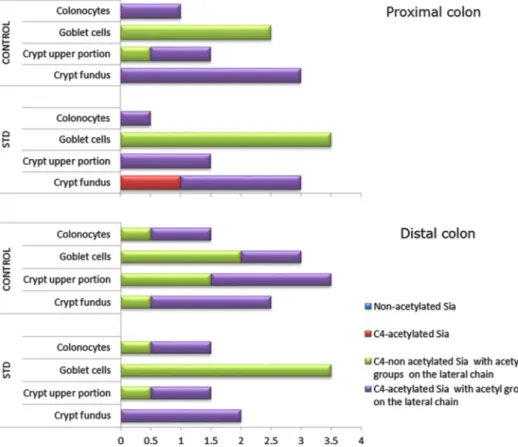
the proximal colon, and more markedly, in the crypts in the distal colon. Conversely, in the surface goblet cells of both colon portions a higher occurrence of these sialylated sequences, with sialic acids mostly 9-O-acetylated, was found in the STD rats, compared with the control group (Table 4; Fig. 4).
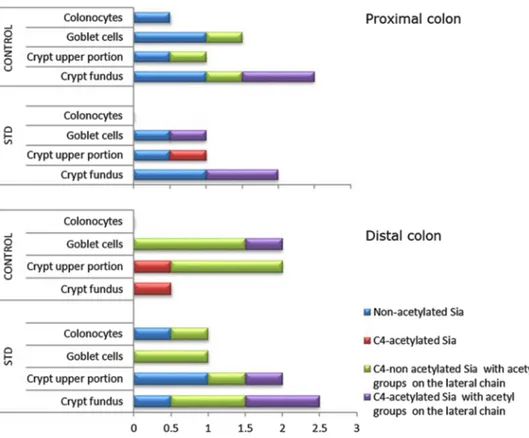
A more marked modification of the expression of sialic acid-D-GalNAc sequences as well as changes in the acety-lation degree and position of the sialic acid residues was induced by the starch-enriched diet at all the reactive sites in both the proximal and distal colon (Table 5). In particu-lar, a weak enhancement of DBA reactivity after sialidase digestion, suggestive of sialic acids linked to GalNAc resi-dues, was found in the goblet cells of the STD rats which, instead, exhibited a more marked staining of the native lec-tin at the same sites, when compared with the control ones (Fig. 5).
A summarizing view of the sialylated sequence distribu-tion in the colon of STD and control rats is presented as a schematic drawing in Figs. 6, 7.
Controls
No staining was detected in sections exposed to unconju-gated lectins or to conjuunconju-gated lectins preincubated with their appropriate inhibitory sugars (data not shown). Con-trols to check the efficacy and specificity of the enzymatic digestions were as expected.
Discussion
The question addressed by this study was whether a long-term feeding on a high starch diet can modulate the gly-cosylation pattern of the rat colonic mucosa. To this aim, a slightly modified AIN-76 rodent diet, with carbohydrates supplied by corn starch (64%), was tested for 9 months. The question arises from the knowledge that dietary factors, by providing substrates for the intestinal microbiota, influence the intestinal microenvironment and the functional effi-ciency of the mucosal barrier [27, 28]. Indeed, bacteria act
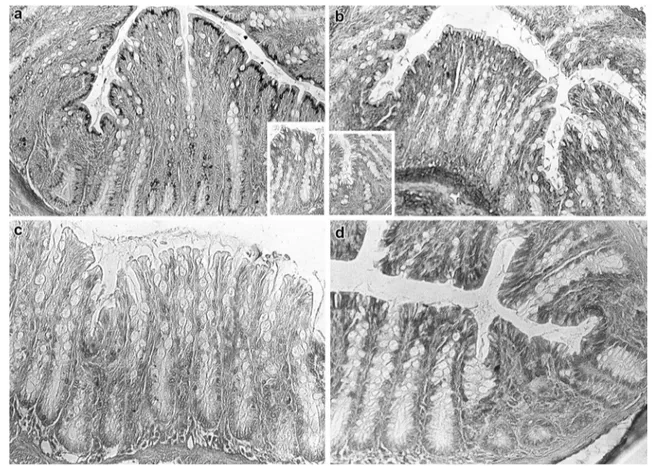
Fig. 2 a–d Histological appearance of the proximal colon of rats. The reactivity produced by LID reaction in control (a) and STD rats (b) shows the distribution of acidic groups. HID reaction, indicative of strongly acidic groups, results in a more reduced distribution of
staining in the controls (c) than in the STD animals (d), when com-pared with the relevant patterns from LID reactions (a and b, respec-tively). Original magnification ×20
not only by interacting directly or indirectly with the com-ponents of the intestinal mucus layer. They also degrade dietary fibres and/or other dietary factors that escape diges-tion to produce further agents that can influence colonic mucus secretion, thus resulting in damage or protection to the underlying mucosa. Since the classic histological stud-ies on germ-free animals, the intestinal bacteria have been recognized to play an essential role in driving the matura-tion of the colonic mucosa and normal mucus producmatura-tion [29, 30]. In addition, previous investigations on germ free and conventional rats fed different diets have proven that dietary factors and microbial flora interact in modifying the glucidic composition of intestinal mucins [3, 31]. In this study, we applied conventional histochemistry for carbohy-drates and lectin labelling to examine whether a high starch diet can affect the expression of glycoconjugates in the rat colon. We found that a starch-enriched diet modulates the glucidic profile and the structural features of glycoconju-gates in the colonic epithelium, goblet cells, and crypts.
In both the experimental groups, a regional distribu-tion of acidic glycoconjugates, distinct as sialomucins and sulfomucins, was evidenced within the colonic mucosa,
showing an increasing gradient of sulfomucins from proxi-mal to distal colon. In the STD rats, compared with the control samples, a reduced expression of acidic glycocon-jugates was observed, mainly in the distal colon. In both portions, most of these acidic glycoconjugates, located in the surface goblet cells, in the crypts, and at the epithelium luminal borders, could be identified as sulfated glycoconju-gates. Thus, in spite of an overall reduction of acidic glyco-components in the colonic secretory products, an increase in sulfation levels was observed in rats fed on the starch-enriched diet versus the control animals. The sulfation of colonic mucins has been previously related to a protective function against colitis in mice and humans [32, 33]. It has been also demonstrated that in colonic epithelial cells of mice the expression of N-acetylglucosamine 6-O-sul-fotransferase, one of the major sulfotransferases responsible for the mucin sulfation, is regulated by butyrate, a metab-olite from anaerobic bacteria in the colon [34] thus indi-cating a relationship between mucin sulfation, commensal bacteria and mucosal defence. The increase of sulfomucins in STD rats, here observed, suggests that dietary carbohy-drates might have an impact on this interaction. The results
Table 3 Lectin binding patterns, with and without fucosidase predigestion, in the rat proximal and distal colon
Results are expressed by a subjective scale ranging from 0 to 4, with 0 being unreactive and 4 being strongly reactive Fuc fucosidase
α Staining in the supranuclear cytoplasm β Staining in few cells
Control group Starch-enriched group
Colonocytes Surface
goblet cells Crypt upper portion Crypt fundus Colonocytes Surface goblet cells Crypt upper portion Crypt fundus Proximal colon GSA I-B4 1 0 0 0 1 0–1 0–1β 0 WGA 0 1–2 1–2 2 0 2 1–2 2–3 GSA II 2 0 0 0 2 0 0 0 Fuc/GSA II 2 0 0 0–1 2 0 0 0–1 PNA 1 0 0 0 1 0 0 0 Fuc/PNA 2α 0 0–1 0–1 1 0 0 0 LTA 0–1 0 0–1 0 2 0 1 0 Con A 1 0 0 0 1 0 0 0 PSA 0–1 0 0 0 1 0 0 0 Distal colon GSA I-B4 1 0 0 0 1 0 0 0 WGA 0 0–1 1–2 2 0 2–3 2 3–4 GSA II 1–2 0 0 1β 1–2 0 0 0–1β Fuc/GSA II 2α 0 0 2β 1–2 0 0 2–3 PNA 1 0 0 0–1 1 0 0 0 Fuc/PNA 3α 0 0–1 2α 2α 0 0 0–1 LTA 0 0 0 0–1 2 0 0 0–1 Con A 0–1 0 0 0 1–2 0 0 0 PSA 1 0 0 0 2 0 0 0
of a previous investigation on rats, fed on the same diet as that one here applied, support such a conclusion [23]. Indeed, variations in faecal microbiota composition were found by Cresci et al. in rats fed on the starch-enriched diet and the analysis of faecal concentrations of short-chain fatty acids pointed out higher values in the faeces of rats fed on the starch-enriched diet compared with those fed on a sucrose-enriched diet [23].
Moreover, the carbohydrate structural analysis of mouse colonic mucins indicated that sulfation was preferential on the C-6 of GlcNAc residues on the core 2 branch of O-gly-cans [34]. Based on these data, our finding that GlcNAc occurrence increases in the goblet cells in response to the starch-enriched diet, as indicated by the WGA reactivity pattern, may account for an enhanced production of highly sulfated mucins.
Also, the widely modified expression of fucosylated and sialylated sequences, that we found in the rat colon, might be related to a possible alteration of the intestinal microbiota induced by the starch rich diet, as previously reported [23]. Indeed, research has indicated that bacteria
may affect mucus production because they partially uti-lize the host-derived carbohydrates. This degradation is a stimulus for goblet cells to modulate mucus production either by direct activation of diverse signalling cascades or through bioactive factors generated by epithelial cells [35–38]. In addition, bacteria can produce specific secre-tory elements, such as lipopolysaccharides and flagelin A in the case of Gram negative bacteria and lipoteichoic acids in the case of Gram-positive bacteria, which act as modulators of mucin production [39]. Here, on the basis of the PNA and GSAII binding patterns obtained with and without fucosidase digestion, we found a reduced distribution or the absence of Fuc-d-Gal-β(1–3)-d
-GalNAc and Fuc-d-GlcNAc sequences in either
colono-cytes and crypts of the STD group, compared with the control one. Notably, the binding patterns produced by fucosidase digestion and PNA lectin showed a distinctive feature at the level of the distal colon of the rats fed on a conventional diet, where Fuc-d-Gal-β(1–3)-d-GalNAc
sequences were visualized in the colonocyte supranu-clear cytoplasm, at the Golgi level. A downregulation
Fig. 3 Histological appearance of the distal colon of rats. In control rats (a), fucosidase digestion induces a marked PNA staining in the colonocyte supranuclear cytoplasm and at the crypt fundus. In STD rats (b), weak sites of PNA reactivity are detectable only in colono-cytes, after fucosidase predigestion. The PNA binding pattern, prior
to digestion, is negative in both rat groups (insets in a, b). c Distal colon of control rats shows no LTA reactivity. d A clear, diffuse LTA staining is detectable in colonocytes of STD rats. Original magnifica-tion ×20
Table 4 PNA lectin binding patterns, with and without sialidase predigestion and chemical treatments, in the rat proximal and distal colon
Results are expressed by a subjective scale ranging from 0 to 4, with 0 being unreactive and 4 being strongly reactive SA sialic acid
*Staining at the cell luminal border
β Staining in few cells
Control group Starch-enriched group
Colonocytes Surface
goblet cells Crypt upper por-tion
Crypt fundus Colonocytes Surface
goblet cells Crypt upper por-tion Crypt fundus Proximal colon PNA 1 0 0 0 1 0 0 0 Sial/PNA 1 2–3β 0–1* 0 1 3–4 0 0 1PO/sial/PNA 1 2–3β 0–1* 0 1 3–4 0 0 44PO/sial/PNA 0 2–3β 0–1* 0 0 2–3 0 0 KOH/sial/PNA 2* 2–3β 1–2 3 1–2* 3–4 1–2 3
1PO/ KOH/ sial/PNA 2* 2–3β 1–2* 3 1–2* 3β 1–2 2
44PO/ KOH/ sial/PNA 2* 2–3β 1–2* 3 1–2* 2β 1–2* 1
Distal colon PNA 1 0 0 0–1 1 0 0 0 Sial/PNA 1–2* 2 1–2β 1β 1–2* 3–4 0–1 0 1PO/sial/PNA 1–2* 2 1–2β 1β 1–2* 3–4 0–1 0 44PO/sial/PNA 1 0–2β 0–1β 0 1 0 0 0 KOH/sial/PNA 2–3* 3 3–4 3* 2–3* 3–4 1–2 2*
1PO/ KOH/ sial/PNA 2–3* 3 3–4 3* 2–3* 3 1–2 2*
44PO/ KOH/ sial/PNA 2–3* 0–2β 1–3* 2–3* 2–3* 0–2β 0–1 2*
Table 5 DBA lectin binding patterns, with and without sialidase predigestion and chemical treatments, in the rat proximal and distal colon
Results are expressed by a subjective scale ranging from 0 to 4, with 0 being unreactive and 4 being strongly reactive SA sialic acid
*Staining at the cell luminal border
β Staining in few cells
Control group Starch-enriched group
Colonocytes Surface
goblet cells Crypt upper por-tion
Bottom crypt
cells Colonocytes Surface goblet cells Crypt upper portion Bottom crypt cells Proximal colon DBA 1 1–2 2 1–2 1–2* 2–3 3 2 Sial/ DBA 1–2* 3β 3 3 1–2* 3 3–4 3 1PO/sial/ DBA 1* 2 2–3 2 1–2* 2–3 3 2 44PO/sial/ DBA 1 2 2–3 2 1 0–1 3 2 KOH/sial/ DBA 1–2* 3 3 4 1–2* 3–4 4 4
1PO/ KOH/ sial/ DBA 1* 2 2–3 3 1–2* 3 3 3
44PO/ KOH/ sial/ DBA 1 2 2–3 3 1–2* 0–1 0–2β 3
Distal colon DBA 1 0 1–2β 0 1 2 2 0–1 Sial/ DBA 1 1–2 3 0 2* 3 3–4 2 1PO/sial/ DBA 1 1–2 3 0 1–2* 3 2–3 1–2 44PO/sial/ DBA 0–1 0–1 0–1 0 1–2* 3 2–3 1–2 KOH/sial/ DBA 1 2 3–4 0–1* 2* 3 4 3
1PO/ KOH/ sial/ DBA 1 2 3 0 1–2* 3 3 2–3
of fucosyltransferases may be suggested to explain the reduction or absence of such a reactivity in STD rats.
In contrast, a stronger reactivity in the colonocytes of rats fed on the starch-enriched diet than in colonocytes of rats fed on a conventional diet was produced by LTA, which suggests an increased expression of glycocompo-nents rich in α-l-Fuc residues at these sites. Since LTA
spe-cifically recognizes α-l-fucose bound via α(1–2) linkage to
Gal-β(1–4)GlcNAc or Fuc-α(1–3) sequences [40], the LTA binding patterns may be suggestive of an enhanced expres-sion of fucosylated sequences in STD rat colonocytes, dis-tinct from Fuc-d-Gal-β(1–3)-d-GalNAc and Fuc-d-GlcNAc
sequences in either the linkage or the type of penultimate sugar. The modulation of the fucose-specific LTA binding profile in the colonic epithelium, upon a long-term feeding on a starch-enriched diet, might be due to a regulation of the fucosylation process, a rather complex process which involves several systems controlling fucosyltransferase activity, also through endogenous protein inhibitor, or sub-strate availability [41]. Indeed, previous data indicate that intestinal fucosylation is modulated during development in response to some nutritional factors [42]. It has also been
reported that fucose, in the gastrointestinal lumen, can affect the expression of microbial metabolic pathways and reduce the expression of bacterial virulence genes. In par-ticular, during pathogen-induced stress, rapid intestine epi-thelial cell fucosylation appears to be a protective mecha-nism that utilizes the host’s resources to maintain host–gut microbiota interactions [43].
A contribution to potentiate the protective function of the intestinal mucosa has been largely attributed to sialyla-tion of the mucus and epithelial cells, too. In our study, the starch-enriched diet proved to affect differently the expres-sion of sialylated sequences, as visualized by combining the PNA and DBA lectins with neuraminidase predigestion. A distinctive feature found in the STD rats, compared with control ones, concerns an increased expression of Sia-d
-Gal-ß(1–3)-d-GalNAc sequences in the goblet cells of both
the proximal and distal colon coupled to a parallel decrease in the content of Sia-d-GalNAc sequences. This finding
suggests a compensatory (“balance”) mechanism in the sialic acid modulation induced by the starch-enriched diet in the goblet cell secretory products. In turn, the decrease in Sia-d-GalNAc expression in the goblet cells within the
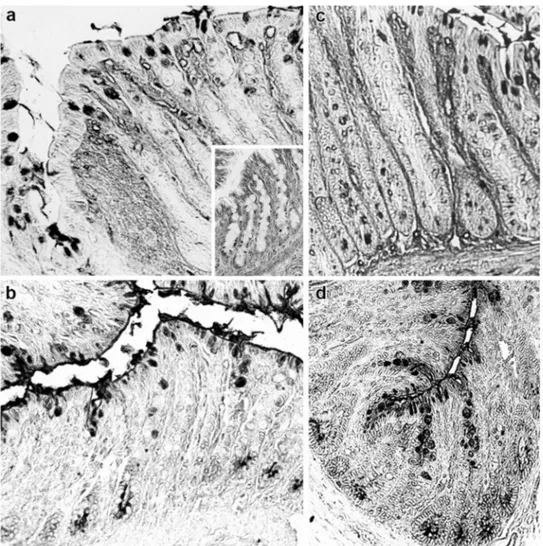
Fig. 4 Histological appear-ance of the distal colon of rats. Sialidase digestion induces new PNA reactivity in surface goblet cells in the distal colon of STD rats (a) when compared with PNA binding pattern at the same sites (inset in a). The staining intensity increases further after KOH pretreatment (b). In the distal colon of con-trol rats, sial/PNA (c) and KOH-Sial/PNA (d) result in a lower increase of the lectin reactivity. Original magnification ×20
Fig. 5 Histological appearance of the distal colon of rats. DBA labe-ling is more marked in surface goblet cells and crypts of STD rats (a) with respect to the control ones (b). Such a reactivity is weakly increased by sialidase digestion (inset in a) that, instead, strongly
enhances the lectin staining in the control rats (inset in b). c, d Dis-tal colon of STD rats. Deacetylation and sialidase digestion increase DBA reactivity mainly in the crypts (c). The staining partly resists after strong oxidation (d). Original magnification ×20
Fig. 6 Distribution and charac-terization of Sia-d -Gal-β(1,3)-d-GalNAc sequences in rat
proximal and distal colon. The scores in the X axis have been derived from the comparative evaluation of the results from the PNA binding patterns (see Table 4)
colonic mucosa of STD rats proved to be in parallel with a higher content in d-GalNAc terminal residues, with respect
to the controls. A possible explanation is that a long-term high-starch feeding in rats could modify the glycoconjugate profile through either endogenous or exogenous signals affecting the sialylation pathways.
The differences in the degree and position of acetylated substituents in sialic acids linked to preterminal d-GalNAc
residues, between the two dietary groups, support the hypothesis of an involvement of sialyltransferases and/or
O-acetyltransferases in contributing to such a modulation.
The previously documented changes in the faecal micro-biota and faecal concentration of SCFAs in rats fed on a starch-enriched diet [23] could indicate an altered intesti-nal microbiota as a possible candidate for the induction of signals and mechanisms that result in the observed changes of the colonic sialylation profile. A role of the intestinal microbiota can be also suspected when considering our failure to identify Sia-d-GalNAc sugars at the luminal
cell borders of the proximal colon epithelium in rats on a starch-enriched diet, in contrast to control rats. This find-ing, taken together with the increased brush border mem-brane staining with DBA, specific for d-GalNAc terminal
residues, suggests that also the activity of microbial siali-dases might be involved in reducing the Sia-d-GalNAc
sequence expression at these sites.
In conclusion, application of lectin histochemistry, combined with enzyme digestion, deacetylation and
differential oxidation, allowed a comprehensive inves-tigation of the effects of a starch-enriched diet on the tissue-specific glucidic profile in the rat colon. The large spectrum of modifications detected, with respect to the control animals, demonstrates the critical contribution of dietary factors to intestinal glycosylation in rats, with a possible impact on the protective function of the intesti-nal mucosa.
Acknowledgements Funding was provided by University of Cam-erino (Grant no: FAR 2014-15).
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
1. Lee YK, Puong KY (2002) Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br J Nutr 88:S101–S108
2. Guglielmetti S, Tamagnini I, Minuzzo M, Arioli S, Parini C, Comelli E, Mora D (2009) Study of the adhesion of Bifidobac-terium bifidum MIMBb75 to human intestinal cell lines. Curr Microbiol 59:167–172. doi:10.1007/s00284-009-9415
3. Sharma R, Schumacher U (1995) The influence of diets and gut microflora on lectin binding patterns of intestinal mucins in rats. Lab Invest 73:558–564
Fig. 7 Distribution and char-acterization of Sia-D-GalNAc sequences in rat proximal and distal colon. The scores in the X axis have been obtained from the comparative analysis of the results from the DBA binding patterns (see Table 5)
4. Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Micro-biol 10:323–335. doi:10.1038/nrmicro2746
5. Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA (2008) Mucins in the mucosal barrier to infection. Mucosal Immunol 1:183–197. doi:10.1038/mi.2008.5
6. Yang Y, Jobin C (2014) Microbial imbalance and intestinal pathologies: connections and contributions. Dis Model Mech 7:1131–1142. doi:10.1242/dmm.016428
7. Campbell BJ, Hounsell E, Finnie IA, Rhodes JM (1995) Direct demonstration of increased expression of Thomsen–Friedenreich antigen (Galβ1-3GalNAc) by mucus in colon cancer and inflam-matory bowel disease. J Clin Invest 95:571–576
8. de Albuquerque Garcia Redondo P, Nakamura CV, de Souza W, Morgado-Díaz JA (2004) Differential expression of Sialic Acid and N-acetylgalactosamine residues on the cell surface of intesti-nal epithelial cells according to normal or metastatic potential. J Histochem Cytochem 52:629–640
9. An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L (2007) Increased susceptibility to colitis and colorec-tal tumors in mice lacking core 3-derived O-glycans. J Exp Med 204:1417–1429
10. Saeland E, Belo AI, Mongera S, van Die I, Meijer GA, van Kooyk Y (2012) Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients. Int J Cancer 131:117–128. doi:10.1002/ijc.26354 11. Park JJ, Lee M (2013) Increasing the α2,6 sialylation of gly-coproteins may contribute to metastatic spread and thera-peutic resistance in colorectal cancer. Gut Liver 7:629–641. doi:10.5009/gnl.2013.7.6.629
12. Kudo T, Ikehara Y, Togayachi A, Morozumi K, Watanabe M, Nakamura M, Nishihara S, Narimatsu H (1998) Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Invest 78:797–811
13. Caderni G, Dolara P, Spagnesi T, Luceri C, Bianchini F, Mas-trandrea V, Morozzi G (1993) Rats fed high starch diets have lower colonic proliferation and fecal bile acids than high sucrose-fed controls. J Nutr 123:704–712
14. Brunsgaard G (1998) Effects of cereal type and feed particle size on morphological characteristics, epithelial cell proliferation, and lectin binding patterns in the large intestine of pigs. J Anim Sci 76:2787–2798
15. Hedemann MS, Theil PK, Bach Knudsen KE (2009) The thick-ness of the intestinal mucous layer in the colon of rats fed vari-ous sources of non-digestible carbohydrates is positively corre-lated with the pool of SCFA but negatively correcorre-lated with the proportion of butyric acid in digesta. Br J Nutr 102:117–125. doi:10.1017/S0007114508143549
16. Mao J, Hu X, Xiao Y, Yang C, Ding Y, Hou N, Wang J, Cheng H, Zhang X (2013) Overnutrition stimulates intestinal epithe-lium proliferation through β-catenin signaling in obese mice. Diabetes 62:3736–3746. doi:10.2337/db13-0035
17. Zhu QC, Gao RY, Wu W, Guo BM, Peng JY, Qin HL (2014) Effect of a high-fat diet in development of colonic adenoma in an animal model. World J Gastroenterol 20:8119–8129. doi:10.3748/wjg.v20.i25.8119
18. Key TJ, Spencer EA (2007) Carbohydrates and cancer: an overview of the epidemiological evidence. Eur J Clin Nutr 61/1:S112–S121
19. Le Leu RK, Hu Y, Brown IL, Young GP (2009) Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr Metab 6:11. doi:10.1186/1743-7075-6-11
20. Wang Z, Uchida K, Ohnaka K, Morita M, Toyomura K, Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Maekawa T, Yasunami Y, Takenaka K, Ichimiya
H, Terasaka R (2014) Sugars, sucrose and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol 49:581–588. doi:10.3109/00365521.2013.822091
21. Roncal-Jimeneza CA, Lanaspaa MA, Rivarda CJ, Nakagawa T, Sanchez-Lozada LG, Jalala D, Andres-Hernando A, Tanabe K, Maderoc M, Lia N, Cicerchia C, Mc Fanna K, Sautin YY, Rich-ard J (2011) Sucrose induces fatty liver and pancreatic inflam-mation in male breeder rats independent of excess energy intake. Metabolism 60:1259–1270. doi:10.1016/j.metabol.2011.01.008 22. Hobden MR, Guérin-Deremaux L, Rowland I, Gibson GR,
Ken-nedy OB (2015) Potential anti-obesogenic properties of non-digestible carbohydrates: specific focus on resistant dextrin. Proc Nutr Soc 74:258–267. doi:10.1017/S0029665115000087 23. Cresci A, Orpianesi C, Silvi S, Mastrandea V, Dolara P (1999)
The effect of sucrose or starch-based diet on short-chain fatty acids and faecal microflora in rats. J Appl Microbiol 86:245–250 24. Schauer R, Srinivasan GV, Wipfler D, Kniep B, Schwartz-Albiez R (2011) O-Acetylated sialic acids and their role in immune defense. Adv Exp Med Biol 705:525–548. doi:10.1007/978-1-4419-7877-6_28
25. Spicer SS (1965) Diamine methods for differentiating mucosub-stances histochemically. J Histochem Cytochem 13:211–234 26. Accili D, Menghi G, Gabrielli MG (2008) Lectin histochemistry
for in situ profiling of rat colon sialoglycoconjugates. Histol His-topathol 23:863–875
27. Kim YS, Ho SB (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroen-terol Rep 12:319–330. doi:10.1007/s11894-010-0131-2
28. Öhman L, Törnblom H, Simrén M (2015) Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol 12:36–49. doi:10.1038/ nrgastro.2014.200
29. Hill RRH, Cowley HM, Andremont A (1990) Influence of colo-nizing micro-flora on the mucin histochemistry of the neonatal mouse colon. Histochem J 22:102–105
30. Enss ML, Grosse-Siestrup H, Schmidt-Wittig U, Gärtner K (1992) Changes in colonic mucins of germfree rats in response to the introduction of a”normal” rat microbial flora. Rat colonic mucin. J Exp Anim Sci 35:110–119
31. Freitas M, Axelsson L, Cayuela C, Midtvedt T, Trugnan G (2002) Microbial–host interactions specifically control the glyco-sylation pattern in intestinal mouse mucosa. Histochem Cell Biol 118:149–161. doi:10.1007/s00418-002-0432-0
32. Raouf AH, Tsai HH, Parker N, Hoffman J, Walker RJ, Rhodes JM (1992) Sulphation of colonic and rectal mucin in inflamma-tory bowel disease: reduced sulphation of rectal mucus in ulcera-tive colitis. Clin Sci (Lond) 83:623–626
33. Boltin D, Perets TT, Vilkin A, Niv Y (2013) Mucin function in inflammatory bowel disease: an update. J Clin Gastroenterol 47:106–111. doi:10.1097/MCG.0b013e3182688e73
34. Tobisawa Y, Imai Y, Fukuda M, Kawashima H (2010) Sul-fation of colonic mucins by N-Acetylglucosamine 6-O-sul-fotransferase-2 and its protective function in experimental colitis in mice. J Biol Chem 285:6750–6760. doi:10.1074/jbc. M109.067082
35. Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM (2008) The mucin degrader Akkermansia muciniphila is an abundant resident of human intestinal tract. Appl Environ Micro-biol 74:1646–1648
36. Turroni F, Bottacini F, Foroni E, Mulder I, Kim JH, Zomer A et al (2010) Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived gly-can foraging. Proc Natl Acad Sci USA 107:19514–19519. doi:10.1073/pnas.1011100107
37. Turroni F, Milani C, van Sinderen D, Ventura M (2011) Genetic strategies for mucin metabolism in Bifidobacterium bifidum
PRL2010: an example of possible human–microbe co-evolution. Gut Microbes 2:183–189
38. Xu X, Xu P, Ma C, Tang J, Zhang X (2013) Gut microbiota, host health, and polysaccharides. Biotechnol Adv 31:318–337. doi:10.1016/j.biotechadv.2012.12.009
39. Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K (2009) Role of intestinal mucins in innate host defense mechanisms against pathogens. J Innate Immun 1:123–135. doi:10.1159/000163037
40. Debray H, Montreuil J (1989) Aleuria aurantia agglutinin. A new isolation procedure and further study of its specificity towards various glycopeptides and oligosaccharides. Carbohydr Res 185:15–26
41. Martin A, Ruggiero-Lopez D, Biol MC, Louisot P (1990) Evi-dence for the presence of an endogenous cytosolic protein inhibi-tor of intestinal fucosyltransferase activities. Biochem Biophys Res Commun 166:1024–1031
42. Biol-N’garagba MC, Louisot P (2003) Regulation of the intes-tinal glycoprotein glycosylation during postnatal development: role of hormonal and nutritional factors. Biochimie 85:331–352 43. Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D,
Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turn-baugh PJ, Chervonsky AV (2014) Rapid fucosylation of intesti-nal epithelium sustains host-commensal symbiosis in sickness. Nature 514:638–641. doi:10.1038/nature13823