1
Master Universitario di II livello
PERCUTANEOUS INTERVENTIONAL TREATMENT OF STRUCTURAL HEART DISESAES
Anno Accademico
2017/2018
SAFE COMBINED APPROACH OF
PERCUTANEOUS LAA CLOSURE
PLUS MITRAL/AORTIC
VALVULOPLASTY: SINGLE
CENTER EXPERIENCE
Autore
Dr. Maria Benedetta Giannico
Tutor Scientifico
Prof. Claudio Passino
Tutor Aziendale
2
Tables of contents
1. Introduction: the burden of the problem Page 3 1.1 Transcatheter LAA occlusion: indication and treatment approaches Page 7 1.2 Mitral stenosis: prevalence, diagnosis and treatment Page 13 1.3 Aortic stenosis: prevalence, diagnosis and treatment Page 17 1.4 Cerebrovascular protection Page 18 1.5 LAmbre device Page 19 2. Cases Presentation
2.1 Case 1 Page 21 2.2. Case 2 Page 25 2.3 Case 3 Page 28 2.4 Index of figures and tables Page 31 3. Conclusions Page 32 4. References Page 34
Abbreviations and Acronyms LAA: Left Atrial Appendage OAC: Oral Anticoagulant NOAC: Novel Oral Anticoagulant VHD: Valvular Heart Disease
ETE: TransEsophageal Echocardiography AF: Atrial Fibrillation
TAVI: Transcatheter Aortic Valve Implantation PMV: Percutaneous mitral valvuloplasty
3
1. Introduction
The burden of the problem
Atrial fibrillation is an extremely common condition with a prevalence of approximately 0.4% and a strong association with age and male sex. The age-adjusted incidence approaches 5% per year in patients greater than 80 years of age, and prevalence in this population is approximately 9%. The lifetime risk of developing atrial fibrillation is approximately 1 in 4.
Mechanistically, atrial fibrillation is characterized by uncoordinated and uncontrolled activation of atrial tissue. Although numerous classification schemes exist, the most commonly employed classification system refers to timing and duration of the arrhythmia ( Figure 1). Paroxysmal atrial fibrillation refers to episodes lasting usually less than 48 hours, although individual paroxysms may last up to 7 days. Persistent atrial fibrillation either lasts longer than 7 days or requires electrical/chemical cardioversion to restore sinus rhythm. Atrial fibrillation lasting greater than one year is either considered long-standing persistent if a rhythm control strategy is pursued or permanent if no further rhythm control methods are planned.
Fig 1
Treatment strategies for atrial fibrillation focuse on two concepts: management of symptoms and prevention of complications. In terms of symptoms, management can either focus on rate or rhythm
4
control. Rate control strategies include beta-blockers, calcium channel blockers, digoxin, and in refractory cases, atrioventricular node ablation with pacemaker implantation. Rhythm control strategies include medical therapies such as sotolol, dofetilide, amiodarone, dronedarone, electrical cardioversion or pulmonary vein isolation.
Regardless of the treatment strategy for rate or rhythm control, treatment efforts must also focus on prevention of thromboembolic events. Atrial fibrillation increases the risk of cerebral ischemic events 5-fold across the entire spectrum of age, and the risk worsens with advancing age, so that it accounts for approximately 1.5% of events in patients under age 50 to greater than 20% of events in patients greater than age 80. (1) Regardless of whether it is paroxysmal or permanent, the annual rate of cerebral ischemic events in patients with atrial fibrillation approaches 4.5% per year.(2) In fact, even subclinical atrial fibrillation lasting 6 minutes or longer is associated with an increased risk of ischemic events.(3)
It is very important to make attempts at individualizing the risk for a particular patient. A number of risk models have been proposed. The first is the CHADS2 score.(4) In this model, a patient is assessed according to 5 established risk factors including age >75, history of hypertension, history of congestive heart failure, diabetes, and prior embolic event. Each risk factor is worth one point with the exception of a prior embolic event, which is worth two points. The resultant score ranges from 0-6 and corresponds to a significant increase in risk for each incremental score. The absolute risk varies by series. In one study of 1733 patients with atrial fibrillation not on warfarin, the annualized risk of cerebral ischemic events ranged from 1.9% for a CHADS2 score of 0 to 18.2% for a CHADS2 score of 6.(4) In another, larger series of 11,526 patients with atrial fibrillation not on warfarin, the annualized risk of events ranged from 0.49% for a CHADS2 score of 0 to 6.9% for a CHADS2 score of 6.
It is uncertain whether this score adequately stratifies low risk patients. This point is highlighted by the fact that even low risk patients (those with CHADS2 scores of 1) can benefit from oral anticoagulation with warfarin or the non-vitamin K oral anticoagulant medications. Furthermore, greater emphasis on certain risk factors and the addition of other known risk factors for cerebral ischemic events may further refine risk stratification. The CHA2DS2VASc score is a 9-point scale incorporating the factors in the CHADS2 system (congestive heart failure, hypertension, age >75, diabetes and prior stroke x2) placing increased emphasis on age (65-74 merits 1 point while 75 merits 2 points). Additionally, it adds as risk factors vascular disease and female gender. Based on an analysis of 1084 patients Lip et al. validated this risk model and demonstrated incremental risk of embolic events with a rising score (5)
It is also important to balance the risk of cerebral ischemic events against the risk of bleeding. Numerous algorithms have been proposed, but the most commonly utilized is the HAS-BLED score
5
incorporating hypertension, abnormal liver/kidney function, prior stroke, prior bleeding, labile INRs, elderly and drug/alcohol abuse on a 9 point scale.[6] Scores of ≥ 3 are associated with increased bleeding risk, but it is important to note that often, similar factors (such as age and hypertension) increase both the bleeding risk and the risk of cerebral ischemic events, so that both the 2012 updated European guidelines and the 2014 ACC/AHA/HRS guidelines recommend thoughtful anticoagulation with close monitoring in patients at elevated risk for bleeding (7)
Multiple anti-coagulation strategies to prevent cerebral ischemic events have been studied, including aspirin, clopidogrel, warfarin and the target specific anticoagulant medications: dabigatran, rivaroxaban, apixaban and edoxaban. In 1999, Hart et al. published a meta-analysis of studies comparing aspirin to placebo, warfarin to placebo and aspirin to warfarin in patients with atrial fibrillation.[8] Aspirin resulted in a relative risk reduction of 22% and an absolute risk reduction of 1.5% per year respectively for primary prevention and 2.5% per year for secondary prevention versus placebo; warfarin resulted in a relative risk reduction of 62% with absolute reductions of 2.7% per year for primary prevention and 8.4% per year for secondary prevention versus placebo. Finally, warfarin resulted in a 34% relative risk reduction, proving superior to aspirin in the reduction of cerebral events; however, it should be noted that the risk of both intracranial and extracranial hemorrhage was higher with warfarin compared to aspirin.[8]
Clopidogrel has also been studied in patients with atrial fibrillation. The Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) trials evaluated the efficacy and safety of dual antiplatelet therapy in patients with atrial fibrillation. The ACTIVE W was stopped early, as therapy with dual antiplatelet therapy was associated with a significantly higher event rate (relative risk 1.44, 95% CI 1.18-1.76, p=0.003) compared to warfarin alone.[9]
In the ACTIVE A trial, 7554 patients with atrial fibrillation at increased risk of stroke who were considered unsuitable candidates for warfarin therapy were randomized to aspirin plus clopidogrel versus aspirin alone. Over a mean of 3.6 years of follow-up, patients treated with dual antiplatelet therapy had a significantly lower risk of the composite primary endpoint consisting of cerebral event, non-cerebral embolic event, myocardial infarction or vascular death (relative risk 0.89, 95% CI 0.81-0.98, p=0.01) at the cost of an increased risk of bleeding (relative risk 1.57, 95% CI 1.29-1.92, p<0.01).[10] As a result of these and other studies, dual antiplatelet therapy has not gained widespread acceptance for thromboembolic prevention in patients with atrial fibrillation except for patients in whom warfarin is contraindicated.
Although long-term anti-coagulation with warfarin is efficacious, numerous drawbacks exist. Warfarin requires frequent blood tests, has numerous food and drug interactions exist, and finally, in patients at risk for falling, anti-coagulation itself may incur more hemorragic risk. Additionally, up to 40% of patients with atrial fibrillation have contraindications to anticoagulation therapy.[11]
6
The non-vitamin K oral anticoagulants, dabigatran, rivaroxaban and apixaban have gained considerable interest for thromboembolic event prevention. The first such agent was dabigatran, studied in the Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) Trial evaluating 18,113 patients with atrial fibrillation and an increased risk of stroke (mean CHADS2 score of 2). Patients were treated with warfarin or dabigatran (110 or 150mg twice daily) with a primary endpoint of cerebral or systemic embolic events in a non-inferiority design. Both doses of dabigatran proved non-inferior to warfarin; however, dabigatran at 150 mg twice daily proved superior to warfarin with regard to both the primary endpoint (relative risk 0.66, 95% CI 0.53-0.82, p<0.001) as well net clinical benefit, including the primary endpoint, bleeding and death (relative risk 0.91, 95% CI 0.82-1.00, p=0.04).[12]
Rivaroxaban was studied in the 14,226 patient Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for the Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Patients were randomized to either warfarin targeted to an INR of 2.0-3.0 or rivaroxaban 20 mg daily (15 mg daily for creatinine clearance 30-49 ml/min) with a primary composite endpoint of stroke and systemic embolism. In the primary, as treated analysis, there were similar rates of stroke and systemic embolism (1.7%/year rivaroxaban vs. 2.2%/year warfarin, HR 0.79, 95% CI 0.66-0.96, p<0.001 for non-inferiority). There were similar rates of bleeding between the two groups, but intracranial (0.5% vs. 0.7%, p=0.02) and fatal bleeding (0.2% vs. 0.5%, p=0.0.03) were both significantly lower in the rivaroxaban treated patients.[13]
The Apixaban for Reduction of Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial evaluated 18,201 patients with atrial fibrillation and at least one risk factor for cerebral embolic events to warfarin targeted to INR 2.0-3.0 or apixaban 5 mg twice daily with a primary endpoint of stroke or ischemic embolism over a median 1.8 year follow-up. In this study, the primary endpoint occurred in 1.27%/yr. in the apixaban group compared to 1.6%/year in the warfarin group (HR 0.79, 95% CI 0.66-0.95, p<0.001 for non-inferiority, p=0.01 for superiority). Apixaban was also associated with reductions in major bleeding (2.1%/year apixaban vs. 3.1 %/year warfarin, p<0.001) and all-cause mortality (3.5% apixaban vs. 3.9% warfarin, p=0.047).[14]
The Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) randomized 21,105 patients with nonvalvular atrial fibrillation to warfarin (n=7036) or edoxaban at either 60 mg (n=7035) or 30 mg (n=7034) with a primary efficacy endpoint of stroke or systemic embolic event and a primary safety endpoint of major bleeding. In this noninferiority designed trial, stroke or systemic embolism occurred in 1.50% patients/year with warfarin versus 1.18% patients/year with edoxaban 60 mg (HR 0.79; 97.5% CI 0.63-0.99; p<0.001 for noninferiority) and 1.61% patients/year with edoxaban 30 mg (HR1.07; 97.5% CI, 0.87-1.31; p=0.005 for noninferiority). Bleeding events occurred at a rate
7
of 3.43% patients/year with warfarin versus 2.75% patients/year with edoxaban 60 mg (HR 0.80; 95% CI, 0.71 to 0.91; p<0.001) and 1.61% patients/year with Edoxaban 30 mg (HR 0.47; 95% CI, 0.41 to 0.55; P<0.001). Additionally, non-cardiovascular death, a prespecified secondary endpoint, occurred in 3.17% patients/year with warfarin versus 2.74% with edoxaban 60 mg (HR 0.86; 95% CI, 0.77 to 0.97; p=0.01), and 2.71% with edoxaban 30 mg (HR 0.85; 95% CI, 0.76 to 0.96; p=0.008).[15]
These agents, since their introduction in clinical practise, are gaining good post-market experience and utility in more general populations with multiple comorbidities..
In conclusion, while for patients with CHA2DS2VASc scores of 0, either aspirin or no therapy is indicated, scores of 1 or greater warrant oral anticoagulation.
When thromboembolic events occur in patients with atrial fibrillation, the majority of thrombi originate in the left atrial appendage (LAA). More than 90% of all thrombi in patients with non-rheumatic atrial fibrillation originate in the LAA.[16] In view of the numerous issues with anti-coagulation therapy and the relative simplicity of this anatomical target, exclusion of the LAA, either by surgical or percutaneous means, has garnered interest in recent years.
The great majority of thromboembolic events in patients with atrial fibrillation arise from thrombus in the left atrial appendage. Occlusion of the left atrial appendage may significantly reduce the risk of events, particularly in patients in whom anti-coagulation is contraindicated. Surgical means of left atrial appendage exclusion have been evaluated with mixed results, particularly owing to incomplete closure and residual flow into and out of the appendage. Percutaneous techniques are emerging as an effective means of LAA occlusion. Currently approved devices for LAA occlusion include Watchman, Amplatzer Amulet, Wavecrest, Cardia and LAmbre.
1.1 Transcatheter Laa Occlusion: Indication And Treatment Approaches
The LAA is an embryonic remnant of the left atrium. This structure is located anterolaterally in the atrioventricular groove and is lined with trabeculated pectinate muscles. There are wide variations in its shape, number of lobes, length, volume and orifice diameter.[17]
With regard to procedural guidance, transesophageal echocardiography (TEE) and fluoroscopy remain the primary modalities for assessment of anatomic variation, device positioning, assessment of successful deployment and monitoring of complications.
Surgical exclusion of the LAA dates back to the 1940’s. In recent years, the procedure has become increasingly common when patients with atrial fibrillation undergo otherwise indicated cardiac surgery. Despite common practice, there exist little published data to support this practice. The only randomized trial to date is the Left Atrial Appendage Occlusion Study (LAAOS) published in 2005.
8
In this trial, 77 patients with atrial fibrillation and risk factors for cerebral events undergoing coronary artery bypass surgery were randomized 2:1 to LAA exclusion or no treatment. Of the 52 patients randomized to exclusion, 44 underwent repeat TEE. Only 5/11 (45%) of patients who had the LAA closed with sutures had successful closure on follow-up TEE, while 24/33 (72%) of patients who underwent combined suture and staple closure had complete closure.[18] The study was not powered to assess events, but the results suggest suboptimal closure rates despite the employment of multiple techniques.
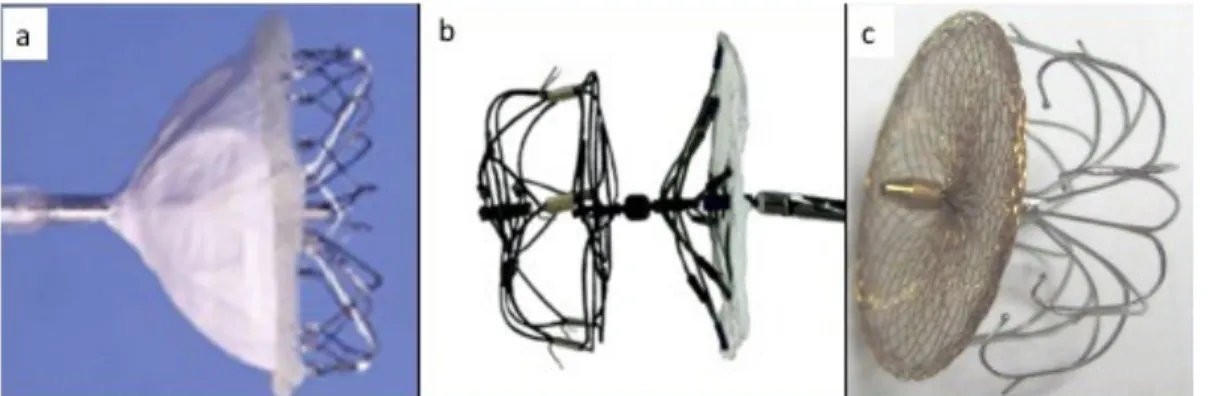
Percutaneous means to occlude the LAA are intuitively attractive as they may be equally (or more) effective with substantially less physiologic insult to the patient. A number of devices have thus far been developed for occlusion of the left atrial appendage. Initial devices included the Percutaneous Left Atrial Appendage Occluder (PLAATO, eV3, Inc., Plymouth, MA, USA) and the Watchman Left Atrial Appendage System (Boston Scientific Corporation, Marlborough MA, USA). Additionally, Amplatzer ASD and VSD devices (St. Jude Medical, St. Paul, MN, USA), although originally not intended to occlude the left atrial appendage, have been utilized to occlude the LAA. The Amplatzer Amulet left atrial appendage occluder is an iterative design of the ACP, specifically developed for atrial appendage occlusion, and it has recently become available in a number of countries. Additionally, the WaveCrest (Coherex Medical, Salt Lake City, UT, USA), UltraSept (Cardia Inc, Eagan, MN, USA) and LAmbre (LifeTech Shenzen, China) devices are three newer devices with CE mark approval. Finally, the Lariat device (SentreHEART, Redwood City, CA) provides an extracardiac means to ligate/occlude the left atrial appendage.
The LAA should be evaluated by TEE for existing thrombus. If thrombus does exist, the procedure should be postponed, and oral anticoagulation should continue until the thrombus resolves. Once the appendage is found to be free of thrombus, transseptal puncture is necessary to gain access to the left atrium. The location of the transseptal puncture is important to ensure coaxial alignment. Once transeptal access is gained, a sheath is placed across the septum and a pigtail catheter is inserted into the LAA. This technique minimizes trauma to the LAA on engagement. Multiple fluoroscopic and echocardiographic measurements are made to accurately define the diameter, angle and depth and specific anatomy of the atrial appendage. After the appropriate size is determined, the device is positioned in the LAA, and the delivery sheath is retracted, thus deploying the device. Upon determination of successful deployment, the delivery sheath is detached from the device. At this point, the sheath is withdrawn into the right atrium.
The PLAATO device, although no longer in production, was the first successfully implanted LAA occlusion device in humans. The device consisted of a self-expandable nitinol cage 18 to 32 mm in diameter and was coated with expanded polytetrafluoroethylene.
9
The PLAATO system was successfully implanted in 97.3% of patients in a nonrandomized multicenter trial in 111 patients with non-rheumatic atrial fibrillation of at least 3 months duration who had contraindications to therapy with warfarin. Four patients developed pericardial effusions or cardiac tamponade, and pericardiocentesis was necessary in three of them. One patient experienced a hemothorax and another a pleural effusion. At 6 months follow-up, successful LAA occlusion was demonstrated in 98% of the patients with a transesophageal echocardiogram. One patient developed a laminar thrombus on the device, detected at routine 6-month follow-up. During a follow-up period of 91 patient-years, two patients had a stroke, leading to an annual stroke rate of 2.2% after successful left atrial appendage occlusion. This represents a 65% relative risk reduction compared to a CHADS2 score predicted stroke rate of 6.3% in this patient cohort.[19-20]
Fig 2 PLAATO device
The Watchman implant consists of a self-expanding Nitinol frame covered by a 160 µm polyester membrane on its left atrial side. Fixation barbs around the mid-perimeter secure the occluder to the wall of the left atrial appendage. The device is available in diameters from 21– 33 mm. Minimum LAA length is 19 mm as each device is of similar length
Measurement of the LAA width enables accurate device sizing, where the goal is to achieve device compression of 8-20% at the inferior ridge. As a result, LAA width can range from 17 mm (20% compression of a 21 mm device, to 31 mm (8% compression of a 33 mm device).
In 2007, Sick et al reported the initial experience of 75 patients.[21] in which was successfully implanted 88% of patients with 93% complete sealing of the LAA at 45 days.
In the PROTECT AF (Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority) trial, the device was successfully implanted in 88% of patients, and at 45 days 86% of these patients were able to discontinue warfarin.[22], the primary endpoint (cerebral events, cardiovascular death and embolic event) was noninferior (2.3% vs. 3.8%, relative risk 0.60, 95% CI 0.41-1.05). Interestingly, all-cause
10
mortality (3.2% vs. 4.8%, relative risk 0.66, 95% CI 0.45-0.98, p = 0.0379) and cardiovascular mortality (1.0% vs. 2.4%; relative risk 0.40, 95% CI 0.23-0.82, p= 0.0045) were lower in Watchman treated patients.[23]
The Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial randomized 407 patients with nonvalvular atrial fibrillation and a mean CHADS2score of 2.6±1.0 to either LAA occlusion with the Watchman device (n=269) or medical therapy (n=138) did not meet the prespecified margin to satisfy noninferiority for primary combined endpoint of stroke, systemic embolism and cardiac/unexplained death.[24]. The second noninferiority endpoint of stroke and system embolism >7 days post randomization was satisfied. The rates of pericardial effusion requiring surgical repair decreased in this trial to 0.4% (compared to 1.6% in PROTECT AF). These findings led to the general conclusion that LAA occlusion is both safe and effective.[24]
A meta-analysis of PROTECT AF and PREVAIL patients over a median 3.1 years demonstrated that apart from the first week[25] overall bleeding rates were significantly less in the Watchman patients (1.8 vs. 3.6 events per 100 patient years, RR 0.49, 95% CI 0.32 – 0.75, p=0.001) with an even more significant difference noted after 6 months. This study demonstrates that the initial procedural risk is more than balanced by long term benefit with regard to bleeding.
Fig 3 Watchmann device
The Amplatzer Cardiac Plug is a device developed specifically for atrial appendage occlusion. It consists of a distal lobe with a proximal disk connected by a flexible central waist. Procedurally, the distal lobe is positioned within the left atrial appendage and the proximal disc is angled to fully cover the orifice of the left atrial appendage. Six pairs of hooks are attached to the distal body enabling the occluder to engage the wall of the left atrial appendage. The device is fully repositionable and recapturable. Device sizing is with reference to the distal lobe and ranges from 16-30 mm in 2 mm increments. The proximal disks are 4 mm larger for lobe sizes 16-22 mm and 6 mm larger for lobe sizes 24-30 mm. Appropriate sizing is 10-20% larger than the minimum appendage orifice measurement by TEE.
11
Recently, Tzikas et al reported experience with the first generation Amplatzer cardiac plug in 22 centers involving 1,047 patients. Procedure success rate was high at 97%, and overall periprocedural complications were low at 4.3% (death, tamponade, stroke, bleeding, myocardial infarction, and device failure). On follow up, the observed stroke rate was 2.3% versus an expected 5.62% based on CHADS2VASc score risk stratification with an overall reduction of 59%.[26]
The Amulet was enriched with added 31 and 34 mm sizes that allows for closure of a wider range of LAA diameters (11- 31 mm), a longer waist and greater number of fixation wires to enhance stability and a recessed to pin to minimize thrombus formation. CE approval was obtained in early 2013. Procedural success was 96% with no procedural complications and complete closure in all patients in first in man study. One patient experienced device related thrombus.[27] More recently, a head-to-head comparison of the Amulet and ACP devices was performed in 59 patients (31 ACP, 28 Amulet). There were no differences in procedural success or device related complications, but less patients in the Amulet group had any form of leak on follow-up TEE (8% Amulet vs. 48% ACP, p=0.01). Currently, enrollment is ongoing for a randomized trial comparing Amulet vs. Watchman devices in 1600 patients in 150 centers globally with atrial fibrillation and a compelling reason for avoiding OACs (www.clinicaltrials.gov: NCT02879448).
Fig 4 ACP and Amulet
12
The WaveCrest (Coherex Medical, Salt Lake City, UT, USA) is a next generation occlusion device that recently received a CE mark in Europe. The device is designed to allow occlusion at the ostium with little device protrusion into the appendage (the device is short). It has retractable distal anchors distributed around the perimeter of the occluded designed to conform to irregular anatomy and maximize stability. The PTFE outer coating is both occlusive and non-thrombogenic. The device is available in 3 sizes: 22, 27 and 32 mm. In the initial trial of 73 patients, 68 (93%) had acute procedural success with 2 cases of pericardial effusion requiring drainage. At 45-day follow-up, successful closure (defined as less than a 3 mm residual leak) was present in 67 (92%) of patients.[28]
The UltraSept device (Cardia Inc, Eagan, MN, USA) is another next generation device recently receiving a CE mark in Europe. The device is available in sizes ranging from 16-32 mm (the outer occluding “sail” is 6 mm larger than the bulb) and is delivered through either a 10 or 12 French sheath. It is articulated with titanium centerposts and a nitinol bulb with the design intended to conform to various LAA shapes with minimal tension on the system. The device is completely repositionable and retrievable.
The LAmbre device (LifeTech, Shenzen, China) is the third novel device (Figure 10cc) with recent CE approval. This device is a dual membrane design (umbrella and cover) available in two configurations depending on the size and lobality of the LAA to be occluded. Seventeen different combinations are available with sizes ranging from 16-36 mm for the umbrella and 22-40 mm for the cover. The occluder is delivered through an 8-10 French sheath depending on the particular size chosen. The CE mark study was based on a 60 patient experience demonstrating 100% success with complete closure in all cases and a 3.3% adverse event rate (1 patient died secondary to LAA wire perforation and another developed a pericardial effusion).[29]
Lariat device (SentreHEART, Redwood City, CA) (Figure 6) occludes the LAA via an epicardial suture. In this procedure, epicardial access is obtained via a subxyphoid approach and a 14F epicardial guide is inserted. Transeptal access is gained via standard techniques and the left atrium is visualized under cineangiography. A 20 mm balloon tipped catheter is advanced over a 0.025” magnet tipped guide wire to the left atrial appendage. Next, a 0.035” magnet tipped epicardial guide wire is advanced through the epicardial sheath to the appendage so that the two magnets connect. Then the Lariat snare is advanced over the 0.35” epicardial guide wire into position around the LAA. A balloon tipped catheter may be advanced to assist in positioning over the ostium of the LAA. As the snare is closed, under TEE and fluoroscopic imaging, flow in the LAA is assessed. When fully occluded, the suture is closed, the snare is removed and a suture cutter is advanced. A pericardial drain is exchanged over the 0.035” guide wire and the remaining equipment is removed. The drain is removed as appropriate.
13
The Lariat device was evaluated in 89 patients; 85 were successfully closed with immediate complete occlusion in 81 with no evidence of flow by TEE. Adverse events included late pericardial effusion (n=1), pericarditis (n=2), sudden death (n=2) and non-embolic stroke (n=2). At one-month follow-up, complete closure rates were 91%.[30] In 2015, the FDA released a serious adverse effects warning for the Lariat device primarily related to pericardial bleeding.
Currently the randomized controlled aMAZE (LAA ligation Adjunctive to PVI for Persistent or Longstanding Persistent Atrial Fibrillation) trial is ongoing (NCT02513797). In this trial, patients with atrial fibrillation are randomized to pulmonary vein isolation alone or pulmonary vein isolation in combination with LARIAT LAA suture closure. The endpoint is the incidence of recurrent atrial fibrillation and safety. The plan is to enroll 600 patients.
Fig 6 LAARIAT device
1.2 Mitral stenosis: prevalence, diagnosis and treatment
Mitral stenosis (MS), is predominantly caused by rheumatic heart disease. The prevalence of rheumatic heart disease in school-age children is estimated at between 1 and 6 per 1,000 in Asia and between 3 and 14% in Africa [31, 32, 33], but recent data based on systematic echocardiographic examination show that the true prevalence is approximately tenfold higher [34]. Conversely, MS is the least frequent valvular disease in industrialised countries: the prevalence of MS was estimated at 0.1% in the US population-based study [35] and MS accounted for 9% of single-valve diseases in the
14
Euro Heart Survey. The other main cause of MS is degenerative calcification of the mitral annulus, which is frequent in the elderly, congenital MS and infiltrative diseases.
Mitral valvuloplasty acts in the same way as surgical commissurotomy, by opening the fused commissures but it is of little or no help in cases where the predominant mechanism of mitral stenosis is restricted valvular mobility caused by valve fibrosis or severe subvalvular disease and where commissural fusion is mild.
The transvenous or antegrade approach is the more widely used, performed through the femoral or jugular vein by transseptal punture in the middle part of the fossa ovalis.
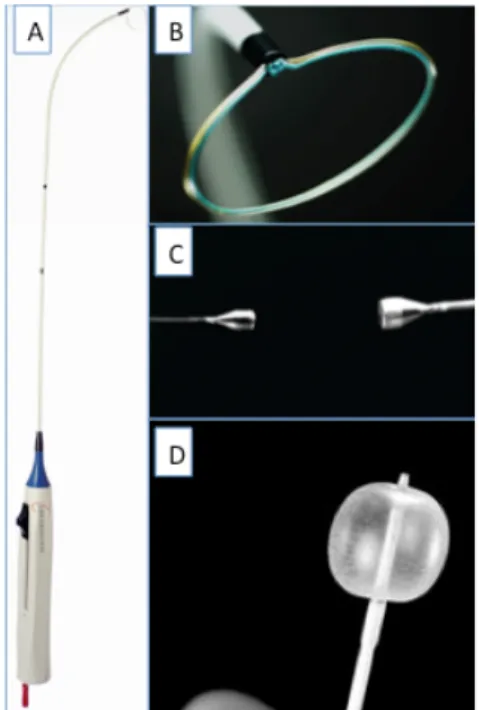
The Inoue balloon, composed of nylon and rubber micromesh, is self-positioning and pressure-extensible. It is large (24 to 30 mm in diameter) and has a low profile (4.5 mm). The balloon has three distinct parts, each with a specific elasticity, enabling them to be inflated sequentially. There are four sizes of Inoue balloon available (24, 26, 28 and 30 mm): each is pressure-dependent, so its diameter can be varied by up to 4 mm as required by circumstances. Balloon size is chosen in accordance with the patient’s height. The main steps are the following:
Before use, the size at full inflation should be checked. A syringe filled with diluted contrast is inflated and deflated in a basin of water in order to “de-bubble” the balloon. The first balloon inflation should be performed 4 mm below the maximal balloon size.
After transseptal catheterisation, the stiff guidewire (0.025, 175m) is introduced into the left atrium. The femoral entry site and the atrial septum are dilated using a rigid dilator (14 Fr).
The Inoue balloon is introduced into the left atrium. If resistance is felt when crossing the skin and subcutaneous tissues, re-dilate the groin entry site with the dilator or, if difficulty persists, inserting a 16 Fr sheath (COOK). If this problem occurs at the level of the septum the balloon should be pulled and rotated in one or other direction and then pushed again. If resistance is still present, it is preferable to dilate the septum with a peripheral angioplasty balloon (6 or 8 mm in diameter, 4 cm long) before another attempt at crossing the inter-atrial septum with the balloon.
When the Inoue balloon is introduced into the LA, its tip is shortened and the balloon stretching tube and guidewire are withdrawn. The permeability of the catheter is then checked by aspiration and pressure recording.
The stylet is introduced in AP view in order to orient the balloon catheter towards the mitral valve. The Inoue balloon is inflated sequentially in RAO 30° view. First, the distal portion is inflated with 1 or 2 mL of a diluted contrast medium (1 contrast-5 saline). This then acts as a floating balloon catheter when crossing the mitral valve. Crossing of mitral valve is performed by a combination of gently pulling the stylet with an anticlockwise rotation while gently pushing the balloon catheter. If entry into the LV is difficult the “loop manoeuvre” could be used. Inflation should be pursued only if the balloon is moving freely in the left ventricular cavity directed to the apex. Further inflation should
15
not be performed if the balloon is directed obliquely towards the base of the heart because in such a case it may become trapped in the subvalvular apparatus. Inflation of the distal portion of the balloon, which is thereafter pulled back and anchored at the mitral valve. Subsequent inflation of the proximal and middle portions of the balloon. At full inflation, the waist of the balloon in its mid portion has disappeared.The inflation/deflation time should be short (3 to 4 sec).
If the echocardiographic evaluation shows that the result is insufficient the balloon size is increased up to the maximum size in 1 mm increments according to echocardiographic monitoring.
After that, the guidewire and the balloon stretching tube should be reintroduced into the Inoue balloon in order to slenderise it fully before withdrawal. This is done in AP view. Before pulling the balloon across the inter-atrial septum, the guidewire needs to be pulled so that only its soft part is left external in order to avoid “a cutting effect” which may occur if the stiff part of the guidewire is out of the tip of the balloon during the manoeuvre.
The balloon and guidewire are withdrawn as a single unit.
Echocardiography is essential for monitoring the procedure to guide transseptal catheterisation, on the course of the mitral opening and finally enables detection of early complications. Transoesophageal approach requires general anaesthesia and should probably be restricted to cases in which technical difficulties are encountered. Intracardiac echocardiography (ICE) is currently considered the imaging tool of choice because it may be used without additional operators or general anaesthesia, although the price of the device is a serious limitation in most places. For evaluating the results of the procedure: use of the mean left atrial pressure and mean valve gradient can be criticised because of variations in the heart rate or cardiac output; the accuracy of Doppler measurements during commissurotomy is low, so planimetry from 2D/3D echocardiography appears to be the method of choice. Colour Doppler assessment is the method of choice for sequential evaluation of changes in the degree of regurgitation. The commissural opening, which is the main parameter, is usually assessed in the parasternal short-axis view during TTE. Real-time three-dimensional echocardiography is the most accurate method for assessing the degree of opening using short-axis views or real-time three-dimensional TEE en face views which may provide further information regarding the extent of the commissural opening. The following criteria have been proposed for the desired endpoint of the procedure:
• Valve area >1 cm²/m² BSA, and
• complete commissural opening in at least 1 commissure, or • appearance or increase of regurgitation >1+
16
To assess stability of results the following days, echo should be performed 1 to 2 days after valvuloplasty when the valve area may be calculated by planimetry or by the half-pressure time or continuity equation method.
Complications:
The main causes of death are massive haemopericardium or the poor condition of the patient that precedes the treatment due to comorbidities.The fatality rate ranges from 0% to 3%.
Haemopericardium may be related to transseptal catheterisation or to left ventricular perforation by the guidewires or the balloons. Its incidence varies from 0.5% to 12%.
Embolism may be due to a thrombus that was pre-existing, usually in the left atrial appendage, or which developed during the procedure. It may also be due to air leaking from the balloon or, very rarely, to calcium. Embolism is encountered in 0.5% to 5% of cases
Severe mitral regurgitation can be due to chordal rupture or to excessive commissural splitting or papillary muscle rupture. The frequency of severe mitral regurgitation ranges from 2% to 19%. If valvuloplasty is initially successful, survival rates are excellent, the need for secondary surgery is infrequent, and functional improvement occurs in most cases. In most cases the improvement in valve function is stable.
Restenosis after valvuloplasty has generally been defined as a loss of more than 50% of the initial gain with a valve area of less than 1.5 cm2. The incidence of restenosis is usually low, between 2% and 40%
The low incidence of embolism during follow-up [35], the progressive decrease in intensity or disappearance of spontaneous echocardiographic contrast [37], and the improved left atrial function [39] after valvuloplasty suggest a beneficial effect of the procedure on left atrial blood stasis [38], from which a lower risk of thromboembolism may be expected. [40]
The indication to the intervention is only considered in symptomatic patients with a mitral valve area <1.5 cm2 . It may be considered in symptomatic patients with a valve area >1.5 cm2 if symptoms cannot be explained by another cause and if the anatomy is favourable. It should be considered in asymptomatic patients without unfavourable clinical and anatomical characteristicsc for mitral commissurotomy (Class II Level A) and:
• high thromboembolic risk (history of systemic embolism, dense spontaneous contrast in the LA, new-onset or paroxysmal atrial fibrillation), and/or
• high risk of haemodynamic decompensation (systolic pulmonary pressure >50 mmHg at rest, need for major non- cardiac surgery, desire for pregnancy
The suitability for valvuloplasty depends on different anatomic features of the mitral valve apparatus. These features are usually combined in a scoring system. The most widely used is the Wilkins score, which ranks each component between 1 and 4 and adds them together to obtain a score between 4
17
and 16 [41, 42]. Another approach is Cormier’s score which consists in an overall approach to the mitral apparatus defining three classes [43]. The three classes of Cormier’s score correspond to the most appropriate surgical alternative: class 1 corresponds to ideal indications for commissurotomy, class 2 to intermediate indications for commissurotomy, and class 3 to indications for prosthetic valve replacement. MC is definitely the preferred treatment in patients with favourable valve anatomy, i.e., with a Wilkins score ≤8 or a Cormier class 1
Contraindications to valvuloplasty before the procedure are: • Mitral valve area >1.5 cm²
• Left atrial thrombus
• More than mild mitral regurgitation • Severe or bicommissural calcification • Absence of commissural fusion
• Severe concomitant aortic valve disease, or severe combined tricuspid stenosis and regurgitation
• Concomitant coronary artery disease requiring bypass surgery
Finally the use of cerebral protection devices may be evaluated in patients with LA thrombosis, contraindications for surgery and urgent need for intervention
1.3 Aortic stenosis: prevalence, diagnosis and treatment
Aortic stenosis (AS) is now the most common valve disease requiring intervention in Europe and North America, and it is increasing in prevalence due to the ageing population. The frequency of aortic valve sclerosis is approximately 25% at 65 years of age, rising to 48% after 75 years, while the frequency of AS is 4–5% in those aged over 65. AS has become the most common indication for valve surgery as well as catheter intervention for structural heart disease.
Echocardiography is the key diagnostic tool. It confirms the presence of AS, assesses the degree of valve calcification, LV function, and wall thickness; detects the presence of other associated valve disease or aortic pathology; and provides prognostic information.
Indications for aortic valvuloplasty include:
• bridge to SAVR or TAVI in haemodynamically unstable patients or in patients with sympto- matic severe aortic stenosis who require urgent major non-cardiac surgery (Class II Level B, 2017 ESC guidelines)
18
• diagnostic means in patients with severe aortic stenosis or other potential causes for symp- toms (i.e. lung disease) and in patients with severe myocardial dysfunction, pre-renal insufficiency or other organ dysfunction that may be reversible with balloon aortic valvotomy when performed in centres that can escalate to TAVI (Class II, Level B, 2017 ESC guidelines) 1.4 Cerebrovascular Protection
The SENTINEL CPS
The Sentinel CPS is a unique device that filters, captures and removes embolic debris released during TAVI. Sentinel CPS is simple to use, with 99% deployment success in a median deployment time of four minutes.1
Delivered through a conventional radial artery approach in the right arm, Sentinel CPS utilizes a proximal embolic filter delivered to the brachiocephalic artery, and a distal embolic filter delivered to the left common carotid artery at the beginning of the TAVI procedure.
The Sentinel CPS consists of a 6 French-compatible catheter with deployable proximal and distal filters, an articulating sheath and an integral handle assembly. Precisely located radiopaque markers enable visualization under fluoroscopy during use. Using the articulating sheath, the curve of the device can be adjusted to accommodate anatomic variations of the aortic arch. CLEAN-TAVI study demonstrated that the use of Claret Medical technology significantly reduced the number and volume of cerebral lesions, with a 65% reduction in the total volume of new brain lesions and a 57% reduction in the number of new brain lesions 7 days after the procedure.MISTRAL-C showed that during TAVI unprotected patients have a statistically significant (p=0.017) worsening in cognition when compared to Sentinel-protected patients at five days post-TAVI, when assessed using the Mini Mental State Exam (MMSE) and a 52 percent reduction in the median total new lesion volume at 5 days post-procedure as assessed using highly sensitive 3-Tesla brain MRI (44, 45).
Sentinel CPS is available in one universal size that adjusts to the vast majority of vascular anatomies. The SENTINEL trial showed that routine use of the Sentinel TCEP does not result in a reduction in new lesion volume on MRI or strokes within 30 days compared with usual management, but atherothrombotic debris is common following TAVR and was noted in 99% of patients in this trial. Of particular importance, the adjudicated 30-day stroke rate for the control arm (unprotected TAVI) was 9.1%, and a majority of the strokes (61%) were identified within 72 hours of the TAVI procedure. The use of the Sentinel CPS was found to reduce strokes by 63% in the first 72 hours after TAVI – when most strokes occur, although in a non significant manner (p=0,25) (46).
In an early study of five consecutive patients undergoing routine LAAO, the Sentinel CPS was used to protect patients’ brains. Even though thrombus in the atrial appendage was ruled out prior to the
19
procedure by TEE, embolic debris was found in 100% of the procedures post-procedure, with the most common type of debris found being acute and organizing thrombus (47).
In the study, the Sentinel CPS showed 100% procedural success, with no periprocedural complications and no neurological abnormalities present after the procedure.
In an initial experience with 14 high surgical risk patients at two German centers using the Sentinel Cerebral Protection System (CPS) during MitraClip implantation, the Sentinel CPS demonstrated 100% procedural success. No transient ischemic attacks, strokes, or deaths occurred peri-procedurally or during a median follow-up interval of 8.4 months in patients protected with the Sentinel CPS (48). In the study, embolic debris was identified in all 14 patients. The most common debris types were acute thrombus and small fragments of non-polarizable basophilic foreign material that were morphologically consistent with hydrogel, a material coating several devices used during the MitraClip procedure, including the transseptal sheath for transseptal puncture and the guide catheter for the MitraClip system. No evidence was found of foreign material arising from the Sentinel CPS or its coating in this study.
1.5 The LAMBRE
The name “LAmbre” is a derivative from “an umbrella in the left atrial appendage”. The LAA closure system consists of an implant and its delivery system. The implant is a nitinol-based, self-expanding device comprising a hook-embedded umbrella and a cover connected with a short central waist.
20
The waist acts as an articulating, compliant connection between the cover and the umbrella, allowing the cover to self-orient to the cardiac wall. The cover is 4 to 6 mm larger in diameter than the umbrella, covering the LAA orifice and provides apposition against the chamber wall under gentle tension. The proximal cover is filled with sewn in polyethylene terephthalate (PET) fabric. The distal umbrella comprises 8 claws with individual stabilizing hooks attaching to them to facilitate anchoring to LAA wall. The umbrella was specially engineered to allow for complete collapse and repositioning. An additional PET membrane has been introduced to the umbrella in the newer version of the implant to ensure LAA sealing in case the cover fails to achieve optimal occlusion. Several sizes of the implants (16–36 mm) have been developed to accommodate the variation of LAA anatomy and they were delivered by sheaths smaller than those used for other devices, ranged 8–10 Fr in size.
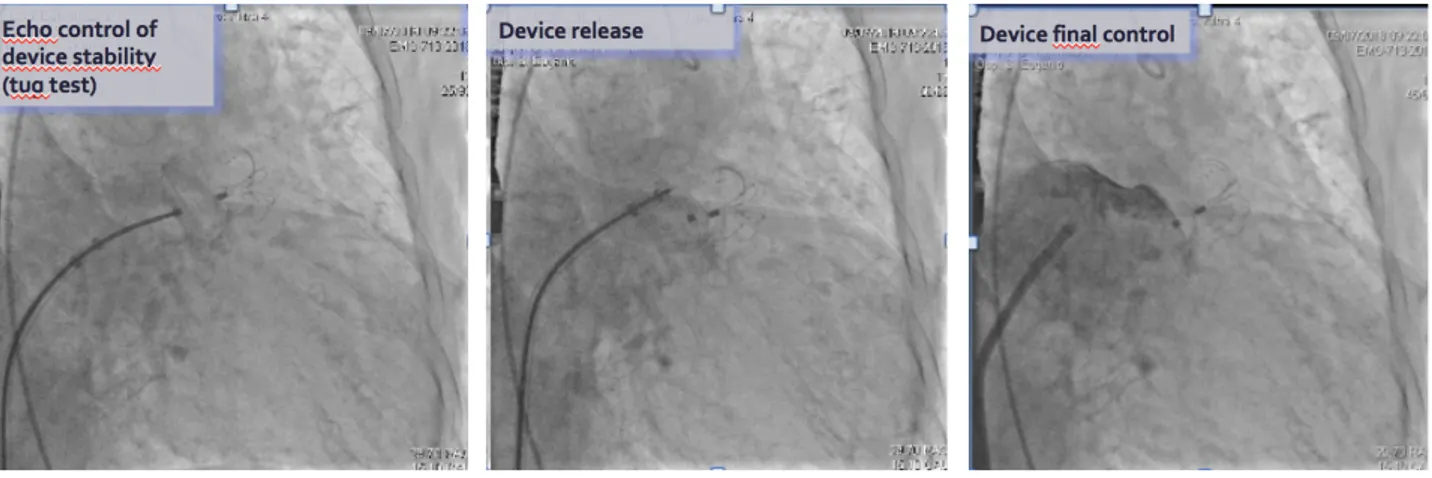
The delivery system consists of a sheath, dilator, delivery cable, loader and vise. The delivery sheath allows for contrast injection both in LAA and proximal to the occluding surface to assess sealing and device positioning at the interface of LAA orifice and left atrial wall. The procedure of implantation is clearly exposed as follow. The delivery sheath containing the implant is placed to the proximal part of LAA. The umbrella of the implant is partially deployed by slowly pushing out the device from the delivery sheath. The whole system is then gently push “en-bloc” forward to the desired landing zone to allow better flowering of the umbrella and grasping of LAA walls by the retention hooks. The sheath is then withdrawn to expose the disc, allowing it to expand in the left atrium and covering the LAA ostium by gently pushing the delivery cable forward. Once the implant is placed in LAA, left atrial angiogram is performed to check device positioning, LAA sealing and impingement on surrounding cardiac structures (circumplex artery, Marshall ligament, mitral valve). Gentle tug test by applying tension to the delivery cable is performed to ensure device stability. The implant can be recaptured, completely retrieved and redeployed. Acute procedural success is defined as proper and stable implant in LAA without peri-device leakage or impingement on surrounding cardiac structure. The implant is released from delivery cable once acute procedural success is achieved. Clinical trials demonstrated an excellent implant success rate, favorable implant property, and very low incidence of complications (49, 50,51,52,53).
21
2. Cases presentation 2.1 Case # 1
69-year-old patient with dyspnea and sudden onset of right hemiplegia and dysarthria was admitted to our institution and rapidly underwent successful mechanical thrombectomy because of a basilar artery occlusion. A good functional outcome was achieved (Figure 8a-d).
Fig 8a 03:48 pm
22 Fig 8c 04:05 pm
Fig 8d
The diagnostic work-up revealed rapid ventricular rate atrial fibrillation and severe mitral valve stenosis, left atrial spontaneous echocardiographic contrast, and a small thrombus formation in the apex of a chicken-wing-morphology left atrial appendage (LAA). Both CHA2DS2-VASc and HAS-BLED scores were 4. CHA2DS2-VASc couldn’t be applied to atrial fibrillation associated to severe mitral stenosis, but it has only been calculated to indicatively estimate the ischemic risk enhanced in heart valve diseases. Because of contraindication to oral anticoagulation, a decision was made to undertake percutaneous LAA closure and combined balloon mitral valvuloplasty with total cerebrovascular protection.
From both transradial access points, 2 Sentinel (Claret Medical, Santa Rosa, California) cerebral protection systems were advanced toward the aortic arch with their embolic filters delivered to all 3 supra- aortic vessels (Fig 9).
23
Fig 9
The right femoral artery was also cannulated for aortography and invasive blood pressure monitoring. Following right femoral vein access and spontaneous breathing sedation, transesophageal echocardiography–guided transseptal puncture was performed at a midposterior site (Fig 10).
Fig 10
After imaging and measurement of the LAA, a 32/36-mm LAmbre (Lifetech Scientific, Shenzhen, China) LAA occlusion system was implanted (Figure 11).
24 Fig 11
Thereafter, balloon mitral valvuloplasty was performed with an Inoue (Toray Medical, Tokyo, Japan) 26 balloon advanced through the same transseptal access, restoring a valve area of 2. 5 cm2 after 3 consecutive dilatations (Figure 12)
Finally, before embolic filter removal, external electric cardioversion (synchronous 360-J shock) restored sinus rhythm (Figure 13). Notably, a small thrombus was found in the left subclavian filter (Figure 14).
25 Fig 14
Routine neurological assessment pre- and post- intervention yielded normal neurocognitive function, and no new lesions were found on post-procedural brain magnetic resonance imaging (Figure 15).
FIG 15
2.2 Case # 2
A 87 yrs old woman was admitted to our hospital for worsening heart failure; cardiac work-up revealed rythmn disturbance such as atrial fibrillation and hypertensive and valvular heart disease
Pre-procedure MR
Post-procedure MR DW-SSH
DW-SSH
T2W-FLAIR T2W-FLAIR
26
with severe aortic stenosis; the patient has been repeatedly hospitalized for serious anemia and was treated with blood transfusions; she had definite contraindication to oral anticoagulant.
After extensive evaluation, the patient was directed to perform a combined procedure of LAA closure and aortic valvuloplasty. In cath lab, following right femoral vein access and spontaneous breathing sedation, transesophageal echocardiography–guided transseptal puncture was performed at a midposterior site.
After imaging and measurement of the LAA, a 28/34-mm LAmbre (Lifetech Scientific, Shenzhen, China) LAA occlusion system was implanted (Fig 16-17).
Fig 16
Fig 17
Subsequently device stability check for 3 min was done and device release with TEE and final angiographic control (Fig 18 and 19).
27 Fig 19
Thereafter, we performed angiographic evaluation of aortic root dimensions (Fig 20); we crossed the stenotic valve using a standard guide supported by Amplatz left 2 catheter, exchanged the standard guide with superstiff guide and positioned the 22x40 mm balloon for valvuloplasty and inflated twice the balloon after activation of the PMK (Fig 21), then removed the balloon and performed haemodynamic and angiographic control (Fig 22) with good result and significant reduction of peak-to-peak gradient (Fig 23).
Fig 20 Fig 21 Fig 22 Aortic valvuloplasty with
a 22x40 mm balloon
28 Fig 23
Subsequent hospital stay was free of complications.
2.3 Case # 3
A 64 yr woman during neurological rehabilitative therapy for a recent ischemic stroke due to the occlusion of the middle cerebral artery, despite anticoagulant therapy with warfarin, and treated with thrombectomy, was admitted to intensive care unit for bronchopneumonia with acute liver failure with poorly controlled values of INR (that rised up until 9,97). Cardiac check-up revealed permanent AF, mitral valvulopathy with severe stenosis and moderate insufficiency and pulmonary hypertension, severe anemia with need for blood transfusions.
At thoracic and abdominal CT performed for confirmation of the diagnosis of bronchopneumonia was reported: …”parietal thrombosis affecting the left LAA and the adjacent portion of the atrium”… Blood chemistry tests showed positivity of antinuclear (ANA) antibodies predisposing an increased thrombotic risk. After multidisciplinary discussion the patient was referred to a combined LAA closure procedure and mitral valvuloplasty.
From right transradial access, Sentinel (Claret Medical, Santa Rosa, California) cerebral protection system was advanced toward the aortic arch with their embolic filters delivered to supra- aortic vessels.
The right femoral artery was also cannulated for aortography and invasive blood pressure monitoring. Following right femoral vein access and spontaneous breathing sedation, transesophageal echocardiography–guided transseptal puncture was performed at a midposterior site.
After imaging and measurement of the LAA, a 32/36-mm LAmbre (Lifetech Scientific, Shenzhen, China) LAA occlusion system was implanted.
Thereafter, balloon mitral valvuloplasty was performed with an Inoue (Toray Medical, Tokyo, Japan) 26 balloon advanced through the same transseptal access, restoring a valve area of 1.88 cm2 after 3 consecutive dilatations. (Fig 24-25)
29 Fig 24
Fig 25
The day after a TEE was repeated and showed:
“At the level of the primitive entrance to the left LAA it was visualized a stratified iso-echogenic deposit material of about 5 mm thickness, from its surface some small mobile frustas are blown out” (Fig 26)
30
Therefore the patient was treated with heparin in infusion i.v. for a further 5 days and then the ultrasound evaluation was repeated which showed the complete resolution of the thrombosis and the correct arrangement of the umbrella to seal the left atrial appendage (Fig 27)
Fig 27
To the cerebral CT scan post-procedure there was no evidence of new encephalic lesions (Fig 28). The patient did not present periprocedural complications and was referred to the rehabilitation clinic after the resolution of the bronchopneumonic process.
31
2.4 Index of figures and tables
Fig 1 Classification of atrial fibrillation Page 3 Fig 2 Plato Device Page 9 Fig 3 Watchmann Device Page 10 Fig 4 ACP and Amulet devices Page 11 Fig 5 WaveCrest (a), UltraSept (b), LAmbre (c) Page 11 Fig 6 LAARIAT device Page 13 Fig 7 LAMBRE device Page 19 Fig 8a and 8b Basilar occlusion Page 21 Fig 8 c and Fig 8d Trombectomy Page 22 Fig 9 Positioning of Sentinel Filters Page 23 Fig 10 Echo imaging of transeptal puncture and LAA misuses Page 23
Fig 11 LAMBRE release Page 24
Fig 12 Mitral valvuloplasty Page 24
Fig 13 Restoring sinus rhythm Page 24
Fig 14 Thrombus from filter Page 25
Fig 15 Cerebral MRI Page 25 Fig 16 Echo monitoring of transeptal puncture and LAA misures Page 26
Fig 17 Angiographic misures Page 26
Fig 18 Echo monitoring of LAMBRE release Page 26
Fig 19 Angiography views of LAMBRE release Page 27
Fig 20 Aortic valvuloplasty Page 27
Fig 21 Hemodynamic results Page 27
Fig 24 Lambre release in third patient Page 29
Fig 25 Echo results of mitral valvuloplasty Page 29
Fig 26 Device thrombosis Page 29
Fig 27 Thrombus resolution after Heparin infusion Page 30
Fig 28 CT cerebral scan Page 30 Fig 29 Schematic figure of LAMBRE modified implantation technique Page 33 Note: the figures 1-7 are taken from the web
32
3. Conclusions
I presented three cases of complex cardiological diseases in which atrial fibrillation is associated with valvular disease that increase the cardioembolic risk. In all three cases there was a contraindication to anticoagulant therapy due to a high risk of bleeding or to the occurrence of major bleeding events. The need to carry out an intervention of LAA closure in the two patients with thrombosis of the auricula was motivated by the desire to prevent new embolic events in patients not susceptible to prolonged treatment with oral anticoagulants. Furthermore, the use of this device has minimized the embolic risk since a new implantation technique is described. While with devices currently in use it is necessary to place a J guidewire in the body of the LAA to allow a proper alignment of the delivery catheter to the LAA, in the case of this LAmbre device it is possible to perform a partial release of the device in the atrium, a subsequent advancement of the device and the delivery catheter towards the LAA; thereafter the retraction of the delivery catheter allows the umbrella to be completely unfolded in the LAA, and subsequently the cover is pushed against the LAA to seal its entry.In this way the dissemination of any thromboembolic detritus present in the LAA is prevented (Fig 29).
33 Fig 29
In conclusion, despite adeguate oral anticoagulation a proportion of patients undergo repeated ischemic events; the rate of these events increases if there are associated concurrent valvular diseases. A combined procedure of LAA closure/valvuloplasty with single transseptal access is feasible, safe and reproducible by experienced operators. Valvuloplasty has the function of preventing low flow conditions predisposing atrial thrombosis. The safety of this procedure has been guaranteed by the preventive positioning of filters in the epiaortic trunks.
More than 40 publications established the emerging role of cerebro-vascular protection from embolic risk during similar aortic procedures (TAVI): on the contrary, only 2 case reports have been reported for mitral valvuloplasty with neuro-embolic protection. Long term follow up in a greater cohort of patients will be required to evaluate the safety and efficacy of this strategy. Finally data comparing percutaneous LAA closure to direct oral anticoagulants do not exist. However, results from ongoing trials evaluating left atrial appendage closure compared with direct oral anticoagulants will hopefully be available in the near future and will allow us to use the best strategy for patients eligible for both anticoagulation or LAA closure.
34
4. References
• 1. Lloyd-Jones D, Adams RJ, Brown TM et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010;121:e46-e215.
• 2. Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183-7.
• 3. Healey JS, Connolly SJ, Gold MR et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-9.
• 4. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285:2864-70.
• 5. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2009;137:263-72.
• 6. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100.
• 7. Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719-47.
• 8. Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501.
• 9. Connolly S, Pogue J, Hart R et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. • 10. Connolly SJ, Pogue J, Hart RG et al. Effect of clopidogrel added to aspirin in patients
with atrial fibrillation. N Engl J Med 2009;360:2066-78.
• 11. Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin?. Arch Intern Med 2000;160:41-6.
• 12. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51.12. Lane DA, Lip GY. Use of the
35
CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 2012;126:860-5.
• 13. Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in atrial fibrillation. N Engl J Med 2011;365:883-91.17. Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007;167:1414-9.
• 14 Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.
• 15. Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104.19. Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke 1997;28:2382-9. • 16. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical
patients with atrial fibrillation. Ann Thorac Surg 1996;61:755-9.
• 17. Hara H, Virmani R, Holmes DR, Jr. et al. Is the left atrial appendage more than a simple appendage?. Catheter Cardiovasc Interv 2009;74:234-42.20. White HD, Gruber M, Feyzi J et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007;167:239-45. • 18. Healey JS, Crystal E, Lamy A et al. Left Atrial Appendage Occlusion Study (LAAOS):
results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005;150:288-93.
• 19. Sievert H, Lesh MD, Trepels T et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002;105:1887-9.
• 20. Ostermayer SH, Reisman M, Kramer PH et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005;46:9-1421. Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis?. J Thromb Haemost 2008;6:1500-6.
• 21. Sick PB, Schuler G, Hauptmann KE et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007;49:1490-522. Gage BF, van Walraven C, Pearce L et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-92.
36
• 22. Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42.
• 23. Reddy VY, Sievert H, Halperin J et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98
• 24. Holmes DR, Jr., Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12.
• 25. Park JW, Bethencourt A, Sievert H et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011;77:700-6.
• 26. Tzikas A, Shakir S, Gafoor S et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016;11:1170-9.
• 27. Freixa X, Abualsaud A, Chan J et al. Left atrial appendage occlusion: initial experience with the Amplatzer Amulet. Int J Cardiol 2014;174:492-6.
• 28. Whisenant B. WaveCrest. Transcatheter Cardiovascular Therapeutics. Washington, DC, 2014.
• 29. Sievert H. Clinical experience with the L’Ambre left atrial appendage occlusion system. Joint Interventional Meeting. Milan, Italy, 2017
• 30. Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18
• 31. Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009;374:1271-83. • 32. Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol.
2014;30:962-70.
• 33. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. Lancet. 2012;379:953-64.
• 34. Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, Paquet C, Jacob S, Sidi D, Jouven X. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470-6.
• 35. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-11.