BACILLUS CALMETTE-GUERIN
131I LABELING
USING SOLID-STATE REAGENT IODOGEN AS
A SOLID-PHASE OXIDIZING AGENT
A.F. SEDDA, G. ROSSIFusion and Technology for Nuclear Safety and Security Department Casaccia Research Centre, Rome
G. ATZEI, S. BOEMI
Nuclear Medicine Division S. Eugenio Hospital, Rome S. Eugenio Hospital, Rome
C. CIPRIANI
Nuclear Medicine Division AlGa Insitute, L'Aquila, Italy
RT/2018/10/ENEA
ITALIAN NATIONAL AGENCY FOR NEW TECHNOLOGIES, ENERGY AND SUSTAINABLE ECONOMIC DEVELOPMENT
A.F. SEDDA
Fusion and Technology for Nuclear Safety and Security Department Casaccia Research Centre, Rome
G. ATZEI, S. BOEMI
Nuclear Medicine Division S. Eugenio Hospital, Rome
BACILLUS CALMETTE-GUERIN
131
I LABELING
USING SOLID-STATE REAGENT IODOGEN AS
A SOLID-PHASE OXIDIZING AGENT
C. CIPRIANI
Nuclear Medicine Division AlGa Insitute, L'Aquila, Italy
RT/2018/10/ENEA
ITALIAN NATIONAL AGENCY FOR NEW TECHNOLOGIES, ENERGY AND SUSTAINABLE ECONOMIC DEVELOPMENT
I rapporti tecnici sono scaricabili in formato pdf dal sito web ENEA alla pagina www.enea.it I contenuti tecnico-scientifici dei rapporti tecnici dell’ENEA rispecchiano
l’opinione degli autori e non necessariamente quella dell’Agenzia
The technical and scientific contents of these reports express the opinion of the authors but not necessarily the opinion of ENEA.
BACILLUS CALMETTE-GUERIN 131I LABELING USING SOLID-STATE REAGENT IODOGEN AS A
SOLID-PHASE OXIDIZING AGENT
A.F. Sedda, G. Rossi, G. Atzei, S. Boemi, C. Cipriani
Abstract
Intravesical instillation of bacillus Calmette-Guerin (BCG) has been found to be an efficacious treatment against recurrences of superficial bladder carcinoma and carcinoma in situ after resection of the pri-mary cancer. The mechanisms by which BCG mediates antitumor activity are not known. In the present paper the radiolabeling with 131I of a strain of attenuated BCG cells, by using IODOGEN as a iodination promoter, has been performed and optimized. This optimization is part of a project aimed to clarify the adhesion mechanisms, the retention times and the optimal dosage of BCG to bladder tumor cells, both in-vitro and in-vivo.
Key words: Bacillus Calmette-Guerin, BCG, IODOGEN, radiolabeling, 131I
Riassunto
L'instillazione intravescicale del bacillo di Calmette-Guerin (BCG) è risultata essere un trattamento ef-ficace contro le recidive del carcinoma superficiale della vescica e del carcinoma in situ dopo la rese-zione del tumore primitivo. I meccanismi con cui BCG media l'attività antitumorale non sono noti. Nel presente lavoro la radiomarcatura con 131I di un ceppo di cellule BCG attenuate, utilizzando IODOGEN
come promotore di iodinazione, è stata eseguita e ottimizzata. Questa ottimizzazione è parte di un progetto volto a chiarire i meccanismi di adesione, i tempi di ritenzione e il dosaggio ottimale di BCG alle cellule tumorali della vescica, sia in-vitro che in-vivo.
Introduction
Material and methods Results Discussion References 7 9 10 12 14
INDEX
Introduction
Bacillus Calmette-Guerin (BCG) is a live attenuated vaccine derived from a strain of Mycobacterium bovis. Based upon the original work by Morales (Morales et al., 1976) , intravesical immunotherapy with Bacillus of Calmette-Guarin (BCG) is a widespread used after- surgery therapy, aimed to prevent recurrences of superficial bladder cancer and of bladder carcinoma in situ, by raising an immunological response towards the bladder tumor cells.
In spite of intensive efforts to clarify the rationale of the technique, the details and exact cellular mechanism of the antitumor activity of BCG remains only partially known (Becich et al. 1991; Garden et al. 1992; Kuroda et al., 1993).
Some studies have suggested that an initial requisite step in mediating the antitumor effect of BCG is attachment of BCG organisms to matrix fibronectin, at sites of urothelial disruption (Cheng et al., 1994; Schneider et al., 1994).
The mechanisms of attachment of BCG on urothelial cells appears mediated by extracellular matrix proteins (Akaza et al, 1993: Zhao et al., 2000). It has been demonstrated that pretreatment of BCG with soluble fibronectin prevents the binding of BCG to matrix fibronectin exposed on the murine bladder wall after mucosal disruption, and the inhibition of intravesical BCG attachment resulted in the loss of antitumor activity. (Ratliff et al., 1987, 1988).
In a paper, bladder urothelial disruption induced by acrolein, adriamycin, or electrocautery resulted in BCG binding in areas of urothelial damage. Binding induced by each method was inhibited by anti-fibronectin (FN) antibodies but not by antibodies to the basement membrane component laminin. Intravesical BCG binding also was inhibited by pretreating BCG with soluble FN. Inhibition of intravesical FN-mediated BCG attachment prevented immunization via the intravesical route.
Moreover, the expression of both delayed hypersensitivity in the bladder of BCG-immunized mice and antitumor activity was inhibited by blocking FN-mediated intravesical BCG attachment. These data suggest that intralumenal attachment of BCG appears to be mediated by FN.
Moreover, these data suggest that intravesical FN mediated attachment of BCG is a requisite step in BCG-mediated antitumor activity in the murine bladder tumor model (Kavoussi et al 1990).
Some papers have tried to clarify the chemical steps and mediators of the therapeutic action of BCG, but no clear evidence of a unique mechanism has up to now been established (Jansson et al., 1998; Patard et al., 1993; Mizurani et al., 1994).
In order to study the adhesion mechanism of BCG cells, it is important to evidence their behaviour in-vivo and in vitro tests, without disrupting their vitality and membrane receptor; one simple and effective way to 7
perform this task can be achieved by radiolabeling the cells. A interesting technique is the use of the gamma tracer 131I, that allows a labeling of the cells in in mild conditions,, without disrupting the biological activity.
The radioiodination of peptides, proteins and cells is a simple, non-invasive and efficient method to follow up their in-vitro and in-vivo behaviour and path. Iodination is a widely performed radiolabeling method, and generally involves the introduction of radioactive iodine (125I or 131I) into certain aminoacids of proteins (tyrosine); in most of the cases iodination takes place at the positions ortho to the hydroxyl group on tyrosine, but mono- or di-substitution can also occur.
It is possible to achieve iodination only when radioactive iodide anion I- is first oxidized to I+, that reacts electrophilically with the aromatic ring of tyrosine protein structure. After incubation, any residual reactive I+ is reduced back to the I- anionic form, and removed from the product solution.
The solid-state reagent IODOGEN (1,3,4,6 tetrachloro-3α,6α-diphenylglycoluril) (Figure1) is an oxidant catalyst for the iodination reaction, that allows a simple and rapid incorporation of iodine with minimal damage for the substrate to be labeled (Fraker et al., 1978; Salacinski et al., 1981; Tuszynski et al., 1983). More than this, this molecule, being water insoluble, can be coated on a reaction test tube from an organic solvent solution, and has the advantage that cannot be internalized by the cells, so hindering the iodination of proteins internal to the cells to be labeled.
Figure 1. Molecular structure of IODOGEN.
For its mild oxidizing power and water-insolubility, IODOGEN is generally preferred to other classical reagents, like chloramines-T and lactoperoxidase.
In the present paper the radiolabeling with 131I of a strain of actenuated BCG cells by using IODOGEN as a iodination promoter has been performed and optimized.
This paper is a preliminary part of a more general project, aimed to clarify the adhesion mechanisms, the retention times and the optimal dosage of BCG to bladder tumor cells, both in-vitro and in-vivo.
Materials and Methods
Among different variables affecting the 131I radiolabeling, the influence of IODOGEN concentration, the amount of inactive carrier, and the reaction time have been investigated, with the aim to obtain the maximum of cell radiodination. IODOGEN was obtained from PIERCE, Rockford, Illinois. BCG (IMMUCYST), 27 mg dry weight (corresponding to 3.4 3.0 x108 Colony Forming Units), was reconstituted with saline sterile solution, according to the manufacturer indications.
Carrier free 1 3 1I (GE Healthcare, USA), has been used for cell labeling. 1 3 1I solution has been diluted with saline sterile solution, obtaining a solution with specific activity of 18,5 MBq/ml. (Solution I).
The IODOGEN reagent (2.5 mg) has been dissolved in trichloromethane (6.25 ml) (Solution A). 5 mM potassium iodide (KI) water solution was used as a carrier, to drive the iodination reaction.
To all the vials containing the IODOGEN, have been added (in this order) the 1 3 1I, the iodine carrier, and the BCG cells (100 μg). The iodination has been stopped after 30 min of incubation at room temperature, with occasional shaking, by adding 1 ml of 5mM KI solution to the labeling vial.
The content of the vials has been transferred in centrifuge test tubes, and centrifugated at 1000 rpm for 5 min, to separate the labeled cells from the supernatant liquid, washed by saline to remove free 1 3 1I, and again centrifugated.
The gamma radiation activity of the vials containing the precipitate and of the supernatant have been measured, to obtain the labeling yields.
Data collection for measurements of the net area of gamma peaks was performed using an ORTEC high- purity germanium detector, coupled with a CANBERRA pulse height/multichannel analyzer system. The obtained spectra have been normalized and the isotope quantified by the software CANBERRA Genie-2K.
All determinations have been carried out in duplicate.
Results
Variable amounts of Solution A (250 μl, 500 μl, 750 μl, corresponding respectively to 100 μg, 200 μg and 300 μg of IODOGEN) have been transferred in dry borosilicate glass vials with conical bottom (1cm diameter, 2.5 cm height), previously cleaned by ethanol and trichloromethane.
The organic solvent was slowly evaporated to dryness at room temperature, under a light argon stream, leaving a fine and homogeneous film on the bottom of the vials.
The cell labeling yields obtained at different IODOGEN concentrations, working at different carrier concentrations, is plotted in Figure 2.
Figure 2 Incorporation of 131I obtained with increasing amounts of IODOGEN and carrier
The best results are obtained whit 200 μg of IODOGEN per vial, (30 μl of carrier Solution A are present in the labeling medium), with a labeling yield of 29.5%. As can be seen in Figure 2, an increase of the IODOGEN up to 300 μg does not produce any increase in the labeling yield curve.
The effect of incubation time on BCG labeling has been studied, using vials containing 100 μg of IODOGEN, obtained according to the above mentioned procedure, and adding to them 50 μl of 1 3 1
I solution and 30 μl of carrier solution.
The reaction has been stopped after 15, 30, 45, 60, 90 min of incubation time. The curve labeling yields vs. incubation time is showed in Figure 3; results show that, after 1 hour of incubation, iodination achieves the maximum value (12.6%).
After this time, the yield starts to decrease.
Figure 3 Iodination yield vs. incubation time
In the radiolabeling by using 1 3 1I, a little amount of inactive carrier is often used, to drive the reaction, and increase the yield of the incorporation of the radioactive isotope, particularly to label inner membrane proteins.
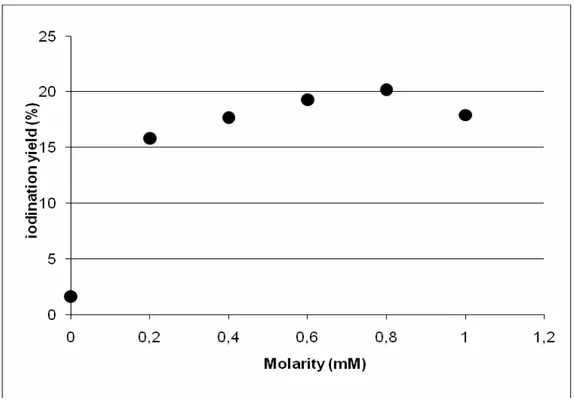
The influence of carrier concentration on BCG labeling has been investigated by varying the KI concentration in the labeling vial. KI concentrations of 0, 0.2, 0.4, 0.6, 0.8, and 1.0 mM have been used, by keeping the IODOGEN amount per vial to 200 μg, the 1 3 1I solution volume to 400 μl, and stopping the reaction after 1 hour of incubation.
Figure 4 summarizes the obtained results; a maximum in the iodination has been obtained using a KI concentration of about 0.8 mM.
Figure 4 Iodination yield vs. carrier concentration
Discussion
The obtained results have demonstrated that BCG iodination is dependent from IODOGEN amount, from incubation time and from carrier concentration: all these parameters can be optimized to achieve maximum 1 3 1I BCG labeling. In the present work, the higher labeling yields have been obtained in the following conditions:
200μg of IODOGEN per vial (IODOGEN was coated on the bottom of glass vials Ø 1cm, height 2.5 cm);
reaction time of 1 hour at room temperature; carrier concentration (KI) 0.8 mM
The procedure has been found simple, easy, and highly reproducible in the labeling yield. The reagents and solution used in the labeling were similar to the ones used in the ordinary intravesical instillation of BCG, in order to reproduce, as much as possible, the standard medical procedures.
It is known that radioisotope labeling of cells may result in reduced survival or function, due to the incorporation of the radioisotope into DNA. In order to avoid this fact, the mildest labeling conditions were applied, that only allows the outer membrane radiolabeling.
The definition of a standard protocol for BCG labeling with 1 3 1I using IODOGEN reagent has been considered preliminary to any study of adhesion mechanism of BCG cells to the tumour cells, and/or extracellular matrix proteins.
Due to the fact that IODOGEN labeling uses a slow and mild oxidation step of iodide ions, without the presence of interfering soluble strongly oxidizing molecule (for example Chloramine T), a preservation of the physicochemical integrity of radiolabeled cells and their biological and immunological activity can be foreseen.
The labeled cells are particularly interesting in the in-vivo studies, allowing the definition of adhesion site, stability, and quantification of the internalization of BCG in the tumour cells, both in animals and in humans, by the use of scintigraphic imaging.
It is a well known fact that in the iodine labeling of living cells a low yield is usually obtained, mainly due to the extensive presence of lipoproteins and poor accessibility of tyrosine residues in the membrane of cells. The best operative parameters combination obtained in the present work furnished us a reproducible yield for labeling of about 30%;, that can be considered a quite satisfactory value.
More than this, a clear preliminary evidence (unpublished results) of an un-disturbed biological activity of the radiolabeled BCG cells has been obtained.
References
Akaza H., Iwasaki A., Ohtani M., Ikeda N., Niijima K., Toida I., Koiso K. : Expression of antitumor response. Role of attachment and viability of bacillus Calmette-Guérin to bladder cells. Cancer, 72 (2), 558-563 (1993)
Becich M. J., Carroll S. and Ratliff T. L.: Internalization of bacille Calmette- Guérin by bladder tumor cells. The Journal of Urology 145, 1316-1324 (1991)
Cheng D.L.W., Shu W.P., Choi J.C.S., Margolis E.J., Droller M.J. and Liu B.C.S.: Bacillus Calmette-Guérin interacts with the carboxyl-terminal heparin binding domain of fibronectin: implications for BCG-mediated antitumor activity. The Journal of Urology 152, 1275-1280 (1994)
Fraker p.J. and Speck J.C. jr : Protein and cell membrane iodinations with a sparingly soluble chloramide, 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril. Biochemical and Biophysical Research Communications, 80 (4), 849-857 (1978)
Garden R.J., Liu B.C.S., Redwood S.M., Weiss R.E. and Droller M.J. :Bacillus Calmette-Guérin abrogates in vitro invasion and motility of human bladder tumor cells via fibronectin interaction. The Journal of Urology, 148 900-905 (1992)
Jansson O.T., Marcos E., Brundin L., Lundberg J.O., Adolfsson J., Soderhall M., Wiklund N.P.: The role of nitric oxide in bacillus Calmette-Guérin mediated anti- tumor effects in human bladder cancer. Br. Jour. Cancer 78 (5), 588-592 (1998)
Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL, Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990 Jan;85(1):62-7.
Kuroda K., Brown E.J., Telle W.B., Russell D.G. and Ratliff T.L. : Characterization of the internalization of bacillus Calmette-Guérin by human bladder tumor cells. Jour. for Clinical Investigation 91, 69-76 (1993)
Mizutani Y., Nio Y., Fukumoto M., Yoshida O. : Enhanced antitumor effect of bacillus Calmette-Guérin in combination with fibrinogen on urinary bladder tumor. Journal of Urology 151 (5), 1420-1426 (1994)
Morales A, Eidinger D, Bruce A.W.: Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumours. J Urol. 116,180–183 (1976)
Patard J.J., Chopin D.K., Boccon-Gibod L., : Mechanism of action of bacillus Calmette-Guérin in the treatment of superficial bladder cancer. World Journal of Urology 11 (3), 165-168 (1993)
Ratliff .L., Palmer J.O. McGarr J.A. and Brown E.J. : Intravesical bacillus Calmette-Guerin therapy for murine bladder tumors: Initiation of the response by fibronectin-mediated attachment of bacillus Calmette-Guerin. Cancer Res 47 (7) 1762- 1766 (1987)
Ratliff T.L., Kavoussi L.R., Catalona W.J. : Role of fibronectin in intravesical BCG therapy for superficial bladder cancer. J Urol. 139 (2), 410–414 (1988)
Salacinski P.R.P., McLean C., Sykes J.E.C., Clement-Jones V.V., and Lowry P.J. : Iodination of Proteins, Glycoproteins, and Peptides using a Solid-Phase Oxidizing Agent, 1,3,4,6-Tetrachloro-3α,6α-diphenylglycoluril (Iodogen. Analytical Biochemistry. 117, 136-146 (1981)
Schneider B., Thanhäuser A., Jocham D., Loppnow H., Vollmer E., Galle J., Flad H.D.: Specific binding of bacillus Calmette-Guérin to urothelial tumor cells in vitro. World Journal of Urology 12, 337-344 (1994)
Tuszynski G.P., Knight L. C., Kornecki E., and Srivastava S.“Labeling of Platelet Surface Proteins with 125I by the Iodogen Method”, Analytical Biochemistry vol. 130, pp 166-170, 1983
Zhao W., Schorey J.S., Bong-Mastek M., Ritchey J., Brown E.J., Ratliff T.L.; Role of a bacillus Calmette-Guérin fibronectin attachment protein in BCG-induced antitumor activity. Int. Jour. Of Cancer 86 (1), 83-88 (2000)
ENEA
Servizio Promozione e Comunicazione
www.enea.it
Stampa: Laboratorio Tecnografico ENEA - C.R. Frascati luglio 2018