UNIVERSITA’ DEGLI STUDI DI PISA
FACOLTA’ DI MEDICINA E CHIRURGIA
Spatial distribution of radiation dose to metastatic skeletal tumor
from
153Sm-EDTMP. A Monte Carlo simulation of a micro-CT
based trabecular bone model
TESI DI SPECIALIZZAZIONE IN MEDICINA NUCLEARE
Il candidato
Dr. A. Lorenzoni
Il relatore
1 “Io stimo più il trovar un vero, benché di cosa leggiera, ché il disputar lungamente delle
massime questioni, senza conseguir verità nissuna” Galileo Galilei
2
TABLE OF CONTEXT
Summary
3Introduction
4The clinical problem of bone metastases 6
Radiometabolic therapy with 153Samarium-lexidronam 9
Dosimetric models of trabecular bone 13
Materials and Methods
16Analysis of trabecular bone structure with micro-computed tomography 16
Monte Carlo simulation 18
Results and Discussion
19Conclusion
243
SUMMARY
Bone metastases are a very common problem in clinical oncology, affecting approximately 70% of patients with prostate or breast cancer. Radiopharmaceutical therapy has proved effective with minimal side effects for treatment of refractory painful skeletal metastases of the blastic or mixed type, but administered activities are generally based on limited series of clinical studies without a proven correlation between the activity and the delivered dose to metastatic lesions. In bone pain palliation therapy the red marrow is generally the dose-limiting organ and due to the complex microstructure of the skeleton it has been difficult to calculate accurately the dose deposited to this region. Thus, the estimation of dose to the skeletal system at the microscopic level has been limited by the lack of a realistic characterization of the trabecular bone architecture. The dose distribution to bone marrow of metastatic lesions for the bone seeking radiopharmaceutical 153Sm EDTMP has been evaluated using a Monte Carlo simulation. In this setting a new dosimetric model has been developed based on the micro-CT analysis of bone metastases to investigate the morphology, topology and texture of bone samples based on assessment of the micro-structural parameters classically evaluated with histomorphometry. The distribution of radiation dose and the mean absorbed dose per unit cumulated activity (S value) were computed for bone marrow space.
4
INTRODUCTION
Bone metastases are a very common problem in clinical oncology, affecting a large number of patients with different primary tumors. Approximately 70% of patients with prostate or breast cancer and 35% of those with advanced lung, thyroid, and kidney cancers will develop painful skeletal metastases which cause considerable morbidity and, ultimately mortality (1). Bone metastases are generally classified as either osteolytic or osteoblastic, reflecting dysregulated bone destruction or bone formation as the predominant mechanism. Certain characteristics of cancer-related pain, including its multifocal nature, severity, heterogeneity, progressive nature, and other special challenges, distinguish it from most other types of chronic pain. The management of bone pain is a challenging clinical problem and involves a multidisciplinary approach which includes analgesics, hormone therapies, bisphosphonates and external beam radiation (2). Systemic radiopharmaceutical therapy, either as monotherapy or combined with radiosensitisers or chemotherapeutics, has proved effective
with minimal side effects for treatment of refractory painful skeletal metastases of the blastic or mixed type (3). The radiopharmaceuticals include 89Sr-chloride (89SrCl2), 186 Re-1-1-hydroethylidene diphosphate (186Re-HEDP) and 153Sm-ethylene diamine tetramethylene phosphonate (153Sm-EDTMP). These agents differ in terms of efficacy, duration of pain palliation, tumoricidal effects, suitability for repeat treatments; toxicity, and cost. Samarium-153-EDTMP is a complex of radioactive Samarium-153 and a tetraphosphonate [ethylenediamine-tetramethylene phosphonic acid (EDTMP)] with high affinity for the skeletal tissue, where it concentrates by chemio-absorption in areas of enhanced bone turnover. The overall response rate ranges between 65%-80% with complete response in 10%-30% of the cases associated with mild and reversible hematologic toxicity (4). The
schedules and radioactivity administered activities, derive from a limited series of clinical studies, and therefore may be considered empirical (5,6,7,8). The limitation of this therapeutic approach is the absence of a clear, well proven relationship between the administered activity and the dose delivered to metastatic lesions. Furthermore an accurate skeletal dosimetry is important because of the high radiosensitivity of the hematopoietic cells contained within the marrow cavities of trabecular bone, as well of the osteogenic cells on the surfaces of the bone trabeculae. The difficulty to calculate accurately the dose deposited to this region is primarily due to the complex microstructure of the skeleton. The choice of optimal administered activities based on dosimetry could lead to a modification in treatment plans, also considering the emerging prospective of combined strategies with anti-tumor
5 agents, such as chemotherapy. The radiation dose to bone from bone-seeking radiopharmaceuticals has been modeled over the last 40 years. Different models of electron transport in trabecular and cortical bone for the adult have been proposed such as the three-dimensional transport model by Bouchet et all (9,10) based on morphometric data originating by the research group of Spiers (11); from these data the mean absorbed dose per unit cumulated activity (S value) has been systematically computed for several bone seeking nuclides and geometries (12). The most commonly employed dosimetry model provides only a first order of approximation based on the assumptions of homogenous lesion morphology, uniform distribution of radioactivity within the lesion and complete energy deposition within the lesion by charged particles. The estimation of microscopic dose to skeletal system has os fast been limited by the lack of a realistic characterization of the trabecular bone architecture, derivable from a direct imaging technique with sufficient spatial resolution to be useful in dosimetric models (13). Micro-computed tomography (μCT; micro-CT) is an important approach to analyze quantitatively the trabecular bone structure in three dimensions, providing direct measurement of trabecular thickness, number thickness and separation thickness with high correlation between morphometric analysis performed with μCT and conventional histology (14). Indeed, dysregulation of the normal bone remodeling process of bone resorption and bone formation that ordinarily maintains skeletal integrity leads to important alterations in bone structure, assessable with bone histomorphometry. Dosimetry is not a trivial issue: the general case requires extensive modelling while patient-specific dosimetry requires actual data on activity uptake and the size of the lesions. Integral dose is, of course, more difficult to estimate than dose rate as more experimental data are required. The Monte Carlo method has provideduseful information through the simulation ofradiation transport and with improved computer technology, more complex methods may be used in radiationtherapy such as EGS4and MCNP-4B (15,16). The MCNP (Monte Carlo N-Particle) code was developed at Los Alamos Laboratory (Los Alamos, NM) originally as a neutron and photon transport for reactor analysis. This method is widely used to solve complex physical problems, particularly those involving multiple independent variables through the generation of random histories of particles.
The aim of this study is estimate the delivered radiation doses to bone marrow of metastatic lesions for the bone-seeking radiopharmaceutical 153Sm EDTMP, using a Monte Carlo simulation. The development of a realist model is based on μCT studies of metastatic bone
6
The clinical problem of bone metastases
Bone metastasis is a major complication of several solid cancers; approximately 70% of patients with prostate or breast cancer and 35%-42% of those with advanced lung, thyroid, and kidney cancers will develop pain skeletal metastases which cause considerable morbidity and mortality. The dimension of the clinical problem is substantial, since cancers of the prostate, breast, and lung account for about 45% of cancers in all sites. Comprehensive evaluations must be made to determine the etiology of the pain and any possible complicating factors, such as cord compression, neuropathic conditions, and impending pathologic fractures. Indeed the annual incidence of skeletal complications is 1.5– 4.0 events per year in patients with bone metastasis (1). Despite its clinical relevance, metastasis remains the most poorly elucidated aspect of carcinogenesis. The biological mechanisms leading to bone metastasis have been referred to a complex network between cancer cells and the bone microenvironment, although the precise molecular mechanisms underlying this process remain to be elucidated (17). The molecular mechanism of bone metastases is an area of active investigation, resulting in the development of several bone-targeted therapies including a monoclonal antibody targeting the receptor activator of nuclear factor (NF)-κB ligand (RANKL). Considering the multistep process of tumor metastasis, several of these steps can represent a potential target for therapeutic intervention.
The pathophysiology of bone metastases
The development of bone metastases is a complex process that occurs as a result of a pathophysiologic interactium between tumor cells and bone microenvironment involving to tumor-cell invasion, cell migration and adhesion, and stimulation of osteoclastic and osteoblastic activity. Each of these discrete steps represents cellular interactions caused by specific determinants of both the tumor and the tissue. Bone metastases most commonly affect the axial skeleton and the proximal ends of the long bones, the ribs, and the spine, that are particularly rich in red bone marrow, this pattern of distribution suggests that properties of the circulation within the bone marrow play an important role. In 1942 Batson et al. (18) suggested that venous blood in the pelvis and breast flowed not only into the vena cava, but also into a vertebral-venous plexus of vessels extending from the pelvis throughout the epidural and perivertebral veins and into the thoraco-abdominal wall, head, and neck; this system could facilitate in the local nesting of tumor cells and also their progression into
7 bone metastases. Liotta and Kohn (19) reported that the distribution of metastases can be predicted by the anatomic distribution of blood flow from the primary site in only 30% of cases, while specific properties of the tumor cell and features at the metastatic site determine where the metastasis occurs in the majority of cases. In physiological conditions bone undergoes dynamic remodeling by a coupled and sequential process of osteoclast-mediated bone resorption followed by osteoblast-mediated bone formation, that maintains the balance between bone formation and degradation. Systemic factors, local osteoclast-activating cytokines and local bone-derived growth factors, such as the parathyroid hormone, thyroxine, prostaglandins, bone morphogenic proteins (BMPs), several local and systemic factors that are important for bone regulation, including receptors for transforming growth factor β (TGFβ), insulin-like growth factor (IGF), and interleukin (IL)-1 and IL-6 contribute to the bone remodeling process (20). Osteoblasts express receptors for PTH, prostaglandins, estrogen, vitamin D3, and cytokines such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and TGF. Moreover they are involved in the regulation of osteoclast differentiation via expression of the receptor activator of nuclear factor (NF)-κB ligand (RANKL) on its cell surface (21). The Wnt pathway (Wnt/β-catenin signaling) has recently been identified as a major regulator of osteoblast function and bone formation inducing an increased bone mass. Several Wnt proteins are expressed by osteoblasts and stimulate osteoblastogenesis via distinct mechanisms, including attenuation of peroxisome proliferator-activated receptor γ (PPARγ)-induced adipocyte differentiation (22). Osteoclasts are derived from precursor cells of the monocyte-macrophage lineage that differentiate into inactive osteoclasts. RANKL is a potent inducer of osteoclast production generally expressed on the surface of osteoblasts and stromal cells or released as a soluble variant by activated T cells. Its production is influenced by osteotropic factors such as parathyroid hormone, 1,25-dihydroxyvitamin D and prostaglandins (23). Osteoclast production is stimulated by 6, IL-1, prostaglandins, and colony-stimulating factors (CSFs) by osteoblasts, while immune cells, such as T cells, produce IL-4, IL-18, and interferon γ, which negatively regulate osteoclasts formation. Moreover, osteoclast-mediated secretion of proteases leads to degradation of the bone matrix, while the adherence of osteoclasts to the bone surface is found to be critical for bone resorption. Metastasis of tumor cells to specific sites in the skeleton is a complex multistep process not completely elucidated which includes tumor cell invasion, migration, cell matrix adhesion or cell-to-cell adhesions, interaction with endothelial cells, regulation of growth factor, and stimulation of osteoclasts and osteoblasts. Cadtherins, integrins,
8 imuunoglobulins, selectins, and CD44 are some of the molecules implicated in loss of cellular adhesion that causes cell matrix detachment, invasion, and migration. The bone microenvironment is a source of immobilized growth factors like TGFβ, IGF-1, FGF, PDGF, BMPs, cytokines, chemokines, calcium ions, and cell adhesion molecules that promote growth and proliferation of metastatic cancer cells. Cancer cells also produce cytokines and growth factors that directly or indirectly impact osteoclastic bone resorption (24,25). Bone lesions are commonly radiographically classified as osteolytic (when bone destruction arises by the action of osteoclasts) or osteoblastic (in which sclerotic processes predominate). However, a mixed pattern is common in many lesions, and marker studies suggest that both resorption and formation occur simultaneously. Osteolytic metastases occur more commonly than osteoblastic lesions and have been described in cancers of breast, lung, thyroid, and renal origins. Frequent complications of osteolytic metastases are severe pain, pathological fractures, life-threatening hypercalcemia, spinal cord compression, and other nerve compression syndromes. Calcium released from the bone matrix in the course of this process can lead to the hypercalcemia of malignancy (HCM), a serious metabolic condition. Osteoblastic metastases, predominant in prostate cancer, are characterized by osteoblast proliferation, differentiation, and bone formation. Patients with osteoblastic metastases have bone pain and pathological fractures resulting from the poor quality of bone produced by the hyperactivated osteoblast (25). A major complication associated with bone involvement is severe pain, with variable intensity and intermittent, which later becomes chronic pain. Bone pain is thought to be distinct from neuropathic or inflammatory pain, where there is upregulation of the glial fibrillary acidic protein in the spinal cord indicating astrogliosis. The exact mechanism of cancer pain is unknown and it is postulated that the pain may be due to the presence of tumor in the bone and linked to the extent of osteoclastic activity. Bone resorption and tumor growth are the most important factors of pain onset (26). The trabecular pattern of bone, indicating connectivity within the trabecular architecture, is increased in bone metastases. The tumor releases osteoclast maturation factors or stimulates osteoblast secretion of RANKL and down-regulation of osteoprotegerin, thus resulting in dominant resorptive activity (27-28). The acidic microenvironment produced by osteoclasts contributes significantly to bone cancer-associated pain through activation of acid-sensitive nociceptors that innervate the marrow, mineralized bone, and periosteum. Sensory neurons express different acid-sensing ion channels: transient receptor potential vanilloid 1 (TRPV1) and acid-sensing ion channel-3 (ASIC-3), sensitized by a decrease in pH in the range of 4.0–5.0.
9 Therapeutic validity would therefore be supported by an appropriate correlation between tumor development and activity profiles versus pain intensity. Furthermore, tumors secrete a variety of factors that sensitize or directly excite primary afferent neurons, causing the sensation of pain. In this setting nerve growth factor (NGF) may directly activate sensory neurons that express the TrkA receptor and/or modulate the expression of proteins. The quality of bone cancer pain is characterized by substantial heterogeneity, either due to the cancer or resulting from the alteration of distinct bone structures. Bone cancer seems to generate a profound reorganization in the dorsal root ganglia (DRG) and spinal cord at both neuronal and glial levels (29). Breakthrough pain episodes frequently occur as the tumor infiltrates the bone matrix. Moreover neoplastic cells seem to secrete a variety of factors that sensitize or directly excite primary afferent neurons causing bone pain. Neuronal hyperactivity accompanying hyperalgesia and allodynia is also detected in the spinal cord, reflecting the central sensitization phenomena frequently observed in chronic pain states. In neuropathic pain, non-nociceptive Aβ fibers are stimulated, increasing neuronal firing towards lamina V-VI of the spinal cord. Therefore, Aβ fiber over-activation may be related to acidic tumor environment or nerve compression, thus contributing to c-Fos activation (a marker of neuronal activity) and the peptide dynorphin (30). In addition to prostaglandins and other factors released by the tumor, bone resorption results in the release of protons that will stimulate nociceptors. The number of osteoclasts is therefore an indicator of potential bone pain onset and should be screened in every bone cancer model.
Radiometabolic therapy with
153Sm-lexidronam
Systemic therapy with radionuclides linked to a bone-seeking agent may be the option of choice for the treatment of patients with multiple skeletal localizations, providing a promising pain-control strategy (31). The efficacy of bone-seeking radiopharmaceuticals relies on their selective uptake and prolonged retention at sites of increased osteoblastic activity. The exact mechanism of action is not fully understood, but it involves the reduction of cytokines and growth factors released by tumor anti-inflammatory cells at the interface between tumor and normal bone and radiation-induced mechanical factors, such as reduction of periosteal swelling (32).
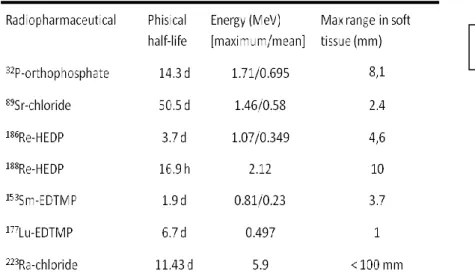
Several radiopharmaceuticals for treating painful bone metastases have been developed (Table 1) and those commercially available include 89Sr, 186Re chelated with
10 hydroxyethylidene diphosphonate (HEDP) and 153Sm chelated with ethylene diamine tetramethylene phosphonate (EDTMP).
The choice of the radiopharmaceutical is based on the physical characteristics of the radionuclide in relation to the extent of metastatic disease, on the bone marrow reserve and on the local availability of the radiopharmaceutical in a given countries. The nature of the emissions (β, internal conversion, or Auger electrons) determines the therapeutic suitability of the radionuclide, while the particle range influences treatment-related toxicity (33). Beta emitters with short half-lives, such as 186Re and 153Sm, deliver their radiation at higher dose rates which may be more therapeutically effective than equivalent doses given at lower dose rates. The sources of radiation within bone differ with the radiopharmaceutical used: the metallic chelated radiotracers tend to chemically absorb to the trabecular surface, whereas 32P and 89Sr (as the chloride) distribute more widely throughout the bone. Due to the heterogeneity of radiopharmaceutical uptake, spicule thickening, and tumor and marrow distribution, there is wide variation in dosimetry estimates described in the literature (34). General requirements for an optimal bone-seeking radiopharmaceutical to be employed for the palliation of painful bone metastases include a selective uptake and prolonged retention at metastatic sites in contrast to normal bone (i.e., a high tumor-to-non tumor ratio), a rapid clearance from soft tissues, a good radiochemical stability and acceptable toxicity. Furthermore, an appropriate patient selection is crucial for the success of the therapy. Other sources of pain, such as vertebral collapse, nerve root entrapment, fracture and visceral pain, will not respond to radionuclide therapy. Response is less predictable in patients with a
Table 1. Main characteristics of bone-seeking radiopharmaceuticals
11 predominantly osteolytic pattern of skeletal metastasis, presumably because poor uptake and retention result in a lower absorbed radiation dose at the metastatic site.
153
Sm is reactor-produced in high radionuclidic purity by neutron bombardment of 152Sm2O3.
During neutron irradiation several low-yield reaction also occur, including spontaneous decay of 153Sm to stable 153Eu, then neutron capture to form 154Eu, a γ-emitting radioisotope.
154
Eu-EDTMP can be detected (35); 153Sm has a complex decay scheme with X-rays, gamma rays and atomic electrons (Auger and conversion) in addition to the dominant beta decay (see Table 2) with a physical half-life of 46.3 h. The maximum energy of the beta emissions is 0.81 MeV and the mean energy is 0.23 MeV, resulting in an average penetration range of 0.83 mm in water and a maximum penetration range of 1.7 mm in bone and of 3.1 mm in soft tissue. The β-particles provide more than 99% of the dose locally, due to the range of electrons of 2-3 mm with energy between 600 and 800 keV, while energy equal to the average of the transition of samarium is less than a millimeter (36). This condition permits an optimal control of the distribution of the dose, which is almost completely delivered in the area of radiotracer uptake. The main gamma emission (103 keV, 29%) allows the evaluation of the radiopharmaceutical biodistribution and dosimetric studies, through gamma-camera acquisition. 153Sm is complexed with EDTMP with a percentage of complexed radionuclide exceeding 99%, resulting in a preparation chemically stable and without any appreciable decomposition for more than 48 hours (37). 153Sm-lexidronam (153Sm-EDTMP) is commercially available (Quadramet®, Schering, Berlin) as single-dose vials with a concentration of 1.3 GBq/ml and a specific activity of 28–65 MBq/µg. The radiopharmaceutical shows a rapid and high uptake into the bone, with a lesion-to-normal bone uptake ratio equal to approximately 5. It concentrates by chemoabsorption in areas of enhanced metabolic activity, associating with the hydroxyapatite crystal of the bone matrix. Washout of the radiopharmaceutical from hydroxyapatite is about 20-30 days and its redistribution to normal skeleton or marrow in normal bone is negligible (38) Generally, 50% or more of the injected dose binds to bone, varying however with on the extent of metastatic disease. 153Sm-EDTMP biodistribution is nearly identical, to the blood clearance properties of
99m
Tc-hydroxymethylene diphosphonate (HDP) and 99mTc methylene diphosphonate (MDP). The lesion-to-normal bone ratios for 153Sm-EDTMP and 99mTc-HDP is about 4 and lesion-to-soft tissue ratios about 6. Similar data have been reported for 99mTc-MDP (39). 153
Sm-12 EDTMP is cleared rapidly from the blood following a bi-phasic or tri-phasic exponential pattern, (50% with T1/2=2 2 min, 30% with T1/2=225 min and 20% with T1/2=2 300 min). One hour after administration, less than 1% of the dose remains in the circulation, without formation of appreciable metabolites (39). Urinary excretion is the main route of elimination and is complete within 6 hours. The bladder dose is inversely related to the extension of
metastatic disease and it shows extremily variability ranging from 12 cGy/GBq to (40) to
0.964 cGy/MBq (41). A moderate hyperhydration of the patient to increase the frequency of
bladder voiding is recommended. The red marrow is generally the dose-limiting organ and
the major toxic effect observed was myelosuppression, thrombocytopenia being dose limiting in 20–42% of patients (42). Major myelotoxic effects occur when the activity administered is one order of magnitude higher than the approved activity, as typically occurs in the treatment of osteosarcoma; in such conditions stem cell transplantation is required (35).
Mild to moderate myelosuppression associated with 153Sm-EDTM therapy is present in
approximately 40%-50% of the patients and WHO grade III and IV toxicity is less than 10%. Blood count nadirs typically occur 3-5 weeks after treatment and the decline is reversible in up to 97% of the patients with complete recovery within 8 weeks (43). Several retrospective studies have shown that bone marrow suppression is primarily related to bone marrow
involvement, old age, marrow atrophy caused by prior treatments and 153Sm-EDTM repeated
therapy (44). The red marrow absorbed dose may be increased in patients with concurrent of radiotherapy or multiple radionuclide treatments. Administration of approved activity (37 MBq/kg) has been selected on the basis of data obtained in relatively small series studies aiming at pain control with an acceptable red marrow toxicity, resulting in a marrow dose that is generally lower than 2 Gy (43). The marrow absorbed dose ranges from 0.3– 2.1 mGy/MBq, while median value of bone surface absorbed dose is 4.4 mGy/MBq (range
2.3-6.8 mGy/MBq) (45). Furthermore the relevant variability in the biodistribution suggests that the fixed administered activity based on patient weight is not sufficient to optimize the treatment (45). A clear relationship between therapeutic efficacy and increase in administered activity (not absorbed dose) has not yet been established.
Localization of the radiopharmaceutical in the bone marrow is minimal due to the rapid plasma clearance that makes the red marrow dose from activity in the blood negligible. The radiation-induced marrow toxicity is primarily produced by the beta particle irradiation from
13
the 153Sm-EDTMP localized on the endosteal surface. The diffusion of radioactivity into the
bone mineral (if any occurs) can be neglected due to the short half-life of 153Sm and thus a
surface model of deposition is most appropriate. Furthermore, the bone marrow irradiation is difficult to establish in the individual patients because of the heterogeneity of the activity within a given bone between trabecular and cortical bone and heterogeneity of red marrow distribution.
Thus, the actual biological response will also be critically dependent on the marrow reserve of the individual patient. A surface model of distribution of 153Sm-EDTMP is most appropriate to describe the localization onto the bone. Published dosimetric estimations have used the MIRD formalism with different S-factors and biokinetic data . The dose estimations
to bone marrow in published studies are 64.1±18.7 cGy/GBq (40), 1,514±261 Gy/MBq (41),
1.86 mGy/MBq (36), and1.03 mGy/MBq (46).
A transient increase in bone pain (flare phenomena) occurs after therapy in about 10–20% of the cases, usually within 72 hours, typically transient, mild and self-limiting and generally
associated with clinical response (43).Palliation of bone pain has been shown in 70–95% of
patients. Several studies have reported on the toxic effects and efficacy of increasing doses of
153
Sm-EDTMP from 27·8 to 1110 MBq/kg. Repeat dosing of 153Sm-EDTMP can improve the duration of pain response and survival without increasing hematological toxicity (47,48). The combination of radionuclide therapy with anti-tumor agents such as chemotherapy with the aim of prolonged survival has been evaluated in several studies. The concurrent administration of docetaxel and repeated administration of 153Sm-lexidronam in patients with castration-resistant prostate cancer has shown encouraging results in term of feasibility and safety (49,50,51).
Dosimetric models of trabecular bone
In radionuclide therapy for bone pain palliation the bone marrow, which is contained within the marrow cavities of trabecular bone, represents the dose-limiting organ. The trabecular bone, characterized by a complex microstructure of network of trabeculae and tissue cavities, is also constituted by the endosteum, a layer of connective tissue, populated with osteoblasts and osteoclasts another important dosimetric tissue. This complex structure of the trabecular bone without a realistic characterization of the bone architecture has limited the accurate estimation of the dose deposited by bone-seeking radiopharmaceuticals. However, recently
14 new imaging techniques have permitted the direct and nondestructive imaging of trabecular bone with sufficient spatial resolution to allow the construction of internal dosimetric bone model (52). The bone models for internal dosimetry have been under investigation over the last 40 years, however based on optical scanning data on skeletal samples from a few individuals.
Previous dosimetric models of the skeleton are based on the chord-length distributions that represent the distribution of distances between entry and exit points of a trabecular bone region, measured by Spiers and colleagues (53,54) The distribution of linear path lengths through marrow cavities and trabeculae skeleton of seven bone sites was used as a quantitative description of 3D structure of the bone. At the present time these data represent the most complete information available on the microstructure of both trabecular and cortical bone used in dosimetry. Subsequently, Whitwell and Spiers calculated dose conversion factors for seven bone surface-seeking radionuclides (14C, 18F, 22Na, 32P, 45Ca, 90Sr and 90Y) (55,56) These dose conversion factors were used for deriving radionuclide S values for skeletal tissues by Snyder et al. (57,58) The S value and the photon-specific absorbed fractions in a heterogeneous phantom, were published in the Oak Ridge National laboratory (ORNL) Report No. 5000 (ORNL5000) and subsequently as a part of Medical Internal Radiation Dose (MIRD) Pamphlet No.11 (58). The method proposed by the MIRD Commitee is a set of general equations which can be adapted to anatomical and kinetic complex models, allowing the calculation of the dose absorbed by each organ as a result to incorporation of one or more radioisotopes.
An important limitation of this approach was the use of the conservation of energy and the uniform isotropic model, valid in homogeneous media. Three source regions (trabecular bone, cortical bone and red marrow) along with two target regions (red marrow and skeletal tissue) were considered. In 1979 the International Commission on Radiological Protection (IRCP) published the Report No. 30 (59) with the recommended absorbed dose fractions of energy. β particles originating on the bone surface, different absorbed doses fraction are recommended based on the energy of particles (mean energy < 0.2 MeV and ≥ 0.2 MeV). Radiopharmaceutical sources were located on the bone surface when the radionuclide physical half-life was less than 15 days or otherwise within the matrix. These absorbed fractions were implemented in the MIRDOSE2. The ICRP 30 model is adequate for radiation protection, but is not useful in predicting marrow doses in radiometabolic therapy.
15 In 1985 Eckerman et al (60) reported a 1-dimensional model of electron transport, extending the methods of Spiers to radionuclides within the marrow to provide for dose conversion factors seven skeletal regions in computational models. This model provided direct calculations of absorbed dose to the bone marrow and the development of dose-volume histograms, which show what fractions of the marrow receive different absorbed doses. The S values derived were implemented in MIRDOSE3. In MIRDOSE3 the target regions are considered the red marrow, the cortical bone volume, the trabecular and cortical bone surface.
This model, similarly as the Spiers’s model, is based on the assumptions that the particle trajectories are linear through both the bone matrix of the trabeculae and the marrow cavities. Furthermore the energy loss within these structures is considered under the continuous slowing-down approximation and the transport of energy by delta rays and bremsstrahlung photons are ignored. Bouchet et al (61) developed a three-dimensional electron transport using the chord length distribution published by Spiers et al, considering the electron backscatter, delta rays and bremsstrahlung photons to derive radionuclide S values for 22 skeletal sites (62). Three source and target regions (the trabecular marrow space, the trabecular bone endosteum, and the trabecular bone volume) are considered. The values calculated were similar to those of the Eckerman model at most energy, when corrected to the same input assumptions. This model differs from the other in the definition of the red marrow region, of the surface source of activity and in the assumption applied in transporting electrons through the trabecular endosteum. The S value of bone marrow considered as source and target calculated by Eckerman are 50% lower than those calculated by Bouchet et al. The different assumption was the consideration of the marrow cellularity in the first case. The dose factors in the Bouchet model for bone surface sources result highest, assuming that the source is distributed in the endosteum, considerated instead part of the red marrow by Eckerman. The most commonly employed dosimetry model provides only a first order of approximation based on the assumptions of the homogenous lesion morphology, uniform distribution of radioactivity within the lesion and complete energy deposition within the lesion by charged particles. The MIRD 11 model gives marrow S values for the reference adult male, while the Eckerman, Bouchet et al., and “revised” models give doses to different regions of the skeleton, and for adult males and females, as well as for children. Trabecular bone dosimetric model designed specifically to calculate the dose from the surface-seeker
186
Re, measuring chord length distributions of cavities and bone trabeculae on 25 samples from skeletal metastases (63). Bone trabeculae were then represented by ellipsoids located in
16 an infinite marrow-tissue medium. The transport of electrons was simulated in a three-dimensional geometry using the Monte Carlo electron transport code EGS4. The 3D radiation transport techniques based upon images acquired with NMR microscopy showed that the Eckerman model underestimates values of the absorbed fraction for self-irradiation of the active marrow at energies below 200 keV and that Bouchet et al. model overestimates these same values at energy exceeding 20 keV. Neither model accurately predicts the absorbed fraction in the energy range of 20 keV to 200 keV. Probably for some radionuclides, depending on physical half-life and radiochemical features , the “bone surface” sources, may be better represented as being distributed in a thin layer close to bone (as in the Eckerman model) or throughout the endosteal space (as in the Bouchet et al. model) (64). At the present time the Eckerman model is recommended, as well as reported by the ICRP in several publications. In 1995 Samaratunga et al (65) developed a complex trabecular model to calculate the dose to bone metastases from 186Re-HEDP. The geometric model was obtained from histomorphometric measures and the deposition pattern of the radiopharmaceutical was evidenced by autoradiographic data.
MATERIALS AND METHODS
The spatial distribution and the delivered radiation doses to bone metastasis for the bone seeking radiopharmaceutical 153Sm-EDTMP were evaluated. Micro-CT of bone metastatic biopsies has allowed the evaluation of the complex bone trabecular morphology. The micro-architectural parameters calculated have been used to develop a geometrical model used for Monte Carlo simulation.
Analysis of trabecular bone structure with micro-computed tomography
Estimation of a realistic bone microstructure is required to better develop radiation dosimetry models. Traditionally, the quantification of structural parameters in vitro, in terms of morphology, topology, and texture, was based on histomorphometry of two-dimensional (2D), with high spatial resolution and high image contrast. However this destructive method does not allow further measurements, such as analysis in different planes. The structural parameters are measured from sections, and the third dimension is added on the basis of stereology. Nondestructive 3D imaging of trabecular bone, such as micro-computed tomography (μCT; micro-CT) and micro magnetic resonance imaging (µMRI), can quantify
17 accurately the structural properties of bone, showing an excellent agreement with the indices assessed from conventional histomorphometry (66). The development of the first micro-CT system was pioneered by Feldkamp et al. (67) for the 3D analysis in vitro of small bone sample with a resolution of 50 µm. The creation of a digital model of bone trabecular with µCT has allowed the comparison of 2D and 3D structural analysis methods and the development of algorithms to calculate the classic structural parameters. Several studies have shown that 3D imaging method, with a resolution ranging from 15 to 30 µm, quantifies with high correlation the structural properties of bone compared to conventional histomorphometry (66,68). A resolution of 20 µm is considered adequate without invalidating a comparison with stereology performed on 2D sections obtained at a section thickness and pixel size of 10 μm.
Several micro-architectural parameters have been developed to describe the bone structure, based on Parfitt’s principles of the “plate and rods” model and derived by the measurements of trabecular surfaces and perimeters of the samples. These descriptors are the trabecular thickness, trabecular number (a measure of plate density) and trabecular separation (a measure between trabeculae) and it can be obtained as direct 3D model-independent measurement (69). Other parameters have been developed based on stereology and 3D imaging, using techniques independent of surfaces and perimeters measures. In this setting the fundamental parameters for describing trabecular bone include the bone volume/tissue volume ratio, the anisotropy, the connectivity density and the structure model index.
The bone volume/tissue volume (BV/TV, %) value estimates the quantity of bone and it is calculated by the number of voxels in the volume of interest (VOI) considering the total numbers of voxels in the VOI. Anisotropy is an important parameter to describe the architecture, defining the orientation of trabeculae. Connectivity density express the number of trabecular connection per cubic millimeter (1/mm3) and it represents the number of the element that may be removed without structural alterations, consisting in the split into two parts. It is derived from the Euler number, as (1-Euler Number)/VOI. The Euler number is the number of particles of a structure plus the number of enclosed cavities minus the connectivity. The structure model index (SMI) estimates the plate-rod characteristics of the structure, ranging from 0, if the network is mainly composed of plates, to 3 when rods prevail.
Twenty one bone samples of metastatic tumors have been analyzed by a micro-CT with resolution 20x20x20 μm3 (X-ray voltage 70 kVp). The primary cancer was prostate carcinoma in 15 cases, colon cancer in 4 cases and breast and lung carcinoma in the
18
remaining 2 samples, respectively. The diagnosis was confirmed histologically. The mean total tissue volume (TV) of the samples, such as the bone volume (BV) and bone volume/tissue volume (BV/TV,%) was directly measured by 3D images. These parameters were calculated using distance triangulation model, without the necessity of a prior model. This approach was also used for the evaluation of the connectivity density (ConnD), the
triangulated Structure Model Index (TRI-SMI), the trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp). The apparent attenuation (total attenuation of x-ray beam through entire biopsy sample) due to bone mineral and bone marrow contents and the bone attenuation due to the attenuation of x-ray beam due to the mineral bone component, were also evaluated for each sample. The triangulate surface of trabeculae/bone volume (TRI-BS/BV), triangulate anisotropy (TRI-DA) and Heigen vectors (TRI-|Hx|) were calculated based on plate model. The micro-structural descriptors of the prostate bone metastases were compared to other primary tumors using the Mann-Whitney U-test and the statistical significance was defined as P <0.05.
Monte Carlo simulation
The Monte Carlo method is a computational technique based on statistical physics, differing from deterministic transport methods which solve the transport equation for the average particle behavior. Therefore Monte Carlo is useful to solving complicated three-dimensional time-dependent problems. The Monte Carlo N-Particle (MCNP) code was developed during the Manhattan Project at Los Alamos Laboratory (Los Alamos, NM) during World War II, originally as a neutron and photon transport for reactor analysis. It is capable to simulate photon and electron transport down to energy of 1 KeV. In this work Monte Carlo based code MCNP5 (70) was used to evaluated the transport of electrons emitted by 153Sm from trabecular bone. The mean dose per unit cumulated activity (S value) was calculated considering the bone marrow space as target region. The marrow space is considered the volume between bone trabeculae excluding the endosteal layers, differing from bone marrow cavity in which the total volume into the trabeculae is considered. The dose distribution within a bone marrow spaces is evaluated considering a discrete model, or rather subdividing the target region in several equal zones and evaluating in each regions the S value. The selected data library cross-section was the EDNF/B-VII (71). The energy deposition function tally *F8 (70) was used to score the doses in unit MeV per starting particle per tally volume,
19 to obtained S value (Gy/decay). The source distribution was modeled using an isotropic distribution with an average value of 0.2246 MeV. Periodic boundary condition were used at the interfaces (70). The elemental compositions are derived from ICRU Reports 44 and 46. The NPS option was used with 1.0x106 electron histories simulated with a standard deviation of 10-4. All the estimated standard deviations resulting from MCNP5 calculations reported hereafter should be intended at the 68% of the confidence level. Only electron were considered in the dose calculation, whereas the contribution of γ-photon to the S value was considered negligible and ignored. 153Sm was assumed to be distributed homogeneously on the bone surface. The simulation time was about 16 hours using current generation Intel CPU (INTEL®, “Quad-Core Intel® Xeon® processor 5400 Series, INTEL® Datasheet).
RESULTS AND DISCUSSION
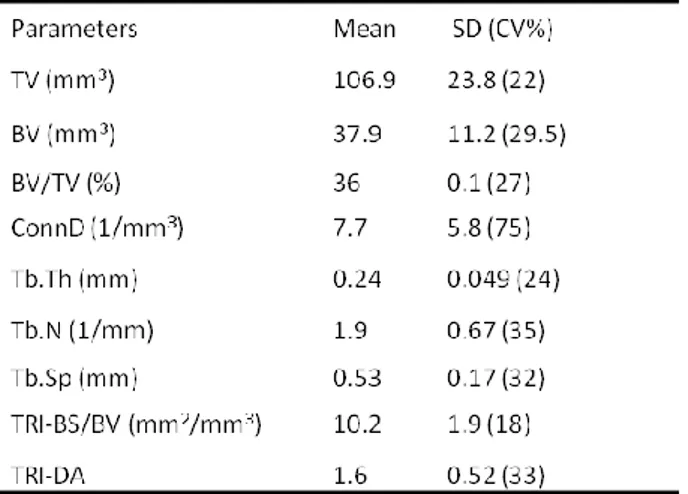
The micro-structural parameters calculated based on µCT analysis of the metastatic bone samples using distance triangulation model are summarized in Table 2. The mean total tissue volume (TV) of the samples was 107 ±24 mm3 with a percent coefficient of variation (CV%) of 9 and the bone volume/tissue volume (BV/TV,%) value estimated was 0.37 ± 0.1, with CV% of 27. The apparent attenuation and bone attenuation/cm was respectively 1.73±0.5 and 3.38±0.74. The triangulate surface of trabeculae/bone volume (TRI-BS/BV), triangulate anisotropy (TRI-DA) and Heigen vectors (TRI-|Hx|) were also calculated.
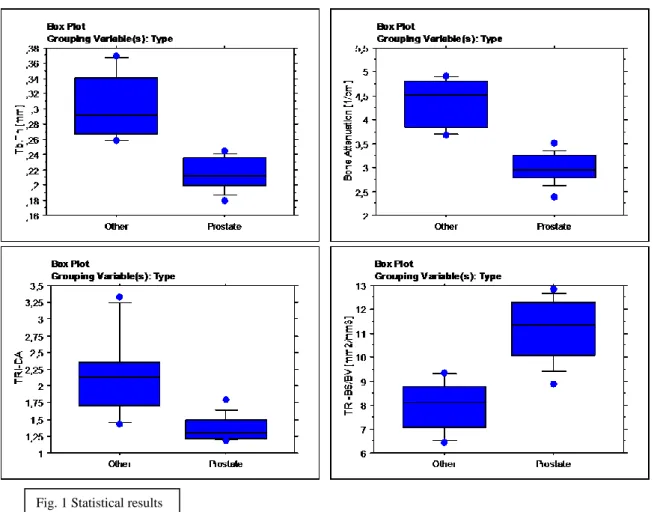
The micro-structural descriptors of the prostate bone metastases were compared to other primary tumors. Two groups differ statistically in terms of Tb.th, apparent and bone attenuation, TRI-BS/BV, and TRI-DA (see fig1). The trabecular thickness in prostate bone
Table .2 Main
microstructural parameters calculated
20 metastases was significantly lower than in the other group (p<0.01), as also were the apparent and bone attenuation (p<0.01). TRI-BS and TRI-BS/BV in prostate bone metastases was higher than in the other group (p<0.01), and anisotropy resulted significantly lowest (p<0.01).
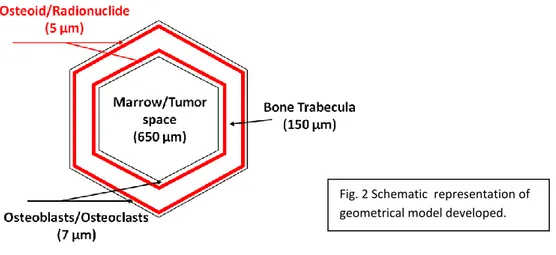
A geometrical model of trabecular bone was developed based on these structural of metastatic trabecular bone, resulting in a set of several concentric hexagonal prisms containing respectively from the center to the periphery the bone marrow, the osteoblast/osteoclast layer, and the bone trabecula. The radionuclide sources were homogeneously included in a thin layer of osteoid of 5 µm. The bone marrow space has a diameter of 650 µm, and the bone trabecular thickness of 150 µm. (see Fig. 2).
21 The electron path length of 153Sm is obtained considering one source point and the symmetry of the model. Indeed the collision track length of electrons emitted from a source of 153Sm in a bone space is the result of the distribution in the contiguous bone trabeculae. The β-particles emitted in all bone trabeculae lead to the collision track in a single cavity which is shown in Figure 3.
Moreover, multiple sources located in different trabeculae have been simulated and the respective track electrons are obtained (see Fig. 4). The electron path length represented in the trabeculae and marrow cavities is the results of the electron irregular paths through the material due to the progressive loss of the energy. This track length is determined by the reduction of the kinetic energy due to ionizations and formation of Bremsstrahlung radiation resulting in changes of directions.
Fig. 2 Schematic representation of geometrical model developed.
Fig .3 Collision track length of electrons (in blue) from a source of 153Sm. Periodic boundary condition is used at the interfaces
22 In this work the dose distribution within a bone marrow spaces is found to be homogenous, without significantly difference increasing the depth from the surface of the radiation source. These results have been obtained with the quantification of the mean dose per unit cumulated activity in discrete areas of the bone marrow space (see Fig. 5).
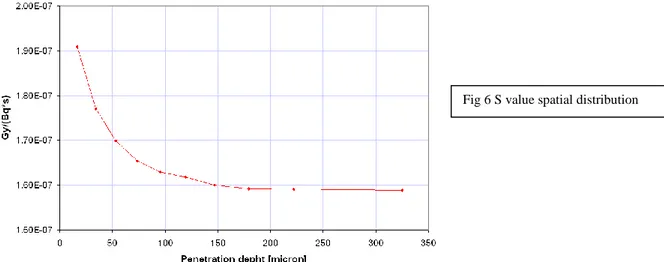
The radiation dose distribution calculated for uniform activity distribution is reported in Figure 6 (mm). Our calculations produced S-values between the maximum of 1.9E-7 Gy/Bq s closed to radionuclide source until the minimum of 1.6E-7 Gy/Bqs, increasing the distance from the source region. The decrease in S value (0.3E-7, 16%) is present in the first 100 µm, becoming substantially constant with the rise of the distance between the source and the center of the target region (1.60E-07). The mean S value calculated in bone marrow space is found to be 1.67e-07 Gy/(Bq*s). The dose distribution within the trabecular bone from high
Fig. 4 Collision track length of electrons (in blue) from multiple sources of radionuclide in several contiguous trabeculae
Fig. 5 Discretized bone marrow cavity used in Monte Carlo simulation
23 energy electrons has been shown to be correlated to the size of bone cavities. Indeed the equality of the absorbed fractions is due to the range of beta particles that results sufficient to traverse several bone space, establishing an energy deposition pattern which is largely independent of the radionuclide source.
On the basis of this work the radiation dose delivered from 153Sm to bone marrow differs considerably compared to this estimated in previous dosimetric model and implemented in MIRDOSE, resulting in a significantly lower value. Moreover, IRCP Publication 30 suggests an energy-independent absorbed fraction of 1.0, grossly overestimating the radiation dose at moderate to high electron energies. Most estimates of absorbed dose are based on calculations made for mathematical phantom of the body with a homogeneous representation of the bone, which is adequate for transport consideration, but does not consider that the transport of energy by secondary electrons. Indeed, the secondary particles generated in mineral regions may contributed significantly to the calculation of absorbed dose, especially for photon energy of about 200 keV. In addition, the problem of an overestimation of the absorbed dose in the active marrow was due to the assumption that the bone marrow absorbs energy as efficiently as the mineral regions. These limitations resulted from the lack of planning the complexity of the bone in a realistic way. Certainly the bone microstructure plays an important role in determining the absorbed dose in a target region. In this setting the influence of the regional microstructure in normal bone on the absorbed fraction dose is clearly showed in the three-dimensional transport model of Bouchet et al. The modification of this parameter is due to the different size of bone trabeculae and marrow cavities. The
24 absorbed fraction dose to the parietal bone compared to cervical vertebra or rib is found to be markedly different because of the larger bone trabeculae and smaller marrow cavities, with a more rapid fall with the increase of electron energy. Our data show that the bone marrow space is significantly smaller than the mean marrow space chord length used in previous dosimetric model and based on the chord-length distributions, representing one of the possibly explanation of the achievement of different results. The geometrical model presented differs substantially from previous dosimetric model in terms of the method used to evaluate the bone structure and especially in the consideration of a bone metastases model. It should be remember that the marrow cavity and trabecular chord length distributions published by Spiers et al. were obtained analyzing only seven bone site in a 44-year old man. Furthermore the chord-length distributions were calculated by optically scanning the trabecular bone under conditions of gamma randomness with the assumption that the track of a particle was straight. The total track could be approximated by alternating the chord lengths in trabeculae and cavities selected randomly from the measured distributions. Moreover, the analysis of the bone microarchitecture of the bone metastases from prostate cancer has shown significant differences in the structural parameters compared to normal tissue and degenerative osteosclerosis. The BV/TV, BS, Tb.N, ConnD in bone metastases was found to be significantly higher than in the normal tissue, indicating evident structural changes in secondary skeletal lesions. The metastatic tissue presents variable alterations leading to a difficult generalization of a dosimetric model, not considering the extreme individual variability in terms of change in bone marrow cellularity and metastatic involvement.
CONCLUSION
In radionuclide bone palliation the hematopoietic cells in the bone marrow are the dose-limiting tissue. The schedules administered activity (37 MBq/kg), derive from data obtained
in a limited number of studies aiming at pain control with an acceptable toxicity. The choice
of optimal activities based on dosimetry could lead to a modification in treatment plans, resulting in the optimization of individual therapy consistent in maximizing dose tumor while minimizing of the marrow toxicity. The bone marrow dose modeling and the methods of calculating the energy deposition from beta-emitters on skeleton have been developed over the last 40 years. However the calculation to the bone marrow represents a challenge due to
25 the complex structural architecture of the trabecular bone. The chord length distributions measured in the work of Spiers had provided the most complete information available on the 3-dimensional microstructure of both trabecular and cortical bone, used in internal dosimetric studies. The one-dimensional computational models of Spiers and Eckerman are based on the assumptions that the particle trajectories are linear such that a given chord length explicitly represents the particle path without angular scatter and that the energy loss within these structures can be treated under the continuous slowing-down approximation. Subsequently three-dimensional electron transport models for assessing absorbed fraction to trabecular bone marrow have been developed and from these the mean absorbed dose per unit cumulated activity (S value) has been systematically computed for several bone-seeking nuclides and different geometries. The most commonly employed dosimetric model provides only a first order of approximation based on the assumptions of the homogenous lesion morphology, uniform distribution of radioactivity within the lesion and complete energy deposition within the lesion by charged particles, considering the chord length distributions as structural information. Indeed the radiation dose delivered from 153Sm to bone marrow metastases considering the variable micro-structural alterations of skeletal secondary lesions is not a trivial issue. In this work the dose distribution of the beta particles originating from
153
Sm-EDTMP through bone marrow cavities and trabeculae are evaluated based on a Monte Carlo simulation in a new dosimetric model. A realistic approach regarding the bone micro-structure was used. The micro-CT analysis has allowed the investigation of morphology, topology, and texture of bone metastases with the calculation of structural parameters classically evaluated with histomorphometry. This preliminary evaluation has lead to the development of a geometrical model of metastatic trabecular bone. The radiation dose distribution appears to be homogeneous in the central region of bone marrow space, while at the periphery of the cavity in the proximity of radiation source seems to be significantly higher. The S value computed for bone marrow space differs considerably compared to this estimated in previous dosimetric model, confirming the limitations of the previous establish data in the evaluation of metastatic lesions. Therefore the update of these models for more patients specific application may provide optimization of administered activity with a reliable dose calculation.
26
References
1. Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010; 40(2):89-104.
2. Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001; 42(6):895-906. 3. Lewington VJ. Bone seeking radionuclides for therapy. J Nucl Med 2005;46:38S–
47S.
4. Serafini AN. Systemic metabolic radiotherapy with samarium-153 EDTMP for the treatment of painful bone metastasis. Q J Nucl Med. 2001 Mar;45(1):91-9.
5. Laing AH, Ackery DM, Bayly RJ, Buchanan RB, Lewington VJ, McEwan AJ, et al. Strontium-89 therapy for pain palliation in prostatic skeletal malignancy. Br J Radiol 1991;64: 816-22.
6. Maxon HR III, Deutsch EA, Thomas SR, Libson K, Lukes SJ, Williams CC et al. Re-186(Sn)-HEDP for treatment of multiple metastatic foci in bone: human biodistribution and dosimetricstudies. Radiology 1988;166:501–7.
7. Collins C, Eary JF, Donaldson G, Vernon C, Bush NE, Petersdorf S, et al. Samarium-153 EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med1993;34:1839–44.
8. Turner JH, Martindale AA, Sorby P, Hetherington EL, Fleay RF, Hoffman RF, et al. Samarium-153 EDTMP therapy of disseminated skeletal metastases. Eur J Nucl Med 1989;15:784–95.
9. Bouchet LG, Jokisch DW, Bolch WE. A three-dimensional transport model for determining absorbed fractions of energy for electrons within trabecular bone. J Nucl Med 1999; 40:1947–66.
10. Bouchet LG, Bolch WE. A three-dimensional transport model for determining absorbed fractions of energy for electrons within cortical bone. J Nucl Med 1999;40:2115–24.
11. Whitwell JR, Spiers FW. Calculated beta-ray dose factors for trabecular bone. Phys Med Biol 1976;21:16–38.
12. Bouchet LG, Bolch WE, Howell RW, Rao DV. S values for radionuclides localized within the skeleton. J Nucl Med 2000;41:189–212.
27 13. Jokisch DW, Patton PW, Inglis BA, Bouchet LG, Rajon DA, Rifkin J, et al. NMR microscopy of trabecular bone and its role in skeletal dosimetry. Health Phys 1998;75:584–96.
14. Müller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and microcomputed tomography. Bone 1998;23:59–66.
15. Nelson WR, Hirayama H, Rogers DWO. The EGS-4 Code System. Report 265. Stanford, CA: Stanford Linear Accelerator Center; 1985.
16. Briesmeister JF. MCNP—A General Monte Carlo N-Particle Transport Code, Version 4B. Report LA-12625-M. Los Alamos, NM: Los Alamos National Laboratory; 1997. 17. Guise T. Examining the metastatic niche: targeting the microenvironment. Semin
Oncol. 2010 Oct;37 Suppl 2:S2-14.
18. Batson OV. The role of the vertebral veins in the metastatic process, Ann Intern Med (1942), 16-38
19. Liotta LA, Kohn E. Cancer invasion and metastases. JAMA.1990;263:1123–1126. 20. Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem.
1999;45:1353–1358.
21. Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone
mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–9.
22. Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone
formation? Mol Cell Endocrinol.2009;310:52–62.
23. Dougall WC, Glaccum M, Charrier K, et al. RANK is essential for osteoclast and
lymph node development. Genes Dev. 1999;13:2412–24.
24. Bussard KM, Gay CV, Mastro AM. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55.
25. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. 26. Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP (2002) Molecular mechanisms of
cancer pain. Nature reviewsCancer 2: 201–209
27. Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, et al. (2009) Bone turnover in bone biopsies of patients with low-energy cortical. fractures receiving bisphosphonates: a case series. Calcif Tissue Int 85: 37–44.
28 28. Halvorson KG, Sevcik MA, Ghilardi JR, Rosol TJ, Mantyh PW (2006) Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain 22: 587–600.
29. Doré-Savard L, Otis V, Belleville K et al. Behavioral, medical imaging and histopathological features of a new rat model of bone cancer pain. PLoS One. 2010 Oct 29;5(10):e13774.
30. Choong PF. The molecular basis of skeletal metastases. Clin Orthop Relat Res Suppl 415 (2003), S19-S30.
31. Bauman G, Charette M, Reid R, Sathya J. Radiopharmaceuticals for the palliation of painful bone metastasis-a systemic review.Radiother Oncol. 2005 Jun;75(3):258-70. 32. Pandit-Taskar N, Batraki M, Divgi CR. Radiopharmaceutical therapy for palliation of
bone pain from osseous metastases.J Nucl Med. 2004 Aug;45(8):1358-65.
33. Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review.Lancet Oncol. 2005 Jun;6(6):392-400.
34. Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001 Jun;42(6):895-906. 35. Anderson P, Nuñez R. Samarium lexidronam (153Sm-EDTMP): skeletal radiation for
osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther. 2007 Nov;7(11):1517-27.
36. Heggie JC. Radiation absorbed dose calculations for samarium-153-EDTMP localized in bone. J Nucl Med. 1991 May;32(5):840-4.
37. Goeckeler WF, Edwards B, Volkert WA, Holmes RA, Simon J, Wilson D. Skeletal localization of samarium-153 chelates: potential therapeutic bone agents. J Nucl Med. 1987 Apr;28(4):495-504.
38. Lamb HM, Faulds D. Samarium 153Sm lexidronam. Drugs Aging. 1997 Nov;11(5):413-8.
39. Singh A, Holmes RA, Farhangi M, Volkert WA, Williams A, Stringham LM, Ketring AR. Human pharmacokinetics of samarium-153 EDTMP in metastatic cancer. J Nucl Med. 1989 Nov;30(11):1814-8.
40. Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of
samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994
29 41. Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M, Hartnett S, Ferency S, Addison SJ, Appelbaum F, et al. Samarium-153-EDTMP biodistribution and dosimetry estimation.J Nucl Med. 1993 Jul;34(7):1031-6.
42. Sartor O. Overview of samarium sm 153 lexidronam in the treatment of painful metastatic bone disease. Rev Urol. 2004;6 Suppl 10:S3-S12.
43. Maini CL, Bergomi S, Romano L, Sciuto R. 153Sm-EDTMP for bone pain palliation in skeletal metastases.Eur J Nucl Med Mol Imaging. 2004 Jun;31 Suppl 1:S171-8. 44. Maini CL, Sciuto R, Romano L, Bergomi S. Radionuclide therapy with bone seeking
radionuclides in palliation of painful bone metastases.J Exp Clin Cancer Res. 2003 Dec;22(4 Suppl):71-4.
45. Vigna L, Matheoud R, Ridone S, Arginelli D, Della Monica P, Rudoni M, Inglese E, Brambilla M. Characterization of the [(153)Sm]Sm-EDTMP pharmacokinetics and Estimation of radiation absorbed dose on an individual Basis. Phys Med. 2011 Jul;27(3):144-52. Epub 2010 Sep 23.
46. Logan KW, Volkert WA, Holmes RA. Radiation dose calculations in persons receiving injection of samarium-153 EDTMP. J Nucl Med. 1987 Apr;28(4):505-9. 47. Sartor O, Reid RH, Bushnell DL, Quick DP, Ell PJ. Safety and efficacy of repeat
administration of samarium Sm-153 lexidronam to patients with metastatic bone pain. Cancer. 2007 Feb 1;109(3):637-43.
48. Menda Y, Bushnell DL, Williams RD, Miller S, Thomas MO.Efficacy and safety of repeated samarium-153 lexidronam treatment in a patient with prostate cancer and metastatic bone pain.) Clin Nucl Med. 2000 Sep;25(9):698-700.
49. Fizazi K, Beuzeboc P, Lumbroso J, Haddad V, Massard C, Gross-Goupil M, Di Palma M, Escudier B, Theodore C, Loriot Y, Tournay E, Bouzy J, Laplanche A. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009 May 20;27(15):2429-35. Epub 2009 Apr 13.
50. Tu SM, Mathew P, Wong FC, Jones D, Johnson MM, Logothetis CJ. Phase I study of concurrent weekly docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer.J Clin Oncol. 2009 Jul 10;27(20):3319-24. Epub 2009 May 4.
51. Morris MJ, Pandit-Taskar N, Carrasquillo J, Divgi CR, Slovin S, Kelly WK, Rathkopf D, Gignac GA, Solit D, Schwartz L, Stephenson RD, Hong C, Delacruz A,
30 Curley T, Heller G, Jia X, O'Donoghue J, Larson S, Scher HI. Phase I study of samarium-153 lexidronam with docetaxel in castration-resistant metastatic prostate cancer.J Clin Oncol. 2009 May 20;27(15):2436-42. Epub 2009 Apr 13)
52. Jokisch DW, Patton PW, Inglis BA, Bouchet LG, Rajon DA, Rifkin J, et al. NMR microscopy of trabecular bone and its role in skeletal dosimetry. Health Phys 1998;75:584–96.
53. Spiers FW. The influence of energy absorption and electron range on dosage inirradiated bone. BrJ of Radial. 1949:22:521-53.
54. Spiers FW, Beddoe AH, Whitwell JR. Mean skeletal dose factors for beta-particle emitters in human bone. Part II: surface-seeking radionuclides. Br J Radial. 1981-,54:500-504.
55. Whitwell JR. Spiers FW. Calculated beta-ray dose factors for trabecular bone. Phys Med Biol. I976;21:16-38.
56. Spiers FW, Whitwell JR. Beddoe AH. Calculated dose factors for the radiosensitive tissues in bone irradiated by surface-deposited radionuclides. Phys Med Biol.1978;23:481-94.
57. Snyder WS, Ford MR, Warner GG, Watson SB. A Tabulation of Dose Equivalent Per Microcurie-Day for Source and Target Organs of an Adult for Various Radionuclides. ORNL-5000. Oak Ridge, TN: Oak Ridge National Laboratory.
58. Snyder WS, Ford MR. Warner GG, Watson SB. "S,"Absorbed Dose per Unit Cumulated Activity for Selected Radionuclides and Organs. MIRD Pamphlet 11. New York, NY: Society of Nuclear Medicine; 1975.1974.
59. International Commission on Radiological Protection. Limits for Intakes of Radionuclides by Workers. Publication 30. Oxford, UK: Pergamon; 1979
60. Eckerman KF. Aspects of the dosimetry of radionuclides within the skeleton with particular emphasis on the active marrow. In: Proceedings of the Fourth International Radiopharmaceutical Dosimetry Symposium. 1985.CONF-85113. Oak Ridge, TN: Oak Ridge Associated Universities; 1985:514-534.
61. Bouchet LG, Jokisch DW, Bolch WE. A three-dimensional transport model for determining absorbed fractions of energy for electrons within trabecular bone. J Nucl Med. 1999 Nov;40(11):1947-66.
62. Bouchet LG, Bolch WE, Howell RW, Rao DV. S values for radionuclides localized within the skeleton.
31 63. Johnson JC, Langhorst SM, Loyalka SK, Volkert WA, Ketring AR. Calculation of radiation dose at a bone-to-marrow interface using Monte Carlo modeling techniques (EGS4). J Nucl Med. 1992 Apr;33(4):623-8.
64. Stabin MG, Eckerman KF, Bolch WE, Bouchet LG, Patton PW. Evolution and status of bone and marrow dose models. Cancer Biother Radiopharm. 2002 Aug;17(4):427-33.
65. Samaratunga RC, Thomas SR, Hinnefeld JD, et al. A Monte Carlo simulation model for radiation dose to metastatic skeletal tumor from rhenium-186(Sn)-HEDP. J Nuc Med. 1995;36:336-50.
66. Müller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, Rüegsegger P. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998 Jul;23(1):59-66.
67. Feldkamp LA, Goldstein SA, Parfitt AM, Jesion G, Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res. 1989 Feb;4(1):3-11.
68. Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc. 2005 May;218(Pt 2):171-9.
69. Genant HK, Jiang Y. Advanced imaging assessment of bone quality. Ann N Y Acad Sci. 2006 Apr;1068:410-28.
70. X-5 Monte Carlo Team, “ MCNP – A General Monte Carlo N-Particle Transport Code, Version 5”, Volume I: Overview and Theory, LA-UR-03-1987, April 24,2003 (Revised 10/03/05), Los Alamos National Laboratory.
71. Chadwick MB et al ENDF/B-VII.0: Next Generation Evaluated Nuclear Data Library for Nuclear Science and Technology Nuclear Data Sheets 107 (2006) 2931–3060.