Duloxetine in the treatment of elderly people
with major depressive disorder
L’uso della duloxetina nel trattamento degli anziani con disturbo
depressivo maggiore
ANTONIO DEL CASALE
1, PAOLO GIRARDI
1,2, ROBERTO BRUGNOLI
1, GABRIELE SANI
1,
SIMONE DI PIETRO
1, CHIARA BRUGNOLI
1, FEDERICA CACCIA
1, GLORIA ANGELETTI
1,
DANIELE SERATA
1,2, CHIARA RAPINESI
1,2, ROBERTO TATARELLI
1, GEORGIOS D. KOTZALIDIS
1E-mail: [email protected]
1School of Medicine and Psychology, NESMOS Department (Neuroscience, Mental Health and Sensory Organs), Sant’Andrea Hospital, Sapienza University of Rome, Italy
2Department of Neuropsychiatry, Villa Rosa Hospital, Suore Ospedaliere del Sacro Cuore di Gesù, Viterbo, Italy
SUMMARY. Introduction. The elderly population is more frequently subjected to depressive mood compared to the
gener-al population and show peculiarities affecting responsiveness; furthermore, aged people need gener-also specigener-al care. Duloxetine is a relatively new antidepressant that proved to be effective in adult depression, but has received little attention in elderly pop-ulations heretofore. Aim. To review the evidence of duloxetine in late-life major depressive disorder (MDD). Method. A sys-tematic review of studies focusing on the use of duloxetine in MDD in the elderly has been carried out through the principal specialized databases, including PubMed, PsycLIT, and Embase. Results. Only a handful of papers were specifically dedicat-ed to this issue. Duloxetine was found to be effective and safe in old-age MDD, to be better than placebo on many clinical measures in all studies, and to better differentiate from placebo with respect to selective serotonin reuptake inhibitors. Com-pared to placebo, its side-effect profile is slightly unfavorable and its drop-out rate is slightly higher. Furthermore, when pain is present in old-age MDD, duloxetine is able to reduce it. Conclusions. The efficacy and safety of duloxetine in old-age de-pression are similar to those encountered in adult MDD. There is a relative lack of comparative studies other than with place-bo. The special needs of elderly patients with MDD must be addressed with close patient contact to avoid the perils of inap-propriate dosing.
KEY WORDS: duloxetine, major depressive disorder, pain, old age, antidepressant pharmacotherapy.
RIASSUNTO. Introduzione. La popolazione anziana è maggiormente soggetta a umore depresso rispetto alla popolazione
generale, con caratteristiche particolari che influenzano la responsività al trattamento farmacologico. Tali pazienti richiedono particolari accorgimenti curativi. La duloxetina è un antidepressivo relativamente nuovo, efficace nella depressione dell’adul-to, ma che non ha finora ricevuto sufficiente attenzione per il suo uso nell’anziano. Scopo. Passare in rassegna l’evidenza ri-guardante l’uso della duloxetina nel disturbo depressivo maggiore (DDM) dell’anziano. Metodo. È stata condotta una ricer-ca sistematiricer-ca di tutti gli studi centrati sull’uso della duloxetina in pazienti anzianti con DDM, attraverso le maggiori basi di dati informatiche, quali PubMed, PsycLIT ed Embase. Risultati. Sono relativamente pochi i lavori dedicati all’argomento, in base ai quali la duloxetina risulta efficace e ben tollerata nel DDM dell’anziano; inoltre, essa si dimostra, in tutti gli studi, su-periore al placebo nel migliorare molti parametri clinici in tutti gli studi, e si differenzia maggiormente dal placebo rispetto agli inibitori selettivi della ricaptazione della serotonina. Il suo profilo di effetti collaterali è solo lievemente sfavorevole ri-spetto al placebo e il suo tasso di drop-out è leggermente superiore a quello del placebo. La duloxetina è efficace nei pazien-ti anziani anche su alcune sintomatologie dolorose organiche in comorbilità con la sintomatologia depressiva. Conclusioni. L’efficacia e la tollerabilità della duloxetina nel paziente anziano con DDM sono simili a quelle riscontrate nel paziente adul-to. Vi è una relativa scarsità di studi comparativi, eccetto che contro placebo. I peculiari bisogni e aspetti clinici dei pazienti anziani con DDM vanno affrontati con attente e costanti visite, per evitare errori nella prescrizione dei dosaggi dei farmaci.
tive effects of androgen replacement on mood. The role
of androgens in the human involves both the regulation
of sexuality and psychological aspects like aggression,
social ranking, general emotionality, and personality.
These effects are also influenced by social factors.
Be-sides this, sex hormones play a cardinal role in the
de-velopment and maintenance of acquired cognitive
abil-ities. At least in part, hormonal changes in androgen
levels in elderly men modulate cognitive changes
oc-curring with aging. Treatment with androgens in
hypog-onadal men improved neurocognition and mentation,
verbal and visual memory, visuomotor scanning and
at-tention, verbal fluency and understanding, and spatial
abilities and memory for both verbal and visual
infor-mation. The behavioral symptoms of PADAM may be
ascribed to multiple factors, including both biological
changes occurring with age and social adaptations
oc-curring during the mid-life transition (5,6).
Similar considerations may be made for
post-menopausal women, a group of persons in which
de-pressive and sexual symptoms are more severe than in
other age groups and are both associated with stressful
life events (8,9). Post-menopausal women with MDD
showed better antidepressant response to selective
serotonin reuptake inhibitors (SSRIs) when this
treat-ment has been administered in combination with
hor-monal substitution therapy (10).
Another important point in elderly depression is the
increased risk of suicide. Various combinations of risk
factors for suicide must be considered in the elderly. The
concomitance of depressive symptoms and stressful life
events, such as feelings of loss, loneliness, and physical
illness in the elderly are to be considered as warning
signs for suicidal behavior (11,12). Loss of libido and
other aspects of sexuality may also constitute a
signifi-cant part of suicidal ideation in the elderly (13).
The physician should carefully assess, for elderly
pa-tients with depression, any underlying organic causes
and comorbidities, including diabetes (14,15),
hyper-tension (16), cardiovascular diseases (17), obesity
(18,19), deficiency of vitamin B12 (20,21), vitamin D
(22,23), folate (20,24,25), homocysteine (20,25,26),
anemia or iron deficiency (27,28) or iron overload
(29-32), and in general, the state of nutrients and
micronu-trients that might influence mood (33).
Duloxetine inhibits the transporters of both
sero-tonin (5-HT) and norepinephrine (NA), thus it belongs
to the class of the Serotonin-Norepinehrine Reuptake
Inhibitors (SNaRIs). A meta-analysis conducted by
Gi-rardi et al. (34) showed that duloxetine has hood
evi-dence of efficacy in acute, adult MDD, especially at
doses of 80-120 mg/day. About six hours after oral
ad-ministration, duloxetine reaches a maximum plasma
INTRODUCTION
Major depressive disorder (MDD) is common in
aged people and is frequently associated with
cogni-tive impairment and disability. Furthermore, clinically
significant depressions, like MDD and other forms of
unipolar depression constitute part of the clinical
course of dementia in the elderly, or complicate it or
are frequently comorbid with it.
MDD affects about 1-4% of the general aged
popula-tion (1), accounting for almost 5% of dementia
syn-dromes (2). Kobayashi (3) introduced a new and more
dynamic approach to investigate the relationship
be-tween depression and dementia, coining the term of
“de-pression-dementia medius”. Within this model,
depres-sion, cognitive impairment, and degenerative dementia
are viewed as an intersecting continuum, in which
pseu-dodementia and depression in dementia are located
in-termediately between depression and dementia.
Apathy is characterized by a partial lack of
motiva-tion, but differently from avolimotiva-tion, it also concerns a
sluggish response to environmental stimuli, a sort of
emotional unresponsiveness. It is primary deficit that
should be distinguished from both cognitive decline
and depressive mood. Apathy may be a common
fea-ture of psychotic disorders, it may be part of a
depres-sive syndrome, and may be extremely common in
de-mentia. It coexists with depression in the same patient
all too frequently, but it should be routinely assessed
during neuropsychiatric evaluation independently
from depression. In distinguishing the two syndromes,
symptoms of sadness and feelings of helplessness,
hopelessness, and worthlessness are helpful in
attribut-ing the case to depression. Accurately differentiatattribut-ing
apathy from depression is basic to achieve appropriate
family education, thus allowing the institution of the
best indicated treatment strategy (4).
Another important aspect in elderly patients with
MDD is the partial androgen deficiency of aging men
(PADAM). It results in a variety of behavioral
symp-toms, like weakness and increased fatigability,
depres-sive mood, lack of motivation, low energy, reduced
per-formance at job and sports, reduced vitality, increased
anxiety and irritability, insomnia, difficulty in
concen-trating, and impaired memory. Decreased libido and
low dominance may reflect reduced testosterone
lev-els; in fact, the syndrome may be reversed through
ex-ogenous testosterone supply (5-7). Psychological and
behavioral aspects of PADAM overlap with signs and
symptoms of MDD. The evidence of the association
between low testosterone (T) levels and depressive
mood in men stems from studies assessing depression
in people with hypogonadism, and reporting the
posi-concentration (C
max) of approximately 47 ng/mL with
40 mg twice-daily dosing, to 110 ng/mL with 80 mg
twice-daily dosing. Its elimination half-life is
approxi-mately 10-12 hours, while its distribution volume is
about 1640 L. Gender, smoking, age, ethnicity,
cy-tochrome P450 (CYP) 2D6 genotype, liver and renal
function were all found to influence its
pharmacokinet-ics. Of these, only impaired liver or renal function
prompted specific warnings or recommendations for
dose adjustment. Drug-drug interaction studies showed
activated charcoal and CYP1A2 inhibition to
signifi-cantly decrease and increase, respectively, plasma levels
of duloxetine. For example, fluvoxamine, an SSRI and
CYP1A2 and CYP2D6 inhibitor, increased the area
under the curve of duloxetine by 460% and its C
maxby
141%. Most of the effect is due to CYP1A2 inhibition,
with CYP2D6 contributing much less. In contrast,
smoking decreases duloxetine plasma concentrations
by 30%. The CYP2D6 poor metabolizer status or
con-comitant administration of CYP2D6 inhibitors does
not require dose adjustment. Duloxetine increases the
exposure to drugs metabolized by CYP2D6, but not by
CYP1A2. Furthermore, duloxetine may enhance the
effects of benzodiazepines, but not those of alcohol or
warfarin (35). Its concentration in human plasma can
be determined by capillary electrophoresis with
laser-induced fluorescence detection (36), or by liquid
chro-matography (37); these techniques proved to be
reli-able for determining the plasma levels of other
antide-pressants (38,39) and antipsychotic drugs (40).
The aim of this paper is to briefly review the main
evidence about the treatment with duloxetine of
elder-ly people with depression.
RESULTS
The combined search yielded 212 papers as of
Sep-tember the 7
th, 2012, of which 8 were highly specific
(keywords appearing in paper title), 11 were most
rel-evant, while 128 were of some relevance. Three of the
highly relevant papers were reviews or metaanalyses
and the other eight were clinical trials. We briefly
dis-cuss their findings.
DISCUSSION
Effectiveness
Duloxetine significantly improved the mean
base-line HAM-D-17 total score of elderly patients with
de-pression (41,42). A summary of the results of studies
using duloxetine in elderly populations is shown in
Table 1.
In previous short-term studies, the comparisons
be-tween duloxetine and placebo of HAM-D-17 subscores
showed differences favoring duloxetine, which were
significant in some items (core, retardation, Maier
sub-scores) or at the limit of the level of significance in
anx-iety (strong trend, as in the study conducted by Nelson
and colleagues) (43). By the 2
ndweek of treatment, a
short-term placebo-controlled study focusing on
elder-ly patients showed significant benefits over placebo in
Geriatric Depression Scale (GDS) total score and
CGI-S, and significantly greater reduction than placebo
in psychic anxiety and in the anxiety somatization
sub-scales (44). In a recent randomized, double-blind,
placebo-controlled trial, duloxetine was used as a
com-parator in a fixed-dose study investigating the efficacy
and safety of Lu AA21004 in elderly patients with
MDD (45). In this sponsored trial, signed by one single
principal investigator and two sponsoring
pharmaceuti-cal company employees, both drugs showed
superiori-ty to placebo at weeks 6 and 8. However, duloxetine
ranked better than the experimental drug on all clinical
measures, but the analysis model used avoided the
di-rect comparison of the two drugs (45).
Another comparison with placebo showed that
du-loxetine has a shorter time to antidepressant response
(46). Additional analysis showed that the effect of
du-loxetine on depression and quality of life was not
sig-nificantly affected by the presence of comorbid
vascu-lar disease, diabetes, arthritis or a combination of more
than one of the above (41,47).
Regarding anxiety, another study conducted by
Davidson and colleagues (48), who pooled
acute-phase data from a subset of patients (≥65 years) with
METHOD
From the 3rdto the 7thof September, current year, we
searched the Medline, Embase, and PsycLIT databases us-ing as terms “duloxetine”, “elderly”, “depression”, “agus-ing” or “ageing”, “aged”, “major depression”, “major depres-sive disorder”, and “major depresdepres-sive episode”. Papers were included if they satisfied standards for adequate methodology and population included and had focused on the subject. Exclusion criteria comprised inadequate methodology (method unspecified and/or inadequately described), not reporting data of treatment efficacy or side effects, and unspecified inclusion of subjects.
Papers published in peer-reviewed journals dealing with duloxetine treatment in elderly people were selected. Almost all of these were published in the last 10 years. Fur-ther papers that did not appear in the above databases were searched from reference lists of retrieved papers.
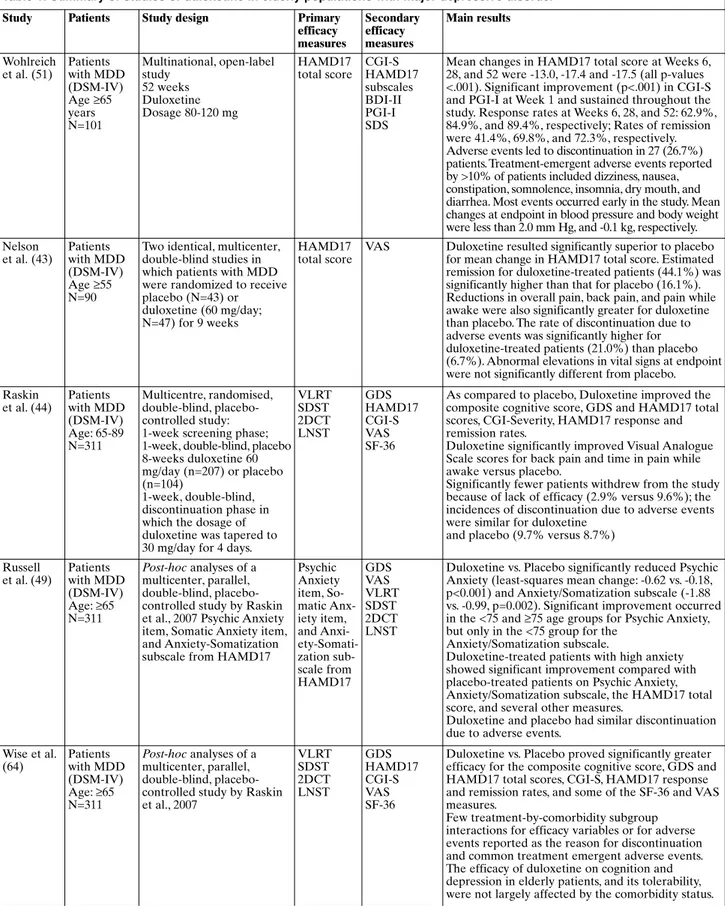
Table 1. Summary of studies of duloxetine in elderly populations with major depressive disorder
Study Patients Study design Primary
efficacy measures Secondary efficacy measures Main results Wohlreich et al. (51) Patients with MDD (DSM-IV) Age ≥65 years N=101 Multinational, open-label study 52 weeks Duloxetine Dosage 80-120 mg HAMD17 total score CGI-S HAMD17 subscales BDI-II PGI-I SDS
Mean changes in HAMD17 total score at Weeks 6, 28, and 52 were -13.0, -17.4 and -17.5 (all p-values <.001). Significant improvement (p<.001) in CGI-S and PGI-I at Week 1 and sustained throughout the study. Response rates at Weeks 6, 28, and 52: 62.9%, 84.9%, and 89.4%, respectively; Rates of remission were 41.4%, 69.8%, and 72.3%, respectively. Adverse events led to discontinuation in 27 (26.7%) patients. Treatment-emergent adverse events reported by >10% of patients included dizziness, nausea, constipation, somnolence, insomnia, dry mouth, and diarrhea. Most events occurred early in the study. Mean changes at endpoint in blood pressure and body weight were less than 2.0 mm Hg, and -0.1 kg, respectively. Nelson et al. (43) Patients with MDD (DSM-IV) Age ≥55 N=90
Two identical, multicenter, double-blind studies in which patients with MDD were randomized to receive placebo (N=43) or duloxetine (60 mg/day; N=47) for 9 weeks
HAMD17 total score
VAS Duloxetine resulted significantly superior to placebo for mean change in HAMD17 total score. Estimated remission for duloxetine-treated patients (44.1%) was significantly higher than that for placebo (16.1%). Reductions in overall pain, back pain, and pain while awake were also significantly greater for duloxetine than placebo. The rate of discontinuation due to adverse events was significantly higher for duloxetine-treated patients (21.0%) than placebo (6.7%). Abnormal elevations in vital signs at endpoint were not significantly different from placebo. Raskin et al. (44) Patients with MDD (DSM-IV) Age: 65-89 N=311 Multicentre, randomised, double-blind, placebo-controlled study: 1-week screening phase; 1-week, double-blind, placebo 8-weeks duloxetine 60 mg/day (n=207) or placebo (n=104)
1-week, double-blind, discontinuation phase in which the dosage of duloxetine was tapered to 30 mg/day for 4 days.
VLRT SDST 2DCT LNST GDS HAMD17 CGI-S VAS SF-36
As compared to placebo, Duloxetine improved the composite cognitive score, GDS and HAMD17 total scores, CGI-Severity, HAMD17 response and remission rates.
Duloxetine significantly improved Visual Analogue Scale scores for back pain and time in pain while awake versus placebo.
Significantly fewer patients withdrew from the study because of lack of efficacy (2.9% versus 9.6%); the incidences of discontinuation due to adverse events were similar for duloxetine
and placebo (9.7% versus 8.7%)
Russell et al. (49) Patients with MDD (DSM-IV) Age: ≥65 N=311 Post-hoc analyses of a multicenter, parallel, double-blind, placebo-controlled study by Raskin et al., 2007 Psychic Anxiety item, Somatic Anxiety item, and Anxiety-Somatization subscale from HAMD17
Psychic Anxiety item, So-matic Anx-iety item, and Anxi- ety-Somati-zation sub-scale from HAMD17 GDS VAS VLRT SDST 2DCT LNST
Duloxetine vs. Placebo significantly reduced Psychic Anxiety (least-squares mean change: -0.62 vs. -0.18, p<0.001) and Anxiety/Somatization subscale (-1.88 vs. -0.99, p=0.002). Significant improvement occurred in the <75 and ≥75 age groups for Psychic Anxiety, but only in the <75 group for the
Anxiety/Somatization subscale.
Duloxetine-treated patients with high anxiety showed significant improvement compared with placebo-treated patients on Psychic Anxiety, Anxiety/Somatization subscale, the HAMD17 total score, and several other measures.
Duloxetine and placebo had similar discontinuation due to adverse events.
Wise et al. (64) Patients with MDD (DSM-IV) Age: ≥65 N=311 Post-hoc analyses of a multicenter, parallel, double-blind, placebo-controlled study by Raskin et al., 2007 VLRT SDST 2DCT LNST GDS HAMD17 CGI-S VAS SF-36
Duloxetine vs. Placebo proved significantly greater efficacy for the composite cognitive score, GDS and HAMD17 total scores, CGI-S, HAMD17 response and remission rates, and some of the SF-36 and VAS measures.
Few treatment-by-comorbidity subgroup interactions for efficacy variables or for adverse events reported as the reason for discontinuation and common treatment emergent adverse events. The efficacy of duloxetine on cognition and depression in elderly patients, and its tolerability, were not largely affected by the comorbidity status.
Continue Table 1.
Study Patients Study design Primary efficacy measures Secondary efficacy measures Main results Karp et al. (52) Patients with MDD (DSM-IV) Age: ≥65 N=274 HAM-D-17 score ≥15, MMSE score ≥18 Data analysis of 40 patients switched from escitalopram to duloxetine Data up to 16.5 weeks HAMD17 total score HRSA MMSE UKU CIRS-G MOS
50% patients met criteria for full response, 17.6% were partial responders and 32.5% did not respond. The median time to response was 12 weeks. 17, 85% responders were females, whereas responders and non-responders did not differ in terms of age, marital status, education, recurrent depression, age on onset of the first depressive episode
Raskin et al. (61) Patients with MDD (DSM-IV) Age: ≥65 N=311 Post-hoc analyses of a multicenter, parallel, double-blind, placebo-controlled study by Raskin et al., 2007 GDS HAMD17 CGI-S VAS The time to response and remission for duloxetine compared with placebo was evaluated using Cox proportional hazards (PH) modeling, Kaplan-Meier estimation, and categorical repeated measures analysis
Discontinuation rates due to adverse events were similar for duloxetine and placebo (9.7% vs 8.7%). Treatment-emergent dry mouth, nausea, and diarrhea occurred significantly (p≤0.05) more frequently with duloxetine. Changes in supine and standing blood pressure, in sustained elevation in BP and treatment-emergent orthostatic hypotension, in pulse and in corrected QT (QTc) interval were not significantly different between duloxetine and placebo, except for change in orthostatic systolic BP (-2.45 vs 0.93 mmHg; p=.017). The duloxetine group showed significant weight loss compared with the placebo group (-0.73 kg vs -0.13 kg; p=0.009). Of 5 hepatic analytes, the only significant difference was an increase in alkaline phosphatase in duloxetine compared with placebo (p=0.017); this difference was not considered clinically relevant. The incidence of 1 or more discontinuation-emergent adverse events was not significantly different between the duloxetine and placebo groups (17.3% vs 11.3%).
Katona et al. (45) Patients with MDD (DSM-IV-TR) Age: ≥65 N=452 MMSE ≥24 Montgomery-Åsberg Depression Rating Scale (MADRS) ≥26 Multicenter, 8-week, 1:1:1 randomized, placebo-controlled, double-blind trial vs. Lu AA21004 Duloxetine 60 mg/day vs. Lu AA21004 5 mg/day or placebo HAMD24 total score at week 8 with analysis of covariance with last observation carried forward and with mixed model for repeated measures Response and remission; HAMD24 at all points; Montgomery-Asberg Depression Rating Scale scores; CGI-S scores; Rey Auditory Verbal Learning Test; Digit Symbol Substitution Test
Duloxetine and Lu AA21004 showed significantly greater improvement vs placebo at weeks 6 and 8. Covariance and mixed-model effects converged. Lu AA21004, but not duloxetine, positively affected cognition.
According to the HAMD, 8-week response was found in 35.2% of patients receiving placebo, 53.2% of patients receiving Lu AA21004, and in 63.3% of patients receiving duloxetine, while remitters were respectively 19.3%, 29.2%, and 34.7%.
Lu AA21004 differed for one adverse event from placebo, while duloxetine differed for six.
Robinson et al. (50) Patients with MDD (DSM-IV) Age: ≥65 N=279 MMSE ≥20 Montgomery-Asberg Depression Rating Scale ≥20 Multicenter, 24-week (12-24-week short-term and 12-week continuation), randomized, placebo-controlled, double-blind trial Duloxetine 60 or 120 mg/day or placebo; placebo rescue possible HAMD17 total score GDS BPI NRS CGI-S PGI-I cognitive measures
Duloxetine vs placebo showed significantly greater improvement at weeks 4, 8, 16, and 20. Similar patterns for Geriatric Depression Scale and Clinical Global Impression-Severity scale emerged, with significance also seen at week 24.
There was a significant treatment effect for all BPI items and 4 of 6 NRS pain measures in the acute phase, most BPI items and half of the NRS measures in the continuation phase.
More duloxetine-treated patients completed the study (63% versus 55%). A significantly higher percentage of duloxetine-treated patients versus placebo discontinued due to adverse event (15.3% versus 5.8%)
Legend: 2DCT: 2 Digit Cancellation Test; BDI-II: Beck Depression Inventory-II; BPI: Brief Pain Inventory; CGI-S: Clinical Global
Impres-sion of Severity scale; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; GDS: Geriatric DepresImpres-sion Scale; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; HAMD17: 17-item Hamilton Rating Scale for Depression; HRSA: Hamilton Rating Scale for Anxiety; LNST: Letter-Number Sequencing Test; MDD: Major Depressive Disorder; MMSE: Mini-Mental State Examination; MOS: Medical Outcomes Study; NRS: Numeric Rating Scales for pain; PGI-I: Patient Global Impression of Improvement scale; SDS: Sheehan Dis-ability Scale; SDST: Symbol Digit Substitution Test; SF-36: 36-Item Short Form Health Survey; UKU: Udvalg for Kliniske Undersogelser-Committee of Clinical Investigations Side Effect Rating Scale; VAS: Visual Analogue Scale for pain; VLRT: Verbal Learning and Recall Test.
generalized anxiety disorder (GAD) from four
ran-domized, double-blind, placebo-controlled trials of
du-loxetine (three using flexible dosing and one using
fixed doses), showed duloxetine 60-120 mg once daily
for 9-10 weeks to be efficacious and tolerable in
elder-ly patients with generalized anxiety disorder, as
com-pared to placebo. It should be remarked that GAD is
a disorder with much symptomatological overlap with
depressive mood disorders which responds in a similar
manner to antidepressant drugs.
Briefly, short-term studies indicate that duloxetine
treatment in aged people with depression improves
depressive symptoms, as assessed through the total
score on the 17-item HAM-D. Furthermore, it reduces
generalized, psychic, and somatization anxiety (43,48,
49). Duloxetine is faster than placebo to show
antide-pressant response and is also effective when there are
comorbid vascular diseases, diabetes, arthritis or more
than one of these.
In a recent multicenter, multinational, 24-week
(12-week short-term and 12-(12-week continuation),
random-ized, placebo-controlled, double-blind trial carried-out
by Robinson et al. (50), patients who received
duloxe-tine showed significantly greater improvement on the
Hamilton’s Maier subscale at weeks 4, 8, 16, and 20.
Furthermore, duloxetine-receiving patients had
signif-icantly lower pain ratings during both the acute and
the continuation phases, but dropped-out three times
as much as their placebo-receiving counterparts.
Long-term data showed that duloxetine maintains
its efficacy over time; HAM-D-17 total score and all
subscales significantly improved up to the 52
ndweek
(51). CGI-S improvement in elderly patients paralleled
that of the remaining included population of
non-eld-erly adults.
Another long-term study showed that 50% of
pa-tients who switched from escitalopram to duloxetine
met criteria for full response, 17.6% of them were
par-tial responders and 32.5% did not respond (52). The
median time to response was 12 weeks. Female gender
was associated with a higher probability of response,
whereas other measures, like history of depression and
baseline MMSE scores did not affect the rate of
re-sponse. Psychological and somatic anxiety improved
over time following treatment with duloxetine (52).
The improvement of depressive symptoms with
dulox-etine was dissimilar to improvements obtained with
placebo, while escitalopram induced changes that
looked like those of placebo-treated patients (53).
Duloxetine proved to be effective also in pain relief
and urinary incontinence (54). In a short-term,
place-bo-controlled study, patients experienced rapid pain
relief, and the comparison versus placebo showed
sta-tistically significant differences in scores on the visual
analogue scale (VAS) pain ratings, in back pain, and in
the amount of pain experienced while awake (55).
Fur-thermore, patients showed faster improvement in
self-reported pain compared to placebo (56). Moreover,
duloxetine produced significant reduction in overall
pain severity, back pain, time in pain while awake, and
interference with daily activities in patients with
de-pression and comorbid arthritis (57). Briefly, the
bene-ficial effects of duloxetine on pain were statistically
significant and clinically meaningful, extending from
the acute to the continuation phase, thus prompting to
hypothesize that they are not subjected to tolerance
with time (50).
In an eight-week, placebo-controlled study (44),
treatment with duloxetine ensued in significant
im-provement, compared to placebo, on the composite
measure of cognitive functions; duloxetine proved to
be the most important determinant of the
improve-ment driven by verbal learning and memory; on the
other hand, measures of focused attention and
execu-tive functioning showed no significant differences
be-tween duloxetine and placebo.
Duloxetine was found to be effective in patients
with urinary stress incontinence (54); however, it may
be associated with a decline in urinary flow in elderly
men (55). At any rate, duloxetine is a safe and effective
treatment for elderly women with symptoms of stress
urinary incontinence or stress-predominant mixed
nary incontinence (56), with elderly women with
uri-nary incontinence showing a safety profile for
duloxe-tine which was comparable to that of their younger
counterparts (57). Duloxetine is also effective in other
pathological conditions of the aged, including
fi-bromyalgia (58), osteoarthritis (47,59), and diabetic
sensory polyneuropathy (60).
Effects on physiological functions
The changes from baseline of heart rate and
stand-ing and supine blood pressure, sustained
hyperten-sion and the number of patients with abnormal
changes did not differ significantly between the
du-loxetine- and placebo-treated patients (44,61). The
rate of treatment-emergent orthostatic hypotension,
defined as standing diastolic blood pressure (BP) at
least 10 mmHg less than supine diastolic BP and
standing systolic BP at least 20 mmHg than supine
systolic BP, was numerically higher in the placebo
group compared to the duloxetine group, though this
did not reach statistical significance. Using an
ortho-static change of 10 mmHg for both systolic and
dias-tolic BP, the rates of treatment-emergent orthostatic
hypotension were similar and not significantly
differ-ent between duloxetine- and placebo-treated
pa-tients, for both diastolic and systolic BP. No
signifi-cant differences in mean changes of QTc interval has
been detected between duloxetine- and
placebo-treated patients, independently from whether the
Bazett or the Fridericia formulas were used (61). In
the analysis at the one-year follow-up,
duloxetine-treated patients exhibited small and nonsignificant
decreases in blood pressure and increases in heart
rate from baseline to end point (51). Patients with
higher baseline BP values had comparatively greater
BP lowering effects, as compared with normotensive
individual. Duloxetine did not appear to prolong the
QTc interval in the elderly, and this is consistent with
what has already been published for nonelderly
pa-tients and healthy volunteers (62,63).
Treatment with duloxetine was associated with a
small (<1 kg) decrease in weight in the short-term.
However, mean body weight slightly increased after
one year, whereas the rate of patients with significant
weight gain was not less than that of patients with
sig-nificant weight decrease (41). Considered cumulatively,
these data do not clarify whether duloxetine increases
or decreases body weight; the drug apparently affects
body weight, albeit in an unpredictable direction.
In the short-term comparative analysis, no
signifi-cant differences between duloxetine and placebo
emerged concerning the incidence of abnormal
labora-tory values (hematology and blood chemistry).
Long-term data did not show clinically significant
abnormal-ities (41).
Duloxetine did not affect sexual function
different-ly from placebo in elderdifferent-ly people, as assessed with the
Arizona Sexual Experience Scale (43). This is
impor-tant, as the presence of sexual alterations are not to be
considered a minor point in such populations, given
their possible repercussions on self-esteem and the
consequent influence on suicidal behavior (13).
Adverse events
Although the rate of discontinuation due to adverse
events in the short-term pooled data (61) was higher in
patients receiving duloxetine than in those on placebo,
direct head-to-head comparisons did not show any
dif-ference in the entire patient sample (9.7% vs 8.7%;
NS), even when the sample was dichotomized in a
<75-year-old and a ≥75-<75-year-old subgroup (44,61). Rates of
dry mouth, nausea and diarrhea were significantly
high-er in patients receiving duloxetine, as compared to
pa-tients receiving placebo. Nausea and fatigue showed
significant heterogeneity when data were analyzed
ac-cording to an age-related stratification (<75 years-old
and ≥75 years-old). In the <75 subgroup, nausea was
significantly more frequent in duloxetine-treated
pa-tients, whereas in the ≥75 subgroup, nausea was more
frequent in the placebo subgroup. Fatigue was
signifi-cantly more frequent for duloxetine-treated patients in
the ≥75 subgroup (61). There was a statistically
signifi-cant greater mean decrease in weight for patients
treat-ed with duloxetine versus patients treattreat-ed with placebo
(-0.73 vs -0.13 kg; p=0.009) (39). Post hoc analyses of
this study also showed duloxetine to be very well
toler-ated in patients with medical comorbidities (64). Dry
mouth and gastrointestinal complaints were the
ad-verse events that were significantly more common with
duloxetine than with placebo in the short-term direct
comparison (44), irrespective of the occurrence of
co-morbidities (64). Other types of adverse events (e.g.,
in-somnia, fatigue, diminished appetite, and reduced
li-bido) in the pooled short-term data (43) were seen
sig-nificantly more often in patients receiving duloxetine,
as compared with those receiving placebo.
Long-term observation showed that most adverse
events occurred early in the study and were of mild or
moderate intensity (51). The rates of events in patients
aged ≥65 years were not significantly higher than in the
18-64 year age range in the same study. In the
compar-ison between age ranges, patients aged ≥65 years
re-ported a significantly lower incidence of insomnia and
headache than younger patients, while no significant
differences between age cohorts were found in the
analysis of all other side effects.
Five (12.5%) patients who switched from
escitalo-pram to duloxetine (52) discontinued the study due
to adverse events. In the analysis of total and
sub-scale scores of the Udvalg Kliniske Undersøgelser
(UKU) scale (65), there were small but statistically
significant decreases (i.e., improvements) in somatic
complaints (as reflected by the total UKU scores),
on the psychological subscale and other systems
sub-scale scores.
Considering the lack of evidence concerning
suici-dal risk in elderly patients with depression receiving
duloxetine, a careful assessment of suicide risk is
es-sential in this category of patients.
The literature reports the following common side
ef-fects associated with duloxetine overdose: nausea,
headache, dry mouth, fatigue, insomnia, dizziness,
som-nolence, constipation, considerable increases in
recum-bent systolic and diastolic blood pressure, and a small
decrease in heart rate (66,67). A review of the annual
reports of the American Association of Poison Control
Centers (AAPCC) shows that there has been an
in-crease in fatal cases in which duloxetine has been
iden-tified: 5 cases in 2005 (68), 11 cases in 2006 (69), 14
cas-es in 2007 (70), 18 cascas-es in 2008 (71), 23 cascas-es in 2009
(72), and 12 in 2010 (73). “Cocktail” drugs caused most
of the poisonings; only a few cases of poisoning were
due to duloxetine alone, which somehow has been
linked to pulmonary embolism (74). However, this
ap-parent increase in duloxetine mortality in the US
should be viewed against the background of the
in-creased use of the drug across the year, hence the
above figures were to be expected and are by no means
excessive with respect to other similar drugs. The recent
decrease in duloxetine-associated mortality is to be
in-terpreted as the result of preventive policies and
cam-paigns carried-out by the US health administration.
The ingestion of high quantities of duloxetine may
have serious outcomes such as complicating
throm-boembolism (75). Elderly patients who are taking
du-loxetine should be advised to avoid overdosage, which
in most cases may be attributed to careless ingestion
by cognitively impaired individuals with depression.
Patients should be prompted to rely on family
mem-bers for the control of their medication intake and
en-couraged to promptly contact their physician in cases
of inadvertent drug ingestion, so to institute timely
detoxifying strategies and interventions. A summary of
the strength and weaknesses of duloxetine use in the
aged is shown in
Table 2.
CONCLUSIONS
Duloxetine is effective, safe and well-tolerated in
elderly populations with depressive disorders in a
sim-ilar manner to adults. Its’ efficacy is simsim-ilar to that of
other antidepressants, like the SSRIs or other SNRIs,
like venlafaxine and milnacipran, although regretfully,
comparisons in elderly populations are lacking. It
might have some advantage with respect to SSRIs, in
terms of dissimilarity with respect to placebo.
Howev-er, there is a relative lack of studies of duloxetine in
aged patients with depressive disorders. Elderly
pa-tients with dementia or pseudodementia must be
closely followed to avoid inappropriate dosing, as this
may expose patients to life-threatening effects.
Acknowledgements. We are grateful to Ms. Mimma Ariano, the late Tiziana Mattei, and Ms. Felicia Proietti, Librarians of the Sant’Andrea Hospital, School of Medicine and Psychology, Sapienza University of Rome, for allowing us to access precious bibliographical material. We also thank Ms. Lucilla Martinelli for skillful assistance in the preparation of this manuscript.
Financial & Competing Interests Disclosure. In the past two years, Paolo Girardi has received research support from Lilly, Janssen, and Springer Healthcare, and has participated in Advisory Boards for Lilly, Otsuka, Pfizer, Schering, and Springer Healthcare and received honoraria from Lilly and Springer Healthcare. All other authors of this paper have no relevant affiliations or financial involvement with any organ-ization or entity with a financial interest in, or financial con-flict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, hono-raria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
This work has not been supported by any funding.
REFERENCES
1. Blazer DG. Depression in late life: review and commentary. Gerontol A Biol Sci Med Sci 2003; 58: 249-65.
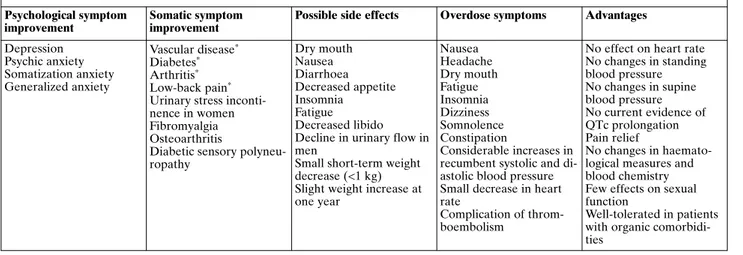
Table 2. Summary of the effects (and the lack of them) of the use of duloxetine in elderly patients Psychological symptom
improvement
Somatic symptom improvement
Possible side effects Overdose symptoms Advantages Depression Psychic anxiety Somatization anxiety Generalized anxiety Vascular disease* Diabetes* Arthritis* Low-back pain* Urinary stress inconti-nence in women Fibromyalgia Osteoarthritis
Diabetic sensory polyneu-ropathy Dry mouth Nausea Diarrhoea Decreased appetite Insomnia Fatigue Decreased libido Decline in urinary flow in men
Small short-term weight decrease (<1 kg) Slight weight increase at one year Nausea Headache Dry mouth Fatigue Insomnia Dizziness Somnolence Constipation Considerable increases in recumbent systolic and di-astolic blood pressure Small decrease in heart rate
Complication of throm-boembolism
No effect on heart rate No changes in standing blood pressure No changes in supine blood pressure No current evidence of QTc prolongation Pain relief No changes in haemato-logical measures and blood chemistry Few effects on sexual function
Well-tolerated in patients with organic comorbidi-ties
2. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med 1988; 109: 476-86.
3. Kobayashi T, Kato S. Depression-dementia medius: between de-pression and the manifestation of dementia symptoms. Psy-chogeriatrics 2011; 11: 177-82.
4. Tagariello P, Girardi P, Amore M. Depression and apathy in de-mentia: same syndrome or different constructs? A critical re-view. Arch Gerontol Geriatr 2009; 49: 246-9.
5. Amore M. Partial androgen deficiency and neuropsychiatric symptoms in aging men. J Endocrinol Invest 2005; 28 (11 suppl proceedings): 49-54.
6. Amore M, Scarlatti F, Quarta AL, Tagariello P. Partial androgen deficiency, depression and testosterone treatment in aging men. Aging Clin Exp Res 2009; 21: 1-8.
7. Amore M, Innamorati M, Costi S, Sher L, Girardi P, Pompili M. Partial androgen deficiency, depression, and testosterone sup-plementation in aging men. Int J Endocrinol 2012; 2012: 280724. 8. Amore M, Di Donato P, Berti A, et al. Sexual and psychological symptoms in the climacteric years. Maturitas 2007; 56: 303-11. 9. Dennerstein L, Soares CN. The unique challenges of managing
depression in mid-life women. World Psychiatry 2008; 7: 137-42. 10. Zanardi R, Rossini D, Magri L, Malaguti A, Colombo C, Smeral-di E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur Neuropsychopharmacol 2007; 17: 400-5.
11. Pompili M, Innamorati M, Masotti V, et al. Suicide in the elder-ly: a psychological autopsy study in a North Italy area (1994-2004). Am J Geriatr Psychiatry 2008: 727-35.
12. Innamorati M, Tamburello A, Lester D, et al. Inequalities in sui-cide rates in the European Union’s elderly: trends and impact of macro-socioeconomic factors between 1980 and 2006. Can J Psy-chiatry 2010; 55: 229-38.
13. Klug A, Lindner R, Fiedler G, Altenhöfer A. Sexualität suizidaler Älterer. Z Gerontol Geriatr 2008; 41: 22-8.
14. Albers B, Kruse J, Giani G, Icks A. Diabetes and incident de-pression: Is the association mediated or modified by sociodemo-graphic factors or co-morbidities? A systematic review. Exp Clin Endocrinol Diabetes 2011; 119: 591-8.
15. Dziemidok P, Makara-Studzi_ska M, Jarosz MJ. Diabetes and depression: a combination of civilization and life-style diseases is more than simple problem adding. Literature review. Ann Agric Environ Med 2011; 18: 318-22.
16. García-Fabela L, Melano-Carranza E, Aguilar-Navarro S, Gar-cía-Lara JM, Gutiérrez-Robledo LM, Avila-Funes JA. Hyper-tension as a risk factor for developing depressive symptoms among community-dwelling elders. Rev Invest Clin 2009; 61: 274-80.
17. Saran RK, Puri A, Agarwal M. Depression and the heart. Indian Heart J 2012; 64: 397-401.
18. Apostolopoulou M, Savopoulos C, Michalakis K, Coppack S, Dardavessis T, Hatzitolios A. Age, weight and obesity. Maturitas 2012; 71: 115-9.
19. Han TS, Tajar A, Lean ME. Obesity and weight management in the elderly. Br Med Bull 2011; 97: 169-96.
20. Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother 2009; 9: 1393-412. 21. Wolters M, Ströhle A, Hahn A. Cobalamin: a critical vitamin in
the elderly. Prev Med 2004; 39: 1256-66.
22. Annweiler C, Souberbielle JC, Schott AM, de Decker L, Berrut G, Beauchet O. Vitamine D chez la personne agée: les 5 points à retenir. Geriatr Psychol Neuropsychiatr Vieil 2011; 9: 259-67. 23. Parker G, Brotchie H. “D” for depression: any role for vitamin
D? “Food for Thought” II. Acta Psychiatr Scand 2011; 124: 243-9.
24. Fava M, Mischoulon D. Folate in depression: efficacy, safety, dif-ferences in formulations, and clinical issues. J Clin Psychiatry 2009; 70 (suppl 5): 12-7.
25. Bottiglieri T. Homocysteine and folate metabolism in depres-sion. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1103-12.
26. Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP, Lipsitz LA. The role of homocysteine in multisystem age-related prob-lems: a systematic review. J Gerontol A Biol Sci Med Sci 2005; 60: 1190-201.
27. Fischbach R, Gastager H, Harrer G. Hyposideremia and en-dogenous depression. Pharmakopsychiatr Neuropsychophar-makol 1973; 6: 252-7.
28. Stewart R, Hirani V. Relationship between depressive symp-toms, anemia, and iron status in older residents from a national survey population. Psychosom Med 2012; 74: 208-13.
29. Cutler P. Iron overload in psychiatric illness. Am J Psychiatry 1991; 148: 147-8.
30. Cutler P. Iron overload and psychiatric illness. Can J Psychiatry 1994; 39: 8-11.
31. Feifel D, Young CW. Iron overload among a psychiatric outpa-tient population. J Clin Psychiatry 1997; 58: 74-8.
32. Serata D, Del Casale A, Rapinesi C, et al. Hemochromatosis-in-duced bipolar disorder: a case report. Gen Hosp Psychiatry 2012; 34: 101.e1-3.
33. Kaplan BJ, Crawford SG, Field CJ, Simpson JS. Vitamins, miner-als, and mood. Psychol Bull 2007; 133: 747-60.
34. Girardi P, Pompili M, Innamorati M, et al. Duloxetine in acute major depression: review of comparisons to placebo and stan-dard antidepressants using dissimilar methods. Hum Psy-chopharmacol 2009; 24: 177-90.
35. Knadler MP, Lobo E, Chappell J, Bergstrom R. Duloxetine: clin-ical pharmacokinetics and drug interactions. Clin Pharma-cokinet 2011; 50: 281-94.
36. Musenga A, Amore M, Mandrioli R, Kenndler E, de Martino L, Raggi MA. Determination of duloxetine in human plasma by capillary electrophoresis with laser-induced fluorescence detec-tion. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 1126-32.
37. Mercolini L, Mandrioli R, Cazzolla R, Amore M, Raggi MA. HPLC analysis of the novel antidepressant duloxetine in human plasma after an original solid-phase extraction procedure. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 856: 81-7. 38. Mandrioli R, Mercolini L, Cesta R, Fanali S, Amore M, Raggi
MA. Analysis of the second generation antidepressant venlafax-ine and its main active metabolite O-desmethylvenlafaxvenlafax-ine in human plasma by HPLC with spectrofluorimetric detection. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 856: 88-94. 39. Musenga A, Kenndler E, Mercolini L, Amore M, Fanali S, Rag-gi MA. Determination of sertraline and N-desmethylsertraline in human plasma by CE with LIF detection. Electrophoresis 2007; 28: 1823-31.
40. Saracino MA, Amore M, Baioni E, Petio C, Raggi MA. Deter-mination of selected phenotiazines in human plasma by solid-phase extraction and liquid chromatography with coulometric detection. Analytica Chimica Acta 2008; 624: 308-16.
41. Mancini M, Gianni W, Rossi A, Amore M. Duloxetine in the management of elderly patients with major depressive disorder: an analysis of published data. Expert Opin Pharmacother 2009; 10: 847-60.
42. Tarolla E, Brugnoli R, Pancheri P. Duloxetina: efficacia clinica, sicurezza e tollerabilità nel trattamento del disturbo depressivo maggiore. Giorn Ital Psicopatol 2007; 13: 258-78.
JG, Kennedy JS. Duloxetine for the treatment of major depres-sive disorder in older patients. Am J Geriatr Psychiatry 2005; 13: 227-35.
44. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-con-trolled trial. Am J Psychiatry 2007; 164: 900-9.227-35.
45. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly pa-tients with major depressive disorder. Int Clin Psychopharmacol 2012; 27: 215-23.
46. Raskin J, Xu JY, Kajdasz DK. Time to response for duloxetine 60 mg once daily versus placebo in elderly patients with major de-pressive disorder. Int Psychogeriatr 2008; 20: 309-27.
47. Wohlreich MM, Sullivan MD, Mallinckrodt CH, et al. Duloxe-tine for the treatment of recurrent major depressive disorder in elderly patients: treatment outcomes in patients with comorbid arthritis. Psychosomatics 2009; 50: 402-12.
48. Davidson J, Allgulander C, Pollack MH, et al. Efficacy and toler-ability of duloxetine in elderly patients with generalized anxiety disorder: a pooled analysis of four randomized, double-blind, placebo-controlled studies. Hum Psychopharmacol 2008; 23: 519-26.
49. Russell J, Raskin J, Wiltse C, Walker D, Brawman-Mintzer O. Ef-ficacy and tolerability of duloxetine treatment in elderly patients with major depressive disorder and concurrent anxiety symp-toms. Psychiatry (Edgmont) 2007; 4: 33-45.
50. Robinson M, Oakes TM, Raskin J, Liu P, Shoemaker S, Nelson JC. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry 2012 Jul 21. Epub ahead of print, doi: 10.1097/JGP. 0b013e 31825d08f1.
51. Wohlreich MM, Mallinckrodt CH, Watkin JG, Hay DP. Duloxe-tine for the long-term treatment of major depressive disorder in patients aged 65 and older: an open-label study. BMC Geriatr 2004; 4: 11.
52. Karp JF, Whyte EM, Lenze EJ, et al. Rescue pharmacotherapy with duloxetine for selective serotonin reuptake inhibitor non-responders in late-life depression: outcome and tolerability. J Clin Psychiatry 2008; 69: 457-63.
53. Dolder C, Nelson M, Stump A. Pharmacological and clinical profile of newer antidepressants: implications for the treatment of elderly patients. Drugs Aging 2010; 27: 625-40.
54. Patel-Gadhia R, Bhal K, Patil P. Retrospective audit on tolera-bility and efficacy of duloxetine for stress urinary incontinence. J Obstet Gynaecol 2011; 31: 258-9.
55. Wade AG, Crawford GM. Urinary flow and urinary symptoms in elderly males exposed to either escitalopram or duloxetine. Curr Med Res Opin 2010; 26: 1031-5.
56. Schagen van Leeuwen JH, Lange RR, Jonasson AF, Chen WJ, Viktrup L. Efficacy and safety of duloxetine in elderly women with stress urinary incontinence or stress-predominant mixed urinary incontinence. Maturitas 2008; 60: 138-47.
57. Skinner MH, Kuan HY, Skerjanec A, et al. Effect of age on the pharmacokinetics of duloxetine in women. Br J Clin Pharmacol 2004; 57: 54-61.
58. Cui Z, Zhao Y, Novick D, Faries D. Predictors of duloxetine ad-herence and persistence in patients with fibromyalgia. J Pain Res 2012; 5: 193-201.
59. Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis:
randomised placebo-controlled trial. Age Ageing 2012; 41: 646-52.
60. Zilliox L, Russell JW. Treatment of diabetic sensory polyneu-ropathy. Curr Treat Options Neurol 2011; 13: 143-59.
61. Raskin J, Wiltse CG, Dinkel JJ, Walker DJ, Desaiah D, Katona C. Safety and tolerability of duloxetine at 60 mg once daily in eld-erly patients with major depressive disorder. J Clin Psychophar-macol 2008; 28: 32-8.
62. Wernicke J, Lledó A, Raskin J, Kajdasz DK, Wang F. An evalua-tion of the cardiovascular safety profile of duloxetine: findings from 42 placebo-controlled studies. Drug Saf 2007; 30: 437-55. 63. Thase ME, Tran PV, Wiltse C, Pangallo BA, Mallinckrodt C,
De-tke MJ. Cardiovascular profile of duloxetine, a dual reuptake in-hibitor of serotonin and norepinephrine. J Clin Psychopharma-col 2005; 25: 132-40.
64. Wise TN, Wiltse CG, Iosifescu DV, Sheridan M, Xu JY, Raskin J. The safety and tolerability of duloxetine in depressed elderly pa-tients with and without medical comorbidity. Int J Clin Pract 2007; 61: 1283-93.
65. Lingjærde O, Ahlfors UG, Bech P, et al. The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotrop-ic drugs, and a cross-sectional study of side effects in neurolep-tic-treated patients. Acta Psychiatr Scand 1987; Suppl 76: 1-100. 66. Baselt RC. Disposition of toxic drugs and chemicals in man, 8th
ed. Foster City (CA) USA: Biomedical Publications, 2008. 67. Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and
safety of duloxetine, a dual-serotonin and norepinephrine reup-take inhibitor. J Clin Pharmacol 2000; 40: 161-7.
68. Lai MW, Klein-Schwartz W, Rodgers GC, et al. 2005 Annual Re-port of the American Association of Poison Control Centers’ na-tional poisoning and exposure database. Clin Toxicol (Phila) 2006; 44: 803-932.
69. Bronstein AC, Spyker DA, Cantilena LR Jr, Green J, Rumack BH, Heard SE. 2006 Annual Report of the American Associa-tion of Poison Control Centers’ NaAssocia-tional Poison Data System (NPDS). Clin Toxicol (Phila) 2007; 45: 815-917.
70. Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Heard SE; American Association of Poison Control Cen-ters. 2007 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008; 46: 927-1057. 71. Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack
BH, Giffin SL. 2008 Annual Report of the American Associa-tion of Poison Control Centers’ NaAssocia-tional Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila) 2009; 47: 911-1084.
72. Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Giffin SL. 2009 Annual Report of the American Associa-tion of Poison Control Centers’ NaAssocia-tional Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila) 2010; 48: 979-1178.
73. Bronstein AC, Spyker DA, Cantilena LR Jr, Green JL, Rumack BH, Dart RC. 2010 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clin Toxicol (Phila) 2011; 49: 910-41.
74. Anderson D, Reed S, Lintemoot J, et al. A first look at duloxe-tine (Cymbalta) in a postmortem laboratory. J Anal Toxicol 2006; 30: 576-80.
75. Mari F, Gualco B, Rensi R, Bertol E. Acute massive pulmonary thromboembolism due to acute intoxication by duloxetine: a case report. Cardiovasc Toxicol 2012; 12: 258-62.