Ph.D. Thesis
Metal nanoparticles for biomedical
applications: engineered coatings for
multifunctionalization and controlled release
Valerio Voliani
Advisors
Prof. Fabio Beltram
Dr. Stefano Luin
General Index
Introduction ... 1
Chapter 1 – Properties and synthesis of metal nanoparticles ... 7
1.1 Nanomaterials and their behavior ... 8
1.1.1 Optical properties ... 8
1.1.2 Cellular uptake ... 17
1.2 Synthetic Processes: State of the Art ... 20
1.2.1 Gold nanospheres: organic media ... 21
1.2.2 Gold nanospheres: aqueous media... 22
1.2.3 Production of other types of nanoparticles ... 25
1.3 Synthetic Processes ... 26
1.3.1 Zhong Method ... 27
1.3.2 Magnetite nanostars ... 29
1.4 Materials & Methods ... 29
Nanoparticles characterization... 29
Turkevich method ... 30
Xia method ... 31 Zhong method ... 31 Platinum nanospheres ... 31 Silver nanocubes ... 32 Gold nanocubes ... 32 Silver nanospheres ... 33 Rhenium nanospheres ... 33
Magnetite, cobalt oxide, and hybrid nanoparticles ... 33
Magnetite “nanostars” ... 33
Chapter 2 – Functional coatings for gold nanospheres ... 39
2.1 Coatings: state of the art ... 40
2.2 Peptidic coatings ... 44
2.3 Bifunctional coating ... 48
2.3.1 Reactivity of the bifunctional coating ... 48
2.3.2 Surface Enhanced Raman Scattering (SERS) ... 52
2.3.3 Nanoparticles as carriers into living cells... 54
2.3.4 Generalization to other metals ... 56
2.4 Multifunctional coating ... 57
2.5 Summary ... 64
2.6 Experimental section ... 64
2.6.1 Peptide substitution ... 64
2.6.2 Reactivity of superficial carboxylic groups ... 65
2.6.3 Reactivity of superficial amine groups... 65
2.6.4 Reactivity of superficial alkynes ... 66
2.6.5 Bifunctionalized AuNsK ... 66
2.6.6 Multifunctionalized AuNs ... 66
2.6.7 SERS measurements ... 67
2.6.8 Glass-slide preparation ... 68
2.6.9 Expression and purification of recombinant Green Fluorescent Protein ... 68
2.6.10 Cell culture and uptake of nanostructures ... 68
2.6.11 Image acquisition ... 69
2.6.12 Synthesis of 5(6)-carboxyfluoresceinamido-ethylazide (FluoresceinAzide, Fn) ... 69
Chapter 3 – Multiphoton controlled photo-release ... 73
3.1 Photo-physical mechanisms ... 74
3.1.1 Photochemistry ... 75
3.1.2 Multiphoton phenomena and their enhancement by metal nanostructures ... 76
3.2 Drug delivery and nanoprobes ... 78
3.3 Modular AuNs for externally controlled photorelease ... 81
3.3.1 The nano-system ... 81
3.3.2 Photorelease in living cells ... 82
3.3.3 Multiphoton cleavage ... 85 3.4 Summary ... 88 3.5 Experimental section ... 88 3.5.1 Fluorescence measurements ... 89 3.5.2 Confocal images ... 89 3.5.3 Cross-section calculation ... 89
Conclusion & Perspectives ... 93
Appendix A -Techniques for the analysis of nanoparticles- ... 97
Appendix B -Interactions of light with nanoparticles- ... 101
Table of Structures... 119 List of Publications ... Acknowledgment ...
Introduction
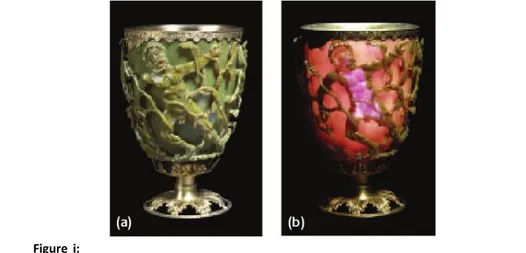
Nanoparticles (Nps) are inorganic structures with dimensions below 1 µm, made of a few hundred up to a few thousand atoms. The first (unintentional) exploitations of nanometric metallic objects ensue from their intense and peculiar interaction with light. The earliest reported example dates back to the fourth century A.D., when the Roman glassmakers produced glasses containing metallic nanoparticles. An artifact from this period is the Lycurgus cup that resides in the British Museum in London (Fig. i). The cup is a soda-lime glass containing silver and gold nanoparticles and changes its color from green to deep red when a light source is placed inside it. Nps were subsequently used to make the vast variety of colors of cathedrals’ windows and photography technologies. In 1857 Michael Faraday[1] was the first to attempt an explanation of the impact of metal nanoparticles on the color of glass[2, 3] (Fig. ii). Many years later, Von Gustav Mie[4] provided a theoretical explanation of the interaction between light and nanometric materials of different size, shape, and composition. The modern era of Nps began in the last half-century[5] with the development of reliable and high-yield methods for the synthesis of
Introduction 2
nanocrystals, both with spherical and non-spherical shape. In the last decade several experimental conditions were investigated that allow the fine tuning of size and morphology of Nps in colloidal solutions. These include the choice of suitable precursors, of stabilizer molecules (surfactants or ligands) and of reducing agents, or temperature modulation and adjustment of the relative concentration ratio of the reactants[6, 7]. The ability to tailor the dimensional and geometrical features of the nanostructures represents a landmark achievement in materials science, since at the nanoscale these properties dictate the peculiar chemical and physical (catalytic, optical and electrical) properties of materials[8, 9].
The peculiar interaction with light of noble-metal nanoparticles stems from the existence of a Localized Surface Plasmon Resonance (LSPR): the particles resonantly absorb and scatter visible and near-infrared light upon excitation of their surface-plasmon oscillation. The plasmon resonance band of Nps can be tuned over a wide spectral range by changing intrinsic parameters such as their composition (considering also bi-metallic or hybrid particles), size, or shape[10] (sphere, rod, cube, triangle, cage, etc.). It depends also on the environment around Nps. The light-scattering signal is usually much brighter than the fluorescence emission available with organic molecules and does not suffer from photobleaching or blinking[11]. This is an advantage for their application to
single-molecule imaging, where the use of dyes, fluorophores, or quantum dots is limited by low signal intensities, complex blinking phenomena, and/or
Figure i: The Lycurgus cup in diffused (a) and transmitted (b) light. The scene shows
Lycurgus being enmeshed by Ambrosia, now transformed into a vine-shoot. Department of Prehistory and Europe, The British Museum. Height: 16.5 cm, diameter: 13.2 cm.
photobleaching. In very small nanoparticles (below 10 nm of diameter for gold nanospheres) absorption phenomena become dominant over scattering. This behavior can lead to applications in photothermal microscopy[12] or photo-thermal-therapy. While the detailed size- and shape-dependent optical and electronic behavior depends on the metallic core, stability and reactivity of these structures depend on surface coating[6]. Indeed several encapsulation processes were developed to improve the water-stability and the versatility of Nps. The most widely used surfactants contain functional groups[5] such as thiols, phosphines, and amines (which exhibit high affinity for metal surfaces) at one end of the molecule, and carboxylic acid or amines at the other end. The peculiar optical properties, the surface reactivity and the potential integration of Nps with existing technologies make colloids a versatile tool useful in many technological fields (such as nanomedicine or environmental sciences).
In particular, gold nanoparticles (AuNps) are very attractive nanomaterials for biological and biomedical applications, especially for their low toxicity for cells[13]. Gold nanoparticles for bio-applications are generally prepared by reduction of gold salts in presence of stabilizing agents (surfactants or ligands), which are needed to prevent nanoparticle agglomeration and to control their final geometry[14-18]. Furthermore, gold nanoparticles can be easily functionalized by anchoring thiol linkers onto the metallic surface. A wide
Introduction 4
variety of functional bio-nanoconjugates has been obtained, including nanoparticles modified with peptides, proteins, antibodies, oligosaccharides, and nucleic acids[19-22]. This allows the use of nanomaterials as multifunctional platforms for both biological and biomedical purposes[23-25]. Examples are bio-imaging, single molecule tracking, biosensing, drug delivery, transfection, and other therapeutic and diagnostic uses. For instance, particles can be engineered to accumulate preferentially in chosen cells using targeting ligands, providing e.g. a tool for cancer diagnosis and gene therapy[26]. Sensor arrays were developed to discriminate normal, cancerous, and metastatic cells using the fluorescence quenching properties of gold nanoparticles[24]. Interactions and fate of a broad range of functionalized nanoparticles are currently under investigation in a wide variety of biological models, ranging from whole organisms to tissues or cells in culture, to yeast[27] and prokaryote bacteria[28]. New possibilities are arising from the interaction of different materials: assembling several components into a single nanostructure is an attractive way to design systems possessing carefully tuned physical and chemical properties. In these multicomponent systems, one can expect multifunctionality[29] and even novel properties originating from synergies between the constituents[13].
This thesis describes and discusses the advancements made in this subject during my PhD. Every chapter reports the state of the art of the relevant technologies before this work, the results obtained on the specific topics, a discussion on their chemical and physical basis and on their use in biological or biomedical experiments or applications. In particular, in Chapter 1 I shall describe some of the synthetic processes used to obtain stable colloidal solutions of gold and other materials with different shapes, emphasizing the dependence of the physical-chemical features upon their geometry. The description of a novel versatile, biocompatible, and multifunctional coating designed for gold nanospheres is discussed in Chapter 2. Chapter 3 reports the presentation of the first multicomponent photo-sensitive nanosystem able to penetrate in living cells and to release (bio)cargos upon visible-light external stimuli. Finally, in the Conclusion, I shall summarize the findings and discuss some perspectives of multicomponent nano-systems.
References
[1] M. Faraday, Philos. Trans. R. Soc. London 1857, 147, 145.
[2] M. Hayat, Colloidal gold: principles, methods, and applications, San Diego, 1989.
[3] P. P. Edwards, J. M. Thomas, Angewandte Chemie-International Edition
2007, 46, 5480.
[4] G. Mie, Ann. der Phys. (Leipzig) 1912, 11, 1.
[5] M. C. Daniel, D. Astruc, Chemical Reviews 2004, 104, 293. [6] Y. Yin, A. P. Alivisatos, Nature 2005, 437, 664.
[7] M. P. Pileni, Nature Materials 2003, 2, 145.
[8] X. G. Peng, L. Manna, W. D. Yang, J. Wickham, E. Scher, A. Kadavanich, A. P. Alivisatos, Nature 2000, 404, 59.
[9] C. Burda, X. B. Chen, R. Narayanan, M. A. El-Sayed, Chemical Reviews
2005, 105, 1025.
[10] C. Sonnichsen, T. Franzl, T. Wilk, G. von Plessen, J. Feldmann, O. Wilson, P. Mulvaney, Physical Review Letters 2002, 88.
[11] J. Yguerabide, E. E. Yguerabide, Analytical Biochemistry 1998, 262, 137. [12] D. Boyer, P. Tamarat, A. Maali, B. Lounis, M. Orrit, Science 2002, 297,
1160.
[13] E. E. Connor, J. Mwamuka, A. Gole, C. J. Murphy, M. D. Wyatt, Small
2005, 1, 325.
[14] M. Brust, M. Walker, D. Bethell, D. J. Schiffrin, R. Whyman, Journal of
the Chemical Society-Chemical Communications 1994, 801.
[15] G. Frens, Nature-Physical Science 1973, 241, 20.
[16] N. Goubet, Y. Ding, M. Brust, Z. L. Wang, M. P. Pileni, ACS Nano 2009, 3, 3622.
[17] M. P. Pileni, Journal of Physical Chemistry C 2007, 111, 9019. [18] B. V. Enustun, J. Turkevich, J Am Chem Soc 1963, 85, 3317.
[19] A. P. Alivisatos, K. P. Johnsson, X. G. Peng, T. E. Wilson, C. J. Loweth, M. P. Bruchez, P. G. Schultz, Nature 1996, 382, 609.
[20] C. A. Mirkin, R. L. Letsinger, R. C. Mucic, J. J. Storhoff, Nature 1996, 382, 607.
[21] R. Levy, N. T. Thanh, R. C. Doty, I. Hussain, R. J. Nichols, D. J. Schiffrin, M. Brust, D. G. Fernig, J Am Chem Soc 2004, 126, 10076.
[22] S. Kumar, J. Aaron, K. Sokolov, Nat Protoc 2008, 3, 314.
[23] P. Ghosh, G. Han, M. De, C. K. Kim, V. M. Rotello, Advanced Drug
Bibliography 6
[24] A. Bajaj, O. R. Miranda, I. B. Kim, R. L. Phillips, D. J. Jerry, U. H. F. Bunz, V. M. Rotello, Proceedings of the National Academy of Sciences of the
United States of America 2009, 106, 10912.
[25] P. S. Ghosh, C. K. Kim, G. Han, N. S. Forbes, V. M. Rotello, ACS Nano
2008, 2, 2213.
[26] I. H. El-Sayed, X. H. Huang, M. A. El-Sayed, Nano Letters 2005, 5, 829. [27] A. Mohammadi, F. Kaminski, V. Sandoghdar, M. Agio, Journal of
Physical Chemistry C 2010, 114, 7372.
[28] R. M. Amin, M. B. Mohamed, M. A. Ramadan, T. Verwanger, B. Krammer, Nanomedicine 2009, 4, 637.
[29] R. Bardhan, W. Chen, M. Bartels, C. Perez-Torres, M. F. Botero, R. W. McAninch, A. Contreras, R. Schiff, R. G. Pautler, N. J. Halas, A. Joshi,
Chapter
1
Properties and synthesis of
metal nanoparticles
In many cases the behavior of matter at the nano-scale is unexpected and can be completely different from that of the corresponding bulk materials. This has stimulated the development of many applications based on nanostructures in virtually all areas of science and technology. In particular gold nanoparticles (AuNps) are very promising candidates for a number of applications in the fields of photonics, optoelectronics, (bio)sense, bio-labeling, and (nano)medicine[1, 2]. Some properties of gold colloids, such as localized surface plasmon resonance (LSPR) modes and catalytic activity depend sensitively on their size and shape; therefore, many efforts are devoted to the development of methods yielding size controllability and surface tunability. Generally, chemically synthesized metal nanoparticles are produced in liquids (wet chemistry) by reduction of metal ions in the presence of stabilizing surfactants.
1.1 Nanomaterials and their behavior
8Control of the reaction is a prerequisite to achieve the desired size and shape of the particles, and, thus, to finely tune their physical-chemical properties[3, 7].
In this chapter I shall start by introducing in section 1.1 the properties of metallic nanostructures, with a special focus on the correlation between size, shape and materials on optical features and biocompatibility. Section 1.2 addresses the general synthetic processes commonly used to yield metallic nanoparticles by wet chemistry, with particular emphasis on gold nanostructures. In section 1.3 I will describe: i) a novel protocol for the synthesis of magnetite stars, and ii) the synthesis used in this PhD project for the production of gold nanospheres (AuNss). Finally, in the Materials &
Methods section I shall describe the experimental procedures.
1.1.
Nanomaterials and their behavior
1.1.1. Optical properties
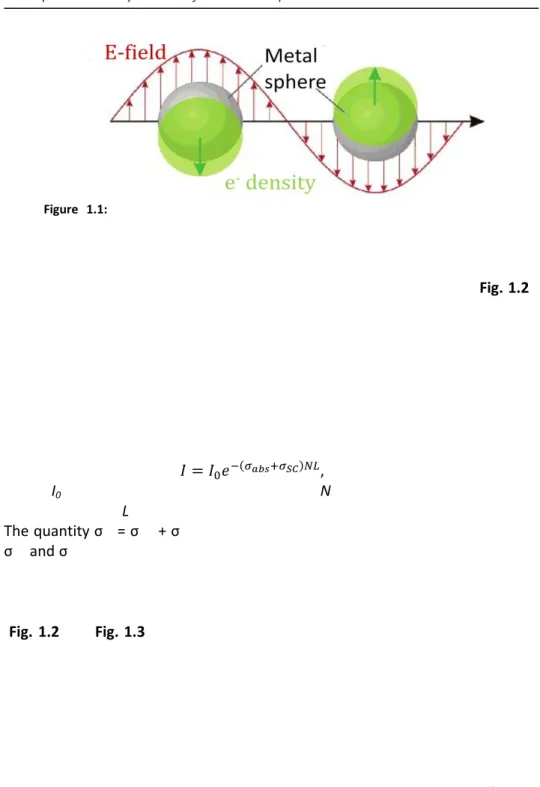
Noble metal colloids are characterized by intense colors caused by light absorption and scattering in the visible region of the spectrum; an example of an early application of this property is in the rose window of the Notre Dame cathedral in Paris, where silver and gold nanoparticles are responsible for the glass colors. These effects are caused by one of the most important type of interaction of metal nanoparticles with the electromagnetic field (Fig. 1.1), the
Localized Surface Plasmon Resonance (LSPR). Metals are characterized by the
presence of “free” electrons and when the diameter of metallic nanostructures is in the 10-100 nm range they interact with the light through[14] (i) collective excitations of free electrons due to intraband transitions, giving rise to LSPR (ii) transitions of electrons from occupied to empty bulk bands of different index, called interband transitions, and (iii) scattering of “free” electrons when their mean free path is comparable to the dimension of the nanostructures. A
resonance occurs when the frequency of an incident electromagnetic (EM) field
matches the frequency of an intrinsic electronic oscillation. This is a collective and coherent oscillation of the electronic density of the metals, called plasmon, which causes a displacement of the electrons from the nuclei. The electronic oscillation lead to the formation of various possible distributions in the nanostructure surface charges (i.e. dipole, quadrupole etc., see Fig. 1.1). Each type of surface charge distribution is characterized by specific resonance energy, the Localized Surface Plasmon Resonance (LSPR, Fig. 1.1). When an incoming radiation of appropriate frequency interacts with the nanostructure, its energy can be stored in the oscillation mode of the nanoparticle and can
result in heat (absorption) and/or in light scattering. Noble metals such as copper, silver, and gold have strong visible-light plasmon resonances (Fig. 1.2), whereas most other transition metals show only a broad and poorly resolved extinction band in the ultraviolet region[15]. The presence of the LSPR in the visible region for noble metals is mainly attributed to the strong coupling between the plasmon resonance and interband excitations. In a dilute sample of nanoparticles, so that each particle behaves independently with respect to the incident radiation, the spectrum is composed of the sum of size-dependent absorption and scattering modes. The intensity of light transmitted through this sample is given by the expression:
(1)
where I0 is the intensity of the incoming light, N is the number of particles per
unit volume, and L is the length of the path travelled by the light in the sample. The quantity σex = σabs + σsc is also known as the extinction cross section, while σabs and σsc are the absorption and scattering cross section of the nanoparticles, respectively. The size and shape of the particles, the dielectric function of the medium, and the presence of other nanostructures in close proximity are the factors that most influence the extinction bands of LSPR in nanostructures[7, 16] (Fig. 1.2 and Fig. 1.3). In the following, I shall discuss the influence of particle size on the position and the width of the spectral band of plasmon modes.
In the case of metal nanospheres (Ns), interband electronic transitions are not very sensitive to particle size (except for the case of sub-2 nm metal clusters, which are made of few atoms) and are located at high energy (UV region of the spectra). For nanoparticles with diameters between 10 and 30 nm, the dominant effect in the visible region is the excitation of plasmon modes. In this size regime, and in the simple case of spherical nanoparticles, a single dominant plasmon mode of dipolar nature is excited. For gold (Fig. 1.2)
Figure 1.1: Scheme of a surface plasmon oscillation for a sphere, showing the
displacement of the conduction electron charge cloud relative to the nuclei. Reproduced from Schatz et al.[3]
1.1 Nanomaterials and their behavior
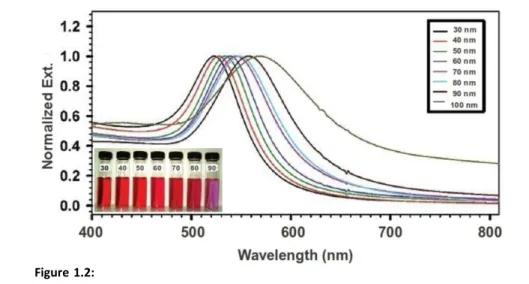
10this mode occurs at about 515 nm, and for silver at 400 nm. On the other hand, scattering effects are more important for Ns with a diameter of more than 30 nm, where electrons are accelerated by the electromagnetic field and radiate energy. Owing to this secondary radiation, electrons lose energy by a damping effect on their motion. It was found (Fig. 1.2) that the spectrum is less intense, wider, and red-shifted when the particle size increases[14]. A depolarization field term leads to a shift to larger wavelengths, while radiation damping causes a decreasing intensity and a spectrum widening[17]. Finally, scattering effects dominate the response of Ns with diameters larger than 100 nm and, in addition, higher order modes (i.e. quadrupolar, octupolar) increasingly contribute to the interaction between light and matter. In a theoretical/experimental work on spherical gold nanoparticles by El-Sayed et al.[16], authors showed that the sum of all these effects causes a red shift on the LSPR max of about 0.7 nm per 1 nm increase in particle radius (for diameter >25 nm). For particle sizes smaller than 25 nm, max is almost diameter-independent.
The basis of the correlation between nanosphere size and the max of the LSPR band was described for the first time by Mie[18]. He solved Maxwell’s equations in the quasi-static regime (he assumed that the field felt by the particle was constant throughout the solid, albeit it can still be time, or frequency, dependent) and obtained, in the dipole approximation (nanoparticles are much smaller than the incident wavelength):
Figure 1.2: Normalized UV-Vis spectra for Au nanospheres with different diameters in
aqueous solution. Inset, photo showing the colors of gold nanospheres with different sizes from 30 to 90 nm. Reproduced from Zhong et al.[6]
, (2)
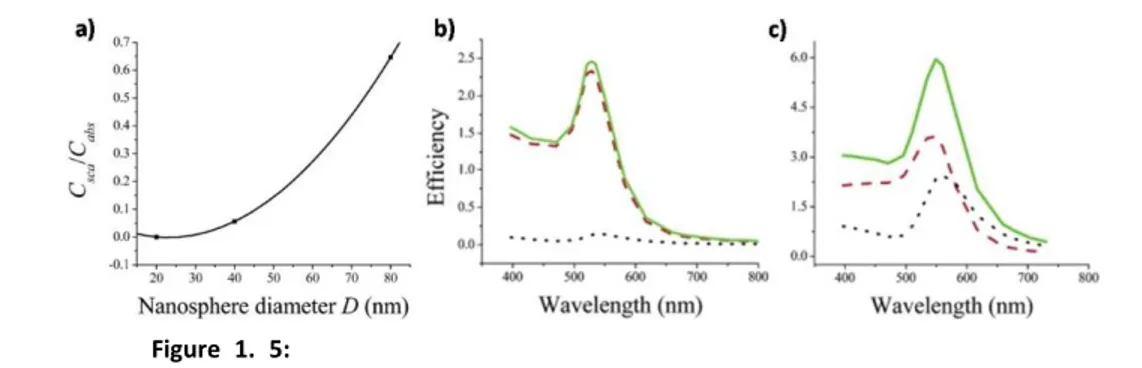
where ex is the extinction cross section, is the angular frequency, V is the volume of each sphere, a is the medium dielectric constant, and 1 and 2 are the real and complex part of the dielectric function of the metal[18]. The resonance condition is fulfilled roughly when 1()=-2a if 2 is small or weakly dependent on ; this links the dependence of LSPR to the dielectric function of the surrounding medium[6]a. In this model the dependence of the LSPR band in Ns of different sizes is a result of the dependence of the refractive index of nanoparticles on R[19]. In fact an intrinsic dependence of the real and imaginary part of the dielectric function of metals[7] on R is indicated in the previous equation. Indeed size-dependence is lost if the dielectric constant of the bulk metal is used to solve Maxwell’s equations. A more quantitative explanation of these features is reported in Appendix B. It is important to note (Fig. 1.4) the direct dependence of nanosphere extinction cross section on the sphere volume and that ex of gold nanospheres are typically 4-5 orders of magnitude higher compared to those of organic dyes[11]. At the same time, the relative contribution of scattering to the total extinction σsc/σabs increases with the square of particle volume as seen in Fig. 1.5. This trend in the ratio of scattering
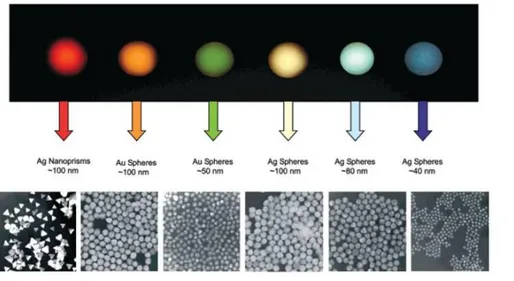
Figure 1.3: Size, shape, and composition of metal nanoparticles can be systematically
varied to produce materials with distinct optical properties. The upper panel reports the color from dark field signals of drops of the nanoparticles shown in the bottom panel. Reproduced from Mirkin et al.[1]
1.1 Nanomaterials and their behavior
12to absorption with the nanoparticle volume was related to an increase in radiative damping in larger particles[11]. Thus, the extinction features of “larger” (>20nm) AuNs were exploited for the selective scattering imaging of cells by using dark field microscopy[13] (DF) and confocal microscopy[20].
On the other hand the “intermediate” size range (3-10 nm) can serve as excellent photoabsorber for laser photothermal therapy and absorption-contrast imaging[21].
The shape of metal nanoparticles has a striking influence on optical properties (Fig. 1.3). The surface plasmon absorption maximum (λmax) of AuNp strongly depends on their aspect ratio[5] r (i.e. the length of the particle divided
Figure 1.4: Variation of extinction cross section (Cext) with nanosphere diameter.
Reproduced from El-Sayed et al.[11]
Figure 1. 5: a) Variation of the ratio between scattering and absorption
cross-sections (Csca/Cabs) with nanosphere diameter D. b, c) Calculated spectra of the efficiency
of absorption Qabs (red dashed), scattering Qsca (black dotted), and extinction Qext (green
solid) for gold nanospheres of diameter (b) D = 40 nm, (c) D = 80 nm. Reproduced from El-Sayed et al.[11]
by its width) for a fixed size:
λmax = 420 + 95r. (3) In equation 3 is reported the case of AuNps with a size of about 20 nm. When AuNp have a spherical shape (with r=1) their surface plasmon absorption band is centered at 515 nm. On the other hand, when AuNps with similar size become elongated on one axis, the surface plasmon absorption band red-shifts with r (Fig. 1.6). Also, in the case of rod-shaped nanoparticles the plasmon appear split into two modes corresponding to the oscillation along and perpendicular to the long axis of the rods[14, 22]. In general all the other geometrical shapes of gold nanoparticles (triangle[23], cube[24], shell[25]) exhibit a red-shifted LSPR band compared to their spherical analogs, since shape affects the electron charge density on the particle surface[5]. Such structural and compositional tuning is very useful for in vivo applications where tissue absorption in the near-infrared window (650–900 nm) is minimal, and thus, favorable to improved light penetration.
The plasmon resonance wavelength of a metal nanoparticle is also affected by the presence of other nanoparticles in its close environment. When two or more nanoparticles are brought into proximity, their dipoles couple, and a shift Figure 1.6: Extinction spectrum of a sample consisting of a colloids of nanorods having
an aspect ratio r of 3.3 and transversal dimension of 22nm (solid line), compared to one of 22 nm nanospheres (dotted line). The inset shows how the maxima of the transverse (squares) and longitudinal (circles) surface plasmon modes vary with aspect ratio. Reproduced from El-Sayed et al.[5]
1.1 Nanomaterials and their behavior
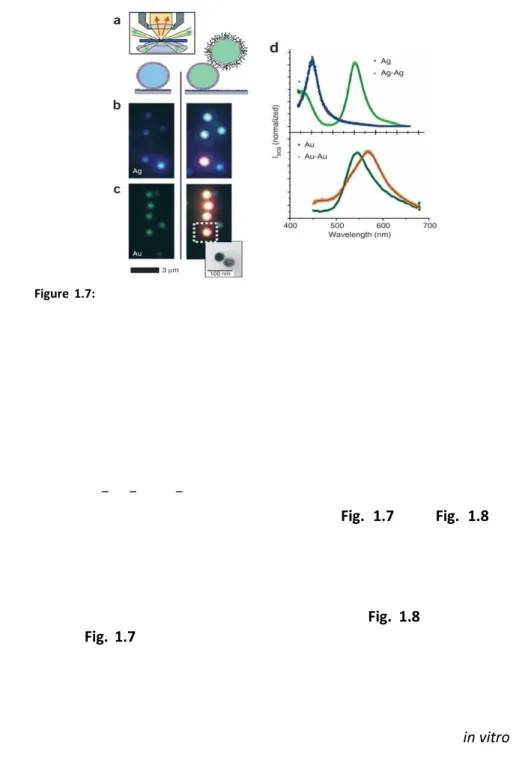
14in the LSPR resonance wavelength takes place (Fig. 1.7 and Fig. 1.8). For example, a colloid of gold nanospheres shows a typical plasmon extinction maximum at 515 nm; if particles agglomerate (for the addition of some analyte or for a change in pH or in salt concentration of the solution) a red-shift and widening in the extinction band is observed[26]. This effect was investigated both theoretically[27] and experimentally for fixed[28] (Fig. 1.8) and non-fixed distances[13] (Fig. 1.7). The magnitude of the assembly-induced plasmon shift depends on the strength of interparticle coupling, which, in turn, depends on the distance between the individual nanoparticles. Therefore, the plasmon shift can give a measure of the distance between pairs of nanoparticles[13]. El-Sayed et al.[29] derived an empirical equation that can be used to estimate the interparticle separation from experimentally observed plasmon shifts in vitro or in biological systems[30]:
Figure 1.7: Effect of coupling of DNA-functionalized gold and silver nanoparticles on
their color when observed in darkfield microscopy. (a) Two gold or silver nanoparticles can be linked together through a biotin-streptavidin bond. Inset: principle of transmission darkfield microscopy. (b) Single silver particles appear blue (left) and particle pair blue-green (right). The orange dot in the bottom comes from an aggregate of more than two particles. (c) Single gold particles appear green (left), gold particle pairs orange (right). Inset: representative transmission electron microscopy image of a particle pair to show that each colored dot comes from light scatted from two closely lying particles, which cannot be separated optically. (d) Representative normalized scattering spectra of single particles and particle pairs for silver (top) and gold (bottom). Silver particles show a larger spectral shift (102 nm) than gold particles (23 nm), stronger light scattering and a smaller plasmon line width. Gold, however, is chemically more stable and is more easily conjugated to biomolecules via –SH, –NH2 or –CN functional groups. Reproduced from Alivisatos et al.[13]
(4) where Δλ/λ0 is the fractional plasmon shift, s is the interparticle edge-to-edge separation, D is the particle diameter, and A and B are two parameters typical of the experimental setup. This equation was deduced for coupled pairs of gold nanoparticles (in 20-100 nm diameter-range) in protein medium at fixed distance in dark field (DF) experiments, by illumination with unpolarized white light. For these functionalized particle dimers randomly oriented in space[29, 30] parameters A and B were estimated[29]: A=0.18 and B=0.23. In particular, based on (4) (the plasmon ruler equation) nanoparticles dimers were indicated as an alternative to Förster Resonance Energy Transfer (FRET) for in vitro single-molecule experiments, especially for applications demanding long observation times (seconds to hours) or large distances (usually up to 2.5 times the diameter of the spheres). Indeed, this effect has several key advantages over rulers based on FRET and should allow a wide range of new single-molecule experiments. In FRET, the observation of single-organic dye fluorescence is often hindered by blinking and rapid photobleaching, limiting the continuous observation time to a few tens of seconds. Furthermore, it is sometimes difficult to distinguish changes in relative dye orientation from changes in distance[31]. The plasmon resonance signal neither blinks nor bleaches and does not depend on the relative probe orientation[30] in experiments that do not use polarized light (in experiments with polarized white light the shift depends on the orientation of the EM field, see Fig. 1.8). In general, gold and silver particles Figure 1.8: Microextinction spectra of Au nanodisc pairs for varying interparticle
separation gap for incident light polarization direction (a) parallel and (b) perpendicular to the interparticle axis. OD: optical density, OD=-log10(T), with T local light transmittivity. c)
SEM image of an array of nanodisks pair used to determine the “plasmon ruler equation”; in this image each nanodisk has a diameter of 88 nm, a thickness of 25 nm, and an interparticle edge-to-edge separation gap of 12 nm. Reproduced from El-Sayed et al.[12]
1.1 Nanomaterials and their behavior
16are more stable under physiological conditions and under laser illumination than organic dyes. The range of distances accessible with plasmon coupling in a pair of nanoparticles depends on the size and coating of the particles. In general, the accessible distance range (≈10-200 nm) is larger than with FRET[31] (2–8 nm). Usually with 40 nm particles and a 0.1 nm spectral resolution for determining the plasmon resonance position, particle separations of up to 70 nm should be accessible with better than 1 nm resolution[27]. Anyway, at least 20-30 nm diameter gold nanoparticles are needed to ensure the collection of scattering signals[11, 13].
The most unexplored feature of gold nanoparticles is their photoluminescence (PL). In addition to the phenomena mentioned above, excitation of LSPR can cause photoluminescence emission of nanomaterials showing sharply angled surfaces (lightning rod effect). In bulk noble metals, the quantum efficiency (the number of photons emitted over the number of absorbed photons) of the photoluminescence is very low, typically[32] of the order of 10-10. The luminescence efficiency (namely, the rate linked to the dissipation of the photon energy in heat) of gold nanorods increases by 6 orders of magnitude from bulk by the lightning rod effect[33] and in gold nanocubes reaches 10-2, about 200 times higher than that of gold nanorods[34]. Luminescence was also found to be absent in 15 nm spherical nanoparticles, whereas an enhancement of the photoluminescence was found for very small gold clusters[35] (<5 nm). The origin of the photoluminescence was attributed to recombination of electron-hole pairs. The electrons from the filled d band of electronic states are promoted to the sp conduction band above the Fermi level Figure 1.9: Major pathways for endocytosis, the process by which cells absorb external
objects. By phagocytosis cells can internalize solid matter larger than 0.75 m, while by pinocytosis the cells can uptake external solutions through 1000-50 nm cell membrane invagination (0.5-1 m for macropinocytosis, about 100 nm for clathrin-mediated, and about 50 nm for caveolae-mediated endocytosis). It is important to notice that phagocytosis and macropinocytosis occur in a non-specific manner, while clathrin- and caveolae-mediated endocytosis happens specifically by membrane receptor-mediated pathways. Reproduced from Stellacci et al.[4]
by incident photons. Both electrons and holes relax by scattering with phonons and recombine radiatively. Thanks to the drastic ex of the gold nanostructures the PL signals are intense enough to perform single molecule experiments and to engineer multicomponent nano-systems of interest for targeting, imaging, and therapeutic or theranostics applications.
1.1.2. Cellular Uptake
The plasma membrane is a selectively permeable lipid bilayer that defines the boundary and maintains the essential intracellular environment of the cell. Small and non-polar molecules such as O2 and CO2 can readily diffuse across the membrane; however, polar or bulkier molecules such as ions, or nanomaterials, are generally unable to cross the plasma membrane on their own. In nature, important ions and nanometer-sized proteins can be transported across the lipid bilayer through specialized membrane-transport protein channels[36]. Most other nanoscale macromolecules and molecular assemblies are internalized through endocytosis (the process of uptaking macromolecules into cells by enclosing them in membrane vesicles that are internalized) upon contact with the cell membrane (Fig. 1.9). Following this process, nanomaterials are confined in endosomes and are usually incapable of reaching the cytosol[37]. They remain trapped in endolysosomal vesicles unless co-internalized with a membrane-disrupting agent. Different approaches are utilized to transport directly nanomaterials into the cytosol of cells: i) disruption of endosomes and entry into cells through the proton sponge-effect mechanism[38] (typical for polycations that show buffering capacity below physiological pH) or the use of chloroquine[39], ii) direct microinjection of nanomaterials into cells[40], and iii) use of electroporation[41].
If no internalization molecules[42] such as cell-penetrating peptides or antibodies, which are able to avoid endocytotic processes, are linked to nanostructure, delivery into a cell usually involves adsorption onto the cell surface, followed by internalization by its endocytosis (Fig. 1.9 and Fig. 1.10).
Indeed the living-cell uptake of AuNps can be strongly influenced by: i) AuNp geometry, and ii) AuNp charge. The charge of AuNps is linked to the functional groups[43] on the nanoparticle surface and drive many important nanomaterial properties, such as colloidal stability, solubility and interactions with macromolecules or cell membrane (Fig. 1.10). While neutral functional groups, such as ethers or azides, are excellent in preventing unwanted interactions between nanomaterials and biological matter (i.e. proteins), most charged functional groups (generally, amines for the positive charged
1.1 Nanomaterials and their behavior
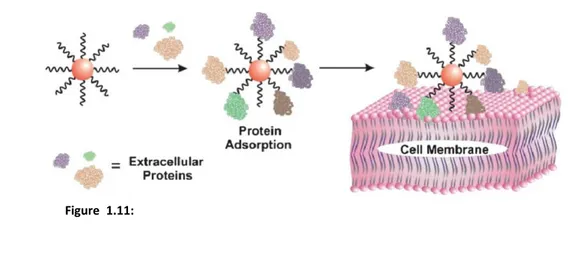
18nanoparticles and carboxylic acid for the negative ones) are responsible for active interactions with cells. Neutral and negatively charged nanoparticles are much less adsorbed on the negatively charged cell-membrane surface, and consequently show lower levels of internalization as compared to the positively charged particles[44], pointing out the role of surface charge in internalization of nanoparticles. However, there is evidence of uptake of negatively charged particles despite the unfavorable interaction between the particles and the negatively charged cell membrane. The internalization of negatively charged nanoparticles is believed to occur through nonspecific binding (and sometimes clustering) of the particles on cationic sites on the plasma membrane followed by their endocytosis[4]. Another mechanism proposed for some negative coatings was based on adsorption of serum proteins[45] on the surface of nanoparticles through electrostatic and hydrophobic interactions, which
Figure 1.10: Schematic illustration of endocytosis pathways for gold nanoparticles
featuring different surface charges. Citrate-coated (negative charge) and PVA-coated (neutral charge) nanoparticles display low affinity of interaction with the cell membrane (A) while a poly(allyamine hydrochloride)-coated nanoparticles (positive charge) show high cell-membrane binding affinity (B). Reproduced from Stellacci et al.[4]
allowed the nanoparticle to interface with the cell membrane (Fig. 1.11). The cellular toxicity of negatively charged nanoparticles is strongly dependent on the type of surface coating[46]; usually, peptide-coated ones do not interfere with cell viability[47]. On the other side, cationic particles are known to bind to negatively charged groups on the cell membrane[36] and translocate across the plasma membrane. However, despite cationic AuNps are the most effective in crossing cell-membrane barriers and localizing in the cytosol or nucleus, they usually show greater cytotoxicity (and a toilsome synthetic process) than negative or neutral nanoparticles.
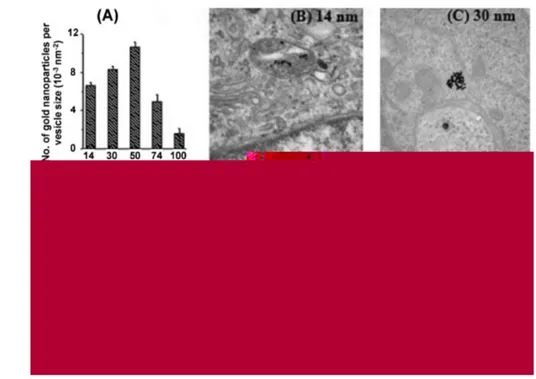
For AuNps with a fixed charge, the geometry (size and shape) of the nanostructure influences greatly the cellular uptake and the vitality of any cells involved. The uptake of 14, 50, and 74 nm gold nanospheres was recently investigated in Hela cells[10] (Fig. 1.12). It was found that the kinetics of uptake as well as the saturation concentration varied with the nanoparticle diameter and that the main internalization is achieved with about 50 nm spheres. This result indicates that there might be an optimal size for efficient nanomaterial uptake into cells. The effect of nanoparticle shape on its internalization was also examined: spherical particles of similar size were taken up 5 times more than rod-shaped particles, which is explained by the longer membrane wrapping time required for the elongated particles. Also it seems that gold nanocubes are uptaken with more difficulty than gold nanospheres, probably for the initial higher surface contact area between the nanostructures and the cell membrane[43]. In other studies, nanoparticle size was shown to strongly affect the binding and activation of membrane receptors and subsequent protein expression[48]. The inherent polydispersity of any batch of nanoparticles may cause unpredictable events, hence different batches of the same
Figure 1.11: Schematic view of the interactions amongst negatively-charged coated
nanoparticles, proteins, and cell membranes. Proteins in the media could adsorb on the functionalized negative gold nanoparticle; this allows interfacing of the nanoparticle with the cell membrane and its subsequent internalization Reproduced from Stellacci et al.[4]
1.2 Synthetic processes: State of the Art
20nanomaterial may display different results in cell studies: in this view the monodispersion in diameter of the nanospheres is a key-goal for developing useful nano-probes.
In summary, negative-coated gold nanospheres are promising materials for living cell application in view of their nontoxicity, nonimmunogenicity, and good tissue permeability[49]. In this view the PhD project was based on aqueous synthesized 30 nm gold nanospheres coated with a novel engineered negatively charged hexa-peptide described in Chapter 2.
1.2.
Synthetic Processes: State of the Art
Optical and biological properties of colloids are the result of a delicate balance of material, size, shape and dispersion of the nanostructures. This has prompted the exploration of many synthetic processes in the last decade in order to achieve the desiderate nanomaterials. Here the most commonly methods to produce nanoparticles (in particular gold nanospheres) will be discussed.
Figure 1.12: TEM images of gold nanoparticles entrapped in vesicles within HeLa cells.
A) Graph showing the number of gold nanoparticles per vesicle diameter vs. nanoparticle size. TEM images of nanoparticles with a diameter of B) 14 nm, C) 30 nm, D) 50 nm, E) 74 nm, and F) 100 nm within vesicles. Reproduced from Chithrani et al.[10]
There are two opposite approaches to the synthesis of nanostructures;
bottom-up or top-down. In the first strategy the synthesis starts from the
interaction of small building blocks (metallic atoms) to form a more complex structure, while in the latter the base material is gradually “eroded” until the desired size and shape are achieved. The production of nanostructures for biological applications is in general bottom-up, because the synthesis techniques (wet chemistry, vapor deposition, pyrolysis) allow to tightly control surface composition. In particular, wet-chemistry methods were the processes used to synthesize gold nanoparticles in this PhD project since they allow to produce AuNps with well-known coating and functionalization. In general, reaction processes are based on the reduction of salts of the metal of interest in the presence of reducing and surfactant agents in aqueous or organic media. By changing the reactants and their relative molar concentrations (and also reaction temperature and stirring velocity), it is possible to control the nucleation and growth processes, achieving colloids with the desired properties. In the following the synthetic strategies are labeled by the name of the first author that reported the method.
1.2.1. Gold nanospheres: organic media
The strategy proposed by Brust[50] (Brust method) consists in growing the metallic clusters with the simultaneous attachment of self-assembled thiol monolayers on the growing nuclei. In order to allow the surface reaction to take place during metal nucleation and growth, the particles are grown in a two-phase system. In this method, AuCl4- is transferred from aqueous solution to toluene using tetraoctylammonium bromide as the phase-transfer reagent and reduced with aqueous sodium borohydride in the presence of dodecanethiol (C12H25SH). Upon addition of the reducing agent, the organic phase changes color from orange to deep brown within a few seconds. The overall reaction is summarized as follows:
AuCl4-(aq) + N(C8H17)4 + (C6H5Me) -> N(C8H17)4 + AuCl4-(C6H5Me)
m AuCl4-(C6H5Me) + n C12H25SH(C6H5Me) + 3m e- -> 4mCl-(aq) + (Aum)(C12H25SH)n(C6H5Me)
where the source of electrons is BH4-. The condition of the reaction is determined by the ratio of thiol to gold, i.e. the ratio n/m. This single reaction yields a surface-functionalized gold colloid in the 2-8 nm diameter range with a dispersion of about 4-6% (Fig. 1.13B). The kinetics of cluster growth is
1.2 Synthetic processes: State of the Art
22determined by thiol surface coverage. As for every 2-phase reaction, cluster size can be controlled by reaction conditions and not by metal-ion reduction kinetics. On the other hand, there is no possibility to grow uniform and regular-shaped colloids with diameter > 25 nm. The production of gold nanoparticles in organic media requires further steps to obtain water-soluble AuNs that are ready for use in live-cell experiments[51] (see Chapter 2).
1.2.2. Gold nanospheres: aqueous media
The method proposed by Turkevich[52] (Turkevich method) is based on the reduction of tetrachloroauric acid (HAuCl4) with sodium citrate in water at
90-100 °C. This is the most commonly used process to synthesize AuNs due to its fairly simple and environmentally benign procedure and its ability to tune the size of nanospheres from 10 to 150 nm by varying the molar ratio of citrate to HAuCl4. The experimental protocol is based on a rapid addition of sodium
citrate solution to hot (90-100 °C) aqueous solution of tetrachloroauric acid.
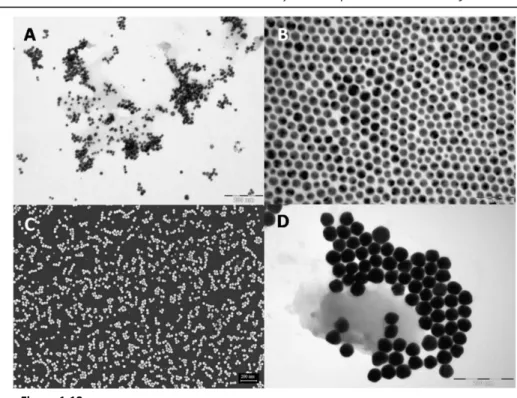
Figure 1.13: A) TEM image of gold nanospheres synthesized by Turkevich method. Average
spheres diameter of 20 nm, scale bar 200 nm. B) TEM image of gold nanospheres synthesized by Brust method. Average spheres diameter of 10 nm, scale bar 50 nm. C) SEM image of gold nanospheres synthesized by Xia method. Average spheres diameter of 15 nm, scale bar 200 nm. D) TEM images of gold nanospheres synthesized by Zhong method. Average spheres diameter of 30 nm, scale bar 200 nm.
When sodium citrate is mixed with HAuCl4 in water at high temperature, the
former is oxidized to sodium acetone dicarboxylate (SADC) while the latter is reduced to AuCl (Fig. 1.14). In parallel, depending on the pH of the reaction solution, AuCl4- ions (pKa 3.3) are hydrolyzed before gold reduction into
different types of auric precursor ions: AuCl3(OH)- (pKa 6.2), AuCl2(OH)2- (pKa
7.1), AuCl(OH)3- (pKa 8.1), and Au(OH)4- (pKa 12.9) ions. Their reactivity decreases in the following sequence[52, 53]: AuCl4- >AuCl3(OH)- >AuCl2(OH)
2->AuCl(OH)3- >Au(OH)4-. Ji et al. highlighted a non-negligible but less-recognized role of citrate that can strongly buffer the pH of the reaction solution[54]. In agreement with these results citrate species besides reducing HAuCl4 to AuCl,
Figure 1.14: Hypothesis of the reactions involved in the formation of colloidal gold in the
1.2 Synthetic processes: State of the Art
24also buffer the pH of the reaction solution from pH ≈ 2 to higher values (even neutral), depending on the amount of citrate added. Taking into account that both nucleation and crystal growth of AuNs are very fast at high temperature (less than 10 min at 100 °C), this buffer effect of citrate plus a possible inhomogeneous mixing of the citrate solution with HAuCl4 solution can cause
inhomogeneous nucleation, leading to a temporal overlap between nucleation and crystal growth and thus to a broad size distribution of the final colloid. The growth of AuNs is catalyzed by the presence of gold clusters, which cause on their surfaces dismutation of AuCl to Au0 and Au3+. It is useful to remember that a dismutation is a redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products. As initially proposed by Turkevich et al. and later supported by others[55], 2,3-SADC and AuCl are coordinated into macromolecular complexes (Fig. 1.14), which consecutively coagulate into colloidal stable precursor particles, in which a number of Au nuclei are formed by dismutation of AuCl. In particular the coagulation of macromolecular SADC/AuCl complexes is induced by concentration fluctuation (in accord to LaMer[56] theory). Matijevic et al. demonstrated that rapid coagulation favors the formation of monodisperse spherical particles[57].
Consequently, the rapid formation of a large amount of SADS should favor the formation of nanoparticles with a narrower size distribution. In parallel to the aforementioned reactions, SADC readily decomposes to acetone at high temperature[58] (>~90 °C), especially at neutral to basic pH. Acetone can reduce auric precursor ions to AuCl, thus leading to a secondary nucleation and in turn broadening the size distribution of the final colloid. This secondary nucleation can be minimized by fast oxidation of citrate to SADC at a lower pH using Ag+ ions[59] under light. Xia et al.[8] improved the Turkevich method adding a catalytic quantity of Ag+ ions during the reaction, obtaining quasi-spherical nanoparticles. Theoretical calculations on the deposition potential for Ag on the Au (111), (100), and (110) facet demonstrated values of 0.12, 0.17, and 0.28 eV[60]. This suggests that Ag atoms, when obtained by citrate reduction of Ag+ ions, deposit preferentially on the (110) and (100) facets on AuNs. The Ag layer is also oxidized and replaced by the Au ions. In this way the deposition of Ag may significantly slow down the growth rate of AuNs on the (110) and (100) facets, thus rendering the shape of AuNs more spherical. Citrate is a weak stabilizing ligand enabling the deposition and decomposition of Ag+ on AuNs, guaranteeing reshaping of the polycrystalline nanostructures to quasi-spherical[55].
In summary, the nanospheres obtained by the Turkevich method have usually a broad distribution of size and shape (Fig. 1.13A). Nanoparticles are
generally produced in the diameter range of 12-60 nm with a relative size distribution of 13-16% and usually with a non-uniform and irregular shape (such as quasi-spheres, ellipsoids, and triangles). This is attributed to the fact that the chemical mechanism of the nucleation and of AuNs crystal growth is governed by many factors such as pH, concentration of reactants, reducing agents and surfactants, stirring speed and temperature. Despite the several improvements to the Turkevich method[8, 61-63], the dispersion of the chemically produced AuNs is still too large for the purpose of this PhD project; thus the decision to synthesize aqueous solutions of gold colloid by the Zhong method described in the following[6].
1.2.3. Production of other types of nanoparticles
As side projects of this PhD, nanostructures with different composition and shape were synthesized in order to develop new hybrid nano-probes and to explore different nano-materials. In particular, in order to achieve high sensitivity in nano-optics, to cover the entire spectral range for
Figure 1.15: A) SEM images of platinum nanospheres. Average spheres diameter of 25
nm, scale bar 20 nm. B) STEM image of a silver nanocubes. Average edge length of 40 nm, scale bar 200 nm. C) STEM image of a gold nanocubes. Average edge lengths of 45 nm, scale bar 200 nm. D) TEM image of silver nanospheres. Average spheres diameter of 18 nm, scale bar 100 nm. E) STEM image of rhenium nanospheres. Average spheres diameter of 60 nm, scale bar 200 nm.
1.3 Synthetic Processes
26(bio)applications or to exploit optical processes such as surface-enhanced Raman spectroscopy (SERS) and fluorescence emission, the geometry (or materials) of the nanostructures were modified. As an example, silver and platinum nanospheres (Fig. 1.15A and D) of different sizes were synthesized to extend the spectral range of the nanocrystals. In this way it was possible to cover all the UV-Vis spectral range from 250 to 600 nm. Some properties of silver nanospheres are greatly attractive: I shall mention the strong enhancement of the Raman signal and of the fluorescence intensity of fluorophores linked to the metallic surface or the giant red-shift of the scattering signal of coupled silver nanospheres. Gold nanocubes (Fig. 1.15C) were also synthesized in particular for their peculiar photo-luminescence and to test their behavior in living cells. On the other hand, silver nanocubes (Fig.
1.15B) were synthesized to produce better probes for SERS and inter alia
because they serve as a sacrificial template to generate gold nanocages with tunable and controllable plasmon resonance peaks in the infra-red (IR) spectral region via a galvanic replacement reaction with tetrachloroauric acid. Gold nanocages were also shown to possess a porosity useful to deliver (bio)molecules into living cells[64, 65]. Magnetite nanospheres and nanopencils
(Fig. 1.16A-C) of cobalt-oxide and hybrid magnetite/cobalt-oxide were synthesized as tools for theranostics (therapeutic and diagnostic nano-tools) and functional NMR studies. Finally, rhenium nanospheres (Fig. 1.15E) were synthesized to achieve a radioactive probe[66] for living cells or organisms, and as a seed to produce novel types of high-temperature superconducting nanowires.
All these colloids were synthesized through wet chemistry methods. Every reaction was realized reducing salts of the metals (i.e. silver nitrate for silver nanospheres or iron oleate for magnetite nanospheres) with a particular reducing agent and in the presence of a well chosen surfactant. The Materials
& Methods section reports the detailed syntheses followed for the production
of all these nanomaterials.
1.3.
Synthetic Processes
In this section I shall introduce the synthesis I followed to produce AuNss for the aims of this thesis work. The second part of the section addresses on the novel synthesis I propose to yield unusual magnetite nanostars.
1.3.1. Zhong Method
We decided to follow the recent Zhong method, although during my PhD project we improve the Turkevich method (by splitting the reaction in multiple steps and obtaining AuNss with a sphericity close to 1 and a size-dispersion of about 6%,). The Zhong method[6] yields water-soluble gold nanoparticles of almost any desired size in the range of 10-100 nm diameter with a size dispersion down to 5% (Fig. 1.13D). This is a two-step reaction that involves the synthesis of gold seeds followed by a seeded growth process in aqueous solutions using sodium acrylate as reducing and capping agent. In the first step, similar to the Turkevich method, the HAuCl4 gold precursor was reduced by
sodium acrylate at 100 °C, yielding 15 nm diameter gold seeds. The major byproducts of the oxidation of sodium acrylate are likely[67] CH3COCO2H,
CHOCH2CO2H, or CH3COCO2. The second step involves a seeded and aggregative
Figure 1.16: TEM images of A) magnetite nanosphere (average diameter 12 nm, scale
bar 50 nm) B) hybrid magnetite/cobalt-oxide nanoparticles (average diameter 8 nm, scale bar 50 nm), and C) cobalt oxide nanopencils (average diameter 15 nm, scale bar 50 nm). D) TEM images of magnetite nanostars worm-like superstructures, scale bar 500 nm. Inset: a particular of the star (average edge 100 nm).
1.3 Synthetic Processes
28growth mechanism, as demonstrated by Njoki et al.[9]. The growth of larger and monodisperse particles arises from the reduction of Au(III) on the surface of Au seeds by a mix of acrylic acid and sodium acrylate agents. The aforementioned reaction occurs at room temperature in about 3 days. The fine-tuned size, the degree of monodispersion and shape uniformity are controlled by Ostwald ripening, a mechanism often considered the general driving force for the growth of particles (Fig. 1.17). In Ostwald ripening, smaller particles dissolve preferentially with subsequent crystallization onto larger particles[68], triggering the growth of larger particles from smaller ones.
On the basis of a spherical model for Au nanoparticle with an initial seed radius r (cm) and a growth thickness d (cm), in order to produce particles of total radius r+d (cm), the mass balance between the grown seed particles and the concentration C of AuCl4- (mM) is:
, (5) where N is the number of Au nanoparticles in the total volume, is density of Au (18.9 g/cm3), M is the molecular weight of Au (197 g/mol), C is the concentration of Au precursor (mol/cm3), and V is the total reaction volume[69]. This yields the following d/r expression:
. (6)
Thanks Equation 6 it is possible to finely choose the molar ratio of the reactants to achieve the desired monodispersed gold nanospheres (Fig. 1.13D). By this strategy, I synthesized (30±1.4)nm AuNss which were the best balance between physical and biological features for the purpose of this PhD project. With respect to the original process I maintained the pH of the second step stable to 7 for all 3 reaction days (adding sodium hydroxide or hydrochloric acid when necessary) and I strictly controlled the variation of temperature in a range of (22±0.5) ˚C. The result were structures with a better sphericity than the original
Figure 1.17: Illustration of the seeded and aggregative growth mechanism for the
controlled growth of gold nanoparticles. In the central picture the coalescence of the smaller particles on the larger one is shown. Reproduced from Zhong et al.[9]
synthetic method. The decision to follow the Zhong method rather than the others presented in this chapter was based on the following points: i) size-monodispersion (and thus uniformity of physical properties) of the colloid, ii) velocity of production of big quantity of colloids, iii) reproducibility of the reaction, and iv) low cytotoxicity of the nanoparticles.
1.3.2. Magnetite nanostars
Magnetic nanostars (Fig. 1.16D) were designed and developed because, thanks to their special shape, they can create 2D worm-like lattices when tethered on chips, whose magnetic properties are currently under study. The synthesis process is based on the thermolysis method proposed by Park et al.[70] to produce high yield magnetite nanospheres at high temperature (about 320 ˚C) from metal-oleate complex (in this case iron-oleate precursor). They observed the formation of hybrid squared nanoparticles by mixing the iron precursor with a cobalt precursor in 1:1 ratio (see Materials & Methods
section). Also, they observed that magnetite nanocubes were obtained if they
increase the reaction temperature to 400 ˚C. On this basis, in order to favorite the kinetics of formation of non-spherical nanostructures, I setup a seven days heating rate of the iron precursor (in presence of ppm of cobalt), from 230 ˚C to 380 ˚C. Probably, this long heating rate promote on one hand the effective separation between the nucleation and the growth processes and on the other hand the growing of the most energetically stable organization of the atoms in the structure. Indeed, while the nucleation process is linked to the dissociation of the iron-oleate species and occurs at temperature down to 260 ˚C, the growing process is time-dependent and starts from 300 ˚C. Studies on thermodynamics and kinetics in order to improve and step-up the production of magnetite nanostars are in progress.
1.4.
Materials & Methods
The metallic precursors were purchased by Alfa Aesar. All the other reactives were purchased by Sigma-Aldrich.
Nanoparticles characterization
In order to observe and analyze the synthesized nanoparticles, SEM/STEM or TEM measurements were performed. For SEM (Scanning Electron Microscopy) analysis, a drop of the colloid was left to dry on an n-doped silicon chip and imaged. For STEM (Scanning-Transmission Electron Microscopy) or TEM (Transmission Electron Microscopy) analysis, the sample was dripped on
1.4 Materials & Methods
30Formvar/Carbon 200 mesh grids and imaged after 20’. ImageJ software was used to analyze the images and compute, usually on 300-500 nanoparticles, the average size and the sphericity (where required) of the nanostructures.
Dynamic Light Scattering (DLS) measurements were performed on 1 mL of the colloidal solutions (at a specific pH defined by the buffer if in water; borate 40 mM pH 9, PBS buffer pH 7.5 or MES 10 mM pH 5.5). The measurements were performed in standard capillary cells DTS 1060 (for size-measurements) or ZEN0040 disposable cuvette (for -analysis) by Zetasizer nano ZS DLS (Malvern Instrument), following the manufacturer instructions. All values reported are the average of five consecutive measurements (see Appendix A).
Electrophoresis analyses were performed on 0.6-1% agarose gels in TBE buffer 0.5x under 90 V bias (see Appendix A).
The UV-visible spectra were measured on a Jasco 550 spectrophotometer (Jasco, Tokyo, Japan) supplied with a Jasco ETC-505T thermostat using 0.5 mL quartz cuvettes; the colloidal concentrations were calculated based on the known molar extinction coefficient[71, 72].
Turkevich method
In the standard procedure to obtain a water colloid of gold nanoparticles[52] with an average diameter of 20 nm, a 50 mL solution of 2.2 mM of trisodium citrate was heated to the boiling point and 1 mL of 25 mM solution of tetrachloro auric acid was added rapidly. In about 25’’ the boiling solution turns from light yellow to faintly blue. After additional approximately 70’’ the blue color suddenly changes into a brilliant red, indicating the formation of spherical gold nanoparticles. The solution was further refluxed for 15’ under stirring to promote sample monodispersion (Fig. 1.13A).
Brust method
An aqueous solution of hydrogen tetrachloroaurate (30 mL, 30 mM) was mixed with a solution of tetraoctylammonium bromide in toluene[50] (80 mL, 50 mM). The two-phase mixture was vigorously stirred until all the tetrachloroaurate was transferred into the organic layer and dodecanethiol (170 mg) was then added to the organic phase. A freshly prepared aqueous solution of sodium borohydride (25 mL, 0.4 M) was slowly added with vigorous stirring. After further stirring for 3 h the organic phase was separated, evaporated to 10 mL in a rotary evaporator and mixed with 400 mL ethanol to remove excess thiol. The mixture was kept for 4 h at - 18 ˚C and the dark brown precipitate was filtered off and washed with ethanol. The crude product was
dissolved in 10 mL toluene and again precipitated with 400 mL ethanol, obtaining a black solid of gold nanospheres with an average diameter of 10 nm (Fig. 1.13B).
Xia method
1 mL of HAuCl4 aqueous solution (0.5 wt %) and 42.5 μL of AgNO3 aqueous
solution (0.1 wt %) were added to a given volume of citrate aqueous solution (1 wt %) under stirring[8]. Water was added to bring the volume of the mixture solution to 2.5 mL. This mixture was incubated for 5’ before addition to the reaction solution at 100 °C. The color of the mixture solutions did not change appreciably during incubation. Note that adding citrate solution to the HAuCl4/AgNO3 mixture solution can cause its color to change from light yellow
to orange, leading to black Au precipitates after the addition of the resulting HAuCl4/AgNO3/citrate mixture solution to boiling water. 47.5 mL of water in a
100 mL flask was placed in the two-necked flask equipped with a condenser and heated to the boiling point. After the water boiled for 10 min, the HAuCl4/citrate/AgNO3 mixture solution was quickly injected into the boiling
water under vigorous stirring. The color of the reaction solution changed quickly from colorless, to grayish blue, to purple, and finally to ruby red within less than 1 min at citrate concentration ranging from 2.97·10-2 to 6.10·10-3 wt %. The reaction solution was further refluxed for 1 h under stirring to warrant formation of uniform quasi-spherical 15 nm diameter AuNs (Fig. 1.13C).
Zhong method
Gold seeds were prepared refluxing for 30 min 100 mL of aqueous solution of HAuCl4 (1 mM) with sodium acrylate[6] (24 mM). The reaction produced a
deep-red colloid characteristic of 14 nm spheres. For the second step all the seed solution was mixed to 15 mL of 25 mM HAuCl4 solution and diluted in 845
mL of MilliQ water. After the mixture was brought to pH 7, 40 mL of 0.5 M acrylic acid solution was added, and in 3 days at 22 °C under continuous stirring the reaction produced a wine-red solution of 30±1.4 nm gold nanospheres (Fig.
1.13D) at a 0.5 nM (2.92·1011 Np/mL) concentration, as measured by ultraviolet-visible (UV-Vis) spectra.
Platinum nanospheres
Platinum seeds[73] of 5 nm in diameter were prepared adding 36 mL of a 0.2% solution of chloroplatinic acid hexahydrate to 464 mL of boiling deionized water. After 1 min, 11 mL of a solution containing 1% sodium citrate and 0.05%
1.4 Materials & Methods
32citric acid was added, followed half a minute later by a quick injection of 5.5 mL of a freshly prepared sodium borohydrate (0.08 wt %) solution containing 1% sodium citrate and 0.05% citric acid. After 10 min, the product was cooled down to room temperature.
To obtain platinum nanoparticles from 10 to 30 nm in diameter (Fig.
1.15A), 1 mL of the platinum seed solution was added at room temperature to
29 mL of deionized water. To tune the final average diameter of the nanospheres, different amount (from 0.020 to 0.200 mL) of a 0.4 M chloroplatinic acid solution was added, followed by the addition of 0.5 mL of a solution containing 1% sodium citrate and 1.25% L-ascorbic acid. Under stirring, the temperature was slowly increased to the boiling point (∼10 °C/min). The reaction time was 30’ in total.
Pt spheres with diameters larger than 30 nm were obtained using the particles described above as seeds. By adding 4 mL of the 30 nm Pt particle solution to 26 mL of deionized water together with 0.045 mL of the chloroplatinic acid solution, followed by the addition of 0.5 mL of the solution containing 1% sodium citrate and 1.25% L-ascorbic acid and slowly increasing the temperature to the boiling point, we obtained spheres with diameter of 48 nm. The same procedure using 1 and 0.25 mL of seed solution in 29 mL of water resulted in spheres of 73 and 107 nm diameter, respectively.
Silver nanocubes
In a typical procedure[24] to obtain silver nanocubes with an average edge length of 40 nm, 6 mL of ethylene glycol was heated for 60’ at 150 °C before the addition of 90 L of a ethylene glycol-solution of sodium sulfide (Na2S) 3
mM. After 10’, 0.03 g of PolyVinylPyrrolidone (PVP) 30000 and 0.5 mL of silver nitrate (AgNO3, 0.3 M) ethylene glycol-solution were added to the reaction
solution and left at 150 °C for 15’. The solution color changed from light yellow to ocher and the reaction was quenched cooling down the temperature to 25 °C. The nanocrystals were washed in centrifuge (and re-dispersed by sonication) one time with acetone, and 3 times with deionized water and stored at room temperature for months (Fig. 1.15B).
Gold nanocubes
Gold nanocubes were grown following a two-step method[34]. In the first step, gold seeds were prepared by the addition of a freshly prepared, ice-cold aqueous NaBH4 solution (0.01 M, 0.6 mL) into an aqueous mixture solution