Chapter 3
Azasugars and Imino sugars
3.1. Azasugars
Azasugars, also known as iminosugars, are structural analogues of “true” sugars, in which the ring oxygen atom is replaced by a nitrogen atom and represent an interesting class of carbohydrate mimics for their ability to inhibit glycosidases, glycosyltransferases, and other enzymes such as glycogen phosphorylases, nucleoside-processing enzymes, metalloproteinases.1
For these important biological properties, recent years have seen an increasing interest in synthetic and naturally occurring azasugars as biological tools and potential therapeutics, to the point that two iminosugars have already been approved as drugs: in 1996, Miglitol, N-hydroxyethyl-1-deoxynojirimycin, (Glyset®) and, in 2003, Miglustat, (Zavesca®). Glyset is used to treat complications associated with type II diabetes, while Zavesca is the first oral treatment for Gaucher’s disease, a severe lysosomial storage disorder.1
The great potency and specifity of these low-molecular-weight compounds can be explained by their structural resemblance to the terminal sugar moiety in the natural substrates. Particurally regarding glycosidases, the inhibitory activity of azasugars is due to their binding with these enzymes by mimicking the shape and the charge, or both, of the glycosidase transition state, that is the postulated oxocarbenium ion intermediate for the glycosidic bond cleavage reaction (structure A, Scheme 3.1).2
In azasugars such as nojirimycin (NJ), bearing an hydroxyl group on C(1) carbon, the half-chair conformation obtained for partial cleavage of the glycosidic bond (structure B, Scheme 3.1) mimics the structure of the oxocarbenium ion intermediate (structure A, Scheme 3.1) involved in the transition state of the enzymatic mechanism. In this case, the presence of the endocyclic nitrogen atom stabilizes the positive charge generated in the oxygen or anomeric carbon of the natural
N OH HO OH HO N OH HO OH HO Miglitol Miglustat OH
glycoside. Other azasugars, such as as 1-deoxynojirimycin (DNJ), can become positively charged on protonation due the presence of basic amino moiety (structure C, Scheme 3.1) mimicking the charge of the oxocarbenium ion intermediate, and could interact with anionic groups in the enzyme active site.
Since glycosyltransferase reactions are thought to proceed through transition states similar to those of glycosidases, iminosugars are also investigated as potential glycosyltransferase inhibitors.3
Actually the main characteristics, consisting of stabilization of the positive charge on the nitrogen atom, trigonal anomeric center, half-chair conformation, and specific configuration of the hydroxyl ions, are crucial for the activity in these alkaloids.
-
Scheme 3.1. Mechanism of action of azasugars on glycosidases
3.1.1. Classification of natural azasugars
Azasugars belong to the polyhydroxylated alkaloid super-family of natural products. The azapyranose motif can be, in fact, recognised in three of the main structural groups of alkaloids: piperidines, indolizidines and nortropanes.1b
- Examples of piperidine azasugars are nojirimycin (3.1) (NJ), the first natural polyhydroxylated alkaloid discovered, isolated from a Streptomyces filtrate, that actively inhibit α- and β-glucosidase, nojirimycin B (also called mannonojirimicyn) (3.2) and galactostatin (3.3). These three compounds, bearing a hydroxyl group at C(1), are relatively difficult to isolate and handle due to the unstable aminal functionality.
NH OH HO HO OH N OH HO HO OH H H H+ NH OH HO HO OH NH OH HO HO OH H+ OH O H H N OH HO HO OH H2O -H+ O OR +H+ - ROH O O A nojirimycin (NJ) B C 1-deoxynojirimycin (DNJ)
The first deoxy-derivative, 1-deoxynojirimycin (DNJ) (3.4), isolated from Mulberry trees as well as Streptomyces cultures, was the first deoxy-derivative synthesized by Inouye et al. by the reduction of the anomeric hydroxyl group. Epimer of DNJ is 1-deoxymannonojirimycin (DMJ) 3.5, first found in Lonchocarpus sericeus.
To piperidine azasugars belong also N-substitued piperidines, as N-metyl-DNJ (3.6), isolated from the leaves of Omphalea diandra (Euphorbiaceae), and C-glycosides, as α-homonojiricin (3.7), a highly potent inhibitor of α-glucosidase, intestinal sucrase and α-galactosidase. A further DNJ analogue is the 1,2-dideoxynojirimycin, fagomine 3.8, isolated from all parts of Mulberry trees.
- Indolizidines alkaloids are bicyclic compounds where the azapyranose ring is fused to a pyrrolidine ring via a N-bridge. The first example found in nature was swainsonine (3.9), isolated in 1979 from the leaves of Swainsona canescens (Legumonosae). Another important indolizidine alkaloide is castanospermine (3.10), a bicyclic equivalent of DNJ having an ethylene bridge between the hydroxymethyl group and nitrogen atom.
H N OH OH HO OH HO H N OH OH HO OH HO H N OH OH HO OH HO Nojirimicin (NJ) Nojirimicin B (NJ) (Mannojirimicin) Galactostatin (Galactonojirimicin) 3.1 3.2 3.3 H N OH HO OH HO H N OH HO OH HO 1-Deoxynojirimycin (DNJ) 1-Deoxymannojirimicyn (DMJ) 3.4 3.5 N OH HO OH HO H N OH HO OH HO N-Methyl-1-deoxynojirimycin !-Homonojirimycin (!-HNJ) CH3 H N HO OH HO OH Fagomine 3.6 3.7 3.8
- Finally nortropanes, called calystegines, are byciclic compounds characterized by a tertiary hydroxyl group at the bicyclic bridgehead, with the exception of calystegine N1 (3.11). They were
isolated primarily from the Atropa belladonna (Solanaceae) and Convolvulus arvenis (Convolvulaceae) and from Moranaceae families. Based on electrophoresis separation they were divided into two groups, calystegines A, as calystegine A3 (3.12) and calystegines B, as calystegine
B1 (3.13).
3.1.2. Therapeutic applications
The diversity of enzymes inhibited by iminosugars, as previously mentioned, promises a generation of medicines in a wide range of diseases, such as diabetes, viral infections, lysosomial storage disorders and tumour metastasis.1
- Anti-diabetic activity
In the epithelial cell of the brush border region of small intestine are present oligosaccharides and disaccharides that breakdown dietary carbohydrates to monosaccharides, which are absorbed through the intestinal wall. Therefore these enzymes are directly responsible for the level of glucose found in the blood and inhibition of some or all of these enzyme activities can lead to the regolation of the carbohydrate adsorption.
Some azasugars were tested to see if they could have this activity, useful, for example, for the treatment of diabetes mellitus, a disease that occurs when the body cannote remove circulating blood glucose properly. substitued azasugars were found to be active for this patology, as N-hydroxyethyl-DNJ (Glyset®) (3.14) and the analogue Miglitate (3.15).
NH H2N HO HO OH NH HO HO HO NH HO HO HO OH Calistegine N1 3.11 Calistegine A3 3.12 Calistegine B1 3.13 N OH OH OH H Swainsonine 3.9 N OH OH OH H Castanospermine 3.10
In 1996, Glyset tablets were grandet market clearance by the US Food Drug Administration (FDA) and introduced into the market in 1999 as a more effective second-generation α-glucosidase inhibitor with fewer gastrointestinal side effects.
- Anti-cancer activity
It is believed that glycosylation plays a key role in the formation and migration of tumor cells (metastasis). It has been shown that high levels of many glycosidase enzymes are present in some tumor cells and interstitial fluids. Furthermore, many tumor cells show abnormal glycosylation due to an altered expression of glycosyltransferases. This can manifest either as a shortening of the carbohydrate chain of glycoproteins or as alteration to their structure.
Since glycosidases are involved in the formation of cancer cells and migration of tumour cells, a line of treatment could involve the specific inhibition of catabolic glycosidases associated with cancer. Castanospermine (3.10) and N-methyl-1-DNJ (3.6) have been shown to have antimetastatic activity, as well as swainsonine (3.9) and its analogues.
- Anti-viral activity
The viral envelop glycoproteins are often essential for viron assembly and secretion and/or infectivity. Compounds that interfere with the glycosylation processes of viral glycoproteins can be expected to be antiviral agents. In fact, α−glucosidase inhibitors such as DNJ (3.4), N-butyl-DNJ (3.16), castanospermine (3.10) inhibit human immunodeficiency virus (HIV) replication in vitro.
N OH HOH2C OH HO OH N O HOH2C OH HO OH 3.14 3.15 CO2CH2CH3 Miglitol (Glyset) Miglitate N HOH2C OH HO OH 3.16 N-butyl-DNJ
- Sphingolipid storage diseases
Sphingolipid storage diseases are hereditary disorders in which the control of sphingolipid biosynthesis or degradation is lost. Most research in the treatment of sphingolipid storage diseases has focused on direct enzyme replacement, bone marrow transplantation or gene therapy. Therefore a more conventional method of treatment was needed. Only recently, it was found that drugs could be used to regulate the biosynthesis of glycosphingolipid, so that the amount of substrate matches the activity of the residual enzyme. In particular N-alkylated azasugars of glucose or galactose stereochemistry are found to inhibit glucosphingolipids biosynthesis.
Gaucher disease is the most common glycosphingolipid lysosomial storage disorder, due to the deficiency of the lysosomal enzyme glucocerebrosidase that leads to build up of glucocerebroside, expecially in the mononuclear phagocyte cell system. N-butyl DNJ (3.16) is a potent inhibitor of the ceramide-specific glucosyltrasferase, which is involved in the glycosphingolipid biosynthetic pathway and catalyses the formation of glucocerebroside. After successful clinical trials, N-butyl-DNJ 3.16 was released for the treatment of Gauchers disease by Oxford Glycoscience.
3.2. State of the art about imino glycals
Imino glycals, azapyranosides incorporating a double bond between C(1) and C(2), represent versatile intermediates for the synthesis of a wide variety of iminosugars and oligosaccharides of iminosugars due to the possibility of manipulating by appropriate functionalization of the present unsaturation (cycloaddition reactions, palladium catalyzed cross-coupling reactions at C(2) and cross coupling reactions in addition to common electrophilic addition reactions). In spite of their potentiality, imino glycals have received rather scant attention and, in literature, there are only systematic studies on this type of compounds, undertaken, mainly, by Dransfieldand Comins,4,5
regarding their reactivity under Ferrier reaction conditions.
In the original application of glycal system, the Ferrier nucleophilic substitution reaction, carried out under the promoting activity of a Lewis acid, determines the introduction of a nucleophile on C(1) carbon with the contemporary displacement of the substituent on C(3), to give corresponding 2,3-unsaturated glycosides as here shown in a tipycal example for tri-O-acetyl-D-glucal (Scheme 3.2)
Scheme 3.2. Ferrier reaction on glycals
O O Lewis Acid (LA) Nu OAc AcO AcO AcO AcO Nu
The first studies directly to the rationalization of the reaction mechanism in imino glycals, under Ferrier reaction conditions, were started at the end of the ‘80s. In an extended concept of imino glycal, Comins and coll., had initially studied the α-alkylation of pyridines, as 3.17 (Scheme 3.3), showing that the nucleophilic attack by an alkyl zinc reagent occurred selectively at the C(2) carbon of the reactive intermediate, the N-acyliminium ion precursor 3.18.4a
Scheme 3.3. α-alkylation of pyridines
Analogous behavior was observed with 1-phenyloxycarbonyl-4-methoxy-tetrahydropyridine 3.20 (Scheme 3.4) in which nucleophilic attack by soft nucleophiles, i.e. alkyl zinc reagents, mainly occurred at C(2). In this case, the reaction proceeded through the formation of the conjugated iminium ion 3.21, formed, in situ, by treatment of 3.20 with a Lewis acid.4a
Scheme 3.4. Regioselective alkylation on C(2) of 1-acyl-4-methoxy-1,2,3,4-tetrahydropyridine 3.20
When the imino glycal substrate presented an alkyl group on C(6) carbon with a defined stereochemistry, as the methyl derivative 3.23, the same reaction led to a mixture of cis and trans-2,6-dialkyl-∆3-piperidines 3.25a and 3.25b in a 4:1 ratio (Scheme 3.5).4b
Scheme 3.5. Synthesis of diasteroisomeric cis and trans-2,6-dialkyl-∆3-piperidines
N PhOCOCl N R COOPh R R'ZnI N R COOPh R' 3.17 3.18 3.19 2 R'= Cl(CH2)4 -N N OMe COOPh N COOPh R' COOPh 3.20 3.21 3.22 BF3.OEt 2 R'ZnI
R' = Me, n-Bu, Cl(CH2)4, EtO2C(CH2)2ZnI
N N OMe COOR N COOR COOR 3.23 3.24 3.25a BF3.OEt Me Me n-PrZnI N COOR 3.25b 2 6 4 : 1
The reason for the observed stereoselectivity towards the cis addition product 3.25a has been rationalized on the basis of stereoelectronic effects. Actually, due to the steric hindrance between the methyl group on C(6) carbon and the N-acyloxy group (A(1,3) allylic strain),4 the reactive
intermediate iminium ion 3.24 reacts through the preferential conformer 3.24’’ in which the methyl group is axial, a position which minimizes the interaction with the N-acyloxy group. Subsequent, stereoelectronically preferred axial attack on 3.24’’ by the alkylzinc iodide gives the cis-product 3.25a, as the main reaction product (Scheme 3.6).
Scheme 3.6. Rationalization of the prevalent formation of the cis diasteroisomer 3.25a
On 1998, Graig and coll.carried out a study on the chemical behavior of 1,4-bis-(arylsulfonyl)-1,4,5,6-tetrahydropyridines 3.26 (Scheme 3.7) bearing different substituents on C(6) carbon in addition reactions of different C-nucleophiles in the presence of a Lewis acid.6 In all cases
examined, the corresponding 2,6-cis diastereoisomer was obtained as the only reaction product (Table 3.1).
Scheme 3.7. Addition of C-nucleophiles on 1,4-bis-(arylsulfonyl)-1,4,5,6- tetrahydropyridines 3.26
Also in this case, the reactions proceeded through the formation of the corresponding conjugated iminium ion 3.27, which preferentially exists as the corresponding conformer in which the R1group
initially present on C(6) is axial. The preferred pseudoaxial attack by the nucleophile on the less hindered α-face, determines, as usual, the observed complete diasteroselectivity (Scheme 3.8).
N 3.24' 3.24'' PhO O Me N PhO O Me n-PrZnI N COOR Me 3.25a axial attack N N Ts Ts N COOR R2 Ts 3.26 3.27 3.28 (see Table 3.1) LA/Nu R1 6 2 R1 R1
TABLE 3.1. Reaction of 3.26 in the presence of C-nucleophile and a Lewis acid
Actually, an X-ray structure determination of the starting tetrahydropyridines 3.26 (Scheme 3.8) have indicated that these compounds exist in the corresponding conformer with the alkyl group axial confirming the clear tendency of these systems to minimize the repulsive interaction between the alkyl group on C(6) and the N-acyloxy group. This tendency for a preferential axial position of the alkyl side chain, can be reasonably extended to the intermediate N-acyloxy iminium ion 3.27, as done in the previous discussion, in order to rationalize the results.
Scheme 3.8. Conformation of 3.27 and x-ray of 3.26
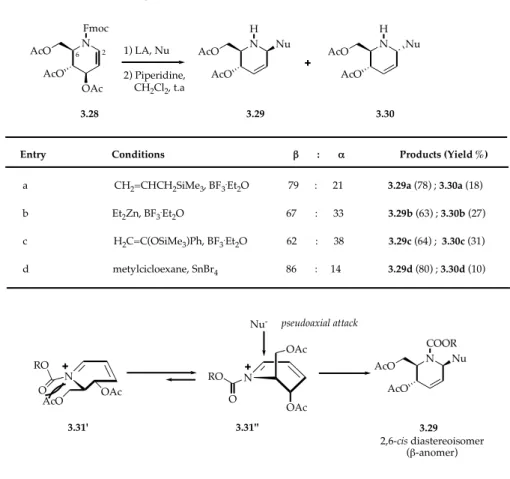
More recently, Dransfield and coll.5 applied the Ferrier reaction conditions with C-nucleophiles to imino glycal 3.28 (Table x.2), an analog of 3,4,6-tri-O-acetyl D-glucal, with the formation of a new C-C bond and contemporary allylic displacement of the C(3) acetate group. As shown in the Table 3.2, even with different types of C-nucleophiles and Lewis acids, the 1,5-cis piperidine 3.29, the β-anomer, was always obtained as the major product. Again the reaction proceed through a
Entry R1 Lewis Acid/Nucleophile R2 Yield
a CH2Ph Me3Al (1.1 equiv) Me 85%
b i-Pr Me3Al (2 equiv) Me 80%
c i-Bu Me3Al (2 equiv) Me 99%
d CH2Ph Et2AlCl (1.1 equiv) Et 86%
e i-Pr Et2AlCl (1.1 equiv) Et 99%
f i-Bu Et2AlCl (1.1 equiv) Et 99%
g CH2Ph SnCl4/ TMSCH2CH=CH2 CH2CH=CH2 99% h i-Pr SnCl4/ TMSCH2CH=CH2 CH2CH=CH2 99% i i-Bu SnCl4/ TMSCH2CH=CH2 CH2CH=CH2 99% N R1 S O O Nu !-attack "-attack Nu 3.27 3.28 2
conjugated iminium ion 3.31’’ upon addition of Lewis acids and the nucleophilic attack occurs regioselectively at C(1) carbon of 3.31’’ (Scheme 3.9). The regiochemical outcome was rationalized on the basis of the kinetic preference for attack at the site of lowest electronic density in the conjugated N-acyl iminium ion. The diastereoselectivity of the reaction was explained considering that steric repulsion between the C(6) acetoxymethyl group and the N-Fmoc group disfavours 3.31’ relative to 3.31’’ (Scheme 3.9), and the stereoelectronically controlled axial attack of the nucleophile to the top face of the favored conformer 3.31’’ led to the observed major product.
TABLE 3.2. C-nucleophilic addition reactions catalyzed by Lewis acid to imino glycal 3.28
Scheme 3.9. Conformation of 3.31 and explanation of the stereochemical outcome of the Ferrier reaction on
imino glycals N N H Fmoc 3.28 3.29 1) LA, Nu 2) Piperidine, CH2Cl2, t.a N H Nu 3.30 2 6 AcO OAc AcO AcO AcO AcO AcO Nu
Entry Conditions ! : " Products (Yield %)
a CH2=CHCH2SiMe3, BF3.Et2O 79 : 21 3.29a (78) ; 3.30a (18)
b Et2Zn, BF3.Et2O 67 : 33 3.29b (63) ; 3.30b (27) c H2C=C(OSiMe3)Ph, BF3.Et2O 62 : 38 3.29c (64) ; 3.30c (31) d metylcicloexane, SnBr4 86 : 14 3.29d (80) ; 3.30d (10) N 3.31' 3.31'' RO O N RO O Nu -N COOR 3.29 pseudoaxial attack AcO OAc OAc OAc AcO Nu 2,6-cis diastereoisomer (!-anomer) AcO
When Ferrier reaction conditions were applied to imino glycal 3.32, the epimer of 3.28, a mixture of the two tetrahydropyridines 3.33 and 3.34 was obtained. Once again the main product was the 2,6-cis diasteroisomer.
Scheme 3.10. Ferrier reaction on imino glycal 3.32, epimer of 3.28
Subsequently, starting from 3.28, Dransfield applied this protocol to the synthesis of the (+)-Deoxoprosophylline (3.36), a natural product, and to the obtainement of C-glycosides of iminosugars (Scheme 3.11).5
Scheme 3.10. Application of Ferrier reaction on imino glycal 3.28
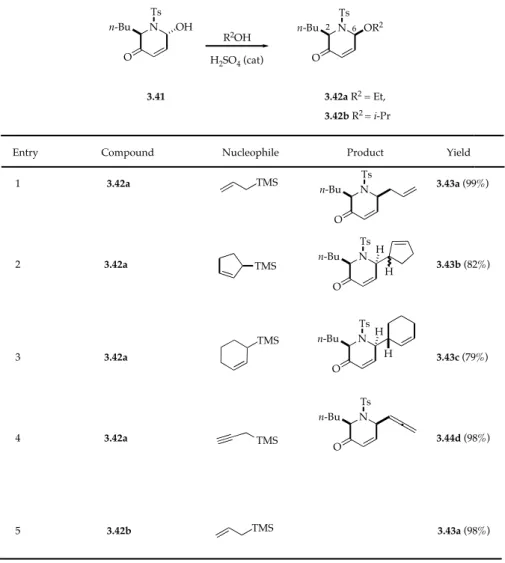
A similar process was realized using 2-alkyl-6-alkoxypyperidin-3-ones 3.42a and 3.42b, prepared from 3.41. Compouds 3.42a and 3.42b were reacted with allyl silanes (C-nucleophiles) in the presence of a Lewis acid yielding, quantitatively the corresponding cis-2,6-disubstituted diastereoisomers 3.43a-3.44d (Table 3.3).7
The structure and stereochemistry of 3.43c was determined by X-ray analysis and extended by analogy to all the other addition products which showed similar spectral data.
As in the previously described examples the preference for the cis-2,6-diastereoisomers was due to a stereoelectronically favored pseudoaxial attack on the corresponding conformer of the intermediate N-sulfonyloxy iminium ion, bearing the butyl group axial.
N N H Fmoc 3.32 3.33 (58%) N H Et 3.34 (42%) 2 6 AcO OAc AcO AcO AcO AcO AcO Et a) Et2Zn, BF3.Et 2O b) piperidine, CH2Cl2, rt N N H Fmoc 3.28 3.35 AcO OAc AcO AcO AcO a) BF3.Et 2O, CH2Cl2, -60°C 0°C CH2=CHCH(SiMe3)(CH2)8CH3 b) piperidine, CH2Cl2, rt N H 3.36 (+)-Deoxoprosophylline AcO AcO BF3.Et2O, Et2Zn, CH2Cl2, -20°C N Fmoc 3.37 AcO AcO Et N Fmoc 3.38 AcO AcO Et N H 3.39 AcO AcO Et N H 3.40 AcO AcO Et OAc OAc OAc OAc (CH2)11CH3
TABLE 3.3. Reactions of 3.42a and 3.42b with allyl silanes in the presence of Lewis acid
Finally, Kozikowski8, on 1990, examined the behavior of hydroxy compound 3.44, with different types of nucleophiles (C-, O- and S-nucleophiles), in the presence of a Lewis acid, by changing reaction temperature and solvent (Scheme 3.11).
N N Ts Ts 3.41 3.42a R2 = Et, R2OH H2SO4 (cat) O n-Bu OH n-Bu OR2 O
Entry Compound Nucleophile Product Yield 1 3.42a 3.43a (99%) 2 3.42a 3.43b (82%) 3 3.42a 3.43c (79%) 4 3.42a 3.44d (98%) 5 3.42b 3.43a (98%) TMS 3.42b R2 = i-Pr TMS TMS TMS TMS N Ts n-Bu O N Ts n-Bu O H H N Ts n-Bu O H H N Ts n-Bu O 2 6
Scheme 3.11. Reactions of compound 3.44 in the presence of C-, O-, S-nucleophiles
With heteroatom-based nucleophiles, as thiophenol and ethanol, the main reaction product was the corresponding α-substituted tetrahydropyridine 3.45 accompanied by only a small amount of the γ-substitued regioisomer 3.46. Actually, in these cases, after the α-attack occurred, a) an anomeric effect weakened by interaction of the nitrogen lone pair with the ethoxy carbonyl substituent, b) the possible nonbonded interactions of the “ortho-related” substituents in the primari reaction product, and c) resonance stabilization of the enamido system resulting from rearrangement, may conspire to promote migration of the heteroatom-based nucleophile to the y-position.
References
1. a) Compain, P.; Martin, O. R. “Imino sugars. From synthesis to therapeutic applications”, CNRS, University of Orleans, France, J. Wiley & Sons, 2007; b) Afarinkia, K.; Bahar, A. Tetrahedron: Asymmetry 2005, 16, 1239 and references therein.
2. Vasella, A.; Davies, G. J.; Bohm, M. Curr. Opin. in Chem. Biol. 2002, 6, 619. 3. Compain, P.; Martin, O. R. Curr. Top. in Med. Chem. 2003, 3, 541.
4. a) Comins, D.; O’Connor, S.; Tetrahedron Lett. 1987, 28, 1843. b) Comins, D. L.; Foley, M. A. Tetrahedron
Lett. 1988, 29, 6711.
5. Dransfield, P. J.; Gore, P. M.; Prokes, I.; Shipman, M.; Slawion, A. M. Z. Org. Biomol. Chem. 2003, 1, 2723. 6 Craig, D.; McCague, R.; Potter, G. A.; Williams, M. R: V. Synlett 1998, 55.
7. Hopman, J. C. P.; van den Berg, E.; Ollero, L.; Hiemstra, H.; Speckamp, W. N. Tetrahedron Lett. 1995, 36, 4315.
8. Kozikovski, A. P.; Park, P. J. Org. Chem. 1990, 55, 4668.
N N COOEt COOEt 3.44 3.45 1) LA, Nu N COOEt 3.46 OH Nu Nu Nu = EtOH, PhSH, (CH3)3CCH2C(CH3)2N C LA = PPTS, Me3SiOTf