NEW PERSPECTIVES FOR VITAMIN D
AND HEART: ROLE IN HEART
FAILURE PATHOGENESIS AND
PROGNOSIS
PhD Programme in: Translational MedicinePhD candidate
Dr. Federica SaponaroTutor
Prof. Claudio Passino Prof. Fabio Recchia
Supervisors
Prof. Riccardo Zucchi Prof. Claudio Marcocci
PhD Programme in: Translational Medicine
Academic years
2016-2017NEW PERSPECTIVES FOR VITAMIN D
AND HEART: ROLE IN HEART
FAILURE PATHOGENESIS AND
PROGNOSIS
PhD candidate
Dr. Federica Saponaro
Tutor
Prof. Claudio Passino Prof. Fabio Recchia
Supervisors
Prof. Riccardo Zucchi Prof. Claudio Marcocci
A
A
m
m
i
i
o
o
p
p
a
a
d
d
r
r
e
e
,
,
c
c
o
o
n
n
a
a
m
m
o
o
r
r
e
e
“
“
O
O
n
n
c
c
e
e
w
w
e
e
a
a
c
c
c
c
e
e
p
p
t
t
o
o
u
u
r
r
l
l
i
i
m
m
i
i
t
t
s
s
,
,
w
w
e
e
g
g
o
o
b
b
e
e
y
y
o
o
n
n
d
d
t
t
h
h
e
e
m
m
”
”
A
A
.
.
E
E
i
i
n
n
s
s
t
t
e
e
i
i
n
n
1
C
CO
ON
NT
TE
EN
NT
T
CONTENT ... 1 SUMMARY ... 3 ABBREVIATIONS ... 5 1. BACKGROUND ... 6 INTRODUCTION ... 6 1.1 VITAMIN D ... 71.1.1 “Classical effects” of Vitamin D ... 7
1.1.2 “Extraskeletal effects” of Vitamin D ... 10
1.2 VITAMIN D AND CARDIOVASCULAR DISEASES ... 14
1.2.1 Cardiovascular effects of vitamin D active hormone ... 14
1.2.2 Observational studies ... 17
1.2.3 Randomized clinical trials (RCT) and vitamin D supplementation in cardiovascular diseases ... 17
1.3 VITAMIN D AND HEART FAILURE ... 20
1.3.1 Modulatory effects of vitamin D on Heart Failure physiopathology ... 20
1.3.2 Observational studies ... 21
1.3.3 Controversies from RCTs ... 23
2. PROJECT AIMS ... 25
2.1 AIMS AND EXPECTATIONS ... 25
2.2 FACILITIES AND CONTRIBUTIONS ... 27
3. METHODS ... 30
3.1 PATIENTS SELECTION CRITERIA ... 30
3.2 CLINICAL, BIOCHEMICAL AND INSTRUMENTAL EVALUATION .. 31
3.2.1 Clinical and biochemical evaluation ... 31
3.2.2 Cardiopulmonary test (CPET) and instrumental evaluation ... 32
3.2.3 Metabolic exercise and cardiac and kidney indexes (MECKI) score ... 33
3.2.4 Vitamin D (25OHD) measurement by Mass Spectrometry coupled to High Performances Liquid Chromatography (HPLC-MS-MS) ... 34
3.3 STATISTICAL ANALYSES ... 37
4. RETROSPECTIVE STUDY ... 38
4.1 INTRODUCTION ... 38
4.2 MATERIALS AND METHODS ... 40
4.2.1. Patients and study design ... 40
4.2.2. Twenty-five hydroxyvitamin D (25OHD) levels ... 41
4.2.3. Statistical analysis ... 41
4.3 RESULTS ... 42
4.4 DISCUSSION ... 46
5. PROSPECTIVE STUDY ... 51
5.1 INTRODUCTION ... 51
5.2 MATERIALS AND METHODS ... 52
5.2.1. Patients and study design ... 52
2
5.2.3. Schedule of visit and primary and secondary endpoints ... 54
5.2.4. Statistical analyses ... 54 5.3 RESULTS ... 55 5.4 DISCUSSION ... 60 6. METHODOLOGICAL STUDY ... 64 6.1 INTRODUCTION ... 64 6.2 MATERIAL E METHODS ... 65
6.2.1 LIAISON 25 OHD Total Assay ... 66
6.2.2 High Performances Liquid Chromatography (HPLC-MS-MS) ... 66
6.2.3 Statistical analyses ... 68 6.3 RESULTS ... 68 6.4 DISCUSSION ... 72 7. IN VIVO EXPERIMENTS ... 76 7.1 INTRODUCTION ... 76 7.2 MATERIAL E METHODS ... 78
7.2.1 Animals and perfusion technique ... 78
7.2.2 Measurement of Vitamin D3 and 25OHD from tissues and perfused samples ... 80
7.3 RESULTS ... 80
7. 4 DISCUSSION ... 83
8. CONCLUSIONS AND PERSPECTIVES ... 85
9. BIBLIOGRAPHY ... 88
10. SUPPLEMENTARY MATERIALS ... 106
10.1 LIST OF PUBLICATIONS RELATED TO THIS WORK ... 106
10.2 LIST OF CONTRIBUTES TO CONGRESSES RELATED TO THIS WORK ... 106
10.3 LIST OF PUBLICATIONS OBTAINED DURING PHD COURSE ... 107
3
S
S
U
U
M
M
M
M
A
A
R
R
Y
Y
Background: Chronic Heart failure (HF) is a major cause of morbidity and mortality
with poor prognosis. Vitamin D shows many extraskeletal functions, including pleiotropic effects on cardiovascular system. Twenty-five hydroxy-vitamin D (25OHD) represents the biomarker of vitamin D levels and reflects vitamin D status. There are evidences that low serum 25OHD levels are associated with increased risk of cardiovascular disease (CVD) and vitamin D could exert some modulatory effects in the pathophysiology of HF.
Retrospective Study: Aim of the present study was to evaluate vitamin D status in HF patients by High Performances Liquid Chromatography coupled to mass spectrometry (HPLC-MS-MS) and to correlate serum 25OHD levels with functional (VO2peak%) and mortality (MECKI score) HF paramethers. 261 consecutive patients with HF underwent a comprehensive clinical, biochemical characterization and serum 25OHD measurement by HPLC-MS-MS. Cardio-pulmonary test (CPET) parameters and MECKI score of mortality risk were measured. The large majority (87%) of patients showed hypovitaminosis D and 25% had severe deficiency (25OHD <10ng/ml). The latter patients had significantly lower CPET VO2/kg, VO2 peak% and significantly higher NT-proBNP and MECKI score, than patients with 25OHD>10 ng/ml. Patients with VO2peak% <50% showed significantly lower 25OHD than those with VO2peak% > 50%. There was a significant, positive correlation (r=0.16, p=0.008) between 25OHD levels and VO2peak% and an inverse correlation with MECKI score (r=-0.21, p<0.001), even when adjusted for age, BMI, MDRD, NT-proBNP. Vitamin D levels are associated with functional and mortality HF prognosis paramethers.
Prospective Study: It was a pilot monocentric, randomized, placebo-controlled trial
with the aim to evaluate vitamin D supplementation effect on biochemical marker, functional capacity and echocardiographic parameters in patients with HF. Twelve patients matched the inclusion criteria and were randomized to placebo or cholecalciferol 25.000 UI once every two weeks. Vitamin D treatment was safe, well tolerated and efficient to reach vitamin D sufficiency. Biochemical, CPET and echocardiographic parameters after six months of follow-up did not show any
4
statistical significance between patients in the placebo and vitamin D group, except for tricuspid annular plane systolic excursion (TAPSE) measurement. TAPSE was higher in the group of patients supplemented with vitamin D compared to the placebo group (TAPSE 23.5±5.8 mm vs 18.3±1.4 mm p=0.04) and showed a higher increase compared to the baseline values in the former group. This pilot study could show an improvement in right ventricular function after 6 months of vitamin D supplementation and encourages to proceed with the complete and statistically powered study.
Methodological Study: 110 consecutive HF patients underwent vitamin D
quantification by chemiluminescence immunoassay (DiaSorin LIAISON) and HPLC-MS-MS at the same moment. A good correlation between 25OHD values measured with the two methods was found. The inter-assay bias was evaluated by Bland-Altman plots: compared to the HPLC-MS-MS method, LIAISON assay demonstrated a mean relative bias of -6.54% with 95% of limits of agreement (-46.52% to +33.44%). 25OHD levels with LIAISON were statistically lower than with HPLC-MS-MS (17.6±8.9 ng/ml vs 18.9 ±9.4, p<0.0001). The prevalence of Vitamin D insufficiency (<50 nmol/l or 20 ng/ml) was statistically lower 59% using HPLC-MS-MS compared to LIAISON 63% (p<0.0001). HPLC-MS-MS is well correlated to the method currently used (CLIA), avoids overestimation of hypovitaminosis D and is a reliable diagnostic tool for 25OHD measurement.
In vivo experiments: we developed and validated a method for Vitamin D3 and
25OHD measurement directly in rat tissues by HPLC-MS-MS, with high accuracy, specificity, sensibility and requiring small amount of tissue. We performed a pharmacokinetic analyses of Vitamin D3 and 25OHD distribution after administration by hepatic portal vein in an ex-vivo preparation. We could detect a significant Vitamin D3 and 25OHD uptake from liver and kidney and a moderate 25OHD store in heart, lung and skeletal muscle. We confirmed that there is a rapid distribution and tissue metabolism of Vitamin D3 and 25OHD after hepatic vein perfusion and we detected vitamin D storage in skeletal muscles and in the heart. Despite the small concentration we can conclude that vitamin D systemic administration can modify vitamin D distribution and levels in these tissues.
5
A
A
B
B
B
B
R
R
E
E
V
V
I
I
A
A
T
T
I
I
O
O
N
N
S
S
BMI, Body Mass Index
CaBP, calcium-binding protein
CLIA, chemiluminescence immunoassay CPET, Cardio-pulmonary test
CRP, C-reactive protein CVD, cardiovascular disease eGFR, glomerular filtration
ESC, European Society of Cardiology FGF23, fibroblast growth factor 23 Hb, hemoglobin
HF, Chronic Heart failure
HPLC-MS-MS, High Performances Liquid Chromatography coupled to mass spectrometry IL6, interleukin 6
LV, left ventricular
LVEF, left ventricular ejection fraction LVDD, left ventricular diastolic diameter LVSD, left ventricular systolic diameter LVDV, left ventricular diastolic volume LVSV, left ventricular systolic volume LXR, liver X receptor
MECKI, Metabolic exercise and cardiac and kidney indexes MS, multiple sclerosis
NHANES, Third National Health and Nutrition Examination Survey NHRs, nuclear hormone receptors
NT-proBNP, N-terminal proBrain natriuretic peptide PPAR, peroxisome proliferators-activated receptor PRA, plasma renin activity
PTH, parathyroid hormone
RANKL, nuclear factor-κB ligand RAS, renin-angiotensin system RCT, randomized clinical trials
TAPSE, tricuspid annular plane systolic excursion T1DM, type 1 diabetes mellitus
T2DM, type 2 diabetes mellitus
TRPV6, transient receptor potential cation channel, subfamily V, member 6 VDR-RXR, vitamin D receptor–retinoic acid x-receptor complex
VE/VCO2, slope of the minute ventilation to carbon dioxide production ratio VO2%, peak oxygen consumption
1,25(OH)2D, 1,25-didroxyvitamin D 25OHD, Twenty-five hydroxy-vitamin D
6
1.
B
B
A
A
C
C
K
K
G
G
R
R
O
O
U
U
N
N
D
D
INTRODUCTION
HF is a progressive condition and, once it has ensued, may require hospitalization because of worsening, arrhythmias or myocardial infarction occurrence. The prognosis of HF remains poor, even if the treatment of this condition has made remarkable progress in the past decades. Indeed, novel targets, biomarkers and therapies are needed. Among different biomarkers proposed for cardiovascular risk evaluation in HF, Vitamin D could be an emerging tool.
Vitamin D is classically involved in bone homeostasis. However, recent studies strongly suggest other extraskeletal functions for vitamin D, including pleiotropic effects on cardiovascular system [1][2][3][4]. Twenty-five hydroxy-vitamin D (25OHD) represents the biomarker of vitamin D status, but it is still controversial which is the optimal level in the adult [5][6][7].
There are evidences that low serum 25OHD levels are associated with increased risk of cardiovascular disease (CVD) [8] including hypertension, coronary artery disease, ischemic heart disease, stroke and type 2 diabetes [9][10][11][12][13]. Data from the Third National Health and Nutrition Examination Survey (NHANES 1988–1994) showed a strong and independent association between 25OHD deficiency and the prevalence of CVD in a large population [14]
Moreover, there are data supporting the hypothesis that vitamin D could exerts some modulatory effects in the pathophysiology of HF, namely downregulation of the renin–angiotensin system, enhancement of insulin secretion and sensitivity, protection against angiogenesis and modulation of inflammatory processes. [1][2][3].
7
Growing data about the condition of poor vitamin D status in patients with HF have been recently published, with main limitations due to 25OHD levels evaluation or a variety of approaches, difficult to be compared. Indeed, in most studies, Vitamin D levels were measured with traditional immunoassay, that often show an unsatisfactory accuracy and a remarkable intra- and inter-assay variability around the cut off limits. Higher specificity and sensitivity can be obtained by High Performances Liquid Chromatography coupled to mass spectrometry (HPLC-MS/MS), that is frequently referred as the new “gold standard” method [15][16][17]. Previous studies investigated the possible relation between serum 25OHD levels and HF markers with various end-points: echocardiographic parameters, inflammatory markers, neurohormones, supporting the hypothesis that vitamin D may have a role in HF pathophysiology and outcome. However, data regarding vitamin D and HF functional capacity and prognosis markers are still scanty and required.
1.1 VITAMIN D
1.1.1 “Classical effects” of Vitamin D
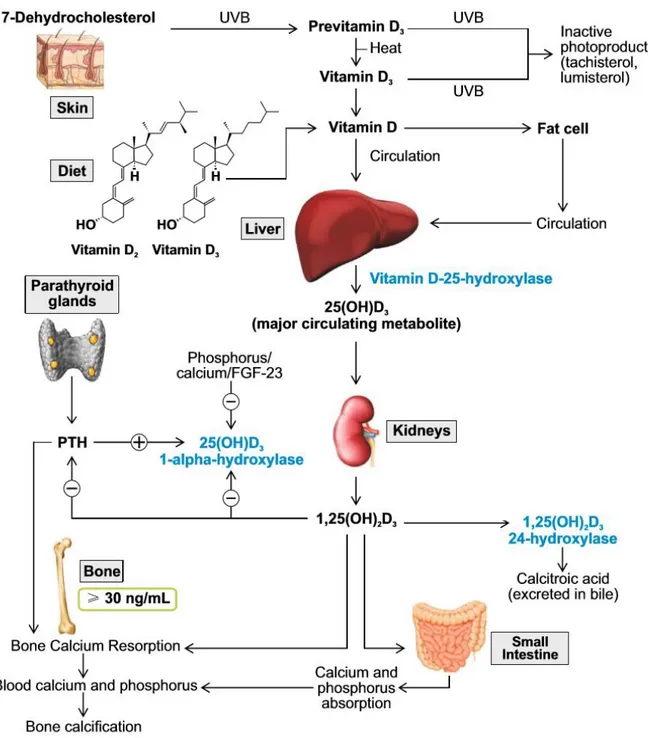
Vitamin D is a fat-soluble vitamin, which functions as a steroid hormone. The two major forms of vitamin D are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Vitamin D3 is mainly produced in the skin upon UV light exposure, while dietary sources are scarce. Vitamin D2 is found in vegetables. Two subsequent hydroxylations of vitamin D are required for the production of the active form 1,25-didroxyvitamin D (1,25(OH)2D) or calcitriol: the hydroxylation in position 25 occurs in the liver and is substrate-dependent; conversely the hydroxylation in position 1 occurs in the kidney,
8
under the control of parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). Extrarenal hydroxylation of 25OHD in position 1 may also occurs and mediates paracrine-autocrine effects.
1,25(OH)2D enhances intestinal calcium absorption in the small intestine by interacting with the vitamin D receptor–retinoic acid x-receptor complex (VDR-RXR) to enhance the expression of the epithelial calcium channel (transient receptor potential cation channel, subfamily V, member 6 [TRPV6]) and calbindin 9K, a calcium-binding protein (CaBP). 1,25(OH)2D is recognized by its receptor in osteoblasts, causing an increase in the expression of the receptor activator of nuclear factor-κB ligand (RANKL). RANK, the receptor for RANKL on preosteoclasts, binds RANKL, which induces preosteoclasts to become mature osteoclasts. Mature osteoclasts remove calcium and phosphorus from the bone, maintaining calcium and phosphorus levels in the blood. Adequate calcium (Ca2+) and phosphorus (HPO42−) levels promote the mineralization of the skeleton [18, 19].
Serum 25 hydroxy-vitamin D (25OHD) represents the biomarker of vitamin D status, but it is still controversial which is the optimal level in the adult. The Institute of Medicine in 2011 recommended a minimum level of 50 nmol/l (≥20 ng/ml), while the Endocrine Society suggests 75 nmol/l (30 ng/ml).
Vitamin D is involved in bone and skeletal homeostasis, in calcium and phosporus absorption and bone matrix mineralization.
9
Figure 1. Metabolism of vitamin D and skeletal classic effects on phosphocalcic metabolism. (Adapted
10
1.1.2 “Extraskeletal effects” of Vitamin D
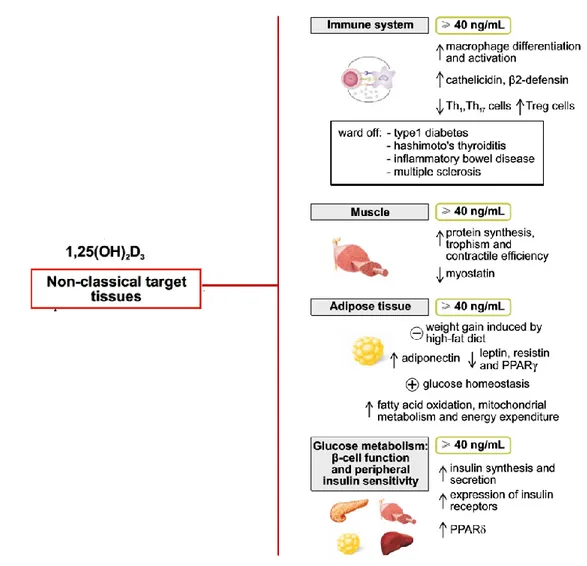
1,25(OH)2D, the active hormone, needs its specific receptor VDR to exert many biological actions. VDR was discovered to be ubiquitous and it is expressed not only in bone system, but also in muscle, myocardium, immune system and many other tissues and organs. Moreover, the machinery for local production and inactivation of 1,25(OH)2D was demonstrated in the brain, in the vasculature, in the adrenal gland, in the colon, in the limphonodes and in the insulin-secreting pancreatic β cells, suggesting the hypothesis that vitamin D and its metabolites play a role in the general health of human being. It is noteworthy that the active form of vitamin D regulates approximately 3% of mice to human genome in a direct or indirect way [20, 21].
Immune system: Vitamin D seems to influence either innate immune system and
acquired immune system with opposite effects. Indeed, it stimulates the differentiation and activation of macrophages and the local production of defensins, as demonstrated in mice with low intake of vitamin D3, in which IL6, TNFα and IL 1 were impaired [22].
On the other hand, vitamin D inhibits the acquired immune system, mainly reducing the expression of MHC class II and co-signaling molecules on antigen presenting cells, decreasing the activity of TH1 and TH17 cells, and up-regulating regulatory T cells. The final result is to promote the regulatory and protective phenotype of T cells [23].
These in vitro data give reason of in vivo data in mouse models, in which vitamin D seemed to prevent insulitis and type 1 diabetes mellitus (T1DM). Moreover, some retrospective studies showed a beneficial effect of vitamin D supplementation in early
11
life on the risk of T1DM [24]. Few clinical trials have been carried on, however some showed that cholecalciferol supplementation improved the suppressive ability of T cells in T1DM patients [25].
In the setting of autoimmune diseases, there is an interesting association between low levels of vitamin D and increased risk of developing multiple sclerosis (MS). Some prospective studies have demonstrated that increasing levels of 25OHD significantly reduce the risk of MS, among Caucasian people [26]; moreover, cholecalciferol supplementation associated with interferon β1b significantly reduced the activity of the disease at magnetic resonance, compared to interferon β1b alone [27].
Adipose tissue and glucose/lipid metabolism: many recent studies showed an
association between low levels of vitamin D and almost all aspects of metabolic syndrome, such as type 2 diabetes mellitus (T2DM), impaired fasting glucose, hypertension, dyslipidaemia, obesity, insulin resistance, therefore it has been speculated a role of vitamin D in adipose tissue biology. Some studies have showed a negative correlation between vitamin D and leptin and resistin and an inverse correlation with adiponectin. [28, 29] Old preclinical studies have demonstrated that a normal activity of pancreatic β cells needs adequate levels of vitamin D and functioning VDR [30], while more recent studies on vitamin D deficient mice, showed and impaired glucose-stimulated insulin secretion in pancreatic islets. [31]
In a recent review by Mathieu, the revision of the available data on vitamin D and T1 and T2 diabetes mellitus, shows that there is a convincing role of the hormone in both the diseases. Indeed, VDR is present in all tissues involved in T1 and T2DM, either in
12
pancreatic islets and immune cells, adipose tissue, liver and muscle and in all these tissues and organs it’s possible to demonstrate the machinery for locally producing 1,25(OH)2D and its metabolites. Moreover, low levels of vitamin D are associated with increased risk of T1 and T2DM and animal models clearly demonstrate that vitamin D supplementation improves islets function and insulin sensitivity. However, convincing randomized prospective studies in humans are still lacking and necessary.
Muscle: it’s ascertain that vitamin D deficiency is responsible of low muscle strength,
balance disorders and with increase in risk of falls. In a model of VDR null mice it was demonstrated a specific phenotype due to immature muscle-specific genes and cardiomyocytes specific VDR knockout mice developed cardiac hypertrophy and defects. [32, 33] Observational studies have showed the association between low levels of vitamin D and muscle weakness in children and in elderly and supplementation has been proved to improve muscle function and time and energy recovery after exercise. [34, 35]
In conclusion, recent literature has focuses on the pivotal role of vitamin D, beyond skeletal wellness, particularly in the field of metabolism and metabolic syndrome. The most important extraskeletal effects of vitamin D are summarized in Figure 2.
13
Figure 2. Extraskeletal effects of vitamin D and assumed 25OHD levels required. (Adapted by Caprio
et al. Eat Weight Disorders 2016)
14
1.2 VITAMIN D AND CARDIOVASCULAR DISEASES
1.2.1 Cardiovascular effects of vitamin D active hormone
In the early 1980s Robert Scragg for the first time proposed the hypothesis that the increase in cardiovascular diseases in winter might be a result of low 25(OH)D levels, due to the reduced sunlight exposure during that period [36]. This idea turned on a great interest in the potential cardiovascular benefits of vitamin D, leading to several publications in this field in the past ten years. However, the physiological role of vitamin D in cardiovascular system is still unclear, despite many evidence of the importance of adequate levels of this hormone for cardiovascular health.
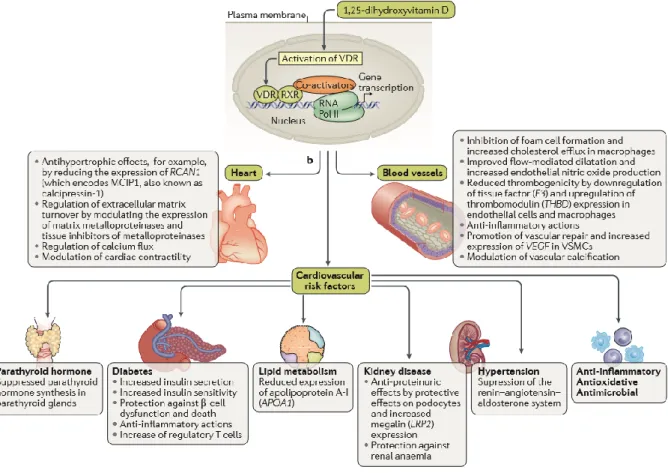
Calcitriol binds to the specific nuclear receptor VDR, which is expressed in rat and human heart tissue and has a potential role as modulators of cardiac hypertrophy and failure. Calcitriol is also directly involved in calcium-dependent cellular processes, including synthesis of calcium-binding protein, activation of adenylate cyclase, rapid activation of voltage-dependent calcium channels, and influx, reuptake, and release of calcium from the sarcoplasmic reticulum. Altered intracellular handling of ionized calcium could also lead to the impaired contractility of the myocardium in HF patients [37].
Another possible mechanism is that calcitriol seem to be a negative regulator of renin-angiotensin system (RAS). Data from literature showed that in both normotensive and hypertensive subjects, 1,25OH2D3 serum levels are inversely associated PRA leading to a potential role of vitamin D in hypertension via renin regulation. Recent evidence showed that nuclear hormone receptors (NHRs) including VDR, liver X receptor
15
(LXR) and peroxisome proliferators-activated receptor (PPAR) mediate renin regulation via specific elements in the renin promoter [37].
Strong support for the involvement of vitamin D in the pathogenesis of cardiovascular diseases comes from vitamin D receptor knockout mice (VDR-/-). These mice develop typical signs of HF including activation of the renin–angiotensin–aldosterone system, cardiac hypertrophy, high blood pressure, and increased levels of atrial natriuretic peptide. Furthermore the development of hypertension in VDR-/- mice can be corrected by administration of ACE inihibitors, but only as long as vitamin D levels are sufficient [1–3].
Some effects of 1,25OH2D3 have been demonstrated on vasculature; indeed, vitamin D can modulate the growth of smooth cells and endothelial cells and can induce the activation of vasodilatory and antithrombotic genes [38]. Moreover, vitamin D seems to suppress inflammation and to reduce update of oxidized LDL, giving potential vascular benefits. VDR-/- mice show hypercoagulability and atherosclerosis [39]. Finally, some indirect actions have been hypothesized from vitamin D effects on cardiovascular health. In a recent review, published on Nature Reviews, Pilz et al. summarized all known mechanisms of vitamin D action on cardiovascular system (Figure 3) [40].
16
Figure 3: Cardiovascular effects of vitamin D receptor activation. On the basis of the major findings from
experimental studies, the effects of vitamin D can be categorized into effects on the blood vessels, the heart, and on cardiovascular risk factors. While most studies indicate beneficial cardiovascular effects of vitamin D receptor activation, the effects of vitamin D on lipid metabolism and vascular calcification are unclear and might be harmful or beneficial. LRP2, low-density lipoprotein receptor- related protein 2; MCIP1, modulatory calcineurin inhibitory protein 1; RNA Pol II, RNA polymerase II; VSMC, vascular smooth muscle cell. (Adapted
17
1.2.2 Observational studies
Several longitudinal cohort studies demonstrated the association between vitamin D deficiency and cardiovascular events. In the Framingham Heart Study published in 2008, low 25OHD levels, less than 15 ng/ml, were associated with 60% increase in the risk of developing cardiovascular events, despite any further adjustment for the most important cardiovascular risk factors [41]. In a study of general healthcare population, conducted in Utah, vitamin D deficiency was associated with an increase of twofold risk for cardiovascular events [42]. The association seems to be valid either for men, as demonstrated by the Health Professional Follow-Up Study and for women as showed in a large Finnish study [43, 44].
A recent meta-analyses by Wang confirmed a strong association between low levels of vitamin D and cardiovascular risk across 19 independent, prospective, observational studies [9].
Moreover, vitamin D deficiency seems to be associated with prevalence and incidence of hypertension, as confirmed by a meta-analyses of cross sectional studies and with incident stroke [45, 46].
All studies confirm a consistent association between low levels of vitamin D and cardiovascular diseases, nevertheless these studies can be limited by residual confounding and reverse causation. Indeed, some authors propose that vitamin D deficiency is a general marker of poor health and that the causative role in the association with cardiovascular diseases can be misleading.
1.2.3 Randomized clinical trials (RCT) and vitamin D supplementation in cardiovascular diseases
18
Since observational studies seem to clearly establish the association between low vitamin D levels and cardiovascular diseases, RCT are required to prove the usefulness of vitamin D supplementation on cardiovascular outcomes. RCT are still few and show some limitations: cardiovascular diseases were not the principal focus of the trial and in some cases, there weren’t prespecified endpoints; different dosage and different preparations of vitamin D were used; 25OHD levels were missing at baseline and at the last follow-up in some studies.
One of the largest RCT is the Women’ Health Initiative was designed for skeletal primary endpoint, but had secondary endpoints for cardiovascular events. In this study, more than 36.000 women were randomized to vitamin D plus calcium (400 UI and 1000 mg) or placebo: there was no difference between the two arms, in term of risk of coronary events and stroke [47]. Vitamin D3 supplementation significantly reduced all-cause mortality compared with placebo or no intervention in a Cochrane review, but vitamin D3 had no significant effect on cardiovascular mortality (risk ratio 0.98, 95% CI 0.90–1.07; n = 47,267) [48]. The hazard ratios for heart failure, myocardial infarction, and stroke were 0.82 (95% CI 0.58–1.15), 0.96 (95% CI 0.83–1.10), and 1.07 (95% CI 0.91–1.29), respectively in a meta-analyses of 21 RCTs of vitamin D and a total of 13,033 participant [49]. Overall, there are still non-convincing data about vitamin D supplementation effects on cardiovascular health, with the main studies limitations that we have above described. Several large RCTs are currently ongoing, with the specific aim to evaluate vitamin D action on cardiovascular end-points, and hopefully they will clarify this important matter. In Figure 4 and 5 all RCTs meta-analyses, either concluded or ongoing, are reported.
19
Figure 4: Meta-analyses of RCTs on vitamin D effects on cardiovascular events and cardiovascular mortality. Direction of comparison is vitamin D versus control. Only meta-analyses of
RCTs testing vitamin D3 (D3) or vitamin D2 (D2); or D3, D2, or active vitamin D (‘mixed’) are shown. Pooled effect estimate and 95% CI were calculated using random effects models. Heterogeneity was assessed by I2. NR, not reported; RCT, randomized controlled trial; RR, relative risk. *Bolland et al. (2014) investigated the effects on myocardial and ischaemic heart disease as well as on stroke and cerebrovascular disease. Adapted from Pilz et. al Nature Reviews 2016
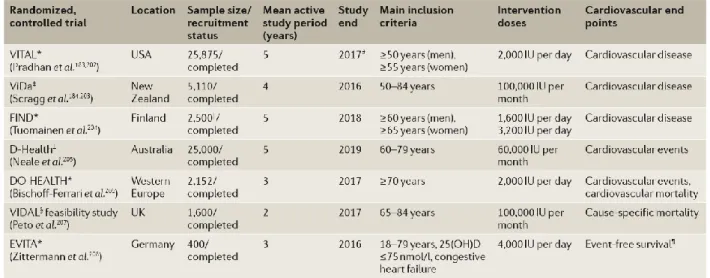
Figure 5: Large, randomized, placebo-controlled trials on vitamin D treatment effect on cardiovascular outcomes still ongoing. Only trials with n ≥400 patients are shown and only the
cardiovascular end points are provided. Study end was approximated from data available at the respective clinical trial registry. 25(OH)D, 25-hydroxyvitamin D; IU, international units. *Registered at ClinicalTrials.gov. ‡Registered at Australian New Zealand Clinical Trials Registry. §Registered at ISRCTN registry. ||Sample size was reduced from 18,000 to 2,500, according to clinical trial registry. ¶Events are defined as cardiac transplantation, high urgent listing for cardiac transplantation,
20
resuscitation, hospitalization, and ventricular assist device implantation. (Adapted from Pilz et. al
Nature Reviews 2016)
1.3 VITAMIN D AND HEART FAILURE
1.3.1 Modulatory effects of vitamin D on Heart Failure physiopathology
Vitamin D shows some well recognized modulatory effects on mechanism involved in the physiopathology of heart failure. We have already discussed in the previous chapter the role of vitamin D as inhibitor of renin expression.
Moreover, animal models have demonstrated that 1,25OH2D3 has an important role in maturation and differentiation of ventricular myocytes isolated from neonatal rat hearts [10]. Increased ventricular mass and higher levels of atrial natriuretic peptide were showed in VDR knockout mice, leading to ventricular dilatation and 1-alpha-hydroxylase enzyme knockout mice had hypertension and ventricular hypertrophy [33, 50–52]. In the latter mice model, the administration of vitamin D was able to normalize serum calcium and phosphorus levels but also to normalize blood pressure, cardiac structure-function and the renin-angiotensin system [52]. Furthermore, administration of vitamin D analogues to Dahl salt-sensitive rats reduces the degree of left ventricular hypertrophy. Vitamin D analogues improve not only left ventricular structure, but also functional parameters of contractility and relaxation. Pre-clinical data suggest that vitamin D therapy might also favourably influence on other pathways of relevance to heart failure, including glucose tolerance, immunity, and vascular remodelling [52].
21
1.3.2 Observational studies
Growing data about the condition of poor vitamin D status in patients with HF have been recently published, with main limitations due to 25OHD levels evaluation or a variety of approaches, difficult to be compared. Indeed, in the majority of studies, Vitamin D levels were measured with traditional immunoassay, that often show an unsatisfactory accuracy and a remarkable intra- and inter-assay variability around the cut off limits. Higher specificity and sensitivity can be obtained by High Performances Liquid Chromatography coupled to mass spectrometry (HPLC-MS/MS), that is frequently referred as the new “gold standard” method, as we will discuss in the dedicated section [15, 16].
Previous studies investigated the possible relation between serum 25OHD levels and HF markers with various end-points: echocardiographic parameters, inflammatory markers, neurohormones, supporting the hypothesis that vitamin D may have a role in HF pathophysiology and outcome. However, data regarding vitamin D and HF functional capacity and prognosis markers are still scanty.
An observational study in 101 HF patients published in 1997, linked low serum 25OH-D levels to a diminished exercise tolerance, resulting in lower peak VO2 [53]. In a study on 88 Caucasian patients, they were divided into three groups: chronic HF patients <50 years, chronic HF patients >50 years and a control group (patients >50 years): HF patients, regardless whether < 50 or >50 years of age, had significantly lower levels of 25OH-D and 1,25(OH)2D3 compared to elderly healthy controls [54]. Subsequent studies demonstrated that 25OH-D and 1,25(OH)2D3 have been associated with left ventricular (LV) dysfunction: patients with a poor LV function
22
showed decreased 25OH-D and 1,25(OH)2D3 levels [55]. Fiscella and Franks reported that 1,25(OH)2D3 levels function as independent predictor of mortality in HF patients [56]. Kilkkinnen et al. demonstrated that an inverse association between serum 25(OH)D levels and cardiovascular disease is present when results are adjusted for age and sex. On the other hand, the risk for coronary heart disease is not significantly related to 25OH-D levels when they are adjusted for potential confounders. Nevertheless, low vitamin D levels statistically increased total cardiovascular disease mortality in a multivariate model [44].
An interesting study from Liu et al. was conducted on 548 patients with HF, in whom plasma renin activity (PRA), interleukin 6 (IL6), C-reactive protein (CRP and the incidence of death or HF rehospitalisation were measured. They showed that in a multivariate linear regression model PRA and CRP were independent predictors of vitamin D levels and patients with low levels of 25OHD had an increased risk of all-cause mortality and HF rehospitalisation [57].
A large observational study was published by Gotsman et al. in 2012. They reported the prevalence of vitamin D deficiency in a large cohort of HF patients, and described the seasonal variation in vitamin D levels and the impact of vitamin D deficiency and supplementation on mortality in this population. They demonstrated that vitamin D levels are low in the general population and even lower in HF patients and vitamin D deficiency was a significant predictor of reduced survival in patients with HF as well as in the control group. Moreover, vitamin D supplementation in HF patients was associated with improved outcome [58].
23
1.3.3 Controversies from RCTs
Some randomized controlled trials were published in the last years, investigating the benefit of vitamin D supplementation in HF patients. The overall conclusions are still controversial, as mentioned in a recent meta-analysis on seven clinical trials [59]. The conclusions of this meta-analyses are that vitamin D therapy seems to have effects in decreasing serum levels of PTH, TNFα and CRP in patients with HF, but the beneficial effect on left ventricular function and exercise tolerance were limited. It is noteworthy that the meta-analyses had some limitations due to the methodological quality of the trials that was less than optimal, to the limited number of patients included and to the heterogeneity of vitamin D levels and supplementation dosages across the studies [59].
Shleithoff et al analysed a quite large cohort (n=123 patients with HF, randomized 61 at treatment and 62 at placebo) and reported that supplementation with vitamin D 2000 UI/daily for nine months decreased proinflammatory cytokines levels, without changes in ventricular function and biochemical markers [4]. Schroten et al. described a significant decrease of PRA and plasma renin concentration with the same scheme of supplementation [60]. Dalbeni et al. found that six months of vitamin D supplementation significantly improved ejection fraction in elderly patients with HF [61] and Shedeed et al indicated that young patients with HF achieved marked improvement in both cardiac function and inflammatory markers after 12 months of vitamin D analogues [62].
The most recent study on the topic, is the VINDICATE study, a randomised double blind trial in which HF patients were supplemented with 4000 IU of vitamin D3/daily
24
for 12 months or placebo. 25OHD levels were appropriately measured by HPLC-MS-MS. The authors could detect a substantial improvement of Left Ventricle (LV) ejection fraction, dimension and volume in the supplemented group, but not on 6-minute walk test, that was the primary end-point [63].
25
2
2
.
.
P
P
R
R
O
O
J
J
E
E
C
C
T
T
A
A
I
I
M
M
S
S
2.1 AIMS AND EXPECTATIONS
The aims of the study and expectations were as follows:
1. To evaluate the relation between serum 25OHD levels and functional capacity in
patients with Heart Failure (HF) and to investigate the relationship between 25OHD
levels and the MECKI score, by a retrospective study.
Based on literature data, we expected to find a positive correlation between 25OHD levels and functional capacity parameters and a negative correlation with the score of mortality MECKI. The novelty of the study was the evaluation of functional capacity using the parameters derived by cardiopulmonary test (CPET), that are well-established tools of risk stratification among HF patients and solid predictors of prognosis. Moreover, we used for the first time the MECKI score, a score of prediction of mortality at 2 years, built from Metabolic Exercise Cardiac Kidney Indexes, that was successfully validated in recent investigations.
Finally, for our purposes, we used a modern and accurate 25OHD measurement method, i.e. HPLC-MS-MS and we performed the analyses in large cohort, studied in a single tertiary referral centre, with the same protocol and pre-analytical procedures. 2. To asses 25 OH-D levels in the HF population by a retrospective analysis.
We expected to reveal and confirm the condition of vitamin-D deficiency or insufficiency in the HF population.
3. To evaluate the effects of vitamin D supplementation on HF outcomes comparing
26
This aim was achieved by a pilot, prospective, placebo-controlled study in a small cohort of patients with HF. The next step, beyond this project, will be to further increase the population sample.
The hypothesis of the study was that an adequate vitamin D status, reached by a “standard care” long-term vitamin D supplementation, can improve cardiovascular outcome. However, there was a real need to explore this hypothesis, since RCTs studies in literature showed controverted results. The primary endpoint was to evaluate cardiopulmonary exercise test (CPET) parameters and MECKI score changes after vitamin D supplementation. Secondary endpoint was to evaluate biochemical markers of HF and echocardiography parameters changes after vitamin D supplementation.
4. To compare levels of 25OHD measured by LIAISON immunoassay and High
Performances Liquid Chromatography (HPLC-MS-MS) at the same moment, in a
population of patients with HF.
An aspect of novelty in this project was the validation and use of a fast isotope dilution HPLC-MS-MS method, for measuring 25OHD in human serum and in tissue specimens (for the basic study). A large proportion of previous studies evaluating vitamin D and cardiovascular outcomes used traditional immunoassays, that often showed an unsatisfactory accuracy and a remarkable intra- and inter-assay variability around the cut off limits. The expectations of this part of the project was to confirm HPLC-MS-MS as the “gold standard” method for 25OHD measurement.
5. To evaluate the vitamin D metabolites levels and distribution in tissues, in rat
27
compare and correlate vitamin D metabolites levels in rat serum and different tissues after sacrifice. The study expectation was to develop and validate a method to quantify levels of Vitamin D3 and 25OHD3 in different rat tissues by HPLC-MS-MS, either in basal condition and after vitamin D perfusion
2.2 FACILITIES AND CONTRIBUTIONS
This project was part of a collaboration between tree referral tertiary Centres in Pisa: - “Fondazione Toscana Gabriele Monasterio (FTGM, U.O.C. Cardiologia e Medicina Cardiovascolare, Pisa)”: provide serum HF patients samples for retrospective study, recruitment of patients for prospective study, HF enrolled patients baseline and follow-up “routine” evaluation.
- Biochemistry Unit (University of Pisa): supported fast isotope dilution Mass Spectrometry coupled to High Performances Liquid Chromatography (HPLC-MS-MS) method vitamin D measurement in retrospective and prospective study and in vivo study
- “Department of Clinical and Experimental Medicine, U.O. Endocrinologia 2”, University Hospital of Pisa: supported prospective study in each part that is not considered “routine” in HF patient evaluation and follow-up, i.e. randomization, drug/placebo providing.
Dr. F. Saponaro was directly responsible for:
- Conception and design of the study
- Retrospective study: retrieving serum samples and samples preparation for
HPLC-MS-MS (serum and solvents mix, centrifuge, withdraw supernatants and prepare for injection) since she has already experienced lab techniques.
28
- Prospective study: bone metabolism markers samples collection at baseline and
6 months follow-up, vitamin D samples collection and samples preparation for HPLC-MS-MS (as previous) at baseline and 6 months follow-up. Titration phase: patient interview, visit and vitamin D dose adjustment at baseline and 6 months’ follow-up, since she has clinical experience in Endocrinology and particularly Bone Biology and Metabolism.
- In vivo study: she performed experiments with rats in all steps, since she has
already experienced in vivo (mice/rats) procedures and techniques during foreign University research training (Boston, Leuven and München Universities).
- Collection, analysis and interpretation of data - Manuscript/thesis drafting
During this three-year project, Dr Saponaro took active part to clinical and research meetings at the Scuola Superiore “Sant’Anna”, and was directly involved in the clinical activity of Endocrinology U.O., as integral parts of his PhD course.
Prof. C. Passino and Dr. G. Vergaro and E. Benelli were involved in the clinical part of the project. Prof. C. Passino contributed to analysis and interpretation of data and revision of manuscripts.
Prof. R. Zucchi, Dr. A. Saba, Dr. S. Frascarelli were involved in the basic part of the project, particularly in the HPLC-MS-MS measurements and in the rat study. Prof. Zucchi contributed to analysis and interpretation of data and revision of manuscripts. Prof. C. Marcocci was involved in the clinical part of the project and contributed to analysis and interpretation of data and revision of manuscripts.
29
Dr. C. Prontera and Prof. A. Clerico were involved in the laboratory measurements for the retrospective and prospective study. Prof. F. Recchia supervised the entire project.
Vitamin D and placebo were provided with the free and impartial contribution of ABIOGEN SPA.
In Figure 6 the summary of the four part of the PHD project are reported.
30
3
3
.
.
M
M
E
E
T
T
H
H
O
O
D
D
S
S
The present research project aimed to investigate different clinical and basic science issues. A general methodological approach has been followed, even though, of course, each study branch has its own methodology. In this Chapter the general methods suitable for all branches are described, while other specific features are reported in the respective chapters, where necessary.
3.1 PATIENTS SELECTION CRITERIA
Reserving the modality of patient’s recruitment, i.e. prospective or retrospective, which is detailed in each single study branch, enrolment criteria were constantly the same along the whole research project.
Inclusion criteria:
- Fulfilled criteria for the diagnosis of HF of the European Society of Cardiology
(ESC) [64]
- HF symptoms (NYHA functional classes I–IV)
- Systolic dysfunction (left ventricular ejection fraction, LVEF <50%)
- Reference to the Division of Cardiovascular Medicine of the Fondazione G.
Monasterio in Pisa for HF management.
- Stable clinical conditions with unchanged medications for at least three months - Ability to perform a CPET
- Age ≥ 18 years
- Gender either males and females - Written informed consent
31
Exclusion criteria
- Body Mass Index (BMI)>30
- Already taking vitamin D supplements
- Primary or secondary hyperparathyroidism or phospho-calcic metabolism
alterations/diseases
- History of pulmonary embolism
- Moderate-to-severe aortic and mitral stenosis - Pericardial disease
- Severe obstructive lung disease
- Exercise-induced angina and significant ECG alterations. - Inability to perform a CPET
- Pregnancy/Lactation - Age < 18 years
- Intolerance to vitamin D medications - Hypercalcemia/Hypercalciuria - Nephrolithiasis/nephrocalcinosis
- Use of barbiturates or anticonvulsant drugs
3.2
CLINICAL,
BIOCHEMICAL
AND
INSTRUMENTAL
EVALUATION
3.2.1 Clinical and biochemical evaluation
All patients underwent a comprehensive clinical and biochemical characterization, including haemoglobin, creatinine and glomerular filtration (eGFR calculated using the MDRD formula), serum electrolytes, PRA, aldosterone, catecholamine, N-terminal
32
proBrain natriuretic peptide (NT-proBNP levels, albumin adjusted serum calcium, Parathormone (PTH). All parameters were measured with standard methods. Glomerular filtration rate was calculated as MDRD using the following formula: 186.3*(crea)-1.154*(Age)-0.203*0.75 for women [65].
3.2.2 Cardiopulmonary test (CPET) and instrumental evaluation
All patients underwent echocardiographic and CPET measurements. Left Ventricular (LF) volumes and ejection fraction (EF) were measured on echocardiography according to standard criteria.
All CPETs were performed using an electronically braked cycle-ergometer. A modified Bruce protocol was applied in CPET with cycle-ergometer. The exercise protocol was set to achieve peak exercise in ~10 min [66]. In the absence of clinical events, CPET was interrupted when patients stated that they had reached maximal effort. We performed breath-by-breath analysis of expiratory gases and ventilation. Anaerobic threshold was measured by V-slope analysis of VO2and VCO2, and it was confirmed by ventilatory equivalents and end-tidal pressures of CO2 and O2. If no agreement was obtained, AT was considered as not identified. Exercise-induced periodic breathing was defined as a cyclic fluctuation of ventilation [67]. VO2/work rate relationship was measured throughout the entire exercise (cycle-ergometer). VE/VCO2 slope was calculated as the slope of the linear relationship between VE and VCO2 from 1 min after the beginning of the loaded exercise and the end of the isocapnic buffering period. Peak exercise oxygen pulse was calculated as peak VO2/peak heart rate (HR). Predicted values of VO2 and HR were calculated as: peak
33
VO2 pred=(Height−Age)*20 if male, = (Height−Age)*14 if female; peak HR pred=(220−Age), if male, = (210−Age) if female [68].
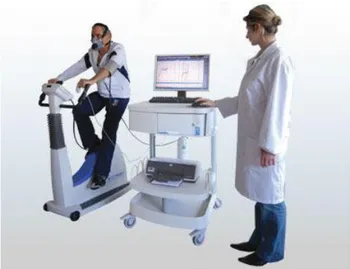
In Figure 7 the machine for Cardiopulmonary test is showed.
Figure 7: Cardiopulmonary test apparatus.
3.2.3 Metabolic exercise and cardiac and kidney indexes (MECKI) score
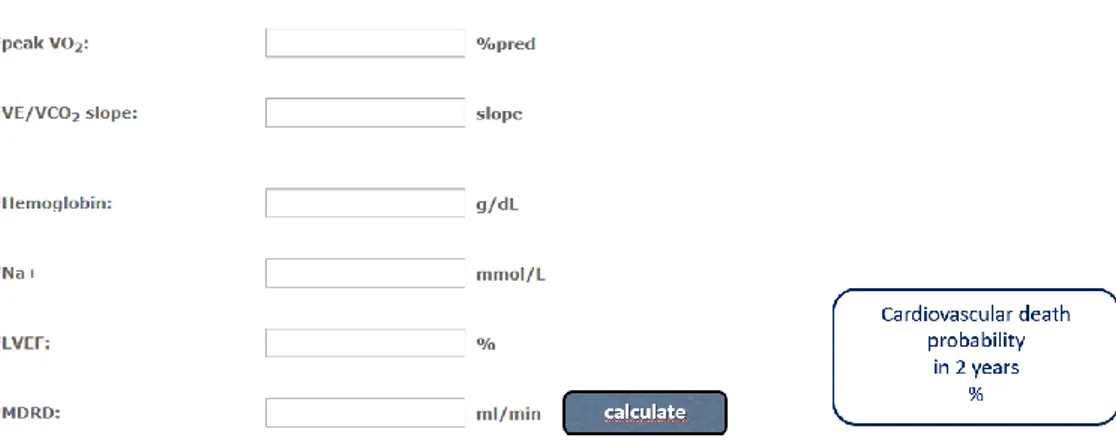
Recently, the metabolic exercise and cardiac and kidney indexes (MECKI) score, a prognostic score combining data from CPET with clinical, laboratory and echocardiographic measurements, was validated in patients with chronic systolic HF [69]. This score includes 6 variables: hemoglobin (Hb), serum sodium, estimated glomerular filtration rate (eGFR; estimated by means of the Modification of Diet in Renal Disease equation, MDRD), left ventricular ejection fraction (LVEF), peak oxygen consumption (calculated as % of predicted value, based on age and gender) and the slope of the minute ventilation to carbon dioxide production ratio (VE/VCO2
34
slope). In a previous study this score had a strong association with risk of death or need for urgent heart transplantation [69].
MECKI score was calculated with the subsequent algorithm, previously reported [70]:
exp(k)/(1+exp(k)) where k=10.3464
0.0262×V̇O2peak(%pred)+0.0472×V̇E/V̇CO2slope–0.1086×Hb(g/dl)– 0.0615×Na+(mmol/L)–0.0699×LVEF(%)–0.0136×MDRD(ml/min). In Figure 8 is showed how to calculate MECKI score.
Figure 8: Software is available online to calculate MECKI score, including the six variables required.
3.2.4 Vitamin D (25OHD) measurement by Mass Spectrometry coupled to High Performances Liquid Chromatography (HPLC-MS-MS)
We used the blood samples (serum) collected at the baseline evaluation and stored at -20°C. The accurate measurement of 25-OH-vitamin D3 status was carried out by
isotope dilution Mass Spectrometry coupled to High Performances Liquid Chromatography (HPLC-MS-MS), by using the MSMS Vitamin D Kit from PerkinElmer (Waltham, MA, USA). It is well known that HPLC-MS-MS offers a good quantification accuracy and the contribution of interfering compounds to the final results is limited (as previously described).
35
Chemicals: solvents for sample preparation and HPLC elution, such as acetonitrile
(LC-MS grade), methanol (LC-MS grade), ultra-pure water (LC-MS grade), and formic acid (for mass spectrometry ≈ 98%), were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
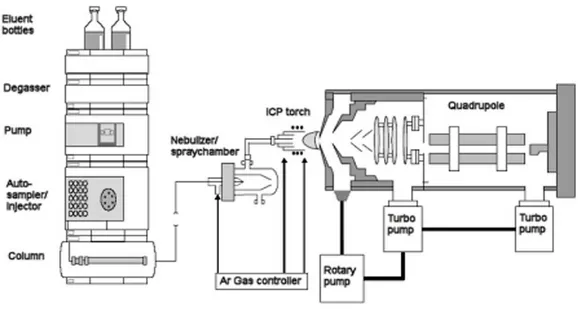
Instrumentation: the instrument consisted in an Agilent 1290 Infinity UHPLC system (Santa Clara, CA, USA), including autosampler, binary pump, and column oven, coupled to an AB Sciex API 4000 triple quadrupole mass spectrometer (Concord, ON, Canada), equipped with an APCI source. Cromatography was performed by a PerkinElmer Brownlee Supra C18 3 µm, 50 x 2.1 mm HPLC column, protected by a PerkinElmer Brownlee Supra C18 Guard Column. (Figure 9)
HPLC-MS-MS Conditions: the separation was carried out under gradient conditions by
using methanol as a solvent A, and water as a solvent B, both containing formic acid 0.1%. The chromatographic gradient program is reported in table x. The MS method, based on positive ion mode Multiple Reaction Monitoring (MRM), made use of the following quantifying transitions: 25-hydroxyvitamin D3 (25(OH)D3), 401.4→159.1; 2H
3-25-hydroxyvitamin D3 (2H3-25(OH)D3, IS), 404.4→162.1; 401.4→159.1; 2H6
-25-hydroxyvitamin D3 (2H6-25(OH)D3). Remarkable is the use of 2H6-25(OH)D3, which
simulates 25(OH)D3 in the real matrices containing this latter compound, used in the
preparation of either calibration standard solutions or quality controls (QC). Calibration curve and QCs: solutions containing 2H6-25(OH)D3 at six different
concentration levels (4.7, 9.4, 18.8, 37.5, 75, 150 ng/ml) were used as standard solutions to build the calibration curve. They were obtained by reconstituting with water the lyophilized calibrators provided with the kit and, following, by adding to a
36
100 µl aliquot of the resulting solution 200 µl of acetonitrile containing 0.1 % formic acid and a suitable amount of IS (Daily Precipitation Solution, DPS). QC were prepared as described for the calibration solutions, starting from the suitable lyophilized matters provided with the kit. In these solutions 2H6-25(OH)D3 was at
three different concentration levels (9.4, 37.5, 75 ng/ml).
Sample Preparation: plasma samples were stored at -20 °C. A 100 µl aliquot of
thawed sample was added with DPS, as for calibrators and QC. The same protocol was applied for tissue specimens.
Figure 9: HPLC-MS-MS: Liquid sample introduction is the standard in ICP-MS, therefore coupling
of liquid chromatography with ICP-MS in the simplest form is just the connection of the column outlet with the nebulizer of the sample introduction system via a transfer tubing. It is not a surprise that the hyphenated system resulting from the coupling of liquid chromatography and ICP-MS is the most often used system for speciation analysis related to the ICP-MS detection. About 3/4 of all publications related to ICP-MS hyphenated techniques for speciation analysis describe the use of LC-ICP-MS.
37
3.3 STATISTICAL ANALYSES
In this Chapter the general statistical methods suitable for all branches are described, while other specific analyses are reported in the respective Chapters, where necessary. Categorical variables were expressed as number of cases and percentages. Continuous variables were expressed as mean values +/- standard deviations or median values and interquartile range (IQR - 25° and 75° quartiles) according to whether they were normally distributed or not. Nonparametric or parametric tests were performed accordingly. Comparisons of qualitative data were performed using the chi-square test, whereas comparisons of quantitative variables among different groups were conducted by the ANOVA one-way test or the Kruskall-Wallis test, as appropriate. Moreover, the Bonferroni correction was used for multiple comparisons. Assessments of any possible differences between the different groups considered were searched using both Kruskal Wallis and Mann Whitney tests. Associations between variables were analyzed using the Pearson correlation coefficient. P values <0.05 were considered as statistically significant. Multivariate linear regression analyses were used to appropriately assess correlation between variables. The statistical package SPSS (16.0) was used for analysis.
38
4
4
.
.
R
R
E
E
T
T
R
R
O
O
S
S
P
P
E
E
C
C
T
T
I
I
V
V
E
E
S
S
T
T
U
U
D
D
Y
Y
4.1 INTRODUCTION
Chronic Heart failure (HF) is a major cause of morbidity and mortality in all industrialized countries. HF is a progressive condition and, once it has ensued, may require hospitalization because of worsening, arrhythmias or myocardial infarction occurrence. The prognosis of HF remains poor, even if the treatment of this condition has made remarkable progress in the past decades. Indeed, novel targets, biomarkers and therapies are needed [71]. Among different biomarkers proposed for cardiovascular risk evaluation in HF, Vitamin D could be an emerging tool.
Vitamin D is classically involved in bone homeostasis. However, recent studies strongly suggest other extraskeletal functions for vitamin D, including pleiotropic effects on cardiovascular system [1][2][3][4]. Twenty-five hydroxy-vitamin D (25OHD) represents the biomarker of vitamin D circulating levels and it’s recommended for reliable measurement of vitamin D status. In 2011 the Endocrine Society provided a clinical practice guideline for evaluation, treatment, and prevention of Vitamin D deficiency, suggesting to define vitamin D deficiency as 25OHD below 20 ng/ml (50 nmol/l) and vitamin D insufficiency as a 25(OH) D of 21–29 ng/ml (52.5– 72.5 nmol/liter) [6]. However, it is still controversial which is the optimal 25OHD level in the adults, to exert the extraskeletal effects [5, 7, 72].
There are evidences that low serum 25OHD levels are associated with increased risk of cardiovascular disease (CVD) [8] including hypertension, coronary artery disease, ischemic heart disease, stroke and type 2 diabetes [9][10][11][12][13]. Data from the Third National Health and Nutrition Examination Survey (NHANES 1988–1994)
39
showed a strong and independent association between 25OHD deficiency and the prevalence of CVD in a large poplation [14]
Moreover, there are data supporting the hypothesis that vitamin D could exerts some modulatory effects in the pathophysiology of HF, namely downregulation of the renin–angiotensin system, enhancement of insulin secretion and sensitivity, protection against angiogenesis and modulation of inflammatory processes. [1][2][3].
Growing data about the condition of poor vitamin D status in patients with HF have been recently published, with main limitations due to 25OHD levels evaluation or a variety of approaches, difficult to be compared. Indeed, in the majority of studies, Vitamin D levels were measured with traditional immuasnoassay, that often show an unsatisfactory accuracy and a remarkable intra- and inter-assay variability around the cut off limits. Higher specificity and sensitivity can be obtained by High Performances Liquid Chromatography coupled to mass spectrometry (HPLC-MS/MS), that is frequently referred as the new “gold standard” method [15][16][17].
Previous studies investigated the possible relation between serum 25OHD levels and HF markers with various end-points: echocardiographic parameters, inflammatory markers, neurohormones, supporting the hypothesis that vitamin D may have a role in HF pathophysiology and outcome. However, data regarding vitamin D and HF functional capacity and prognosis markers are still scanty.
Aim of the present study was to investigated the relationship between 25OHD levels, measured using HPLC-MS/MS, and functional parameters derived from the cardiopulmonary test (CPET), that is a good, routinely used tool of risk stratification in HF patients [73][74][75]. Furthermore, we analysed the prognostic value of serum
40
25OHD levels, using an emerging score of mortality risk for HF: the Metabolic Exercise Cardiac Kidney Index or MECKI score[76][70].
4.2 MATERIALS AND METHODS
4.2.1. Patients and study design: From January 2012, up to October 2014 we
enrolled 261 consecutive patients with systolic dysfunction (left ventricular ejection fraction, LVEF <50%), referred to the Division of Cardiovascular Medicine of the Fondazione G. Monasterio in Pisa for HF management. The diagnosis of HF was based upon history, symptoms, physical and instrumental findings for the assessment of structural myocardial involvement. Cardiac morphology and function were assessed by two-dimensional Doppler echocardiography.
All patients fulfilled the European Society of Cardiology (ESC) criteria for the diagnosis of HF. All patients underwent a comprehensive clinical and biochemical characterization, including haemoglobin, creatinine and glomerular filtration (eGFR calculated using the MDRD formula), serum electrolytes, PRA, aldosterone, catecholamine, N-terminalproBrain natriuretic peptide (NT-proBNP levels, albumin adjusted serum calcium, Parathormone (PTH). All parameters were measured with standard methods. Glomerular filtration rate was calculated as MDRD using the following formula: 186.3*(crea)-1.154*(Age)-0.203*0.75 for women.
All patients underwent echocardiographic and CPET measurements. Left Ventricular (LF) volumes and ejection fraction (EF) were measured on echocardiography according to standard criteria. CPET was performed using an electronically braked cycle-ergometer by a ramp protocol with increments of 10 W/min. We measured VO2, CO2 production, and minute ventilation using breath-to-breath gas analysis (Vmax,
41
Sensormedics, Conshohocken, Pennsylvania). Peak VO2 (the highest value at end-exercise, as a 20-s average expressed as percentage of predicted value (VO2peak% or normalized by body weight VO2/kg) and ventilatory efficiency on exercise (slope of the ventilation vs. VCO2 relation in its linear part) were determined. MECKI score was calculated with the subsequent algorithm, previously reported [70]:
exp(k)/(1+exp(k)) where k=10.3464
0.0262×V̇O2peak(%pred)+0.0472×V̇E/V̇CO2slope–0.1086×Hb(g/dl)– 0.0615×Na+(mmol/L)–0.0699×LVEF(%)–0.0136×MDRD(ml/min).
4.2.2. Twenty-five hydroxyvitamin D (25OHD) levels: Blood samples were
collected at the baseline evaluation and serum was stored at -80°C until use. 25-OH-vitamin D3 status was measured by isotope dilution (HPLC-MS/MS), by using the MSMS Vitamin D Kit from PerkinElmer (Waltham, MA, USA).
HPLC-MS/MS details: We used Agilent 1290 Infinity UHPLC system (Santa Clara, CA, USA), including autosampler, binary pump, and column oven, coupled to an AB Sciex API 4000 triple quadrupole mass spectrometer (Concord, ON, Canada), equipped with an APCI source. Cromatography was performed by a PerkinElmer Brownlee Supra C18 3 µm, 50 x 2.1 mm HPLC column, protected by a PerkinElmer Brownlee Supra C18 Guard Column.
4.2.3. Statistical analysis
Categorical variables were expressed as number of cases and percentages. Continuous variables were expressed as mean values +/- standard deviations or median values and interquartile range (IQR - 25° and 75° quartiles) according to whether they were normally distributed or not. Nonparametric or parametric tests were performed
42
accordingly. Comparisons of qualitative data were performed using the chi-square test, whereas comparisons of quantitative variables among different groups were conducted by the ANOVA one-way test or the Kruskall-Wallis test, as appropriate. Moreover, the Bonferroni correction was used for multiple comparisons. Assessments of any possible differences between the different groups considered were searched using both Kruskal Wallis and Mann Whitney tests. Associations between serum 25OH D levels and VO2 peak and MECKI score were analyzed using the Pearson correlation coefficient. P values <0.05 were considered as statistically significant.
Multivariate linear regression analyses, adjusted for age, BMI, MDRD, NT-proBNP, EF, gender or age, BMI, NT-proBNP, EF, gender was used to test if VO2 peak or MECKI score, respectively, were independently related to serum 25OHD levels, expressed as natural logarithm (ln25OHD). The statistical package SPSS (16.0) was used for analysis.
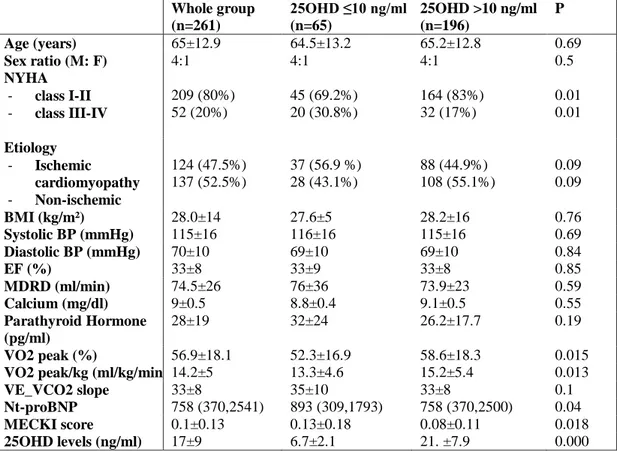
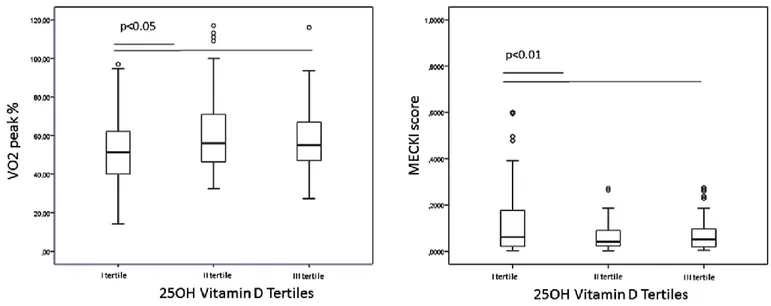
4.3 RESULTS
The study group included 214 males and 47 females (M:F ratio =4:1), with a mean age of 65±12 years and mean BMI of 28±14. Patients were all Caucasian. All patients had stable HF disease, mostly in the NYHA II class (57.5%, n=150). The HF etiologies were ischemic cardiomyopathy in 124 (47.5%) and non-ischemic cardiomyopathy in 137 (52.5%). Mean EF was 33±8%; no patients had severe kidney failure (MDRD <15 ml/min) and/or secondary hyperparathyroidism, with a mean PTH 22.6 (15.8-34.1) pg/ml and albumin-adjusted total serum calcium 9.0±0.5 mg/dl. The clinical, biochemical and instrumental parameters of the whole group of patients are shown in Table 1.