www.journalpulmonology.org
ORIGINAL
ARTICLE
Pirfenidone
and
Nintedanib
in
idiopathic
pulmonary
fibrosis:
Real-life
experience
in
an
Italian
referral
centre
E.
Bargagli
a,∗,
C.
Piccioli
a,
E.
Rosi
a,
E.
Torricelli
a,
L.
Turi
a,
E.
Piccioli
a,
M.
Pistolesi
a,
K.
Ferrari
a,
L.
Voltolini
baDepartmentofClinicalandExperimentalMedicine,UniversityHospitalCareggi,LargoBrambilla1,50134Florence,Italy bThoracicSurgeryUnit,CareggiUniversityHospital,LargoBrambilla1,50134Florence,Italy
Received17January2018;accepted3June2018
KEYWORDS Idiopathicpulmonary fibrosis; Pirfenidone; Nintedanib Abstract
Background: Idiopathicpulmonaryfibrosishasamediansurvivaltimeafterdiagnosis of2---5 years.ThemaingoaloftreatingIPFistostabilizeorreducetherateofdiseaseprogression. NintedanibandPirfenidonehavebeenabreakthroughinthemanagementofIPF.Herewe eval-uatedtheeffectivenessofPirfenidoneandNintedanibinapopulationofIPFpatientsdiagnosed inthelast12monthsatFlorenceILDReferralCentre.
Methods:In thelast12months,82IPFpatients(66male,meanage78.3±23.8years)were diagnosedandstartedantifibrotictherapywithPirfenidoneorNintedanib.Theirclinicaland functionaldetailswereanalyzedretrospectivelyattime0andafter6and12monthsoftherapy. Results:ThemedianageofthepatientstreatedwithNintedanibwashigherthanthatofthe Pirfenidonegroup(p<0.0001).Themostcommonsymptomsatdiseaseonsetwereexertional dyspnoeaanddrycoughwithnodifferencesbetweenthetwogroups(p<0.05).AllIPFpatients manifestedbibasalcracklesatthetimeofdiagnosis.NosignificantdifferencesinFVC,FEV1, TLCandDLCOwerefoundattime0orafter6monthsbetweenpatientstreatedwithPirfenidone andNintedanib(p>0.05).After1year,lungfunctiontestparametersofpatientstreatedwith Pirfenidonehadremainedstablefrombaseline.
Discussion: Thisstudyemphasizesthatbothantifibroticdrugsappearedtobeagood therapeu-ticchoiceintermsoffunctionalstabilization,alsoinolderpatients.
PublishedbyElsevierEspaña,S.L.U.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abbreviations: HRCT,highresolutioncomputedtomography;ILD,interstitiallungdiseases;LFT,lungfunctiontests.
∗Correspondingauthor.
E-mailaddress:[email protected](E.Bargagli).
https://doi.org/10.1016/j.pulmoe.2018.06.003
2531-0437/Published by Elsevier España, S.L.U. This is an open access article under the CC BY-NC-ND license
Introduction
Idiopathicpulmonaryfibrosis (IPF)is achronicprogressive interstitiallungdisease(ILD)ofunknownaetiology,limited tothelungs,andcharacterizedbyausualinterstitial pneu-monia (UIP) histopathological pattern.1,2 IPF accounts for approximately55%ofallidiopathicinterstitialpneumonias (IIP)and25%ofallinterstitiallungdiseases(ILD)withabout 35,000newdiagnosesperyearinEuropeand5millioninthe world.3Ithasanannualincidenceof0.22---7.4per100,000 personsandaprevalenceof1.25---23.4casesper100,000of populationandismorefrequentinpatientsbetween65and 79yearsofage.3Itisadisablingandfataldiseasewithapoor prognosis.1---11 Five-year survivalis 20---40%, which islower than for breast cancer, colorectal cancer and pulmonary arterialhypertension.1---5 Because thereis noknown cure, thegoalofIPFtreatmentistostabilizeorreducetherateof diseaseprogression.6TwotherapiesforIPF,Nintedaniband Pirfenidone,wererecentlyapprovedbyEMArespectivelyin ... andFDA4yearsago.Thenewdrugshavebeena break-throughinthemanagementofIPF.6Theworld-widephaseIII clinicaltrialsASCEND,CAPACITY004and006demonstrated forthefirsttimethatPirfenidonereducedIPF progression andbrought long-term benefits tothese patients.6---10 The ASCENDtrialassessedPirfenidoneefficacyafter52weeks, whiletheCAPACITYstudydeterminedtheeffectsofthedrug overanobservationperiodof72weeks.8Thesetrialsfound thatPirfenidonetreatmentsignificantlyreducedthedecline inforced vitalcapacity, improving the 6-minwalking test distance(6MWD)by50m.7Inaddition,progression-free sur-vival analysisshowed that the risk of IPF progressionfell by38%inthePirfenidonegroupcomparedtothe placebo-group.The riskofIPFmortalityduringtreatmentdeclined by60% in thegroup of patients treatedwith Pirfenidone, comparedtotheplacebo group,while the risk of mortal-ity for all causes fell by 37%.10 Likewise, phase II and III clinicaltrialsrevealed thatNintedanibslowed progression ofthedisease,reducing thedeclinein FVC,12,13 whilethe TOMORROW study showed a 68% reduction in annual FVC declinerate for Nintedanib-treatedpatients compared to a placebo group and a reduction in the number of acute exacerbationsin treatedcomparedtountreatedpatients. The INPULSIS I and II studies consistently demonstrated theefficacy ofNintedanibin reducingdisease progression with a significant slowing down in the annual decline in FVC.12,13 The Italian Medicines Agency AIFA approved Pir-fenidone for use in Italy in June 2013, while Nintedanib was authorized in April 2016. Regional Referral Centres for ILD can prescribe these therapies for patients with IPF.
Inthe presentstudy we evaluatedtheeffectivenessof PirfenidoneandNintedanibinapopulationofpatients fol-lowedat ourhospitallastyear.Theclinical andfunctional characteristicsof IPF patientstreated withPirfenidoneor Nintedanibwererecordedatthestart(time0)andafter6 and12monthsoftreatmenttodefine theeffectivenessof antifibrotictherapyandcompareitwithdatainthe litera-ture.
Population
and
methods
In the last 12 months, 82 IPF patients (66 male, mean age 78.3±23.8 years) have been diagnosed with intersti-tiallungdiseases(ILD)atAOUCCareggiReferralCentreand startedantifibrotictherapywithPirfenidoneorNintedanib. The clinical and functional details were analyzed ret-rospectively from demographic data, family history for pulmonary fibrosis, smoking, occupational/environmental exposureandcomorbidities.Thefollowingparameterswere recorded:age,sex,BALdifferentialcellcount,BAL lympho-cyte phenotype, lung function tests, oxygen desaturation during 6min walking test, and PaO2 values measured by
blood gas analysis. All theseparameters were entered in adatabasetogetherwithfunctional,radiological, histologi-calandimmunologicaldata.Lungfunctiontestparameters wereperformedaccordingtoATS/ERSguidelinescollecting percentpredictedFEV1,FVC,TLCandDLCO.14 IPF diagno-siswasbasedonclinical radiological parametersin 70/82 IPFpatients.ChestX-raysintheposterior-anteriorand lat-eralprojectionsandhigh-resolutioncomputedtomography ofthechestweredoneinallpatients.Diagnosiswas formu-latedinthecontextofamultidisciplinarymeeting.
Histological diagnosis of IPF/UIP was obtained in 12 patients with HRCT signs of IPF with no clear radiologi-calhoneycombing;fourpatientsunderwenttransbronchial lung cryobiopsy,asinglepatientunderwentnon-intubated thoracic surgeryperformed under spontaneousventilation (AWAKEVATS)andsevenpatientsaVATSlungbiopsy.
Inasubgroupofpatientsbronchoscopywith bronchoalve-olarlavagewasperformedwithinformedconsentofpatients inordertoexcludeotherinterstitiallungdiseases.Briefly, samples were obtained by instillation of four aliquots of 50mlsalinesolutionbyvideofiberopticbronchoscope.Each aliquot was immediately gently aspirated. The first BAL sample was kept separate from the others and was not usedforimmunologicaltests.Sub-sampleswereculturedfor microbes,fungiandvirusestoexcludeinfections.Cellswere separated bycentrifuge and thefluid fractionwasfrozen forenzymeassays.Differentialcellcountsweredone. Lym-phocyte phenotypewasanalyzed by flow cytometryusing anti-CD3,CD4andCD8monoclonalantibodies.
Thepatientsincludedinthestudyhadnotbeentreated withsteroids or otherimmunosuppressants at the timeof BAL. They were monitored every 3 monthsfrom onsetat ourregionalinterstitiallungdiseasereferralcentre.Allgave writteninformedconsenttoparticipationinthestudy.
Patients with IPF were treated with Pirfenidone or Nintedanib according to Italian national drug inclu-sion/exclusioncriteria.NintedanibinclusioncriteriainItaly were age ≥40 years, diagnosis of idiopathic pulmonary fibrosis accordingtointernational guidelines,FVC>50% of predictedandDLCO>30%.ExclusioncriteriaforNintedanib treatment in Italy were ALT,AST >1.5× ULN,total biliru-bin>1.5×ULN,highriskofbleeding,INR>2,PT,PTT>150% of ULN, majorsurgeryscheduled in the next3 monthsor highriskofthrombosis.PirfenidoneinclusioncriteriainItaly were: age 40---80 years,IPF diagnosed in past 48 months,
Table1 ClinicalfeaturesofIPFpatientstreatedwith Pir-fenidone(n=52)orNintedanib(n=30). Pirfenidone (n=52) Nintedanib (n=30) Age(M±SD) 71.0±6.1 78.3±5.5** Male 81%(42/52) 80%(24/30) Dyspnoea 81%(42/52) 85%(25/30) Bibasalcrackles 100% 100% Drycough 83.9±32.6 85.8±37.2 BalTotcells(×104) 12.5±11.8 13.6±12.9 Macrophages(%) 69.1±29.3 68±31.2 Lymphocytes(%) 18.3±10.5 17.4±11.1 Neutrophils(%) 8.5±7.4 8.5±8 Eosinophils(%) 5.9±4.8 6.2±4.6 PaO2(mmHg) 70.6±38.1 68.4±49.9 PaCO2(mmHg) 29.3±11 28.6±18.7 Oxygendesaturation6MWT 55%(29/52) 63%(19/30)
Nosignificantdifferenceswerefoundbetweenthetwogroups,
exceptthatmeanagewassignificantlyhigherintheNintedanib
thanthePirfenidonegroup(**p<0.001).
BAL=bronchoalveolar lavage, M=macrophages,
L=lymphocytes,N=neutrophils,E=eosinophils.Theresultsare
reportedasmedium±standarddeviation.
FVC≥50%oftheoreticalvalueandDLCO≥35%ofpredicted value. Exclusion criteria for Pirfenidone treatment were hypersensitivity to the active substance or to any of the excipients,severeliverfunctionorliverdiseaseinterminal stage, severe renal impairment (CrCl <30ml/min) or kid-ney diseaseinterminal stagerequiring dialysis.When the patientsfulfilledcriteriaforuseofbothdrugs(Nintedanib andPirfenidone)thefinaldecisionwasbasedon contraindi-cationsand patientchoice when facedwiththe different side-effectprofiles.Arelevantaspectwasthedosage (Pir-fenidoneisadministeredasthreecapsulestakenthreetimes aday,whereasNintedanibonlyonecapsuletwiceaday).
Alltheclinical,radiological,immunologicalparameters were entered in a database and updated every 3 months duringclinicalassessment.
Statisticalanalysis
Alldatawasexpressedasmean±standard deviation.The WilcoxontestwasusedtodescribethetrendofPFRpatients inthetwotreatmentgroups.Analysisofvariance(with Bon-ferronitestforinternalcomparisons)wasusedtocompare groups.Statisticalsignificancewassetatp<0.05. Statisti-calanalysiswascarriedoutusingStatSoft(2001)andGraph PadPrismsoftware.
Results
Table1showstheclinicalcharacteristicsof82IPFpatients enrolledinthisstudyinthelast12months:52patientswere treatedwithPirfenidoneand30patientswithNintedanib. The mean age of those treated with Pirfenidone was 71.04±6.1 years compared to 78.33±5.5 years of those treatedwithNintedanib.Themedianageofpatientstreated withNintedanibwassignificantlyhigherthanthatofpatients
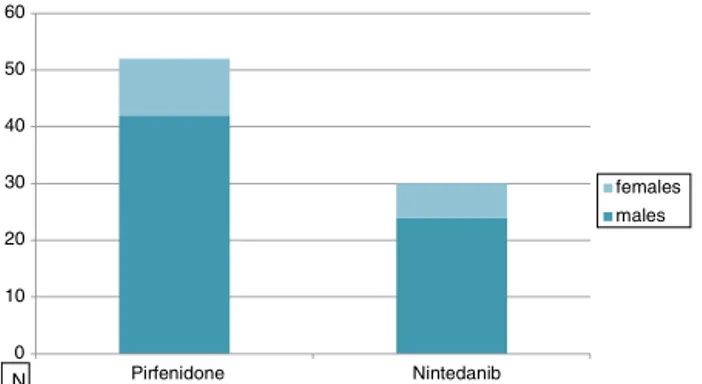
0 10 20 30 40 50 60 Pirfenidone Nintedanib females males N
Figure1 MalesandfemalesinthepopulationsofPirfenidone (81%)versusNintedanib(80%). 83 78 51 68 88 83 50 71 86 81 70 46 0 10 20 30 40 50 60 70 80 90 100 FEV1% FVC% TLC% DLCO% T0 6months 12 months
Figure2 AveragevaluesofFVC, FEV1, TLC andDLCO per-centagesofpredictedattime0(n=52),after6months(n=35) and12 months (n=25) inpatients treatedwith Pirfenidone. Nostatisticallysignificantdifferencesamongthegroupswere observed(p>0.05).
intheothergroup(p<0.0001)duetothePirfenidonegroup inclusioncriteria(inItalyonlypatientsunder80yearsold canbe treated withthis drug while there is noage limit forNintedanib).Asexpected,therewasaclearprevalence of males in our IPF population (80%) with no difference betweengroups (Fig. 1).Smokinghistory revealed 74% of smokersinthePirfenidonegroupand52%intheNintedanib group.Table1alsoshowsthatthemostcommonsymptoms atdiseaseonsetwereexertionaldyspnoeaanddry cough, withnodifferencebetweengroups.Allpatientsmanifested bibasalcracklesduringphysical examination,andclubbing wasevidentin35%ofcases(Table1).
Fig. 2 indicates lung function test parameters at time 0 and after 6 and 12 months in the population of IPF patients treated with Pirfenidone. At time 0, FVC, FEV1, DLCO and TLC percentages were 78.7±16.9% predicted values,83.3±16.5%predicted,51.2±15.3%predictedand 68.7±14.7%predicted,respectively.
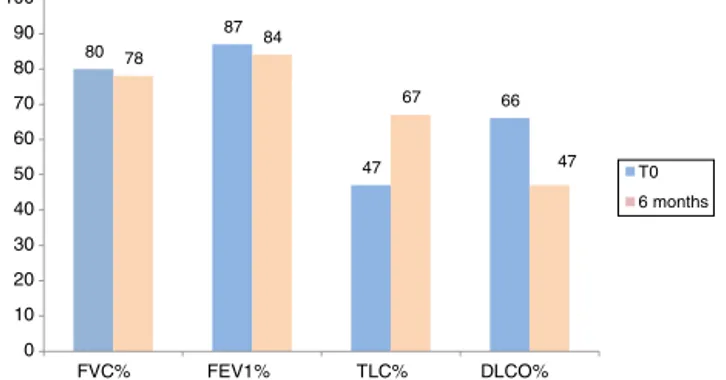
Fig. 3 shows lung function test parameters at time 0 and after 6 and 12 months in the population of IPF patients treated with Nintedanib. At time 0, FVC, FEV1, DLCO and TLC percentages were 80.9±19.6% predicted, 87±19%predicted,47±15%predictedand66.3±13% pre-dicted,respectively.NosignificantdifferencesinFVC,FEV1, TLCandDLCOpercentages werefound attime0between patients treated with the two drugs (p=0.59, p=0.37, p=0.21,p=0.48,respectively).
80 87 47 66 78 84 67 47 0 10 20 30 40 50 60 70 80 90 100 FVC% FEV1% TLC% DLCO% T0 6 months
Figure3 Averagevalues ofFVC, FEV1, TLC andDLCO per-centage predictedat time 0(n=30),after 6 months (n=16) inpatients treatedwithNintedanib.No statisticalsignificant differencesamongthesegroupswereobserved(p>0.05).
At 6-month follow-up, FVC, FEV1, DLCO and TLC percentages of predicted values were 83±17.3% pre-dicted,88.6±18.3%predicted,50.7±13.3%predictedand 71.3±15% predicted, respectively, in patients treated with Pirfenidone, and 78.3±18% predicted, 84.3±17.8% predicted, 47.6±15.3% predicted and 67.15±16% pre-dicted, respectively, in patients treated with Nintedanib (Figs.2 and 3).Interestingly, nosignificant differences in FVC,FEV1,DLCOor TLC%ofpredictedvalueswerefound betweenpatientstreatedwithPirfenidoneandNintedanib at 6-month follow-up(p=0.54, p=0.38, p=0.76,p=0.31, respectively).
OnlyIPF patients treatedwithPirfenidonereached 12-monthfollow-upbecauseNintedanibwasapprovedinItaly morerecentlyandonlyalimitednumberofpatientstreated withthis drughad completed 1 year of treatment at the timeofwriting.At12-monthfollow-up,FVC,FEV1,TLCand DLCO % of predicted values of IPF patients treated with Pirfenidonewere81.2±20.7%predicted,86.7±19.7% pre-dicted,70.2±19.2%predicted and 46.7±9.8% predicted, respectively. No statistical difference in any lung func-tion test parameter wasobserved between time 0 and 6 or 12 months in the Pirfenidone therapy group (p>0.05) (Figs.2and3).
Pirfenidone showed a good tolerability profile in the majorityof IPFpatients. Itwasmanageableandthemost frequentsideeffectsincludedgastrointestinaldisordersand skinreactions.Inourpopulationdyspepsiaandnauseawere themostfrequentadverse eventoccurringin35%ofcases followedbydiarrhea(28%ofpatients)andcutaneousrash (19%ofcases).Theuseofprokineticdrugsandproton-pump inhibitorsallowed toimprovegastrointestinal sideeffects inthemajority ofpatients aswellastheapplication ofa broad-spectrum sunscreen. Inthreepatients therush and thephotosensitivityweresevereconsequenttothe persis-tentsunexposureforworkreasons.
Nintedanib therapy was well tolerated. The most fre-quentsideeffectwasdiarroheaoccurringin40%ofpatients andrequiringin18%ofcasesaprematurediscontinuation. Treatmentinterruptionwasnecessaryonlyin5%ofpatients, theothersubjectsimprovedafterdosereductionfrom150 to 100mg twice a day. 8% of patients treated with this drugpresentedalteredliverenzymes,reversibleafterdose reduction.Infourpatientstheelevationofaminotransferase
concentrationstendedtopersistaftersixmonthsof treat-ment and liver alteration were not reversible with dose modification anddiscontinuation.Boththesepatients had apersistingmildliverdysfunctionsecondarytolithiasis.
Discussion
SafetyandefficacyoutcomesofPirfenidoneandNintedanib treatment in patients with IPF are available from many international clinical trials.7,10---13 Very little data is cur-rentlyavailableaboutreal-lifeexperiencewithantifibrotic drugsinIPFpatients.15---17 Thepresent studyevaluatedthe effectiveness of Pirfenidone and Nintedanib therapy in a populationofIPF patientsdiagnosedinthelast12months at Florence ILD Referral Centre. This observational study offersinformationondrugeffectivenessintherealclinical settingofauniversityhospital,butcanonlypartlybe com-paredwithinternationalclinicaltrialsandit hasthelimit thatdidnotshow12monthsfollow-upforpatientstreated withNintedanib.Asexpected,therewasahighprevalence of male over female patients: while ILDs associated with connectivetissuedisordersareprevalentinwomen,IPF is typical of males over 65 years of age.18 The patients in theNintedanibsubgroupwereolder thanthoseinthe Pir-fenidonegroup,becauseinItalyIPFpatientsover80years oldcanonly betreatedwithNintedanibandnotwith Pir-fenidone.
Inlinewiththeliterature,allpatients(100%)presented bibasalcracklesandrestrictivefunctionaldeficitwitha sig-nificantreductioninDLCOatdiseaseonset.19,20At6-month follow-upbothdrugsappeared tostabilizethespirometric profileof adiseasetypicallyassociatedwithdeterioration of lung function in the period of a few months. In the Pirfenidone group,stabilization wasalsoconfirmed at 12-monthfollow-up,inlinewithreportsontheliterature.Inour real-experience study, irrespective of baseline functional status,treatment withthe twodrugs wasassociatedwith functional benefits thatwere similartothosereported in clinicaltrials9andwithsimilarratesofFVCdecline. Stabi-lization of lung function parameterswasalso observed in theolderpopulationofpatientstreatedwithNintedanib.
This preliminary study assessing the therapeutic bene-fit of Pirfenidone and Nintedanib in an Italian cohort of IPF patients revealed a stabilization of lung functiontest parameters.Theantifibroticdrugsalsoappearedtoprovide functionalstabilizationinolderIPF/UIPpatients.
Compliance
with
ethical
standards
Thisstudywasunfunded.Noneoftheauthorshaveaconflict ofinteresttodeclare.
Ethicalapproval:Allprocedureswereinaccordancewith the ethical standards of the institutional and/or national researchcommitteeandwiththe1964Helsinkideclaration anditslateramendmentsorcomparableethicalstandards. Thisarticledoesnotcontainanystudieswithanimals per-formedbyanyoftheauthors.
Informedconsent: Informedconsentwasobtainedfrom allindividualparticipantsincludedinthestudy.
Conflict
of
interest
Theauthorshavenoconflictsofinteresttodeclare.
Appendix
A.
Supplementary
data
Supplementary data associated with this arti-cle can be found, in the online version, at doi:10.1016/j.pulmoe.2018.06.003.
References
1.CottinV,CapronF,GrenierP,CordierJF.Diffuseidiopathic inter-stitial pneumonias, International multidisciplinary consensus classificationbytheAmericanThoracicSocietyandthe Euro-peanRespiratorySociety,principalclinico-pathologicalentities, anddiagnosis.RevMalRespir.2004;212Pt1:299---318.
2.RaghuG,CollardHR,EganJJ,MartinezFJ,BehrJ,BrownKK, etal.AnofficialATS/ERS/JRS/ALATstatement:idiopathic pul-monaryfibrosis: evidence-based guidelines for diagnosis and management.AmJRespirCritCareMed.2011;183:788---824.
3.NalysnykL, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalenceofidiopathicpulmonaryfibrosis:reviewofthe lit-erature.EurRespirRev.2012;21:355---61.
4.RicheldiL,KreuterM,SelmanM,CrestaniB,KirstenAM,Wuyts WA,etal.Long-termtreatmentofpatientswithidiopathic pul-monaryfibrosiswithNintedanib:resultsfromtheTOMORROW trialanditsopen-labelextension.Thorax.2017[inpress].
5.KingTEJr,BradfordWZ,Castro-BernardiniS,FaganEA,Glaspole I, Glassberg MK, et al. A phase 3 trial of Pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083---92.
6.NathanSD.Evaluatingnewtreatmentoptions.AmJManagCare. 2017;2311Suppl:S183---90.
7.TaniguchiH, EbinaM,Kondoh Y, OguraT, Azuma A, SugaM, etal.Pirfenidoneinidiopathicpulmonaryfibrosis.EurRespirJ. 2010;35:821---9.
8.NoblePW,AlberaC,BradfordWZ,CostabelU,GlassbergMK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonaryfibrosis(CAPACITY):tworandomisedtrials.Lancet. 2011;377:1760---9.
9.Robalo-CordeiroC,CamposP,CarvalhoL,BorbaA,Clemente S,FreitasS,etal.Idiopathicpulmonaryfibrosisintheeraof
antifibrotictherapy:searchingfornewopportunitiesgrounded inevidence.RevPortPneumol.2017;23:287---93.
10.AzumaA,NukiwaT,TsuboiE,SugaM,AbeS,NakataK,etal. Double-blind,placebo-controlledtrialofPirfenidoneinpatients withidiopathicpulmonaryfibrosis.AmJRespirCritCareMed. 2005;171:1040---7.
11.CottinV.‘Nintedanib:anewtreatmentforidiopathicpulmonary fibrosis.ClinInvest.2015;5:621---32.
12.RicheldiL,CostabelU,SelmanM,KimDS,HansellDM,Nicholson AG,etal.Efficacyofatyrosinekinaseinhibitorinidiopathic pulmonaryfibrosis.NEnglJMed.2011;365:1079---87.
13.RicheldiL,duBoisRM,RaghuG,AzumaA,BrownKK,CostabelU, etal.EfficacyandsafetyofNintedanibinidiopathicpulmonary fibrosis’.NEnglJMed.2014;370:2071---82.
14.Brusasco V, Crapo R, Viegi G, American Thoracic Society; European RespiratorySociety.Coming together: theATS/ERS consensusonclinicalpulmonaryfunctiontesting.EurRespirJ. 2005;26:1---2.
15.HarariS,CaminatiA,AlberaC,VancheriC,PolettiV,PesciA, etal.EfficacyofPirfenidoneforidiopathicpulmonaryfibrosis: anItalianreallifestudy.RespirMed.2015;109:904---13.
16.HughesG,ToellnerH,MorrisH,LeonardC,ChaudhuriN.Real
world experiences: Pirfenidone and Nintedanib are effective
andwelltoleratedtreatmentsforidiopathicpulmonaryfibrosis.
JClinMed.2016;5,http://dx.doi.org/10.3390/jcm5090078.
17.Aiello M, Bertorelli G, Bocchino M, Chetta A,
Fiore-Donati A, Fois A, et al. The earlier, the better: impact
of early diagnosis on clinical outcome in idiopathic
pul-monary fibrosis. Pulm Pharmacol Ther. 2017;44:7---15,
http://dx.doi.org/10.1016/j.pupt.2017.02.005.
18.Carleo A, Bargagli E, Landi C, Bennett D, Bianchi L,
Gagliardi A, et al. Comparative proteomic analysis of
bronchoalveolar lavage of familial and sporadic cases of
idiopathic pulmonary fibrosis. JBreath Res. 2016;10:026007,
http://dx.doi.org/10.1088/1752-7155/10/2/026007.
19.Purokivi M, Hodgson U, Myllärniemi M, Salomaa ER,
Kaar-teenaho R. Are physicians in primary health care able to
recognizepulmonaryfibrosis?EurClinRespirJ.2017;4:1290339,
http://dx.doi.org/10.1080/20018525.2017.1290339.
20.Sellarés J, Hernández-González F, Lucena CM, Paradela
M, Brito-Zerón P, Prieto-González S, et al. Auscultation
of Velcro crackles is associated with usual
intersti-tial pneumonia. Medicine (Baltimore). 2016;95:e2573,