CHAPTER 6
PALAEOECOLOGY
6.1. INTRODUCTION
Sirenians are the only herbivorous marine mammals now living, and the only herbivorous mammals ever to have become totally aquatic. They are today only represented by four species, which have the crucial ecological role of principal seagrass consumers in tropical and subtropical shallow waters: the only species of Dugongidae (Dugong dugon) is strictly marine, lives in the Indian Ocean and in the southwestern Pacific Ocean and is a specialized hypergrazer feeding almost exclusively on seagrass leaves and rhizomes; while the three species of Trichechidae (Trichechus senegalensis,
T. inunguis and T. manatus) live in fresh and coastal waters in the Atlantic region and
are mixed-feeders consuming over 60 species of aquatic and terrestrial plants including seagrasses and true grasses (Gramineae) (Domning, 2001a; Werth, 2000)
Sirenians are considered to have been herbivores since their origin, and to have depended on seagrasses and other aquatic plants for food. The strong relationship between sirenians and seagrasses is well documented in the fossil record, and sirenians appear to have had the crucial ecological role of principal seagrass consumers in the past as well. This condition determined their tropical and subtropical distribution in fresh and coastal waters where these plants lived (Domning, 1981; Domning, 2001a; Clementz et al., 2006). The only exception seems to be represented by the Hydrodamalinae, an endemic northern Pacific subfamily including the Steller’s sea cow (Hydrodamalis gigas), which became extinct in 1768 and was secondarily adapted to eat algae in colder waters (Domning, 1978).
Domning (2001a) studied the sirenian palaeoecology of the Cenozoic Western Atlantic-Caribbean. He defined the aquatic megaherbivore adaptive zone; classified the seagrasses on the basis of the diametre of their rhizomes (small: <2 mm; medium: 2-3 mm; large: 3-10 mm; very large: >15 mm); and proposed morphological indicators of feeding-niche partitioning among fossil sirenians: width of the rostrum and of the mandibular symphysis; degree of rostral deflection; size and morphology of tusks; and body size. Finally, he presented a list of Western Atlantic-Caribbean fossil and living sirenians and showed what suites of species have occurred sympatrically. Domning, through this study, followed the history of individual feeding niches within the sirenian
guild through geological time, and, in turn, reconstructed gross aspects of Caribbean seagrass communities and their changes through time.
These palaeoecological hypotheses based on morphological and biostratigraphic data have been tested and other palaeoecological aspects have been reconstructed by analyses of carbon and oxygen stable-isotope composition of tooth enamel bioapatite, considered as a proxy for reconstructing respectively dietary and habitat preferences (MacFadden et al., 2004).
In this chapter I follow the criteria proposed by Domning (2001a) and by MacFadden et al. (2004) in order to propose a reconstruction of Euro-North African sirenian palaeoecology during the Neogene in relation to the seagrass communities and to the palaeoclimatic and geological evolution of the region. This aim is achieved by combining data from:
1. Seagrass evolution and ecology. 2. Sirenian distribution and morphology.
3. Stable isotope analyses of sirenian tooth enamel.
6.2 SEAGRASSES
6.2.1. GENERALITIES
Seagrasses are the only angiosperms that have successfully invaded the marine environment. They are characterized by ability to grow whilst completely submerged; adaptation to survive in high, and often varying, salinity; an anchoring system to withstand water movements; a submarine pollination mechanism; and ability to compete with other species in the marine environment (Spalding et al., 2003). They form an ecological, polyphyletic group and not a single monophyletic group. The taxa regarded as seagrasses belong to the subclass Alismatidae (Monocotyledonae) and are constituted by all the species belonging to the families Zosteraceae, Cymodoceaceae and Posidoniaceae, and by three genera of the family Hydrocharitaceae (Hartog & Kuo, 2006). Moreover also Ruppia tuberosa (Ruppiaceae) and Lepilaena marina (Zannichelliaceae) occur exclusively in marine habitats and are regarded as seagrasses by Hartog and Kuo (2006); while the other 14 genera of Hydrocharitaceae are confined to fresh-water habitats (Hartog & Kuo, 2006).
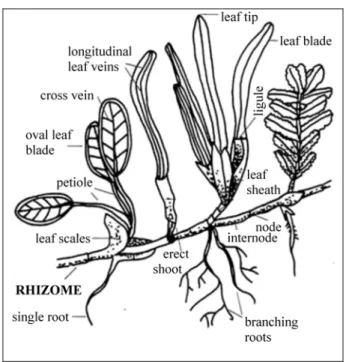
Seagrasses generally are mostly small plants with a similar external morphology, consisting of a well-developed creeping rhizome bearing at each node one or more branched or unbranched adventitious roots and a short, erect shoot bearing several
foliage leaves, each with a sheath at the base (Fig. 1). There are exceptions: Halophila has each shoot carrying a pair of petiolate leaves which do not have sheaths; Amphibolis and Thalassodendron have distinct erect stems carrying foliage leaves (Kuo & McComb, 1989).
Fig. 1: Morphological features of seagrasses.
The rhizomes store nutrients in the form of carbohydrates and typically comprise half or more of the plant biomass in a seagrass bed. For this reason, the rhizomes tend to assume special importance in the diets of any sirenian that can excavate and consume them (e.g. Erftemeijer et al., 1993). The rhizomes are usually herbaceous, cylindrical to laterally compressed, and monopodially or irregularly branched; however in Amphibolis and Thalassodendron, the rhizome branches sympodially and becomes woody. Rhizomes are almost always buried under sediment, and are usually covered with the persistent fibrous remains of old leaf sheaths, for example in Posidonia and Enhalus (Kuo & McComb, 1989). The growth of rhizomes has greater importance than seed production in extension of seagrass meadows (e.g. Tomlinson, 1974).
The rhizosphere of many seagrasses has been found to support a diversity of microorganisms, especially bacteria (Kuo & McComb, 1989). The seagrass meadows, with their associated communities, constitute an important tropical to temperate marine shallow-water ecosystem (e.g. Larkum & Hartog, 1989). Seagrass communities also play a significant role in sediment baffling and trapping and in the production of
carbonate benthic biogenic particles (e.g. Perry & Beavington -Penney, 2005); they support major detritus-based food chains in many coastal waters, providing nutrients for a number of invertebrates and fish (e.g. Phillips & McRoy, 1980). Direct consumption of seagrasses is much less common and probably accounts for only a small percentage of seagrass production. McRoy and Helfferich (1980) observed that empty niche space for large herbivores exists in the seagrass communities today, since most of the primary production of modern seagrass beds ends up as detritus.
At the present day, besides sirenians, only two other large herbivores, the green turtle (Chelonia mydas) and the hawksbill turtle (Eretmochelys imbricata), include seagrasses in their diet, but they do not disturb the rhizomes. Moreover, Chelonia mydas also eats large amounts of algae and uses a much wider range of habitats than sirenians; and
Eretmochelys imbricata usually occurs at only low population densities in seagrass
habitats, being more frequently found associated with reefs (Garnett et al., 1985).
The specialized hypergrazer Dugong dugon feeds on nine of the ten genera of seagrasses occuring in its habitat: it feeds largely on species of Halophila and Halodule (small rhizomes: <2 mm in diametre following Domning, 2001a), and less frequently species of Cymodocea, Zostera and Syringodium (small and medium rhizomes: 1-3 mm in diametre), Thalassia (medium and large rhizomes: 2-5 mm in diametre), and just leaves of Thalassodendron and Amphibolis, which have woody rhizomes, and leaves of
Enhalus which has large to very large rhizomes (10-15 mm in diametre). Dugong dugon
has not been observed feeding on Posidonia (large rhizomes: 3-10 mm in diametre) (Lanyon et al., 1989; Domning & Beatty, 2007). It seems to feed according to food availability within selected feeding areas and seems to prefer seagrass beds of low density (Lanyon et al., 1989). The low nutritive values of seagrasses, particulary at certain times of the year, coupled with their often low biomass, means that a dugong may need to spend a major proportion of its time feeding (Marsh et al., 1982).
The three species of Trichechus show a wider diet, eating blades, leaves, stems, and sometimes also rhizomes of over 60 species of aquatic and terrestrial plants, including seagrasses, true grasses (Gramineae), forbs, herbs, and mangroves. Water hyacinth (Eichhornia crassipes) and Hydrilla verticillata are eaten in enormous quantities where these plants have been introduced and have become the dominant aquatic vegetation (Werth, 2000). T. inunguis is specialized for exclusively freshwater habitats; while T.
manatus and T. senegalensis are euryhaline, ranging widely in coastal marine waters
Concerning the past, it is usually not possible to establish which seagrass species lived in sirenian habitats and which were fed on by sirenians. Seagrasses have very poor preservation potential and so are rarely found in the fossil record; therefore it is not possible to arrive at firm conclusions concerning their origin, past distribution and biodiversity. However, there are abundant evidences from many parts of the world that seagrass beds harbour characteristic assemblages of organisms not represented in neighbouring unvegetated sediments. Evidences of these characteristic assemblages are observed in the fossil record too, and provide an indirect approach to investigate seagrass history and to reconstruct ancient seagrass habitats. Besides sirenian remains themselves, some indirect evidences of seagrass beds in the fossil record are produced by several groups of organisms: calcareous algae (e.g. Perry & Beavington-Penney, 2005), foraminifers (e.g. Wright & Murray, 1972; Brasier, 1975; Hoffman, 1979; Eva, 1980; Blanc-Vernet, 1984; Langer, 1993; Haunold et al., 1997; Lukasik et al., 2000; Saint-Martin et al., 2000), mollusks (e.g. Hoffman, 1979; Russo et al., 1989; Lukasik et al., 2000), bryozoans (Voigt, 1981; Lukasik et al., 2000), ostracodes (e.g. Hajjaji et al., 1998; Saint-Martin et al., 2000); and by sedimentary characteristics (e.g. Wanless, 1981; Fornos et al., 1992; Perry, 1999; Pomar, 2001; Pomar et al., 2002; Falco et al., 2003).
Among these indirect evidences, benthic foraminifers or other invertebrates associations appear to be the easiest to observe in deposits with sirenian remains. Many of the marine sediments where sirenian specimens have been found also contain benthic foraminifers or other invertebrates characteristic of seagrass beds, as pointed out in chapter 3. Unfortunately no such indirect evidences permit us to establish the exact species composition of the ancient seagrass beds.
6.2.2. ORIGIN; FOSSIL RECORD AND PRESENT DISTRIBUTION
Seagrasses are the only higher plants to have returned to a completely submerged marine existence. They probably evolved from freshwater monocots during the Late Cretaceous; while the monocot diversification took place during the Early Cretaceous (Janssen & Bremer, 2004).
Fossil seagrasses are rare. The earliest fossils which can be referred confidently to seagrasses come from the Early Campanian (Late Cretaceous) of The Netherlands (Ham et al., 2007). Ham et al. (2007) provided a detailed description of the morphology and anatomy of the leaves from the Cretaceous of The Netherlands and referred the Maastrichtian and Danian specimens to a single species of seagrass Thalassotaenia
debeyi and the Campanian specimens to Zosterites, which could not be a seagrass.
Moreover, a poorly known record of Posidonia cretacea comes from the Cretaceous deposits of Germany (Larkum & Hartog, 1989) and a fine specimen close to Posidonia
oceanica has been found in the Cretaceous deposits of The Netherlands (Hartog & Kuo,
2006). Furthermore, there are many other fossil remains of less certain attribution which can be assigned to a wide range of plant groups (Larkum & Hartog, 1989). Therefore there is good evidence that seagrasses arose in the Middle to Late Cretaceous along the shores of the Tethys Sea and later of the Paratethys, reaching westwards to the Neotropics (present Caribbean) with the help of tropical currents. This scenario appears to be consistent with present biogeographical evidences. It is also reasonable to assume that primitive protoseagrass stocks spread relatively quickly and produced many of the modern genera, or genera which were closely allied to modern genera (Larkum & Hartog, 1989).
Dispersal of these genera seems to have been rapid, since by the Eocene the fossil and other evidences indicate a wide range in the Indo-Pacific and Neotropics, with most of the modern genera widely distributed at that time, and with some genera such as
Cymnodocea, Posidonia and Thalassodendron having wider distributions than at the
present time (Larkum & Hartog, 1989).
These fossils closely resemble extant species, which indicates little diversification in the Cenozoic (Larkum & Hartog, 1989): from the Paris Basin (Eocene) come Posidonia
parisiensis; Cymodocea serrulata and C. nodosa (Fritel, 1909, 1913; Stockmans, 1932;
Hartog, 1970; Phillips & Meñez, 1988); from the Avon Park Formation of Florida (late Middle Eocene) come Thalassodendron auricula-leporis, Cymodocea floridana,
Thalassia testudinum, and three other records assigned to Cymodocea sp., Halodule sp.
and an unknown “Zosteroid” (Randazzo & Saroop, 1976; Lumbert et al., 1984). During the Palaeogene there is definitive evidence that Cymodocea, Thalassodendron,
Thalassia and Posidonia were present and may have been widespread, initially with
warm water affinities (Larkum & Hartog, 1989).
Neogene seagrass fossils are rare: fossils of Cymodocea micheloti from the Miocene deposits of Makassar, Celebes, Indonesia (Laurent & Laurent, 1926); Zostera from the Miocene deposits of the Borsod Basin, Hungary (Radócz, 1972); leaves referred to
Posidonia oceanica above the Messinian gypsum bed of the Piedmont Basin, Italy
(Sturani, 1976); seagrasses and seagrass-associated communities from the Pliocene deposits of Rhodes, Greece (Moisette et al., 2007); fruiting Cymodocea nodosa from Pliocene and Pleistocene deposits of Emilia-Romagna, Italy (Ruggieri, 1952);
Posidonia remains from the lower Pleistocene of Sicily, Italy (Geronimo, 1984); Posidonia remains and associated gastropod assemblages from the upper Quaternary of
Ischia, Naples, Italy (Russo et al., 1989); and root casts of Thalassia testudinum from late Holocene of south Florida (Froede, 2002).
At the present time, seagrasses occur in all regions of the globe except Antarctica and form an important part of the ecosystems of shallow inshore regions, but it is remarkable that while there are several hundred thousand species of angiosperms in existence today there are only about sixty known species of seagrasses (Larkum & Hartog, 1989; Hartog & Kuo, 2006). Hartog and Kuo (2006) recognized 64 living seagrass species belonging to four families: Cymodoceaceae (Amphibolis, Cymodocea,
Halodule, Syringodium and Thalassodendron), Hydrocharitaceae (Enhalus, Halophila
and Thalassia), Posidoniaceae (Posidonia) and Zosteraceae (Heterozostera,
Phyllospadix and Zostera). Moreover, these authors regarded as seagrasses also Ruppia tuberosa (Ruppiaceae) and Lepilaena marina (Zannichelliaceae)
Seagrasses are adapted to tropical and temperate habitats: 7 genera are characteristic of the tropics (Cymodocea, Enhalus, Halodule, Halophila, Syringodium, Thalassia and
Thalassodendron), while the other 5 are confined to temperate waters (Amphibolis, Heterozostera, Phyllospadix, Posidonia and Zostera). Obviously the boundaries are not
clear-cut, especially where there are north- or south-flowing currents of warm or cold waters (Kuo & McComb, 1989).
Seagrasses tolerate a large range of salinities above and below that of normal seawater (34.3‰), with individual species differing in the extremes of the range: for example
Amphibolis antarctica has a range from 37‰ to 64‰ (Walker, 1985); Zostera capricorni from 3‰ to 37‰ (Harris et al. 1980), and Posidonia australis from 13‰ to
57‰ (Tyerman et al., 1984). Their productivity can be affected by salinity (e.g. Walker, 1985 for Amphibolis antarctica) or not (e.g. Tyerman et al., 1984 for Posidonia
australis; Wium-Anderson & Borum, 1984 for Zostera marina). Differences between
species in tolerance to either low salinity, high salinity or frequently changing salinity has often been given as the reason for species distributions in salinity gradients. However, the apparent effect of salinity on distribution of species could be due to another factor associated with environments in which changes in salinity frequently occur (e.g. turbidity), or to the effect of salinity on a phase in the plant’s life cycle other than vegetative growth (e.g. seed germination). Tolerance to variations in salinity
depends on certain characteristics of the ion and water relations of the leaf cells (Tyerman, 1989).
The lack of speciation of seagrasses therefore appears inconsistent with their apparent success; in fact, seagrasses (like sirenians) are species-poor. Seagrasses (like sirenians) exploited their present niche successfully at an early stage of evolution and, because of lack of competition, they have changed very little (Larkum & Hartog, 1989). Vermeij (1978) asserted that “physiologically stressful environments are occupied for the most part by long-lived species that are little affected by predation and that are adapted to chronic stress; they are highly resistant to extinction and little prone to speciation”. This seems to well describe seagrass evolution as well as that of sirenians.
6.2.3 THE EURO-NORTH AFRICAN SEAGRASSES AND THEIR FOSSIL RECORD
The Mediterranean seagrasses today comprise only 5 species: Cymodocea nodosa,
Halophila stipulacea, Posidonia oceanica, Zostera marina and Zostera noltii. Cymodocea nodosa and Posidonia oceanica are the two common species. The first is a
typical pioneer species that tends to be replaced by the second, the climax species, dominant in terms of biomass and standing crop. Posidonia oceanica displays a comparatively more stable architectural complexity throughout the year, while the
Cymodocea nodosa–Zostera noltii complex is characterised by marked seasonal
changes of the main structural variables (e.g. shoot density); the leaf canopy of C.
nodosa and Z. noltii is well developed during the warmer months and decreases or
completely disappears at the onset of the colder season. Zostera noltii and Zostera
marina are rare or localized in distribution. These four species all occur in the
Northeastern Atlantic too, while Halophila stipulacea has newly come into the Mediterranean from the western Indian Ocean by way of the Suez Canal, opened in 1869 (Hily et al., 2003; Lipkin et al., 2003; Procaccini et al., 2003).
A much more diverse seagrass flora exists in the Red Sea and Indian Ocean. In the Red Sea in particular, eleven species occur: Cymodocea serrulata, C. rotundata, Enhalus
acoroides, Halodule uninervis, Halophila stipulacea, H. decipiens, H. minor, H. ovalis, Syringodium isoetifolium, Thalassia hemprichii, and Thalassodendron ciliatum (Lipkin
et al., 2003).
In the Black Sea Zostera marina, Z. noltii and Ruppia maritima occur, associated with
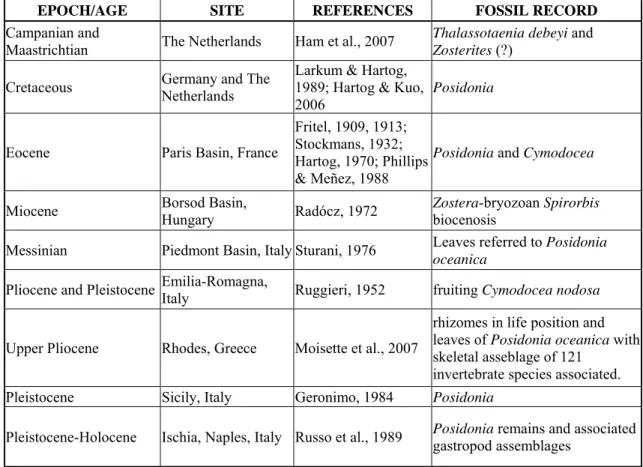
Concerning the Euro-North African seagrass fossil record, the main bibliographical data are summarized in Table 1.
EPOCH/AGE SITE REFERENCES FOSSIL RECORD
Campanian and
Maastrichtian The Netherlands Ham et al., 2007 Thalassotaenia debeyi and Zosterites (?)
Cretaceous Germany and The Netherlands
Larkum & Hartog, 1989; Hartog & Kuo,
2006 Posidonia
Eocene Paris Basin, France
Fritel, 1909, 1913; Stockmans, 1932; Hartog, 1970; Phillips & Meñez, 1988
Posidonia and Cymodocea
Miocene Borsod Basin, Hungary Radócz, 1972 Zostera-bryozoan Spirorbis biocenosis Messinian Piedmont Basin, Italy Sturani, 1976 Leaves referred to Posidonia
oceanica
Pliocene and Pleistocene Emilia-Romagna, Italy Ruggieri, 1952 fruiting Cymodocea nodosa
Upper Pliocene Rhodes, Greece Moisette et al., 2007
rhizomes in life position and leaves of Posidonia oceanica with skeletal asseblage of 121
invertebrate species associated. Pleistocene Sicily, Italy Geronimo, 1984 Posidonia
Pleistocene-Holocene Ischia, Naples, Italy Russo et al., 1989 Posidonia remains and associated gastropod assemblages
Table 1: Main Euro-North African seagrass records.
The genus Posidonia was much more widely distributed in the Palaeogene and its disjunct distribution at the present time (Mediterranean and southern Australia) is certainly a result of localised past extinctions. However, the current lack of any evidence for the occurrence of this genus in the Neotropics, at any period, may indicate a much more limited temperature tolerance (Larkum & Hartog, 1989). Both Posidonia and Zostera may have suffered local extinction in the tropics as the temperature of these waters rose in the Miocene leading to their present bipolar and more temperate distribution. Also, four of the eight extant species of the subgenus Zosterella have populations in the tropics, thus it is readily apparent that the subgenus Zosterella is composed of eurybiontic species made up of populations with broad adaptive tolerances to temperature (Larkum & Hartog, 1989).
On the basis of present-day seagrass biogeography and of the fossil record, more diverse seagrass beds in the Mediterranean Basin during the Miocene are here supposed, constituted by the genera Posidonia (large rhizomes: >10 mm in diametre), Cymodocea
(medium rhizomes: 2-3 mm in diametre), Zostera (small and medium rhizomes: 1-3 mm in diametre), and, most probably, by other species now limited to the Red Sea; with possible fluctuations in composition and extension due to palaecological changes.
6.3 MORPHOLOGICAL STUDIES
6.3.1 INTRODUCTION
The Neogene sirenians are completely aquatic animals swimming with the tail only. Their main morphological adaptations to aquatic life, observed in the fossil record, include (Domning, 2000):
• Short neck, which allows a more anterior position and consequently greater turning moments of forelimbs.
• Forelimbs modified as paddles or hydrodynamic control surfaces to produce turning or steering movements.
• A single sacral vertebra, with short pleurapophyses that do not articulate with any other vertebra to make sacral region more flexible.
• Pelvis without a pubic symphysis, pubis and acetabulum reduced, obturator foramen absent and hind limbs absent or with just a vestigial femur, to reduce weight of bones and muscles that lay behind the center of gravity, thereby improving horizontal trim.
• Caudal vertebrae with broad transverse processes which support an enlarged and powerful tail modified as main propulsive organ.
• Heavy skeleton with pachyosteosclerotic bones, especially in the ribcage, as an adaptation to shallow diving and slow swimming speeds.
Therefore all the Neogene sirenians show similar swimming adaptations and a similar body shape; they differ mainly in some cranial features and in body size.
Among the different cranial features: deflection of rostrum and mandible, shape of zygomatic arch, premaxilla-frontal contact, shape and size of tusks, molar cusp patterns and mental foramen size seem to represent feeding adaptations; even if tusks could be involved or not in feeding behaviour.
In this section I analyze these cranial features in the Neogene Euro-North African sirenian species, including the Late Oligocene “Halitherium” bellunense, in order to reconstruct their diets and feeding behaviour.
Moreover, I analyze the body size and the tusk size evolution of the Euro-North African
Metaxytherium lineage, in order to reconstruct the history of this genus in relation to the
palaeoclimatic and palaeogeographic changes.
The age of an individual is determined on the basis of the cranial sutures and the tooth development, following Mitchell (1973). The sutures considered are: parietal-supraoccipital; interparietal; interfrontal; exoccipital-basioccipital; basisphenoid-basioccipital and exoccipital-supraoccipital. Individuals with basisphenoid-basisphenoid-basioccipital suture fused and with third molar completely erupted are considered adult. Measurements used are listed in Appendix 4.
Due to the small samples any statistical study has been precluded.
6.3.2 TUSKS AND OTHER CRANIAL FEATURES
All the Neogene Euro-North African sirenians, including the Late Oligocene
“Halitherium” bellunense, show more or less developed incisor tusks. These tusks
differ among species in size and shape and among specimens belonging to the same species in degree of eruption and wear.
• The only known specimen referred to “Halitherium” bellunense is a very juvenile specimen with a tusk that is unworn and probably not erupted, not strongly curved and with enamel on the entire tooth surface. Due to the very young age of the holotype and the loss of the anterior portion of its premaxilla, no observation can be made on the exact tusk size and alveolus length; however, I can observe that the tusk alveolus appears to be at least about half the length of the symphysis and the tusk shows a certain specialization, being not oval in cross-section, but lens-shaped with sharp anterior and posterior edges.
• Rytiodus has specialized broad, mediolaterally compressed, bladelike tusks whose roots extend the full length of the premaxillary symphysis and whose medial surfaces are covered by thin enamel which provides a self-sharpening edge.
• The only known specimen referred with confidence to Miosiren kocki has large, subelliptic, nearly straight, and slightly anteriorly-divergent tusks, extending nearly the entire length of the premaxillary symphysis, with the crown about 84 mm long, about 14 mm protruded, distinct from the root, and entirely covered by thin enamel.
• Metaxytherium has simple subconical enamelled tusks. The Euro-North African
subapenninum represent a single evolutionary lineage, as supposed by Domning
and Thomas (1987). This lineage was characterized by an increase in tusk size: the tusks extend less than half the length of premaxillary symphysis in M.
krahuletzi and M. medium, about half the length of the symphysis in M. serresii
and about or more than half the length of the symphysis in M. subapenninum (see Bianucci et al., in press). M. petersi, considered a short-lived and localized offshoot of the M. krahuletzi-M. medium stem, does not preserve any trace of tusk alveoli in the available specimens; if tusks were present they were at least as small as in other Early and Middle Miocene Metaxytherium species (Domning, pers. comm.).
Studies on sirenian tusks are difficult because only one tusked sirenian is presently living, the dugongine Dugong dugon, and it appears to be morphologically and perhaps behaviourally aberrant (Domning & Beatty, 2007). Dugong dugon has a pair of deciduous incisors and a pair of permanent, ever-growing incisors enlarged as tusks; and it presents a slight sexual dimorphism in tusk development. The tusks grow in the same way in both sexes until about 10 years of age; they then grow posteriorly through the premaxilla and a hole appears in the lateral side of the premaxilla at the root of the tusk. In the female, the tusk continues to grow posteriorly through the premaxilla. The increase in length of the tusk is accompanied by a corresponding increase in the length of the alveolus, which continues to extend up the premaxilla, the hole in the premaxilla marking the base of the alveolus. In old females the tusks can be erupted and worn, presumably because they reach the base of the premaxilla and can not grow posteriorly any farther. In the male, the tusks erupt after about 12 years and the length of the tusk alveolus remains relatively constant thereafter and never reaches the base of the premaxilla. The premaxillary bones are much thicker than in the female and the hole in each premaxilla at the base of each tusk usually disappears around the time that the tusks erupt. The tip of the tusk is constituted by postnatal dentine deposited in a prolonged series of coaxial cone-shaped increments covered by a layer of enamel up to about 330 µm thick. The enamel becomes thinner on the sides of the tusk and disappears altogether after a few years except on the ventromesial side, where a layer of enamel about 330 µm thick continues beneath the cementum to the base of the tusk in both males and females. Cementum covers almost the entire surface of the tusk of older animals, but is absent from the tip of the tooth in young animals. The anterior erupted end of the tusk wears quickly on the lateral surface into a chisel shape, the cutting edge being reinforced by an enamel layer below the cementum (Marsh, 1980).
Erupted tusks are not essential equipment for feeding in D. dugong, as they are absent in almost all females and in young males; males of D. dugon with erupted tusks do not consume more rhizomes than females without erupted tusks. The tooth wear seems to be due to passing through the substrate during normal feeding bouts, with occasional events of vigorous cutting of rhizomes by anterior and posterior movements of the tusks in the substrate (Domning & Beatty, 2007).
Domning (1989b; 2001a) noted that where different sirenian species occur sympatrically in the fossil record they show differences in tusk morphology, and these differences should be interpreted as adaptative feeding features that served to partition the feeding-niches. Moreover Domning (1989b; 2001a) noted that the big specialized tusks should represent an adaptation for excavating seagrass rhizomes. This hypothesis has been tested in a recent study by Domning and Beatty (2007). These authors used plastic casts of fossil tusks as hand-tools for excavating seagrass rhizomes, and they inferred that larger, more bladelike tusks are more effective at harvesting rhizomes; therefore they supposed that dugongines like the Early Miocene Euro-North African
Rytiodus, the Late Miocene - Early Pliocene Caribbean Corystosiren and probably the
Late Miocene or Early Pliocene Caribbean Xenosiren, which show this type of specialized tusk, could use tusks in excavation of seagrass rhizomes. These dugongines also show a modified nasal process of the premaxilla: it became shortened, with the posterior end broadened and thickened, and the premaxilla-frontal joint surface became vertical and transverse. This cranial feature is interpreted by Domning and Beatty (2007) as an adaptation to support a compressive stress field along the dorsal side of the skull. This type of stress could be due to downward and backward movements of the rostrum against resistance, as would occur in cutting motions of their tusks (Fig. 2). It is interesting to note that also the juvenile specimen referred to “Halitherium”
bellunense, here considered the sister-taxon of the Rytiodus + Corystosiren clade (see
Chapter 5), shows a modified nasal process together with a certain specialization in the tusk; in fact, the tusk alveolus appears to be at least about half the length of the symphysis and the tusk is not oval in cross-section, but lens-shaped with sharp anterior and posterior edges. The specimen is very young as indicated by the tusk being unworn and not erupted and the second molar likewise being unworn and not completely erupted; no other observation can be made on the tusk development. But the modified nasal process of “Halitherium” bellunense indicates downward and backward movements of the rostrum against resistance, and I can not rule out that its tusks would have increased in size and become bladelike in adults, useful in excavation of seagrass
rhizomes as in Rytiodus and Corystosiren. Therefore, I can suppose that this mode of feeding could have evolved by the Late Oligocene in this derived line of dugongines.
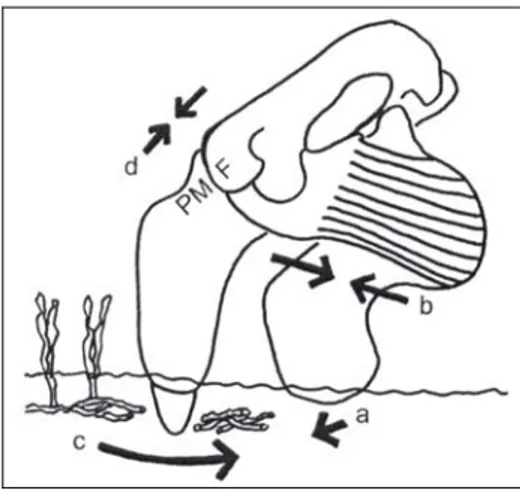
Fig. 2: Lateral view of skull, mandible, and masseter muscle of a derived dugongine, illustrating the hypothesized manner of tusk use in harvesting seagrass rhizomes. With the front of the lower jaw braced against the substrate (a), contraction of the masseter to close the jaw (b) drags the bladelike tusk backward (c), severing rhizomes with the posterior edge of the tusk. The resistance of the substrate results in a compressive force field along the dorsum of the skull (d), which is countered in part by a butt joint between the premaxilla (PM) and frontal (F) (from Domning & Beatty, 2007).
In the light of the study carried out by Domning and Beatty (2007), I conclude that the simple Dugong-like tusks of Miosiren kocki and of Metaxytherium species were not involved in feeding.
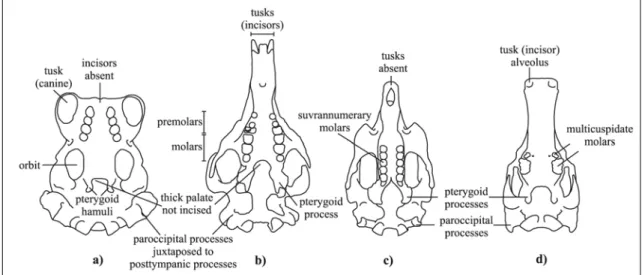
Since its discovery, Miosiren kocki has been considered an aberrant sirenian. Sickenberg (1934), on the basis of its anatomical features, suggested that it fed on molluscs. Indeed, Miosiren kocki is characterized by a thick palate with the posterior border not incised, an extraordinarily thick lamina orbitalis of the frontal, pterygoid processes relatively short and thick, a somewhat elongated paroccipital process juxtaposed to a massive, clublike posttympanic process, and a well-developed cheek dentition, with permanent premolars present and with thick enamel (having a maximum thickness of about 2.2 mm on the lingual side of the M2). On the whole, these cranial features indicate a hard and/or abrasive diet that could consist of molluscs with shells or plants rich in silica such as true grasses (Gramineae). A molluscan diet would constitute an exception among the herbivorous sirenians. Modern sirenians also eat animals on occasion, but never, apparently, as a major part of the diet (e.g. Powell, 1978; Preen,
1995). At any rate they could have had the capability to evolve feeding behaviour and physiological adaptations for eating animals.
A comparison with living species eating molluscs could be made with the walrus (Odobenus rosmarus), which is a benthic suction feeder. The mechanics and functional morphology of its suction feeding are summarized by Adam and Berta (2002): “once a walrus has exhumed its bivalve prey using a jet of water followed by vibrissal inspection, the whole bivalve is positioned between the gum and the upper lip. The tongue is pressed against the hard palate and the lips pressed tightly against each other. The walrus then retracts its tongue, generating negative intraoral pressure. The foot, body, and siphon are torn from the shell and swallowed. The shell, plus any soft remains, are not ingested and simply dropped”. The anatomical adaptations which characterize this suction-feeding are: elongation and vaulting of the hard palate, enlargement of the pterygoid hamuli, reduction and disappearance of incisors, and fusion of the mandibular symphysis (Adam & Berta, 2002).
The bizarre fossil cetacean Odobenocetops peruvianus from the Lower Pliocene deposits of Peru is also regarded as a benthic suction feeder, resembling the walrus in having an hourglass-shaped skull, a vaulted palate, enlarged paroccipital processes, possible vibrissae and sensitive upper lip, and possible horny covering of part of the upper lip (Muizon et al., 2002). It is interesting to observe that, apart from these functional convergences, the living walrus and Odobenocetops peruvianus also resemble each other in having well-developed tusks (Muizon et al., 2002). Adam and Berta (2002) concluded that the enlarged canine tusks of walruses are not involved in feeding. On the contrary, Muizon et al. (2002) observed that if structures resembling walrus tusks evolved in an animal clearly convergent on walruses in other characters that are unquestionably feeding adaptations, tusk-like structures can be considered an integral part of the “benthic suction-feeding” character complex in both species. In conclusion, Muizon et al. (2002) proposed that the tusk-like structures are primarily involved in feeding (as orientation guides for the mystacium) and only secondarily in social interactions.
Miosiren kocki has a thick palate, but it is flat and not concave. Moreover, it shows a
long, worn and well-developed cheek dentition with very thick enamel, features that seem not to support a benthic suction-feeding behaviour. Nonetheless, the palate is much thicker than those of other sirenian species, the pterygoid processes (corresponding to the pterygoid hamuli of the walrus) are relatively short and thick, and the well-developed cheek dentition is consistent with a diet including hard and/or
abrasive food. Miosiren kocki could smash mollusc shells or chew abrasive plants like true grasses. The second hypothesis would be consistent with the systematic position of
Miosiren kocki. Miosiren kocki, in fact, belongs to the Trichechidae, a family now
represented by the manatees. Manatees live in fresh and marine waters and are mixed feeders; they are adapted to abrasive foods by supernumerary molars with horizontal replacement. Miosiren could have resolved the same problem by developing thick enamel and a very thick palate; also the thick lamina orbitalis of the frontal could be an adaptation to dorsoventral compression due to strong mastication movements. These features appear to be present in the Oligocene miosirenine Anomotherium langewieschei as well (Fig. 3).
Also the Desmostylia show adaptations for an abrasive diet (in the Family Desmostylidae) and they are commonly considered herbivorous, adapted to several types of seagrasses and aquatic plants (Inuzukaet al., 1995).
It is interesting to observe that Miosiren kocki is characterized by tusks, which are absent in living manatees (Trichechus spp.) and are unknown in the other fossil trichechids (Anomotherium langewieschei, Potamosiren magdalenensis and Ribodon
limbatus). The forward-directed incisive tusks of Miosiren kocki are not comparable
with the large, downward-directed canine tusks of Odobenus rosmarus or even the backward-directed incisive tusks of Odobenocetops peruvianus, and therefore the hypothesis of Muizon et al. (2002), concerning the use of tusks as orientation guides for the mystacium, appears not applicable for the tusks of Miosiren kocki.
Just three sirenian species appear to evolve independently subconical, unmodified, large tusks, extending the entire length of the premaxillary symphysis: the living dugongine
Dugong dugon, the Pliocene halitheriine Metaxytherium subapenninum, and the Early
Miocene trichechid Miosiren kocki. Dugong dugon tusks are sexually dimorphic and, probably, so are those of Metaxytherium subapenninum. It would not be surprising if
Miosiren kocki also showed sexual dimorphism in the tusks. Only new discoveries
Fig. 3: Schematic line drawings of crania in ventral view: a) Living Pinnipedia Odobenidae Odobenus rosmarus (modified from Adam & Berta, 2002); b) Miocene Trichechidae Miosireninae Miosiren kocki (RBINS M.136); c) Living Trichechidae Trichechinae Trichechus senegalensis d) Miocene Desmostylia Desmostylus hesperus (modified from Domning, 2002b). Not in scale.
Metaxytherium species have simple small or medium-sized tusks, rather strongly
downturned snouts and a primitive long nasal process of the premaxilla. Following the model proposed by Domning (2001a) and the study carried out by Domning and Beatty (2007), they appear to be generalized bottom feeders, consumers of seagrass blades and rhizomes of smaller seagrass species, without using the tusks in feeding.
It is interesting to note that the Euro-North African Metaxytherium lineage (M.
krahuletzi, M. medium, M serresii and M. subapenninum) shows an increase in tusk
size, together with an enlargement of the rostrum, an increase in the height of the horizontal ramus of the mandible, an increase in enamel thickness of the molars, and a slight tendency to increase complication in cusp patterns of the molariform teeth (Domning & Thomas, 1987). These trends culminate in the latest species,
Metaxytherium subapenninum. This species, in particular, shows large tusks, probably
sexually dimorphic (see Chapter 4), extending half or more the length of the premaxillary symphysis, and a dorsal enlargement of the nasal process of the premaxilla (Fig. 4) that could be interpreted as an adaptation to support a compressive stress field along the dorsal side of the skull.
The nasal process of the premaxilla is primitively long, thin and tapering at the posterior end, having a long overlap with frontal and/or nasal. It appears to be modified in at least three lineages of sirenians (see Chapter 5): 1) in the Oligo-Miocene Indian genus
Bharatisiren it becomes thickened at the posterior end, but it preserves a long overlap
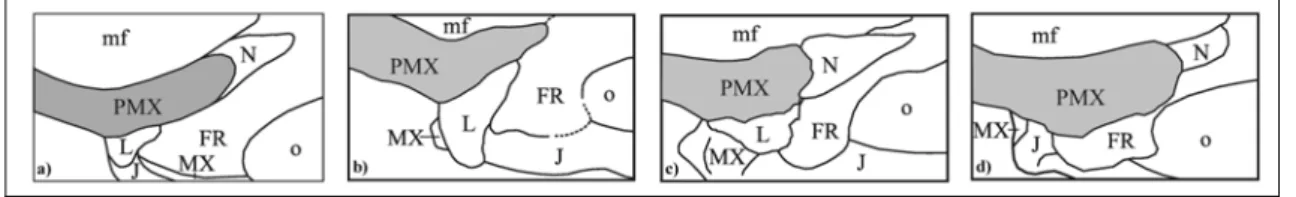
with the frontal; 2) in derived dugongines it becomes broadened and bulbous at the posterior end, having a more or less vertical joint surface in contact with the frontal, and in the “Halitherium” bellunense, Corystosiren varguezi and Rytiodus spp. clade it also becomes shortened; 3) in Metaxytherium subapenninum it becomes dorsally enlarged but not thickened, and it preserves a long overlap with the frontal (Fig. 4). This change could be interpreted as an adaptation to movement of the rostrum or simply to the increase in tusk size.
Fig. 4: Line drawings of nasal process of premaxilla (in grey) in dorsal view with contacts: a) Metaxytherium medium; b) Bharatisiren kachchhensis; c) Rytiodus sp. from Libya; d) Metaxytherium subapenninum. Abbreviations: FR frontal; J jugal; L lacrimal; mf mesorostral fossa; MX maxilla; N nasal; o orbit; PMX premaxilla. Not in scale. The supposed sexual dimorphism of tusks of M. subapenninum is mainly supported by two very old specimens which differ only in tusk size (see Appendix 4 for measurements):
1. IGF 13747 preserves only the very worn M2-M3, and its exoccipitals meet in the midline. This specimen has relatively small enamelled tusks (23 x 27 mm in diametres), fixed in the alveoli with only 58 mm protruded; elliptical in cross section, unworn except for a very small apical portion on the lateral side.
2. MC unnum. (the holotype of “Felsinotherium Gastaldi”) preserves only the very worn M1-M3 on the left side and M2-M3 on the right side, and its exoccipitals meet in the midline. This specimen has a large right tusk (35 x 54 mm in diametres), about 200 mm long and about 70 mm protruded, mediolaterally compressed, slightly flattened on medial side; slightly sigmoidal in cross section. This tusk has a closed root and a crown with a nearly triangular flat wear surface on the lateral side of the apex, nearly completely covered by cementum, as in old dugongs. MC unnum. is nearly complete and very well preserved; nevertheless the left tusk is missing, and the alveolus is empty,
partially filled by sediments. Therefore, the left tusk was evidently lost after death but before burial.
This difference in tusk size and shape could be interpreted simply as intraspecific variability. However, among extant mammals, variability in tusk size and morphology is usually associated with sexual dimorphism. This is the case with elephants, narwhals, walruses, and the extant sirenian Dugong dugon. So, it is not surprising that a
Metaxytherium species might also have evolved sexually dimorphic tusks, with a
development similar to those observed in the living dugongs.
6.3.3 THE EURO-NORTH AFRICAN METAXYTHERIUM BODY SIZE AND TUSK SIZE EVOLUTION
The Metaxytherium body size and tusk size evolution have been observed and studied by Bianucci et al. (in press) and the preliminary results have been expounded by me at the “Euro-African Biotic Evolution in the Neogene, Research Conference and Workshop” (Athens, November 2006).
The Euro-North African Metaxytherium lineage shows an increase in tusk size beginning with the peri-Messinian Metaxytherium serresii and culminating with the Pliocene Metaxytherium subapenninum, and an intriguing trend in body size evolution with a gradual increase during the Miocene, subsequently accelerated and culminating in the Pliocene but abruptly interrupted by the peri-Messinian dwarfing.
Concerning the body size evolution, in no case, unfortunately, is a perfectly complete axial skeleton available to provide a precise measurement of body length. However, some nearly complete or composite skeletons of M. krahuletzi, M. medium and M.
serresii are preserved to provide estimated body lengths:
• A composite, partly restored skeleton of M. krahuletzi from Austria 3.25 m long (Domning & Pervesler, 2001).
• Two nearly complete skeletons of M. medium, one from the Loire Valley (MNHN 1921-10) and the other from southern Italy (MPUN 18403), about 2.8 m and 2.9 m long respectively, exclusive of the posterior portions of the tails. • A nearly complete skeleton of M. serresii from Libya (7P64A) about 2.3-2.4 m
long as observed and estimated in the field (Domning & Thomas, 1987). Moreover, some complete crania of all the species are available:
• Just two nearly complete crania referred to M. krahuletzi are known: one from southern France (MPNRL-MAN2000) and the other from southern Spain (MV 1210), about 46 cm and 40 cm long respectively.
• M. medium crania range from 47 to 50 cm in length.
• M. serresii crania range from 40 to 45 cm in length (Domning & Thomas, 1987; Carone & Domning, 2007).
• The three nearly complete crania of M. subapenninum from Italy (MC unnum., MGPUB unnum. and IGF 13747) measure about 59 cm, 52 cm and 52 cm in length respectively.
In Dusisiren jordani (Domning, 1978: Tab. 2), which may have had a smaller head/body ratio than Metaxytherium, a complete axial skeleton of an old adult with a skull 63 cm long had a total body length of 4.32 m. This suggests that M. subapenninum probably grew to lengths approaching 4 m.
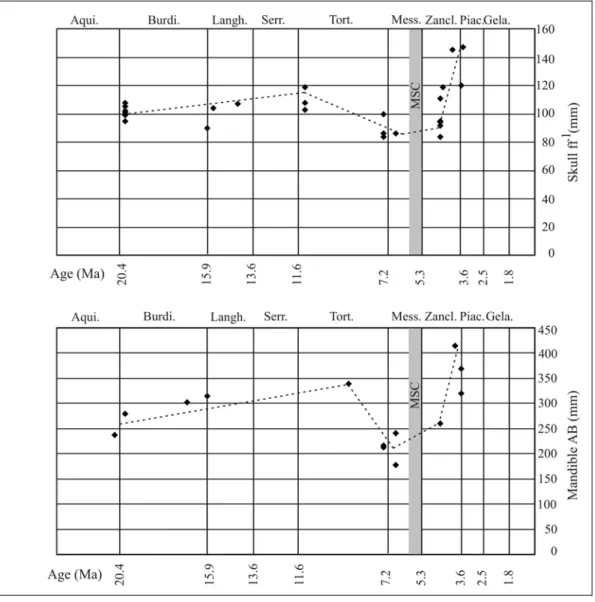
These measurements show the same trend in body size evolution. In order to corroborate these few data other linear measurements, available for a greater number of specimens, have been taken, and all of them have shown the same body size changes (Bianucci et al., in press). In particular, the breadth across occipital condyles (measurement ff' ~ breadth across anterior cotyles of atlas) and the total length of the mandible (measurement AB) have been chosen to illustrate this pattern of changes, because they were available for several stratigraphic samples including the most critical Late Miocene samples. Moreover, the measurement ff’ is of particular interest, as it has recently been advocated as a proxy for body size in studies of fossil cetaceans (Pyenson & Lindberg, 2003).
Fossil samples for which at least one of these dimensions could be used to characterize the body size of Metaxytherium are listed in table 2, together with localities and determinations of their geochronological ages; and the values of these dimensions are graphed against age of sample in figure 5. Although it would be desirable to attach measures of statistical significance to these results, the small sample sizes presently available from most of the stratigraphic horizons preclude this, so the presented data are considered as a preliminary indication of the apparent pattern in size change. In any case, the size differences found are gross enough to be apparent despite the small statistical samples.
a)
LOCALITIES SPECIMENS Ma ff'
Bra MC unnum. 3.5 147
Riosto MGPUB unnum. 3.6 120
Ruffolo IGF13747 4.0 145 Genoa DSTG 2534 (atlas) 4.5 119 Alicante MCNV unnum. 4.6 111 Montpellier MNHN 1868-234 (atlas) 4.6 94 Montpellier NMB MP 215 4.6 84 Montpellier NMB MP 994 4.6 92 Montpellier FSM SM10 4.6 95 Sahabi 69P66A 6.8 86 Cessaniti GPT 30(ces)VM7 7.4 84 Cessaniti GPT "Cranio C" 7.4 86 Cessaniti MBC 37 7.4 100 Loire MNHN Fs2740 11.2 108 Loire MNHN Fs5001 11.2 119 Loire MNHN Fs2718 (atlas) 11.2 103 Vienna Basin BLLM 22816 14.5 107 Brest LPB 16001 15.7 104 Olèrdola MV 1210 16.0 90
Eggenburg KME unnum. 20.0 100
Eggenburg SON 95/109 20.0 105 Eggenburg SON 95/51 20.0 102 Eggenburg HMH unnum. 20.0 101 Eggenburg HMH unnum. 20.0 99 Eggenburg KÜH 87 20.0 95 Eggenburg SON 96/41 20.0 108 b) LOCALITIES SPECIMENS Ma AB
Case il Poggio MUSNAF 4960 3.6 320
Riosto MGPUB unnum. 3.6 370
S. Quirico d'Orcia IGPS 213 3.9 415
Montpellier NHMBs M.P. 145 4.6 260
Sahabi GUDGb 45P15A 6.8 178
Sahabi GUDGb 7P64A 6.8 240e
Sahabi GUDGb 3P66A 6.8 240e
Cessaniti MBC 005 7.4 217
Cessaniti DSTC CMS 21 7.4 213e
S. Domenica di Ricadi MPUN 18403 9.1 340e
Olèrdola MV 1210 16.0 315
Manosque PNRL unnum. 17.0 302
Eggenburg KÜH 95/3 20.0 280e
Léognan MHNBo unnum. 20.5 238
Table 2: Data on a) width across occipital condyles (measurement ff’); b) total length of mandibles (AB) of Euro-Mediterranean Metaxytherium spp., used here as proxies for body size; from Bianucci et al. (in press). e = estimated. Ages of localities are approximated as roughly the midpoints of the age ranges listed Appendix 2.
Fig. 5: Scatterplots of measurements and ages of specimens in Table 2. The grey zone marks the Messinian Salinity Crisis (MSC) extending from 5.96 to 5.32 Ma. The dotted lines are drawn to represent the interpretation of the pattern of body size change in this lineage.
The two graphs obtained are largely superimposable and show an overall pattern of body size change that appears to be a gradual increase during the Miocene, accelerated and culminating in the Pliocene. This trend is abruptly interrupted by the peri-Messinian dwarfing.
Bianucci et al. (in press) provided an initial interpretation of this pattern and considered the gradual increase in size as due to long-term climatic cooling and the peri-Messinian dwarfing as ecophenotypic dwarfing attributed to the degradation of the near-shore habitat and food resources coincident with the MSC.
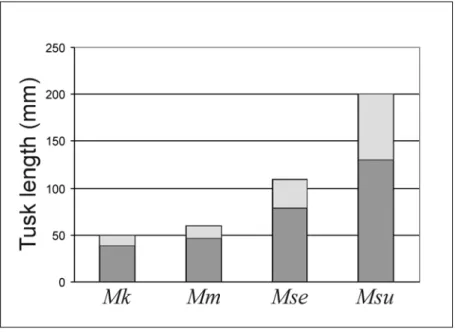
Concerning the Euro-North African Metaxytherium tusk size evolution, data available indicate that:
• No M. krahuletzi specimens preserve tusks, but judging from the alveoli, they should have been small, about 50 mm long, extending less than half the length of the premaxillary symphysis (Domning & Pervesler, 2001).
• M. medium specimens have tusks about 60 mm long, extending less than half the length of the premaxillary symphysis; with the enamel crown about 20 mm long, extending 10-15 mm outside its alveolus and maximum diametres at base of crown of about 23 x 15 mm.
• M. serresii specimens have tusks ranging from 70 mm to 110 mm in length, extending about half the length of the premaxillary symphysis; with maximum diametres at base of crown 27 x 17.5 mm and 10 – 30 mm erupted beyond tip of premaxilla (Carone & Domning, 2007).
• M. subapenninum specimens have tusks ranging from 22 x 23 mm to 35 x 54 mm in diametres of crown and 20 – 70 mm erupted beyond tip of premaxilla, about or more than half the length of the symphysis. No complete tusk, extracted from the alveoulus, is available in order to determinate the total length, except for the cast of MC unnum. This cast represents a large complete tusk, 200 mm long and 35.2 x 54.8 mm wide.
Fig. 6: The increase in tusk size in the Euro-North African Metaxytherium lineage: M.
krahuletzi (Mk), M. medium (Mm), M. serresii (Mse) and M. subapenninum (Msu).
Maximum values for each species are graphed. Light grey rectangles indicate the length of the tusk protruded outside the alveolus.
I excluded from these considerations the isolated tusk above the average, signalled in chapter 4: the 200 mm long tusk from the upper Burdigalian – Langhian deposits of Son Morellό (Majorca, South Spain), referred by Cañigueral (1952) to Metaxytherium; because this specimen is now lost and, judging by Cañigueral’s illustrations, it could belong to a sirenian species unlike Metaxytherium.
The increase in Metaxytherium tusk size (represented in figure 6) was explained by Bianucci et al. (in press) as a shift into a diet richer in rhizomes, especially the medium-sized ones, correlated with the degradation of the near-shore habitat and seagrass beds due to the Messinian Salinity Crisis (MSC). This hypothesis could be plausible if the tusks were effectively involved in feeding in M. serresii and in M. subapenninum, but they are Dugong-like in shape and in size, and dugong tusks are not involved in feeding (Domning & Beatty, 2007), so I can suppose that tusks of M. serresii and M.
subapenninum also were not.
The increase in tusk size and the size evolution trend could be explained without implication of feeding changes, by considering the whole evolution of Metaxytherium (see discussion and conclusion below).
6.4 STABLE ISOTOPE ANALYSES
6.4.1 INTRODUCTION
The stable-isotope (C and O) composition of tooth enamel bioapatite is widely used in reconstructing the palaeoecology and the palaeoenvironment of fossil terrestrial and marine vertebrates (e.g. Koch et al., 1994; Koch, 1998; Roe et al., 1998; Kohn & Cerling, 2002), including sirenians (MacFadden et al., 2004; Clementz et al., 2006). These geochemical studies have allowed testing of previous hypotheses based on morphological and biostratigraphic data and reconstructing various aspects of palaeoecology of vertebrate species and communities not previously discernible with conventional data. The differences in the variation and means of carbon and oxygen isotopes are used as proxies for reconstructing respectively dietary and habitat preferences.
The isotope ratios of the samples are compared with the internationally accepted standard isotope ratios, which for carbon is V-PDB (Pee Dee Belemnite) and for oxygen is V-SMOW (Standard Mean Ocean Water), following the Vienna convention (indicated by the “V-”). The conventional delta (δ) notation, expressed in parts per mil (‰), indicates for carbon: δ13C = [(13C/12Csample/ 13C/12Cstandard) – 1] x 1000 and for
oxygen δ18O = [(18O/16Osample/ 18O/16Ostandard) – 1] x 1000 (e.g. MacFadden et al., 2004).
Oxygen values are initially calculated using the V-PDB values for oxygen, because the samples and standards are both carbonates and therefore follow the same reactions and fractionations; afterwards, they are converted to V-SMOW, which is the standard used for water samples. In this study the MacFadden et al. (2004) correction is applied: δ18OV-SMOW = 1.03091(δ18OV-PDB) + 30.91.
CARBON - The δ13C value in an aquatic mammal is directly controlled by the δ13C values of its foods (Ambrose & Norr, 1993; Tieszen & Fagre, 1993). Sirenians are herbivorous aquatic mammals, so the δ13C value in their body depends on the type of plant foods; when isotopes of carbon are incorporated into skeletal tissues, there is an isotopic enrichment (ε*) in 13C relative to the type of plants eaten (Cerling & Harris, 1999).
Plants incorporate carbon by using one of three different photosynthetic pathways: C3,
C4 and CAM. Most plants (about 85% of the world’s plant biomass), including
seagrasses, use the Calvin Cycle in which carbon is incorporated into three-carbon chain compounds, hence the term C3; tropical and temperate grasses (about 10%) incorporate
percentage of plants, represented by Crassulaceae and Euphorbiaceae, uses the Crassulacean Acid Metabolism, hence the term CAM (Ehleringer et al., 1991). Terrestrial C3 plants have a mean δ13C value of 27‰, and a broad range of 32‰ to
-23‰; while terrestrial C4 plants have a mean δ13C value of -13‰, and a narrow range of
-15‰ to -10‰ (Dienes, 1980; Farquhar et al., 1989; Boutton, 1991). Most fully marine plants, including seagrasses, use the C3 pathway, but their carbon can be derived not
only from atmospheric CO2, but also from bicarbonate; therefore their δ13C values can
be more enriched than their terrestrial counterparts (Boutton, 1991). The coastal aquatic plants, living along the land/water interface, dispose of multiple sources of carbon and incorporate it by using either the C3 or the C4 pathway depending upon species;
therefore their δ13C values are more complicated (Boutton, 1991).
Sirenians eat C3 and C4 plants; following the model proposed by MacFadden et al.
(2004), mean δ13C values can be used to discriminate their diets, from a specialized seagrass diet (δ13C ~ 4‰) to a diet predominantly of C3 plants (δ13C ~ -13‰).
Living manatees are mixed-feeders, consuming over 60 plant species including both seagrasses and true grasses, and they have a mean δ13C of –5.7‰ with a broad range between –13.9‰ and 0.5‰. On the contrary, living dugongs are specialized hypergrazer seagrass feeders, and they have a mean δ13C of 1.2‰ with a narrow range
between –0.6‰ and 1.7‰ (MacFadden et al., 2004) (Fig. 9).
OXYGEN - The δ18O value in the environment is a function of temperature, and thus
the classic use of δ18O as a palaeothermometre. However, aquatic mammals are
homeothermic animals: their body temperature is constant and so it does not influence the δ18O value. The majority (>98%) of oxygen flux in their body is the result of passive diffusion of environmental water across the skin (Anderson & Nielsen, 1983), therefore the δ18O value of an aquatic mammal’s body water should correlate strongly with the δ18O value of the waters in which it lives (Yoshida & Miyazaki, 1991).
Following the models proposed by Roe et al. (1998) and MacFadden et al. (2004), the mean δ18O values for sirenians can be used to discriminate between marine (δ18O ~ 30‰) and freshwater (δ18O ~ 25‰) species.
Living manatees inhabit rivers, estuaries and marine coasts, and they have a mean δ18O of 29.1‰ with a range between 27.3‰ and 31.2‰, indicating a mixture of freshwater to marine habitats. On the contrary, living dugongs are strictly marine, and they have a mean δ18O of 29.8‰ with a narrow range between 29.6‰ and 30.1‰, indicating a marine environment (MacFadden et al., 2004) (Fig. 9).
In this chapter, I present the results of the stable isotope analyses performed on 30 samples of tooth enamel of Euro-North African sirenians, spanning the Late Oligocene to the Middle Pliocene, in order to investigate their dietary and habitat preferences and to corroborate the observations based on morphological characters and biostratigraphic data. A comparison with the data from Florida sirenians obtained by MacFadden et al. (2004) is also considered.
6.4.2 MATERIAL AND METHODS
The stable isotopes can be extracted from the carbonate present in bones, tooth dentine or tooth enamel. Tooth enamel is better than bones or dentine, because it is nearly free of organic impurities, consisting of ~ 96% (by weight) hydroxyapatite ([Ca5 (PO4)3
(OH)]) and just ~ 3% water and ~ 1% organic matter; it is more coarsely crystalline (crystallite length is > 1600nm) and more resistant to diagenetic alteration. The hydroxyapatite composition is characterized by complex vacancy-balanced substitutions of CO3 and HPO4 for PO4 and smaller amounts of CO3 and Cl substitutions in the OH
site, and Na and Mg in the Ca site (Kohn et al., 1996).
The enamel is formed by accretion over a limited interval during the animal’s life and, once formed, it does not turn over and so its stable-isotope composition remains fixed, providing a nearly continuous record that may cover a period of months to years and can be retained for millions of years after fossilization (Fricke & O’Neil, 1996; Passey & Cerling, 2002).
The teeth formed shortly after birth retain an isotopic signature of the mother’s milk, which introduces additional fractionation factors that can significantly offset the enamel isotope values from those of weaned animals and confound dietary interpretation, so it is better to sample the teeth forming later in life, such as the second or (better) the third molars (MacFadden et al., 2004).
The enamel samples were mechanically drilled from teeth, by using a Foredom hand drill outfitted with 1.5 mm-diametre diamond drill bits.
The specimens were sampled by collecting a small fragment of enamel (about 2x2 mm) or a small sample of powdered enamel (10-100 mg) from some points along the tooth. The exact location and type of sampling (powder or fragment) were based on tooth preservation, in order to minimize the destruction of the tooth morphology and to not alter the anatomic characters.
The samples were analyzed by Mark Todd Clementz (Department of Geology and Geophysics, University of Wyoming, USA) following the standard protocol (see MacFadden & Cerling, 1996).
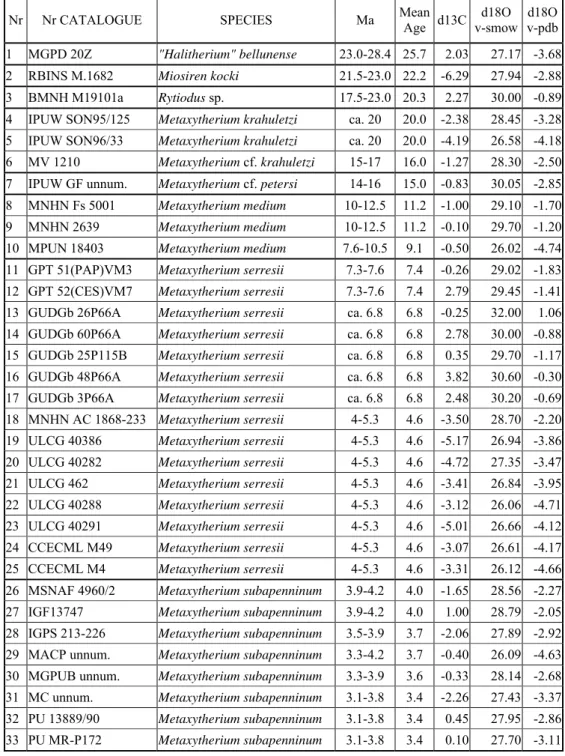
Of the 33 specimens here analyzed (listed in table 3), 26 were personally sampled, 2 (GPT specimens) were sampled by Giuseppe Carone (Gruppo Palaeontologico Tropeano, Calabria, Italy) and 5 (GUDGb specimens) come from Clementz’s database.
Nr Nr CATALOGUE SPECIES TOOTH TYPE SITE Ma
1 MGPD 20Z "Halitherium" bellunense PM5 Cavarzano, Belluno, ITALY 23.0-28.4
2 RBINS M.1682 Miosiren kocki M1 Boom, BELGIUM 21.5-23.0
3 BMNH M19101a Rytiodus sp. M3 Gebel Zelten, LIBYA 17.5-23.0
4 IPUW SON95/125 Metaxytherium krahuletzi frag. Sonndorf, AUSTRIA ca. 20 5 IPUW SON96/33 Metaxytherium krahuletzi M1 Sonndorf, AUSTRIA ca. 20 6 MV 1210 Metaxytherium cf. krahuletzi M3 Olèrdola, Mas Romeu, SPAIN 15-17 7 IPUW GF unnum. Metaxytherium cf. petersi m2 Gainfarn, AUSTRIA 14-16 8 MNHN Fs 5001 Metaxytherium medium M2 Chaze-Henry, Loire Basin, FRANCE 10-12.5 9 MNHN 2639 Metaxytherium medium m3 Noyant, Loire Basin, FRANCE 10-12.5 10 MPUN 18403 Metaxytherium medium m3 S. Domenica di Ricadi, Calabria, ITALY 7.6-10.5 11 GPT 51(PAP)VM3 Metaxytherium serresii M2 Cessaniti, Vibo Valentia, ITALY 7.3-7.6 12 GPT 52(CES)VM7 Metaxytherium serresii m2 Cessaniti, Vibo Valentia, ITALY 7.3-7.6 13 GUDGb 26P66A Metaxytherium serresii molar desert of Cyrenaica; Ajdābiyā, LIBYA ca. 6.8 14 GUDGb 60P66A Metaxytherium serresii m3 desert of Cyrenaica; Ajdābiyā, LIBYA ca. 6.8 15 GUDGb 25P115B Metaxytherium serresii molar desert of Cyrenaica; Ajdābiyā, LIBYA ca. 6.8 16 GUDGb 48P66A Metaxytherium serresii molar desert of Cyrenaica; Ajdābiyā, LIBYA ca. 6.8 17 GUDGb 3P66A Metaxytherium serresii molar desert of Cyrenaica; Ajdābiyā, LIBYA ca. 6.8 18 MNHN AC1868-233 Metaxytherium serresii m2 Montpellier, FRANCE 4-5.3 19 ULCG 40386 Metaxytherium serresii m1 Montpellier, FRANCE 4-5.3 20 ULCG 40282 Metaxytherium serresii M2 Montpellier, FRANCE 4-5.3 21 ULCG 462 Metaxytherium serresii M2 Montpellier, FRANCE 4-5.3 22 ULCG 40288 Metaxytherium serresii M2 Montpellier, FRANCE 4-5.3 23 ULCG 40291 Metaxytherium serresii m2 Montpellier, FRANCE 4-5.3 24 CCECML M49 Metaxytherium serresii M3 Montpellier, FRANCE 4-5.3 25 CCECML M4 Metaxytherium serresii m3 Montpellier, FRANCE 4-5.3 26 MSNAF 4960/2 Metaxytherium subapenninum m3 Case il Poggio, Val di Pugna, Siena, ITALY 3.9-4.2 27 IGF13747 Metaxytherium subapenninum M3 Ruffolo, Val di Pugna, Siena, ITALY 3.9-4.2 28 IGPS 213-226 Metaxytherium subapenninum dp5 Fornaci, S. Quirico d'Orcia, Siena, ITALY 3.5-3.9 29 MACP unnum. Metaxytherium subapenninum M2 El Alamillo, Mazarrón, Murcia, SPAIN 3.3-4.2 30 MGPUB unnum. Metaxytherium subapenninum M2 Riosto, Bologna, ITALY 3.3-3.9 31 MC unnum. Metaxytherium subapenninum M3 Bra, Cuneo, ITALY 3.1-3.8 32 PU 13889/90 Metaxytherium subapenninum M3 Montiglio, Casale Monferrato, Asti, ITALY 3.1-3.8 33 PU MR-P172 Metaxytherium subapenninum M2 Nizza Monferrato, Asti, ITALY 3.1-3.8
Table 3: List of the sirenian specimens analyzed in this study. Specimens are listed in chronostratigraphic sequence.
6.4.3 RESULTS
The results of the analyses carried out by Clementz are presented in table 4. and graphed in figures 7, 8 and 9.
Nr Nr CATALOGUE SPECIES Ma Mean Age d13C v-smow d18O v-pdbd18O 1 MGPD 20Z "Halitherium" bellunense 23.0-28.4 25.7 2.03 27.17 -3.68 2 RBINS M.1682 Miosiren kocki 21.5-23.0 22.2 -6.29 27.94 -2.88 3 BMNH M19101a Rytiodus sp. 17.5-23.0 20.3 2.27 30.00 -0.89 4 IPUW SON95/125 Metaxytherium krahuletzi ca. 20 20.0 -2.38 28.45 -3.28 5 IPUW SON96/33 Metaxytherium krahuletzi ca. 20 20.0 -4.19 26.58 -4.18 6 MV 1210 Metaxytherium cf. krahuletzi 15-17 16.0 -1.27 28.30 -2.50 7 IPUW GF unnum. Metaxytherium cf. petersi 14-16 15.0 -0.83 30.05 -2.85 8 MNHN Fs 5001 Metaxytherium medium 10-12.5 11.2 -1.00 29.10 -1.70 9 MNHN 2639 Metaxytherium medium 10-12.5 11.2 -0.10 29.70 -1.20 10 MPUN 18403 Metaxytherium medium 7.6-10.5 9.1 -0.50 26.02 -4.74 11 GPT 51(PAP)VM3 Metaxytherium serresii 7.3-7.6 7.4 -0.26 29.02 -1.83 12 GPT 52(CES)VM7 Metaxytherium serresii 7.3-7.6 7.4 2.79 29.45 -1.41 13 GUDGb 26P66A Metaxytherium serresii ca. 6.8 6.8 -0.25 32.00 1.06 14 GUDGb 60P66A Metaxytherium serresii ca. 6.8 6.8 2.78 30.00 -0.88 15 GUDGb 25P115B Metaxytherium serresii ca. 6.8 6.8 0.35 29.70 -1.17 16 GUDGb 48P66A Metaxytherium serresii ca. 6.8 6.8 3.82 30.60 -0.30 17 GUDGb 3P66A Metaxytherium serresii ca. 6.8 6.8 2.48 30.20 -0.69 18 MNHN AC 1868-233 Metaxytherium serresii 4-5.3 4.6 -3.50 28.70 -2.20 19 ULCG 40386 Metaxytherium serresii 4-5.3 4.6 -5.17 26.94 -3.86 20 ULCG 40282 Metaxytherium serresii 4-5.3 4.6 -4.72 27.35 -3.47 21 ULCG 462 Metaxytherium serresii 4-5.3 4.6 -3.41 26.84 -3.95 22 ULCG 40288 Metaxytherium serresii 4-5.3 4.6 -3.12 26.06 -4.71 23 ULCG 40291 Metaxytherium serresii 4-5.3 4.6 -5.01 26.66 -4.12 24 CCECML M49 Metaxytherium serresii 4-5.3 4.6 -3.07 26.61 -4.17 25 CCECML M4 Metaxytherium serresii 4-5.3 4.6 -3.31 26.12 -4.66 26 MSNAF 4960/2 Metaxytherium subapenninum 3.9-4.2 4.0 -1.65 28.56 -2.27 27 IGF13747 Metaxytherium subapenninum 3.9-4.2 4.0 1.00 28.79 -2.05 28 IGPS 213-226 Metaxytherium subapenninum 3.5-3.9 3.7 -2.06 27.89 -2.92 29 MACP unnum. Metaxytherium subapenninum 3.3-4.2 3.7 -0.40 26.09 -4.63 30 MGPUB unnum. Metaxytherium subapenninum 3.3-3.9 3.6 -0.33 28.14 -2.68 31 MC unnum. Metaxytherium subapenninum 3.1-3.8 3.4 -2.26 27.43 -3.37 32 PU 13889/90 Metaxytherium subapenninum 3.1-3.8 3.4 0.45 27.95 -2.86 33 PU MR-P172 Metaxytherium subapenninum 3.1-3.8 3.4 0.10 27.70 -3.11
Table 4: Stable isotope values for sirenian specimens analyzed in this study and listed in table 3.
Fig. 7: Plot of δ18O against time for all the sirenian teeth sampled in this study. Hb:
“Halitherium” bellunense, Mko: Miosiren kocki, R: Rytiodus sp., Mk: Metaxytherium krahuletzi; Mp: M. petersi, Mm: M. medium, Mse: M. serresii, Msu: M. subapenninum.
Fig. 8: Plot of δ13C against time for all the sirenian teeth sampled in this study. Hb:
“Halitherium” bellunense, Mko: Miosiren kocki, R: Rytiodus sp., Mk: Metaxytherium krahuletzi; Mp: M. petersi, Mm: M. medium, Mse: M. serresii, Msu: M. subapenninum.
Fig. 9: Plot of δ13C against δ18O for all the sirenian teeth sampled in this study, compared with the δ13C and δ18O values range of living manatees (broken square) and with the δ13C and δ18O values range of living dugong (plain square) from MacFadden et
al. (2004).
The results appear to be consistent with the morphological characters and associated floral and faunal remains.
“Halitherium” bellunense – The δ13C and δ18O values for “Halitherium” bellunense
are respectively 2.03‰ and 27.17‰, indicating a specialized seagrass diet and a mixture of freshwater to marine habitats.
Miosiren kocki – The δ13C and δ18O values for Miosiren kocki are respectively -6.29‰
and 27.94‰, indicating a mixed diet and a mixture of freshwater to marine habitats. Values for Miosiren kocki have been obtained by a first molar; and it is important to consider that the δ13C value could be influenced by the mother's milk; in fact, usually, the first molars have much lower carbon isotope values than those for the third molars. This is a little disturbing because it suggests that the first molars may be capturing a nursing signal, instead of adult diet. Thus, the extremely low carbon isotope value obtained may be the result of the mother's milk, rather than a truly mixed adult diet. Moreover, the carbon isotope result doesn't rule out the possibility of eating molluscs. The carbon isotope values track the foodwebs in which an animal feeds, but can not be used to easily distinguish between animals feeding at different trophic levels. Therefore the carbon value for Miosiren kocki suggests either a mixed diet of freshwater and marine vegetation or consumption of primary consumers or producers in marine
algae-based foodwebs. In conclusion, the carbon isotope analysis does not provide a certain interpretation of the diet of Miosiren kocki.
Rytiodus sp. – The δ13C and δ18O values for Rytiodus sp. are respectively 2.27‰ and
30.00‰, indicating a specialized seagrass diet and a predominantly marine environment.
Metaxytherium – The δ13C and δ18O values for Metaxytherium specimens show an
intriguingly wide variability:
The three specimens of M. krahuletzi have δ13C values: -1.27‰, -2.38‰ and –4.19‰ (mean δ13C = -2.61‰), suggesting a mixed diet consisting of seagrasses and also other C3 plants; and relatively low δ18O values: 26.58‰, 28.30‰ and 28.45‰ (mean δ18O =
27.77‰), indicating a mixture of freshwater to marine habitats.
The only specimen of M. petersi analyzed has – 0.83‰ δ13C and 30.5‰ δ18O indicating a predominantly seagrass diet and a marine environment.
The Mediterranean specimen of M. medium analyzed has –0.50‰ δ13C and 26.02‰ δ18O, indicating a predominantly seagrass diet and a mixture of freshwater to marine habitats; while the two Northeastern Atlantic specimens have one –1.00‰ δ13C and 29.10‰ δ18O and the other –0.10‰ δ13C and 29.70‰ δ18O, indicating a predominantly seagrass diet and a marine environment.
The δ13C and δ18O values for the Upper Miocene M. serresii specimens (n = 7) have respectively a mean of 1.74‰ (range between –0.26‰ and 3.82‰) and 30.13‰ (range between 29.02‰ and 32.00‰). These data indicate a specialized seagrass diet and a marine environment.
On the contrary, δ13C and δ18O values for the Lower Pliocene M. serresii specimens (n = 8) have respectively a mean of –3.97‰ (range between –5.17‰ and –3.07‰) and 26.65‰ (range between 26.12‰ and 27.35‰). These low values indicate a mixed diet and predominantly freshwater habitats.
The subsequent M. subapenninum specimens (n = 8) show higher δ13C and δ18O values: a mean δ13C of –0.64‰ (range between –2.26‰ and 1.00‰) and a mean δ18O 27.81‰ (range between 26.09‰ and 28.79‰). These data indicate a diet relatively specialized in seagrasses and a mixture of freshwater to marine habitats.