7
CHAPTER I
8 ANELLOVIRUS
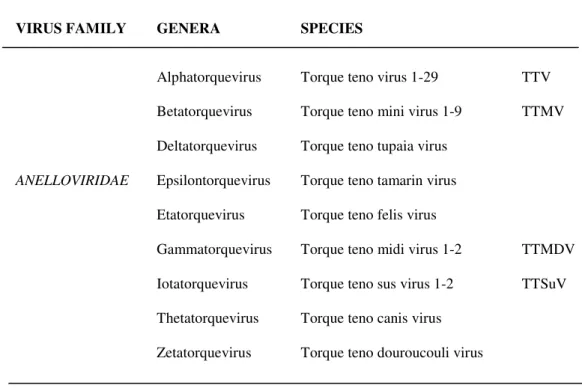
Ten years after the discovery of the first partial sequence of Torque teno virus (TTV), more than 200 partial or full-length related genomes have been characterized in humans and in several animal species. All these sequences belong to a group of novel nonenveloped DNA viruses with a circular, single-stranded (negative sense) DNA genome of 3.6-3.9 kilobases (Kb), 3.2 Kb, or 2.8-2.9 Kb in size. A considerable genetic variability characterizes TTV and related viruses, previously considered members of the floating genus Anellovirus (according to the official International Committee on Taxonomy of Viruses, ICTV, 2005). Since information related to anelloviruses diversity is in constant evolution contrasting to the lack of precise morphologic and structural data, a growing number of sequences have been submitted in databases, and new TTV-related forms have been identified. Taking into account the new data collected in the literature, anelloviruses classification is currently under evaluation by the ICTV. Indeed, according to the new ICTV classification (http://www.ictvonline.org/virusTaxonomy.asp, 2010), members of the family Anelloviridae have been divided into 9 genera, each of them including a different number of species (Table 1).
9
VIRUS FAMILY GENERA SPECIES
ANELLOVIRIDAE Alphatorquevirus Betatorquevirus Deltatorquevirus Epsilontorquevirus Etatorquevirus Gammatorquevirus Iotatorquevirus Thetatorquevirus Zetatorquevirus
Torque teno virus 1-29 Torque teno mini virus 1-9 Torque teno tupaia virus Torque teno tamarin virus Torque teno felis virus Torque teno midi virus 1-2 Torque teno sus virus 1-2 Torque teno canis virus Torque teno douroucouli virus
TTV TTMV
TTMDV TTSuV
Table 1. Taxonomy of family Anelloviridae (ICTV 2010).
Anelloviruses from different species share a similar genome organization but have low nucleotide sequence identity. Diversity among genera is higher than 56% within the ORF1 (putative capsid protein) region of the genome and among species within a genus ranges between 20% to 55% (Biagini, 2009). However, short conserved nucleotide sequences are located in the untranslated region (UTR) of the genome close to the TATA box (Okamoto et al., 2000a).
The most representative and characterized member of the family Anelloviridae is TTV, discovered in 1997 in a Japanese patient (initials T.T.) with posttransfusion hepatitis of unknown etiology (Nishizawa et al., 1997). After discovering the
10 original TTV isolate, using primers based on the UTR, many TTV variants presenting marked genetic variability were identified (Hallet et al., 2000; Hijikata et al., 1999b; Okamoto et al., 1999a, c, d, 2000d, 2001a; Peng et al., 2002; Takahashi et al., 2000a; Ukita et al., 2000) and separated into at least 39 genotypes with a difference greater then 30%, all grouped in five major genetic groups with a difference greater than 50% among them (Okamoto et al., 2004: Peng et al., 2002). Although the described high genetic variability, the genomic organization of TTVs is structurally similar, with a length approximately of 3,800 nt, three or more partially overlapping ORF regions and a UTR containing G-C rich sequences. Furthermore to ubiquitous distribution of many TTV-like variants in various tissues and body fluids of humans, multiple genotypes of TTV may be found within an infected individual, often with different genotype combinations predominating in a tissue or in the other (Okamoto et al., 2001a). In 2000, a small virus distantly related to TTV was discovered by PCR using TTV-specific primers, and provisionally named TTV-like mini virus (TLMV) (Takahashi et al., 2000b). TLMV, following named Torque teno mini virus (TTMV) according to ICTV, presents a full-length genome of approximately 2,800-2,900 nt and resembles TTV in structure (Biagini et al., 2001b, 2007; Okamoto et al., 2000a; Takahashi et al., 2000b). In 2005, two new TTV-like viruses of approximately 2,200 and 2,600 nt, named small anellovirus (SAV) 1 and 2, respectively, were detected (Jones et al., 2005). In 2007, sequences related to SAV were demonstrated belonging to a new anellovirus, called Torque teno midi virus (TTMDV) (Ninomiya et al., 2007a, 2007b). Anelloviruses have been also isolated from nonhuman primates such as
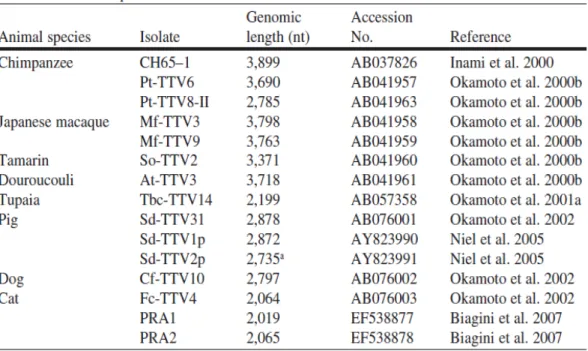
11 chimpanzees, macaques, tamarins and douroucouli (Cong et al., 2000; Verschoor et al., 1999). In addition, other TTV isolates obtained from mammalian species as tupaia, pig, dog and cat have been detected (Abe et al., 2000; Biagini et al., 2007; Bigarre et al., 2005; Leary et al., 1999; McKeown et al., 2004; Okamoto et al., 2001b, 2002). Anelloviruses in animal species are highly divergent from those in human, both in nucleotide sequence and genome size, although phylogenetically some animal TTVs cluster with the human TTVs (Fig. 1; Biagini et al., 2009).
12 Fig. 1 Phylogenetic tree of the human anelloviruses based on the isolates for which the complete genome is present in GenBank. The numbers on the main branches represent the genogroups in which the human TTVs species have been grouped. One asterisk indicates some animal TTVs that cluster with the human TTVs. Two asterisks denote the sequence of the animal TTV Torque teno tupaia virus (accession number: AB057358) used as outgroup.
1. Torque teno virus (TTV)
The genus Alphatorquevirus contains 29 TTV species with a genome length of 3.8 Kb. Due to their extensive genetic heterogeneity, human TTVs have been operatively dividedinto 5 genogroups and more than 40 genotypes (Biagini, 2009). Epidemiologically, TTV infection has been suggested to be associated to many diseases including respiratory disorders, liver diseases, hematological disorders, and cancer (Asim et al., 2010; de Villiers et al., 2007; Pifferi et al., 2008; Tokita et al., 2002). However, due to its global distribution and often persistent viremia in human populations, there is no definitive causal association of TTV infection with the diseases investigated. It may be possible that TTVs do not cause any disease and do not have any adverse effect on human health, being commensal in normal conditions and unable to exceed the threshold of disease-causing load (Griffiths, 1999; Simmonds et al., 1999). On the other side, diseases may be generated only under exceptional circumstances or some isolates/genotypes could be more
13 pathogenic than others, as suggested (Maggi et al., 2007; Okamura et al., 2000; Sugiyama et al., 2000; Tokita et al., 2001).
1.1. TTV detection and characterization
In 1997, while searching for an as-yet-unidentified hepatitis virus, Nishizawa et al. detected a novel DNA virus in a Japanese patient (initials T.T.) with posttransfusional hepatitis of unknown etiology (Nishizawa et al., 1997). Representational difference analysis (RDA) (Lysitsyn et al., 1993) was performed for specific amplification of nucleotide sequences present in the serum of the patient during the period of his acute hepatitis, but which were absent before transfusion. After visualization on electrophoresis of a band 0,5 Kb in size, the fragment was subjected to molecular cloning.
Among the 36 clones obtained, 9 clones of 500 bp in length were similar to each other and their sequence was detectable only during the period of acute hepatitis in the index patient. A representative clone (N22) with the consensus sequence showed poor homology to any other sequences deposited in the GenBank databases as of 2 October 1997 (Nishizawa et al., 1997). The N22 clone was found to originate from the genome of a nonenveloped, single-stranded DNA virus. The virus was provisionally named “TT” virus (TTV) after the initials of the index patient (Nishizawa et al., 1997). Since the N22 sequence was not amplified from any other human genomic DNA sample, a viral origin of N22 was attested. In addition, serum-derived TTV DNA was sensitive to Mung-bean nuclease but resistant to RNase A and restriction enzymes. Therefore, TTV was believed to be a
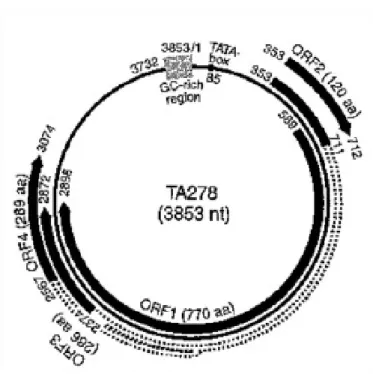
14 DNA virus that had a single-stranded genome (Nishizawa et al., 1997; Okamoto et al., 1998b). Taking into account that the density of Tween 80-treated TTV remained unchanged in sucrose gradient, TTV was considered a nonenveloped virus (Okamoto et al., 1998b). The N22 sequence was extended to 3,739 nt in the prototype TTV isolate (TA278) amplified by PCR with nested N22 primers, but its extreme 5’-and 3’-end sequences remained undetermined at that time (Okamoto et al., 1998b). In 1999, the presence of a GC-rich sequence of approximately 120 nt was reported (Miyata et al., 1999; Mushawar et al., 1999), allowing the recognition of the circular nature of the TTV genome with negative polarity. The genomic full-length of the TA278 isolate was finally determined to be 3,853 nt, presenting the unique stem-and-loop structures in the GC-rich region, which would play a key role in viral replication (Okamoto et al., 1999b, 1999c). Following studies revealed the other characteristics of TTV here consecutively reported. The buoyant density in cesium chloride (CsCl) was found to be 1,31-1,33 g/cm3 for TTV in serum and 1,33-1,35 g/cm3 for TTV in feces (Okamoto et al., 1998a). TTV particles in the circulation were bound to immunoglobulin G (IgG), forming immune complexes (Itoh et al., 2000). So, TTV-associated particles with a diameter of 30-32 nm recovered from the sera of infected humans were observed as aggregates of different sizes on electron microscopy. On the contrary, TTV particles in feces exist as free virions (Itoh et al., 2000; Tsuda et al., 1999). The messenger RNAs (mRNAs) analyzed were transcribed from a plasmid containing the complete genome construct of TTV in COS1 cells (Kamahora et al., 2000). Three spliced mRNAs of 3,0 Kb, 1,2 Kb and 1,0 Kb with common 5’-and 3’-termini were
15 recovered, and it was demonstrated that the splicing sites link distant ORFs to create two new ORFs capable of encoding 286 aminoacids (aa) and 289aa (Kamahora et al., 2000). Spliced mRNAs of TTV have been also observed in actively replicating cells including bone marrow cells in infected humans (Okamoto et al., 2000d). Recently, a more detailed TTV transcription profile was characterized by transfection of a clone of TTV genotype 6, HEL32 isolate in human cells (Qiu et al., 2005). Furthermore, Leppik et al. (2007) demonstrated the existence of additional splice variants in L428 Hodgkin’s lymphoma cells transfected with tth7 and tth8 isolates (genogroup 5). All the three TTV mRNAs are transcribed from a single promoter, which is located in the region -154/-76 (the initial RNA site is denoted as position +1) (Kamada et al., 2004) and they are polyadenylated at a single site at nt 2978 in HEL32. All three TTV mRNAs use alternative AUGs for translation and therefore at least six TTV proteins are expressed in genotype 6 (Qiu et al., 2005). The abundant 2.8 Kb mRNA of HEL32 expresses the ORF1 and ORF2 proteins by alternative AUG usage. The TTV ORF1 protein (~81 kDa) is the largest encoded by TTV mRNA; the ORF2 protein (~13 kDa) is encoded by the 2,8 Kb mRNA in the second ORF, initiating at the AUG in nt position 354 (O2AUG) and extending for 117aa (Qiu et al., 2005). The small 1,2 Kb mRNA expresses TTV proteins ORF2/2 and ORF1/1 with sizes of 31 and 22 kDa, respectively; the smallest 1,0 Kb mRNA expresses TTV proteins ORF2/3 and ORF1/2 with sizes of 30 and 16 kDa, respectively. Transfection of the TTV clone in 293 cells confirmed the expression of these proteins (Qiu et al., 2005). Nowadays the functions of the TTV proteins are poorly understood since neither a
16 virus culture system nor an animal model is available. Additionally, there is not any report of TTV purification yet, whereby the structural proteins forming the virus capsid have not been identified. Chicken anemia virus (CAV), an animal circovirus causing anemia, severe immunosuppression and thrombocytopenia (Noteborn et al., 1991), has a structural protein, VP1. The TTV ORF1 and ORF2 encoded proteins have been shown to be predominantly localized into the cytoplasm (Qiu et al., 2005), and the ORF1 protein is shown to be a structural and replication–associated protein based on similarities with CAV VP1 (Erker et al., 1999; Mushahwar et al., 1999; Tanaka et al., 2001). The TTV ORF2 protein has been studied in cells transfected with ORF2 complementary DNA (cDNA) (Zheng et al., 2007). Subsequent transfection into human cell lines demonstrated that the TTV ORF2 protein suppressed both the canonical and the noncanonical nuclear factor (NF)-Kb pathways, probably avoiding his displacement into the cell nucleus (Peters et al., 2002). Therefore, by hindering NF-Kb from reaching the nucleus, the TTV ORF2 protein indirectly decreased the expression of the inflammation factors interleukin (IL)-6, IL-8 and cyclo-oxygenase (COX)-2. Thus, the role of the ORF2 protein in immune evasion may represent an important aspect of TTV biology (Peters et al., 2002). CAV VP2 possesses a functional protein-tyrosine phosphatase (PTPase)-like domain (Peters et al., 2002; Takahashi et al., 2000a). The ORF2 protein of TTV also shares a considerable similarity with the CAV VP2 (Andreoli et al., 2006; Biagini et al., 2001a). Protein phosphatases are known to have important functions in the regulation of gene transcription, signal transduction, mitogenesis and in cytokine responses of lymphocytes (Ong et al., 1997; Schievella et al., 1993). In
17 TTV genotype 1 (TA278 isolate, Fig. 2), an additional protein was proposed to be encoded from the N-terminus of the 3,0 Kb mRNA. Due to the similarity to apoptin, the main apoptosis-inducing agent of CAV (Kooistra et al., 2004; Miyata et al., 1999), this protein (~12 kDa) was named TTV-derived apoptosis-inducing protein (TAIP). Both TAIP and apoptin induced p53-independent apoptosis in human hepatocellular carcinoma (HCC) cell lines (Kooistra et al., 2004). In general, our knowledge of the function of TTV proteins is limited by the lack of an efficient cell culture system. However, it is possible that, for example, the multifunctional nature of TTV ORF2 proteins could confer pathogenetic diversity to the genus Alphatorquevirus.
Fig. 2 Genomic organization of the prototype TTV (TA278 isolate) (Okamoto, 2009a, b).
18 1.2. Variability
Several TTV variants with high genetic diversity have been detected by using conserved UTR-specific primers (Hallet et al., 2000; Hijikata et al., 1999b; Khudyakov et al., 2000; Muljono et al., 2001; Okamoto et al., 1999a, c, d, 2001a; Peng et al., 2002; Takahashi et al., 2000a; Ukita et al., 2000). They have been separated into at least 39 genotypes having a genetic diversity higher than 30%, and at least 5 principal genogroups presenting a genetic diversity higher than 50% (Okamoto et al., 2004; Peng et al., 2002). Especially, this genetic variability concerns the coding region of TTV, and it is maximum in the middle of TTV ORF1. Here, there are at least three hypervariable regions (HVRs) characterized by several insertions and deletions and an aminoacidic diversity among TTV isolates higher than 70% (Erker et al., 1999; Hijikata et al., 1999b; Takahashi et al., 1998). The reasons, on which this genetic diversity is based, are unknown yet. Although few TTV isolates (i.e., genotypes 12 and 13) seem to not have HVRs (Ukita et al., 2000), the HVRs of the all TTVs show a high variability in patients with chronic infection, mainly during the period of acute infection (Nishizawa et al., 1999). In some region of TTV genome the frequency of nucleotide substitutions (7,3x10-4 per site per year in the HVRs of SENV) can be compared to the frequency observed in RNA viruses and seems to be 10 times higher respect than the frequency observed in DNA viruses as hepatitis B virus (HBV) (Umemura et al., 2002). Both in TTV isolates belonging to the same genotype and in isolates belonging to different genotypes have been identified at least 19 recombination sites, whose 13 located into the UTR. This latter aspect could play an important role in increasing the
19 genetic variability (Worobey, 2000; Biagini et al., 2001a; Manni et al., 2002; Erker et al., 1999; Hijikata et al., 1999b), especially considering that the co-infection with different isolates is very common in the infected host.
1.3. Replication
TTV is present in several tissues and organs, thus demonstrating a potentially wide host cell tropism. By several methods (DNA quantification, circular double-stranded PCR detection, potentially replicating TTV has been detected in a variety of tissues, including lung, stimulated peripheral blood mononuclear cells (PBMCs), bone marrow, spleen, liver, pancreas, kidney, thyroid gland and lymphnode (Bando et al., 2001; Mariscal et al., 2002; Okamoto et al., 2000d, e, 2001a). It has been suggested that some TTV genotypes could have preference for PBMCs (Okamoto et al., 1999a) and that PBMCs could represent a reservoir for TTV (Chan et al., 2001; Garbuglia et al., 2003; Maggi et al., 2001b). The precursors for hematopoietic cells reside in bone marrow. During myelosuppression in bone marrow-transplant recipients a reduction of TTV levels has been observed, thus suggesting that hematopoietic cells could sustain TTV replication (Kanda et al., 1999). Additionally, the presence of high TTV loads in saliva (Deng et al., 2000; Gallian et al., 2000) has led to investigations of oropharyngeal tissue as a putative site of TTV persistence. TTV DNA has been detected in the cytoplasm of oral ephithelial cells (Rodriguez-Inigo et al., 2001) and TTV loads in the nose exceed those in serum, suggesting that the nasal cavity could represent the primary site of TTV infection (Maggi et al., 2003b). TTV DNA has been also found in nucleus
20 and/or cytoplasm of hepatocytes of patients with liver damage (Cheng et al., 2000; Comar et al., 2002), supporting the concept that liver may play an important role in the dynamics of TTV replication. TTV replication has been also studied in several cells: to date the best candidates for supporting TTV propagation are cells of hepatic and erythroid origin, as well as the ciliary cells of the respiratory tract. However, it is also possible that only a specific subset of these cells or not yet identified cell types may be the main targets for TTV infection and replication. Furthermore, TTV genotypes can differ in host cell tropism thus heavily complicating the picture.
The most of small DNA viruses depends on the host cell machinery for their replication. Based on sequence analysis TTV is not assumed to encode a DNA polymerase, but probably it uses cellular polymerases. Indeed, in the presence of a drug blocking the cellular DNA polymerase, TTV replication of HEL32 isolate (genotype 6) did not occur, thus clearly demonstrating the fundamental role of the cell replication machinery for virus replication (Kakkola et al., 2007). The cell mechanism of TTV replication is not known yet. It is hypothesized, based on similarities with other circular single-stranded (ss)DNA viruses, that TTV could use the rolling circle mechanism (Mushahwar et al., 1999). Maybe, viral proteins are needed to interact with cellular proteins for starting the replication. For this role, circoviruses encode replication-associated proteins with specific Rep-motifs that bind to the replication initiation site (Mankertz et al., 1998). Based on the amino acid sequence, TTV ORF1 seems to contain similar Rep-motifs (Erker et al., 1999; Mushahwar et al., 1999; Tanaka et al., 2001). As it happens for animal circoviruses,
21 also conserved sequences and genomic structures have been discovered involving the replication (Mankertz et al., 2004; Niagro et al., 1998; Todd et al., 2004). Indeed, TTV UTR contains sequences that could form similar structures (Hijikata et al., 1999b; Mushahwar et al., 1999; Peng et al., 2002). Nowadays, if these structures and which TTV proteins are utilized in viral replication mechanism is not known yet.
1.4. Pathogenesis
TTV penetrates into the host cell by several ways, including respiratory tract, gastro-intestinal system, genital system and placenta, being detected in blood in few weeks. After natural or experimental TTV infection, viremia is observable in about 5-8 weeks, often persisting for a long time (Mushahwar et al., 1999; Tawara et al., 2000). Infection with TTV is characterized by persistent lifelong viremia in humans, with circulating levels of up to 108 copies/mL in the general population (Hu et al., 2005; Pistello et al., 2001). A study on the kinetics of clearance of TTV suggested that a daily production rate of >1010 virions is necessary to maintain the observed levels of viremia (Maggi et al., 2001b). This rate is a clear indication of the great replicative capacity of TTV in vivo. As demonstrated by in situ ibridization and/or quantitative PCR, TTV replicates in the liver (Ohbayashi et al., 2001; Okamoto et al., 2000c; Rodriguez-Inigo et al., 2000), and high levels of TTV excreted in bile were detected (Luo et al., 2000; Nakagawa et al., 2000; Ukita et al., 1999). Excretion of TTV in bile may be the main source of the virus in the gastrointestinal tract and also of its fecal shedding. Anyway, TTV replication is not
22 restricted to the liver. As reported above, high viral loads, double-stranded replicative forms and mRNA transcripts have been also detected in lung tissues and pancreas (Okamoto et al., 2001a), bone marrow (Fanci et al., 2004; Kikuchi et al., 2000; Okamoto et al., 2000b), spleen (Okamoto et al., 2001a; Jelcic et al., 2004) and other lymphoid tissues (Kakkola et al., 2004). TTV DNA is frequently present in PBMCs (Barril et al., 2000; Lopez-Alcorocho et al., 2000; Okamoto et al., 1999a, 2000c; Okamura et al., 1999), where TTV shows a really wide tropism with viral DNA detected not only in T and B limphocytes, monocytes and natural killer (NK) cells (Maggi et al., 2001a; Takahashi et al., 2002; Zhong et al., 2002) but also in granulocytes and other polymorphonuclear cells (Maggi et al., 2001a; Takahashi et al., 2002). TTV replication proceeds with a kinetic flow which is comparable to those of hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). TTV infection is frequently acquired early in infancy, which may lead to substantial immune tolerance, as it is known for HBV also. The persistent nature of infection and co-infection of multiple TTV variants in the circulation may suppose the presence of mechanisms of immune evasion that have developed in TTV the capability to establish persistent infection in immunocompetent individuals. However, recombinant ORF1 proteins (Handa et al., 2000; Ott et al., 2000) or antibodies against native TTV virions (Tsuda et al., 1999) have been detected in viremic and non viremic individuals, and TTV particles in the circulation are frequently bound to IgG, forming immune complexes (Itoh et al., 2000). Even so, nowadays there is no evidence indicating an association with diseases evoked by the deposition of immune complex, such as
23 glomerulonephritis. TTV viral loads have been demonstrated to increase in HIV-infected patients who are progressing towards AIDS, and a high TTV viral load was associated with a low CD4 cell count, suggesting a potential role of the immune system in controlling TTV replication (Christensen et al., 2000; Shibayama et al., 2001; Thom and Petrik, 2007; Touinssi et al., 2001; Zhong et al., 2002). Although it remains not clear which role the immune system plays in the natural course of TTV infection, TTV may work as an opportunistic pathogen in immune-compromised hosts, such as human cytomegalovirus (CMV) in AIDS patients.
1.5. Association with disease
Although TTV is potentially related to many diseases, contrasting opinions exist on its disease-causing potential due to its nearly universal presence in human populations. It is possible that certain genotypes/genogroups of TTV may be specifically pathogenic. Interestingly, the expression of genotype 1-ORF1 in transgenic mice, leading to production of a spliced protein, caused pathological changes in kidney. This protein seemed to interfere with the differentiation of renal ephitelial cells (Yokoyama et al., 2002). Recently, it has been also suggested that subgenomic fragments of TTV identified in human sera could have some role in diseases as it is known for plant geminiviruses (Leppik et al., 2007). However, there are only few reports which support the disease-inducing potential of TTV.
24 1.5.1. TTV and Liver Diseases
When discovering the original TTV isolate, it was found in three of five patients with posttransfusion acute hepatitis of unknown etiology, and the presence of TTV was closely associated with the serum ALT level (Nishizawa et al., 1997). When a serum sample from an 11-month-old infant with acute hepatitis of unknown etiology, who had been infected with genotype 1 TTV, was intravenously inoculated into a native chimpanzee, TTV was transiently detected in the animal at 5-15 weeks postinoculation (PI), with the titer peaking at 12-13 weeks PI (Tawara et al., 2000). At the peak of TTV viremia, an unexpected elevation of the serum α-glutathione-S-transferase level and mild elevation of ALT level were observed. In association with the drop of TTV titer, appearance of IgM and IgG-class anti-TTV (genotype 1) antibodies and histological changes in biopsied liver samples were observed. They suggested that TTV genotype 1 shows hepatitis-inducing capacity. Indeed, studies reported that TTV genotype 1 may play a role in the pathogenesis of non-A, -B, or -C fulminant hepatic failure (Shibata et al., 2000), and also that TTV genotype 1 may be more pathogenic than the others in children with liver disease of unknown etiology (Okamura et al., 2000; Sugiyama et al., 2000). Foschini et al. (2001) reported an Italian case of TTV (genotype 13)-related acute recurrent hepatitis, with clinicopathological findings enhancing the suggestion that TTV can be responsible for a mild form of liver disease. Other studies also suggested an association between the prevalence of TTV and/or TTV load and several hepatic disorders (Charlton et al., 1998; Ikeda et al., 1999; Kanda et al., 1999; Okamura et al., 2000; Tanaka et al., 1998, 2000). Takayama et al. (1999) reported that
25 persistent TTV infections could contribute to cryptogenic hepatic failure in hemophiliac individuals. However, opposite results demonstrating that TTV is not correalated with any form of hepatitis have been also presented (Hijikata et al., 1999a; Hsieh et al., 1999; Niel et al., 1999; Prati et al., 1999). Tokita et al. (2002) demonstrated that high TTV viral loads were independently associated with the complication of hepatocellular carcinoma (HCC) and that it may have prognostic significance in patients with hepatitis C virus (HCV)-related chronic liver disease. Indeed, it has been reported that high TTV viremia has an adverse effect on the progression of chronic liver disease in concert with concurrent HCV infection and may be correlated to the development of HCC. Zein et al. (1999) reported that TTV infection was more prevalent among patients presenting advanced HCV-associated liver disease respect than among those with stable disease. Moriyama et al. (2001) demonstrated that the score of irregular regeneration of hepatocytes among TTV-infected cirrhotic patients with chronic hepatitis C was higher than that among patients who were not infected with TTV. These results suggest that TTV plays a role in the development of cirrhosis and following complications. However, a correlation between high TTV load and low CD4 cell count among patients infected with HIV type 1, and the supposed prognostic significance of TTV viral load in immunocompromised patients, has been reported (Christensen et al., 2000; Shibayama et al., 2001). Therefore, it is probable that an impaired immune system or suppression of the immune system is involved in elevated TTV viremia in HCC patients.
26 1.5.2. TTV and Respiratory Diseases
TTV infection has been suggested to have a potential role in children with respiratory diseases. Indeed, TTV replication has been shown to occur in lung tissues (Okamoto et al., 2001a; Bando et al., 2001). Biagini et al. (2003) reported that infection with TTV coincided with mild rhinitis in a neonate, and children hospitalized with acute respiratory diseases or bronchiectasis presented higher TTV viral loads respect than those in the control children (Maggi et al., 2003a; Pifferi et al., 2006). Additionally, children with high TTV loads in nasal specimens were shown to have worse spirometric values, and TTV was supposed to contribute to the pathogenesis of asthma (Pifferi et al., 2005). Bando et al. (2001) reported the influence of TTV infection on the disease activity and prognosis of idiopathic pulmonary fibrosis. Furthermore, the correlation between TTV infection and the complication of lung cancer in patiens with idiopathic pulmonary fibrosis has been reported (Bando et al., 2008). Although these observations arouse interesting questions about the significance of TTV in the respiratory tract of infected humans, it remains undetermined if TTV is the cause or the consequence of the disease. Interestingly, it was supposed that TTV replication could modulate the immunobalance towards the T helper 2 cell (Th2) response, which is known to have a role in the pathogenesis of asthma (Pifferi et al., 2005).
1.5.3. TTV and Hematological Disorders
A high level of TTV replication in bone marrow has been suggested to be responsible for hepatitis-associated aplastic anemia of enexplained etiology
27 (Kikuchi et al., 2000). In addition, a possible correlation between TTV infection and aplastic anemia has been also suggested (Miyamoto et al., 2000). However, opposite results indicated that TTV is not associated with post-hepatitis aplastic anemia (Safadi et al., 2001). As for the correlation of TTV with hematopoietic malignancies, TTV DNA was found in lymphocytes circulating in the lymphnodes with B-cell lymphomas and those with Hodgkin’s disease (Garbuglia et al., 2003). It was suggested that TTV could modulate the infected T cells and thus play some role in the pathogenesis of lymphomas.
1.5.4. TTV and Cancer
Additionally to HCC, the possible participation of TTV infection in other malignant disorders such as lung cancer and hematopoietic malignancies has been suggested. TTV DNA has been detected in an extensive variety of neoplastic tissues (de Villiers et al., 2002). However, there is no plausible causal association of TTV infection with tumorigenesis or malignant transformation of cells.
1.6. Laboratory diagnosis
Due to the lack of an efficient culture system supporting TTV replication (Kakkola et al., 2007; Leppik et al., 2007) and specific tests for the determination of immunoglobulins and viral antigens, the techniques used for the identification of TTV DNA are based on polymerase reaction chain (PCR o real-time PCR) assays. The high genetic variability of TTV has obstructed the choice of which specific region had to be used for the amplification. Indeed, although N22 (ORF 1) was the
28 region initially amplified by N22-derived primers (N22 PCR), it only allowed the detection of genogroups 1 to 6 (Muljono et al., 2001). Therefore, at present, for the detection and quantification of TTV, conserved sequences belonging to the UTR are used for the amplification of all available TTV isolates (Okamoto et Mayumi, 2001c) by real-time quantitative PCR (Maggi et al., 2001a, 2001b; Moen et al., 2002a; Nakagawa et al., 2000). TTV isolates detected by quantitative PCR can be subsequently characterized by genogroup-specific PCR system using as targets several regions of the viral genome (Maggi et al., 2001b). To furtherly characterize the TTV isolate amplified, sequencing methods have to be performed. By using entire TTV particles or fragments of ORF 1 or ORF 2 proteins expressed in bacterial cells as antigens in immunoblotting and/or immunoprecipitation assays combined with PCR system, IgG towards the virus have been detected in human sera. Therefore, it was possible to determine the percentage of TTV forming immune complexes (Itoh et al., 2000). TTV-associated particles with a diameter of 30-32 nm were observed as aggregates of various sizes on electron microscopy. In contrast, by immune electron microscopy using γ-globulins from human plasma containing TTV genotype 1a-specific antibodies, TTV particles in fecal supernatant have been visualized as free virions (Itoh et al., 2000; Tsuda et al., 1999).
1.7. Epidemiology
TTV DNA is diffused worldwide and its chronic infection can be detected in human sera of 2/3 of the general population independent of ethnic origin, age, socioeconomic conditions and other factors (Biagini et al., 2000, 2001a; Matsubara
29 et al., 2001; Niel et Lampe, 2001). Initial studies about the prevalence of TTV, using the ORF 1 region as template for PCR amplification, demonstrated a considerable diffusion of the virus, showing different percentages of distribution in distinct geographic areas (Niel et al., 1999): very low in USA and Northern Europe and extremely high in Africa and South America (Prescott et Simmonds, 1998). However, the subsequent use of PCR system based on the conserved UTR showed a global distribution of TTV without any significant differences among geographic areas (Biagini et al., 2000, 2001a; Matsubara et al., 2001; Niel et Lampe, 2001). Although the levels of prevalence of TTV simultaneously increase with the age of the individuals (Chen et al., 1999; Davidson et al., 1999; Hsieh et al., 1999; Kazi et al., 2000; Lin et al., 2002; Maggi et al., 1999, 2003a; Ninomiya et al., 2008; Oguchi et al., 1999; Toyoda et al., 1999; Vasconcelos et al., 2002), none of the performed studies have detected a correlation between the prevalence of TTV with clinical data of the individuals, their sex, or other features of the selected population. A such high distribution of TTV infection suggests that the virus is extremely infectious and its diffusion occurs through several forms of transmission, including parenteral route, oro-faecal tract and maternal-fetal way (Hsieh et al., 1999; Morrica et al., 2000; Okamura et al., 1999). Furthermore, the detection of TTV DNA in saliva, semen, vaginal fluid, secretions from nasopharyngeal tract and mother’s milk, suggests the existence of other forms of viral transmission including sexual transmission (Biagini et al., 2001a; Chan et al., 2001; Davidson et al., 1999; Fornai et al., 2001; Gerner et al., 2000; Inami et al., 2000; Kazi et al., 2000; Krekulova et al., 2001; Liu et al., 2000; Martinez et al., 2000; Matsubara et al.,
30 2000, 2001; Pirovano et al., 2002a; Schroter et al., 2000; Stark et al., 2000). Only few studies have demonstrated the distribution of the different genogroups/genotypes of TTV. While it has not been reported yet that their transmission occurs with different efficiencies, it is certainly known that their distribution depends, at least in part, on the geographic area (Devalle et Niel, 2004; Gallian et al., 2000; Mikuni et al., 2002; Pirovano et al., 2002b) and/or the selected population. Indeed, TTV genotype 1 seems to be more diffused in Asia than in occidental countries (Shibata et al., 2001; Umemura et al., 2001) and TTV genotypes 22 and 23 are highly prevalent in Indonesia but almost entirely absent in Japan (Muljono et al., 2001). This information suggests that different genogroups/genotypes show a differential transmission.
2. TTV related viruses
After TTV discovery, by using primers based on the conserved UTR of the viral genome, many TTV variants with high genetic variability were detected (Hallett et al., 2000; Hijikata et al., 1999b; Muljono et al., 2001; Okamoto et al., 1999a, c, d, 2000d, 2001a; Peng et al., 2002; Takahashi et al., 2000a; Ukita et al., 2000). They were segregated into at least 39 genotypes with a difference of greater than 30% and into 5 major genetic groups with a difference of greater than 50% (Okamoto et al., 2004; Peng et al., 2002).
31 2.1. Torque teno mini virus (TTMV)
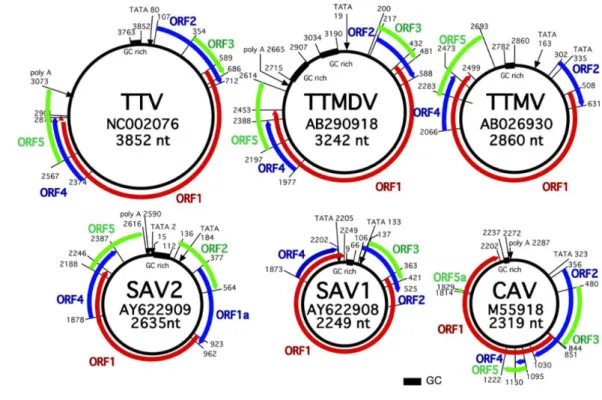
In 2000, a small virus distantly related to TTV was accidentally detected by PCR from human plasma samples using TTV-specific primers which partially matched homologous sequences but generated a shorter amplicon than expected for TTV. This new small virus was provisionally named as TTV-like mini virus (TLMV) (Takahashi et al., 2000b) and consisted of a circular, single-stranded DNA genome of approximately 2,800-2,900 nt with negative polarity (Fig. 3). The size of a TLMV virion was estimated to be less than 30 nm in diameter (Takahashi et al., 2000b). In genomic structure, TLMV resembles TTV, containing also an arginine-rich N-terminus as well as Rep-motifs in the ORF 1 region and a chicken anemia virus (CAV)-like motif in the ORF 2 region (Biagini et al., 2001b, 2007; Okamoto et al., 2000a; Takahashi et al., 2000b). TLMV is also highly divergent. Indeed, the first three TLMV sequences reported by Takahashi et al. (2000b) differed from each other by 42% at the nucleotide level and by 67% at the aminoacid level. The distribution of TLMV is worldwide among healthy individuals (Biagini et al., 2001b; Niel et al., 2001). The prevalence of TLMV DNA among blood donors is reported to be 48%-72% (Biagini et al., 2006b; Moen et al., 2002b; Niel et al., 2001). TLMV has been isolated from various body fluids and tissues, such as plasma/serum, feces, saliva, bone marrow, spleen, cervical swabs and PBMCs (Biagini et al., 2001b; Fornai et al., 2001; Thom et al., 2003; Vasconcelos et al., 2002). In 2005 the ICTV officially designated TTV and TLMV as Torque teno virus (TTV) and Torque teno mini virus (TTMV), respectively, deriving from the Latin terms torque meaning “necklace” and tenuis meaning “thin”. At present, no
32 diseases are certainly correlated with TTMV, although it has been suggested a potential role of TTMV in some severe kidney disease. The only diagnostic assays that are possible to use for the detection of TTMV are PCR systems (qualitative PCR or real-time quantitative PCR), which are based on the amplification of nt sequences belonging to the UTR (Biagini et al., 2001a).
33
2.2
. Torque teno midi virus (TTMDV)By DNase-sequence independent single primer amplification (SISPA) method, two new TTV-like viruses named small anellovirus 1 (SAV1) and small anellovirus 2 (SAV2) were isolated from sera of patients with acute viral infection syndrome (Jones et al., 2005). SAV1 consisted of a genomic DNA of 2,249 nt with three putative ORFs, while SAV2 showed a genomic DNA of 2,635 nt with five ORFs. The SAV ORF 2 region was shown to have a similar CAV-like motif as TTVs and TTMVs (Andreoli et al., 2006). Similar to TTVs and TTMVs, SAV isolates had wide genomic variation of up to 41%. SAV has been also isolated from several body fluids and tissues, including saliva and PBMC (Biagini et al., 2006a) as well as nasopharyngeal aspirates (Chung et al., 2007). As for TTVs and TTMVs, also SAVs were found to be common among healthy individuals and were present in 20% of French blood donors (Biagini et al., 2006a) and in 34.5% of Korean children (Chung et al., 2007). Additionally, using a combined rolling-circle amplification (RCA) and SISPA approach, isolates related to SAV and differing from it by approximately 40% have been identified (Biagini et al., 2007). Recently, in attempt to amplify SAV sequences in human sera, amplicons longer than expected were obtained and the corresponding full-length clones were 3,242-3,253 nt, showing all the features of TTV-like viruses (Fig. 3). These newly identified isolates were named Torque teno midi virus (TTMDV) (Ninomiya et al., 2007a). Furthermore, analyzing 15 additional TTMDV sequences over the whole genome, it was found that they represent a large clade of isolates differing in length (3,175-3,230 nt) and in sequence (up to 33% divergence at the nucleotide level and 61%
34 divergence at the aminoacid level of ORF 1 (Ninomiya et al., 2007b). In addition, three Rep-motifs were also identified in the ORF 1 region, as well as putative stem-loop structures in the GC-rich region.
3. Animal TTV
TTV infection is not restricted to humans only. Using highly conserved primers derived from UTR of the human TTV genome, a variety of TTV-like viruses have been detected circulating in nonhuman primates such as chimpanzees, macaques, tamarins, and douroucoulis (Okamoto et al., 2000e). Although some genetic groups of human and chimpanzee TTVs cluster to make human/chimpanzee clades, it is known that TTV variants in nonhuman primates are species-specific (Abe et al., 2000; Cong et al., 2000; Leary et al., 1999; Okamoto et al., 2000a, b, 2001a). TTVs from macaques and tamarins are increasingly divergent from TTV variants infecting humans and chimpanzees (Okamoto et al., 2000a). Farm animals are also naturally infected with species-specific TTVs: several TTV isolates have been detected in cats, dogs, chickens, sheeps and cows (Biagini et al., 2007; Brassard et al., 2008; Leary et al., 1999; Okamoto et al., 2002). Additionally, other mammals including tupaias (Okamoto et al., 2001b) and pigs (Bigarré et al., 2005; Brassard et al., 2008; Kekarainen et al., 2006, 2008; Martelli et al., 2006; McKeown et al., 2004; Niel et al., 2005; Okamoto et al., 2002) have species-specific TTVs. Both genomic organization and proposed transcriptional profile of animal TTVs are similar to those of human TTVs (Biagini et al., 2007; Inami et al., 2000; Niel et al.,
35 2005; Okamoto et al., 2000a, 2001b, 2002; Table 2; Fig. 4). However, the TTVs in animals have not been completely characterized as yet.
Table 2. TTV isolates from nonhuman primates and other mammalian species where the full nucleotide sequence is known.
36 Fig. 4 Genomic organization of 12 members of the family Anelloviridae from humans and animals.
37 Fig. 4 (continued).
38 3.1. Torque teno sus virus (TTSuV)
Genus Iotatorquevirus contains two known swine TTV species, namely Torque
teno sus virus 1 (TTSuV1) and Torque teno sus virus 2 (TTSuV2), with a genome length of approximately 2.8-2.9 Kb in size (Cortey et al., 2010; Huang et al., 2010; Niel et al., 2005; Okamoto et al., 2002). The Sd-TTV31 and Sd-TTV83 isolates represent the prototypes of these species, respectively. The Sd-TTV31 isolate had a circular genomic structure of 2,878 nt and by digestion with S1 nuclease or Mung-bean nuclease it was deduced to be single-stranded. The Sd-TTV31 isolate presents three ORFs encoding 635aa (ORF 1), 73aa (ORF 2) and 224aa (ORF 3), but lacking ORF 4 similar to tupaia TTV (Fig. 4). ORF 3 is supposed to arise from a splicing of TTV mRNA, similar to human prototype TTV. At present, the full-length genomic sequences of three TTSuV isolates have been published (Niel et al., 2005; Okamoto et al., 2002; Table 2). Two of the strains, TTV31 and Sd-TTV1p, share relatively high nucleotide sequence identity (70%), whereas strain Sd-TTV2p presents approximately 44% sequence identity to the other two strains described (Niel et al., 2005). Due to the wide genetic variability of these isolates, it has recently been suggested that Sd-TTV2p would be the prototype of a novel genogroup 2, and Sd-TTV31 the prototype of genogroup 1 (Niel et al., 2005; Okamoto et al., 2002). The Sd-TTV83 isolate, from previous studies demonstrated belonging to the second group of swine TTV (Okamoto et al., 2002), was found to consist of 2,796 nt and was 94% identical to Sd-TTV2p. These results suggest that Sd-TTV83 is classifiable into genogroup 2. TTV infection in pigs is diffused worldwide (Brassard et al., 2008; Kekarainen et Segalés, 2008). TTSuV1
39 prevalence is ranging from 24% to 100% in sera collected from different geographical regions (Bigarré et al., 2005; Gallei et al., 2010; Segalés et al., 2009; Martelli et al., 2006; McKeown et al., 2004; Taira et al., 2009; Takács et al., 2008). The prevalence of TTSuV2 has been demonstrated to be from 31% to 90% (Gallei et al., 2010; Kekarainen et al., 2006; Lee et al., 2010; Taira et al., 2009). Furthermore, in Spain, 58% and 66% of wild boars were found to be infected with TTSuV1 and TTSuV2, respectively (Martinez et al., 2006). It is probable that more than two species may exist in swine as shown for the human counterpart (Kekarainen et Segalés, 2008; Leary et al., 1999; Mushahwar et al., 1999).
TTSuV has not been shown to be pathogenic yet; however, its role during co-infection with other pathogens remains unknown. Recently, TTSuVs have been linked to economically important swine diseases, such as porcine circovirus (PCV) diseases (Ellis et al., 2008; Kekarainen et al., 2006; Krakowka et al., 2008). Post-weaning multisystemic wasting syndrome (PMWS), an important swine disease worldwide etiologically associated with porcine circovirus type 2 (PCV2) (Chae, 2005), has been correlated to the presence of TTSuV genogroup 2. Indeed, a higher prevalence of TTV infection was observed in pigs with PMWS than in those without PMWS (97% vs 78%). Of interest, pigs with PMWS resulted more likely to be infected with TTSuV 2 than the nonaffected pigs (Kekarainen et al., 2006). Nevertheless, the biological importance of the described finding has to be elucidated.
40