27
Chapter 2
NITRIC OXIDE MODULATES TISSUE PLASMINOGEN ACTIVATOR RELEASE IN NORMOTENSIVE SUBJECTS AND HYPERTENSIVE PATIENTS
28 Introduction
Vascular endothelium plays a primary role in the modulation of several important functions including the regulation of vascular tone and fibrinolysis.1 In particular, mechanisms whereby endothelium modulates fibrinolysis include the production of tissue plasminogen activator (t-PA) and its main inhibitor, the plasminogen activator inhibitor type 1 (PAI-1).1, 2 In healthy vessels, the activation of platelets or coagulation system induces a rapid release of t-PA from endothelial cells, which is a counter-regulatory mechanism to clear intravascular fibrin and therefore preventing thrombus extension. 3 It follows that, if acute t-PA release is defective, fibrinolytic activity is impaired and this alteration may contribute to atherothrombotic events. 4 Patients with essential hypertension show an increased risk of atherosclerosis and thrombotic-related complications, and a dysfunctioning endothelium represents an early major mechanism promoting the atherosclerotic process.5, 6 In hypertensive patients, endothelial dysfunction is mainly caused by an increased production of oxidative stress, leading to impaired nitric oxide (NO) availability.7 In addition to its well-documented vasoactive properties, NO also participates in maintaining the antithrombotic properties of endothelial surface by different mechanisms including the inhibition of platelet adhesion and aggregation. 1, 6
The role of NO pathway in the regulation of vascular tone is well documented.6 In contrast, its effects in promoting endogenous fibrinolysis by the regulation of endothelial t-PA release is less clear and only scanty investigated, with not conclusive results.8-10 Indeed, at the present time whether NO promotes endothelial t-PA release is still not clarified. Therefore, the first aim of the present study was to assess whether NO is directly involved in local t-PA release in the forearm microcirculation of normotensive subjects. The second aim was to evaluate whether NO availability might be involved in impaired t-PA release in essential hypertension, a clinical condition characterized by reduced NO availability 7. We believe the latter as a crucial aim with clinical
29
impact, because the restoration of NO availability might improve fibrinolysis in essential hypertension.
Material and Methods
Subjects
The study population included 34 men with essential hypertension and 30 age-matched healthy male volunteers. Patients were recruited among newly diagnosed cases in our outpatient clinic. Inclusion criteria were age between 30 and 65 years, sitting clinical blood pressure (after 10 minutes of rest) values between 140-90 and 160-99 mmHg, confirmed in two separate occasions within one month and absence of target organ damage according to European guidelines. 11 Each patient underwent a history, physical examination, ECG and routine laboratory analysis. None had evidence of hyperlipidemia, diabetes, body mass index (BMI) > 30 Kg/m2, impaired renal function or cardiovascular disease other than essential hypertension. Secondary forms of hypertension were excluded by routine diagnostic procedures. According to inclusion criteria, all were non-smokers. Patients did not take any medications for at least one month before the study. The study protocol was approved by the local ethics committee and performed in accordance to guidelines of our institution. All patients were aware of the nature, purpose and potential risks of the study and gave written consent to it. After the preliminary analysis of results obtained in 20 patients and 16 healthy subjects, power calculation of the study indicated the need to reach a sample size of 30 patients and 30 healthy subjects.
Experimental Procedure
The perfused-forearm model, which has previously been described in detail, 7 was used. Studies were performed in the morning after an overnight fast, in a temperature-controlled room.
Intravenous catheters were placed in deep antecubital veins in both arms (experimental and controlateral forearm). After subdermal administration of 1% mepivacaine, a 20-gauge
30
polyurethane catheter (BD AngiocathTM, Becton Dickinson Infusion Theraphy Systems Inc. Sandy, Utah, USA) was inserted into the brachial artery of the non-dominant arm for intra-arterial administration of drugs at systemically ineffective rates and intra-arterial blood pressure and heart rate monitoring using a digital monitor (Entour, Mennem Medical Ltd, USA).
After the placement of intravenous and intra-arterial catheters, subjects were allowed to rest 30 minutes before baseline measurement were made.
Forearm blood flow (FBF) was measured in both forearms by strain gauge plethysmography; the strain gauge was connected to plethysmography (EC-6, D.E. HokansonInc., Bellevue, WA, USA) and for each measurement, a cuff placed around the upper arm was inflated to 40 mmHg with a rapid cuff inflater (model E-10, Hokanson Inc., Bellevue, WA, USA) to occlude venous outflow from the extremity. Circulation to the hand was excluded 1 minute before FBF measurement by inflation of a pediatric cuff around the wrist at suprasystolic blood pressure. Forearm volume was measured according to the water displacement method. 12
Experimental Design
Protocol 1: Effect of acetylcholine on FBF and local t-PA and PAI-1 release
In 12 healthy subjects and 16 hypertensive patients endothelium-dependent vasodilation was estimated by performing a dose-response curve to intra-arterial acetylcholine (0.45-1.5 µg/100mL/min). A dose response curve to intra-arterial sodium nitroprusside ( 0.5-1.0 µg/100mL/min), a direct smooth muscle cell relaxant compound, was performed to assess endothelium-independent vasodilation. These rates were selected to induce a comparable vasodilation to that obtained with acetylcholine. Acetylcholine and sodium nitroprusside were infused in random order and each dose was administered for 15 minutes. A free interval of 30 minutes was allowed between the different substances.
31
In order to evaluate the effect of NO on vasodilation and endothelial release of t-PA, in 12 healthy subject and 12 hypertensive patients acetylcholine (1.5 µg/100mL/min) was infused in the absence and in the presence of intra-arterial infusion of NG-monomethyl-L-arginine (L-NMMA), a nitric oxide synthase (NOS) inhibitor (Clinalfa AG, Laufelfingen, Switzerland; 100 µg/100mL/ min). Because L-NMMA modifies blood flow, the effects of acetylcholine were evaluated in the presence of NO-clamp, which allows assessment of endothelial agonists in the presence of NOS blockade without changes in basal blood flow, thus avoiding any perturbation possibly altering net balance of t-PA. Briefly, following 10 min of L-NMMA infusion, sodium nitroprusside was co-infused at adjusted dose (0.3 and 0.4 µg/100mL/min) to neutralize the L-NMMA-induced vasoconstriction and restore baseline FBF, as previously described in detail. 13
Protocol 3: Sham study
As a control study, in an adjunctive group of 6 healthy subjects and 6 essential hypertensive patients intra-arterial saline and glucose solution 5% were injected at the same infusion rates than those utilized for acetylcholine and sodium nitroprusside, respectively.
Blood Sampling and Biochemical Measurements
At baseline, venous blood samples were collected in lithium-heparin or EDTA tubes and immediately placed on ice. Plasma was immediately centrifuged and stored at –70°C until assayed. Total serum cholesterol, triglycerides, HDL-cholesterol and plasma glucose were assessed by enzymatic methods (Roche, Diagnostic, Mannheim, Germany). LDL-cholesterol was calculated with Friedewalds’ equation.
Before measurement of FBF, simultaneous arterial and venous samples were obtained from the infused arm before and after each dose of study drugs or vehicles. Infusions were interrupted during arterial sampling. All samples were obtained after the first 4 mL of blood were discarded. Blood samples were collected in tubes containing 1/9 vol 0.45mol/L sodium citrate buffer (Vacutainer), kept on ice and centrifuged immediately at 4°C and 3000g for 15 minutes; plasma was stored at
-32
70°C until time of assay. Plasma concentration of t-PA and PAI-1 antigens were determined by enzyme-linked immunosorbent assay (ELISA, Tecnoclone GmbH, Vien, Austria). All samples were assayed in duplicate on the same test plate. Intra-assay and inter-assay variation coefficients were <10%.
Data analysis
Forearm plasma flow was determined by FBF and hematocrit. Net release or uptake rates for t-PA were calculated by the following formula: net release=(Cv-Ca) x [FBF x (101-hematocrit)/100], where Cv and Ca are the venous and arterial concentrations, respectively. The total amount of t-PA released was calculated using the area under curve formula for repeated measure. 14 Study population characteristics, basal venous, arterial, venous-arterial concentrations and t-PA balance at baseline were compared using Wilcoxon test. Responses to acetylcholine and sodium nitroprusside were analyzed by one-way (infusion) and two-way (group and infusion) ANOVA for repeated measures. Results were expressed as mean±SEM, except for those shown in table 1 (mean±SD). Findings were considered significant at p<0.05. Computations for the power calculation and for the statistical methods were performed with the use of the SAS System.
Drugs
Acetylcholine HCl (Farmigea SpA), L-NMMA (Clinalfa AG) and sodium nitroprusside (Malesci SpA) were obtained from commercially available sources and diluted to the desired concentration by the addition of normal saline. Sodium nitroprusside was dissolved in glucose solution 5% and protected from light by aluminum foil.
33 Results
Clinical hemodynamic and humoral characteristics of the study population are shown in Table 1.
Table 1. Clinical Characteristics of Study Group
Parameters Normotensive Subjects
(n=30) Hypertensive Patients (n=34) Age (years) 44±7 48±10 Gender (male/female) 30/0 34/0 Smokers (yes/no) No No SBP (mmHg) 129±8 156±11* DBP (mmHg) 81±6 95±4* BMI (Kg/m2) 25.4±2.4 26.2±3.4 Total cholesterol (mg/dl) 210±34 194±37† HDL cholesterol (mg/dl) 46±14 49±13 LDL cholesterol (mg/dl) 123±28 118±21 Plasma glucose (mg/dl) 90±10 87±12
Plasma homocysteine (µmol/l) 10.3±4.4 9.5±7.9
Plasma folate (ng/ml) 6.7±3.4 6.5±2.4
Plasma vitamin B12 (pg/ml) 311.5±124.9 470.5±354.5
CRP (mg/l) 4.4±3.3 4.6±3.6
Data are presented as the mean value ± SD or number of subjects. SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; HDL: high density lipoprotein; LDL: low density lipoprotein; CRP: C Reactive Protein. *p<0.0001 vs. normotensive subjects; † p<0.05 vs.
normotensive subjects
Protocol 1: Effect of acetylcholine on FBF and local t-PA and PAI-1 release Forearm Blood Flow
Vasodilation to acetylcholine was significantly (p<0.001) reduced in hypertensive patients, as compared to controls (Figure 1). The vascular response to sodium nitroprusside was found to be similar between the two groups (Figure 1). In both normotensive subjects and essential hypertensive patients, contralateral FBF did not change throughout the study (data not shown).
34
Figure 1. Effect of acetylcholine and sodium nitroprusside on forearm blood flow (FBF) and t-PA release in normotensive subjects and essential hypertensive patients.
Acetylcholine (A) and sodium nitroprusside-induced (B) increase in FBF in normotensive subjects (solid circles) and hypertensive patients (open circles). Effect on t-PA balance following the infusion of acetylcholine (C) and sodium nitroprusside (D) in normotensive subjects (solid circles) and hypertensive patients (open circles). Data are shown as mean±SEM. *p<0.05;† p<0.01 vs baseline.
Fibrinolytic components release
At baseline, arterial and venous concentrations of t-PA antigen were lower in hypertensive patients as compared to normotensive subjects (Table 2; p<0.01), with a lower venous–arterial difference among hypertensive patients as compared to controls (Table 2; p<0.05). As a consequence, the basal release of t-PA across the forearm was significantly reduced in hypertensive patients when compared to normotensive subjects (Figure 1; p<0.01).
35
Table 2. Arterial (A), venous (V) and venous-arterial (V-A) concentrations gradient of t-PA and PAI-1 at baseline and following the infusion of acetylcholine and sodium nitroprusside.
Drug doses (µµµµg/100ml tissue/min) Normotensive Subjects (n=12) Hypertensive Patients (n=16) t-PA A t-PA V t-PA V-A PAI-1 A PAI-1 V PAI-1 V-A t-PA A t-PA V t-PA V-A PAI-1 A PAI-1 V PAI-1 V-A Baseline 6.7±0.5 7.2±0.6 0.5±0.1 36.6±3.0 39.9±2.4 3.5±0.4 1.6±0.2 1.6±0.2* 0.1±0.0* 39.4±3.1 43.7±3.0 4.3±0.4 ACh (0.45) 6.8±0.6 7.3±0.5 0.6±0.2 30.8±2.5 32.7±2.5 1.8±0.9 1.3±0.3 1.4±0.2 0.1±0.1 35.2±2.6 37.1±2.3 2.0±0.7 ACh (1.5) 6.8±0.6 8.0±0.3† 1.3±0.1† 30.2±2.6 31.3±1.9 1.0±0.6 1.5±0.3 1.6±0.3‡ 0.1±0.1‡ 30.2±1.9 31.8±2.4 1.7±0.8 Baseline 7.1±0.4 7.6±0.5 0.4±0.2 36.4±2.5 39.4±2.0 3.0±0.4 1.4±0.3 1.6±0.3 0.2±0.1* 35.8±1.6 39.5±2.9 3.7±0.4 SNP (0.5) 7.2±0.5 7.5±0.6 0.3±0.1 34.1±2.8 35.6±1.8 1.6±0.6 1.3±0.2 1.5±0.3 0.2±0.1 34.3±2.5 36.2±2.5 2.0±1.0 SNP (1.0) 7.2±0.6 7.5±0.5 0.2±0.1 34.4±2.3 35.4±2.7 0.9±0.3 1.4±0.3 1.5±0.2 0.1±0.1 32.1±2.0 33.0±3.0 1.0±0.8
Data are presented as the mean value ± SEM. Ach: acetylcholine; PAI-1: plasminogen activator inhibitor type; SNP: sodium nitroprusside; t-PA: tissue-type plasminogen activator. * p<0.05 vs. normotensive subjects; † p<0.05 vs. basal levels; ‡ p<0.01 vs. normotensive subjects.
36
Venous concentration of t-PA antigen significantly progressively increased during the infusion of acetylcholine in normotensive subjects, an effect not observed in hypertensive patients (Table 2). Since the arterial t-PA antigen concentrations in both normotensive and hypertensive groups did not alter significantly under agonists administration, during acetylcholine infusion the venous-arterial concentration gradient of t-PA antigen significantly increased in normotensive subjects but not in hypertensive patients (Table 2). No increase of both venous and venous-arterial concentrations of t-PA was observed during sodium nitroprusside infusion in both groups (Table 2).
Concerning t-PA balance, acetylcholine infusion induced a significant, dose-dependent increase in net t-PA release across the forearm in normotensive subjects but not in hypertensive patients (Figure 1).
The cumulative amount of t-PA antigen secreted during acetylcholine infusion was also significantly reduced in the hypertensive as compared to the normotensive group (Figure 2).
During sodium nitroprusside infusion, no significant increase in t-PA release in both normotensive and hypertensive group was seen (Figure 1).
Arterial and venous concentrations of PAI-1 antigen at baseline were similar in both normotensive and hypertensive subjects and no significant difference in arterial, venous and venous-arterial concentration gradient of PAI-1 was observed after the infusion of acetylcholine and sodium nitroprusside in both groups (Table 2). No significant difference in PAI-1 antigen balance resulted after the infusion of acetylcholine and sodium nitroprusside, in both normotensive and hypertensive groups (Figure 3). Contralateral venous-arterial concentrations of fibrinolytic components did not change throughout the study in both normotensive subjects and essential hypertensive patients (data not shown).
37
Figure 2. Amount of t-PA release in response to acetylcholine.
Cumulative amount of t-PA antigen (area under the curve) secreted during acetylcholine infusion in normotensive subjects and essential hypertensive patients. Data are expressed as the mean value ± SEM. *p<0.001 vs normotensive controls.
38
Figure 3. PAI-1 balance during acetylcholine and sodium nitroprusside infusions.
Effect of acetylcholine and sodium nitroprusside on PAI-1 balance in normotensive subjects (solid circles) and essential hypertensive patients (open circles). Data are expressed as mean value ± SEM.
Protocol 2: Effect of NOS inhibition on stimulated t-PA release Forearm blood flow
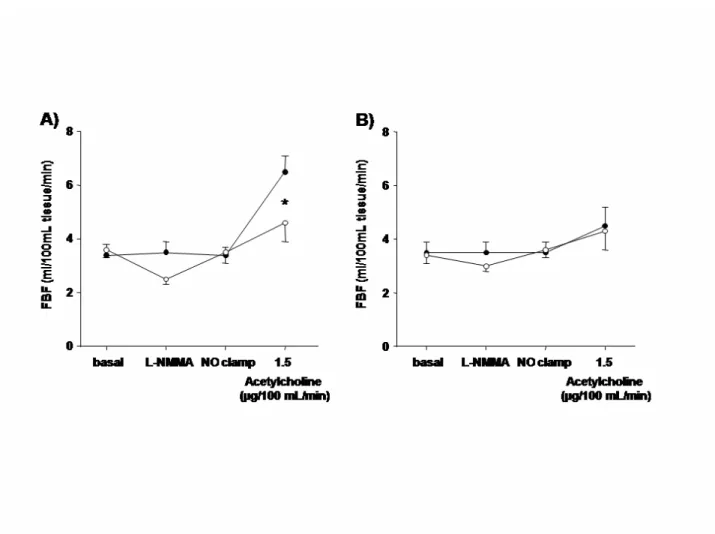
In normotensive subjects, L-NMMA infusion, which reduced basal FBF, significantly blunted the vasodilation to acetylcholine (Figure 4). In contrast, in hypertensive patients, the NOS inhibitor caused a smaller decrease in FBF and failed to affect the response to acetylcholine (Figure 4). In both normotensive subjects and essential hypertensive patients, contralateral FBF did not change throughout the study (data not shown).
39
Figure 4. Vasodilation to acetylcholine in the absence and presence of NOS inhibition.
Acetylcholine-induced increase in FBF during saline (solid circles) or L-NMMA (open circles) in normotensive subjects (A) and hypertensive patients (B). * p<0.05 vs saline infusion
Fibrinolytic components release
In normotensive subjects, L-NMMA infusion decreased basal t-PA release (Figure 5). Moreover, in the presence of L-NMMA, acetylcholine induced t-PA release was impaired (Figure 5). In contrast, in hypertensive patients, no significant change on t-PA release was observed at the end of L-NMMA alone infusion (Figure 5) as well as during L-L-NMMA plus acetylcholine (Figure 5). In both normotensive subjects and essential hypertensive patients, contralateral venous-arterial concentrations of t-PA antigen did not change throughout the study (data not shown).
40
Figure 5. Tonic and stimulated t-PA balance in the absence and presence of NOS inhibition.
A) Tonic t-PA balance before (solid bars) and after (empty bars) L-NMMA infusion in normotensive subjects and hypertensive patients. * p<0.05 vs baseline.
B) Stimulated t-PA balance following acetylcholine (ACh) alone (solid bars) or in the presence (empty bars) of L-NMMA in normotensive subjects and essential hypertensive patients (right). Data are expressed as mean value ± SEM. * p<0.05 vs ACh alone.
Protocol 3: Sham study
FBF did not change significantly during the infusions of saline and glucose solution 5% in both normotensive subjects (FBF, saline: from 3.3±0.4 to 3.4±0.5 ml/100mL/min; glucose: from 3.2±0.5 to 3.4±0.5 ml/100mL/min) and hypertensive patients (FBF, saline: from 3.3±0.3 to 3.4±0.3 ml/100mL/min; glucose: from 3.4±0.5 to 3.5±0.6 ml/100mL/min).
No significant difference in t-PA balance was observed after the infusion of saline and glucose solution 5% in both normotensive subjects (saline: from 1.6±0.1 to 1.7±0.2 ml/100mL/min; glucose: from 1.5±0.2 to 1.6±0.3 ml/100mL/min) and hypertensive patients (saline: from 0.4±0.1 to
41
0.5±0.3 ml/100mL/min; glucose: from 0.5±0.2 to 0.6±0.1 ml/100mL/min). Similarly, no effect on PAI-1 balance was observed during the infusions in both groups (data not shown).
Discussion
The present study shows that local intra-arterial infusion of acetylcholine, an endothelium-dependent agonist, induces a rapid release of t-PA across the forearm in normotensive subjects. Since sodium nitroprusside administration, despite a similar blood flow increment, had no such effect, these results confirm that the t-PA release is an endothelial property not dependent on flow increase.15-17 Worth noting, sodium nitroprusside is an exogenous NO donor, acting directly on vascular smooth muscle cells. 18 Our finding that this compound failed to stimulate t-PA release reinforces the concept that the relationship between NO and fibrinolysis mainly occurs within endothelial cells. While evidence is concordant on the possibility to release t-PA by receptor-operated agonists, including metacholine 15, 17 and bradykinin, 16, 19 results with acetylcholine are discordant. 16, 19 However, a likely explanation is the short duration of acetylcholine infusion in negative papers (5 min per dose) 16 as compared to our experimental conditions (15 min per dose). On the other hand, both metacholine (a stable derivative of acetylcholine) and acetylcholine stimulate the same muscarinic receptors 20 and therefore any possible difference in results should be related to methodological problems.
In the present study we also observed that in our hypertensive patients, characterized by a reduced endothelium-dependent vasodilation, acetylcholine administration failed to increase t-PA release, thus indicating an impairment of t-PA release in essential hypertension.
The concept of impaired t-PA release in response to endothelial stimuli in essential hypertension was previously assessed in a small population demonstrating an impaired endothelial t-PA release following desmopressin infusion. 21 In addition, Ridderstrale et al showed an impaired release of t-PA in response to substance P in essential hypertension patients. 22
42
The demonstration that different endothelial agonists revealed an impaired endothelial t-PA release, suggests that this fibrinolytic alteration is not related to a specific defect of the muscarinic receptor for acetylcholine or to an abnormality of a single intracellular signal-transduction pathway but to a more generalized abnormality of endothelial fibrinolytic properties in essential hypertension. However, our data are partially in contrast with results obtained from Hrafnekelsdottir et al. 21 who observed no difference in terms of t-PA release between normotensive controls and hypertensive patients when metacholine was utilized. A likely explanation for this discrepancy could be that a smaller group of hypertensive patients (n=7) was evaluated in the Hrafnekelsdottir report, by utilizing a weaker stimulus.21
The major novel finding of the present study concerns the demonstration in humans that endothelial NO is involved in t-PA release modulation. To test NO availability we infused L-NMMA to block NO synthase. In normotensive subjects, as previously demonstrated, 7 vasodilation to acetylcholine is blunted by L-NMMA, confirming the preservation of NO availability in healthy conditions. It is worth noting that in these subjects, when L-NMMA was infused, we also observed a decrease in the venous t-PA concentration. Since FBF did not change because of the application of the NO-clamp technique, these results indicate a reduced basal t-PA release across the forearm vascular bed. In addition, L-NMMA infusion, which blocked agonist induced NO release, blunted stimulated t-PA release in the forearm vascular bed. Taken together, these findings indicate a positive modulating effect of NO on tonic and stimulated endothelial t-PA release in healthy subjects.
In contrast, in essential hypertensive patients, vasoconstriction to L-NMMA was significantly reduced as compared to controls, confirming the presence of impairment basal NO release. 23 Interestingly, in hypertensive patients basal t-PA release was almost abolished and therefore it was not possible to detect any eventual further reduction induced by L-NMMA administration. In addition, in essential hypertensive patients the reduced vascular response to acetylcholine was totally resistant to L-NMMA, thus confirming the evidence of impaired NO availability in essential hypertension. 7 Moreover, no modification on acetylcholine-induced t-PA release was observed
43
under the simultaneous L-NMMA infusion. These data confirm and strengthen the concept of a strong relationship between NO and t-PA release. Indeed, in a clinical condition characterized by a reduced NO availability, such as essential hypertension, 7 a blunted profibrinolytic property of endothelial cells occurs. Our results are in line with those obtained in previous animal models, 24, 25 where a strict relationship between endothelial t-PA release and NO pathway was documented. This hypothesis was already assessed in humans, with conflicting results. According to our results, Newby et al 8 reported a blunted t-PA release secondary to substance P administration in the presence of L-NMMA in the forearm microcirculation of healthy subjects. In contrast it was reported an increased t-PA release induced by bradykinin in the presence of L-NMMA. 9 These conflicting results may be related to the higher dose of L-NMMA used in the latter study (5 mg/min) as compared to that used in our and Newby’s report (4 µmol/min ≈ 1 mg/min). 8
Since the calculation of net t-PA balance is a function of plasma flow, it is conceivable that the greater perturbation in blood flow secondary to high L-NMMA dose might account for the increase of t-PA balance in these patients. 9 It is worth noting that in our experimental condition, the utilization of NO-clamp, which restored basal FBF after L-NMMA-induced vasoconstriction, overwhelmed this methodological limit. Finally, Brown et al 10 observed a lacking effect of L-NMMA infusion on bradykinin-stimulated t-PA release in healthy subjects, arguing against a modulatory influence of NO on endothelial fibrinolytic capacity. However, in this study L-NMMA infusion induced only a slight reduction of vascular response to bradykinin, 10 thus suggesting an incomplete eNOS inhibition, a finding which does not provide conclusive results.
Perspectives
The results of the present study demonstrate that both tonic and agonist-induced release of NO are directly involved on t-PA release by endothelial cells. Essential hypertension, characterized by a reduction in tonic and stimulated NO availability, is also associated with impaired capacity of t-PA release, suggesting a major role of impaired NO availability in worsening both vasodilation and
44
endothelial t-PA release. When considering that NO plays a prominent role in the modulation of vascular tone and of endothelial fibrinolytic properties as well, it is conceivable that both these functions may contribute to the risk of atherothrombotic events in essential hypertensive patients. 2, 26
The definition of additional aspects characterizing endothelial dysfunction may increase the knowledge of the pathophysiology of atherothrombosis and provide further information for the development of specific strategies of treatment in essential hypertension.
45 References
1. Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:329-341.
2. Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470-2479. 3. Emeis JJ. Regulation of the acute release of tissue-type plasminogen activator from the
endothelium by coagulation activation products. Ann N Y Acad Sci. 1992;667:249-258.
4. Newby DE, McLeod AL, Uren NG, Flint L, Ludlam CA, Webb DJ, Fox KA, Boon NA. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001;103:1936-1941.
5. Taddei S, Salvetti A. Endothelial dysfunction in essential hypertension: clinical implications. J Hypertens. 2002;20:1671-1674.
6. Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233-246.
7. Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222-2229.
46
8. Newby DE, Wright RA, Dawson P, Ludlam CA, Boon NA, Fox KA, Webb DJ. The L-arginine/nitric oxide pathway contributes to the acute release of tissue plasminogen activator in vivo in man. Cardiovasc Res. 1998;38:485-492.
9. Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial release of tissue-type plasminogen activator in the human forearm: role of nitric oxide. J Cardiovasc Pharmacol. 2003;42:311-314.
10. Brown NJ, Gainer JV, Murphey LJ, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation. 2000;102:2190-2196.
11. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011-1053.
12. Whitney RJ. The measurement of volume changes in human limbs. J Physiol. 1953;121:1-27. 13. Virdis A, Ghiadoni L, Cardinal H, Favilla S, Duranti P, Birindelli R, Magagna A, Bernini G,
Salvetti G, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction induced by fasting hyperhomocystinemia in normotensive subjects and patients with essential hypertension. J Am Coll Cardiol. 2001;38:1106-1115.
14. Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916-931.
15. Jern S, Selin L, Bergbrant A, Jern C. Release of tissue-type plasminogen activator in response to muscarinic receptor stimulation in human forearm. Thromb Haemost. 1994;72:588-594. 16. Brown NJ, Gainer JV, Stein CM, Vaughan DE. Bradykinin stimulates tissue plasminogen
47
17. Stein CM, Brown N, Vaughan DE, Lang CC, Wood AJ. Regulation of local tissue-type plasminogen activator release by endothelium-dependent and endothelium-independent agonists in human vasculature. J Am Coll Cardiol. 1998;32:117-122.
18. Schultz K, Schultz K, Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977;265:750-751.
19. Rosenbaum DA, Pretorius M, Gainer JV, Byrne D, Murphey LJ, Painter CA, Vaughan DE, Brown NJ. Ethnicity affects vasodilation, but not endothelial tissue plasminogen activator release, in response to bradykinin. Arterioscler Thromb Vasc Biol. 2002;22:1023-1028.
20. Bruning TA, Chang PC, Kemme MJ, Vermeij P, Pfaffendorf M, van Zwieten PA. Comparison of cholinergic vasodilator responses to acetylcholine and methacholine in the human forearm. Blood Press. 1996;5:333-341.
21. Hrafnkelsdottir T, Wall U, Jern C, Jern S. Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet. 1998;352:1597-1598.
22. Ridderstrale W, Ulfhammer E, Jern S, Hrafnkelsdottir T. Impaired capacity for stimulated fibrinolysis in primary hypertension is restored by antihypertensive therapy. Hypertension. 2006;47:686-691.
23. Taddei S, Ghiadoni L, Virdis A, Buralli S, Salvetti A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100:1400-1405.
24. Klocking HP. Release of plasminogen activator by acetylcholine from the isolated perfused pig ear. Thromb Res. 1979;16:261-264.
25. Emeis JJ. Perfused rat hindlegs. A model to study plasminogen activator release. Thromb Res. 1983;30:195-203.