Accademic Year
2016/2017
PhD Course Agrobiosciences
Exploiting the genetic diversity of Triticum urartu
for the identification of genes involved in
adaptation to climate change
Author
Alice Brunazzi
Supervisor
1
To Francesca
The secret of improved plant breeding, apart from scientific knowledge, is love
2
OUTLINE
ABSTRACT
4
CHAPTER I
6
WILD WHEAT RELATIVES: THE KEY FOR GLOBAL FOOD
SECURITY AND RESPONSE TO CLIMATE CHANGE
6
WHY WILD WHEAT RELATIVES 9
WHEAT EVOLUTION AND DOMESTICATION 10
Triticum urartu 12
GENETIC VARIATION AND PLANT BREEDING 14
DOUBLED HAPLOID TECHNOLOGY 15
REFERENCES 22
CHAPTER II
30
AIMS OF THE THESIS
30
REFERENCES 32
CHAPTER III
34
ASSEMBLY OF THE COLLECTION OF T. URARTU AND
GEOGRAPHIC CHARACTERIZATION OF THE
COLLECTION SITES
34
MATERIALS AND METHODS 35
RESULTS AND DISCUSSION 37
REFERENCES 44
3
LANDSCAPE GENOMICS
46
ABSTRACT 47 INTRODUCTION 48 RESULTS 57 DISCUSSION 62 REFERENCES 66CHAPTER V
109
SEED ANALYSIS
109
MATERIAL AND METHODS 109
RESULT AND DISCUSSION 113
CONCLUSION 117
CHAPTER VI
119
NEW TOOLS FOR WHEAT IMPROVEMENT
119
DOUBLED HAPLOIDS TECHNOLOGY 119
PROCEDURES 120 CONCLUSIONS 126 REFERENCES 128
CHAPTER VII
129
PUBLICATIONS 129 DIDACTIC ACTIVITIES 130 ACKNOWLEDGEMENTS 1314
ABSTRACT
The adaptation of agriculture to climate change is one of the most important challenges of our time and requires a broadening of the bases of crop genetic diversity. This is well true for wheat where new allelic variation is necessary to breeding to produce varieties withstanding new abiotic and biotic stresses in a sustainable way. Natural populations of Triticum urartu, the wild donor of the Au genome to both common and durum wheat, may represent an important reservoir of adaptive genes/alleles useful for wheat improvement that diffused to diverse environments and adapted locally under natural selection.
This PhD thesis focuses on the assembly and characterization of the most complete collection of 428 natural accessions of T. urartu, representing the whole distribution of this species across the Fertile Crescent, where wheat was domesticated. Sampling sites of the collection were used in a geographic information systems (GIS) analysis in order to extract environmental information relative to T.
urartu populations. The observed environmental heterogeneity in both temperature
and precipitations of the sampling area confirms that our T. urartu collection is representative of several microclimates in which this species is still growing. The molecular variation existing within the collection was evaluated by a double-enzyme RAD sequencing protocol, producing tens of thousands of molecular markers that were used to study the phylogenic relationship and genetic structure existing among the T. urartu accessions of our collection. The analysis revealed a complex pattern for T. urartu genetic diversity: accessions are in fact highly differentiated and poorly
5
structured. Geographic and molecular data were combined and used to identify adaptive alleles through a landscape genomics approach. The genomic loci resulting from this analysis represent promising tools to study and exploit the genetic basis of environmental adaptation in wheat. As a first glimpse into the phenotypic variation, we investigate the genetic diversity for grain shape of our T .urartu collection. Ten mature spikelets of each accession were hand-threshed and the obtained seeds were scanned to collect the corresponding perimeter, area, minor and maximum axis. A broad seeds variation was observed in all the analyzed traits and accessions sampled in Iraq are always differentiated from all the others suggesting that may belong to a different sub- species of T. urartu. Thanks to this preliminary analysis, the basis for future genome-wide association studies were set. In parallel to these discoveries, this thesis reviews the current status of research on doubled haploid production in wheat considering the methodology currently applied at the Heartland Plant Innovation Center Inc. in Kansas (USA). Doubled haploid production in fact bears the promise to speed up the production of wheat lines from segregating material. Together, these approaches will empower wheat breeding with new tools to speed up the production and release of new improved varieties addressing the challenges posed by climate change.
6
CHAPTER I
WILD WHEAT RELATIVES: THE KEY FOR GLOBAL FOOD SECURITY AND RESPONSE TO CLIMATE CHANGE
Food security is a very flexible concept as reflected in the many attempts at definition in research, social sciences and policy usage. See for instance to the definition proposed by the World Food Summit in 1996:
“Food security exists when all people, at all times, have physical, social and economic access to sufficient safe and nutritious food that meets their dietary needs and food preferences for an active and healthy life”1
Based on that definition, four main dimensions of food security can be identified: food availability, access, utilization and stability2 (Fig. 1).
Figure 1. The four dimensions of food security and their determinants (adapted from
FAO)
According to the World Food Programme (http://www1.wfp.org/), climate change, a long-term change in the earth's climate, is one of a variety of threats to food security and affects all dimensions of food security and nutrition, as indicated here below.
7
• Food availability: switches in meteorological conditions have already afflicted the production of several world key crops especially cereals, and imminent climate changes threaten to aggravate this troubling scenario3.
Higher temperatures will have a significant brunt on yield although changes in precipitations could affect both crop quality and quantity4.
• Food access: climate change could influence the prices of main crops in various lands of the world. For the most susceptible communities, lower agricultural gains means lower incomes. Under these circumstances, the poorest people — who already use most of their livelihood on food — loss additional income and other resources to meet their alimentary requirements5.
• Food utilization: climate-related risks affect calorie intake, particularly in areas where costant food insecurity is already a significant issue. Changing climatic conditions could also create a violent cycle of disease and hunger. Nutrition is likely to be affected by climate change through related impacts on food security, dietary diversity, care habits/traditions and health6.
• Food stability: the atmospheric variability produced by more persistent and severe unfavorable weather events can defeat the stability/solidity of individuals and government food security actions, resulting in fluctuations in food availability, access and utilization7,8.
Nowadays, the diversification of production could be considered as one way to increase resilience of farming systems to shocks in an environment of increasing uncertainties9. For an efficient and decisive adaptation to climate change it will be
8
also required access (both physical and legal through appropriate intellectual property rules) to plant genetic resources, both of existing crops, livestock and their
wild relatives, as well as old varieties that may be used in the near future10. For this
reason, farmers, public and private sector institutions, research communities, and governments need to increase cooperation and ensure dissemination, distribution and creation of knowledge and transfer of technologies to characterize, conserve and curate genetic resources both in situ and in germplasm banks and related facilities to support adaptation to climate change11–13. These actions will be vital to improve the living conditions of farmers and rural populations across the globe.
Wheat (Triticum spp.) is considered a major food crop critical to global food security. The current increase in wheat production of around 1% per year is not keeping pace with the rate of yield growth required to achieve the target of doubling crop production by 205014. Future challenges of crop improvement could be seriously advanced by an effective utilization of the vast genetic resources for abiotic and biotic adaptive tolerance available in natural populations of crop progenitors. Using artificial populations of established crops (elite varieties) as a starting point for breeding, in fact, dramatically reduces the genetic variation among which to search for adaptive loci because of historical and present diversity bottlenecks15. Focusing on natural populations permits to exploit at the same time their diversity and its relation to pedo-climatic features, in order to discover alleles of agronomic relevance.
9
The main focus of this PhD thesis activities is to address the issues of the use and the exploitation of Crop Wild Relatives (CWR) in modern wheat breeding highlighting the reasons why they are so important to conserve and characterized. Particularly, we focused on the Wild Wheat Relative (WWR) Triticum urartu pointing out its role in wheat evolution and overviewing its botanical and genetic features. However, since being able to transfer the genetic variation present in the wild relative T. urartu still remains a laborious process and, for complex characters, largely unfulfilled16, the last section of this chapter is dedicated to analyze the peculiar properties of the doubled haploids technology that allows the development of large number of homozygous lines and the development of markers for useful traits in much less time17.
WHY WILD WHEAT RELATIVES
The acronym CWR refers to crop progenitors as well as other plant species more or less closely related to crops18. CWR are an inestimable source of genes/alleles for
resistance to both biotic stresses, such as diseases and pests, and abiotic stresses such as drought, salt, flooding and extreme temperatures. For the exploitation of CWR in plant breeding, few points are highlighted. First of all, it is crucial to cross the selected crop under study with a wild relative that show the character(s) we are looking for. Then, after collecting the hybrid progeny, is necessary to apply the backcrossing technique over various (usually 6-7) generations with the aim of collecting a new line with the requested new character introgressed19. However,
10
plant breeders and geneticists generally have to deal with some major hurdles. In fact, it is not easy to eradicate in the new hybrids and in all the successive generations of offspring, unwanted traits derived from the native relative and surely additional backcrossing could seriously slow down and delay the development of new plant varieties. Another underestimate factor is the fact that wild relatives showed poor interspecific crossability: many CWR are difficult to cross with their relative crop and sometimes, even when a specific and working-crossing protocol is developed, the hybrid offspring may be infertile. Thanks to recent advances in plant biotechnologies, specific and successfully tissue culture protocols and hybridization techniques can be now used to go beyond these troubles and help the obtained new lines of any particular cross throughout the primary critical step of crossing and then backcrossing.
CWR are used by scientist to create new varieties containing valuable traits that were thought to have been completely lost forever especially by thousands of years of farmer selection and then by the last century of more intense plant breeding mostly focused on yield performance. Triticum spp. was on of the first domesticated species and was the result of spontaneous crossings of different wild monocots grasses, the wild relatives of wheat. Thus, the next paragraph is dedicated to analyze how modern wheat evolved.
WHEAT EVOLUTION AND DOMESTICATION
The evolution of wheat was mostly dependent on the phenomenon of natural hybridization and surely cytogenetics has made a major contribution to our present
11
understanding. Previous studies relying mostly on disciplines such as botany, genetics and archeology demonstrated that all the cultivated wheat belongs to the genus Triticum, which was divided by Schultz20 into three major taxonomic groups:
• einkorn (2n = 2x = 14)
• emmer (2n = 4x = 28)
• dinkel (2n = 6x = 42)
all with the basic chromosome number x = 7. Soon after, based on specific analysis by Sakamura21, it was coined the genome formula for einkorn (Ae. aegilopoides L.,
T. monococcum L. and T. urartu, 2n = 2x = 14), emmer (T. turgidum L., 2n = 4x =
28), and dinkel (T. aestivum L., 2n = 6x = 42) as AmAm, AuAuBB, and AuAuBBDD, respectively21. The most recent and accepted studies22 described T. aegilopoides, formerly Ae. boeoticum L. as the wild progenitor of T. monococcum. Instead, T.
aestivum as an allopolyploid produced from two autonoumus hybridization events
occurred without the involvement of the Am genome (Fig. 2). Each hybridization was followed by chromosome doubling in the fertile plants. The initial hybridization, that occurred approximately 500,000 years ago, is now believed to have been between the two grass species T. urartu (the Au genome donor), and presumably Ae.
speltoides (the B genome donor) or a wild species today extinct23,24,25. This new species is tetraploid (four complete genome complements). Hexaploid wheat arose as a result of a second hybridization between the new tetraploid species and a third diploid species, Ae. tauschii var. strangulata (previously denominated as T. taushii, the D genome donor). Again, chromosome doubling must have occurred in order to
12
produce fertile plants. This new species has 42 chromosomes that is, six complete genomes each with a complement of 7 chromosomes.
Figure 2. Evolution and genome relationship between the Triticum taxa (adapted
from Matsuoka25).
Triticum urartu
Triticum urartu L. (2n=2x=14; genome AuAu) thus is the A-genome donor of cultivated tetraploid (T. turgidum L.; 2n=2x=28; genome AuAuBB) and hexaploid wheat (T. aestivum L.; 2n=2x=42; genome AuAuBBDD), it was never domesticated26 and is still found in the Near East27 as natural populations (Fig. 3).
13
Figure 3. A: Detail of the Triticum urartu spike. B: Triticum urartu spikelet and its
seeds
Botanically, T. urartu is a herbaceous annual plant up to 1.80 m in height. The stems are erect and hollow inside except at the nodes. Stems growth is apical and also produced by stretching of the meristems above the nodes (tillering). Leaves grow from the nodes. Flowers are gathered in spikes and each consisting of a main axis called rachis on which the spikelets are distributed laterally.Each spikelet comprises an axis, the rachilla, which bears two glumes and a specific number of florets. Within each spikelet, there are usually two (from two to four in wheat) potentially fertile florets. The floret has two sheathing structures, the outer lemma and the inner palea that envelope two lodicules, three stamens and the carpel. Each stamen is made up of a filament, which is very short and a anther. The anther is about 3 to 5 mm long and has four chambers or loculi were micro-sporogenesis occurs. The spherical pollen grain has a small circular pore and contains a single nucleus and starch
14
grains28. The basal part of the carpel, the ovary, can be obconical or obovate and white in color with a smooth surface except at the tip, which has numerous unicellular hairs. The ovary contains a single ovule oriented so that the nucellar apex (micropyle) is slightly below the horizontal mid-plane of the ovule.
Triticum urartu chormosomes, being the donor of Au genome, are homoeologous to the chromosomes of the AuAu genome of durum and bread wheat, making it a good candidate for the discovery of loci that could be useful for cultivated wheat. This can be done either by targeting homologous sequences, or by direct hybridization, enabling an efficient transfer of desirable alleles from wild chromosomes into their cultivated homoeologues29.Therefore T. urartu is crucial to
characterize and preserve. Untended by humans, T. urartu continued to evolve in the wild and developed traits, such as resistance to fungal diseases including powdery mildew and leaf rust30, that farmers and breeders can exploit and pass to
domesticated wheat in order to produce new improved varieties. The availability of
the T. urartu genome sequence31 and the application of high-throughput sequencing
technologies allows to use this resource as a benchmark for the identification of loci of agronomic interest
GENETIC VARIATION AND PLANT BREEDING
The presence of genetic variation among plant species is an essential condition for setting up all plant breeding programs. The main task of the plant breeder is indeed to create an improved variety for specific trait(s) starting form a collection of plants with high genetic diversity. By selecting two parents that are genetically similar, a
15
breeder restricts the amount of variation that will be evaluated in the offspring. Contrarily, by crossing two genetically divergent parents, the range of phenotypic variation will be much more extensive. Thus, if a plant breeder is interested in innovation and wishes to generate maximum wide crosses wild relatives or landraces are a very valuable option32. With time and thanks to recent advances in plant technology, breeders have developed different methods to fix and produce highly homozygous genotypes from such generated variation, instead of using conventional inbreeding methods such as, for instance, single seed descent and backcrossing that are extremely time consuming and expensive. Furthermore, in conventional plant breeding, truly homozygous lines are quite rare and most selections still contain a number heterozygous loci33.
The Doubled Haploids (DH) technology can be considered as one of the most efficient method that could help plant breeders and geneticists to reduce the time they need to develop homozygous lines and implicitly create new platforms for the identification of genes responsible for the variation of adaptation and agronomic traits. In the next section, the state of the art of this innovative technology focusing on the DH production in wheat is discussed.
DOUBLED HAPLOID TECHNOLOGY What are haploids?
The term haploid refers to a plant or an embryo or a cell that contains a gametic (haploid, n) set of chromosomes34. All their genes are hemizygous and each gene has
16
Blakeslee first described this phenomenon in Datura Stramonium35,36. Subsequently,
the phenomenon was found to occur in many crop species such as cotton, tomato, soybean etc.. In general, haploids can be divided into three types37:
• maternal haploid: they contain only nuclear material and cytoplasm from the maternal parent, and either result from the elimination of the chromosome provided by the paternal parent during the embryo development or by paternal pollen that are incapable of fertilization;
• in vitro androgenic haploid: they can be obtained through anther or microspore culture and contain both the cytoplasm and nucleus of the developing microsporocyte;
• in vivo androgenic haploid: they arise by in vivo embryogenesis. This class of haploids develops from an egg cell or any other cell of the embryo sac with the chromosomes of the maternal parent being lost during embryogenesis. Such haploids contain the cytoplasm of the maternal plant and the chromosomes of only the paternal parent.
However, spontaneous occurrence is a rare event and therefore of limited practical value.
Why haploids and doubled haploids?
The production of haploid plants from hybrids (heterozygous parents), followed by chromosome doubling and thus producing DH, provides wheat breeders with a means to accelerate the process of true breeding line development allowing a single-step development of complete homozygous lines (Fig. 4).
17
Figure 4. Doubled haploid wheat breeding scheme
In fact, in a traditional breeding program, pure lines of wheat are developed after several generations of selfing and still may not be 100% homozygous. Moreover, because of their homozygousity, in doubled haploid plants it is possible to observe the direct expression of both dominant and recessive mutations. Finally, DH can also influence the efficiency of crop breeding programs, particularly of genome mapping. They, in fact, provide an excellent platform to obtain solid information on the location of major genes and QTL for economically important traits38.
Haploid induction techniques
The two systems of anthers culture and maize pollination are the most commonly used methods to induce the haploid state in wheat.
Anthers culture
The process of anthers culture is quite simple and begins with the selection of primary wheat spikes which contain anthers with fresh microsporocytes at the mid–
18
late uni-nucleate stage of development39–41. The stage of pollen development is very
important as minor deviations can lead to major decreases in yield. Accordingly to the most used protocol42, anthers are aseptically dissected and cultured on induction
medium and incubated in darkness at 26–28°C for 4–6 weeks, after which calli are transferred to a solid plant regeneration medium and incubated in a culture room at 25°C and 16 h day length for about 30 days. Green plantlets are transferred to culture tubes containing 20 ml of modified plantlet regeneration medium. After 1–2 months of hardening, vigorous seedlings are transplanted into pots contain autoclaved soil mixture and kept in a plastic house43. Unfortunately, at least two important
difficulties still challenge commercial DH production with the anther culture techniques in wheat. Especially in durum wheat, green plants are regenerated at a low frequency because most of the genotypes do not respond at all to anthers culture, or because most plantlets regenerated might be albino44. Additionally, when green
plants are obtained45 high numbers of aneuploids (cells with the presence of an abnormal number of chromosomes) are regenerated from the microspore-derived calluses.
Maize pollination technique
The use of wide crosses has been broadly utilized for the production of haploids for plant breeding and genetic studies. Wheat DH can be produced by various crosses with
• maize46,47 (Zea mais L.);
19
• barley49 (Hordeum vulgare L.).
The production of wheat DH through wheat x maize crosses is widely used because following the fertilization, the maize chromosomes are almost immediately eliminated47. Because of the absence of adequate endosperm development, spikes
are treated with plant growth regulators (PGR), which stimulate embryo growth to a stage where mature embryos can be rescued onto nutrient medium (12-16 days post-pollination). The resulting haploid seedlings are then treated with colchicine to restore their diploid chromosome number. The final products are anticipated to be fertile true-breeding wheat plants. The main disadvantages of using the wheat x maize system are the low ratio of embryo caryopses produced and the loss of embryo viability. To counter this, research has focused on the manipulation of PGR treatments and embryo rescue conditions to optimise the efficiency of the technique46,47,50.
Colchicine treatment
In general, haploid plants are infertile because sexual fertility depends upon meiotic division of the diploid chromosome number. Spontaneous rate of chromosome doubling among plants derived from microspores of wheat is relatively low. In fact, in many experiments only a small portion (15–20%) of the plants obtained are capable of seed set by selfing without a treatment for chromosome doubling51–53. Colchicine (Fig. 5), which is a water-soluble alkaloid produced from the bulbs of
Colchicum autumnale, is the most frequently used compound for chromosome
20
during mitosis and disturbs normal polar segregation of sister chromatids to form a restitution nucleus (Fig. 6).
Figure 5. Colchicine chemical formula.
There are different methods of colchicine treatment to obtain diploidization for the production of homozygous plants.
• When the plants are mature, colchicine in the form of a paste is applied to the axils of leaves. Now, the main axis is beheaded. This stimulates the axillary buds to grow into diploid and fertile branches.
• The young plantlets are directly treated with colchicine solution, washed thoroughly and replanted.
• The axillary buds can be repeatedly treated with colchicine cotton wool for about 2-3 weeks.
21
Figure 6. Colchicine treatment prevents spindle formation and results in doubling of
chromosome numbers (from Leland56)
Upon mitotic divisions of such affected cells, chromosome-doubled chimera sectors are formed which lead to partial fertility of the plant if they comprise sexual organs. Colchicine is traditionally administered to the young plants established in soil at the 3–5 tiller stages. The colchicine treatment procedure of Inagaki57 is very powerful. According to this method, roots of the haploid seedling are pruned leaving a zone of 2–3 cm and submerged in a 0.1% colchicine solution supplemented with 2% dimethyl sulfoxide (DMSO) and ca. 0.05% Tween-20 at 20°C for 4-5 h. After this treatment the roots are washed free from residual colchicine and potted in normal peat soil. The entire procedure results in completely homozygous plants useful.
22
REFERENCES
1. World Food Summit. in (1996).
2. Pinstrup-Andersen, P. Food security: definition and measurement. Food
Secur. 1, 5–7 (2009).
3. Özdoğan, M. Modeling the impacts of climate change on wheat yields in Northwestern Turkey. Agric. Ecosyst. Environ. 141, 1–12 (2011).
4. Mann, M. L. & Warner, J. M. Ethiopian wheat yield and yield gap estimation: A spatially explicit small area integrated data approach. F. Crop. Res. 201, 60–74 (2017).
5. Schmidhuber, J. & Tubiello, F. N. Global food security under climate change.
Proc. Natl. Acad. Sci. U. S. A. 104, 19703–8 (2007).
6. Madgwick, J. W. et al. Impacts of climate change on wheat anthesis and fusarium ear blight in the UK. Eur. J. Plant Pathol. 130, 117–131 (2011).
7. Costion, C. M. et al. Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biol. Conserv. 191, 322–330 (2015).
8. Meza, F. J. & Silva, D. Dynamic adaptation of maize and wheat production to climate change. Clim. Change 94, 143–156 (2009).
9. Lemaire, G., Gastal, F., Franzluebbers, A. & Chabbi, A. Grassland Cropping Rotations: An Avenue for Agricultural Diversification to Reconcile High
23
Production with Environmental Quality. Environ. Manage. 56, 1065–1077 (2015).
10. Mochida, K. & Shinozaki, K. Unlocking triticeae genomics to sustainably feed the future. Plant Cell Physiol. 54, 1931–1950 (2013).
11. Sanderson, T., Hertzler, G., Capon, T. & Hayman, P. A real options analysis of Australian wheat production under climate change. Aust. J. Agric. Resour.
Econ. 60, 79–96 (2016).
12. Luo, Q., Wen, L., McGregor, J. L. & Timbal, B. A comparison of downscaling techniques in the projection of local climate change and wheat yields. Clim.
Change 120, 249–261 (2013).
13. Semenov, M. A., Stratonovitch, P., Alghabari, F. & Gooding, M. J. Adapting wheat in Europe for climate change. J. Cereal Sci. 59, 245–256 (2014).
14. Shiferaw, B. et al. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 5, 291–317 (2013).
15. Dell’Acqua, M., Zuccolo, A., Tuna, M., Gianfranceschi, L. & Pè, M. E. Targeting environmental adaptation in the monocot model Brachypodium distachyon: a multi-faceted approach. BMC Genomics 15, 801 (2014).
16. Preece, C. et al. How did the domestication of Fertile Crescent grain crops increase their yields? Funct. Ecol. 31, 387–397 (2017).
24
17. Wingen, L. U. et al. Wheat Landrace Genome Diversity. Genetics 205, 1657– 1676 (2017).
18. Jarvis, S., Fielder, H., Hopkins, J., Maxted, N. & Smart, S. Distribution of crop wild relatives of conservation priority in the UK landscape. Biol.
Conserv. 191, 444–451 (2015).
19. Uauy, C., Wulff, B. B. H., Zhou, J., Krasileva, K. & Clark, M. D. Genomic innovation for crop improvement. Nature 543, 20–28 (2017).
20. A, S. Die Geschiehte der kultivierten Getreide. Nebert Halle (1913).
21. Sakamura, T. Kurze Mitteilung über die Chromosomenzahlen und die Verwandtschaftsverhältnisse der Triticum-Arten. Shokubutsugaku
Zasshi 32, 150–153 (1918).
22. Peleg, Z., Fahima, T., Korol, A. B., Abbo, S. & Saranga, Y. Genetic analysis of wheat domestication and evolution under domestication. J. Exp. Bot. 62, 5051–5061 (2011).
23. Dvořák, J., Terlizzi, P. di, Zhang, H.-B. & Resta, P. The evolution of polyploid wheats: identification of the A genome donor species. Genome 36, 21–31 (1993).
24. Deng, P. et al. Genome-wide characterization of microsatellites in Triticeae species: abundance, distribution and evolution. Sci. Rep. 6, 32224 (2016).
25
25. Matsuoka, Y. Evolution of polyploid triticum wheats under cultivation: The role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol. 52, 750–764 (2011).
26. Feldman, M. & Levy, A. A. Allopolyploidy – a shaping force in the evolution of wheat genomes. Cytogenet. Genome Res. 109, 250–258 (2005).
27. Tanno, K. I. & Willcox, G. Distinguishing wild and domestic wheat and barley spikelets from early Holocene sites in the Near East. Veg. Hist.
Archaeobot. 21, 107–115 (2012).
28. López-Merino, L., Leroy, S. A. G., Haldorsen, S., Heun, M. & Reynolds, A. Can Triticum urartu (Poaceae) be identified by pollen analysis? Implications for detecting the ancestor of the extinct two-grained einkorn-like wheat. Bot.
J. Linn. Soc. 177, 278–289 (2015).
29. Fricano, A. et al. Crossability of Triticum urartu and Triticum monococcum Wheats, Homoeologous Recombination, and Description of a Panel of Interspecific Introgression Lines. G3 (Bethesda). 4, 1931–41 (2014).
30. Rouse, M. N., Wanyera, R., Njau, P. & Jin, Y. Sources of Resistance to Stem Rust Race Ug99 in Spring Wheat Germplasm. Plant Dis. 95, 762–766 (2011).
31. Ling, H.-Q. et al. Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 496, 87–90 (2013).
26
32. McCouch, S. Diversifying selection in plant breeding. PLoS Biol. 2, (2004).
33. Stephen Baenziger, P. & Depauw, R. M. Wheat Breeding: Procedures and
Strategies. Wheat Science and Trade (2009).
34. Dwivedi, S. L. et al. Haploids: Constraints and opportunities in plant breeding.
Biotechnol. Adv. 33, 812–829 (2015).
35. Belling, B. and. From ten of the primary 25-chromosome forms Trom eiqft of the secondary 25-chrom . fbrms. 116–120 (1924).
36. Blakeslee, A. F., Belling, J., Farnham, M. E. & Bergner, A. D. A HAPLOID MUTANT IN THE JIMSON WEED, ‘DATURA STRAMONIUM’. Science
55, 646–647 (1922).
37. Dwivedi, S. L. et al. Haploids: Constraints and opportunities in plant breeding.
Biotechnol. Adv. 33, 812–829 (2015)..
38. Tan, X. L., Vanavichit, A., Amornsilpa, S. & Trangoonrung, S. Genetic analysis of rice CMS-WA fertility restoration based on QTL mapping. Theor.
Appl. Genet. 97, 994–999 (1998).
39. Gómez, J. F., Talle, B. & Wilson, Z. A. Anther and pollen development: A conserved developmental pathway. Journal of Integrative Plant Biology 57, 876–891 (2015).
27
on bread wheat anther culture: ovary genotype and developmental stage, and candidate gene association. Frontiers in Plant Science 6, (2015).
41. Liu, W., Zheng, M. Y., Polle, E. A. & Konzak, C. F. Highly Efficient Doubled-Haploid Production in Wheat (Triticum aestivum L.) via Induced Microspore Embryogenesis. Crop Sci. 42, 686–692 (2002).
42. Ling, S. et al. The mature anther-preferentially expressed genes are associated with pollen fertility, pollen germination and anther dehiscence in rice. BMC
Genomics 16, 101 (2015).
43. Grauda, D. et al. Anther Culture Effectiveness in Producing Doubled Haploids of Cereals/ Putekðòu Kultûras Efektivitâte Graudaugu Dubultoto Haploîdu Izveidoðanâ. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact, Appl. Sci.
68, 142–147 (2014).
44. Esteves, P., Clermont, I., Marchand, S. & Belzile, F. Improving the efficiency of isolated microspore culture in six-row spring barley: II-exploring novel growth regulators to maximize embryogenesis and reduce albinism. Plant Cell
Rep. 33, 871–879 (2014).
45. Dogramaci-Altuntepe, M. & Jauhar, P. P. Production of durum wheat substitution haploids from durum x maize crosses and their cytological characterization. Genome 44, 137–142 (2001).
28
aestivum) through crosses between Japanese wheat and maize (Zea mays).
Plant Cell Rep. 8, 263–266 (1989).
47. Laurie, D. A. & Bennett, M. D. The production of haploid wheat plants from wheat x maize crosses. Theor. Appl. Genet. 76, 393–397 (1988).
48. Ishii, T., Ueda, T., Tanaka, H. & Tsujimoto, H. Chromosome elimination by wide hybridization between Triticeae or oat plant and pearl millet: pearl millet chromosome dynamics in hybrid embryo cells. Chromosome Res. 18, 821– 831 (2010).
49. Polgari, D. et al. High-frequency generation and characterization of intergeneric hybrids and haploids from new wheat-barley crosses. Plant Cell
Rep. 33, 1323–1331 (2014).
50. O’Donoughue, L. S. & Bennett, M. D. Comparative responses of tetraploid wheats pollinated with Zea mays L. and Hordeum bulbosum L. Theor. Appl.
Genet. 87, 673–680 (1994).
51. Redha, A. et al. Improved production of doubled haploids by colchicine application to wheat (Triticum aestivum L.) anther culture. Plant Cell Rep. 17, 974–979 (1998).
52. Kim, K.-M. & Baenziger, P. S. A simple wheat haploid and doubled haploid production system using anther culture. Vitr. Cell. Dev. Biol. - Plant 41, 22– 27 (2005).
29
53. Mishra, R. & Rao, G. J. N. In-vitro Androgenesis in Rice: Advantages, Constraints and Future Prospects. Rice Sci. 23, 57–68 (2016).
54. Soriano, M., Cistué, L. & Castillo, A. M. Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant Cell Rep. 27, 805–811 (2008).
55. Inagaki, M. N., Hash, C. T. & N, M. Short Communication crossed with pearl miUet. Plant Breed. 487, 485–488 (1998).
56. Leland H. Hartwell , Michael L. Goldberg, Janice A. Fischer, Leroy Hood, C. F. A. genetics: from genes to genomes. (2014).
57. Inagaki, M. N. in Doubled Haploid Production In Crop Plants. A Manual 53– 58 (Kluwer Academic Publishers, 2003).
30
CHAPTER II
AIMS OF THE THESIS
Plant genetic variability is the key pillar of biodiversity and it is the most important resource needed to achieve any plant breeding objectives1. Despite CWR harbor vast genetic diversity, new crop varieties, including modern varieties of wheat keep being developed for the most part through the rearranging of alleles present mostly in the elite gene pool that exhibits a relatively limited genetic diversity2. Even though
practically exploiting WWR in the breeding process is still challenging3, it has provided notable successes in cereal improvement, suggesting that it is extremely important to conserve and characterize them as a potential novel source of useful alleles4,5.
The main goal of this PhD thesis is to provide a comprehensive characterization of the genetic variability present in a large collection of Triticum urartu populations sampled from different regions throughout the Fertile Crescent: an arc that extends from the eastern part of the Mediterranean to the lower Zagros Mountains (Iraq - Iran) around the Tigris and Euphrates rivers. By using next-generation sequencing technologies (genotyping–by-sequencing approach6,7) and geographic information
systems (GIS), this thesis aimed at tracking genes involved in environmental adaptation, whilst providing the most throughout characterization of a WWR species to-date. The territory under study is, according to Vavilov8, home to the eight Neolithic founder crops important in early agriculture including einkorn wheat (Triticum monococcum and T. urartu). Currently, different collections of T. urartu
31
are maintained at diverse germplasm resource centers. We reproduced and characterized a large proportion of those populations at three different levels:
• geographic, studying the sampling collection area and relating environmental data to genetic diversity through landscape genomics for the discovery of adaptive alleles9 (CHAPTER III and IV);
• molecular, using genotyping-by-sequencing technology, on e of the latest application of next-generation sequencing for the purpose of discovering SNPs and concurrently genotype individuals10 (CHAPTER IV )
• phenotypic, focusing on the seed morphology11 (CHAPTER V).
The importance of the genes/alleles from wild relative has been already well demonstrated: indeed many important cultivars, not only in cereals, have genes introgressed from CWR obtained through the doubled haploids technology12. Thus, CHAPTER VI is dedicated to the results of the works and the experiences done for six months at Kansas State University (Plant Pathology Department, Jesse Poland Lab) and the Heartland Plant Innovation Center Inc. both located in Manhattan (Kansas, USA) learning the wheat doubled haploid technology. The last chapter of this thesis, is dedicated to all the didactic activities done during the PhD Program at Scuola Superiore Sant’Anna.
32
REFERENCES
1. Govindaraj, M., Vetriventhan, M. & Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 1–14 (2015).
2. Lopes, M. S. et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 66, 3477–3486 (2015).
3. Tiwari, V. K. et al. SNP Discovery for mapping alien introgressions in wheat. BMC Genomics 15, 273 (2014).
4. Liu, H. et al. Physiological and Comparative Proteomic Analysis Reveals Different Drought Responses in Roots and Leaves of Drought-Tolerant Wild Wheat (Triticum boeoticum). PLoS One 10, e0121852 (2015).
5. Nygren, J., Shad, N., Kvarnheden, A. & Westerbergh, A. Variation in Susceptibility to Wheat dwarf virus among Wild and Domesticated Wheat.
PLoS One 10, e0121580 (2015).
6. Elshire, R. J. et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6, e19379 (2011).
7. Poland, J. a & Rife, T. W. Genotyping-by-Sequencing for Plant Breeding and Genetics. Plant Genome 5, 92–102 (2012).
33
Breed. 14, 1–245 (1926).
9. Manel, S., Schwartz, M. K., Luikart, G. & Taberlet, P. Landscape genetics: combining landscape ecology and population genetics. Trends Ecol. Evol.
18, 189–197 (2003).
10. Poland, J. A., Brown, P. J., Sorrells, M. E. & Jannink, J.-L. Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS One 7, e32253 (2012).
11. Gegas, V. C. et al. A genetic framework for grain size and shape variation in wheat. Plant Cell 22, 1046–1056 (2010).
12. Feuillet, C., Langridge, P. & Waugh, R. Cereal breeding takes a walk on the wild side. Trends Genet. 24, 24–32 (2008).
34
CHAPTER III
ASSEMBLY OF THE COLLECTION OF T. URARTU AND GEOGRAPHIC CHARACTERIZATION OF THE COLLECTION SITES
Today, in the Era of climate change, the assembly and the characterization of a collection of genetic resources is an essential but complex and intricate scientific operation, because it implies different key steps: from measuring the status of plant biodiversity, selecting the most vulnerable species and the relative most threatened habitat, to the implementation of specific conservation/breeding acitons that can transfer the results to all the conservation institution involved1–3. Seeds of many crop, landraces and wild relatives, as well as wild wheat relatives (WWR) have been collected across the respective centres of origin and are now conserved in different germplasm resources centers. These centers are delegated to generate different types of specific ouptus, including information on the genealogy (pedigree data), and all the plant peculiarity of the germplasm4 (plant botanical description, evaluation and passport data). For some natural populations the exact point of sampling can be specified if is availabe or derived by ethnobotanical studies if a geographical gazetteer is present. When this information is included in the passport data. the germplasm become also geo-referenced, and thus suitable to fruitful spatial and geographical analyses. Different kind of tools were developed by scientists in order to make spatial analysis and one of them is the use of the geographic information system (GIS). GIS can be defined as a cogent database management system, which can at the same time operate with spatial data in graphics form and influence the use
35
of the material by concentrating the attention of the researchers on the most hopeful/suitable material for their peculiar breeding objectives. We focused on the one of the 12 Vavilovian centers of origin and diversity of many WWR including
Triticum urartu. The Fertile Crescent is characterized by an impressive range of
altitudes, spanning from 400 m below sea level in areas of the Jordan Valley to 3,000 meter above sea level (m.a.s.l.) at Qurnat as Sawdā’, the highest elevation in Lebanon and all the Levant region. Furthermore within the the Fertile Crescent big temperature and rainfall differences are present, which are coupled with highly variable physiographic and edaphic factors. These diverse pedo-climatic conditions make the actual Middel East a model environment to explore the genetic basis of ecological adaptations in plants. The aim of this chapter is to gather relevant information about the sampling collection area of different accessions of Triticum
urartu from the Fertile Crescent, which is the collection that we characterized in this
PhD thesis project.
MATERIALS AND METHODS Plant material
Plant materials used in this study consist of different populations of Triticum urartu L. (AA, 2n=2x=14) representing the whole set of collected T. urartu accessions publicly available. These populations were collected throughout the Fertile Crescent in the current states of Armenia (ARM), Iran (IRN), Iraq (IRQ), Jordan (JOR), Lebanon (LBN), Syria (SYR), and Turkey (TUR). Most of them were collected by B. L. Johnson in 1962 and 19635. Seeds from these populations were obtained from:
36
• Germplasm Resources Information Network in USA ( http://www.ars-grin.gov /);
• the National Agriculture and Food Research Organisation (NARO) in Japan, ex NIAS (http://www.naro.affrc.go.jp/ );
• CREA - Unità di ricerca per la selezone dei cereali e la valorizzazione delle varietà vegetali in Italy (http://sito.entecra.it/ ).
Geographic data
The sampling collection area was analysed with GIS by using two open-source programs: QGIS 2.4.0 (http://www.qgis.org/) and DIVA-GIS 7.5 ( http://www.diva-gis.org/), coupled with BioClim data derived from Worldclim 2.5 minutes data6 (years ~1950-2000, ~5 Km). Variables included are: monthly total precipitation, and monthly mean, minimum and maximum temperature, and 19 derived bioclimatic variables (Tab. 1).
Through GIS, these data are related to T. urartu accessions as quantitative variables. The software R (https://cran.r-project.org/) was used to extract such data and to analyze the sampling collection area by a correlation matrix and Principal Component Analysis (PCA)7. The study of the most limiting factors that could influence the growth of T. urartu in the Fertile Crescent was done by exploiting the EcoCrop modelling in DIVA-GIS.
37
Code Definition
BIO1 Annual Mean Temperature
BIO2 Mean Diurnal Range (Mean of monthly (max temp - min temp))
BIO3 Isothermality (BIO2/BIO7) (* 100)
BIO4 Temperature Seasonality (standard deviation *100)
BIO5 Max Temperature of Warmest Month
BIO6 Min Temperature of Coldest Month
BIO7 Temperature Annual Range (BIO5-BIO6)
BIO8 Mean Temperature of Wettest Quarter
BIO9 Mean Temperature of Driest Quarter
BIO10 Mean Temperature of Warmest Quarter BIO11 Mean Temperature of Coldest Quarter BIO12 Annual Precipitation
BIO13 Precipitation of Wettest Month BIO14 Precipitation of Driest Month
BIO15 Precipitation Seasonality (Coefficient of Variation) BIO16 Precipitation of Wettest Quarter
BIO17 Precipitation of Driest Quarter BIO18 Precipitation of Warmest Quarter BIO19 Precipitation of Coldest Quarter
Table 1. Bioclimatic variables. RESULTS AND DISCUSSION Collection assembly
The theory of isolation by distance8 states that there is a regular decrease of genetic similarity with the increase of geographic distance. Data on molecular polymorphisms have also confirmed a strong association between genetic and geographic distance9. Based on that, the accessions included in this collection were first chosen depending on the geographical information available from the germplasm resource centers but cuncurrently trying to maintain a high representation across the entire Fertile Crescent. In particular, by looking at the information present
38
in the Genesys portal (https://www.genesys-pgr.org) and in previous works10 we selected 428 accessions of Triticum urartu (Fig. 1).
Figure 4 Origin of the Triticum urartu collection used in this study. A: Barplot for
country representation. B: Pie chart of the contribution of the germplasm resources centers.
All the accessions were sorted by the country of origin and the international progressive identifier (PI) and renamed with a progressive identifier number (SSA_1, SSA_2,…, SSA_428).
Geographic characterization
We analysed the sampling collection area considering altitude, annual mean temperature and annual precipitations (Fig. 2), coupled with the BioClim data set.
39
Samples were collected from a wide range of altitudinal values, from 150 to 2,300 meters above sea level across the Fertile Crescent. The sampling sitse are also characterizad by very different annual temperature, clearly influenced by the altitude.
Figure 5. Depiction of sampling area in the Fertile Crescent: GIS survey obtained
through QGIS coupled with BioClim data derived from Worldclim 2.5 minutes data (years ~1950-2000, ~5 Km. A: Altitude - green=0 to red=2400 m.a.s.l. B: Annual mean temperature – white=0 to orange=27°C C: Annual precipitations – white=0 to blue=884 mm.
As reported in Figure 2C, altitude influences rainfall as well, though not completely accounting for its variation. This information is best described by the boxplots reporting the distribution of environmental variables in the T. urartu collection (Fig. 3).
B
A
40
Figure 6 A: Representation of all the bioclimatic variables derived from the monthly
temperature BIO1 (see Tab. 1 for more details). B: Representation of all the bioclimatic variables derived from the rainfall values (see Tab. 1 for more details).
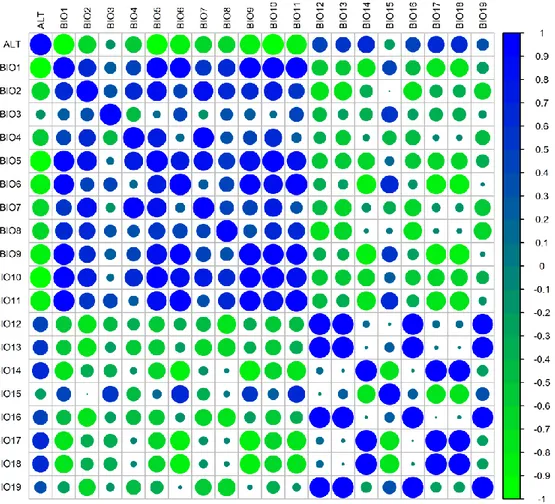
Taking into consideration the annual mean temperature (BIO1, Fig. 3A) we noticed that most of the accessions were sampled in sites characterized by a high temperature values (spanning 18 - 25°C). However, considering the min temperature of the coldest month (BIO6, Fig. 3A), there are some accessions that were sampled even in sites whose temperature was below zero. Regarding the annual mean precipitation (BIO12, Fig. 3B), as expected, the majority of the collection is distributed throughout arid and semi-arid regions (<500 mm). Both temperature and precipitations are influenced by altitude. In particular, altitude is negatively correlated with almost all the temperature variables (BIO1 to BIO11) although with different strength. In contrast, a positive correlation between altitude and precipitation (BIO12 to BIO19) was found (Fig. 4).
41
Figure 7. Correlation plot of all the bioclimatc variables. Blue circle positive
correlation, green circle green correlation.
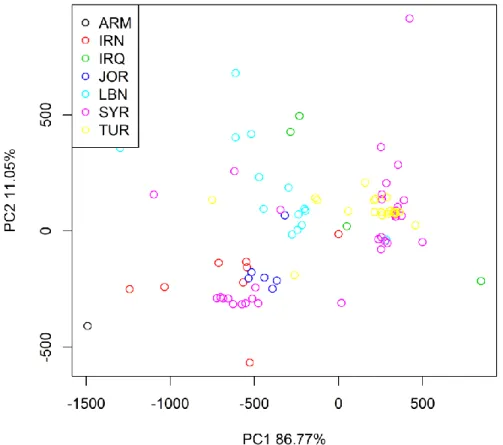
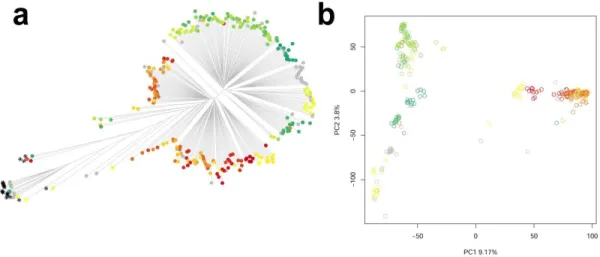
The first two principal components computed by a PCA (Fig. 5) account for 86% and 11% of the total variance respectively. The plot shows that the T. urartu samples are not strongly separated following a strict geographical criterion and samples collected in the same country show a lot of variation. This confirms the high environmental heterogeneity present in the Fertile Crescent.
42
Figure 8. Two main PC of all the bioclimatic variables.
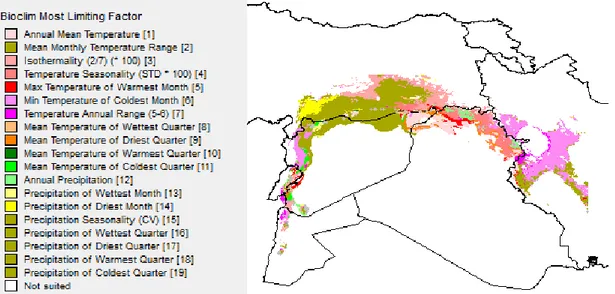
The environmental diversity in both temperature and rainfall of the sampling area confirms that our collection of T. urartu is representative of several microclimates in which this species is growing. To have a general depiction of which are the most limiting factors that can influence the growth of T. urartu in the Fertile Crescent, we have exploited the EcoCrop modelling in DIVA-GIS (Fig. 6). This analysis takes into account sites of sampling and all the variables available in the BioClim data, and calculate the so-called most limiting factor based on the environmental niches in
43
which T. urartu was collected. In most part of the region, the climate variable that appear to limit T. urartu growth is precipitation seasonality (dark green color). In the Eastern part of the region, where altitude severely increases, the limiting factor is related to the minimum temperature of the coldest month (purple color). Altogether, our analysis suggests that different T. urartu natural populations should be adapted to different pedo-climatic conditions.
This preliminary study on the sampling collection area of the T. urartu collection, put basis for the next chapter on landscape genomics.
Figure 6.GIS image obtained through DIVA-GIS. False colours were generated to represent the BioClim most limiting factors throughout the Fertile Crescent.
44
REFERENCES
1. Maxted, N. & Kell, S. P. Establishment of a Global Network for the. Situ
Conserv. Crop Wild Relat. Status Needs. FAO Comm. Genet. Resour. Food Agric. Rome, Italy (2009).
2. Jiang, J. et al. Reprint of: The wild relatives of grape in China: Diversity, conservation gaps and impact of climate change. Agric. Ecosyst. Environ. 209, 155–163 (2015).
3. Dempewolf, H. et al. Adapting Agriculture to Climate Change: A Global Initiative to Collect, Conserve, and Use Crop Wild Relatives. Agroecol.
Sustain. Food Syst. 38, 369–377 (2014).
4. Khoury, C., Laliberté, B. & Guarino, L. Trends in ex situ conservation of plant genetic resources: A review of global crop and regional conservation strategies. Genet. Resour. Crop Evol. 57, 625–639 (2010).
5. Johnson, B. L., and 0. H. Analysis of phylogenetic affinities in the Triticinae by protein electrophoresis. Amer. J. Bot. 506–513 (1965).
6. Hijmans R. J.; Cameron S. E.; Parra J. L.; Jones P. G.; Jarvis A. The WorldClim interpolated global terrestrial climate surfaces.
http://www.worldclim.org (2005).
7. Rodriguez, M. et al. Landscape genetics, adaptive diversity and population structure in Phaseolus vulgaris. New Phytol. 209, 1781–1794 (2016).
8. Ramachandran, S. et al. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect
45
originating in Africa. Proc. Natl. Acad. Sci. U. S. A. 102, 15942–15947 (2005).
9. Oleksa, A., Chybicki, I. J., Gawroński, R., Svensson, G. P. & Burczyk, J. Isolation by distance in saproxylic beetles may increase with niche specialization. J. Insect Conserv. 17, 219–233 (2013).
10. Fricano, A. et al. Crossability of Triticum urartu and Triticum monococcum Wheats, Homoeologous Recombination, and Description of a Panel of Interspecific Introgression Lines. G3 (Bethesda). 4, 1931–41 (2014).
46
CHAPTER IV
LANDSCAPE GENOMICS
This work of landscape genomics was submitted as a manuscript at Molecular Ecology (MEC-17-0877). 18-Jul-2017
Molecular Diversity and Landscape Genomics of the Crop Wild
Relative Triticum urartu Across the Fertile Crescent
Brunazzi A.1, Scaglione D.2, Talini R.F.1, Magni F.2, Poland J.3, Pè M.E.1, Brandolini A.4, Dell’Acqua M.1*
1 Institute of Life Sciences, Scuola Superiore Sant'Anna, Pisa, Italy 2 Institute of Applied Genomics, Udine, Italy
3 Wheat Genetics Resource Center, Department of Plant Pathology, Kansas State
University, Manhattan, KS, USA
4 Consiglio per la ricerca e la sperimentazione in agricoltura e l'analisi
dell'economia agraria, Unità di Ricerca per la Selezione dei Cereali e la
Valorizzazione delle varietà vegetali (CREA-SCV), S. Angelo Lodigiano, Italy
Keywords: Wheat, Adaptation, Landscape Genetics, GWAS, GBS, RAD
47
ABSTRACT
Modern plant breeding can benefit from the allelic variation existing in natural populations of crop wild relatives that evolved under natural selection in varying pedoclimatic conditions. In this study, next-generation sequencing was used to characterize the most complete ex situ collections of Triticum urartu L., the wild donor of the A genome of modern wheat. More than 1.3 million SNPs were produced on 298 T. urartu accessions collected throughout the Fertile Crescent. The T. urartu collection showed a complex pattern of genetic diversity, with two main genetic groups distributed sequentially from West to East. The incorporation of geographic information of sampling points showed that genetic diversity was partially influenced by the spatial features of the T. urartu sampling area. An outlier loci detection method incorporating spatial information was used, reporting 0.60% of the SNP loci under directional selection. Bioclimatic information derived from grid data was joined with allele frequencies in a genome wide association study that identified 1,912 marker-environment associations at a high significance level. We advocate the application of genomics and landscape genomics on ex situ collections of crop wild relatives to efficiently identify promising genetic materials and genomic loci in modern plant breeding.
48
INTRODUCTION
The adaptation of agriculture to climate change is one of the most urgent and important challenges of our times. Ensuring that the crops feeding humanity will be able to thrive in new climates is a key step to achieve food security in the future (Lipper et al. 2014), but limited allele pools in elite crop varieties may limit future breeding achievements. A crossing the best - hoping for the best strategy (Acquaah 2012) is not sufficient to address emerging threats (Jarvis et al., 2008), and new genetic variation is needed to overcome shifting biotic (Saintenac et al. 2013; Bebber
et al. 2013) and abiotic pressures (Abberton et al. 2016). Crop wild relatives (CWR),
having diffused to diverse environments and adapted locally under natural selection (Vavilov & Dorofeev 1992), harbor vast genetic diversity. Their use in breeding has long been advocated to provide favorable alleles to cultivated elite lines (Harlan 1976), however, the utilization of such favorable alleles has been limited by the poor genetic background of the CWR relative to modern varieties. Nowadays, the increasing availability of genomic tools bears the promise to ‘mine’ wild alleles with increased efficiency, and use this information to produce better crops (Brozynska et
al. 2016). In the midst of climate change, the conservation and classification of CWR
is therefore a global priority (Maxted et al. 2012; Dempewolf et al. 2017) which are already altering their spatial distribution and availability (Jarvis et al. 2008). Although comprehensive germplasm collections exist, much remains to be done especially for ex situ CWR taxonomic and geographic representation (Castañeda-Álvarez et al. 2016).
49
The molecular, geographic and phenotypic characterization of existing CWR collections may provide useful information to support their use in modern plant breeding. Recent approaches in statistics and genomics merge genotypic and bioclimatic information to identify genomic loci responsible to environmental adaptation (Rellstab et al. 2015; Rissler 2016). These ‘landscape genomics’ approaches have found application in several research fields, including evolutionary studies (Sork et al. 2013), conservation efforts (Vincent et al. 2013), and epidemiology (Schwabl et al. 2017). In an agronomic perspective, landscape genomics may be applied on model species to derive detailed information on candidate genes for environmental adaptation (Dell’Acqua et al. 2014; Mattila et al. 2016). When focusing on crop landraces, landscape genomics may yield genomic loci for stress adaptation readily available for breeding (Pallotta et al. 2014; Lasky
et al. 2015; Russell et al. 2016). Both these approaches, however, do not capture the
allelic diversity that is available in natural populations of CWR, superior to that of landraces and even more of elite cultivars (Zhou et al. 2015).
Modern wheats (Triticum aestivum L. and Triticum durum Desf.) are much less diverse than their ancestors as a result of a series of demographic and selective bottlenecks occurring since the initial domestication of wild emmer (Haudry et al. 2007). During the second half of the 20th century, breeding heavily focused on elite germplasm, further narrowing wheat diversity and increasing field uniformity (Cox
et al. 1986). This trend toward loss of genetic diversity is currently slowing down,
50
breeding alleles (Reif et al. 2005). Wheat landraces and CWR are in fact strategic reservoirs of allelic diversity to produce better lines (Reynolds et al. 2007; Hairat & Khurana 2015; Mengistu et al. 2016), and landscape genomics approaches could be used to exploit the environmental adaptation developed by wheat CWR during evolutionary times.
Triticum urartu L. (2n=2x=14; genome AuAu) is the A-genome donor of cultivated tetraploid (2n=2x=28; genome AABB) and hexaploid wheat ( 2n=2x=42; genome AABBDD). It was never domesticated and thus it provides an excellent system to investigate local adaptation to environment. Triticum urartu is broadly distributed across the Fertile Crescent, where it contributed to originate the first wild forms of tetraploid wheat subsequently domesticated thousands of years ago (Özkan
et al. 2002). Genes from Triticum urartu can be transferred to cultivated wheat by
biotechnology approaches, by amphiploids production (Ahmed et al. 2014), or by direct hybridization with the polyploid (Qiu et al. 2005) or diploid wheat (Fricano et
al. 2014). The most recent genome editing approaches (Zhang et al. 2016) may also
help transfer in cultivated wheat discoveries made in CWR. Triticum urartu alleles may be expressed in cultivated wheat complementing their homeologs and provide enhanced functionality (Gao et al. 2017). Resistance genes have been already mapped in T. urartu (Qiu et al. 2005) and other diploid A genomes (Chhuneja et al. 2008) and transferred to hexaploid wheat. Triticum urartu has been also used as a model to study gliadin alleles (Zhang et al. 2015), and showed a variety of glutenin alleles of promising use in wheat breeding (Cuesta et al. 2015). The availability of
51
a draft genome sequence (Ling et al. 2013) makes T. urartu a promising tool for generating and pushing genomic discoveries on CWR into wheat breeding pipelines. In this study, we report the characterization of the most complete ex situ collection of wild T. urartu accessions currently available. A genotyping by sequencing approach allowed the description of the extant molecular variation among T. urartu natural populations distributed across the Fertile Crescent. The incorporation of spatial information about sampling points provided insights into the genomic signatures of environmental adaptation, leading to the discovery of several loci whose allele frequencies are related to the spatial and climatic distribution of this species.
MATERIALS AND METHODS Plant materials and DNA extraction
The plant materials used in this study (Table S1) were 298 accessions of Triticum
urartu L. in the U.S. Department of Agriculture (USDA) National Plant Germplasm
System, USA, and the Consiglio per la Ricerca e la Sperimentazione in Agricoltura e l'Analisi dell'Economia Agraria (CREA-SCV), Italy. The collection assembled for this study represents the entirety of T. urartu accessions available from ex situ germplasm banks at the time of the experiment. One accessions of Triticum
monococcum L. (var. MONLIS) and two accessions of Triticum boeoticum L. (ID
1094 and ID 948 from CREA-SCV genebank) were included as outgroups. Five seeds per accession were germinated in individual petri dishes, and green tissues were pooled and used to extract genomic DNA with a GeneElute Plant Genomic
52
DNA Miniprep Kit (Sigma-Aldrich, St Louis, MO) following manufacturer instructions. DNA was checked for quality and quantity using agarose gels and spectrophotometry.
Genotyping
Genomic DNA was shipped to IGA Technology Services (Udine, Italy) to perform genotyping using a custom double-digestion RAD sequencing (Baird et al. 2008) protocol. To define the enzymes to be used in the genomic DNA digestion and size selection, restriction simulations were carried on the reference genome, as available from EnsemblPlants (GCA_000347455.1.26 build), using custom scripts. The combination of SphI and BstYI enzymes together with a size selection of fragments in the range of 230-330 bp predicted to generate loci in the order of 100,000. For each sample 250 ng of genomic DNA were digested in a 30 µL reaction with 2U of each SphI and BstYI enzymes (New England Biolabs) in SmartCut buffer for 1 h at 37 °C, followed by 1 h at °60 C and heat inactivation at 65 °C for 15 min. One and a half volumes of AmpureXP beads (Agencourt) were added to the reaction mix and put on a magnetic rack. Beads pellet was washed twice with 70% ethanol and DNA was re-suspended in 20 µL of Tris-HCl 10 mM (pH 8.5). For each sample 10 µL of restriction product were mixed with 2 and 5 pmoles of adapters P1 and P2 respectively (Table S3; variable length inline barcodes on both sides) and 200 U of T4 DNA ligase (New England Biolabs) in a final reaction volume of 30 µL and incubated for 1 h at 23 °C and 1 h at 20 °C. Purification was done as described above. Samples were pooled in 24-plex by means of P1 inline barcodes and concentrated