Chapter III - Results and Discussion
Part I Aphids
This chapter contains the results of a research aimed at characterising the OBPs of aphids. As reported in the Introduction, recent evidence has been provided on the essential and important role of OBPs in semiochemicals perception and discrimination in insects.
The choice of aphids was mostly motivated by the enormous economical interest of these insects that represent one of the major pests for crops worldwide. Also the on-going sequencing of the genome of pea aphid Acyrthosiphon pisum provided useful information to start a research at the biochemical level.
Identification of OBPs in aphids
When I started my work, OBPs had not been reported in aphids, although a number of CSPs had been identified. Although the genome of the pea aphid Acythosiphon pisum had not yet been published, the ongoing project had already produced a great number of sequences that were available in the EST database.
We therefore performed a first search in the EST database, using the BLAST programme and the locust OBP-1 (Genbank acc.no. AF542076) as a template. This was not an exhaustive mining of aphid OBPs in the EST database. We obtained 36 aphid sequences, 9 of them from the pea aphid
A. pisum, that encoded proteins with the typical 6-cysteine signature of OBPs (Figure 12). Some
of them (1, 3, 7 and 8) can be regarded as “classic” OBPs, containing only the six conseved cysteines, while others (2, 4, 5, 6 and 9) contained additional cysteines. In particular, OBP2 also presented a much longer N-terminus and can be classified among the C-plus OBPs. The subsequent sequencing of the genome of the pea aphid A. pisum provided 15 genes encoding OBPs, two of them belonging to the sub-group of C-plus OBPs (Zhou et al., 2010b).
Fig. 12. Predicted amino acid sequences of A. pisum OBPs obtained from DNA fragments found in the EST database. These nine sequences appear very different from each other, but all share the six-cysteine conserved motif of insect OBPs.
OBP1 10 OBP2 11 12 OBP3 14 12 11 OBP4 8 7 15 4 OBP5 4 10 9 7 10 OBP6 10 16 11 10 5 7 OBP7 35 20 11 9 2 9 6 OBP8 5 17 9 8 8 17 15 9 OBP9
Table 2. Percent of identical amino acids between pairs of
A. pisum OBPs. The values
are calculated on the mature proteins. ApisOBP2 MKVSAATAVLVALVATVQSSDPCNISTCYKSGTTKPPMAVTPTHLPVQSSSTQTSHPQTTYAKDHVHGSTT ApisOBP5 MSANSATIKCIAVAAILLQISVI ApisOBP4 MRGNYSSMRV ApisOBP1 MLNLKVMMFLCLSVIVVYCESDQVPINSSAAVESCLLETNMTRDEFEDMLT ApisOBP8 MFALKVACLCLSVAVVFGENNQQNGPSDRSATIFQSCIAETKLSGDA ApisOBP2 TKSGVNATVTTASGASVNGTEPPAVVKSSAGVTGNSTTPKPTMTEGHVALKQKLNTIAVKCKDELHAPQEI ApisOBP9 MIIKKTLLLSVFVLFGCLFSINKADDADAKDKELMSKLFTVVFKCFKDADWGTCGEM ApisOBP7 MVARKRMYNMLPTTVLFAIIAATVLKDCDAYLSEAAIKKTQQMLKTVCSKKHSVEEDVF ApisOBP3 MISSTFYITLVFGIAMLISCGHGRFTTEQIDYYGKACNASEDD ApisOBP5 FADAGHHRRGKELLDTEDSDFFRCKQASRKSCCGPENAMKRFGDKDKVAADECYAQVAEKFATVTATTPKQ ApisOBP6 MPNILPNLDSTWEKCFETFKQFKDK ApisOBP4 FLLFAIGFQDIFCQKQETSGKCRAPDKAPLNLEIIINTCQEEIKSALLQEALDIFNDGNVEQNTPNYSSSK ApisOBP1 SPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEVLSKYVLNFYGRPDYKRRLIIKDVEHIVDVC---ApisOBP8 LKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVAQKYYG----TNEAMMKKAKDLIDVC---ApisOBP2 MALVSNTVVPQNEQQRCYLECVYKNLNLIKNNKFSVEDGKAMARIRFA----NQPEEHKKAVTIIETC---ApisOBP9 ITTKYDITQAKYKQCTCHMACAGEELGMINASGQPEPAKFLEYVNKIN---NPDIKSQLQLIYDKC---ApisOBP7 TNIKKGIFPEDNNNIKCYFACNFKTMQLINQKGVIDKKMFKDKMSMMA---PPNVYKILLPVIEQCTGK ApisOBP3 LVVVKSYKVPTTETGKCLMKCMITKLGLLNDDGSYNKTGMEAGLKKYW---SEWSTEKIESINNKCYEE ApisOBP5 DLFSAEAVKITKKKQFCLHECIGKKNNLLTEDGSLNKTFIADYAMKSVFK---EQWQKQVGQKALDKCLEE ApisOBP6 PETKEYKEMAHGKEPPCLFQC----FMQSGLTTSDGKLNEDAITKKMSEGINNDEKWKSIWQNSLNKCFDD ApisOBP4 READEDLTNEERRVAGCLLQCVYKKVKAVDETGFPVVDGLMKLYNEGV----QDRNYYIATLSAVRHCISI ApisOBP1 ---AKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI ApisOBP8 ---AKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM ApisOBP2 ---EKEAVIDPKTTEKCAAGRVIRNCFVKNGEKINFFPKA ApisOBP9 ---QNVKGSEKCDLAEQFAICAFKESPALKERVSTLMEMLVKMKPKSK ApisOBP7 DK---GEELCQSSYNVIKCAHSVDPKSLEFLPL ApisOBP3 ALLVS---KEVVATCNYSYTVMACLNKQLDLDKST ApisOBP5 TYIPWPAEDKENVCNPVYVQFQHCLWLQYESNCPANKIKITKKCEKTRNRYRMQKSTSN ApisOBP6 VKQ--EDKKQILIMNTPAGRLMKCFLRDMYMSCPKNVWVESSECLNMKDLVQKCPEMPPPVFKSPPKLI ApisOBP4 AQQLKQQQPSKS---FDDGQTCDLAYEMFECVSEKIEENCGVENKSNN ApisOBP2 MKVSAATAVLVALVATVQSSDPCNISTCYKSGTTKPPMAVTPTHLPVQSSSTQTSHPQTTYAKDHVHGSTT ApisOBP5 MSANSATIKCIAVAAILLQISVI ApisOBP4 MRGNYSSMRV ApisOBP1 MLNLKVMMFLCLSVIVVYCESDQVPINSSAAVESCLLETNMTRDEFEDMLT ApisOBP8 MFALKVACLCLSVAVVFGENNQQNGPSDRSATIFQSCIAETKLSGDA ApisOBP2 TKSGVNATVTTASGASVNGTEPPAVVKSSAGVTGNSTTPKPTMTEGHVALKQKLNTIAVKCKDELHAPQEI ApisOBP9 MIIKKTLLLSVFVLFGCLFSINKADDADAKDKELMSKLFTVVFKCFKDADWGTCGEM ApisOBP7 MVARKRMYNMLPTTVLFAIIAATVLKDCDAYLSEAAIKKTQQMLKTVCSKKHSVEEDVF ApisOBP3 MISSTFYITLVFGIAMLISCGHGRFTTEQIDYYGKACNASEDD ApisOBP5 FADAGHHRRGKELLDTEDSDFFRCKQASRKSCCGPENAMKRFGDKDKVAADECYAQVAEKFATVTATTPKQ ApisOBP6 MPNILPNLDSTWEKCFETFKQFKDK ApisOBP4 FLLFAIGFQDIFCQKQETSGKCRAPDKAPLNLEIIINTCQEEIKSALLQEALDIFNDGNVEQNTPNYSSSK ApisOBP1 SPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEVLSKYVLNFYGRPDYKRRLIIKDVEHIVDVC---ApisOBP8 LKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVAQKYYG----TNEAMMKKAKDLIDVC---ApisOBP2 MALVSNTVVPQNEQQRCYLECVYKNLNLIKNNKFSVEDGKAMARIRFA----NQPEEHKKAVTIIETC---ApisOBP9 ITTKYDITQAKYKQCTCHMACAGEELGMINASGQPEPAKFLEYVNKIN---NPDIKSQLQLIYDKC---ApisOBP7 TNIKKGIFPEDNNNIKCYFACNFKTMQLINQKGVIDKKMFKDKMSMMA---PPNVYKILLPVIEQCTGK ApisOBP3 LVVVKSYKVPTTETGKCLMKCMITKLGLLNDDGSYNKTGMEAGLKKYW---SEWSTEKIESINNKCYEE ApisOBP5 DLFSAEAVKITKKKQFCLHECIGKKNNLLTEDGSLNKTFIADYAMKSVFK---EQWQKQVGQKALDKCLEE ApisOBP6 PETKEYKEMAHGKEPPCLFQC----FMQSGLTTSDGKLNEDAITKKMSEGINNDEKWKSIWQNSLNKCFDD ApisOBP4 READEDLTNEERRVAGCLLQCVYKKVKAVDETGFPVVDGLMKLYNEGV----QDRNYYIATLSAVRHCISI ApisOBP1 ---AKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI ApisOBP8 ---AKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM ApisOBP2 ---EKEAVIDPKTTEKCAAGRVIRNCFVKNGEKINFFPKA ApisOBP9 ---QNVKGSEKCDLAEQFAICAFKESPALKERVSTLMEMLVKMKPKSK ApisOBP7 DK---GEELCQSSYNVIKCAHSVDPKSLEFLPL ApisOBP3 ALLVS---KEVVATCNYSYTVMACLNKQLDLDKST ApisOBP5 TYIPWPAEDKENVCNPVYVQFQHCLWLQYESNCPANKIKITKKCEKTRNRYRMQKSTSN ApisOBP6 VKQ--EDKKQILIMNTPAGRLMKCFLRDMYMSCPKNVWVESSECLNMKDLVQKCPEMPPPVFKSPPKLI ApisOBP4 AQQLKQQQPSKS---FDDGQTCDLAYEMFECVSEKIEENCGVENKSNN
low, less than what reported for OBPs of other insects. Table 2 reports the percent of amino acid identities between pairs of OBPs, calculated on the mature sequences.
This unusual divergence of OBP sequences in A. pisum can also be visualised in the alignment of Figure 12 that contains many gaps in order to get most of the cysteines aligned.
Fig. 13. Alignement of OBP1 and OBP8 sequences in different aphid species. Similarity across even phylogenetically distant species is extremely high. The different amino acids are highlighted.
A.pisumOBP1 ESDQVPINSSAAVESCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEV P.salicisOBP1 ESDQVPINSSAAVENCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEV T.salignusOBP1 ESDQVPMNSSAAVENCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMLGCVMRKNHIVNDGVVSKEV A.pisumOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI P.salicisOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI T.salignusOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI A.pisumOBP8 ENNQQNGPSDRSATIFQSCIAETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.persicaeOBP8 ENNQQN-SSDRSATIFQSCIAETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA N.ribisnigriOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.viciaeOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.dirhodumOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA A.fabaeOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA A.pisumOBP8 QKYYGTNEAMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM M.persicaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTAEECALAGIVTTCIVEEAQKAGLAGGPGSRSRRTVSPKFRRNSM N.ribisnigriOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEGQKAGLTGGPGGRSRRTVSPKFRRNSM M.viciaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM M.dirhodumOBP8 QKYYGTNGTMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM A.fabaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEGQKVGLTGGPGGRSRRTVSPKFRRNSM A.pisumOBP1 ESDQVPINSSAAVESCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEV P.salicisOBP1 ESDQVPINSSAAVENCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMFGCVMRKNHIVNDGVVSKEV T.salignusOBP1 ESDQVPMNSSAAVENCLLETNMTRDEFEDMLTSPNARELTILKSHAHKCMLGCVMRKNHIVNDGVVSKEV A.pisumOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI P.salicisOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI T.salignusOBP1 LSKYVLNFYGRPDYKRRLIIKDVEHIVDVCAKKVADESETDECELAATLVTCIVLEANKAGLVDDPARQI A.pisumOBP8 ENNQQNGPSDRSATIFQSCIAETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.persicaeOBP8 ENNQQN-SSDRSATIFQSCIAETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA N.ribisnigriOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.viciaeOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA M.dirhodumOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA A.fabaeOBP8 ENNQQN-SNDRSATIFQSCISETKLSGDALKGFRSMSIPKTQAEKCMMGCLMRKVNVINKGKFSVEEATKVA A.pisumOBP8 QKYYGTNEAMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM M.persicaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTAEECALAGIVTTCIVEEAQKAGLAGGPGSRSRRTVSPKFRRNSM N.ribisnigriOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEGQKAGLTGGPGGRSRRTVSPKFRRNSM M.viciaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM M.dirhodumOBP8 QKYYGTNGTMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEAQKAGLSGGPGSRSRRTVSPKFRRNSM A.fabaeOBP8 QKYYGTNETMMKKAKDLIDVCAKKAQSTTEECALAGIVTTCIVEEGQKVGLTGGPGGRSRRTVSPKFRRNSM Next we wanted to verify whether such diversity would be present also between the same type of OBP in different aphid species.
Using specific primers designed on the ends of the sequences encoding the mature proteins, we were able to amplify homologous genes from other aphid species, obtaining in total 3 sequences for OBP1, 7 for OBP2 and 6 for OBP8 in Myzus persicae (Mper), Megoura viciae (Mvic),
Nasonovia ribisnigri (Nrib), Aphis fabae (Afab), Aphis craccivora (Acra), Metopolophium dirhodum (Mdir), Tuberolachnus salignus (Tsal) and Pterocomma salicis (Psal). GenBank
acc.no. for all the sequences obtained are: ApisOBP1: FM242527, TsalOBP1: FM242558, PsalOBP1: FM242559, ApisOBP2: FM242528, MvicOBP2: FM242550, NribOBP2: FM242553, AfabOBP2: FM242555, AcraOBP2: FM242557, MdirOBP2: FM242538, PsalOBP2: FM242560, ApisOBP3: FM242529, ApisOBP8: FM242534, MperOBP8: FM242547, MvicOBP8: FM242552, NribOBP8: FM242554, AfabOBP8: FM242556, MdirOBP8: FM242542.
In constrast with the high diversity found between OBPs of A. pisum, homology between OBPs of the same type in different aphid species is very high, with no more than 2-3 amino acid substitutions, even between phylogenetically distant species. Figure 13 and Figure 14 report the sequences of OBPs in the aphid species studied in this work.
Fig. 14. Alignement of OBP2 sequences in different aphid species. Similarity is very high also for this non-classic OBP.
Such high conservation of the sequences, unusual among insect OBPs (Pelosi et al., 2006), may be related to the fact that several aphid species, although phylogenetically distant, utilise the same pheromone components, namely (E)-β-farnesene as the alarm pheromone (Pickett et al., 1992; Bowers et al., 1972), and nepetalactone and/or one of the isomeric nepetalactols as the sex pheromones (Birkett and Pickett, 2003; Dawson et al., 1996), as reported in the Introduction. The great similarity (or even identity) of OBPs in different species of aphids could present interesting advantages, both in the study of their structural and functional properties, and in prospective applications that may result from such research. In fact, any semiochemical to be used in population control, that could be designed on the basis of structural parameters of OBPs, would likely be effective with the majority of aphid species.
Expression of aphids OBPs
A first selection of OBPs to be expressed and functionally studied included OBP1, OBP3 and OBP8. All are classic OBPs containing only the six conserved cysteines. (Figure 15)
Fig. 15. Amino acid sequences of three classical OBPs of A. pisum (OBP1, OBP3, OBP8) expressed in bacteria. Signal peptides are underlined. In addition to the six cysteines (asterisks), only six amino acids are conserved in all three proteins (indicated by dots).
Fig. 16. Bacterial expression and purification of A. pisum OBP1, OBP3 and OBP8. Electrophoretic analysis (SDS-PAGE) of crude bacterial pellets before (pI) and after (In) induction with IPTG, and of the purified proteins (P). Molecular weight of markers (M) are from the top: 66, 45, 36. 29, 20 and 14 kDa.
As far as the length of OBPs is concerned, a classification of these proteins into three groups has been recently proposed. The first group includes OBPs of about 160 residues, with a C-terminus long enough to fold back into the binding pocket and compete with the ligand, as observed in the B.mori PBP (Wojtasek and Leal, 1999). In medium length OBPs (≈120 residues), the C-terminus can only block the entrance to the binding site, while in short OBPs (≈110 residues), no conformational change seems to occur (Pesenti et al., 2008). According to this classification, OBP3 with 118 residues, seems to fall in the group of medium OBPs, while OBP1 (140 aa) and OBP8 (144 aa) could belong to the group of long OBPs.
The three OBPs were expressed in a bacterial system that proved highly efficient for the production of similar proteins. We used a construct encoding the mature protein with the addition of an initial methionine, as described in the “Materials and Methods”.
The recombinant proteins, as is the case for most insect OBPs, were expressed in high yields (more than 20 mg of protein per litre of culture), but were produced as inclusion bodies and had to be denatured and renatured in order to obtain them in a soluble form. This process has been
reported to yield the OBPs in their native folding with the six cysteines correctly paired (Ban et
al., 2003; Kruse et al., 2003). Figure 16 shows the electrophoretic analysis of the crude bacterial
pellets containing the recombinant proteins and samples of the purified OBPs.
Ligand-binding experiments
The three purified aphid OBPs were used in binding experiments to define the structural requirements of ligands for effective interactions with the protein and investigate their specificity to odorants including pheromones.
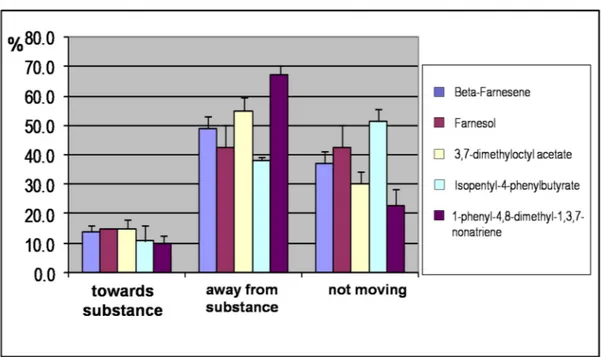
All three proteins bound the fluorescent probe 1-NPN with micromolar dissociation constants in the micromolar range as other insect OBPs (OBP1: 6.9 ±1.0 mM, OBP3: 5.9±1.4 mM, OBP8: 5.5± 0.6 mM). The upper left panel of Figure 17 shows the data for OBP3, while similar results were obtained with the other two proteins. Using 1-NPN as the fluorescent reporter, we then measured the affinity of twelve selected compounds in competitive binding experiments. The lower panels of Figure 17 report the displacement of 1-NPN from the complex with OBP3 by those compounds. Parallel experiments were performed with the other two proteins and the dissociation constants were calculated for each compound from the concentration value that halved the initial 1-NPN fluorescence ([IC50]). The data are reported in Table 3.
In the upper right panel of Figure 17, the affinities of the ligands for the three proteins are compared. A first series of compounds, including linear alkyl benzoates of different chain length, were chosen to evaluate the size requirements for a ligand. The ideal length of the chain appeared to be between four and six carbon atoms, with a rapid loss of activity for shorter (ethyl benzoate) as well as longer chains (n-octyl benzoate). Interestingly, 3,7-dimethyloctyl benzoate exhibited better affinity than the n-octyl derivative. The binding was not significantly affected by removal of the benzene ring, as shown by the behaviour of 3,7-dimethyloctyl acetate. A second series of ligands included compounds of different structures, both aliphatic terpenoids and aromatic compounds, bearing some structural similarity to the aromatic fluorescent probe 1-NPN. Some of the chemicals tested exhibited good affinity to all three proteins, whereas others were more selective. In particular, three ligands showed good affinity only to OBP3: (E)-β-farnesene, the alarm pheromone for most aphid species, its corresponding alcohol farnesol
Fig. 17. Upper left panel: Binding curve of 1-NPN to recombinant A. pisum OBP3 and Scatchard plot (inset). A 2 μM solution of the protein in Tris buffer was titrated with 1 mM solution of 1-NPN in methanol to final concentrations of 2–16 μM. Binding curves were measured also for OBP1 and OBP8 (data not shown) and dissociation constants (average of three replicates) were: OBP1: 6.9 ±1.0 μM, OBP3: 5.9± 1.4 μM, OBP8: 5.5± 0.6 μM. Lower panels: Competitive binding of selected ligands to A.
pisum OBP3. A mixture of the protein and 1-NPN in Tris, both at the concentration of 2 μM, was
titrated with 1 mM solutions of each competing ligand to final concentrations of 2–16 μM. Fluorescence intensities are reported as percent of the values in the absence of competitor. The same ligands were also used with OBP1 and OBP8 (data not shown). Upper right panel: Comparative binding properties of A. pisum OBP1, OBP3 and OBP8. For a more clear presentation, 1/Ki values have been plotted. Ligands and dissociations constants (μM in brackets, in the order for OBP1, OBP3, OBP8) were: (1) Ethyl benzoate (>100, >100, >100); (2) n-Butyl benzoate (12.4, 8.9, 5.9); (3) n-Hexyl benzoate (18.6, 5.2, 14.7); (4) n-Octyl benzoate (>100, >100, >100); (5) 3,7-Dimethyloctyl benzoate (>100, 14.9, 7.4); (6) 3,7-Dimethyloctyl acetate (>100, 13.4, >100); (7) Farnesol (>100, 4.5, >100); (8)
(E)-β-Farnesene (>100, 9.0, >100); (9) Butylbenzophenone (3.1, 3.7, 2.9); (10)
p-tert-Butylphenyl benzoate (9.3, 11.9, 4.4); (11) a-Amylcinnamaldehyde (4.7, 6.0, 4.4); (12) N-Benzyl-N’-phenylhydrazine (6.2, 14.9, 2.9). Ki could not be calculated when IC50values were >100.
and 3,7-dimethyloctyl acetate. This latter compound, although of a different chemical nature, has close similarity to (E)-β-farnesene, when comparing the shapes of the two molecules. The good affinity of these terpenoid structures to OBP3 could also be related to the apparently intriguing observation that 3,7-dimethyloctyl benzoate binds the same protein better than octyl benzoate.
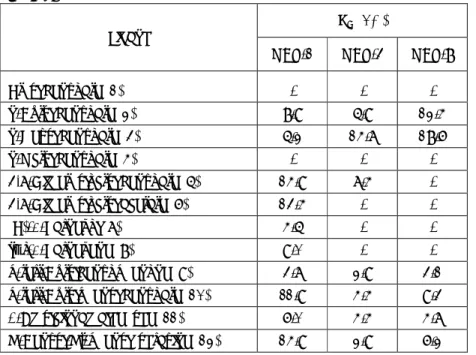
Table 3. Dissociation constants of complexes between recombinant A. pisum OBP1, OBP3, OBP8 and different ligands. Numbers 1-12 are as in
Figure 17.
Fig. 18. Model of A. pisum OBP3. The model was generated with the programme Swissmodel (Schwede et
al., 2003; Guex and Peitsch, 1997;
Arnold et al., 2006), using the PBP of
Leucophaea maderae (PDB: 1ORG_A;
Lartigue et al., 2003) as a template. In the alignment of the two proteins, identical residues are highlighted, those lining the binding site are in red font. Three of the hydrophobic residues in the binding site (Ile43, Leu48 and Val104) have their counterparts in the structure of L. maderae PBP.
Ligand
KD(μM)
OBP-1 OBP-3 OBP-8
Ethyl benzoate (1) - - -n-Butyl benzoate (2) 8.9 5.9 12.4 n-Hexyl benzoate (3) 5.2 14.7 18.6 n-Octyl benzoate (4) - - -3,7-Dimethyloctyl benzoate (5) 14.9 7.4 -3,7-Dimethyloctyl acetate (6) 13.4 - -(E)-β-Farnesol (7) 4.5 - -(E)-β-Farnesene (8) 9.0 - -p-tert-Butylbenzophenone (9) 3.7 2.9 3.1 p-tert-Butylphenyl benzoate (10) 11.9 4.4 9.3 α-Amylcinnamaldehyde (11) 6.0 4.4 4.7 N-Benzyl-N’-phenylhydrazine (12) 14.9 2.9 6.2
Based on the structure of the PBP of the cockroach L. maderae (acc no. 1orgA), we have modelled the folding of OBP3 of A. pisum. Figure 18 reports the alignment of the two proteins and the model of A. pisum OBP3 with some of the residues lining the binding pocket.
It is interesting to observe that five hydrophobic amino acids (Ile43, Leu48, Leu64, Val104 and Leu108) on opposite α-helical domains could well interact with the branched chains of
terpenoids, like β-farnesene. A sixth residue, Tyr84 could establish some interaction with the π electrons of the two double bonds at one end of β-farnesene or a hydrogen bond with the hydroxy group of farnesol, that also proved to be a good ligand.
We can observe that although each protein exhibits a broad spectrum of binding, the system is clearly capable of discrimination. Such a system is at the same time specific and yet not limited in the number of potential ligands it can detect. On the other hand, a strong affinity of OBPs towards semiochemicals is not required when we consider that these proteins are highly concentrated in the sensillar lymph. In fact, with a protein concentration of 1 mM (below the values estimated in the sensillum that can be as high as 10 mM), even a dissociation constant of 10 μM is enough to keep 99% of the ligand bound to the protein. These data indicate that the aphid OBP3 is involved in detecting and recognising the alarm pheromone (E)-β-farnesene. The view that OBPs might also be responsible for recognising the chemical stimuli in addition to membrane-bound receptors, is consistent with recent publications on the essential role of OBPs in insect chemoreception (Xu et al., 2005; Matsuo et al., 2007; Laughlin et al., 2008). In particular, the latest report shows that OBPs are able to stimulate olfactory receptors even in the absence of a ligand, if they adopt a conformation mimicking that of the complex (Laughlin et al., 2008). It is possible that several different chemicals bind the same OBP, but perhaps only one or a few are able to induce the specific conformational change in the protein for an efficient interaction with the receptor. We should also be aware that, besides OBPs, olfactory receptors (Clyne et al., 1999; Vosshall et al., 2000), as well as membrane-bound proteins, called SNMPs (Rogers et al., 1997; Vogt, 2003), whose function is still unclear, are required for a correct functioning of the olfactory system in insects (Ha and Smith, 2006; Benton et al., 2007; Jin et
al., 2008).
Finally, it is also important to consider that current OBP/ligand-binding model is based on binding measurements performed at concentrations of OBPs about three orders of magnitude lower than those present in the sensilla. It is difficult to predict what the behaviour of these proteins would be in their natural environment.
In any case, the active and essential roles of OBPs demonstrated in insect olfaction indicate the possibility of using these proteins as potential targets for interfering with the olfactory perception of insects as a way to control pest populations in the field. In particular, the detailed
Fig. 19. Comparison of the structures of (from the back) farnesol, (E)-β-farnesene and 3,7-dimethyloctyl acetate. In this last ligand, the carbonyl group replaces one of the double bonds of (E)-β-farnesene, while the branched chain can be superimposed in the three molecules. OH
O
O
OHO
O
study of the three-dimensional structure of OBP3 and its interaction with (E)-β-farnesene could help in the design of new compounds to mimic alarm pheromones and affect aphid behaviours in the field.
A comparison between the structures of the three ligands specific for OBP3, (E)-β-farnesene, farnesol and dimethyloctyl acetate, reveals interesting similarities between the last compound and the former two (Figure 19). In fact, the carbonyl group of the acetate moiety of the molecule could replace one of the terminal double bonds of (E)-β-farnesene, providing a similar electron-rich area, while most of the branched chains in the two molecules result superimposed.
Design and synthesis of new potential alarm pheromones
Based on the above considerations and in particular on the structural comparison of the three specific ligands for OBP3, we designed new molecules mimicking the structures of (E)-β-farnesene, but much easier and economical to make, as well as more stable in the environment.
The leading idea was to maintain the terpene structure of the branced chain, while replacing the system of the two terminal conjugated double bonds with a more stable electron-rich moiety, such as an ester or an aromatic ring.
Therefore, some new molecules were synthesised in our lab, starting from inexpensive natural compounds, such as citronellal, citral or citronellol, that provided the terpene structure and could be easily transformed in the desired products.
Figure 20 briefly shows the synthetic routes used for their preparation, while in Figure 21 one of these structures is compared with that of (E)-β-farnesene.
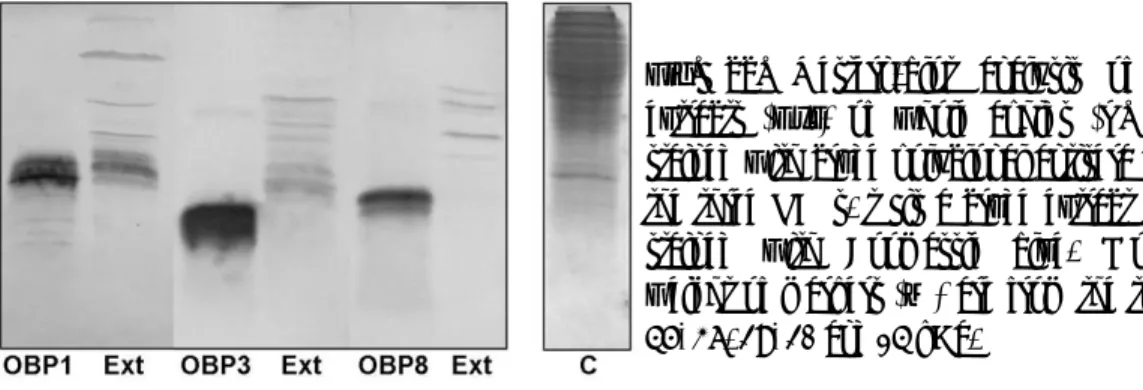
Fig. 22. Western-blot analysis of crude extracts (Ext) of whole aphids (A. pisum) stained with crude polyclonal antisera against the three OBPs. C is a crude extract sample stained with Coomassie blue. Molecular weight of markers (M) are from the top: 66, 45, 36. 29, 20 and 14 kDa. O OH P Ph Ph Ph O O Br+P(Ph)3 + + CH3-COCl Br - + Wittig
Fig. 20. Synthetic routes utilised for preparing new putative aphid repellents. The first route was applied to the preparation of several esters of similar structure.
Fig. 21. Comparison between the structure of (E)-β-farnesene and the synthetic compound
1-phenyl-4,8-dimethyl-1,3,7-nonatriene. In this molecule the two conjugated double bonds of (E)-β-farnesene are replaced by a more stable benzene ring.
Immunodetection
Polyclonal antibodies raised against the recombinant OBPs were then used in Western blot experiments. Due to the small size of aphids, we decided to utilise extracts from whole insects, rather than to dissect different parts of the body. The results are shown in Figure 22.
The antiserum against OBP1 gave a strongly reacting band in the crude extract at about the same molecular weight as the recombinant protein, the other two antisera cross-reacted with a number of bands within a wide range of molecular masses. Whilst these experiments demonstrate that all
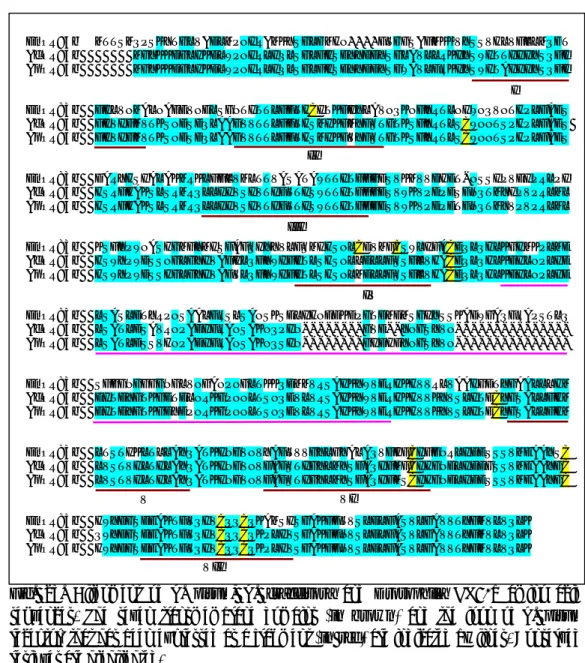
DmOR83b MTTSMQPSKYTGLVADLMPNIRAMKYSGLFMHN----FTGGSAFMKKVYSSVHLVFLLMQFT AcOR83b MGYKKDGLIKDLWPNIRLIQLSGLFISEYYDDYSGLAVLLRKIYSWITTIIIYSQFI ApOR83b MGYKKDGLIKDLWPNIRLIQLSGLFISEYYDDYSGWAVLFRKIYSWITAIIIYSQFI DmOR83b FILVNMALNAEEVNELSGNTITTLFFTHCITKFIYLAVNQKNFYRTLNIWNQVNTHPLFAES AcOR83b FIVIFMVTKSNDSDQLAAGVVTTLFFTHSMIKFMYFSTGTKSFYRTLSCWNNTSPHPLFAES ApOR83b FIVIFMVTKSNDSDQLAAGVVTTLFFTHSMIKFVYFSTGTKSFYRTLSCWNNTSPHPLFAES DmOR83b DARYHSIALAKMRKLFFLVMLTTVASATAWTTITFFGDSVKMVVDHET-NSSIPVEIPRLPI AcOR83b HSRFHAKSLSRMRQLLIIVSIVTIFTTISWTTITFFGESVWKVPDPESFNQTMYIPVPRLML ApOR83b HSRFHAKSLSRMRQLLIIVSIVTIFTTISWTTITFFGESVWKVPDPETFNQTMYVPVPRLML DmOR83b KSFYPWNASHGMFYMISFAFQIYYVLFSMIHSNLCDVMFCSWLIFACEQLQHLKGIMKPLME AcOR83b HSWYPWDSSNGLGYIVAFILQFYWIFITLSHSNLLELLFSSFLVHACEQLQHLKEILNPLIE ApOR83b HSWYPWDSSHGLGYIVAFVLQFYWIFITLSHSNLMELLFSSFLVHACEQLQHLKEILNPLIE DmOR83b LSASLDTYRPNSAALFRSLSANSKSELIHNEEKDPGTDMDMSGIYSSKADWGAQFRAPSTLQ AcOR83b LSATLDSAVRNPAEIFRANSAKNQPIN---GVD--YNGSYVN---ApOR83b LSATLDSSVHNPAEIFRANSAKNQSIN---GIDHDYNGSYVN---DmOR83b SFGGNGGGGNGLVNGANPNGLTKKQEMMVRSAIKYWVERHKHVVRLVAAIGDTYGAALLLHM AcOR83b EITEYGTKGETELNRKGPNNLTSNQEVLVRSAIKYWVERHKHVVKYVSLITDCYGSALLFHM ApOR83b EITEYGTKGEYEPNRKGPNNLTSNQEVLVRSAIKYWVERHKHVVKYVSLITECYGSALLFHM DmOR83b LTSTIKLTLLAYQATKINGVNVYAFTVVGYLGYALAQVFHFCIFGNRLIEESSSVMEAAYSC AcOR83b LVSTVILTILAYQATKINGVNVFAFSTIGYLMYSFAQIFMFCIHGNELIEEGSSVMEAAYGC ApOR83b LVSTVILTILAYQATKINGVNVFAFSTIGYLMYSFAQIFMSCIHGNELIEESSSVMEAAYGC DmOR83b HWYDGSEEAKTFVQIVCQQCQKAMSISGAKFFTVSLDLFASVLGAVVTYFMVLVQLK AcOR83b QWYDGSEEAKTFVQIVCQQCQKPLIVSGAKFFNVSLDLFASVLGAVVTYFMVLVQLK ApOR83b HWYDGSEEAKTFVQIVCQQCQKPLIVSGAKFFNVSLDLFASVLGAVVTYFMVLVQLK I VII II III IV V VI
Fig. 23. Alignment of A. pisum, A. craccivora and Drosophila OR83b amino acid sequences. The seven transmembrane domains (in brown) and the loop of A. pisum receptor, that has been expressed as a fragment (in red) are indicated by lines. Conserved residues are highlighted.
three OBPs are expressed in the adult aphid, they also suggest the occurrence of modified forms of these proteins. Moreover, we cannot exclude the presence of other proteins that might cross-react with our polyclonal antisera.
Identification of aphids Olfactory Receptors
Besides OBPs, also olfactory receptors (ORs) are required for a correct functioning of the olfactory system in insects. It is known that each OR of insect works in tandem with a special OR, first named OR83b in Drosophila, very likely establishing a heterodimeric complex. This special receptor is highly conserved in all insect species so far examined, particularly in its C-terminal region.
Fig. 24. Hydrophobic profile of Olfactory receptor 83b of A. pisum. There are seven putative transnmembrane domains clearly identified. The same programme also predicts that the N-terminus of the protein is intracellular and the C-terminus extracellular.
Therefore, as a first contribution to a future study of ORs in aphids, we decided to clone the orthologue of OR83b in the pea aphid and express a fragment for the preparation of antibodies. We searched for aphids ORs in the aphid genome database, using the BLAST programme and the Drosophila melanogaster 83b receptor sequence (Genebank acc.no. AAT71306) as a template. A gene similar to DOr83b was found in Acyrthosiphon pisum and cloned to confirm the sequence.
Using the same specific primers, we managed to amplify a PCR product of the correct size in
Aphis craccivora, and obtain the full length sequence of OR83b in this specie. Figure 23 shows
the deduced amino acid sequences of OR83b in the two aphid species compared with that of
Drosophila. The similarity of A. pisum and D. melanoglaster is 54%; A. craccivora and D. melanoglaster is 53%; A. pisum and A. craccivora is 95%. The C-terminal region being the most
conserved.
Figure 24 shows the position of predicted transmembrane helices in A. pisum OR83b and the orientation of the sequence. As with most olfactory receptors of insects, this also would present its N-terminus inside the cell and its C-terminus outside.
Fig. 25. Expression and purification of A. pisum OR83b loop. Electrophoretic
analysis (SDS-PAGE) of crude bacterial pellets before (pI) and after (In) induction with IPTG, and of purified samples (P) of this protein. Molecular weight of markers (M) are from the top: 66, 45, 36. 29, 20 and 14 kDa.
Expression of a fragment of OR83b
In order to detect the presence and label the sites of expression of OR83b in A. pisum and other aphids, as well as to confirm the predicted orientation of this receptor in the membrane, we expressed a fragment in a bacterial system, and chose a large loop (235aa.-321aa.) of 87 amino acids predicted to be located intracellularly (Figure 25). The expressed product was obtained in high yield all in its soluble form and purified using conventional chromatographic methods. The purified polypeptide was then used for the production of polyclonal antibodies in rabbit.
Recent report also shows that the topology of olfactory receptors in insect presents its the N-terminus inside the membrane and the C-terminus outside (Benton et al., 2006). If this configuration will be demonstrated also in aphids, the large loop we cloned would be located inside the cell. In any case, this will not prevent an efficient immunostaining, if the tissue is previously permeabilised with detergents. Therefore, performing immunolabelling experiment before and after permeabilization will clarify the topology of this olfactory receptor in aphids. Moreover, we hope that the use of polyclonal antibodies, raised against a relatively large area of the receptor, could allow labelling of orthologous receptors also in other orders of insects.
OBP1
OBP3 OBP1
OBP3
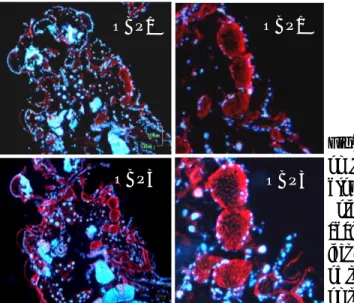
Fig. 26. Immunostaining of chemosensilla on the legs of A. pisum, obtained by the group of Patrizia Falabella, University of Potenza, Italy, using polyclonal antisera against recombinant OBP1 and OBP3. The left images show sections of the upper part of the aphid body, while the enlargements on the right show details of a leg.
Results obtained by collaborators
In this paragraph, I report some results obtained by the groups of Patrizia Falabella and Donatella Battaglia at the University of Potenza, Italy, as part of a collaborative project, using antibodies and chemicals prepared during my thesis.
Immunofluorescence localisation
The polyclonal antisera prepared against the recombinant OBPs were used in immunofluorescence experiments on slices of entire aphids. As can be seen from Figure 26, both antisera against OBP1 and OBP3 efficiently stained the surface of legs. Other experiments are in progress to map the expression of the three OBPs described in this thesis on the aphid body.
Behaviour experiments
The new compounds designed on the structure of (E)-β-farnesene, as reported above, and synthesised in our lab, were used in behaviour experiments with the pea aphid to verify their repellent activity, using (E)-β-farnesene as a reference.
Fig. 27. Behaviour experiments performed in the laboratory of Donatella Battaglia, University of Potenza, Italy, with the compounds synthesised during my thesis. The repellent effect of all the compounds synthesised is comparable with that of the natural alarm pheromone
(E)-β-farnesene.
efficient aphid repellents, at least as good as (E)-β-farnesene. The best chemical in this respect proved to be 1-phenyl-4,8-dimethyl-1,3,7-nonatriene (PDN), that exhibited a repellent effect even stronger than the natural compound.
All these new compounds can be easily prepared, are inexpensive and very stable in the environment. Apart from PDN, that had not been described in the literature before, the other compounds are natully occurring in plants and generally non toxic.
Fig. 28. Two-dimensional gel electrophoretic separation of a crude extract from antennae of 1100 male
An. gambiae. Spots within the
marked area were analysed by MS/MS TOP3 Orbitrap analyzer and the corresponding proteins were identified by their mass and short segments of sequence at the ends. Among the proteins involved in olfaction, a single OBP (OBP9) and two CSPs (SAP1 and SAP3) could be identified in our experimental conditions.
Part II Mosquitoes
Identification of Anopheles gambiae OBPs
The genome of the main malaria vector Anopheles gambiae contains 60 genes encoding putative odorant-binding proteins (OBPs) (Biessmann et al., 2005).
In order to verify which of the 60 OBPs predicted by the genome were most abundantly expressed in the antennae of An. gambiae, we applied proteomics techniques to crude antennal extracts.
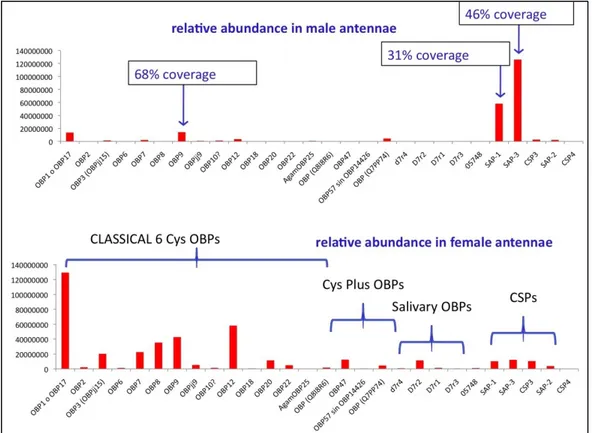
In a first experiment, the antennal proteins of male An. gambiae were separated on a 2D gel and the spots analysed as described in the “Materials and Methods”. The 2D electrophoretic separation is shown in Figure 28. The only OBP found in quantities high enough to be detected in our experimental protocol was OBP9, marked with circles on the gel. Besides, two CSPs (Chemosensory Proteins) could also be identified, marked with squares.
Fig. 29. Olfactory proteins identified in the antennae of male and female An. gambiae, using a shot-gun approach. 600 insects of each sex were used for three replicates. Crude extracts were digested and analysed on a LC-MS/MS system (Eksigent nanoLC and ESI LTQ-Orbitrap XL, Thermo), using a C18 (75μm i.d. × 15cm, 1.8 μm, 100Å) column eluted at the flow rate of 250 nl/min. MS scans were recorded in the Orbitrap analyzer in the mass range mass range 300-1700 Th at a 30,000 nominal resolution at 400 m/z, then up to 20 MS/MS experiments (Top20) were performed in the Velos LTQ Orbitrap.
The same analysis could not be used with female antennae of the same species, due to their much smaller size. Therefore, we applied a shot-gun approach to antennal extracts of both sexes. This method requires much smaller amounts of biological sample compared to the analysis using a 2D gel. Accordingly, the total protein pool was digested with trypsin and LysC after reduction and alkylation of cysteines and separated on nano-HPLC system coupled to a nano ESI-LTQ ORBITRAP XL mass spectrometer. The acquired data were searched using MaxQuant software v1.0.13.13 and Mascot search algorithm (MatrixScience) against An.
gambiae genome together with the entries reporting the Anopheles proteins in UniProtKB.
These experiments were performed by Guido Mastrobuoni at the University of Berlin, Germany in collaboration with Francesca Dani (CISM, University of Firenze) on the samples we have collected and prepared.
Figure 29 reports the proteins identified with this approach as a histogram. The amount of each protein present has been evaluated using a label-free technique, based on the intensity of a number of peaks in the mass spectra of selected peptides for each protein.
We can observe that:
female antennae are much richer than males in the number of OBPs expressed, as previously suggested by studies at the RNA level;
with male antennae, the results of shot-gun experiments are in good agreement with those obtained from 2D-gel, with only an additional major OBP (OBP1 or OBP17: the two proteins are very similar and cannot be distinguished) detected in the present experiment and a couple of other OBPs observed in very low amounts;
with female antennae, the expression of OBPs is in fairly good agreement with those of PCR and microarrays at the RNA level; however, two important proteins, whose genes are abundant in the female antennae, are missing from our results, OBP4 and OBP5. We are currently investigating this aspect to understand whether the expression products are really absent from our extracts or they might occur in modified forms, thus preventing their identification by the software utilised.
Expression and purification of selected OBPs in E.coli
Besides OBP9, that had been recognised as the most abundantly expressed OBPs, we have also selected 6 additional An. gambiae OBPs to be expressed and characterised. The genes encoding these proteins, named as OBP1, 3, 4, 12, 19 and 47, were reported to be up- or down-regulated after a blood meal (Biessmann et al. 2005). They are all classic OBPs (with the conserved pattern of six cysteines), except for OBP47, which is much longer (173 amino acids instead of 119 to 132 for the others) and contains 13 cysteines (Figure 30). The genes encoding these OBPs have all been shown to be expressed at relatively high levels by Northern blot and microarray experiments (Biessmann et al., 2002; 2005; Justice et al., 2003b), with all, except for OBP12 and OBP19, being in the top 10 OBPs most expressed in female antennae. OBP12 was included because its expression is almost three times higher in female antennae than in males and OBP19, together with OBP47, is one of the few OBPs selectively expressed in the head.
Fig. 30. The amino acid sequences of the seven selected Anopheles gambiae OBPs. The conserved cystines are highlighted.
>OBP1 DTTPRRDAEYPPPELLEALKPLHDICLGKTGVTEEAIKKFSDEEIHEDEKLKCYMNCLFHEAKVVD DNGDVHLEKLHDSLPSSMHDIAMHMGKRCLYPEGETLCDKAFWLHKCWKQSDPKHYFLV >OBP3 DEPRRDANYPPPELLEKMKPMHDACVAETGASEDAIKRFSDQEIHEDDKLKCYMNCLFHQAGVV NDKGEFHYVKIQDFLPESMHLITLNWFKRCLYPEGENGCEKAFWLNKCWKTRDPVHYFLP >OBP4 AMTMKQLTNSMDMMRQACAPKFKVEEAELHGLRKSIFPANPDKELKCYAMCIAQMAGTMTKKG EISFSKTMAQIEAMLPPEMKTMAKEALTHCKDTQTSYKDPCDKAYFSAKCAADFTPDTFMFP >OBP9 EFVVQTREDLLAYRAECVKSLGVSDELVEKYKSWNFPEDDTTQCYIKCIFNKMQLFDDTNGPIVD NLVVQLAHGRDANEVREEIVKCAGSNTDGNVCHWAFRGFQCFQKNNLSLIKASVKKD >OBP12 LDISKVTLDAAFYPLFGCARDLVVPEDLIELYKKRIFPDDQLTCCVFRCLGMRLGIYDDVKGFDVDK QYERVKDRLSVDEDTYKRGVKNCIRNVLRGRTLNNCEKAYLILNQCQGNTITNSLNQQLNEIRCN >OBP19 YITQEQLEKTARTFRQVCQPKHKISDEVADAVNRGVFADTKDFKCYVSCLLDIMQVARKGKVNYE KSLKQIDTMLPDHMKPAFRAGLEACKSAAQGVKDHCEAATILLQCFYKNNPKFVFP >OBP47 DNPCLKGPPVPKNAAECCVTPFLVEPSAFMTCHSKWIGQTKRQMAMEGIPRGCCVAECVMNSTSL YSNGKIDREALTKLYLDSTKSMAPEWNKITLDAIDGCFKMADSIKDEIEAGAKLTPAFDGEQICHPI SGTILACMGMTLFAECPAKLFTVNDDCNKLKSYHSKCPFL
Some of the OBPs chosen have interesting structural relationships with their counterparts in other mosquito species and in Drosophila. In particular, OBP1 and OBP3 are the orthologues of
D. melanogaster OS-E and OS-F respectively, and OBP4 is structurally similar to Drosophila
LUSH. It is particularly interesting that these three proteins of Drosophila have been observed to be expressed in the same chemosensilla (Shanbhag et al., 2001). OBP9 is also the orthologue of OBP22 of dengue vector mosquito Aedes aegypti, where this OBP, besides being expressed in the antennae, is also abundantly found in the sperm and transferred to the female during copulation (Li et al., 2008; Sirot et al., 2008).
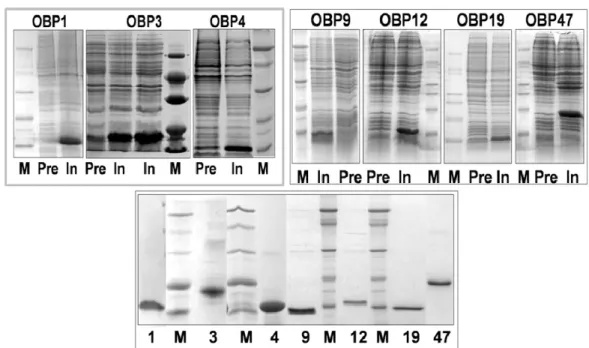
All seven An. gambiae OBPs were expressed in E. coli with good yields (20 mg/L). To minimize the effect of His-tags (or other additional segments sometimes introduced to facilitate purification by affinity chromatography) on ligand-binding assays, our constructs contain only a starting methionine in addition to the mature protein sequence. As observed with most insect OBPs, our recombinant proteins were present in the bacterial cells as insoluble inclusion bodies and had to be solubilised by denaturation and reduction. Although the conditions used in this process are particularly harsh, the unfolded OBPs can be renatured correctly as demonstrated in several cases by binding experiments, pairing of disulphide bridges and crystal structure
Fig. 31. Expression and purification of seven OBPs of An. gambiae. Upper two panels: electrophoretic analysis (SDS-PAGE) of crude bacterial pellets, each expressing one of the OBPs, before (Pre) and after (In) induction of the bacterial culture with IPTG. Lower panel: samples of purified OBPs. M: molecular weight markers of 66, 45, 29, 20 and 14 kDa.
(Prestwich, 1993; Ban et al., 2003; Kruse et al., 2003). OBP47, despite its length and the presence of 13 cysteine residues, was refolded into its active conformation as judged from its binding properties.
Purification of each OBP was accomplished using conventional techniques, combining anion-exchange chromatography with gel filtration. Figure 31 shows the electrophoretic analysis of both the crude expression products and samples of purified proteins for each of the seven OBPs.
Expression of OBPs in yeast
One common problem encountered with bacterial expression of insect OBPs is that often the protein is synthesised as inclusion bodies and has to be denatured in order to be solubilised. Although in several cases it has been demonstrated that correct refolding occurs easily, it is also desirable to set up a method for expressing these proteins in soluble form. The availability of extremely pure proteins, also with respect to misfolding, is specially required for crystallization experiments and identification of three-dimensional structure.
Fig. 32. Expression and purification of AgamOBP9 in yeast. Left: SDS-PAGE and Western blot of OBP9 in yeast culture expressed at different time. Right: SDS-PAGE of fractions obtained by gel filtration of a crude culture medium expressing OBP9. M: molecular weigth markers are from the top: 66, 45, 36, 29, 20 and 14 kDa.
Therefore, we chose the yeast Kluveromyces lactis as a host to express some of our proteins. This host is now commercially available as a complete kit. We decided to express OBP9 and OBP47, the first being the most abundant in mosquito antennae, the second likely to present problems in bacterial expression, due to the great number of cysteines present.
We have successfully expressed both OBPs with yields of about 20 mg/L of culture. In this case, the protein was secreted in the medium in its soluble form and could be easily purified, representing by far the major protein component of the culture medium. Figure 32 shows the electrophoretic analysis of the crude culture medium of yeast strains expressing OBP9. The identity of the expressed protein is confirmed by the Western blot performed with the antiserum against the orthologous protein OBP22 of the mosquito Aedes aegypti, that shares 70% of its residues with An. gambiae OBP9. The same figure also reports the SDS-PAGE analysis of a sample of OBP9 purified by anion-exchange chromatography on DE-52, followed by gel filtration. The MALDI spectrum is in agreement with the calculated molecular weight, thus indicating that the protein does not contain post-translational modifications apart from disulfide bridges (Figure 33).
Fig. 33. MALDI spectrum of OBP9 expressed in the yeast K. lactis. The measured molecular weight is in good agreement with the calculated value (taking into account three disulfide bridges) and indicates that the protein does not contain other post-translational modifications.
Fig. 34. Post-translational modification of OBP47. The protein produced in the yeast K. lactis (1) migrates with an apparent molecular weight of about 22 kDa, whilst
bacterial-expressed OBP47 (3) shows a mass of about 19 kDa, in agreement with the calculated value of 18,918 Da. Treatment of the yeast-expressed OBP47 with PNGase F (2) generates a band migrating with an apparent mass identical with that of the bacterial sample, thus indicating that the yeast produced protein is glycosilated.
Unlike OBP9, the OBP47 prepared in yeast migrated with a higher molecular weight on SDS-PAGE, compared to the protein expressed in the bacteria, suggesting that glycosylation could have been introduced. To verify this hypothesis, a sample of the yeast-produced protein was digested with PNGase and the product analysed by SDS-PAGE followed by Western blot
Fig. 35. (A) MALDI spectrum of OBP47 expressed in K. lactis after treatment with PNGase; both the glycosylated and the deglycosylated forms are present, the first being predominant. (B) MALDI spectrum of OBP47 expressed in E. coli. (C) MALDI spectrum of OBP47 expressed in E. coli after cysteine carboamidomethylation. The mass difference corresponds to a single carboamidomethyl group introduced in the derivatised sample. Calculated masses for OBP47 expressed in E. coli (assuming the presence of six disulfide bridges) before and after carboamidomethylation are 18, 918 and 18,975 respectively.
(Figure 34), as well as by MALDI mass spectrometry (Figure 35). The Western blot analysis, using a crude polyclonal antiserum against the bacterially-expressed OBP47, showed the presence of a band in the digested sample, migrating with the same apparent mass as the bacterial OBP47 and confirming that the protein produced in yeast is glycosylated (Figure 34). Likewise, the MALDI spectrum showed a main broad peak around mass 21,517.6, indicating a heterogeneous population of glycosylated OBP47, and a minor peak at mass 18,922.5 in agreement with the calculated molecular weight of the de-glycosylated protein, assuming the presence of 6 disulphide bridges (18,918). The same antiserum was also used to probe the expression of OBP47 in different parts of the adult mosquito and the results clearly indicate that expression is restricted to the head of both sexes (Figure 36). We can also observe that the OBP is present in mosquitoes in its glycosylated form, migrating with an apparent molecular weight of about 22 kDa. A second experiment showed that the expression of OBP47 is only a barely detectable in the antennae, with most of the reactivity being associated with heads without antennae (Figure 36).
Fig. 36. Left: Expression of OBP47 in head (H), thorax (Th) and abdomen (Ab) of An. gambiae (mixed sexes). Western blot analysis reveals the exclusive presence of OBP47 in the
head. Right: OBP47 is mostly expressed in heads without antennae of male (mH) and female (fH) mosquitoes and is only barely detectable in the antennae of both sexes (mA and fA). M: molecular weight markers of 66, 45, 29, 20 and 14 kDa.
Fig. 37. Comparison of (D) the experimental spectrum of the 12+charge state of OBP47 expressed in E. coli and (E) the theoretical 12+charge state spectrum obtained by considering the protein to
have 6 (in red) or 5 (in green) disulfide bridges in a ratio 7:3 (sum of the two spectra in black). Data were recorded on LTQ Orbitrap high resolution mass spectrometer.
As a first contribution to a structural characterisation of OBP47, we investigated the number of free cysteines in the refolded protein. Derivatization of the bacteria-produced OBP with iodoacetamide gave a single molecular species whose MALDI mass spectrum showed a single peak of mass 18,980.6 (Figure 35). The difference with respect to the non-derivatized protein (measured mass 18,922.5) is 58 units, accounting for a single free cysteine residue. To confirm this hypothesis and rule out the possibility of additional free cysteines buried in the core of the protein and hence not affected by the reagent, we also performed an exact measurement of the mass of the protein. In Figure 37 the portion of the electrospray mass spectrum relative to the 12 charge ion is compared with the theoretical spectra calculated for the formulae
C825H1309N215O249S22and C825H1311N215O249S22 relative to the protein with respectively one and
three free cysteines in a ratio of 7:3. This indicates that the most probable configuration of OBP47 is the one with a single free cysteine, in agreement with the results of the derivatized protein.
Ligand-binding experiments
The 4 recombinant OBPs (OBP9, OBP12, OBP19, OBP47) we have purifed and 3 other OBPs (OBP1, OBP3, OBP4) purified by a collaborator (He Xiaoli of Rothamsted Research in UK), were exchanged and used in ligand-binding experiments to characterise their binding properties, with a number of small organic compounds in fluorescent displacement assays. First, affinity constants were measured for each protein to the fluorescent probe 1-NPN (N-phenyl-1-naphthylamine). All the OBPs used, except OBP9, bind reversibly 1-NPN with dissociation constants in the micromolar range. We could not measure appreciable binding of 1-NPN to OBP9, nor of other fluorescent probes, such as 2-NPN, 1-AMA, 2-AMA and ANS. The possibility that such lack of binding activity could be due to an uncorrectly refolded protein was ruled out by the fact that also the sample of OBP9 expressed in K. lactis (that being soluble did not need any refolding step) showed the same behaviour towards the fluorescent ligands. It is possible that the binding cavity is too small to accommodate the relatively large fluorescent probes or that the probe does bind to the protein, but its emission spectrum does not undergo any change. This second hypothesis can be probably ruled out as we could not observe any quenching of the tryptophan intrinsic fluorescence upon addition of 1-NPN. Figure 38 shows the binding curves, and their relative linearisation, using Scatchard plots.
In most cases the points fit a straight line, but for OBP47 and OBP19 the plot is curved upwards, allowing the evaluation of two binding constants. This phenomenon cannot be attributed to the presence of two binding sites on the same protein unit, as structural studies have clearly shown a compact structure for insect OBPs enclosing a single binding pocket. However, most of these OBPs are present as dimers in the conditions used for the binding experiments (Pelosi et al., 2006) and it has been shown that when OBPs crystallize as dimers, the two monomeric
Fig. 38. Binding curves of 1-NPN and relative Scatchard plot analyses. A 2 μM solution of the protein in Tris-buffer was titrated with 1mM solution of 1-NPN in methanol to final concentrations of 2-16 μM. Dissociation constants (average of three replicates) were OBP1: 4.7 μM (SD 2.5); OBP3, 2.3 μM (SD 1.6); OBP4, 1.6 μM (SD 1.2); OBP12, 8.9 μM (SD 0.3); OBP19, 2.1 μM (SD 0.6); OBP47, 2.7 μM (SD 0.2).
structures, and therefore their binding pockets, cannot be considered equivalent (Tegoni et al., 2004). Whether or not the phenomenon of two binding constants is observed in ligand-binding experiments will depend on (a) the degree of asymmetry of the dimer and (b) the dissociation constant for the equilibrium monomer/dimer. In this respect it is worth recalling that the concentration of OBPs in the insect’s sensillum lymph is extremely high (in the millimolar range), making any equilibrium strongly shifted towards the formation of dimers.
In a second series of experiments we measured the affinity of each OBP for a number of potential ligands in competitive binding assays, using 1-NPN as the fluorescent reporter. We used both volatiles present in the environment, including some reported, or supposed, to be
active on mosquitoes, as well as homologous series of synthetic ligands, such as benzoates, in order to define the structural requirements of an ideal ligand for each protein. The natural compounds we tested included (E)-2-hexenal, citronellal, geranyl acetate and indole, which have all been reported to specifically activate olfactory receptors of An. gambiae (Allison et al., 2010). In particular, indole has been reported as a ligand for OBP1, on the basis that silencing the gene encoding OBP1 suppresses electrophysiological response to indole, but not to other odorants (Biessmann et al., 2010). Other compounds, such as 1-octen-3-ol, decanal, 6-methyl-5-hepten-2-one, menthol, linalool and p-cresol, were found to be constituents of human sweat and to produce electrophysiological and behavioural responses in mosquitoes (Logan et al., 2007, 2008). DEET, Icaridin and thymol were also tested as representatives of mosquito repellents. Table 4 show the IC50 values (the concentration of the ligand halving the
initial fluorescence value) and the calculated inhibition constant (Ki) where possible for the OBP/ligand combinations. For weaker ligands, where IC50 values could not be measured, we
report fluorescence intensity measured at the highest concentration of ligand (16 μM) as percent of the initial value. Figure 39 shows the displacement curves obtained with a selected set of ligands for each OBP.
As expected, on the basis of similar studies performed with other OBPs, a broad specificity can be observed for the OBPs of An. gambiae, with each protein preferentially binding to several related compounds. OBP1, OBP3, OBP4 and, to some extent, OBP47 show some similarities in their binding spectra, being focused on terpenoids of medium size and some structurally related molecules. Figure 39 shows the affinities of these four OBPs to the same set of ligands. Thus, the best ligand for OBP1 is citronellal, OBP3 binds 2-octenal and 2-nonenal, while several compounds bind rather strongly to OBP4 with menthol being the best among the natural volatiles. Menthol is also the best natural ligand of OBP47, despite the marked structural differences between these two OBPs. We can also observe that the structures of citronellal and menthol have the same number of carbon atoms, arranged in the same terpene topology, the main difference being an additional bond, which closes the open chain of citronellal into the ring of menthol. 2-Octenal, on the other hand, differs from citronellal by the absence of the two side methyl groups, apart from the position of the double bond.
Fig. 39. Binding of selected ligands to recombinant OBPs of An. gambiae. A mixture of the protein and 1-NPN in Tris-buffer, both at a concentration of 2 μM, was titrated with 1 mM solutions of each competing ligand to final concentrations of 2-16 μM. Fluorescence intensities are reported as percent of the values in the absence of competitor. The calculated dissociation constants and the binding data relative to all the ligands tested are reported in Table 4.
Ligand OBP1 OBP3 OBP4 OBP12 OBP19 OBP47 IC50 Int Ki IC50 Int Ki IC50 Int Ki IC50 Int Ki IC50 Int Ki IC50 Int Ki
Alcohols (Z)-3-Hexenol 82 67 72 1-Octanol 91 66 91 91 1-Octen-3-ol 82 71 71 71 1-Nonanol 88 62 65 1-Decanol 73 63 58 Linalool 78 66 73 73 Menthol 21 57 20.2 17 51 13.9 6 31 4.6 6 31 5.0 1-Dodecanol 77 64 14 46 10.7 14 46 11.8 3,7-Dimethyloctanol 61 a-Pentylcinnamyl alcohol 77 70 Farnesol 12 52 9.2 112 2.2 26 1.8 Retinol 4 26 3.1 55 5.2 30 4.2
Aldehydes and ketones
Hexanal 83 61 97 85 (E)-2-Hexenal 84 64 73 Octanal 102 66 8 42 6.1 73 73 (E)-2-Octenal 68 12 43 9.8 68 Nonanal 17 51 16.3 16 50 13.1 10 44 7.6 130 54 71 (E)-2-Nonenal 13 46 12.5 12 41 9.8 20 55 15.3 72 Decanal 80 86 17 51 13.0 17 51 14.3 (-)-citronellal 5.5 40 5.3 14 45 11.5 18 53 13.7 16 51 12.9 18 53 15.1 Benzaldehyde 96 60 65 93 69 -Pentyl cinnamaldehyde 3.5 16 2.7 7.4 27 7.3 6-Methyl-5-hepten-2-one 88 63 78 87 Jasmone 80 64 78 Geranylacetone 67 72 93 Retinal 73 110 72 Carboxylic acids Pentanoic acid 71 Heptanoic acid 80 Octanoic acid 60 7-Octenoic acid 92 68 77 77
Table 4. Binding of pure organic compounds to selected recombinant OBPs of An. gambiae. Solutions of protein and fluorescent probe 1-NPN, both at the concentration of 2 μM were titrated with 1 mM solution of each ligand in methanol to final concentrations of 2-16 μM. For each protein, we report the fluorescence intensity (Int) measured at the maximal ligand concentration (16 μM) as percent of the initial fluorescence, the concentration of ligand halving the initial fluorescence intensity (IC50), where applicable,
Nonanoic acid 80 Undecanoic acid 67 Benzoates Ethyl benzoate 59 121 17 52 13.7 Butyl benzoate 62 82 1.3 17 1.0 Hexyl benzoate 3.5 19 2.7 61 6.5 31 5.2 Octyl benzoate 20 54 15.3 92 16 50 12.9 3,7-Dimethyloctyl benzoate 3 30 2.3 20 53 19.8 1.7 29 1.4 3-Hexyl benzoate 11.7 39 11.6 20 52 16.1 4-Methylpentyl benzoate 1.8 12 1.4 12 43 11.9 6 25 4.8 p-Isopropylphenyl benzoate 10 39 7.6 15.5 49 15.3 5.8 36 4.7 Butyl p-tert-butylbenzoate 15 48 14.9 1.4 19 1.1 Phenyl benzoate 104 16 51 12.9 p-Tolyl benzoate 82 55 2-Phenylethyl benzoate 9.5 42 7.7 Benzyl benzoate 87 p-tert-Butylphenyl benzoate 6 29 4.6 10 41 9.9 5 30 4.0 Butyl p-nitrobenzoate 56 11 46 8.9 Isobutyl p-nitrobenzoate 84 64 Aromatic compounds m-Cresol 62 p-Cresol 71 71 71 p-tert-Butylbenzophenone 4 17 3.1 6 21 5.9 7.5 21 6.0 3.2 17 2.7 Phenylbenzylhydrazine 4.7 28 4.7 Indole 87 70 20 58 15.3 86 60 20 58 16.8 Methyl cinnamate 67 Butyl cinnamate 60 57 o-Hydroxybenzaldehyde 58 p-tert-butyl benzaldehyde 88 57 N-p-isopropylphenyl-p-hyd roxybenzimine 8 34 6.1 4-Hydroxy-4’-isopropylazo benzene 2.5 6 1.9 1.1 1 1.1 5.2 28 4.2 1.4 5 1.2 2-Pyrrolyl-p-methyl-azobe nzene 2 2.5 2.0 3.5 15 2.8 5 16 4.2 N,N-diethyl-m-toluamide (DEET) 85 69 85 85 Others β-Farnesene 73 3,7-Dimethyloctyl acetate 108 12 45 9.7 Icaridin 65
Fig. 40. Three-dimensional structure of OBP1 (Wogulis et al., 2006) and a model structure of OBP4. The model of OBP3 is very similar to the structure of OBP1, while the folding of OBP4 appears significantly different, particularly with reference to the residues lining the binding sites. The alignment of the three proteins shows high similarity between OBP1 and OBP3. Asterisks indicate amino acids present in the binding pockets. The sequence and folding differences between OBP1 and OBP4 provide structural support to the different spectra of binding observed with the two proteins.
Fig. 41. Sequence alignment of An. gambiae OBP1, OBP3 and OBP4 with their orthologue proteins OS-E, OS-F and LUSH of Drosophila. Identical amino acids are highlighted. The similarity of OBP1 and OS-E is 62%; OBP3 and OS-F is 54%; OBP4 and LUSH is 41%.
Figure 40 shows an alignment of the amino acid sequences of OBPs 1, 3 and 4, together with the three-dimensional structure of OBP1 (Wogulis et al., 2006) and the model of OBP4. The sequences of OBP1 and OBP3 are very similar to each other, as are their Drosophila orthologues OS-E and OS-F, these in turn differ markedly from the mosquito OBP4 and its
Drosophila orthologue LUSH (Figure 41). The binding cavity of OBP1 is lined with several
branched amino acid residues, most of them conserved in OBP3 (also predicted to fold in a similar way) and which can interact with the terpene skeleton of ligands, such as citronellal and menthol. The two tyrosine residues, conserved in OBP1 and OBP3 and located near the amino and carboxyl termini, and at the entrance of the binding cavity, might establish hydrogen bonds with the terpenoids and related compounds. Two other OBPs, 12 and 19, seem to be tuned to larger molecules. In particular, OBP12 seems to prefer aromatic compounds and OBP19 binds terpenoids of larger size with farnesol being its best natural ligand.
When analysing the results of our binding experiments, we were surprised that none of the OBPs showed significant affinity to indole. Only with OBP4 and OBP47, we were able to calculate dissociation constants of around 16 μM, whilst with the other proteins indole could not displace the fluorescent probe more than 30% even at the highest concentration used. This is not consistent with the previously reported observation that silencing the gene for OBP1 suppresses response to indole in An. gambiae (Biessmann et al., 2010). One possible explaination was that the OBPs can associate in heterodimers, with binding properties different from those of their components. Association between OBPs have been observed in An. gambiae. In particular, co-immunoprecipitation has indicated that there is some sort of interaction between OBP1 and OBP4 (Andronopoulou et al., 2006), although there was no evidence, at the time of that report, that the two proteins were expressed in the same sensilla. Co-localization of OBPs, however, has been reported in D. melanogaster, where OS-E, OS-F and LUSH are found in the same sensilla (Hekmat-Scafe et al., 1997; Shanbhag et al., 2001). Since these three OBPs are the orthologues of An. gambiae OBP1, OBP3 and OBP4, respectively (Vogt, 2002b; Zhou et al., 2008), we decided to measure the affinity of 1-NPN to binary mixtures of the three proteins.
The results for the binding of 1-NPN to binary mixtures of two OBPs are shown in Figure 42. A mixture of equimolar amounts of OBP1 and OBP3 gave a binding curve not different from the sum of those obtained with the individual proteins, and a rather linear Scatchard plot indicating
Fig. 42. Binding curves of 1-NPN and relative Scatchard plot analysis measured with binary mixtures of OBPs 1, 3 and 4. A solution of two proteins in Tris-buffer, both at the concentration of 2 μM was titrated with 1mM solution of 1-NPN in methanol to final concentrations of 2-16 μM. The mixture of OBP1 and OBP3 shows a regular behaviour with a binding curve in agreement with that predicted and a linear Scatchard plot. Mixtures containing OBP4 have binding curves with a maximum around 4 μM of free probe and Scatchard plot that cannot be analysed. Such behaviour indicates cooperative interactions between OBP1 and OBP4 and between OBP3 and OBP4, as predicted in previous reports (Andronopoulou et al., 2006).
that there is no cooperativity effect. However, when one of the partner proteins was OBP4, the binding curve exhibited an unexpected behaviour, rising very sharply at the beginning, reaching a maximum at low concentrations of ligand and then decreasing significantly at higher concentrations. The relative Scatchard analyses are also unusual and do not allow any calculation of affinity constants. The decrease in bound 1-NPN, whilst its total concentration is increasing can only be explained if there is interaction between the two OBPs. From the experiments performed with individual proteins, we have measured values for binding of OBP4 to 1-NPN that are nearly double those of OBP1 or OBP3 (similar between the two). Thus the Scatchard plots seen in Figure 42 can be best explained by assuming that 1-NPN binds first to OBP4 (with the stronger affinity), then, as the concentration of the free probe increases, the ligand is transferred from OBP4 to the other partner protein. The total amount of bound ligand, therefore, does not change, or could even increase, but the observed fluorescence decreases. This mechanism requires an active interaction between the two OBPs.
Although the binding of a ligand, such as indole, cannot be detected easily by measuring the fluorescence of 1-NPN, it seems likely that indole is detected not by OBP1 alone, but rather by the heterodimer OBP1/OBP4. This would require that, like OS-E and LUSH of Drosophila, the
Fig. 43. Co-expression of OBP1 and OBP4 in the antenna of female An. gambiae. Two colour WM-FISH was performed on antennae using DIG-labeled OBP1 and biotin-labeled OBP4 antisense RNAs. Hybridization signals were visualized using detection systems indicating OBP1-positive cells by red fluorescense (C, G) and OBP4-labeled cells by green fluorescence (B, F). In most cases the OBP1- and the OBP4-probes label the same cells (A, D, E and H) indicating a co-expression of the two proteins. OBP1- and OBP4-expressing cells are shown on segment 8 (A-D) and segment 13 (E-H) of two different female antennae. All pictures represent single optical planes taken by a confocal laser scanning microscope. In A and E the red and green fluorescence channels have been overlaid with the transmitted light channel. The boxed areas in A and E, respectively, are shown at higher magnifications below the corresponding image with the green (B, F) and the red (C, G) fluorescence channels presented separately or as an overlay (D, H). Scale bars: 20µm.
An. gambiae OBP1 and OBP4 should be present in the same sensilla. Another group,
collaborating with our laboratory, Jurgen Krieger, University of Hohenheim in Germany, tested the co-localisation of OBP1 and OBP4 in the same sensillum, using two-colour WM-FISH.
Female antennae were simultaneously probed with specific DIG-labeled OBP1- and biotin-labeled OBP4-antisense RNA probes. Then using detection systems leading to red (DIG) and green (Biotin) fluorescence allowed visualization of cells, with both transcripts for the two OBPs by means of confocal laser scanning microscopy (Figure 43A and E). Both red-labelled cells expressing OBP1 (Figure 43C and G) and green-labelled cells positive for OBP4 (Figure 43B and F) were detected and in the overlay of the red and green fluorescence in distinct optical