Table of Contents
1 – INTRODUCTION
1.1. Artificial cultivation of medicinal plants 2
1.2. In vitro tissue culture 4
1.3. Hydroponic culture 6
1.4. Basil (Ocimum spp) 9
1.5. Rosmarinic acid 12
1.6. Aim of the work 14
1.7. Literature 15
2 – ROSMARINIC ACID CONTENT IN BASIL PLANTS GROWN IN VITRO AND IN HYDROPONICS
2.1. Introduction 21
2.2. Materials and Methods 23
2.3. Results 27
2.4. Discussion 37
2.5. Literature 42
3 – INFLUENCE OF NITROGEN NUTRITION ON GROWTH AND
ACCUMULATION OF ROSMARINIC ACID IN SWEET BASIL (OCIMUM BASILICUM L.) GROWN IN HYDROPONIC CULTURE
3.1. Introduction 47
3.2. Materials and Methods 49
3.3. Results 52
3.4. Discussion 57
3.5. Conclusions 59
3.6. Literature 60
4 – INFLUENCE OF HYPOXIA AND NACL SALINITY ON SWEET BASIL
(OCIMUM BASILICUM L.) GROWN HYDROPONICALLY FOR THE PRODUCTION OF ROSMARINIC ACID
4.1. Introduction 64
4.2. Materials and Methods 66
4.3. Results and Discussion 68
4.4. Conclusions 74
4.5. Literature 75
5 - PHOTOMIXOTROPHIC GROWTH AND ROSMARINIC ACID PRODUCTION IN BASIL (OCIMUM BASILICUM L.) MICROPROPAGATED PLANTLETS
5.1. Introduction 79
5.2. Materials and Methods 81
5.3. Results 86
5.4. Discussion 94
5.5. Literature 98
CHAPTER 1 – INTRODUCTION
1.1 ARTIFICIAL CULTIVATION OF MEDICINAL PLANTS
The use of plants as a source of products with therapeutic properties is as ancient as human civilisation but, during the last years, the interest towards the use of herbal remedies has noticeably increased in developed countries, especially in North America and Europe (Briskin, 2000; Rates, 2001; Canter et al., 2005), where approximately 25% of the molecules used by pharmaceutical industry are obtained from either wild or cultivated plants (Rates, 2001). According to WHO, in developing countries above 80% of the total population rely almost exclusively on plant-derived medicines for their healthcare (Gurib-Fakim, 2006). Moreover, several bioactive compounds (e.g. atropine from Atropa belladonna; morphine and codeine from Papaver somniferum) cannot yet be synthesised economically and are generally extracted from plant tissues (Rates, 2001).
Medicinal properties in plants are mainly due to the presence of secondary metabolites, which play a crucial role in the plant protection against both biotic and abiotic stresses, such as pests and pathogens, high temperature, drought, nutrient deficiency etc. (Briskin 2000; Orcutt and Nilsen, 2000). For instance, salinity stress was found to decrease the production of essential oils in Matricaria chamomilla (Razmjoo et al., 2008) and Melissa officialis (Ozturk et al., 2004), while low temperatures increased the morphine accumulation in Papaver somniferum (McChesney, 1999).
Approximately two thirds of 50 thousand medicinal plant species in use are collected from the wild and, in Europe, only 10% of medicinal species used by industry are cultivated (Canter et al., 2005). Two main approaches are generally used for medicinal plant cultivation. The first consists of intensive cropping system in order to produce high quantities of biomass at low-cost. These cultivations are generally controlled directly or indirectly by pharmaceutical companies. Another approach involves organic cultivation and is principally directed to herbal market (Verlet,
1994). The market of organically-certified natural remedies has increased in developed countries during the last years (Craker, 2007).
The adoption of artificial cultivation systems may represent an interesting alternative for the production of medicinal plants, as they allow a strict control of the growing conditions, thus ensuring high and constant yield of the metabolites of interest (Canter et al, 2005; Mensuali-Sodi et al., 2006). In this sense, both in vitro and in
vivo (greenhouse hydroponic culture) systems can be adopted for artificial cultivation
of medicinal plants. The main advantages of both systems include: 1) secondary products can be generated on a year-round basis; 2) production is reliable, predictable and independent of climate and soil conditions; 3) minimal or no occurrence of pest infestation, pathogen diseases and contamination with polluting agents (e.g. heavy methals, pesticides etc.) 4) the extraction of bioactive compounds from plant tissue is easier, in particular because the material is much cleaner compared to that sourced by soil cultivation in the field (Mulabagal and Tsay, 2004; Pardossi et al., 2006).
In order to produce large quantities of metabolites of interest, high amounts of plant biomass are generally required. Once biomass accumulation has been optimized, several strategies can be applied in artificial cultivation systems in order to stimulate the production of active substances. For instance, the use of elicitors (e.g. methyl jasmonate, salicylic acid and yeast extract) are known to enhance the accumulation of bioactive compounds in tissue cultures (Zhao et al., 2005). Similarly, in hydroponic cultivation, the biosynthesis of secondary metabolites can be stimulated by the alteration of the nutrient solution composition (e.g. Zheng et al., 2006; Montanari et al., 2008).
Nevertheless, in vitro tissue culture represents a complex technology that requires expert operators and is not cost-effective in most cases (Ahloowalia and Prakash, 2002). Several efforts have been done to increase the production efficiency and reduction the investment and running costs of in vitro systems, for instance by partial mechanization of some cultural steps or the use of bioreactors (Ahloowalia and
Savangikar, 2002, Levin and Tanny, 2002). It is estimated that in vitro production of any compounds with a market price lower than US$ 1000 per kilogram is not economically sustainable (Rao and Ravishankar, 2002). For instance, plant cell culture has been recognized as a potential alternative of producing taxol, a highly effective anticancer drug produced by Taxus spp. and some microorganisms (Zhong, 2002). The compound is accumulated in the bark of yew trees at very low concentrations (about 0.01DW) and plant growth is very slow (Zhong, 2002). Therefore, Taxus cell culture has been developed in order to produce taxol in higher amounts (more than 100mg/l) and in a relative short period (Zhong, 2002).
Compared to in vitro growing systems, greenhouse hydroponic technique offers the advantages of higher rate of biomass production per unit area and lower investment cost expensive growing structures, although the growing conditions are not so strictly controlled as in the in vitro system.
1.2. IN VITRO TISSUE CULTURE
Plant tissue culture is based on the concept of totipotency and consists in the aseptic culture of cells, tissues, organs and their components under controlled physical and chemical conditions (Thorpe, 2007).
Different methodologies can be employed for in vitro culture of medicinal plants, based on: cell suspension; callus; hairy roots; shoots. During the last few years, numerous important medicinal species have been micro-propagated using different types of explant (Karuppusamy, 2009; Rao and Ravishankaral, 2002). Micropropagation protocols is crucial to propagate genotypes that have been selected for high productivity of active principles (Lucchesini and Mensuali-Sodi, 2010). Five critical stages to obtain a satisfactory micropropagation process were recognized (Tab. 1.1) (Debergh and Maene, 1981). Successful organogenesis in many plant species can be achieved by correct selection of growin medium and explant and the control of micro-environment (Brown and Thorpe, 1995).
Micropropagation protocols could be adopted also to produce of plant material for the extraction of active principles. During the cultural cycle, the induction of bioactive compounds may be stimulated by modulation of several parameters, for instance: the pH and/or content of nutrients, sugar and plant growth regulators in the culture medium (Karuppusamy, 2009; Rao and Ravishankar, 2002); climatic conditions within the culture vessels (Rao and Ravishankar, 2002).
Tab. 1.1. The stages of the plant micropropagation (Debergh and Maene, 1981)
Stage 0, Preparation of the plant stock in health conditions. Stage 1, Beginning of the aseptic cultivation.
Stage 2 Induction, development and multiplication. Stage 3a Shoot elongation.
Stage 3b Rooting.
Stage 4 Acclimatization.
In vitro-culture plants grow normally under heterotrophic conditions and exhibit low
photosynthetic rate due to low light intensity and CO2 concentrations as well as the
presence of sugars in the medium (Kozai, 1991). Moreover, the reduction of gas exchanges between the cultivation vessels and external atmosphere may lead to an accumulation of ethylene (Mensuali et al., 1992). Both heterotrophic growth (Ikemeyer and Barz, 1989) and the accumulation of ethylene (Lee-Parsons, 2007) were found to increase the production of different secondary metabolites. Therefore, understanding the effects of gas composition inside the vessel, as affected by the gas exchange characteristics of the vessel in use, may open new possibilities to the control of secondary metabolites production in in vitro cultivation of medicinal plants.
Micropropagation of Sweet basil plantlets during the multiplication phase.
1.3. HYDROPONIC CULTURE
Hydroponic cultivation is a method of growing plants in medium other than agricultural soil or in pure nutrient solutions (Pardossi et al., 2005). Several different hydroponic systems are applicable for plant cultivation; their principal features are sumarized in Tab. 1.2. Hydroponics offers several advantages over soil culture such as: more efficient use of water and fertilizers; higher yield per unit ground area; all-year round production; higher quality and ease of processing of harvested material as a result of minimal contamination from pollutants, pests and pathogens (Pardossi et al., 2006; Raviv and Lieth, 2007).
Among the hydroponic system listed in Tab. 1.2, the floating raft system represents a low-cost hydroponic technology suitable for growing leafy vegetables under
greenhouse conditions. Plants are cultivated in 'rafts' of lightweight plastic material, like expanded polystyrene, floating on a bed of stagnant nutrient solution. Floating systems is currently used for growing leafy vegetables and herbs, such as rocket salad and basil, in particular for the fresh-cuts (minimally-processed products) market for minimally-processed vegetables (Rodríguez-Hidalgo et al. 2010).
Tab. 1.2. Main characteristics of hydroponic growing systems.
NFT (nutrient film technique)
Plants are grown directly on an impermeable surface to which a thin film of wastewater is continuously or periodically applied.
Floating raft system
Plants are cultivated in 'rafts' of lightweight plastic material, floating on a bed of large volume of stagnant nutrient solution.
Aereoponic system
Plants are suspended in air and the roots are continuously or periodically sprayed with a nutrient solution.
Flood and drain system
The nutrient solution rises periodically to immerse plant roots grown in substrate and then recedes exposing the roots to moist atmosphere. Bag (substrate) culture Plants are grown in an inert porous medium.
Deep water culture
Hydroponic method of plant production by means of suspending the plant roots in a solution of nutrient-rich, oxygenated water.
Floating system can also be used for the cultivation of medicinal plants, such as Echinacea angustifolia DC, which are cultivated mainly for the roots and are difficult to grow in the open field (Montanari et al., 2008; Zheng et al., 2006; Maggini et al., 2011).
One of the main advantages of the floating system is its cost-effectiveness, as the investment costs are quite low (less than 5-10 $/m2) and then it could be employed for low-technology protected crops with short cycle (Incrocci et al.) http://www.fao.org/hortivar/scis/scis.htm?TRX=Redirect&TO=DOC&ID=1
Greenhouse hydroponic cultivation of Echinacea angustifolia. The seedlings are grown in polystyrene rafts floating in fairly stagnant nutrient solution and harvested within 12-16 weeks from sowing for extraction of caffeic acid derivatives from root tissues.
In greenhouse hydroponic culture, the accumulation of secondary metabolites of pharmaceutical interest can be stimulated by modifying the composition of the nutrient solutions (e.g. Maia et al., 2001; Montanari et al. 2008; Zheng et al., 2006) or light conditions, using for instance UV light (Pedneault et al., 2002). Some authors reported that treating hydroponically-grown plants with growth regulators (Wikremesinhe and Arteca, 1996) or bio-stimulants (Parađiković et al., 2011) resulted in the production of secondary metabolites.
1.4. BASIL (OCIMUM SPP.)
Basil is the common name used for identify species that belong to the genus
Ocimum, in the Lamiaceae. In Greek the ‘Okimum’ means smell due to the aromatic
properties of the plants in this genus. Basil species are native to tropical and subtropical areas of India, Africa and southern Asia and is now naturalized almost all over the world, especially in the regions characterized by temperate-warm climate (Putievsky and Galambosi, 1999).
The genus Ocimum enclose a huge number of economic and medicinal species and varieties, characterized by a large variability in the morphological traits, flavours, scents, and uses (Putievsky and Galambosi, 1999). At least 65 species belong to genus Ocimum according to Paton et al. (1999), while Sahoo et al. (1997) listed more than 150 different genotypes in this taxonomic group. The controversial botanical identity of basil is due to the large variability of the genus resulting from cross-pollination and interspecific hybridization (Hardley et al., 1992). Furthermore, the aromatic and morphological characters are greatly influenced by environmental conditions and agronomic techniques (Marotti et al., 1996). Finally, the taxonomy of the genus is complicated also because within the species is possible to count a huge number of varieties, cultivars and chemotypes that do not differ significantly in the morphology (Simon et al., 1990).
The genus Ocimum includes annuals plants, non-woody perennials and even shrubs. Typical characteristics of basil plants are opposite leaves, branching square stems, brown or black seeds and flower spikes. The root system consists of simple tap root in annual plants or in perrennating rhizome. Among different species, there is a big variability in the colour, size, shape and texture of leaves and flowers. Leaves size varied from 1 to 15 cm, their textures range from smooth and shiny to curled and hairy, and the colour can be green or tending to blue/purple. The colour of bilabiate flowers, included in inflorescences, range from white to lavender/purple. The habit of basils is very variable, and the plants can grow to from 30 cm to 3 m in height, depending on the species (Paton et al, 1999; Wetzel et al, 2002).
Plants of the genus Ocimum are cultivated for many purposes: the main application regards the use of fresh, dry or frozen leaves for culinary preparations. Moreover, due to the high content of natural compounds, such as essential oils and antioxidant phenolics, basil is increasingly used for cosmetical, herbal, medicinal preparations as well as for plant protection products (e.g. insecticide) (Simon et al. 1999; Makri and Kintzios, 2007). Different basil species and cultivars are also used as ornamental plants, for instance, due to the attractive purple colour of the leaves (‘Dark Opal’ and ‘Rubin Purple Leaf’) or the dwarf habitus (‘Bush’, ‘Green globe’ or ‘Spicy globe’; they are used as border plants) (Makri and Kintzios, 2007; Putievsky and Galambosi, 1999; Simon et al., 1999).
Sweet basil (Ocimum basilicum L.) is a tender herbaceous annual plant that enclose a huge number of varieties and cultivars that differs for morphology and chemotypes (Simon et al, 1999). At least seven types of different cultivars were classified by Darrah (1980, 1984), ranging from the typical green leaf genotypes, such as ‘Genovese’, ‘Lettuce leaf’ and ‘Gigante’ to purple colored basil, as ‘Dark Opal’ and ‘Red Rubin’ or lemon-flavored cultivar, like ‘Citriodorum’ one.
Sweet basil represents one of the most important species as it is largely cultivated worldwide (also hydroponically – Miceli et al., 2003) mainly for food preparations (Makri and Kintzios, 2007). Fresh green leaves of some cultivars of this species (e.g. ‘Genovese Gigante’) are commonly used for the preparation of the well-known Italian ‘pesto’ sauce, now largely diffused all over the world (Miele et al., 2001).
Sweet basil is considered an important source of essential oils, which are extensively used in food and pharmaceutical industry, perfumery and herbal medicine (Makri and Kintzios, 2007; Hussain et al., 2008). Although this class of compounds is largely variable for composition and concentration in dependence of sweet basil cultivars and growing conditions, the prevalent components are linalool, methyl chavicol, eugenol, methyl-eugenol, chavicol, estragole, methyl-cinnamante, geraniol, geranial and neral (Lee et al., 2005; Makri and Kintzios, 2007).
Greenhouse hydroponic cultivation of sweet basil for fresh-cuts market in Italy. The seedlings are grown in polystyrene rafts floating in fairly stagnant nutrient solution and harvested within 4-6 weeks from sowing.
Sweet basil is also an interesting source of phenolic compounds, which are one of the most numerous and ubiquitously distributed group of plant secondary metabolites, with more than 8000 structures currently known (Soobrattee et al., 2005). Phenolics have a large number of therapeutic applications, for instance for the prevention and treatment of cardiovascular, neurodegenerative, diabetes, cancer and infiammatory diseases (Soobrattee et al., 2005, Hinneburg et al. 2006). Medicinal actions of phenolics is mostly ascribed to their antioxidant capacity, chelation of redox active metal ions, modulation of gene expression and interaction with the cell signalling pathways (Soobrattee et al., 2005, Hinneburg et al. 2006). The free radical scavenging and antioxidant activities of phenolics are mainly dependent upon the number and configuration of hydroxyl groups about the nuclear structure (Soobrattee et al., 2005, Hinneburg et al. 2006).
Among phenolics, caffeic acid derivatives (CADs), such as caffeic, chicoric, cinnamic, p-coumaric, ferulic, rosmarinic and sinapic acids, are largely contained in sweet basil tissues (Jayasinghe et al., 2003; Lee and Scagel 2009; Makri and Kintzios, 2007). In particular, rosmarinic acid (RA), is one of the most abundant phenolic compound accumulated in this species (Li et al., 2007; Juliani et al., 2008; Jayasinghe et al., 2003).
1.5. ROSMARINIC ACID
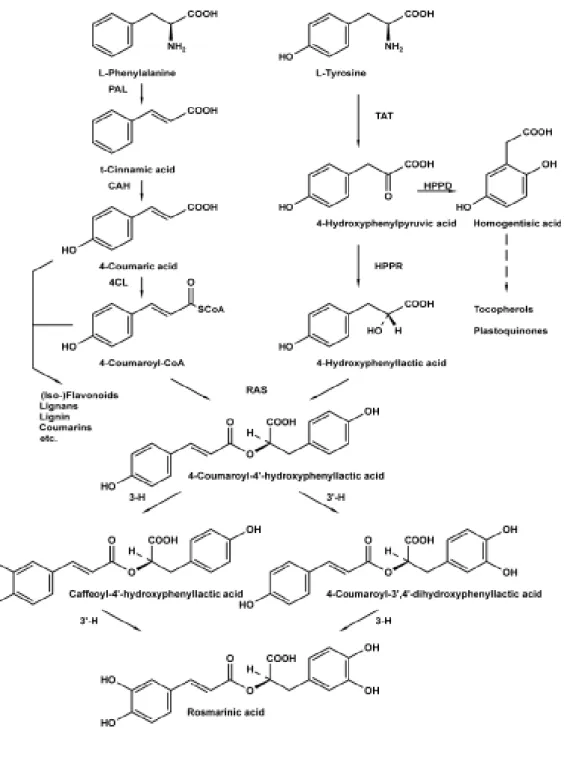
Rosmarinic acid is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid. The pure compound was isolated for the first time by Scarpati and Oriente (1958) in
Rosmarinus officinalis, and the complete biosynthetic pathway was fully elucidated
only recently (Petersen and Simmonds, 2003). The two precursor involved in RA biosynthesis are tyrosine and phenylalanine (Fig. 1.1).
Rosmarinic acid is widely distributed in the plant kingdom, but represents a characteristic compound of several medicinal plants in the Boraginaceae and
Lamiaceae families (e.g. Salvia officinalis, Coleus blumei, Mentha x piperita, Thymus vulgaris, Melissa officinalis, Symphytum officinale), even if not all members
of the Lamiaceae synthetize this metabolite (Petersen and Simmonds, 2003; Petersen et al., 2009). Likewise the vast majority of plant secondary metabolites, RA accumulation is strongly affected by many factors, including growing and environmental conditions, development stage, tissue type, ecc. (del Baño et al., 2003; Juliani et al., 2008; Shiga et al., 2009).
A multitude of biological activities have been described for RA: adstringent, anti-oxidative, anti-inflammatory, anti-mutagen, anti-bacterial and anti-viral activities (Petersen and Simmonds, 2003; Juliani et al., 2008). Rosmarinic acid is a strong scavenging agent due to the presence of four hydroxyl groups located in ortho-position of the benzene rings that act as hydrogen or electron donor (Fig. 1.1).
PAL= phenylalanine ammonia-lyase; CAH= cinnamic acid 4-hydroxylase, 4CL= hydroxycinnamate: coenzyme A ligase; TAT= tyrosine aminotransferase; HPPR= hydroxyphenylpyruvate reductase; HPPD= hydroxyphenylpyruvate dioxygenase; RAS= hydroxycinnamoyl-CoA:hydroxyphenyllactate hydroxycinnamoyl transferase; 3-H,30-H= hydroxycinnamoyl-hydroxyphenyllactate 3- and 30 –hydroxylases.
1.6. AIM OF THE WORK
During the last decades, several studies have been conducted to develop an artificial production system of RA from sweet basil using in vitro culture (e.g. Kintzios et al., 2003; 2004; Rady and Nazif, 2005; Park et al. 2008). Other studies were recently conducted to investigate the effects of NaCl salinity (Tarchoune et al., 2009) or nitrogen concentration (Nguyen and Niemeyer, 2008) on the accumulation of RA and other CADs in sweet basil grown in hydroponic system under greenhouse conditions. The general work of this thesis was to develop an artificial cultivation systems of sweet basil for the extraction of RA. Both in vitro and in vivo (greenhouse hydroponics) systems were evaluated. A number of experiments were conducted from 2008 to 2011 with sweet basil seedlings grown in floating system or in vitro.
A common substrate of RA and anthocyanins pathways is 4-coumaroyl-CoA (Petersen et al., 2010) and a competition between their individual biosynthesis was hypothesized by Yamazaki et al., (2003) in Perilla frutescens. On the other hand, in cell suspensions of Dark Opal basil recently Strazzer et al. (2011) found a positive correlation between the content of RA and anthocyanins. Therefore, in our study we compared sweet basil cultivars with different leaf colour: green-leaved “Genovese” and “Superbo”, and purple-leaved “Dark Opal”. In preliminary experiments, we found that the content of anthocyanins in ‘Dark Opal’ leaves was more than 10-fold higher than the content of green-leaved cultivars.
The specific goals of each experiment were to investigate:
the feasibility of both in vitro or in vivo (floating system) techniques for the biomass and RA production of three different sweet basil cultivars. In hydroponic, the effect of the plant developmental stage on RA accumulation was evaluated, whereas in vitro, the RA content was determined in plantlets of different micropropagation phases and grown at different hormonal composition of the culture medium (Chapter 2).
the effects of N concentration and form (NO3- and NH4+) on biomass
production and RA accumulation in different organs of sweet basil (cv. Genovese) grown in floating system (Chapter 3).
the influence of hypoxia and NaCl salinity on biomass production and RA accumulation in root and leaf tissues of sweet basil (cv. Genovese) seedlings grown in floating system (Chapter 4).
the effects of the cultivation vessel on the growth and RA production in green-leaved (cv. Genovese) and purple-leaved (cv. Dark Opal) micropropagated plantlets of sweet basil (Chapter 5).1.7. LITERATURE
Ahloowalia, B.S., Prakash, J. (2002). Physical components of tissue culture technology. In: Low cost options for tissue culture technology in developing countries. Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and held in Vienna, 26–30 August 2002. Pp.17-29.
Ahloowalia, B.S., Savangikar, V.A. (2002). Low cost options for energy and labour. In: Low cost options for tissue culture technology in developing countries. Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and held in Vienna, 26–30 August 2002. Pp.41-47.
Briskin, D.P. (2000). Medicinal Plants and Phytomedicines. Linking Plant Biochemistry and Physiology to Human Health. Plant Physiol. 124: 507–514. Brown, D.C.W., Thorpe, T.A. (1995). Crop improvement through tissue culture.
World J. Microbiol. and Biotechnol. 4: 409-415.
Canter, P.H., Thomas, H., Ernst, E. (2005). Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol. 23: 180-185.
Craker, L.E. (2007). Medicinal and Aromatic Plants—Future Opportunities. Reprinted from: Issues in new crops and new uses. Janick J. and Whipkey A. (eds.). ASHS Press, Alexandria, VA.
Debergh, P.C., Maene, L.J. (1981). A scheme for commercial propagation of ornamental plants by tissue culture. Scientia Hort. 14: 335-345.
A., Quirin, K.W., Gerard, D. (2003). Phenolicditerpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 51: 4247- 4253.
Gurib-Fakim, A. (2006). Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 27: 1–93.
Harley, M.M., A. Paton, R.M. Harley, and P.G. Cade. (1992). Pollen morphological studies in the tribe Ocimeae (Nepetoideae: Labiatae): Ocimum L. Grana 31:161–176.
Hinneburg, I., Dorman, H.J.D., Hiltunen, R. (2006). Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 97: 122–129.
Hussain, A.I., Anwar, F., Hussain Sherazi, S.T., Przybylski, R. (2008). Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum
basilicum) essential oils depends on seasonal variations. Food Chem. 108:
986-995.
Ikemeyer, D., Barz, W. (1989). Comparison of secondary product accumulation in photoautotrophic, photomixitrophic and heterotrophic Nicotiana tabacum cell suspension cultures. Plant Cell Rep. 8: 479-482.
Incrocci, L., Pardossi, A., Tognoni, F. Growing vegetables in floating sysyem. http://www.fao.org/hortivar/scis/scis.htm?TRX=Redirect&TO=DOC&ID=1. Jayasinghe, C., Gotoh, N., Aoki, T., Wada, S. (2003). Phenolics Composition and
Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 51: 4442−4449.
Juliani, H.R., Koroch, A.R., Simon, J.E. (2008). Basil: a new source of rosmarinic acid, in: Ho, C.T., Simon, J.E., Shahidi, F., Shao, Y. (Eds). Dietary Supplements, American Chemical Society Symposium Series 987. A.C.S. Washington D.C., USA, pp. 129-143.
Karuppusamy, S. (2009). A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants. Res. 3: 1222-1239.
Kintzios, S., Kollias, H., Straitouris, E., Makri O. (2004). Scale-up micropropagation of sweet basil (Ocimum basilicum L.) in an airlift bioreactor and accumulation of rosmarinic acid. Biotechnol. Lett. 26: 521-523.
Kintzios, S., Makri, O., Panagiotopoulos, E., Scapeti, M. (2003). In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol. Lett. 25: 405-408.
Kozai, T. (1991). Autotrophic micropropagation. In: Bajaj YPS, ed. Biotechnology in Agriculture and Forestry: High Tech and Micropropagation I. Berlin Heidelberg, Germany: Springer‑Verlag. Pp. 313‑343.
leaves. Food Chem. 115: 650–656.
Lee, S.J., Umano, K., Shibamoto, T., Lee, K.G. (2005). Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 91: 131–137.
Lee-Parsons, C.W.T. (2007). Gas composition strategies for the successful scale-up of Catharanthus roseus cell cultures for the production of ajmalicine. Phytochem. Rev. 6: 419-433.
Levin, R., Tanny, G. (2002). Bioreactors as a low cost option for tissue culture. In: Low cost options for tissue culture technology in developing countries. Proceedings of a Technical Meeting organized by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture and held in Vienna, 26–30 August 2002. Pp. 47-55.
Li, Z., Wang, X., Chen, F., Kim, H.J. (2007). Chemical Changes and Overexpressed Genes in Sweet Basil (Ocimum basilicum L.) upon Methyl Jasmonate Treatment. J. Agric. Food Chem. 55: 706−713.
Lucchesini, M, Mensuali-Sodi, A. (2010). Plant tissue culture - an opportunity for the production of nutraceuticals. In: Bio-Farms for Nutraceuticals. Functional Food and Safety Control by Biosensor (Giardi, M.T., Rea, G., Berra, B. Eds.). Advances in Experimental Medicine and Biology 698. Pp.185-203.
Maggini R, Tozzini L, Pacifici S, Raffaelli A, Pardossi A (2011) Growth and accumulation of caffeic acid derivatives in Echinacea angustifolia DC. var angustifolia grown in hydroponic culture. Ind. Crop Prod. 35: 269-273
Maia, N.B., Bovi, O.A., Marques, M.O M., Granja, N,. Do, P., Carmello, Q.A.C. (2001). Essential oil production and quality of Mentha arvensis L. grown in nutrient solutions. Acta Hortic. 548: 181-187.
Makri, O., Kintzios, K. (2007). Ocimum sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs Spices Med. Plants 13: 123-150.
Marotti, M. Piccaglia R., Giovanelli E. (1996). Differences in Essential Oil Composition of Basil (Ocimum basilicum L.) Italian Cultivars Related to Morphological Characteristics. J. Agric. Food Chem. 44: 3926−3929.
McChesney, J.D. (1999) Quality of botanical preparations: environmental issues and methodology for detecting environmental contaminants. In Botanical Medicine: Efficacy, quality assurance and regulation (Eskinazi, D., ed.), pp. 127–131, Mary Ann Liebert, Inc publishers.
Mensuali-Sodi, A., Lucchesini, M., Pacifici, S., Pipino, L., Tognoni, F. (2006). Tecniche alternative per la coltivazione di piante medicinali. Erboristeria domani 306: 67-77.
Mensuali-Sodi, A., Panizza, M., Tognoni, F. (1992). Quantification of ethylene losses in different container-seal systems and comparison of biotic and abiotic
contributions to ethylene accumulation in cultured tissues. Physiol. Plant. 84, 472–476.
Miceli, A., Moncada A., Vetrano F., D’Anna F. (2003). First results on yield and quality response of basil (Ocimum basilicum L.) grown in a floating system, Acta Hort. 609: 377-381.
Miele, M., Dondero, R., Ciarallo, G., Mazzei, M. (2001). Methyleugenol in Ocimum
basilicum L. Cv Genovese Gigante. J. Agric. Food Chem. 49: 517-521.
Montanari, M., Degl’Innocenti, E., Maggini, R., Pacifici, S., Pardossi, A., Guidi, L. (2008). Effect of nitrate fertilization and saline stress on the contents of active constituents of Echinacea angustifolia DC. Food Chem. 107: 1461–1466.
Mulabagal, V., Tsay, H.S. (2004). Plant Cell Cultures - An Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. Int. J. Appl. Sci. Eng. 2: 29-48.
Nguyen P., Niemeyer E. (2008). Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.), J. Agric. Food Chem. 56, 8685–8691.
Orcutt, D.M., Nilsen, E.T. (2000). The physiology of plants under stress soil and biotic factors, John Wiley and Sons Inc., New York.
Ozturk, A, Unlukara A, Ipek A, Gurbuz B (2004). Effects of salt stress and water deficit on plant growth and essential oil content of Lemon Balm (Melissa
officialis L.). Pak. J. Bot. 36: 787-792.
Parađiković, N., Vinković, T., Vinković Vrček, I., Žuntar, I., Bojić, M. and Medić-Šarić, M. (2011), Effect of natural biostimulants on yield and nutritional quality: an example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 91: 2146–2152.
Pardossi, A., Malorgio, F., Incrocci, L., Tognoni, F. (2006). Hydroponic technologies for greenhouse crops, In: Dris R., (Ed.), Crops: Quality, Growth and Biotechnology, WFL Publisher, Helsinki, 23. Pp. 360-378.
Pardossi, A., Malorgio, F., Incrocci, L., Tognoni, F. (2005). Hydroponic technologies for greenhouse crops. In: Ramdane, D. (Ed.), Crops: Quality, Growth and Biotechnology. WFL Publisher, Helsinki, pp. 360–378.
Park, S.U., Uddin, M.R., Xu, H., Kim, Y.K., Lee, S.Y. (2008). Biotechnological applications for rosmarinic acid production in plant. Afr. J. Biotechnol. 7: 4959-4965.
Paton, A., Harley, R.M., Harley, M.M.. (1999). Ocimum-an overview of relationships and classification. In: Basil, the Genus Ocimum. Medicinal and Aromatic Plants-Industrial Profiles. Holm, Y. and Hiltunen, R. (eds.) Harwood Academic publishers, Amsterdam, The Netherlands. pp.1-8.
Pedneault, K., Léonhart, S., Gosselin, A., Papadopoulos, A.P., Dorais, M., Angers, P. (2002). Variations in concentration of active compounds in four
hydroponically-and field-grown medicinal plant species. Acta Hort. (ISHS) 580: 255-26.
Petersen, M., Hans, J., Matern, U. (2010). Biosynthesis of Phenylpropanoids and Related Compounds, In: Wink M. (Ed), Annual Plant Reviews: Biochemistry of Plant Secondary Metabolism, Vol. 40, 2nd Edition , Wiley-Blackwell, Oxford, UK., pp.182-230. doi: 10.1002/9781444320503.ch4.
Petersen, M., Abdullah, Y., Benner, J., Eberle, D., Gehlen, K., Hücherig, S., Janiak, V., Kim, K.H., Sander, M., Weitzel, C., Wolters, S. (2009). Evolution of rosmarinic acid biosynthesis. Phytochem. 70: 1663-1679.
Petersen, M., Simmonds, M.S.J. (2003). Molecules of interest: rosmarinic acid. Pytochem., 62: 121–125.
Putievsky, E., Galambosi, B. (1999). Production systems of sweet basil. In: Basil, the Genus Ocimum. Medicinal and Aromatic Plants-Industrial Profiles. Holm, Y. and Hiltunen, R. eds. Harwood Academic publishers, Amsterdam, The Netherlands. Pp. 37-64.
Rady, M.R., Nazif, N.M. (2005). Rosmarinic acid content and RAPD analysis of in vitro regenerated basil (Ocimum basilicum L.) plants. Fitoterapia. 76: 525-533. Rao, R.S., Ravishankar G.A. (2002). Plant cell cultures: Chemical factories of
secondary metabolites. Biotechnol. Adv. 20: 101–153.
Rates, S.M.K. (2001). Plants as source of drugs. Toxicon 39: 603–613.
Raviv, M., and Lieth, H. (2007). Soilless Culture: Theory and Practice. Elsevier Science Publishing Company December.
Razmjoo, K., Heydarizadeh, P., Sabzalian, M.R. (2008). Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria
chamomilla. Int. J. Agric. Biol. 10: 451-454.
Rodríguez-Hidalgo, S., Artés-Hernández, F., Gómez, P. A., Fernández, J. A., Artés, F. (2010). Quality of fresh-cut baby spinach grown under a floating trays system as affected by nitrogen fertilisation and innovative packaging treatments. J. Sci. Food Agric. 90: 1089–1097.
Sahoo, Y., Pattnaik, S.K., Chand, P.K. (1997). In vitro clonal propagation of an aromatic medicinal herb Ocimum basilicum L. (sweet basil) by axillary shoot proliferation. In Vitro Plant 33: 193-296.
Scarpati, M.L., Oriente, G. (1958). Isolamento e costituzione dell’acido rosmarinico (dal rosmarinus off.). Ric. Sci. 28: 2329–2333.
Shiga, T., Shoji, K., Shimada, H., Hashida, S., Goto, F., Yoshihara, T. (2009). Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol. 26: 255–259.
Simon, J.E., Morales, M.R., Phippen, W.B., Vieira, R.F., Hao, Z. (1999). Basil: a source of aroma compounds and a popular culinary and ornamental herb. In Perspectives on new crops and new uses; Janck, J., Simon, J. E. (eds).; ASHS Press: Alexandria, VA. pp 499–505.
Simon, J.E., Quinn J., Murray R.G. (1990). Basil: A source of essential oils. In: Janick J. and Simon J.E. (eds.), Advances in new crops. Timber Press, Portland, OR. Pp. 484-489.
Soobrattee, M.A., Neergheen, V.S., Luximon-Ramma, A., Aruoma, O.I., Bahorun, T. (2005). Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 579: 200–213.
Strazzer, P., Guzzo F., Levi M. (2011). Correlated accumulation of anthocyanins and rosmarinic acid in mechanically stressed red cell suspension of basil (Ocimum
basilicum). J. Plant Physiol. 168: 288-293.
Tarchoune, I., Incerti A., Lachaal M, Ouerghi Z, Izzo R., Navari F., (2009). Relations between antioxidant activity and salinity in basil (Ocimum basilicum Mill.). Agrochimica 53: 56-65.
Thorpe, T.A. (2007). History of plant tissue culture. Mol. Biotechnol. 37: 169–180. Verlet, N. (1994). An overview of the medicinal and aromatic plant industry.
Proceedings International Meeting on “Cultivation and improvement of medicinal and aromatic plants”. Trento 2-3 June, 1994, Italy.
Wetzel, S.B., Krüger, H., Hammer, K., Bachmann, K. (2002). Investigations on Morphological, Biochemical and Molecular Variability of Ocimum L. Species. J. Herbs Spices Med. Plants. 9: 183-187.
Wickremesinhe, W.R.M., Arteca, R.N. (1996). Effects of plant growth regulators applied to the roots of hydroponically grown Taxus x media plants on the production of taxol and related taxanes. Plant Sci. 121: 29-38.
World Health Organization (WHO), (2008)."Traditional medicine" Fact sheet number: 134 (December)".http://www.who.int/mediacentre/factsheets/fs134/en/ Yamazaki, M., Nakajima J., Yamanashi M., Sugiyama M., Makita Y., Springob K.,
Awazuhara M., Saito K. (2003). Metabolomics and differential gene expression in anthocyanins chemo-varietal forms of Perilla frutescens. Phytochem. 62: 987–95.
Zhao, J., Davis, T.L.C., Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 23: 283–333.
Zheng, Y., Dixon, M., Saxena, P.K. (2006). Greenhouse production of Echinacea
purpurea (L.) and E. angustifolia using different growing media, NO3-/NH4+
ratios and watering regimes. Can. J. Plant Sci. 86: 809–815.
Zhong, J.J. (2002). Plant Cell Culture for Production of Paclitaxel and Other Taxanes. J. Biosci. Bioeng. 94: 591-599.