4. Genus Muscari Miller
4.1 Generalities
Description
The genus Muscari is called commonly grape-hyacinth (from Greek "moschos", musk, alluding to the scent of the flowers). It contains perennial, scapose herbs with ovoid bulbs, with or without bulblets; cataphylls from greysh to brown (Straley & Utech, 2003). Leaves (1–) 2–7, basal; blade linear, sometimes sulcate, glabrous, rather fleshy. Scape terete. Inflorescences terminally racemose, many-flowered, dense, bracteate, usually elongating in fruit; distal flowers smaller, sterile, differing in color, forming a tuft; bracts minute. Flowers fragrant; perianth tubular to urceolate, usually constricted basally; tepals 6, connate most of their length, distal portions distinct, reflexed, short, toothlike; stamens 6, epitepalous, in 2 rows, included; anthers dark blue, dorsifixed, globose; ovary superior, green, 3-locular, inner tepals with nectaries; style 1; stigma lobed. Fruits capsular, obtusely 3-angled, papery, dehiscence loculicidal. Seeds 6, black, globose, wrinkled to reticulate. x = 9.
Distribution
This genus has a widespread distribution. It is present in the whole Mediterranean basin as far as Caucasus; temperate Europe, north Africa, southwest Asia (Garbari 1973). It is expected introduced elsewhere and likewise contain a number of ornamentals (Dahlgren & al., 1985).
Systematic frame
The genus Muscari has been segregated into a separate family, Hyacinthaceae, in respect to the Liliaceae sensu lato, according to Dahlgren (Dahlgren & al., 1985). The family Hyacinthaceae is included in the order Asparagales, superorder
Liliiflorae.
The taxonomic history of this taxon is very articulate. Kunth (1843), thinking to attribuite to the genus Muscari Mill. only the species M. moschatum (L.)
species B. vulgaris, B. odorus, B. commutatus and B. parviflorus. Other species have been considered by Kunt as belonging to the genus Bellevalia Lapeyr: B. comosa and
B. maritima (Garbari, 1968). Parlatore (1845) agreed confirming the institution of the
genus Botryanthus by Kunth (1843) and the extension of the genus Muscari, but he wasn’t in agreement to insert in the genus Bellevalia the species M. comosum and B.
maritima, even admitting the separation from Muscari Mill. So it was necessary the
institution of the new genus Leopoldia (Parlatore, 1845), to honour the Grand Duke of Tuscany Leopoldo II. When Parlatore (1845) described Leopoldia, he based the genus on three different species belonging to three different genera; Hyacinthus
comosus L. (which was selected as lectotype for the genus Leopoldia by Garbari and
Greuter, 1970), Muscari maritimum Desf., and Bellevalia pinardii Boiss (Bentzer, 1973).
In more recent times other nomenclatural and taxonomic vicissitudes followed. Garbari (1968) accepted the validity of the genus of Leopoldia, considered the genus
Moscharia Salisb. as a synonym of Muscarimia Kostel. to which two species were
assigned, M. muscari (L.) A. Los. and M. macrocarpa (Sweet) Garbari. Finally, he assigned all the species previously referred to Botryanthus (Kunth) Bak. to the genus
Muscari, following other authors. Furthermore, in the frame of a cytotaxonomical revision, Garbari (1968) pointed out the biosystematic value of the three generic units (Leopoldia, Muscarimia and Muscari), also confirmed - in the author's opinion- by karyological studies.
4.2 Muscari comosum (L.) Miller
4.2.1 Generality
Muscari comosum (L.)
Miller [ =Leopoldia comosa (L.) Parl.; Hyacinthus comosus L.] is a unit
distributed in the whole Mediterranean basin (Garbari, 1973). It is also found in Canary islands, Madeira, in southern Russia and the Middle East (Bentzer, 1973). It has a wide ecological tolerance, appearing to have no special demands on soil condition, and it’s generally
found also in extensively cultivated areas.
Concerning the karyological aspect, it is a diploid unit with 2n=18. Its karyotype consists of four long chromosomes with the first two markedly telocentric, six of medium size metacentric-submetacentric and eight short metacentric chromosomes. Small satellites are normally present on two short chromosomes, but often they escape notice because of their small size (Bentzer, 1972). The chromosome size ranges from 1,8 to 8,8 µm. The karyotype is markedly asymmetrical because of the stable chromosomal polymorphism in the second pair of chromosomes, as confirmed by several authors (Bentzer & Ellmer, 1975; Bentzer & Landström, 1975,) from studies in the Greek Aegean Islands, Kapasa & al. (2001) from the Greek mainland, as well as from Italy (Garbari, 1969; 1973), from Turkey (Dalgic, 1991), from North Africa (Corsi, Garbari & Ghelardi, 1996), and from other territories.
(L) m-st, because of the long size and their r-values variable from metacentric to subtelocentric. Bentzer and Ellmer (1975) indicated four different types of these (L) m-st chromosomes. Two of these types have rather long, short arms, the one having a long arm almost twice as long as the other; the r-values for these two chromosome types are c. 1.1-1.6 for the one referred to as “normal” and indicated with (+), and c. 2.0-2.5 for the other one referred to as “normal with extralong, long arm” (+1). The other two types of (L) m-st chromosomes have short, short arms of similar size, and the long arms differing in size. The r-values for these chromosome types are c. 2.5-3.5 for the “inversion type” (i), and 2.5-3.5-5.0 for the “inversion type with extralong, long arm” (il).
Bentzer and Ellmer (1975) reported the (+) and (i) types of chromosomes as the most frequent ones, while the (+1) and (il) types of chromosomes were found in low frequencies, in plants from Kithnos and Rhodos, Aegean Islands.
This structural heterozygosity has been explained by a pericentric inversion by Bentzer and Ellmer (1975); it was assumed that the longer types of (L) m-st chromosomes are the older ones from which the shorter (+) and (i) types have evolved later on. So the distinctive karyotype of Muscari comosum originated from a more symmetrical karyotype; in this event the production of the shorter form of the heteromorphic pair could have involved the traslocation of material from the long homologues, as explained by Ruiz Rejon et al. (1987).
The low frequencies in which the (+1) and (il) types may reflect a less suitable adaptiveness (or fitness) compared to the short types during certain periods of time. Moreover, Bentzer & Ellmer (1975) pointed out that the (+1) and (il) type are always in company with a (+) or (i) chromosome, supporting their adaptive role. The different chromosome type frequencies between populations within an island, as well as differences in chromosome-type frequencies between islands, can be explained by the founder effect or genetic drift.
Additionally, concerning the general karyotype of Muscari comosum, some authors (Bentzer, 1972; Ruiz Rejón et al., 1986) have also reported the presence of 1-2 B chromosomes, a trisomic karyotype with 1-2n = 18+1 = 19, triploid with 1-2n = 1-2x = 27 and tetraploid karyotypes with 2n = 4x = 36 chromosomes.
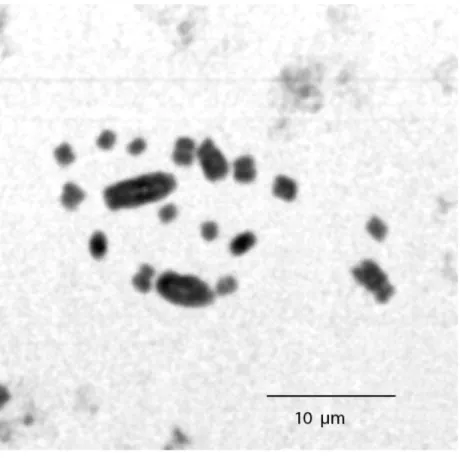
4.2.2 Results
2n=2x=18: 2t + 2sm + 14m
Fig. 18: Muscari comosum (L.) Miller, K424: microphotograph of a somatic metaphasic
plate (up) and its corresponding idiogram and idiogrammatic formula (down).
4.2.3 Discussion
The chromosome number of 2n=18 is confirmed for the population of Muscari
comosum from Thessalia, in the East of the Greek mainland. Also the general
karyotypical structure is confirmed, being the idiogrammatic formula obtained the following:
2n=2x=18: 2t + 2sm + 14m
The second chromosome pair of the population studied represents the case of structural heterozygosity, as reported by many authors above mentioned for different populations studied in the Mediterranean countries.
In particular, the second chromosome pair shows a chromosome with a r-value of 3.0 and the other with a r-value of 2.2. Following the nomenclature adopted by Bentzer and Ellmer (1975) about the polymorphic chromosome pair, the types observed are of the “inversion type”, indicated with (i), and of the “normal with extralong, long arm” type (+1). So, the chromosome pair in object results to be (i)(+1) type.
It could be pointed out that the (+1) chromosome type is generally present at low frequencies, as resulted in the populations from the two Aegean islands (Bentzer and Ellmer, 1975), and its presence has been observed in our investigation. Furthermore, the adaptive role of the (i) type can be deduced also from the present study, because of its presence together with the (+1) type that is indicated to have a less suitable adaptiveness.
The result here obtained is lightly in contrast with another study on a population of Muscari comosum from the same territory of Greece, Thessalia, Nomos Larissis (Kapasa & al., 2001), in which the chromosome type of the heterozygotic pair results to be as (+)(i) type, that means the most common type. Anyway the two habitats of the populations in object are different: the one of the present study consists of uncultivated fields in the surrounding of the Pinios river, the other is a wood of
Fraxinus angustifolia. As affirmed by Bentzer (1972), since the heteromorphism is widely distributed in different kinds of population structures and biotopes it may be of adaptive significance, or it may be selectively neutral.
4.3 Muscari commutatum Guss.
4.3.1 Generality
Muscari commutatum Guss. is a
species widely distributed over the central and eastern Mediterranean area (Garbari, 1984).
According to a recent investigation (Del Guacchio & Nazzaro, 2002), the species includes also Muscari lafarinae (Lojac.) Garbari, that was previously considered as a separate unit, endemic of Sicily (Italy).
The taxonomic position of Muscari lafarinae has been controversed; Parlatore (1852) indicated it simply as a variety of Muscari commutatum Guss., while Lojacono (1908) described it as a distinct species under Botryanthus, that was considered a separate subgenus of Muscari. Garbari (1984) indicated it as a distinct species, pointing out differences between the two units; these consist in the morphology of the plants, as the breadth of the leaves, in the phenology, as the blooming period, and in the colour of the anthocyanic pigmentation of the ovary.
The morphometric investigation of Del Guacchio and Nazzaro (2002), showed that the variation of these characters may be considered as a natural variation among the populations of the species, being these variations nearly continuous. Consequently the authors proposed the synonymization of Muscari commutatum Guss. and Muscari lafarinae (Lojac.) Garbari. This conclusion agrees with the karyological aspect, that hasn’t showed differences between the two units, as affirmed by Garbari (1966).
The karyotype of the species is usually diploid with 2n=18, as indicated by Garbari (1966, 1968) for material from Italy; the same result is indicated by Karlén (1984), considering 36 populations all over Greece. Only two of these populations,
showing also a different flower size; curiously, no diploids have been found on this island. Also from Turkey is reported a triploid chromosome number, in addition to the diploid one (Özhatay, 2002). Finally a tetraploid karyotype 2n=36 is indicated for Cyprus (Heiser, 1978). The karyotype shows relatively short metacentric to submetacentric chromosomes, being the total length of the complement about 65 µm (Karlén, 1984). A satellite is reported by some authors (Garbari, 1968; Karlén, 1984) on the short arm of the second chromosome pair.
4.3.2 Results
10 µm
2n = 2x = 18: 2m + 2sm-SAT + 2m + 4sm + 6m + 2M
Fig. 20. Muscari commutatum Guss.: microphotograph of a somatic metaphasic plate
The present karyological investigation shows a diploid chromosome number, 2n=18, for the population of Muscari commutatum studied from the south east of Peloponnese, in Mount Parnon. This result agrees with the authors over mentioned, especially with Karlén (1984), that studied also populations from the same territory of Greece. The idiogrammatic formula obtained is:
2n = 2x = 18: 2m + 2sm-SAT + 2m + 4sm + 6m + 2M
The karyotype complement confirms the one of other authors (Garbari, 1968; Karlén, 1984), differing only slightly in some details. In the idiogram (fig. 20), the 5 longer metacentric to submetacentric chromosomes decrease more or less continuously in length from the first to the fifth. The 3 following shorter pairs are metacentic and the last one have arms with the same length. These observations agree with the karyotype of Karlén (1984). The only difference consists in the fifth chromosome pair that is indicated from the author as metacentric; in the present study it looks submetacentric, in agreement with the karyotype obtained by Garbari (1968). It is hard to say if these differences are due to various degrees of contraction, depending on details of pretreatments, or if structural changes are really involved, as affirmed by Karlén (1984).
The thesis of structural changes could be supported by their occurring in other species of the genus, e.g. Muscari comosum, in which also the present study confirms the well known structural heterozygosity.
As regards the satellite, its presence on the short arm of the second pair is confirmed as reported previously by the authors. Anyway, it’s pointed out that, analyzing the metaphasic plates, more than one chromosome pair could appear satellited, and mostly on the long arms. This observation can be explained by the fact that the distal regions of some long arms are in some cases less stainable, possibly due to heterochromatic conditions.