Chapter 4
Reissert-like reactions of dialkyl alk-1-enyl
aluminum-pyridine complexes with acid
chlorides
4.1 Introduction
Heteroaromatic systems are very valuable building blocks in the synthesis of biologically active compounds,192,193,194,195 and extensive efforts have been devoted during the past century to finding efficient methods for the addition of nucleophiles to quinoline and isoquinoline derivatives.196,197,198 One of the oldest protocol in this field is the Reissert reaction, first described in 1905. The reaction allows the cyanation of quinoline ring in excellent yields; due to its potential, this protocol has been thoroughly developed.198,199 In this context, nucleophilic 1,2 addition of organometallic reagents to pyridine derivatives has received considerable attention; the reactions with lithium derivatives,200 Grignard reagents201,202,203,204 alkenyl stannanes197,205,206,207,208 and silyl derivatives209 have been extensively studied. Applications to the synthesis of natural products204,210 and liquid crystals203 have been recorded. Regioselectivity of the reaction often depends on the nature of the alkylating agent and is sometimes incomplete.
In the previous chapters it was remarked that complexation of dialkyl alk-1-enyl aluminum reagents with nitrogen ligands strongly affects the reactivity of the alane; it was shown that these complexes present a completely different reactivity
from their uncomplexed counterparts, and this observation led to selective and efficient synthetic routes to unsaturated sulfones and sulfoxides. This complexation is expected to modulate not only the reactivity of the alane, but also of the pyridine; it was in fact reported that aluminum-based Lewis acids could effectively catalyze the Reissert reaction.199 In the light of these reports, it was interesting to investigate the reactivity on pyridine-alane complexes with acid chlorides; two different pathways are conceivable:
1. The reaction could afford unsaturated ketones, by formal substitution of chlorine atom on the acid chloride with alkenyl chain, with or without the formation of an intermediate acid chloride-pyridine adduct.
2. The pyridine could be activated toward a Reissert-like reaction.
The second hypothesis was the more intriguing one: indeed, in spite of the large number of synthetic approaches to the alkenylation of nitrogen bases, the use of alanes is unprecedented. To verify which of the two pathways may be operational, some preliminary studies were performed and the results will be discussed in this chapter.
4.2 Preliminary
experiments
A preliminary experiment, involving reaction of acid chlorides with pyridine-alane complexes, was performed in order to acquire some information on the reactivity of this system. When the di-i-butyl hex-1-enyl aluminum-pyridine complex 18 was reacted at 0 oC with an equimolar amount of benzoyl chloride 32, no trace of phenyl 1-(E)-hex-1-en-1-yl ketone was detected; the reaction product was 1-benzoyl-2-[1-(E)-hex-1-en-1-yl]-1,2-dihydropyridine 33, recovered in a nearly quantitative (94%) yield (Scheme 4.1).
N (i-Bu)2Al Bu-n + O Cl CH2Cl2 0 oC, 5min N O
Bu-n + (i-Bu)2AlCl
18 32 33
formation of a single product. The addition of an alkenyl chain to the pyridine system by means of dialkyl alk-1-enyl aluminum reagents opened interesting synthetic possibilities, in particular for the excellent yield and the simple work-up procedure. Attention was directed to the synthesis of other substrates, in order to better understand the scope and limitations of the reaction.
4.3 Study of the reaction
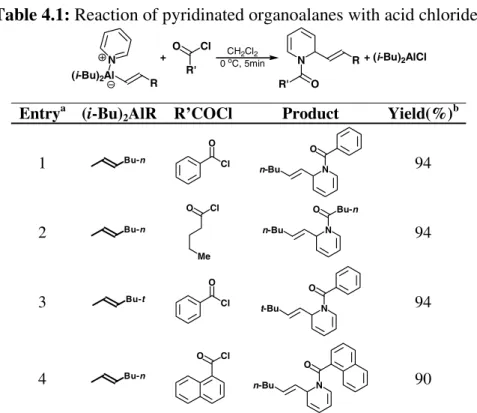
The generality of this protocol was evaluated using different acid chlorides and organo aluminum compounds, obtained from hydroalumination of terminal alkynes. It is clear from reported data (Table 4.1) that the reaction affords the desired products in similar yields regardless of the starting alkyne or the acid chloride employed.
Steric hindrance in the alkenyl chain in position β to the aluminum does not have any effect on reaction times nor on the yield (entries 1 vs. 3). Furthermore, both aryl and alkyl acid chlorides can be successfully employed in this protocol, without any effect on yields and reaction times (entries 1,2,4).
Considering the good results obtained with the simple pyridine system, the study was widened to more complex systems, such isoquinoline (Scheme 4.2).
(i-Bu)2Al Bu-n + N Cl O CH2Cl2 0 oC, 5min N O Ph Bu-n N O Ph Me + Me 85% 10% 34 35
Scheme 4.2: Reaction between isoquinoline-di-i-butyl hex-1-enyl aluminum complex and benzoyl chloride
A preliminary reaction was carried out under the same experimental conditions used for pyridine. In this case product 34 was isolated in a satisfactory (85%) yield, but only partial selectivity was observed, and appreciable amounts (10%) of the correponding alkylation product 35 were obtained. Compound 34 was, however, the sole product (93%) when reaction was performed at -20 oC (Scheme 4.3).
Table 4.1: Reaction of pyridinated organoalanes with acid chlorides R' Cl (i-Bu)2Al R N + 0 CHoC, 5min2Cl2 O N O R' R + (i-Bu)2AlCl Entrya (i-Bu)
2AlR R’COCl Product Yield(%)b
1 Bu-n Cl O N n-Bu O 94 2 Bu-n Cl O Me N O n-Bu Bu-n 94 3 Bu-t Cl O N t-Bu O 94 4 Bu-n Cl O N n-Bu O 90
a All reactions were performed in CH
2Cl2 at 0 oC; b Calculated on the
isolated, chemically pure product
Different ligands as well as acid chlorides were employed to prepare a wider range of targets (Table 4.2). It is evident that the nature of the acid chloride employed does not affect the reaction. Furthermore, the applicability of this reaction seems to be very broad: both alkynyl and alkenyl aluminum reagents are reactive under the reported conditions (entries 2,3), and it is possible to obtain, in satisfactory yields, even products bearing groups potentially reactive towards alanes (entry 4). (i-Bu)2Al Et + N Cl O CH2Cl2 -20 oC, 5min N O Ph 55% 36 (i-Bu)2AlCl + Et
Scheme 4.3: Reaction of di-i-butyl 1’-ethylbut-1-enyl aluminum-isoquinoline complex with benzoyl chloride
The major limitation emerging from this study is represented by the results obtained employing α-substituted alkenyl alanes. In this case the sole product isolated was 36, arising from a reduction of the aromatic system (Scheme 4.3).
Table 4.2: Reaction of alane-isoquinoline complexes with acid halides Entrya (i-Bu)
2AlR RCOCl Product Yield(%)b
1 Bu-n Cl O Me N Bu-n n-Bu O 93 2 Bu-n Cl O N n-Bu O 93 3 Bu-n Cl O N O n-Bu 92 4 Bu-n O Cl O Me N O n-Bu O Me 60 5 Bu-n Cl (CH2)8 Cl O O N (CH2)8 N O O n-Bu Bu-n 54 6 Bu-n Br O Br N n-Bu O Br 55 7 Bu-n Cl O Cl O N O N O n-Bu Bu-n 58
a All reactions were performed in CH
2Cl2 solution at 0 oC; b Calculated on the
isolated, chemically pure product.
It is known that 2,3-dihydro-1H-imidazole derivatives are often obtained by complex or low-yielding approaches, which limits the availability of these compounds.211,212,213 On the contrary, use of an N-acyl imidazole as ligand to the
organoalane would afford these products with a simple and straightforward procedure. A preliminary reaction was carried out on N-benzoyl imidazole 37, which after complexation with di-i-butyl hex-1-enyl aluminum to form intermediate 38 was reacted with benzoyl chloride. The reaction afforded the
(E)-2-hex-1’-enyl-1,3-dibenzoyl-2,3-dihydroimidazole 39 in good (68%) yield, (Scheme 4.4), thus demonstrating that this methodology is easily applicable to the synthesis of 2,3-dihydro imidazole derivatives.
(i-Bu)2Al N Ph O Cl CH2Cl2 -20 oC, 5min 68% Bu-n N O Ph 2) H3O+ N N Ph O Ph O Bu-n N N O Ph (i-Bu)2Al Bu-n 37 38 39 1)
Scheme 4.4: Application of our new reaction to an imidazole derivative
Finally, a more complex reaction sequence was examined, which involved formation of a tricyclic derivative. Nitrogen-containing tricyclic systems are valuable in the synthesis of natural and bioactive products, but their synthesis is often difficult to achieve; in view of the flexibility of the reaction under study, extension to the synthesis of tricyclic derivatives seemed possible.214,215,216
Indeed, 8-trimethyl silyloxy quinoline 40, obtained from 8-hydroxyquinoline and trimethyl silyl chloride, could be employed as ligand in this reaction and complexed with (E) di-i-butyl hex-1-enyl aluminum to form intermediate 41 (Scheme 4.5); bromo acetyl bromide was added at -20 oC, and upon warming to room temperature the labile intermediate 42 was obtained.a Compound 42 cyclized both thermally and in the presence of KF or NaOH under phase-transfer conditions to afford 5-[(E)-hex-1’-enyl]-2H-[1,4]oxazino[2,3,4-ij]quinolin-3(5H)-one 43. Closely related compounds have been investigated214 in view of their microbiocidal215 and fungicidal216 activity. The efficient synthesis of 43 bodes well for the convenient preparation of a host of synthetically related derivatives.
aCompound 42 was too labile to record appropriate 1H and 13C NMR spectra. It was however
(i-Bu)2Al N Me3SiO O Br CH2Cl2 -20 oC to r.t. 20min Br N Me3SiO O Br N O O 80% Bu-n Bu-n (i-Bu)2Al Bu-n N Me3SiO KF Et2O/H2O r.t., 45min Bu-n 40 41 42 43
Scheme 4.5: Synthesis of a tricyclic system by alkenylation and subsequent deprotection of substituted quinoline ring
4.4 Mechanistic
considerations
Although a mechanistic study has not been performed on this reaction, some reasonable hypotheses can be advanced; the most reasonable reaction mechanism involves the formation of an acid chloride-heterocycle adduct, and subsequent transfer of the unsaturated chain from the alane to the position α to the nitrogen atom; to account for the tendency of alanes to form stable complexes with Lewis bases, intermediate 44 is proposed (Scheme 4.6). This intermediate, similar to compound 25 described in Scheme 2.11, is unstable and spontaneously reacts to afford the products.
R' X O CH2Cl2 (i-Bu)2Al R N R' N O (i-Bu)2Al R Cl R' N O (i-Bu)2Al R 44 Cl
Scheme 4.6: Plausible reaction pathway between heteroaromatic nitrogen ligand-acid chloride complexes and di-i-butyl alk-1-enyl alanes.
In support of this hypothesis, di-i-butyl hex-1-enyl aluminum was reacted with preformed pyridine-benzoyl chloride complex (Scheme 4.7, path a) and isoquinoline-benzoyl chloride complex (Scheme 4.7, path b). The expected product were obtained in lower yields than those obtained with complexed alanes, (Table 4.1, entry 1 and Table 4.2, entry 2 respectively); this suggests the mechanism is more subtle than one involving the uncomplexed alane and the acylated pyridine, indicating that perhaps slow decomplexation of the pyridine-alane complex is a key factor in a successful reaction.
Cl O Ph Cl O CH2Cl2 0 oC, 5min CH2Cl2 0 oC, 5min + + N N N O Cl Ph N O Cl N O Ph n-Bu + 70% N O Ph 12% N O Ph n-Bu 25% + productsSide (i-Bu)2Al Bu-n (i-Bu)2Al Bu-n
Scheme 4.7: Reaction between benzoyl chloride-heteroaromatic nitrogen ligand and di-i-butyl hex-1-enyl aluminum
In conclusion, reaction of heteroaromatic nitrogen systems/alane complexes with acid halides affords products arising from the addition of the alkenyl chain in position 2 of the heteroaromatic ring; products are obtained in excellent yields, with simple work-up procedures. This protocol is amenable to the preparation of many synthetically useful intermediates; in particular, synthesis of (E)-2-hex-1’-enyl-1,3-dibenzoyl-2,3-dihydroimidazole 39 and of the tricyclic product 5-[(E)-hex-1’-enyl]-2H-[1,4]oxazino[2,3,4-ij]quinolin-3(5H)-one 43 has been achieved in high yields; this last compound is of potential interest, due to its close relationship with derivatives having fungicidal and herbicidal activity. It has to be pointed out that, as already seen for the preparation of sulfones and sulfoxides, and as usual in the chemistry of dialkyl alk-1-enyl aluminum reagents, products are always obtained as single isomer, because the geometry of the double bond is