137
6. Experimental section
6.1 Chemistry Methods
Melting points were determined on a Kofler hot-stage apparatus and are uncorrected. 1H and 13C NMR spectra were obtained with a Varian Gemini 200 MHz spectrometer.
Chemical shifts (δ) are reported in parts per million downfield from tetramethylsilane and referenced from solvent references; coupling constants J are reported in hertz; 13
C NMR spectra were fully decoupled. The following abbreviations are used: singlet (s), doublet (d), triplet (t), double-doublet (dd), and multiplet (m). Mass spectra were obtained on a Hewlett-Packard 5988 A spectrometer by using a direct injection probe and an electron beam energy of 70 eV.
The elemental compositions of the compounds agreed to within (0.4% of the calculated value.
Chromatographic separation was performed on silica gel columns by flash (Kieselgel 40, 0.040–0.063 mm; Merck). Reactions were followed by thin-layer chromatography (TLC) on Merck aluminum silica gel (60 F254) sheets that were visualized under a UV lamp.
Evaporation was performed in vacuo (rotating evaporator). Sodium sulfate was always used as the drying agent. The microwave-assisted procedures were carried out with a CEM Discover LabMate microwave. Commercially available chemicals were purchased from Sigma-Aldrich.
138 General Procedure To Synthesize Final Compounds 1a-d, 2, 3a-c, 4a-c, 5a-c, 6a-c, 7a-c. To a solution of the compound 12, 13-15 or 18-20 (0.39 mmol) in ethanol (7 mL) was added the appropriate carboxaldehyde (0.43 mmol) and a catalytic amount of piperidine. The resulting solution was stirred and refluxed for 12 h, then the solution was evaporated to dryness. The residual material was purified by crystallization.
(3Z)-2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2-(1H)-yl)-N-(2-oxo-3-(thiophen 2-ylmethylene) indolin-5-yl)acetamide (1a). The residual material was purified by crystallization from AcOEt/hexane, affording 1a as the Z-isomer (62 mg, 0.13 mmol, 30% yield): mp 158-160°C. 1H NMR (CDCl3): δ 2.90-2.99 (m, 4H, CH2); 3.34 (s, 2H, CH2); 3.76 (s, 2H, CH2); 3.84 (s, 3H, OMe); 3.88 (s, 3H, OMe); 6.54 (s, 1H, Ar); 6.66 (s, 1H, Ar); 6.77-6.85 (m, 1H, Ar); 7.15-7.21 (m, 2H, Ar); 7.65 (s, 1H, H-vinyl); 7.76-7.98(m, 2H, Ar); 8.09 (s, 1H, Ar); 8.82 (br s, 1H, NH); 9.20 (br s, 1H, NH) ppm. 13C NMR (CDCl3): δ 170.60, 168.47, 148.03, 147.84, 139.07, 138.05, 137.45, 135.70, 134.02, 132.48, 129.33, 127.60, 125.94, 125.59, 120.06, 116.22, 115.88, 109.77, 62.01, 56.24, 55.97, 51.79, 29.09 ppm. Anal. (C26H25N3O4S) C, H, N.
(3E)-2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2-(1H)-yl)-N-(3-((1-methyl-1H-imidazol-2yl) methylene)-2-oxoindolin-5-yl)acetamide (1b). The residual material was purified by crystallization from AcOEt/hexane, affording 1b as the E-isomer (71. mg, 0.15 mmol, 38% yield): mp 147-149°C. 1H NMR (CDCl3): δ 2.90-3.00 (m, 4H, CH2); 3.35 (s, 2H, CH2); 3.78 (s, 2H, CH2); 3.84 (s, 3H, NCH3); 3.87 (s, 3H, OMe); 3.88 (s, 3H, OMe); 6.55 (s, 1H, Ar); 6.67 (s, 1H, Ar); 6.82 (d, 1H, J = 8.2 Hz, Ar); 7.05 (s, 1H, Ar); 7.25 (s, 1H, Ar); 7.47 (s, 1H, H-vinyl); 7.73-7.81 (m, 2H, Ar, NH); 9.27 (br s, 1H, Ar); 9.38 (br s, 1H, NH) ppm. 13C NMR (CDCl3): δ 170.79, 168.53, 148.43, 148.10, 138.49, 132.84, 131.24, 127.00, 126.47, 126.07, 124.34, 122.83, 120.61, 118.71, 112.34, 110.24, 109.66, 62.12, 56.49, 56.09, 51.91, 34.01, 29.35 ppm. Anal. (C26H27N5O4) C, H, N.
(3Z)-N-(3-((1H-imidazol-5yl)-(2-oxoindolin-5-yl)-2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2 (1H)-yl)acetamide (1c). The residual material was purified by crystallization from AcOEt/hexane, affording 1c as the Z-isomer (73 mg, 0.16 mmol, 42% yield): mp 169-171°C. 1H NMR (CDCl3): δ 2.85-2.95 (m, 4H, CH2); 3.34 (s, 2H, CH2); 3.76 (s, 2H, CH2); 3.85 (s, 3H, OMe); 3.88 (s, 3H, OMe); 6.54 (s, 1H, Ar);
139 6.66 (s, 1H, Ar); 6.83 (d, 1H, J = 8.2 Hz, Ar); 7.21-7.28 (m, 1H, Ar); 7.49 (s, 1H, Ar); 7.60 (s, 1H, H-vinyl); 7.84 (s, 1H, Ar); 7.93 (br s, 1H, Ar); 8.08 (br s, 1H, NH); 9.23 (br s, 1H, NH) ppm. 13C NMR (CDCl3): δ 170.80, 169.58, 148.00, 147.93, 138.99, 135.30, 133.10, 128.67, 125.70, 125.61, 124.90, 123.73, 119.91, 112.01, 111.60, 110.01, 109.92, 62.03, 56.27, 55.90, 51.83, 29.09 ppm. Anal. (C25H25N5O4) C, H, N.
(3E)-2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2-(1H)-yl)-N-(2-oxo-3-(pyridin-2-ylmethylene) indolin-5-yl)acetamide (1d). The residual material was purified by crystallization from AcOEt/hexane, affording 1d as the E-isomer (84.69 mg, 0.18 mmol, 45% yield): mp 170-172°C. 1H NMR (CDCl3): δ 2.82-2.95 (m, 4H, CH2); 3.33 (s, 2H, CH2); 3.70 (s, 2H, CH2); 3.83 (s, 3H, OMe); 3.87 (s, 3H, OMe); 6.51 (s, 1H, Ar); 6.64 (s, 1H, Ar); 6.82 (d, 1H, J = 8.2 Hz, Ar); 7.20-7.28 (m, 1H, Ar); 7.38-7.52 (m, 2H, Ar, H-vinyl); 7.74-7.76 (m, 1H, Ar); 7.96-8.10 (m, 2H, Ar); 8.63 (br s, 1H, NH); 8.88 (br s, 1H, Ar); 9.08 (br s, 1H) ppm.13C NMR (CD3OD-d4): δ 179.08, 176.09, 164.60, 158.52, 151.02, 150.15, 149.10, 148.84, 144.83, 138.33, 127.97, 122.45, 120.34, 118.02, 116.70, 115.07, 113.15, 111.42, 57.52, 56.87, 54.79, 52.33, 25.79 ppm. Anal. (C27H26N4O4) C, H, N.
(3Z)-2-((3,4-dimethoxybenzyl)amino)-N-(2-oxo-3-(thiophen-2-ylmethylene) indolin-5-yl)acetamide (2). The residual material was purified by crystallization from AcOEt/ hexane, affording 2 as the Z-isomer (36 mg, 0.08 mmol, 20% yield): mp 167-169°C. 1H NMR (CDCl3): δ 3.37 (dd, 1H, J = 1.8; 15.1 Hz, CH2); 3.55 (d, 1H, J = 12.9 Hz, CH2); 3.75 (d, 1H, J = 15.1 Hz, CH2); 3.86 (s, 3H, OMe); 3.89 (s, 3H, OMe); 4.00 (d, 1H, J =12.9 Hz, CH2); 6.66 (d, 1H, J = 8.2 Hz, Ar); 6.79-6.96 (m, 3H, Ar); 7.11 (dd, 1H, J = 3.6, 5.1 Hz, Ar); 7.32-7.35 (m, 1H, Ar); 7.58 (s, 1H, H-vinyl); 7.62 (d, 1H, J = 5.1 Hz); 7.76 (d, 1H, J = 3.6 Hz, Ar); 8.00 (s, 1H, Ar) ppm. 13C NMR (CDCl3): δ 169.84, 167.85, 148.76, 148.14, 141.84, 137.67, 137.03, 133.48, 128.95, 128.94, 128.93, 128.16, 126.94, 126.09, 124.97, 120.22, 116.49, 111.23, 110.63, 109.22, 78.63, 55.49, 54.44 ppm. Anal. (C24H23N3O4S) C, H, N. (3Z)-N-(2-oxo-3-(thiophen-2-ylmethylene)indolin-5-yl)methanesulfonamide (3a). The residual material was purified by crystallization from EtOH, affording 3a as the Z-isomer (48 mg, 0.15 mmol, 38% yield): mp 185-187°C. 1H NMR (DMSO-d6): δ 2.94 (s, 3H, Me); 6.73-6.90 (m, 1H, Ar); 7.00-7.36 (m, 2H, Ar); 7.54 (s, 1H, Ar);
140 7.79-8.29 (m, 3H, H-vinyl, Ar); 9.38 (br s, 1H); 10.57 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.09, 137.83, 137.66, 137.01, 134.27, 131.29, 128.47, 127.25, 124.77, 123.46, 121.27, 114.54, 109.60, 38.26 ppm. Anal. (C14H12N2O3S2) C, H, N. (3E)-N-(3-((1-methyl-1H-imidazol-2yl)methylene)-2-oxoindolin-5-yl)
methanesulfonamide (3b). The residual material was purified by crystallization from EtOH, affording 3b as the E-isomer (51 mg, 0.16 mmol, 40% yield): mp 197-199°C. 1H NMR (DMSO-d6): δ 2.99 (s, 3H, Me); 3.90 (s, 3H, OMe); 6.82 (d, 1H, J = 8.2 Hz, Ar); 7.11 (dd, 1H, J = 2.0, 8.2 Hz, Ar); 7.34 (s, 1H, Ar); 7.38 (s, 1H, Ar); 7.52 (s, 1H, H-vinyl); 9.37 (d, 1H, J = 2.0 Hz, Ar); 9.42 (br s, 1H); 10.57 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.92, 139.65, 136.81, 135.45, 131.89, 131.56, 127.46, 123.59, 123.24, 121.13, 117.67, 110.02, 45.07, 38.20 ppm. Anal. (C14H14N4O3S) C, H, N.
(3Z)-N-(3-((1H-imidazol-5yl)methylene)-2-oxoindolin-5-yl)methanesulfonamide (3c). The residual material was purified by crystallization from EtOH, affording 4c as the Z-isomer (55 mg, 0.18 mmol, 45% yield): mp 205-207°C. 1H NMR (DMSO-d6): δ 2.95 (s, 3H, Me); 6.87 (d, 1H, J = 8.1 Hz, Ar); 7.06 (dd, 1H, J = 1.7, 8.1 Hz, Ar); 7.51 (d, 1H, J = 1.7 Hz, Ar); 7.73 (s, 1H, Ar); 7.84 (s, 1H, Ar); 8.03 (s, 1H, H-vinyl); 9.45 (br s, 1H); 11.03 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 170.04, 139.14, 137.77, 131.18, 127.85, 125.79, 123.73, 123.11, 122.29, 114.14, 110.22, 109.17, 38.27 ppm. Anal. (C13H12N4O3S) C, H, N.
(3Z)-4-methyl-N-(2-oxo-3-(thiophen-2-ylmethylene)indolin-5-yl)
benzenesulfonamide (4a). The residual material was purified by crystallization from EtOH, affording 4a as the Z-isomer (76 mg, 0.20 mmol, 50% yield): mp 210-212°C. 1
H NMR (DMSO-d6): δ 2.33 (s, 3H, Me); 6.68 (d, 1H, J = 8.1 Hz, Ar); 6.77 (d, 1H, J = 8.1 Hz, Ar); 7.21-7.26 (m, 1H, Ar); 7.33 (d, 1H, J = 7.3 Hz, Ar); 7.45 (s, 1H, Ar); 7.59 (d, 1H, J = 7.3 Hz, Ar); 7.91 (d, 1H, J = 4.9 Hz, Ar); 7.80-8.10 (m, 1H, Ar); 8.03 (s, 1H, H-vinyl); 9.87 (br s, 1H); 10.56 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 166.99, 142.65, 137.83, 137.64, 136.93, 136.61, 134.35, 130.80, 129.25, 128.39, 127.19, 126.54, 124.57, 122.97, 121.13, 114.28, 109.42, 20.77 ppm. Anal. (C20H16N2O3S2) C, H, N.
(3E)-4-methyl-N-(3-((1-methyl-1H-imidazol-2yl)methylene)-2-oxoindolin-5-yl) benzenesulfonamid (4b). The residual material was purified by crystallization from
141 EtOH, affording 4b as the E-isomer (71 mg, 0.18 mmol, 45% yield): mp 198-200°C. 1
H NMR (CD3OD-d4): δ 2.29 (s, 3H, Me); 3.88 (s, 3H, OMe); 6.74 (d, 1H, J = 8.2 Hz, Ar); 7.00 (dd, 1H, J = 2.0, 8.2 Hz, Ar); 7.22 (d, 2H, J = 8.1 Hz, Ar); 7.32 (s, 2H, Ar); 7.46 (s, 1H, H-vinyl); 7.63 (d, 2H, J = 8.1 Hz, Ar); 8.95 (d, 1H, J = 2.0 Hz, Ar); 8.92 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 170.01, 169.28, 144.54, 144.08, 138.16, 133.18, 131.66, 130.35, 128.39, 126.88, 125.91, 124.18, 123.67, 120.80, 120.10, 110.58, 58.32, 26.71 ppm. Anal. (C20H18N4O3S) C, H, N.
(3Z)-N-(3-((1H-imidazol-5yl)-methylene)-2-oxoindolin-5-yl)-4-methyl
benzenesulfonamide (4c). The residual material was purified by crystallization from EtOH, affording 4c as the Z-isomer (79 mg, 0.21 mmol, 53% yield): mp 219-217°C. 1
H NMR (DMSO-d6): δ 2.31 (s, 3H, Me); 6.70-6.82 (m, 2H, Ar); 7.32 (d, 1H, J = 8.1 Hz, Ar); 7.40 (s, 1H, Ar); 7.59 (d, 1H, J = 8.1 Hz, Ar); 7.73 (s, 1H, Ar); 7.77 (s, 1H, Ar); 8.02 (s, 1H, H-vinyl); 9.94 (br s, 1H); 10.95 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.90, 139.05, 136.74, 135.45, 134.95, 131.88, 131.40, 130.72, 128.46, 127.59, 127.44, 126.68, 121.33, 117.95, 111.01, 110.12, 21.30 ppm. Anal. (C19H16N4O3S) C, H, N.
(3Z)-N-(4-methoxybenzyl)-2-oxo-3-(thiophen-2-ylmethylene)indoline-5-
sulfonamide (5a). The residual material was purified by crystallization from MeOH, affording 5a as the Z-isomer (95 mg, 0.23 mmol, 60% yield): mp 200-202°C. 1H NMR (DMSO-d6): δ 3.66 (s, 3H, OMe); 3.94 (d, 1H, J = 5.5 Hz, CH2); 6.80 (d, 2H, J = 8.6 Hz, Ar); 6.98 (d, 1H, J = 8.2 Hz, Ar); 7.15 (d, 2H, J = 8.6 Hz, Ar); 7.25-7.30 (m, 1H, Ar); 7.64 (dd, 1H, J = 1.5, 8.2 Hz, Ar) ;7.85-7.91 (m, 1H, Ar); 7.98 (d, 1H, J = 5.1 Hz, NH); 8.02 (d, 1H, J = 3.1 Hz, Ar); 8.07 (d, 1H, J = 1.5 Hz, NH); 8.33 (s, 1H, H-vinyl); 11.03 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.29, 157.28, 146.68, 141.94, 135.27, 133.42, 130.89, 131.5, 129.62, 128.79, 128.49, 125.71, 123.80, 121.59, 119.11, 113.67, 108.61; 55.00; 45.51 ppm.Anal. (C21H18N2O4S2) C, H, N.
(3E)-N-(4-methoxybenzyl)-3-((1-methyl-1H-imidazol-2-yl)methylene)-2-oxoindoline-5-sulfonamide (5b). The residual material was purified by crystallization from MeOH, affording 5b as the E-isomer (106 mg, 0.25 mmol, 64% yield): mp 195-197°C. 1H NMR (DMSO-d6): δ 3.69 (s, 3H, OMe); 3.93 (s, 3H, NMe); 3.97 (d, 1H, J = 5.9 Hz, CH2); 6.79 (d, 2H, J = 8.6 Hz, Ar); 6.99 (d, 1H, J = 8.2 Hz, Ar); 7.16 (d, 2H, J = 8.6 Hz, Ar); 7.39 (s, 1H, Ar); 7.47 (s, 1H, Ar); 7.57 (s,
142 1H, H-vinyl); 7.70 (dd, 1H, J = 1.8, 8.2 Hz, Ar); 7.92 (br t, 1H, J = 5.9 Hz); 9.93 (d, 1H, J = 1.8 Hz, Ar); 10.98 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.27, 158.15, 145.26, 142.14, 133.66, 130.58, 129.61, 128.67, 128.52, 125.70, 123.84, 121.53, 119.38, 113.37, 108.97; 54.89; 45.67; 33.20 ppm. Anal. (C21H20N4O4S) C, H, N. (3Z)-3-((1H-imidazol-5-yl)methylene)-N-(4-methoxybenzyl)-2-oxoindoline-5-sulfonamide (5c). The residual material was purified by crystallization from MeOH, affording 5c as the Z-isomer (99 mg, 0.24 mmol, 62% yield): mp 209-211°C. 1H NMR (DMSO-d6): δ 3.65 (s, 3H, OMe); 3.96 (d, 1H, J = 5.9 Hz, CH2); 6.78 (d, 2H, J = 8.5 Hz, Ar); 7.04 (d, 1H, J = 8.2 Hz, Ar); 7.14 (d, 2H, J = 8.5 Hz, Ar); 7.40 (s, 1H, H-vinyl); 7.61 (d, 1H, J =1.4 Hz, Ar); 7.67 (dd, 1H, J = 1.5, 8.2 Hz, Ar); 7.84-7.92 (m, 2H, Ar); 8.16 (d, 1H, J =1.5 Hz, Ar); 10.65 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.34, 158.15, 145.09, 142.54, 133.69, 132.85, 129.63, 128.67, 128.34, 125.48, 123.35, 121.91, 121.67, 113.35, 108.91; 54.89; 45.69 ppm. Anal. (C20H18N4O4S) C, H, N.
(3Z)-N-(3,4-dimethoxybenzyl)-2-oxo-3-(thiophen-2-ylmethylene)indoline-5-sulfonamide (6a). The residual material was purified by crystallization from EtOH, affording 6a as the Z-isomer (110 mg, 0.23 mmol, 60% yield): mp 246-248C. 1H NMR (DMSO-d6): δ 3.62 (s, 6H, OMe); 3.96 (s, 2H, CH2); 6.68-6.82 (m, 3H, Ar); 6.90 (d, 1H, J = 8.1 Hz, Ar); 7.27 (t, 1H, J = 4.2 Hz, Ar); 7.62 (d, 1H, J = 8.1 Hz, Ar); 7.82-7.94 (m, 1H, Ar); 7.82-7.84 (m, 2H, Ar, NH); 8.04 (s, 1H, H-vinyl); 8.31 (s, 1H, Ar); 10.99 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.23, 148.39, 147.77, 143.00, 138.66, 137.10, 135.39, 133.36, 130.38, 129.74, 127.65, 127.08, 124.41, 121.53, 119.82, 118.09, 117.69, 111.31, 109.26; 55.40; 55.20; 46.18 ppm. Anal. (C22H20N2O5S2) C, H, N.
(3E)-N-(3,4-dimethoxybenzyl)-3-((1-methyl-1H-imidazol-2yl)methylene)-2- oxoindoline-5-sulfonamide (6b). The residual material was purified by crystallization from EtOH, affording 6b as the E-isomer (109 mg, 0.24 mmol, 61% yield): mp 235-237°C. 1H NMR (DMSO-d6): δ 3.59 (s, 3H, OMe); 3.67 (s, 3H, OMe); 3.92 (s, 3H, Me); 3.98 (d, 2H, J = 6.1 Hz, CH2); 6.68-6.82 (m, 3H, Ar); 6.96 (d, 1H, J = 8.2 Hz, Ar); 7.39 (s, 1H, Ar); 7.46 (s, 1H, Ar); 7.56 (s, 1H, H-vinyl); 7.68 (dd, 1H, J = 1.8, 8.2 Hz, Ar); 7.92 (br t, 1H, J = 6.1 Hz); 9.92 (d, 1H, J = 1.8 Hz, Ar); 10.99 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.27, 148.39, 147.77, 145.27,
143 142.14, 133.73, 130.60, 130.01, 128.59, 125.72, 123.84, 121.53, 119.69, 119.36, 111.57, 108.93; 55.49; 55.18; 46.04; 33.20 ppm. Anal. (C22H22N4O5S) C, H, N. (3Z)-3-(1-(1H-imidazol-5-yl)-ethylidene)-N-(3,4-dimethoxybenzyl)-2-oxoindoline -5-sulfonamide (6c). The residual material was purified by crystallization from EtOH, affording 6c as the Z-isomer (110 mg, 0.24 mmol, 62% yield): mp 315-320°C. 1
H NMR (DMSO-d6): δ 3.34 (s, 6H, OMe); 3.98 (d, 2H, J = 8.1 Hz, CH2); 6.68-6.80 (m, 3H, Ar); 7.02 (d, 1H, J = 8.0 Hz, Ar); 7.40 (s, 1H, Ar); 7.56-7.70 (m, 2H, Ar); 7.80-7.93 (m, 2H, H-vinyl, NH); 8.12 (s, 1H, Ar); 10.00 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.96, 148.33, 147.73, 143.18, 134.33, 133.73, 133.02, 129.96, 128.58, 127.41, 126.08, 125.63, 124.10, 122.02, 119.82, 111.28, 109.74; 55.45; 55.33; 46.15 ppm. Anal. (C21H20N4O5S) C, H, N.
(3Z)-1-(4-(methylthio)phenyl)-3-(2-oxo-3-(thiophen-2-ylmethylene)indolin-5-yl)urea (7a). The residual material was purified by crystallization from EtOH, affording 7a as the Z-isomer (94 mg, 0.23 mmol, 60% yield): mp 246-248C. 1H NMR (DMSO-d6): δ 2.44 (s, 3H, SMe); 6.78 (d, 1H, J = 8.2 Hz, Ar); 7.11 (d, 1H, J = 8.2 Hz, Ar); 7.22 (d, 2H, J = 8.4 Hz, Ar); 7.44 (d, 2H, J = 8.4 Hz, Ar); 7.84-7.92 (m, 3H, Ar, H-vinyl); 7.96-8.01 (m, 1H, Ar); 8.03 (s, 1H, Ar); 8.48 (br s, 1H); 8.68 (br s, 1H); 10.49 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.16, 152.65, 137.53, 137.11, 135.69, 133.93, 133.16, 129.54, 127.81, 127.16, 124.93, 121.87, 120.71, 119.91, 118.78, 111.17, 110.27, 109.27; 16.10 ppm. Anal. (C21H17N3O2S2) C, H, N.
(3E)-1-(3-((1-methyl-1H-imidazol-2-yl)methylene)-2-oxoindolin-5-yl)-3-(4-(methylthio)phenyl)urea (7b). The residual material was purified by crystallization from EtOH, affording 7b as the E-isomer (101 mg, 0.25 mmol, 64% yield): mp 235-237°C. 1H NMR (DMSO-d6): δ 2.43 (s, 3H, SMe); 3.90 (s, 3H, NMe); 6.78 (d, 1H, J = 8.3 Hz, Ar); 7.21 (d, 2H, J = 8.8 Hz, Ar); 7.36 (s, 2H, Ar); 7.42 (d, 2H, J = 8.8 Hz, Ar); 7.53 (s, 1H, H-vinyl); 7.65 (dd, 1H, J = 2.0, 8.3 Hz, Ar); 8.49 (br s, 1H); 8.64 (br s, 1H); 9.12 (d, 1H, J = 2.0 Hz, Ar); 10.47 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.27, 157.45, 140.26, 139.14, 137.00, 138.86, 133.66, 130.76, 129.42, 128.27, 127.52, 123.70, 122.84, 121.55, 119.78, 112.57, 108.97; 34.25, 14.20 ppm. Anal. (C21H19N5O2S) C, H, N.
144
(3Z)-1-(3-((1H-imidazol-5-yl)methylene)-2-oxoindolin-5-yl)-3-(4-(methylthio)phenyl)urea (7c). The residual material was purified by crystallization from EtOH, affording 7c as the Z-isomer (98 mg, 0.25 mmol, 63% yield): mp 215-217°C. 1H NMR (DMSO-d6): δ 2.44 (s, 3H, SMe); 6.82 (d, 1H, J = 8.3 Hz, Ar); 7.12 (dd, 1H, J = 1.3, 8.3 Hz, Ar); 7.22 (d, 2H, J = 8.6 Hz, Ar); 7.44 (d, 2H, J = 8.6 Hz, Ar); 7.71 (s, 1H, H-vinyl); 7.79 (s, 1H, Ar); 7.86 (d, 1H, J = 1.3 Hz, Ar); 8.02 (s, 1H, Ar); 8.54 (br s, 1H); 8.70 (br s, 1H); 10.90 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.58, 153.00, 138.60, 137.01, 135.82, 134.00, 133.27, 128.54, 127.41, 127.03 124.93, 122.07, 120.49, 120.01, 118.58, 111.35, 109.15; 17.18 ppm. Anal. (C20H17N5O2S) C, H, N.
General Procedure To Synthesize Final Compounds 8a-c. A solution of oxone (215
mg, 0.35 mmol) in water was added to a cooled (0°C) solution of the appropriate derivative 7a-c (0.29 mmol) in 1:1 MeOH-THF (2 mL). The resulting mixture was stirred at room temperature for 12 h. Then the solid was filtered off, and the solution was evaporated. The residual material was diluted with MeOH and the solid product was separated out from the mixture. It was filtered, washed with diethyl ether, and air dried.
(3Z)-1-(4-(methylsulfonyl)phenyl)-3-(2-oxo-3-(thiophen-2-ylmethylene)indolin-5-yl)urea (8a) (76 mg, 0.17 mmol, 60 % yield): mp 213-215°C. 1H NMR (DMSO-d6): δ 3.16 (s, 3H, SMe); 6.79 (d, 1H, J = 8.3 Hz, Ar); 7.14 (d, 1H, J = 8.3 Hz, Ar); 7.23 (t, 1H, J = 4.3 Hz, Ar); 7.60-7.92 (m, 6H, Ar); 7.98-8.10 (m, 2H, Ar, H-vinyl); 8.75 (br s, 1H); 9.29 (br s, 1H); 10.52 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.40, 152.56, 148.00, 137.71, 136.09, 134.12, 133.18, 129.54, 127.75, 127.14, 125.03, 121.87, 120.98, 118.94, 118.75, 111.17, 110.27, 109.27; 52.70 ppm. Anal. (C21H17N3O4S2) C, H, N.
(3E)-1-(3-((1-methyl-1H-imidazol-2-yl)methylene)-2-oxoindolin-5-yl)-3-(4-(methylsulfonyl)phenyl)urea (8b) (79 mg, 0.18 mmol, 62% yield): mp 229-231°C. 1
H NMR (DMSO-d6): δ 3.15 (s, 3H, SMe); 3.90 (s, 3H, NMe); 6.81 (d, 1H, J = 8.2 Hz, Ar); 7.37 (s, 2H, Ar); 7.53 (s, 1H, H-vinyl); 7.61-7.71 (m, 3H, Ar); 7.81 (d, 2H, J = 8.8 Hz, Ar); 8.70 (br s, 1H); 9.10-9.18 (m, 2H, Ar, NH); 10.51 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.15, 153.45, 143.56, 139.24, 138.50, 137.86, 134.56, 130.17,
145 129.71, 128.05, 127.52, 122.73, 122.19, 121.55, 119.53, 112.17, 109.04; 54.25, 35.20 ppm. Anal. (C21H19N5O4S) C, H, N.
(3Z)-1-(3-((1H-imidazol-5-yl)methylene)-2-oxoindolin-5-yl)-3-(4-
(methylsulfonyl)phenyl)urea (8c) (75 mg, 0.18 mmol, 61% yield): mp 215-217°C. 1
H NMR (DMSO-d6): δ 3.16 (s, 3H, SMe); 6.84 (d, 1H, J = 8.2 Hz, Ar); 7.15 (d, 1H, J = 8.3 Hz, Ar); 7.62-7.85 (m, 6H, H-vinyl, Ar); 7.89 (s, 1H, Ar); 8.02 (s, 1H, Ar); 8.74 (br s, 1H); 9.24 (br s, 1H); 10.93 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.85, 152.25, 144.49, 139.28, 138.46, 135.20, 133.22, 132.82, 128.01, 127.68 124.50, 122.70, 120.11, 119.45, 117.41, 110.91, 109.73; 55.89 ppm. Anal. (C20H17N5O4S) C, H, N.
General Procedure To Synthesize Final Compounds 9a-c. To the appropriate compound 22a-c (1.0 mmol) in 10 mL dichloromethane/methanol (90:10), 4-methoxybenzenesulfonyl isocyanate (235 mg, 1.1 mmol) was added slowly at 10 °C. The mixture was stirred at room temperature for 12 h. Solid product 9a-c was separated out from the mixture. It was filtered, washed with dichloromethane, and air dried.
(3Z)-4-methyl-N-((2-oxo-3-(thiophen-2-ylmethylene)indolin-5-yl)carbamoyl) benzenesulfonamide (9a). (382 mg, 0.87 mmol, 87% yield): mp 212-214C. 1H NMR (DMSO-d6): δ 2.34 (s, 3H, Me); 6.98 (d, 1H, J = 8.2 Hz, Ar); 7.12 (d, 1H, J = 8.2 Hz, Ar); 7.36 (d, 2H, J = 8.4 Hz, Ar); 7.65 (d, 2H, J = 8.4 Hz, Ar); 7.84-7.92 (m, 3H, Ar, H-vinyl); 7.97-8.02 (m, 1H, Ar); 8.03 (s, 1H, Ar); 8.48 (br s, 1H); 8.68 (br s, 1H); 10.49 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 170.17, 152.60, 138.53, 137.01, 135.18, 134.93, 133.16, 130.54, 130.01, 129.35, 129.23, 128.78, 128.46, 127.91, 122.75, 121.08, 120.87, 110.28; 21.10 ppm. Anal. (C21H17N3O4S2) C, H, N.
3E)-4-methoxy-N-((3-((1-methyl-1H-imidazol-2-yl)methylene)-2-oxoindolin-5-yl)carbamoyl)benzenesulfonamide (9b). (367 mg, 0.84 mmol, 84% yield): mp 225-227°C. 1H NMR (DMSO-d6): δ 2.43 (s, 3H, Me); 3.90 (s, 3H, NMe); 6.79 (d, 1H, J = 8.3 Hz, Ar); 7.25 (d, 2H, J = 8.8 Hz, Ar); 7.37 (s, 2H, Ar); 7.48 (d, 2H, J = 8.8 Hz, Ar); 7.52 (s, 1H, H-vinyl); 7.68 (dd, 1H, J = 2.0, 8.3 Hz, Ar); 8.49 (br s, 1H); 8.64 (br s, 1H); 9.15 (d, 1H, J = 2.0 Hz, Ar); 10.45 (br s, 1H) ppm. 13C NMR (DMSO-d6):
146 δ 169.27, 153.45, 139.86, 139.14, 137.80, 136.86, 135.66, 130.56, 129.42, 128.77, 128.52, 127.70, 122.04, 121.95, 120.78, 119.57, 115.97; 35.25, 21.20 ppm. Anal. (C21H19N5O4S) C, H, N.
(3Z)-N-((3-((1H-imidazol-5-yl)methylene)-2-oxoindolin-5-yl)carbamoyl)-4-methylbenzenesulfonamide (9c). (339 mg, 0.80 mmol, 80% yield): mp 219-221°C. 1
H NMR (DMSO-d6): δ 2.46 (s, 3H, Me); 6.88 (d, 1H, J = 8.2 Hz, Ar); 7.12 (dd, 1H, J = 1.2, 8.2 Hz, Ar); 7.42 (d, 2H, J = 8.4 Hz, Ar); 7.54 (d, 2H, J = 8.4 Hz, Ar); 7.72 (s, 1H, H-vinyl); 7.81 (s, 1H, Ar); 7.85 (d, 1H, J = 1.2 Hz, Ar); 8.04 (s, 1H, Ar); 8.54 (br s, 1H); 8.70 (br s, 1H); 10.90 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 170.58, 152.08, 139.60, 137.91, 137.42, 136.90, 136.27, 135.54, 130.41, 129.03 128.93, 128.07, 125.49, 122.01, 120.58, 119.35, 112.15; 21.03 ppm. Anal. (C20H17N5O4S) C, H, N.
5-amino-1,3-dihydro-2H-indol-2-one (10). The commercial 5-nitro-1,3-dihydro-2H-indol-2-one (1.00 g, 5.61 mmol) was hydrogenated in EtOH (70 mL) in the presence of 10% Pd-C (315 mg, 2.97 mmol) for 4 h. Then the catalyst was filtered off, and the solution was evaporated, to give 11 (698 mg, 4.71 mmol, 84% yield). 1H NMR (CD3OD-d4): δ 3.43 (s, 2H, CH2); 6.63-6.65 (m, 2H, Ar); 6.73-6.74 (m, 1H, Ar) ppm.
2´-chloro-N-(2-oxo-2,3-dihydro-1H-indol-5-yl)acetamide (11). To a stirred solution of 5-amino-2-oxindole 10 (122 mg, 0.82 mmol) in acetone (5 mL) and DMF (1.2 mL), cooled to 0°C, was added dropwise 2-chloroacetyl chloride (93 mg, 0.82 mmol). The reaction mixture was stirred at room temperature for additional 3 h, then the solvent was evaporated affording compound 11 (180 mg, 0.80 mmol, 98% yield). 1
H NMR (DMSO-d6): δ 3.47 (s, 2H, CH2); 4.21 (s, 2H, CH2Cl); 6.73-6.78 (m, 1H, Ar); 7.33 (d, 1H, J = 8.2 Hz, Ar); 7.49 (s, 1H, Ar); 10.15 (br s, 1H, NH); 10.33 (br s, 1H, NH) ppm.13C NMR (DMSO-d6): δ 175.92, 163.90, 139.70, 132.22, 125.95, 118.82, 116.61, 108.73, 43.36, 35.90 ppm. Anal. (C10H9ClN2O2) C, H, N.
147 General Procedure To Synthesize Compounds 12 and 13. A solution of K2CO3 (1.19 g, 8.61 mmol) in acetonitrile (8 mL), heated at 80°C, was treated with a solution of compound 11 (879 mg, 3.91 mmol) in DMF (1.3 mL) and with a solution of appropriate amine (3.91 mmol) in acetonitrile (2 mL). The suspension was stirred at 80°C for 12 h, then the potassium carbonate was filtered off and the solvent removed. The residue was diluted with CH2Cl2 and washed with water and brine. The organic layer was dried and concentrated.
2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)-N-(2-oxo-2,3-dihydro-1H-indol-5-yl) acetamide (12). The crude product was purified by crystallization from AcOEt/ hexane, affording compound 12 (538 mg, 1.41 mmol, 36 % yield) as a yellow solid. 1H NMR (CDCl3): δ 2.87-2.96 (m, 4H, CH2); 3.32 (s, 2H, CH2); 3.52 (s, 2H, CH2); 3.75 (s, 2H, CH2); 3.84 (s, 3H, OMe); 3.87 (s, 3H, OMe); 6.53 (s, 1H, Ar); 6.64 (s, 1H, Ar); 6.79 (d, 1H, J = 8.2 Hz, Ar); 7.25-7.30 (m, 1H, Ar); 7.62 (s, 1H, Ar); 8.10 (br s, 1H, NH); 9.19 (br s, 1H, NH) ppm. 13C NMR (CDCl3): δ 177.05, 168.44, 148.23, 147.86, 139.07, 132.82, 126.16, 125.92, 125.59, 119.55, 117.45, 111.96, 109.83, 109.66, 61.94, 56.24, 55.93, 51.76, 36.56, 29.05 ppm. Anal. (C21H23N3O4) C, H, N.
2-((3,4-dimethoxybenzyl)amino)-N-(2-oxoindolin-5-yl)acetamide (13). The crude product was purified by crystallization from AcOEt/ hexane, affording compound 13 (462 mg, 1.30 mmol, 33 % yield)as a yellow solid. 1H NMR (CDCl3): δ 3.52 (s, 2H, CH2); 3.78 (s, 2H, CH2); 3.81 (s, 2H, CH2); 3.86 (s, 3H, OMe); 3.88 (s, 3H, OMe); 6.76-7.93 (m, 4H, Ar); 7.22 (d, 1H, J = 8.6 Hz, Ar); 7.58 (s, 1H, Ar); 8.02 (br s, 1H, NH); 9.18 (br s, 1H, NH) ppm. 13C NMR (CDCl3): δ 178.05, 169.00, 149.13, 148.86, 139.07, 132.92, 129.16, 125.05, 123.00, 122.55, 120.45, 111.93, 110.66, 56.94, 56.23, 53.70, 51.80, 36.56 ppm. Anal. (C19H21N3O4) C, H, N.
General Procedure To Synthesize Compounds 14 and 15.To a stirred solution of 5-amino-2-oxindole 10 (148 mg, 1 mmol) in water (10 mL) was added MsCl or TsCl (1.2 mmol) at room temperature and stirring was continued until the reaction was complete. Then the solution was evaporated to dryness. The residual material was
148
diluted with MeOH and the solid product was separated out from the mixture. It was filtered, washed with diethyl ether, and air dried.
N-(2-oxoindolin-5-yl)methanesulfonamide (14). (86 mg, 0.38 mmol, 38 % yield):
mp 196-198°C. 1H NMR (DMSO-d6): δ 2.88 (s, 3H, Me); 3.47 (s, 2H, CH2); 6.77 (d, 1H, J = 8.2 Hz, Ar); 7.04 (d, 1H, J = 8.2 Hz, Ar); 7.09 (s, 1H, Ar); 9.37 (br s, 1H); 10.37 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.74, 135.61, 133.17, 131.18, 126.85, 123.51, 114.84, 112.82, 40.73, 37.41 ppm. Anal. (C9H10N2O3S) C, H, N. 4-methyl-N-(2-oxoindolin-5-yl)benzenesulfonamide (15). (109 mg, 0.36 mmol, 36 % yield): mp 210-212°C. 1H NMR (DMSO-d6): δ 2.37 (s, 3H, Me); 3.44 (s, 2H, CH2); 6.65 (d, 1H, J = 8.2 Hz, Ar); 6.84 (d, 1H, J = 8.2 Hz, Ar); 6.95 (s, 1H, Ar); 7.32 (d, 1H, J = 8.1 Hz, Ar); 7.58 (d, 1H, J = 8.1 Hz, Ar); 9.89 (br s, 1H); 10.30 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 170.06, 141.81, 137.46, 135.73, 133.61, 128.85, 128.11, 127.25, 121.99, 117.19, 110.54, 36.42, 21.87 ppm. Anal. (C15H14N2O3S) C, H, N.
Indolin-2-one (16). Iron chips (1.6 g, 29.40 mmol) was added in one portion to commercial 2-nitrophenylacetic acid (2.0 g, 11.04 mmol) in 25 mL of glacial acetic acid. The resulting mixture was heated to 100 °C for 4 h, then concentrated to dryness, sonicated in ethyl acetate and filtered to remove the insolubles. The filtrate was washed twice with 1 N hydrochloric acid, water, brine, dried over anhydrous sodium sulfate and concentrated, affording compound 16. (1.40 g, 10.49 mmol, 95% yield): mp 92-94C. 1H NMR (CDCl3): δ 3.56 (s, 2H, CH2); 6.90 (d, 1H, J = 7.7 Hz, Ar); 6.98-7.06 (m, 1H, Ar); 7.19-7.26 (m, 2H, Ar); 8.95 (br s, 1H) ppm. 13C NMR (CDCl3): δ 179.25, 143.38, 128.54, 125.98, 125.12, 122.93, 110.66; 37.10 ppm. Anal. (C8H7NO2) C, H, N.
2-oxoindoline-5-sulfonyl chloride (17). Chlorosulfonic acid (4.38 g, 37.61 mmoli) was slowly added to indolin-2-one (13.3 g, 100 mmol). The reaction temperature was maintained below 30 °C during the addition. After the addition, the reaction mixture was stirred at room temperature for 1.5 h, and then at 68 °C for 1 h, cooled, and poured into water. The precipitate was washed with water and dried in a vacuum oven to give compound 17, which was used without further purification. (3.02 g,
149 13.03 mmol, 98% yield): mp 180-185°C. 1H NMR (DMSO-d6): δ 3.46 (s, 2H, CH2); 6.74 (d, 1H, J = 7.7 Hz, Ar); 7.42-7.46 (m, 2H, Ar); 10.48 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 175.89, 143.68, 139.71, 124.55, 121.32, 107.39; 35.16 ppm. Anal. (C8H6ClNO3S) C, H, N.
General Procedure To Synthesize Compounds 18 and 19. To a stirred solution of 2-oxoindoline-5-sulfonyl chloride 17 (530 mg, 2.29 mmol) in ethanol (10 mL) was added a solution of appropriate benzylamine (2.50 mmol) in ethanol (2 mL). The suspension was stirred at room temperature for 4 h, then the mixture was concentrated and the solid collected by vacuum filtration to give 18 or 19.
N-(4-methoxybenzyl)-2-oxoindoline-5-sulfonamide (18). The residual material was
purified by crystallization from MeOH, affording 18 (624 mg, 1.88 mmol, 82% yield): mp 218-220C. 1H NMR (DMSO-d6): δ 3.54 (s, 2H, CH2); 3.70 (s, 3H, OMe); 3.86 (d, 1H, J = 6.2 Hz, CH2); 6.81 (d, 2H, J = 8.2 Hz, Ar); 6.92 (d, 1H, J = 8.2 Hz, Ar); 7.12 (d, 2H, J = 8.2 Hz, Ar); 7.54 (s, 1H, Ar); 7.61 (d, 1H, J = 8.2 Hz, Ar); 7.89 (br t, 1H, J = 6.2 Hz); 10.74 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.92, 158.60, 145.13, 142.75, 133.50, 129.47, 128.65, 127.67, 126.15, 113.89, 109.00; 54.90; 45.71; 35.10 ppm. Anal. (C16H16N2O4S) C, H, N.
N-(3,4-dimethoxybenzyl)-2-oxoindoline-5-sulfonamide (19). The residual material
was purified by crystallization from MeOH, affording 19 (664 mg, 1.83 mmol, 80% yield): mp 227-232C. 1H NMR (DMSO-d6): δ 3.52 (s, 2H, CH2); 3.65 (s, 3H, OMe); 3.69 (s, 3H, OMe); 3.88 (d, 1H, J = 6.2 Hz, CH2); 6.68-6.82 (m, 3H, Ar); 6.90 (d, 1H, J = 8.1 Hz, Ar); 7.51 (s, 1H, Ar); 7.60 (d, 1H, J = 8.1 Hz, Ar); 7.89 (br t, 1H, J = 6.2 Hz); 10.76 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.20, 148.35, 147.75, 143.02, 134.39, 133.50, 128.04, 127.67, 126.08, 122.41, 111.89, 111.31, 109.46; 55.49; 55.20; 46.10; 34.10 ppm. Anal. (C17H18N2O5S) C, H, N.
1-(4-(methylthio)phenyl)-3-(2-oxoindolin-5-yl)urea (20). To a solution of 5-amino-1,3-dihydro-2H-indol-2-one 10 (148 mg, 1.0 mmol) in 10 mL dichloromethane/methanol (90:10), 1-isocyanato-4-(methylsulfanyl)benzene (182 mg, 1.1 mmol) was added slowly at 10 °C. The mixture was stirred at the room
150 temperature for 4 h. Solid product 20 was separated out from the mixture. It was filtered, washed with dichloromethane, and air dried. (330 mg, 0.96 mmol, 87% yield): mp 212-214C. 1H NMR (DMSO-d6): δ 2.43 (s, 3H, SMe); 3.52 (s, 2H, CH2); 6.72 (d, 1H, J = 8.2 Hz, Ar); 7.12-7.23 (m, 3H, Ar); 7.37 (s, 1H, Ar); 7.41 (d, 2H, J = 8.8 Hz, Ar); 8.48 (br s, 1H); 8.62 (br s, 1H); 10.24 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.93, 158.66, 145.19, 142.75, 133.57, 129.87, 128.45, 127.67, 126.25, 113.33, 109.09; 108.90; 36.71; 15.10 ppm. Anal. (C16H15N3O2S) C, H, N.
General Procedure To Synthesize Compounds 21a-c.
A catalytic amount of piperidine (0.85mg, 0.01 mmol) was added into a solution of commercial 5-nitro-1,3-dihydro-2H-indol-2-one (178mg, 1 mmol) and the appropriate carboxaldehyde (1.1 mmol) in ethanol (5 mL). The mixture was heated at reflux for 12 h. After cooling at room temperature, the crude product precipitated from the solution and was collected by filtration, washed with ethanol, and air dried. (3Z)-5-nitro-3-(thiophen-2-ylmethylene)indolin-2-one (21a). (223 mg, 0.82 mmol, 82% yield): mp 215-217°C. 1H NMR (DMSO-d6): δ 7.02 (d, 1H, J = 8.6 Hz, Ar); 7.27-7.31 (m, 1H, Ar); 7.99-8.03 (m, 2H, Ar); 8.15 (d, 1H, J = 8.6 Hz, Ar); 8.57 (s, 1H, H-vinyl); 8.67 (s, 1H, Ar); 11.31 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 167.40, 145.60, 141.85, 139.15, 137.00, 136.04, 131.85, 127.81, 125.12, 124.57, 119.18, 115.10, 109.33ppm. Anal. (C13H8N2O3S) C, H, N.
(3E)-3-((1-methyl-1H-imidazol-2-yl)methylene)-5-nitroindolin-2-one (21b). (218 mg, 0.81 mmol, 81% yield): mp 210-212°C. 1H NMR (DMSO-d6): δ 3.94 (s, 3H, NMe); 7.04 (d, 1H, J = 8.7 Hz, Ar); 7.49 (s, 1H, Ar); 7.51 (s, 1H, H-vinyl); 7.61 (s, 1H, Ar); 8.22 (dd, 1H, J =2.4, 8.7 Hz, Ar); 10.40 (d, 1H, J = 2.4 Hz, Ar); 11.30 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.49, 147.95, 142.07, 141.87, 130.98, 126.12, 122.89, 122.46, 121.85, 120.33, 109.93, 33.30 ppm. Anal. (C13H10N4O3) C, H, N (3Z)-3-((1H-imidazol-5-yl)methylene)-5-nitroindolin-2-one (21c). (205 mg, 0.80 mmol, 80% yield): mp 214-216°C. 1H NMR (DMSO-d6): δ 7.05 (d, 1H, J = 8.3 Hz, Ar); 7.15-7.16 (m, 1H, Ar); 7.57 (s, 1H, H-vinyl); 7.68 (s, 1H, Ar); 8.10 (s, 1H, Ar); 8.40 (s, 1H, Ar); 8.62 (s, 1H, Ar); 11.27 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ
151 168.40, 145.85, 142.15, 136.90, 135.08, 129.85, 127.80, 125.15, 123.57, 122.18, 120.10, 118.33 ppm. Anal. (C12H8N4O3) C, H, N.
General Procedure To Synthesize Compounds 22a-c. To a solution of appropriate compound 21a-c (4 mmol) in MeOH (50 mL) was added carbon (0.216 g) and FeCl3. The reaction mixture was warmed to 60 °C, then hydrazine monohydrate was added dropwise (3.22 mL, 66 mmol). The mixture was refluxed overnight then filtered on a celite pad. Methanol was concentrated, the residue diluted with CHCl3, dried over Na2SO4 and evaporated.
(3Z)-5-amino-3-(thiophen-2-ylmethylene)indolin-2-one (22a). (580 mg, 2.40 mmol, 60% yield): mp 190-192°C. 1H NMR (DMSO-d6): δ 6.63-6.65 (m, 2H, Ar); 7.07-7.10 (m, 1H, Ar); 7.12 (s, 1H, Ar); 7.58.-7.64 (m, 2H, Ar); 8.05 (s, 1H, H-vinyl); 10.20 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.50, 144.92, 140.85, 139.15, 138.07, 136.04, 131.75, 127.71, 124.12, 123.47, 119.10, 114.10, 109.15 ppm. Anal. (C13H10N2OS) C, H, N.
(3E)-5-amino-3- ((1-methyl-1H-imidazol-2-yl) methylene) indolin-2-one (22b). (625 mg, 2.60 mmol, 65% yield): mp 200-202°C. 1H NMR (DMSO-d6): δ 3.96 (s, 3H, NMe); 7.04 (d, 1H, J = 8.4 Hz, Ar); 7.51 (s, 1H, Ar); 7.63 (s, 1H, H-vinyl); 7.71 (s, 1H, Ar); 7.92 (dd, 1H, J =1.9, 8.4 Hz, Ar); 10.39 (d, 1H, J = 2.4 Hz, Ar); 11.30 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 168.18, 147.82, 142.07, 141.87, 130.98, 126.12, 122.89, 122.46, 121.85, 120.73, 108.95, 33.29 ppm. Anal. (C13H12N4O) C, H, N
(3Z)-3-((1H-imidazol-5-yl)methylene)-5-aminoindolin-2-one (22c). (533 mg, 2.36 mmol, 59% yield): mp 194-196°C. 1H NMR (DMSO-d6): δ 6.95 (d, 1H, J = 8.2 Hz, Ar); 7.13-7.15 (m, 1H, Ar); 7.27 (s, 1H, H-vinyl); 7.42 (s, 1H, Ar); 7.97 (s, 1H, Ar); 8.04 (s, 1H, Ar); 8.42 (s, 1H, Ar); 10.97 (br s, 1H) ppm. 13C NMR (DMSO-d6): δ 169.01, 146.50, 142.15, 136.65, 134.89, 129.72, 127.80, 124.95, 123.72, 123.01, 120.10, 119.33 ppm. Anal. (C12H10N4O) C, H, N.
152 6.1.1. Elemental Analysis Compound Formula Calculated % Found % C H N C H N 1a C26H25N3O4S 65.67 5.30 8.84 65.50 4.98 8.89 1b C26H27N5O4 65.95 5.75 14.79 66.11 5.50 14.59 1c C25H25N5O4 65.35 5.48 15.24 65.00 5.39 14.99 1d C27H26N4O4 68.92 5.57 11.91 68.99 5.73 11.90 2 C24H23N3O4S 64.13 5.16 9.35 64.25 5.27 9.58 3a C14H12N2O3S2 52.48 3.78 8.74 52.19 3.51 8.63 3b C14H14N4O3S 52.82 4.43 17.60 52.96 4.75 17.86 3c C13H12N4O3S 51.31 3.97 18.41 51.63 4.13 18.68 4a C20H16N2O3S2 60.59 4.07 7.07 60.75 4.16 7.24 4b C20H18N4O3S 60.90 4.60 14.20 61.03 4.91 14.43 4c C19H16N4O3S 59.99 4.24 14.73 60.12 4.46 17.87 5a C21H18N2O4S2 59.14 4.25 6.57 59.10 4.53 6.74 5b C21H20N4O4S 59.42 4.75 13.20 59.58 5.01 13.46 5c C20H18N4O4S 58.53 4.42 13.65 58.33 4.15 13.37 6a C22H20O5N2S2 57.88 4.42 6.14 58.10 4.40 6.08 6b C22H22O5N4S 58.14 4.88 12.33 58.02 5.16 12.06 6c C21H20O5N4S 57.26 4.58 12.72 57.21 4.73 12.74 7a C21H17N3O2S2 61.89 4.20 10.31 62.08 4.36 10.54 7b C21H19N5O2S 62.21 4.72 17.27 62.03 4.65 17.18 7c C20H17N5O2S 61.37 4.38 17.89 61.48 4.56 17.98 8a C21H17N3O4S2 57.39 3.90 9.56 57.51 3.94 9.68 8b C21H19N5O4S 57.66 4.38 16.01 57.94 4.46 16.32
153 8c C20H17N5O4S 56.73 4.05 16.54 57.00 4.37 16.59 9a C21H17N3O4S2 57.39 3.90 9.56 57.58 3.92 9.37 9b C21H19N5O4S 57.66 4.38 16.01 57.40 4.62 16.36 9c C20H17N5O4S 56.73 4.05 16.54 56.52 3.97 16.09 10 C8H8N2O 64.85 5.44 18.91 65.01 5.73 19.08 11 C10H9ClN2O2 53.47 4.04 12.47 53.51 4.32 12.66 12 C21H23N3O4 66.13 6.08 11.02 66.30 5.89 10.95 13 C19H21N3O4 64.21 5.96 11.82 63.97 5.59 11.59 14 C9H10N2O3S 47.78 4.45 12.38 47.91 4.68 12.51 15 C15H14N2O3S 59.59 4.67 9.27 59.38 4.46 9.03 16 C8H7NO2 72.16 5.30 10.52 72.32 5.35 10.48 17 C8H6ClNO3S 41.48 2.61 6.05 41.55 2.48 6.01 18 C16H16N2O4S 57.82 4.85 8.43 57.54 4.70 8.53 19 C17H18 N2O5S 56.43 5.01 7.73 56.26 5.32 7.80 20 C16H15N3O2S 61.32 4.82 13.41 61.05 4.67 13.23 21a C13H8N2O3S 57.35 2.96 10.29 57.02 2.85 10.11 21b C13H10N4O3 57.78 3.73 20.73 58.06 3.98 20.79 21c C12H8N4O3 56.25 3.15 21.87 56.20 3.12 21.76 22a C13H10N2OS 64.44 4.16 11.56 64.81 4.32 11.84 22b C13H12N4O 64.99 5.03 23.32 65.05 5.09 23.41 22c C12H10N4O 63.71 4.46 24.76 63.40 4.06 24.14
154
6.2 Biological Methods
6.2.1. Drugs and chemicals
Gemcitabine was generously supplied by Eli Lilly (Indianapolis, IN, USA). Gemcitabine was dissolved in sterile distilled water and the compounds 1a-d and 10 in DMSO; the compounds were then stored at 4 °C and −20 °C, respectively, and diluted in culture medium immediately before use. RPMI and DMEM media, foetal bovine serum, horse serum, l-glutamine (2 mM), penicillin (50 IU/ml) and streptomycin (50 g/ml) were from Life Technologies, Inc. (Gaithersburg, MD, USA). All other chemicals were from Sigma (St. Louis, MO, USA).
6.2.2. Cell lines
Human lung cancer cells A549 (adenocarcinoma), and HCC827 (adenocarcinomas) were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were grown in RPMI (A549 and HCC827) with 10% foetal bovine serum, 1% glutamine and 1% penicillin–streptomycin. Cells were cultured in 75-cm2 flasks (Costar, Cambridge, MA, USA), at 37 °C in 5% CO2 and 95% air, and were harvested with EDTA when they were in logarithmic growth.
6.2.3. Assay of cytotoxicity
Cells will be plated in 24-well sterile plastic plates (Sarstedt) at 2 X 104 cells/well and will be allowed to attach for 24 hours. Cells will be treated with 1) perifosine (0.01–100 µM) for 72 hours2) OXDs derivatives (0.01–100 µM) for 72 hours, 3) perifosine for 1h, followed by a 24-h washout in drug-free medium and then temozolomide or radiotherapy for 24h., At the end of drug exposure, the cell growth inhibition will be assessed by counting cells. Cytotoxicity will be expressed as the percentage of cells surviving relative to untreated cultures; the 50% inhibitory concentration of cell growth (IC50) will be calculated by non-linear least squares curve fitting.
155 6.2.4. Cell-cycle analysis
After treatment with the new compounds synthesised, perifosine and combination with temozolomide at their IC50 levels followed by a 24h washout, cells will be harvested with tripsin/EDTA and washed with PBS. Cytofluorimetry will be performed using a FACScan (BD Biosciences, San Jose, CA) and data analysis will be carried out with CELLQuest software, whereas the cell-cycle distribution will be determined using ModFit software (Verity Software House, Topsham, ME).
6.2.5. Analysis of apoptosis
Cells will be treated with perifosine, the OXDs derivatives and their combination with chemotherapeutics (i.e. temozolomide) at their IC50 levels, and at the end of incubation, they will be washedtwice with PBS and fixed in 4% buffered p-formaldehyde for15 minutes. Cells will be spotted on glass slides and examinedby fluorescence microscopy (Leica, Wetzlar, Germany, DE). A totalof 200 cells from randomly selected microscopic fields will be counted and the percentage of cells displaying chromatin condensation and nuclear fragmentation relative to the total number of countedcells (apoptotic index) will be calculated. Apoptosis induced by the compounds synthesised, the reference drug, and their combination with temozolomide will be also study by flow cytometry.
6.2.6. Akt Phosphorylation
The phosphorylation of threonine and serine residues at position 308 and 473, respectively, of aminoacid chain will be analysed using p-Thr308 and p-Ser473 specific ELISA and normalized to total Akt content (BioSource International, Camarillo, CA). To calculate p-Thr308 Akt, p-Ser473 Akt and full length Akt concentrations, a standard curve method will be used. Values of Thr308 Akt and p-Ser473 Akt calculated from the standard curve will be then normalized for total Akt and protein content, as measured with the Lowry reagent (Sigma Chemical Co. St
156 Louis, USA). Western blot analysis of phosphorylated downstream effectors (Bad, GSK3, Foxo and Caspase-9) will be performed.
6.2.7. Statistical analysis
All experiments were performed in triplicate and repeated three times. Data were expressed as mean values ± S.D. and analyzed by Student’s t test or ANOVA as appropriate; the level of significance was set at P < 0.05.
6.2.8. Kinase-specific assay
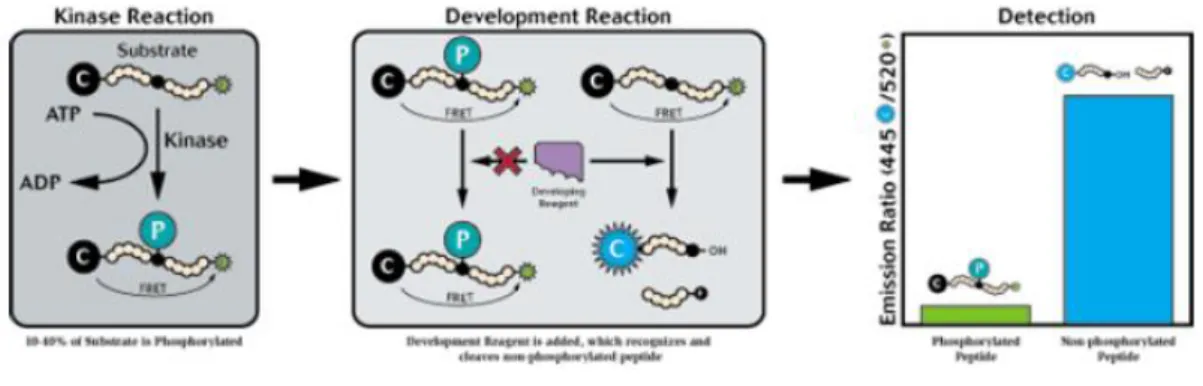
The Z´-LYTE® biochemical assay employs a fluorescence-based, coupled-enzyme format and is based on the differential sensitivity of phosphorylated and non-phosphorylated peptides to proteolytic cleavage (Figure 6.1). The peptide substrate is labeled with two fluorophores—one at each end—that make up a FRET pair.
Figure 6.1: Z'-LYTE® Illustration
In the primary reaction, the kinase transfers the gamma-phosphate of ATP to a single serine or threonine residue in a synthetic FRET-peptide. In the secondary reaction, a site-specific protease recognizes and cleaves non-phosphorylated FRET-peptides. Phosphorylation of FRET-peptides suppresses cleavage by the Development Reagent. Cleavage disrupts FRET between the donor (i.e., coumarin) and acceptor (i.e., fluorescein) fluorophores on the FRET-peptide, whereas uncleaved, phosphorylated FRET-peptides maintain FRET. A ratiometric method, which calculates the ratio (the Emission Ratio) of donor emission to acceptor emission after excitation of the donor fluorophore at 400 nm, is used to quantitate reaction progress, as shown in the equation below.
157
A significant benefit of this ratiometric method for quantitating reaction progress is the elimination of wellto- well variations in FRET-peptide concentration and signal intensities. As a result, the assay yields very high Z´-factor values (>0.7) at a low percent phosphorylation.
Both cleaved and uncleaved FRET-peptides contribute to the fluorescence signals and therefore to the Emission Ratio. The extent of phosphorylation of the FRET-peptide can be calculated from the Emission Ratio. The Emission Ratio will remain low if the FRET-peptide is phosphorylated (i.e., no kinase inhibition) and will be high if the FRET-peptide is non-phosphorylated (i.e., kinase inhibition).
- AKT1 (PKB alpha)
The 2X AKT1 (PKB alpha) / Ser/Thr 06 mixture is prepared in 50 mM HEPES pH 7.5, 0.01% BRIJ-35, 10 mM MgCl2, 1 mM EGTA. The final 10 μL kinase reaction consists of 1.15 - 25 ng AKT1 (PKB alpha) and 2 μM Ser/Thr 06 in 50 mM HEPES pH 7.5, 0.01% BRIJ-35, 10 mM MgCl2, 1 mM EGTA. After the 1 hour kinase reaction incubation, 5 μL of a 1:2048 dilution of Development Reagent A is added. - PDK1 Direct
The 2X PDK1 Direct / Ser/Thr 07 mixture is prepared in 50 mM Tris pH 8.5, 0.01% BRIJ-35, 10 mM MgCl2, 1 mM EGTA, 0.02% NaN3. The final 10 μL kinase reaction consists of 7.36 - 38.7 ng PDK1 Direct and 2 μM Ser/Thr 07 in 50 mM Tris / HEPES pH 8.0, 0.01% BRIJ-35, 10 mM MgCl2, 1 mM EGTA, 0.01% NaN3. After the 1 hour kinase reaction incubation, 5 μL of a 1:32768 dilution of development reagent A is added.