ABSTRACT
In endothelial cells, Erythropoietin receptors (EPO-R) mediate the protective, proliferative and angiogenic effects of EPO and its analogues, which act on EPO-R as receptor agonists. Since hormonal receptors are known to undergo functional changes upon chronic exposure to their agonists, and since erythropoiesis stimulating agents (ESAs) are used for the long-term treatment of anemic states, it is crucial to dissect how the responsiveness of these receptors is regulated at vascular level after prolonged exposure to ESAs.
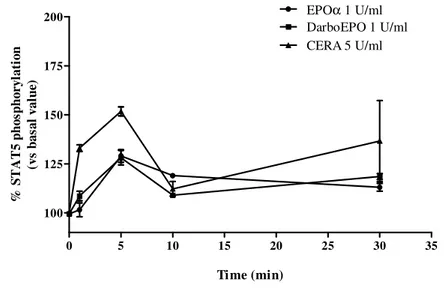
In the present research, we investigated EPO-R desensitization/resensitization in human umbilical vein endothelial cells (HUVEC) upon exposure to three ESAs with different pharmacokinetic profiles: epoetin alpha (EPOα), darbepoetin alpha (DarboEPO) and continuous EPO-R activator (CERA). All these agonists induced the activation of the transcription factor STAT5, the intracellular pathway associated with EPO-R, with monophasic or biphasic kinetics for EPOα/DarboEPO and CERA, respectively. All epoetins induced EPO-R desensitization with a kinetics faster for CERA, with respect to EPOα and DarboEPO. However, recovery of receptor responsiveness was strictly dependent on the type of epoetin, the agonist concentration and the time of exposure to agonist (desensitization time). EPO-R resensitization occurred with faster kinetics after exposure to low epoetins concentrations for a short desensitization time. When the highest concentration of agonists was tested, recovery of receptor responsiveness was faster with CERA with respect to EPOα, and was completely absent with DarboEPO. Our results demonstrate the three ESAs regulate EPO-R resensitization in a highly different way, demonstrating that the type of molecule and the length of EPO-R stimulation are crucial factors in the control of EPO-R function in endothelial cells.
INDEX
Chapter 1: Erythropoietin Receptor. 1
1.1. Subunit structure and activation of EPO Receptor. 2 1.2. Mechanism of action and intracellular signalling of EPO Receptor . 4
1.3. EPO Receptor in endothelial cells. 13
1.4. Inactivation mechanisms of EPO Receptor. 15
1.5. EPO and EPO derivatives. 19
1.6. Therapeutic uses of EPO derivatives. 23
Aims. 31
Chapter 2: Experimental section.
2.1. Materials. 33
2.2. Drugs. 33
2.3. Cell line and growth conditions. 34
2.4. EPO-R Immunoblot Analysis 34
2.5. STAT5 phosphorylation assay. 35
2.6. Epoetin-mediated STAT5 phosphorylation:
Concentration- and time- dependence. 36
2.7. EPO-R desensitization. 36
2.8. EPO-R resensitisation. 37
Chapter 3: Results.
3.1. EPO-R phosphorylation 38
3.2. Experimental condition optimization. 39
3.3. Epoetin-mediated STAT5 phosphorylation:
concentration- and time- dependence. 40
3.4. EPO-R desensitization. 42
3.5. EPO-R resensitization. 44
Chapter 4: Discussion. 49
CHAPTER 1
Erythropoietin Receptor.
Erythropoietin (EPO) is a 34-KDa glycoprotein hormone (Figure 1), that acts on erythroid stem cells of the bone marrow to stimulate proliferation and differentiation. A DNA encoding EPO has been isolated (Jacobs K et al., 1985), and recombinant EPO with oligosaccharide moieties identical to the natural material is available (Sasaki H et al., 1988).
Figure 1. EPO structure. (=: disulphide bond; : Aminoacid; : Mannose; :Galactose; : N-acetylglucosamine; : Sialin acid; : Fucose; : N-acetylgalactosamine.
EPO is synthesized and released by the kidney in the adult and in the liver in the fetus. It circulates to the bone marrow where it stimulates resident erythroid progenitors via a specific receptor (Erslev AJ, 1987). EPO causes a rapid rise in hematocrit in 7-10 days and, as a pharmacologic agent, has clearly demonstrated efficacy in anemic states such as kidney failure (Eschbach JW et al., 1987 and 1989).
The Erythropoietin receptor (EPO-R) is encoded by EPOR gene and it is expressed as a protein that ranges from 66 to 78 KDa. The EPO-R is a 476 amino acid protein containing a transmembrane segment that divides the molecule into extracellular and cytosolic domains of equal length. It belongs to the family of cytokine receptors, as indicated by the lack of endogenous kinase activity and by small regions of homology in its extracellular domain. These homologous regions include four conserved cysteines forming two disulfide bonds, as well as the WSXWS motif near the membrane-spanning segment (Cohen J et al., 2002).
Through specific binding to EPO-R (D’Andrea et al., 1989 a,b), EPO triggers a chain of intracellular signaling events, including activation of the receptor-associated
Signal Transducer and Activator of Transcription 5 (STAT5), leading to progenitor cell proliferation and differentiation (Watowich et al., 1996).
Several studies suggest that the functions of EPO and EPO-R are not strictly limited to erythroid or hematopoietic lineages. For instance, EPO-R expression has been detected in umbilical cord and placental endothelial cell lines, and EPO was capable of stimulating endothelial cell proliferation in vitro (Anagnostou et al., 1990, 1994). In addition, EPO was known to induce a pro-angiogenic phenotype in cultured endothelial cells and stimulated neo-vascularization in the chick chorioallantoic membrane (Ribatti et al., 1999). Moreover, EPO plays an important role in cardiac morphogenesis (Wu et al., 1999), in myoblast proliferation (Ogilvie et al., 2000), and in neurogenesis (Yu et al., 2002).
Despite the availability of recombinant EPO, little is known regarding the interaction of EPO and EPO-R or the physiologic mechanisms by which EPO causes cells to undergo proliferation or differentiation. This is largely due to the lack of adequate quantities of EPO-R for in-depth biochemical study. Only small numbers of surface EPO-R are present on normal erythroblasts and erythro-leukemia cells. Further advances were obtained in understanding of EPO-R physiology, using the cloning of the EPO-R cDNA (D'Andrea AD and Zon L, 1990). Moreover, despite these advances, a detailed analysis of EPO and EPO-R expression patterns and their in vivo functions in the non-erythroid lineages are currently lacking.
1.1 Subunit structure and activation of EPO Receptor.
Before the cloning of the EPO-R cDNA, investigators have used radiolabeled EPO to demonstrate specific binding to cells derived from the erythroid lineage. These cells include normal erythroid progenitors, virally transformed spleen cells (i.e., Friend cells), murine and human erythroleukemia cells, and cells from human fetal liver. Since there is 80% protein homology between the murine and human EPO polypeptide, most of the studies described used iodinated recombinant human EPO. A high-affinity (Kd in pM range) and a low-affinity (Kd in nM range) binding site for EPO has been demonstrated in the extracellular domain of the EPO-R (Philo JS et al., 1996; Mayeux P et al., 1991; Qiu H et al., 1998). The EPO receptor is expressed primarily on erythroid cells between the colony-forming unit erythroid (CFU-E) and the pro-normoblast stage of erythroid cell development (Sawada K et al., 1990; Wickrema A et al.,1992). Small
numbers of EPO receptors are expressed on burst-forming unit erythroid (BFU-E) (Sawada K et al., 1990). The highest number of EPO receptors is seen on the CFU-E and the pronormoblasts (Sawada K et al., 1990; Sawyer ST and Koury MJ, 1987). The number of EPO receptors per cell gradually decreases during erythroid cell differentiation, and studies have shown that the reticulocyte and mature erythrocyte do not contain EPO receptors (Sawada K et al., 1990, Wickrema A et al.,1992; Sawyer ST and Koury MJ, 1987).
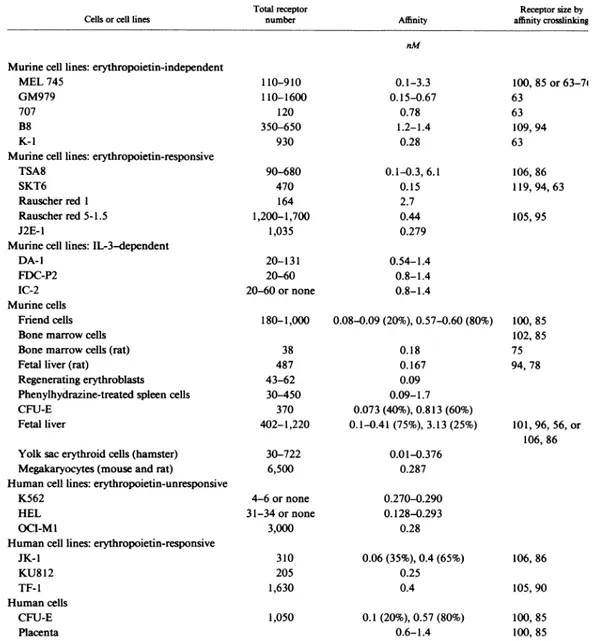
In table 1, the total number of EPO-R per cell, present on the cell surface of purified normal erythroid progenitors, is shown (D'Andrea AD and Zon L, 1990).
Table 1: The listed cells or cell lines have been examined for EPO-R expression using radiolabeled 125I-EPO in standard binding conditions. The total receptor number and affinity were determined by Scatchard analysis. If two distinct affinities were determined, both affinity constants and the relative percentages of each affinity is listed. Affinity cross-linking studies used 125I-EPO for binding and disuccinimidyl suberate for cross-linking.
Even if on certain cell lines the number can increase to about 1000 per cell, the number of EPO-R is relatively low according to other receptors for hematopoietic cytokines such as G-CSF, GM-CSF, interleukin 3 and 6. Scatchard analysis revealed that certain erythroid cells, such as MEL745 cells, express only low-affinity receptors whereas other cell lines express high- and low-affinity receptors. Of note, the higher-affinity receptor has an higher-affinity constant in the 100-200 pM range and therefore still represents an intimate interaction between receptor and ligand. Although functional differences between the higher- and lower-affinity EPO-R have not been determined, the two affinities may account for different cellular responses to EPO. Friend virus-infected cells respond to EPO with proliferation and differentiation, whereas MEL745 cells do not appear to respond to the hormone.
Affinity linking experiments using radiolabeled EPO reveal two cross-linked EPO-R complexes. The complexes appear either as two bands of sizes 145 and 120 KDa or as one band of 110 KDa, whose the size is the sum of the size of EPO and the size of the receptor. Different size bands testify the presence of higher- and lower-molecular-weight species. There is also disagreement on the effect of reducing agent on the cross-linking experiments. Some investigators have demonstrated one large molecular species of 250 KDa which resolves to two bands when reduced (McCaffery PJ et al., 1989), while others have not evidenced this high-molecular-weight band (Sawyer ST. 1989). A relationship between the number of bands and the presence of high- and low-affinity receptors is not evident.
1.2 Mechanism of action and intracellular signalling of EPO
Receptor.
Several studies have investigated the cellular action of the erythropoietin ligand-receptor complex. Using the intracellular calcium chelators, quin-2 and fura-2, EPO has been shown to induce a rapid increase in intracellular free calcium (Bonanou-Tzedaki SA et al., 1987; Miller BA et al., 1988). EPO has also been demonstrated to activate Mg2+, Ca2+, and Na+/K+ ATPases. Monesin, a sodium specific ionophore, potentiates erythroid growth, whereas valinomycin, a potassium specific ionophore, suppresses growth. Investigators have reported that EPO activates adenylate cyclase and increases cyclic AMP levels, independent of activation by β-adrenergic receptors
(Bonanou-Tzedaki SA et al., 1986; Setchenska MS et al. 1988). Conflicting results have been found concerning the role of guanidine nucleotide-binding proteins in EPO stimulation. Phospholipase C and phosphoinositol mechanisms do not appear to mediate the noted calcium flux. Choi et al. (Choi HS et al., 1987) have described a protein of 43 KDa which is dephosphorylated on serine residues in erythroid cell lines in response to EPO.
As discussed previously, the major difficulty encountered in studies of EPO-induced signal transduction has been the small number of surface receptors and the unavailability of a purely EPO-dependent cell line. The EPO-R, although expressed in high numbers in COS cell transfectants (D’andrea AD et al., 1989b), is not functional in these cells. The addition of EPO to these transfectants does not change the growth kinetics of the cells or induce erythroid differentiation. In contrast, transfection of the EPO-R cDNA into Ba/F3 cells does confer EPO dependence for cell growth (Li JP et al., 1990). An EPO-dependent clone of Ba/F3 cells (Ba/F3-EPO-R) has been isolated which expresses cell surface EPO-R and which has absolute dependence on EPO for growth. The availability of this homogeneous EPO-dependent cell line should greatly facilitate the study of the EPO-R structure and signalling mechanisms.
Since the EPO-R is a member of a large hematopoietic growth factor superfamily, any molecular insight into the EPO-R subunit structure, its multiple affinities for EPO, or its signaling mechanism may be generalizable to the other family members. Insight has been derived from the observation that the EPO-R binds to and is activated by the membrane glycoprotein gp55 of the Friend spleen focus-forming virus (SFFV) (Li JP et al., 1990). SFFV is a defective murine C-type retrovirus that causes a multistage erythroleukemia in mice and erythroblastosis in bone marrow cultures (Friend CJ. 1957; Wolff and Ruscetti. 1985). The SFFV gene encodes the gp55, that is located on the cell surface and within rough endoplasmic reticula (Dresler SM et al., 1978); it is essential for the induction of leukemia in vivo (Li JP et al., 1987) and the erythropoietin-independent erythroblast proliferation in vitro (Hankins and Troxler. 1980). By co-transfecting both EPO-R and the gp55 into an interleukin-3-dependent lymphocyte cell line, it was shown that the physical interaction of these two proteins gives rise to autocrine cell growth. The interaction between the EPO-R and gp55 is shown schematically in Figure 2.
Figure 2. Schematic model of the physical interaction between the EPO-R and the gp55 of the Friend spleen focus-forming virus. The EPO-R (507 amino acids) and the gp55 (409 amino acids) are type I membranespanning proteins. Protein-protein interaction may occur either in the membrane-spanning regions of the two proteins or in the extracytoplasmic domains of the two proteins. The interaction stimulates the EPO-R signalling and therefore mimicks EPO binding.
The interaction between the EPO-R and gp55 could occur at several different sites along the molecules (Fig. 2) and in several possible subcellular compartments. Through direct binding of the EPO-R, gp55 can stimulate the receptor, causing a prolonged proliferation of infected erythroid cells. The gp55 may mimic EPO itself, although there is no amino acid homology shared between these two proteins. Alternatively, gp55 may have sequences derived from the normal cellular second subunit of the EPO-R. The interaction between the EPO-R and gp55 is also observed in MEL cells (Li JP et al., 1990) and probably accounts for the absence of high-affinity binding sites on MEL cells and for the EPO unresponsiveness of these cells (D’andrea AD et al., 1989b). Evidence suggests that the interaction between the EPO-R and the gp55 occurs within the endoplasmic reticulum (Li JP et al., 1990; Yoshimura AA et al., 1990). The interaction of these two proteins is a mechanism of viral transformation leading to growth factor independence. It is possible that analogous retroviruses transform other cell types using, for instance, the IL-2 receptor or the IL-3 receptor, other members of the EPO-R superfamily.
Protein fragment complementation assays and crystallographic studies indicate that the EPO-R exists as a preformed dimer (Livuah O et al., 1999; Remy I et al., 1999). One molecule has been demonstrated to activate EPO-R by dimerization of two EPO-R (Philo JS et al., 1996). EPO binding to the receptor changes the conformation of the EPO-R, which is necessary for JAK2 activation by a mechanism of self dimerization (Kubatzky KK et al., 2001; Constantinescu JN et al., 2001) (Figure 3)
Figure 3. Schematic representation of the intracellular part of the EPO-R and the identified binding sites for signaling proteins. (Lacombe C and Mayeux P, 1999).
EPO-induced intracellular signalling occurs through a rapid tyrosine phosphorylation of several proteins even though the EPO receptor does not possess endogenous tyrosine kinase activity (Koury MJ et al., 2002). First, intracellular signalling occurs by activating the JAK2 tyrosine kinase, which is associated with the EPO-R constitutively (Witthuhn B et al., 1996). JAK2 is associated with the EPO-R in the transmembrane region (Hilton CJ, Berridge MV. 1995.). After EPO activates the receptor, eight tyrosine residues in the cytoplasmic domain of the EPO-R are phosphorylated (Miura O et al., 1991; Yoshimura A and Lodish HF. 1992). Docking sites for several intracellular proteins that have Src homology 2 (SH2) domains are provided by these phosphorylated tyrosines. Constantinescu et al. (Constantinescu SN et al., 2001) have provided evidence that a hydrophobic juxtamembrane domain in the EPO-R is required for intracellular signalling. These data suggest that phosphorylation of EPO-R, which probably provides docking sites for intracellular signalling molecules, is more important in EPO signalling than JAK2 kinase activation. However, Zang et al. (Zang H et al., 2001) reported that transgenic mice without cytoplasmic tyrosine residues have normal erythropoiesis. Von Lindern et al. (Von Lindern M et al., 2000) have challenged
the notion that EPO-R dimerization alone is sufficient to activate JAK2 tyrosine protein kinase, and they report that protein kinase C is required for the EPO-R to activate JAK2.
EPO is also well known to activate STAT1, STAT3, STAT5A, and STAT5B (Pallard C et al., 1995; Wakao H et al., 1995; Penta K and Sawyer ST. 1995; Watowich SS, et al., 2000; Ihle JN. 1995; Damen JE et al., 1995), especially in cytokine-induced signaling pathways (Ihle JN. 1995.). The precise mechanism of STAT5 activation in EPO-induced signalling pathways is somewhat controversial in that some investigators have shown a correlation between STAT5 activation and cell proliferation (Damen JE et al., 1995; Chretien S et al., 1996), whereas others have not been able to confirm this correlation (Klingmuller U et al., 1996; Quelle FW et al., 1996). Phosphorylation of SH2 in response to EPO activation has been demonstrated, which results in stimulation of erythroid cell proliferation (Tauchi T et al., 1996), whereas SH1 leads to dephosphorylation of JAK2 after EPO-R activation (Klingmuller U et al., 1995). The stem cell factor (SCF) or c-kit is also known to interact with the EPO-R, probably through phosphorylation of the EPO-R, resulting in enhanced erythroid cell differentiation and proliferation (Wu H et al., 1995). The several steps in EPO activation of the receptor are outlined in Figure 4.
The first line of evidence for the mode of activation of the EPO-R, subsequently confirmed by additional experimental strategies, was provided by the formation of an intermolecular disulfide bond in the membrane proximal region of R129C EPO-R, rendering it constitutively active (Yoshimura A et al., 1990). Structural analysis suggested that unliganded EPO-R exists as a preformed homodimer in an open scissor-like conformation (Livnah O et al., 1998) and that dimerization induced by the transmembrane domain keeps the unliganded EPO-R in an inactive state. Physiologic amounts of EPO, by binding to the receptor, can then switch the receptor to an activated state (Kubatzky KF et al., 2001). EPO-mediated receptor dimerization brings two receptor-associated JAK2 molecules into close proximity (Remy I et al., 1999). This enables them to transphosphorylate and activate each other, after prior phosphorylation of some or all of the eight Tyr residues in the intracellular domain of EPO-R. Gene targeting studies have demonstrated that JAK2 plays a pivotal role in signal transduction via cytokine receptors, which are required for definitive erythro- poiesis (Neubauer H et al., 1998). Tyr phosphorylation of STAT proteins precedes JAK2 activation and links growth factor receptors to gene transcription. Tyr phosphorylation of the EPO-R generates binding sites for SH2 domains, thus eliciting cellular responses via several signaling pathways (Wojchowski DM et al., 1999; Yoshimura A. 1998; Kubota Y et al., 2001). Binding of EPO to the receptor activates three different STAT proteins. STAT5 binds to P-Tyr 343 and P-Tyr 401 of the EPO-R (Barber DL et al., 2001) and is phosphorylated by JAK2. Tyr-phosphorylated STAT5 dissociates from the receptor and forms dimers, which are translocated to the nucleus to activate target genes. STAT5 is essential for the high rate of erythropoiesis during fetal development, and promotes the anti-apoptotic effect of EPO-R by inducing Bcl-xL expression (Socolovsky M et al., 1999), as demonstrated in STAT5 -/- mice. Activation of STAT1 and STAT3, on the other hand, occurs via P-Tyr 432 of the human EPO-R (Kirito K et al., 2002). Specificity of EPO-R STAT signaling is not essential for red blood cell development, as demonstrated by EPO- R mutagenesis targeted to STAT5 binding sites for which STAT3 binding sites have been substituted (Watowich SS et al., 2000). STAT proteins regulate various target genes (e.g., cytokine-inducible SH2 containing proteins). The range of genes activated by each STAT protein and the specific mechanism by which they are activated are important areas for research. Besides activating JAK2, EPO activates the protein Tyr kinases Lyn, Syc, and Fes. The specificity of these Tyr kinases in EPO signalling is
demonstrated by the fact that STAT1 and STAT3 are activated by Fes but not by Lyn (Kirito K et al., 2002). Lyn associates with the EPO-R and participates in EPO-mediated differentiation of erythroid progenitor cells (Tilbrook PA et al., 2001). Since Lyn -/- mice do not exhibit obvious hematologic defects, the exact role of Lyn and other src kinases in EPO signalling is not clear. It is possible that they function in fine tuning of the EPO response.
Figure 5 illustrates the downstream signal transduction pathways for the intracellular part of the EPO receptor.
EPO actives the Ras/MAP kinase pathway, which is involved with cell proliferation (Carrol MP et al. 1991; Gobert S et al., 1995; Miura Y et al., 1994). Phosphatidylinositol 3-kinase (PI3-K) and the EPO-R have been shown to involve the SH2 domains of the p85 subunit of PI3-K and the last tyrosine of the EPO-R (Damen JE et al., 1996; Damen J et al., 1995). Tyrosine phosphorylation of the adaptor protein IRS2 and its association with PI3-K se may also be involved as an alternate pathway of activation (Verdier F, et al., 1997). Shan et al. (Shan R et al., 1999) reported that JNKs/p38 MAP kinase and ERKs play distinct roles in apoptosis and survival of HCD-57 cells induced by withdrawal or addition of EPO. On the other hand, Jacobs- Helber et al. (Jacobs-Helber SM et al., 2000) reported that JNK and p38 are activated by EPO, but are not induced in apoptosis after EPO withdrawal in EPO-dependent HCD 57 cells. The work reported by Shan et al. (Shan R et al., 1999) has been retracted (Krantz SB, Zhao ZJ. 2001).
Binding of EPO to the receptor also activates the GTPase switch protein Ras; such activation is largely facilitated by two cytosolic proteins, Grb2 and Sos. An SH2 domain in the adaptor protein Grb2 binds to P-Tyr EPO-R, either directly, or indirectly via protein Tyr phosphatase SHP2 or Shc (Mason JM et al., 2000). Shc is Tyr-phosphorylated by JAK2 in an EPO-dependent manner, promoting complex formation between EPO-R, Grb2, and the guanine nucleotide exchange factor Sos (Odai H et al., 1997). As a result Sos translocates to the plasma membrane, where it activates Ras, leading to the activation of the downstream raf-1 and MAP kinases. The key participation of Ras in EPO-mediated signal transduction was demonstrated by the increased proliferation and differentiation of CD34 cord blood cells upon transduction of Ras and EPO-R cDNAs (Lu L et al., 2000). Stimulation of hematopoietic cells by EPO induces recruitment of the cytosolic adaptor CrkL to the EPO-R and its physical association with Shc, SHP2, and Cbl (Chin H et al., 1997). Receptor-associated Lyn probably phosphorylates CrkL, leading to the activation of Ras/Erk signaling pathways (Arai A et al., 2001). Several lines of evidence point to the participation of PI3-K in signal transduction via the EPO-R. Antisense oligonucleotide of p85alpha (the regulatory subunit of PI3-K) or LY294002 (a selective inhibitor of PI3-K) independently inhibits the formation of EPO-dependent colonies, a process in which Src activation has been implicated (Kubota Y et al., 2001). Moreover, a constitutively active form of STAT5 interacts with p85, activating the PI3-K pathway. Other activated proteins
include the serine/ threonine kinase Akt (PKB), which directly phosphorylates FKHRL1, a member of the transcription factor Forkhead family (Kashii Y et al., 2000), and p70 S6 kinase, required for cell cycle progression. Future analysis of the molecular basis of cytokine action should determine how JAK/STAT signals are integrated with signals from other downstream pathways such as those originating from Ras/Raf/ MAPK, Akt, and p70 S6 kinase.
Recently, activation of endothelial nitric oxide synthase (eNOS) by EPO has been identified to be of particular importance for its endothelial and vascular effects (Beleslin-Cokic BB et al., 2004; d’Uscio LV et al., 2007). d’Uscio et al. (d’Uscio LV et al., 2007 ) have used a murine model of wire induced injury of the carotid artery in order to examine the effect of EPO on endothelial repair and arterial wall architecture. They administered EPO (1000 U/kg) subcutaneously bi-weekly for two weeks in wild type and eNOS deficient mice, after which reactivity of isolated carotid arteries was studied in vitro, and the vasculature was histologically assessed. Injured arteries exhibited impairment of endothelium-dependent relaxations to acetylcholine, and this was associated with an increased medial cross sectional area. Treatment with EPO up-regulated expression of eNOS and normalized the vasodilator response to acetylcholine. Furthermore, it prevented the injury induced increase in the medial cross sectional area. These vascular protective effects were abolished in eNOS deficient mice. Moreover, the authors observed a significant increase in systolic blood pressure and enhanced medial thickening of injured carotid arteries in eNOS deficient mice. These results demonstrate that the vasculo-protective effects of EPO are critically dependent on activation of eNOS. In line with this assumption, recently, it has been published that the endogenous NO inhibitor asymmetric dimethyl-arginine (ADMA) inhibits renal vasodilatatory effects of EPO in mice (Heeschen C et al., 2003).
1.3 EPO Receptor in endothelial cells.
EPO is increasingly coming into the focus of cardiovascular medicine, since a steadily growing body of evidence indicates that recombinant human EPO (rHuEPO) and its analogues could be mediator of vasculo-protective effects (Goldman SA, Nedergaard M. 2002). EPO is a major regulator of vascular formation and organ growth in the embryo, and EPO-R have been found in almost every embryonic tissue (Frank SJ. 2000; Juul SE et al., 1998; Kertesz N et al., 2004). Kertesz et al. (Kertesz N et al., 2004)
have clearly demonstrated that both EPO and EPO-R are expressed in the vasculature during embryogenesis, and that deletion of either in knockout animals leads to severe angiogenic defects resulting in an embryonic lethal phenotype. These angiogenesis defects can be partially rescued by expressing human EPO during embryogenesis (Kertesz N et al., 2004). Furthermore, EPO-R have been discovered in a variety of cell types including endothelial cells. Of particular interest for vascular medicine could be the observation that EPO has important direct biological effects on mature endothelial cells. EPO stabilizes endothelial structures and vascular integrity such as cell-cell and cell-matrix contacts. The addition of rHuEPO to the culture medium increases endothelial cell proliferation and protects cells against ischaemia and apoptosis (Anagnostou A et al., 1990; Carlini RG et al., 1999; Chong ZZ et al., 2002). The latter effect is mediated via EPO-R and the PI3K/AKT pathway (Chong ZZ et al., 2002). Since mature endothelial cells obviously do not lose their EPO-R, antiapoptotic signalling persists much longer than in erythrocytes thus rendering endothelial cells more resistant to ischaemia induced cell death. Moreover, mature human endothelial cell lines also respond to rHuEPO by differentiating into primitive vascular structures (Carlini RG et al., 1995; Ribatti D et al., 1999). It is therefore tempting to speculate that EPO preserves its role as a key regulator of vascular protection and even vascular formation (neoangiogenesis) in the adult organism (Bahlmann FH et al. 2004).
This hypothesis is supported by observation that administration of rHuEPO or the hyperglycosylated long acting EPO analogue, Darbepoetin alpha, significantly enhances mobilization of bone marrow derived endothelial progenitor cells (EPCs) in humans (Bahlmann FH et al., 2004; Bahlmann FH et al., 2003). These cells are thought to orchestrate vascular reparative processes and promote endothelial regeneration (Asahara T et al., 1999; Dzau VJ et al., 2005). Notably, it has been observed the effect on EPCs with standard therapeutic doses of rHuEPO contrasting most previous observations from in-vitro studies, in which supra-therapeutic doses had been used (Anagnostou A et al., 1990; Carlini RG et al., 1999; Chong ZZ et al., 2002; Carlini RG et al., 1995). These findings, together with results from animal and cell culture experiments, permit the conclusion that EPO is a potent regulator of EPC proliferation and differentiation (Bahlmann FH et al., 2004; Bahlmann FH et al., 2003; Heeschen C et al., 2003). In line with this assumption is the observation in patients with acute coronary syndrome in whom EPO blood levels were strongly and independently related to the number of EPC’s in peripheral blood (Heeschen C et al., 2003). It has been also
demonstrate that the effect of rHuEPO on EPCs is mediated, at least in part, via AKT activation (Bahlmann FH et al., 2004; Bahlmann FH et al., 2003; Heeschen C et al., 2003). This effect is probably related to the activation of the EPO-R on the surface of EPCs. In addition, activation of eNOS in EPCs may contribute to EPO’s effects in endothelial regeneration, since recent work clearly demonstrated that NO production is a key feature of EPCs required for endothelial repair (Urao N et al., 2006; Westenbrink BD et al., 2007; Sorrentino SA et al., 2007). For example, Urao et al. (Urao N et al., 2006) investigated whether rHuEPO mobilizes EPCs and thus promotes the repair of injured endothelium in a wire injury model of the femoral artery in mice. Not only was neointimal formation inhibited by rHuEPO in an NO-dependent manner, but administration of rHuEPO induced a 1·4-fold increase in re-endothelialized area. Part of the regenerative potential was derived from bone marrow cells. In fact, using green fluorescence protein or beta-galactosidase-overexpressing cells they could demonstrate that rHuEPO stimulated both differentiation of bone marrow derived EPCs and proliferation of resident endothelial cells. Finally, rHuEPO induced AKT/eNOS phosphorylation and NO synthesis in EPCs, confirming our results in human EPCs. The results of Urao et al. (Urao N et al., 2006) were recently confirmed in a model of post-myocardial infarction heart failure, were rHuEPO induced neovascularization was also mediated through a combination of EPC recruitment from the bone marrow (Westenbrink BD et al., 2007). Collectively, these data support the hypothesis that EPO is a key molecule in the process of endothelial repair and neoangiogenesis.
1.4 Inactivation mechanism of EPO Receptor.
The low cell surface expression of EPO-R suggests that a tight control mechanism operates to regulate erythroid proliferation and differentiation in response to physiological levels of EPO under normal or hypoxic conditions. Receptor internalisation and ligand-mediated endocytosis are pivotal processes, which regulate cell surface receptors (Flint-Ashtamker G et al., 2002). After transient phosphorylation of the EPO-R and activation of intracellular signalling pathways, inactivation mechanisms are simultaneously turned on, and EPO-induced signaling pathways return to nearly basal levels after 30–60 min of stimulation (Verdier F et al., 2000).
Accumulating evidence suggests that endocytosis of membrane proteins is mediated by specific sequences in their cytosolic domains, which interact with auxiliary
cellular molecules (Trowbridge IS et al., 1993; Mukherjee S et al. 1997; Mellman I. 1996). The sequences include di-Leu-based motifs (Dittrich E et al., 1996; Hamer I et al., 1997; Kirchhausen, T. 1999), Tyr-based motifs (Mukherjee S et al., 1997; Honing S and Hunziker W. 1995.) and a third group of sequences containing various features such as clustered acidic residues (Voorhees P et al., 1995), or mono amino acid-based motifs (Stroh A et al., 1999). The capacity of a series of cytoplasmic tail truncated EPO-R variants to internalize 125I-EPO was measured (Levin I et al., 1998; Beckman, DL et al., 1999), demonstrating that a membrane proximal cytosolic domain of the EPO-R was the minimal region required for receptor-mediated ligand internalization. Ligand binding to the EPO-R was found to accelerate the rate of receptor internalization of cell surface receptor from T1/2 of 3 h in the absence of ligand to 15 min in its presence (Sawyer ST
and Hankins WD. 1993). The contribution of selected EPO-R cytosolic regions to ligand-independent internalization of the EPO-R is not known. Truncated EPO-R containing 267 amino acids or less were defective in internalization of 125I-EPO, whereas internalization via a receptor derivative containing 276 amino acids was unaffected, thus directing focus to the nine amino acid residues FEGLFTTHK at positions 268-276 (Levin et al., 1998). EPO-R mutants were generated to determine the role of these residues in EPO endocytosis, down regulation of cell surface receptors and EPO-mediated signaling. The addition of amino acid residues 268-276 to an EPO-R which is devoid of the cytosolic domain improved the capacity of the respective receptor to endocytose 125I-EPO as well as conferred its constitutive down regulation from the cell surface. Mutation of Phe to Ala at position 272 adversely affected receptor-mediated ligand endocytosis, but did not alter the T1/2 of cell surface receptor in the absence of
ligand, suggesting that these two processes require distinct structural features of the receptor. Excision of amino acids 268-276 from wild type EPO-R did not impair 125 I-EPO internalisation via the receptor, nor alter the T1/2 of cell surface receptor in the
absence of ligand, yet it abrogated binding of the receptor to JAK2 and EPO-mediated proliferation of Ba/F3 cells. Phe residues within the segment 268-276 seemed to play a major role in EPO-mediated signal transduction (Flint-Ashtamker G et al., 2002).
EPO-R signaling may be terminated also as a result of the interaction of certain proteins with the EPO-R or with signalling molecules (table 2).
Table 2. Negative regulators of EPO-R signalling: major proteins that participate in signaling via EPO-R are color-coded and classified by their role in the process.
Among these proteins a major player is SHP1, which is recruited via its SH2 domains to P-Tyr 429 of the EPO-R, leading to dephosphorylation of JAK2 and down-regulation of positive signals (Klingmuller U et al., 1995). Another group of negative regulatory proteins is the CIS protein family, also known as SOCS (suppressor of cytokine signaling) or SSI (STAT-induced STAT inhibitor), whose members are activated via the JAK/STAT pathway and have been implicated in regulating the signal transduction of a variety of cytokines including EPO (Yoshimura A. 1998). CIS1 binds P-Tyr 401 of the EPO-R, which serves as one of the major binding sites for STAT5, thereby negatively regulating STAT5 activation (Matsumoto A et al., 1995). A second member of CIS family was cloned independently by three groups and was termed JAB (JAK-binding protein), SOCS1, or SSI1 (Hilton DJ et al., 1998). Mutation analysis and biochemical characterization revealed that JAB binds specifically to Tyr at position 1007 in the activation loop of JAK2, whose phosphorylation is required for kinase activation (Yasukawa H et al., 1999). The SH2 domain and a C-terminal 40 amino acid region, designated the CIS homology domain, are highly conserved in this family, whereas the N-terminal regions of these proteins share little similarity (Hilton DJ et al., 1998). While both CIS3 and JAB inhibit EPO-mediated proliferation and STAT5 activation (Sasaki A et al., 2000), the in vitro affinity of CIS3 for JAK2 is significantly lower than that of JAB (Yoshimura A. 1998), further illustrating the complexity of negative regulation of cytokine signaling by CIS proteins.
Unusual features of the EPO-R are its short half-life (t1\2 1±2 h), its degradation via multiple pathways and the fact that less than 1% of total cellular EPO-R molecules are found on the cell surface. The contribution of EPO-R structural determinants to the regulation of its intracellular metabolism is still unclear. To determine which EPO-R cytosolic domains are involved in intracellular degradation, chimeric receptor molecules constructed of epidermal growth factor receptor (EGF-R) extracellular and transmembrane parts, linked to the full length or truncated cytosolic part of the EPO-R, were studied. The EGF-R, unlike the EPO-R, is efficiently transported to the cell surface and displays a much longer metabolic half-life. The chimeras were expressed in transiently transfected COS 7 cells and stably expressed in Ba/F3 cells. Such experiments indicated that the cytosolic part of the EPO-R contains determinants that mark it for rapid degradation, in association with the endoplasmic reticulum. This degradation was insensitive to brefeldin A and was inhibited by specific proteasomal inhibitors. A truncated EGF-R/EPO-R chimera containing only 50 amino acids of the EPO-R membrane-proximal cytosolic part was also rapidly degraded suggesting that these 50 amino acids are involved in receptor degradation (Supino-Rosin L et al., 1999).
Although hormone-receptor complexes are most generally believed to be degraded through the endosome-lysosome pathway (Smythe E and Warren G. 1991), the involvement of the ubiquitin-proteasome proteolytic pathway in the down-regulation of the hepatocyte growth factor receptor (Jeffers M et al., 1997) and the platelet-derived growth factor receptor (Mori S et al., 1995) has been documented. In both cases, ligand binding appears to induce the poly-ubiquitination and the degradation of the receptor by a process involving the proteasome. Moreover, proteasome inhibitors have been shown to prolong the activation of JAK tyrosine kinases and of STAT5 transcription factors in cells stimulated by IL-2 (Yu CL and Burakoff SJ. 1997), IL-3 (Callus BA and Mathey-Prevot B. 1998), ciliary neurotrophic factor (Malek RL and Halvorsen SW. 1999), and growth hormone (Gebert CA et al., 1999). It has been reported that these inhibitors also prolonged the duration of EPO-R and STAT5 tyrosine phosphorylation in response to EPO (Verdier F et al., 1998). The mechanisms leading to these prolonged activations have not been determined; neither the JAK nor the STAT5 molecules appear to be degraded during the deactivation process. It has been suggested that the proteasome could modulate a phosphatase activity (Yu CL and Burakoff SJ. 1997; Gebert CA et al., 1999). Further studies evidenced the proteasome control in the EPO-R inactivation
mechanisms. Verdier and colleagues evidenced that in cells treated with the proteasome inhibitors N-Ac-Leu-Leu-norleucinal or lactacystin, EPO-R phosphorylation and activation of intracellular signaling pathways were sustained for at least 2 h (Verdier F et al., 2000). In the same study, the authors showed that such effect was due to the continuous replenishment of the cell surface pool of EPO-R in cells treated with proteasome inhibitors. Proteasome inhibitors did not modify the internalization and degradation of EPO/EPO-R complexes, but they allowed the continuous replacement of the internalized receptors by newly synthesized receptors. Proteasome inhibitors did not modify the synthesis of EPO-R, but they allowed their transport to the cell surface. N-Ac-Leu-Leu-norleucinal, but not lactacystin, also inhibited the degradation of internalised EPO/EPO-R complexes, most probably through cathepsin inhibition. The internalized EPO-R were not tyrosine phosphorylated, and they did not activate intracellular signaling pathways.
1.5 EPO and EPO derivatives.
It has been almost a century since Carnot and Deflandre (Carnot P, DeFlandre C. 1906) postulated that a humeral factor, which they called “hemopoietine” regulates red blood cell production. Their experiments were carried out in rabbits, where they removed plasma from a donor rabbit after a bleeding stimulus and found that, when this plasma was injected into a normal recipient rabbit, a prompt reticulocytosis occurred. Several investigators confirmed the Carnot and Deflandre experiments (Gibelli C. 1911; Sandor G. 1932). However, Erling Hjort (Hjort E. 1936) reported the most convincing confirmation of Carnot and Deflandre’s work up to that time. Hjort reported that erythropoietically active plasma from bled rabbits produced a reticulocytosis when injected into normal recipients in 18 experiments. Krumdieck (Krumdieck N. 1943) published very similar findings as Carnot and Hjort: they found erythropoietic activity in plasma from bled rabbits when injected into recipient rabbits. Allan Erslev, a pioneer in EPO research, must be given much credit for publishing his work in the journal Blood in 1953 (Erslev AJ. 1953.) where he injected large volumes of plasma (50 ml per day for 4 days) from donor rats after a bleeding stimulus into normal recipient rats and produced a marked reticulocytosis. One of the most important papers in EPO research is the work of Kurt Reissmann (Reissmann KR. 1950), who reported his work in parabiotic rats, when one partner breathed an atmosphere of low oxygen tension and the other partner
breathed normal air, both partners developed a reticulocytosis, increase in hemoglobin, and bone marrow hyperplasia. These findings reawakened interest in EPO.
Proof that the kidney is the primary site of EPO production (Jacobson LO et al., 1957; Fisher JW, Birdwell BJ. 1961; Kuratowska Z et al., 1961; Erslev AJ. 1974), that peritubular interstitial cells in the kidney are the renal cells that produce EPO (Koury ST et al., 1988; Fisher JW et al., 1996), and that the liver is a secondary site of EPO production (Zanjani ED et al., 1977; Koury ST et al., 1991) are major advances in EPO research. One of the most important advances in this field occurred when Miyake
et al. (Miyake T et al., 1977) reported purification to homogeneity of human EPO. This made it possible for Lin et al. (Lin FK et al., 1985) and Jones et al. (Jones SS et al., 1985) to clone the gene for EPO and to develop a transfected cell line in Chinese hamster ovary cells that provided recombinant EPO for use in clinical anemias.
New molecules have recently been synthesized called novel erythropoiesis stimulating protein (NESP), which contain a higher content of carbohydrate and provides a new antianemia agents with a longer circulating plasma half-life in vivo than native EPO (Egrie JC, Browne JK. 2001; MacDougall IC. 2000). It was well known from previous experiments that sialic acid residues on EPO are responsible for maintaining in vivo biological activity of EPO (Egrie JC, et al., 1993). EPO is known to be desialylated in vivo, cleared from plasma, and is bound to galactose receptors in the liver (Macdougall IC et al., 1999). Thus, there is a direct correlation between the amount of sialic acid-containing carbohydrates, plasma half-life, and in vivo biological activity (Egrie JC, et al., 1993; Macdougall IC et al., 1999). With this knowledge, it was conjectured that increasing the carbohydrate content of EPO would result in a longer plasma half-life and enhanced biological activity. Thus, NESP was synthesized using the latest techniques of DNA technology (Egrie JC, Browne JK. 2001; MacDougall IC. 2000). An overview of the efficacy and safety of NESP has been reported by Macdougall (Macdougall IC. 2001). NESP contains five N-linked oligosaccharide chains, whereas native EPO contains three oligosaccharide chains. The amino acid sequence of NESP differs from that of native human EPO at five positions (Ala30Asn, His32Thr, Pro87Val, Trp88Asn, and Pro90Thr), which allows for attachment of additional oligosaccharides at asparagine residue positions 30 and 88 (Egrie JC, Browne JK. 2001). NESP contains 22 sialic acid residues compared with 14 sialic acid residues for native EPO. The molecular weight of NESP is 38,50 KDa and native EPO is 30,40 KDa; the total carbohydrate for NESP is 52% and native EPO is 40%. NESP binds to the
EPO receptor in an identical manner as native EPO to induce intracellular signaling involving tyrosine phosphorylation by JAK2 kinase and the same intracellular molecules Ras/MAP-k, PI3-K, and STAT5 as native EPO. In comparing the i.v. pharmacokinetics of NESP and rHuEPO in patients on dialysis, the mean terminal half life for NESP was three times longer than for rHuEPO. The clearance of NESP was significantly less than that of rHuEPO; the volume of distribution for NESP and rHuEPO were equivalent. Given s.c., the mean terminal half-life for NESP was 48.8 hr. Apparently, the half-life for SC rHuEPO is 18–24 hr. When given once weekly i.v., NESP reaches a steady state within 4 weeks and does not accumulate. The s.c. and i.v. pharmacokinetic profiles of NESP in patients on dialysis support the hypothesis that patients with anemia of chronic renal failure (CRF) require less frequent doses of NESP when compared with rHuEPO (Locatelli F et al., 2001; Macdougall IC et al., 1991; Macdougall IC et al., 1999). Three safety and efficacy studies of NESP in patients with anemia of CRF have been completed (Macdougall IC, 1998; Korbert SM. 1993; Vanrenterghem Y et al., 1999). The first two multicenter studies were to evaluate the efficacy of NESP in treating the anemia of CRF in patients on dialysis (Macdougall IC, 1998; Korbert SM. 1993). The third study was to evaluate the safety and effectiveness of NESP in maintaining haemoglobin when administered at less frequent dosing compared with rHuEPO (Vanrenterghem Y et al., 1999).The authors concluded from these studies that NESP is effective in the treatment of anemia of CRF and is safe. It was concluded that NESP can maintain the Hb concentration just as effectively as rHuEPO at less frequent dosing. The adverse effects, withdrawals, and deaths were the same for the NESP as that reported for rHuEPO treatment (Korbert SM. 1993). There have been no reports thus far of antibody production in patients treated with NESP. The NESP Darbepoetin has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of anemia caused by CRF, and may be approved soon for use in cancer patients with anemia associated with chemotherapy (The Medical Letter on Drugs and Therapeutics).
Recently, a new class of third-generation erythropoeietic-stimulating agents was approved by FDA, the continuous erythropoietin receptor activator (CERA). CERA has an extended half-life and a mechanism of action that promotes increased stimulation of EPO-R compared with other agents. It is similar to previous synthetic EPO drugs in terms of structure, except that it is connected to a polyethylene glycol (PEG) chain, which makes it last longer in the body (up to 6 times longer than darbepoetin alpha and
up to 20 times longer than EPO). CERA has generally been well tolerated in clinical trials.
Concerning drug concentration, EPO activities are usually expressed in international units (IU). One unit of EPO was originally defined as the activity that produced the same erythropoietin-stimulating effect as 5 micromoles of cobalt. The first International Reference Preparation (IRP) of Erythropoietin, Human, Urinary, for Bioassay became depleted around 1970. The availability of the Second International Reference Preparation of Erythropoietin, Human, Urinary, for Bioassay (SIRP) was reported in 1972. It was derived from the urine of anemic humans and was standardized by the Committee on Biological Standards of the World Health Organization. It is still available in ampoules containing 10 IU.6 Internal standards or commercial preparations that have been standardized against the SIRP are also suitable for use in EPO assays. In 1991, the International Standard for Recombinant DNA-derived Erythropoietin 87/684 was reported to be 86 IU Erythropoietin r-DNA derived per ampoule. Significant differences in reactivity were seen between in vivo, in vitro biological, and various immunological assays with each of the above standards. These discrepant results suggested that a rDNA EPO standard would be more suitable for assays for therapeutic doses of rDNA EPO, while SIRP would be more appropriate as the reference for diagnostic assays for naturally occurring human EPO (Guidance for Industry Document for Special Controls for Erythropoietin Assay Premarket, Notifications [510(k)s], Document issued on: April 28, 1999, U. U.S. Department Of Health And Human Services, Food and Drug Administration, Center for Devices and Radiological Health Immunology Branch, Division of Clinical Laboratory Devices, Office of Device Evaluation)
Notably, annual worldwide sales of recombinant EPO products are $12 billion. In figure 6 the worldwide sales of same EPO brands (from 1997 to 2005) are shown.
Figure 6. Worldwide sales of same EPO brands (from 1997 to 2005).
1.6 Therapeutic uses of EPO derivatives.
The cloning of the EPO gene led to production of the recombinant hormone, allowing its introduction into clinical practice. In 1987, Eschbach et al. (Eschbach JW et al., 1987) reported the results of combined phase I and II clinical trials of rHuEPO in which the anemia of end-stage renal disease was corrected. rHuEPO was approved for human use in patients with CRF by the FDA in June 1989. The National Kidney Foundation Dialysis Outcomes Quality Initiative (NKF-DOQI) reported, in 1997 (Eschbach J et al., 1987), the results of a work group that established evidence based guidelines for the management of the anemia of CRF. In 2000, Eschbach (Eschbach JW. 2000) outlined several principles related to anemia management of patients with CRF being treated with rHuEPO. The clinical response of the patient with CRF to rHuEPO does not occur unless doses of >15 U/kg i.v., three times weekly, are administered. Further increase in response is not likely to occur at doses above 500 U/kg i.v. three times weekly (Eschbach JW. 2000). Moreover, on the average, subcutaneous (s.c.) administration is more effective than i.v. or i.p. injections even though only 25% of the s.c. administered dose is absorbed (Kaufman JS et al., 1998). Pharmacokinetic studies show that rHuEPO has a half-life (T1⁄2) after i.v. administration of 4–9 hr, whereas the T1⁄2 after s.c. injection is >24 hr (Egrie JC et al., 1988). It is important not to discontinue rHuEPO just because the target haemoglobin has been achieved. The haemoglobin level may fall more abruptly than anticipated if therapy is stopped because the rising haemoglobin suppresses endogenous EPO production (Eschbach JW et al.,
1989). Furthermore, the treatment with RHuEPO leads to iron deficiency and it is essential that the patient with anemia of CRF being treated with rHuEPO be followed for symptoms of iron deficiency, e.g., serum ferritin, transferring saturation, etc. Even though the best target haemoglobin level that should be achieved in patients with CRF has been somewhat controversial, the European Best Practice Guidelines has recommended that a target hemoglobin of >11g/dl be achieved for 85% of patients with CRF (Jacobs C et al., 2000). To attain this target, the population median will be 12.0– 12.5g/dl. They recommend that the target haemoglobin may need to be varied for patients with CRF with specific comorbidities (Jacobs C et al., 2000). A National Kidney Foundation ad hoc committee recommended a target hematocrit of 33–38% (Fisher JW et al., 1989). However, the FDA in the U.S. recommended a hematocrit range up to 36%, and this is the basis for reimbursements of patients treated with EPO in the U.S. Other investigators reported that current evidence-based recommendations suggest a target hematocrit range of 33–36%, but caution that normalization of the hematocrit in hemodialysis patients with symptomatic heart disease has shown an increase in both mortality and the rate of vascular access thrombosis (Murphy ST, Parfrey PS. 1999). The value of early management of patients with CRF has been stressed (Kessler M. 1997). The objective of early referral in patients with CRF is apparently to prevent complications as the disease progresses to the stage where dialysis or transplantation becomes necessary (Kessler M. 1997). When dialysis is initiated after the development of uremia, the poor nutritional status, severe acidosis, and anemia and poorly controlled hypertension makes the patient more difficult to manage. It has been suggested that nephrology referral should be centered around controlling anemia with rHuEPO, high blood pressure with appropriate, nutritional status with adapted protein-calorie intake, and renal osteo-dystrophy with calcium carbonate or recombinant growth hormone in children (Kessler M. 1997). It has been suggested that chronic renal disease progression may be slower when anemia is reversed, emphasizing the benefits of early correction of the anemia with EPO (Ritz E, Eisenhart A. 2000). The mechanism of the anemia of CRF is probably due to the inability of the kidneys with compromised renal function to produce sufficient amounts of EPO to meet the demands for new red cell production in patients with uremia. Over the years, there has been some controversy over the role of the bone marrow response to endogenous EPO in patients with uremic end-stage renal disease. Obviously, the primary cause of the anemia in CRF is due to the lack of sufficient amounts of EPO to maintain steady-state erythropoiesis due to the
reduced red cell life span, deficiency of iron and folic acid, hyperparathyroidism with myelofibrosis, aluminium toxicity, blood loss, and inhibition of erythropoiesis caused by “uremic toxins.” However, serum levels of EPO in normal human subjects range between 1 and 27mu/ml (mean 6.2 ± 4.3 mu/ml, n=53). On the other hand, serum levels of EPO in patients with CRF were between 4.2 and 102 mu/ml (mean 29.5 ± 4.0 mu/ml,
n=36)( Garcia MM et al., 1990). Thus, it seems that even though EPO deficiency is the primary cause of the anemia of CRF, the uremic state may blunt the bone marrow response to EPO. Serum levels of EPO in patients with CRF were about five times as high in patients with CRF than in normal human subjects (Garcia MM et al., 1990). McGonigle et al. (McGonigle RJS et al., 1985) and Radtke et al. (Radtke HW et al., 1980) reported a suppressed response of bone marrow cultures to EPO in the presence of plasma from patients with anemia. Macdougall (Macdougall IC. 2001) has recently summarized the role of “uremic toxins” in exacerbating the anemia in patients with CRF. Candidates suggested to play a role in uremic inhibition of erythropoiesis are polyamines, parathyroid hormone, and some inflammatory cytokines (Macdougall IC. 2001). Radtke et al. (Radtke HW et al., 1980) and Kushner et al. (Kushner DS et al. 1991) provided some support for polyamines as one of several uremic toxins that could be responsible for the suppressed response of the bone marrow to EPO in the patient with anemic CRF. Allen et al. (Allen DA et al., 1999) reported that when bone marrow from patients with uremia with and without inflammatory disease was cultured with autologous serum, the optimal response to EPO was significantly inhibited. Treatment of parallel cultures with a combination of antibodies to interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) almost completely restored the response to EPO. They conclude that CFU-E colony formation is inhibited by soluble factors present in the sera of patients with uremia with or without inflammatory disease (Allen DA et al., 1999). Erslev and Besarab (Erslev AJ, Besareb A. 1995) compared the rate and control of baseline red cell production in haematologically stable patients and uremic patients with anemia. The erythrokinetic rates in the anemic uremic patients were about one-half the rate in normal hematologically stable individuals even though the serum EPO levels were the same or higher in the anemic uremic patients (Erslev AJ, Besareb A. 1995). It is also of interest that patients with CRF undergoing ambulatory peritoneal dialysis maintain significantly higher hematocrits than patients on hemodialysis (Steiner RW. 1984; McGonigle RJS et al., 1984). Thus, it seems possible that pharmacological doses of EPO in patients with CRF may overwhelm the bone marrow and correct both the EPO
deficiency and the suppressed bone marrow response to EPO. The cardiovascular effects of EPO and anemia correction has been summarized by Vaziri (Vaziri NA. 2001). It appears that EPO-induced hypertension is not due to the amelioration of anemia because a similar rise in blood pressure occurs in EPO-treated iron-deficient animals and humans even though the anemia persists (Vaziri ND et al., 1996; Koupke CJ et al., 1994). Chronic administration of EPO results in hematocrit independent, vasoconstriction- dependent hypertension that is largely due to elevated resting and agonist-stimulated cytoplasmic calcium concentrations, which leads to resistance to the vasodilatation of nitric oxide (Vaziri ND et al., 1996; Vaziri ND et al., 1995). Other factors that may be involved in the hypertensive action of EPO are increased endothelin production (Bode-Boger SM et al., 1996; Brochu E et al., 1999), upregulation of tissue rennin and angiotensinogen production (Eggena P et al., 1991), and changes in vascular tissue prostaglandins (Bode-Boger SM et al., 1996; Bode-Boger SM et al., 1992). EPO has also been demonstrated to promote angiogenesis (Pelletier L et al., 2000; Ribatti D et al., 1999; Carlini RG et al., 1995) and to stimulate endothelial and vascular smooth muscle cells (Iimura O et al., 2000). In addition, correction of anemia in patients with CRF may partly prevent or reverse left ventricular hypertrophy in dependent and dialysis-independent patients with chronic renal insufficiency (Jereen-Sturgic B et al., 2000). It has also been reported that inborn erythrocytosis leads to cardiac dysfunction and premature death in mice overexpressing EPO (Wagner KF et al., 2001). Finally, it has been reported that neutralizing anti- EPO antibodies and pure red cell aplasia developed in 13 patients with anemia of CRF during treatment with epoetin (Casadevall N et al., 2002). Suppression of erythroid progenitor cells in the bone marrow (Gogue SR et al., 1995) and anemia (Henry DH et al., 1992; Fischl M et al., 1990) are frequently seen as side effects of Zidovudine treatment of patients with AIDS. Patients with AIDS with endogenous serum EPO levels <500 mu/ml who are receiving doses of Zidovudine usually respond to rHuEPO therapy (Henry DH et al., 1992). However, patients with serum EPO levels >500 mu/ml usually do not respond to RHuEPO therapy (Fischl M et al., 1990). RHuEPO is being used to treat patients with anemia associated with non-myeloid malignancies where the anemia is due to the effects of concomitantly administered chemotherapy. RHuEPO was reported to be effective in the treatment of patients with cancer receiving cisplatin (Smith DH et al., 1988). A study of 3012 patients with non-myeloid malignancies who were receiving chemotherapy was carried out in a multicenter, open label, non-randomized study conducted in 600 United States
community-based practices (Gabrilove JL et al., 2001). Of the study patients, 819 (27.6%) received taxane chemotherapy, 349 (11.8%) received cisplatin chemotherapy, and 682 (23.0%) received carboplatin chemotherapy. Patients were administered 40,000 units of rHuEPO once weekly by the s.c. route, which could be increased to 60,000 units one time per week after 4 weeks, depending upon the hemoglobin response. Treatment was continued for a maximum of 16 weeks. Patients who were accessible for efficacy evaluation (2,964) were observed to have significant increases in Hb levels, decreases in transfusion requirements, and improvements in functional status and fatigue. In another randomized, double-blind, placebo controlled study of 375 patients with anemia receiving nonplatin chemotherapy with solid or non-myeloid hematologic malignancies were treated with rHuEPO at dosages of 150–300 I.U./kg three times per week s.c. for 12–24 weeks. rHuEPO significantly decreased transfusion requirements and increased Hb (De Andrade JR, Jove M. 1996). An increase in energy level and ability to do daily activities were significantly greater in the rHuEPO group when compared with the placebo patients (Vercameen E, Rappaport B. 2001). rHuEPO was studied in a double-blind clinical trail in 316 patients prior to elective orthopedic hip or knee surgery (De Andrade JR, Jove M. 1996). The patients were randomly assigned according to pretreatment Hb levels to receive 300 u/kg, 100 u/kg, or placebo s.c. for 10 days before surgery, on the day of surgery, and for 4 days after surgery (Goldberg MA, McCutchen JW. 1996). The dosage of 300 u/kg rHuEPO significantly reduced the risk of allogenic transfusions. In patients with pretreatment Hb of >10 to <13 g/dl, there was not a significant difference in the transfusion requirements between 100 u/kg rHuEPO and the placebo group. In another study of 145 patients scheduled for orthopedic hip or knee surgery who received 600 u/kg rHuEPO s.c. once weekly for 3 weeks prior to surgery and on the day of surgery or 300 u/kg once daily for 10 days prior to surgery, on the day of surgery, and for 4 days after surgery (Di Raimondo F et al., 1996), the increase in Hb in the 600 u/kg weekly group was significantly greater than that observed for the 300 u/kg group. rHuEPO has been investigated for use in myelodysplastic syndrome (Di Raimondo F et al., 1996), preoperative autologous blood donation (Thomas MJG et al., 1996; Price TR et al. 1996), anemia of pregnancy (Harris SA et al., 1996), rheumatoid arthritis (Pincus T et al., 1990), anemia of prematurity (Messer J et al., 1993; Bader B et al., 1996), bone marrow transplantation alone (Steegman JL et al., 1992), and in combination with G-CSF (Pierelli L et al., 1996). Evidence-based guidelines for anemia management in patients with CRF have been outlined by the National Kidney
Foundation Dialysis outcomes Quality Initiative (Eschbach JW. 2000), which supplements guidelines for the clinical use of RHuEPO previously reported (Fisher JW et al., 1989; Goodnough LT et al., 1993). In a Phase III multicenter clinical trial (Eschbach JW et al., 1989) of rHuEPO in 333 hemodialysis patients with a median maintenance dose of 75 u/kg i.v. three times a week for approximately 13 months, the adverse effects noted were myalgias, 5%; iron deficiency, 43%; increased blood pressure, 35%; and seizures, 5.4% (Eschbach JW et al., 1989). The patients that did not respond had complicating causes for the anemia, such as myelofibrosis, osteitis fibrosa, osteomyelitis, and acute or chronic blood loss (Eschbach JW et al., 1989). If delayed or diminished response to doses of rHuEPO within the recommended dosing range occurs, the following etiologies should be considered:iIron deficiency (all patients will eventually require supplemental iron therapy), underlying infections, inflammatory or malignant processes, occult blood loss, underlying hematologic disease, vitamin deficiencies (folic acid or vitamin B-12), hemolysis, aluminum intoxication, and osteitis fibrosa cystica.
To benefit from recombinant human erythropoietin are, as expected, patients with end-stage renal failure (Eschbach JW et al. 1989), in whom the diseased kidneys do not produce endogenous EPO. Indicators of successful therapy are increased levels of hemoglobin and hematocrit, reduction in the blood transfusion requirements, and a significant improvement in the quality of life and performance status. The successful results in over 90% of these patients have encouraged investigators to treat other types of anemia with rHuEPO. Cancer patients often suffer from anemia. Patients with various malignancies respond to anemia with an increase in EPO production, which however is still inadequate and less than the expected normal amount in relation to the degree of anemia (`relative EPO deficiency') (Miller CB et al., 1990). This finding constitutes the scientific basis for clinical trials on the use of rHuEPO to treat patients suffering from various types of neoplasms with anemia (Itri LM. 2002). The overall response rate has been about 60%. Patients with multiple myeloma or head and neck cancer achieve a success rate of about 70±80%, whereas the response rate of patients with myelodysplastic syndromes is around 20%. More experimental designs are required to estimate the benefits of rHuEPO in the wide range of cancer patients undergoing treatment. Anemias in hematologic and other disorders rHuEPO was also shown to be effective in the treatment of other types of anemia, such as that seen in patients with AIDS, and the anemia that accompanies chronic diseases such as systemic lupus
erythematosus, rheumatoid arthritis, and inflammatory bowel disease (Bieber E. 2001). EPO can ameliorate anemia of infancy, hemoglobinopathies, and Gaucher's disease. EPO has become an important component of the bone marrow transplantation procedure, as a measure to increase progenitor cell recruitment prior to transplan- tation, as well as after the procedure when it promotes proliferation of the erythroid series (Rizzo JD et al., 2001). Additional clinical indications for rHuEPO include its perisurgical use. Several studies have shown that injection of EPO before and immediately after elective surgery, especially cardiac and orthopedic operations, may minimize the need for blood transfusions (Stovall TG. 2001).
Futhermore data suggest that EPO may exert non-erythroid effects, such as neuroprotection (Cerami A et al., 2002). EPO has been reported to activate specific receptors in the central nervous system and was found to be neurotrophic and neuroprotective in both in vitro and in vivo models (Marti HH, Bernaudin M. 2002; Juul S. 2002). EPO and the EPO-R have both been reported in the brain cortex, cerebellum, hippocampus, pituitary gland and spinal cord (Marti HH, Bernaudin M. 2002). The mechanisms which have been proposed by which EPO produces a neuroprotective effect are (Juul S. 2002): reduction in glutamate toxicity, increased production of neuronal anti-apoptotic factors, reduced nitric oxide mediated injury, anti-inflammatory effects, and anti-oxidant properties.
Other non-erythroid effects are the vasculo-protective ones (Fliser and Bahlmann. 2008). Above all the encouraging experimental results in application of rHuEPO and analogues in vascular medicine have prepared the ground for studies exploring the therapeutic potential of rHuEPO in humans, but before that some safety issues must be resolved. Particularly the dose dependent effects on the number and activation state of thrombocytes, and the stimulation of platelet adherence to endothelium, could be of concern (Fuste B et al., 2002). However, the effects of EPO on thrombocytes obviously may also depend on the presence of an intact eNOS driven NO formation of the endothelium. Lindenblatt and colleagues (Lindenblatt N et al., 2007) recently showed that in mice a 4 week treatment with darbepoetin actually reduced endothelial activation and platelet reactivity, possibly through its effect on eNOS. Furthermore, using a murine intravital microscopic thrombosis model of the cremaster muscle they could show that in wild type mice darbepoetin did not exert prothrombogenic effects, whereas it significantly accelerated thrombus formation in eNOS knockout (eNOS–/–) mice. They concluded that the increase in haematocrit