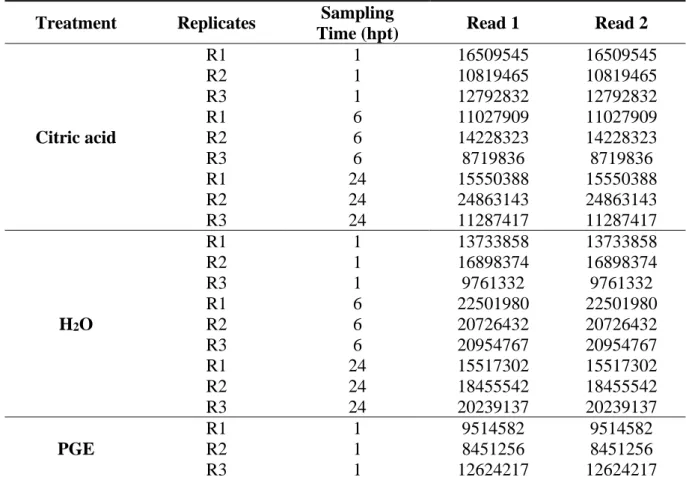
Ph.D. in Agricultural, Food and Forestry Sciences
(XXXII CYCLE)
University ‘Mediterranea’ of Reggio Calabria
Department of AGRARIA
Imen Belgacem
DEVELOPMENT AND APPLICATION OF ALTERNATIVE
CONTROL METHODS TO CONTROL POSTHARVEST
ROTS OF FRESH FRUIT AND VEGETABLES
Tutor:
Prof. Leonardo Schena Ph.D. Coordinator:
Prof. Marco Poiana
Co-Tutor:
Dr. Maria Giulia Li Destri Nicosia Co-tutor:
Dr. Ahmed Abdelfattah
1
Table of content
Abstract ... 3
Riassunto ... 5
Chapter 1. General Introduction ... 7
1. Plant extracts ... 8
1.1. Plant extracts antimicrobial activity ... 8
1.2. Plant induced resistance ... 9
2. Pomegranate peel extracts ... 10
References ... 14
Chapter 2. Pre- and postharvest applications of a pomegranate peel extract to control decay of citrus fruit during storage and shelf life ... 19
Abstract ... 19
1. Introduction ... 20
2. Material and Methods ... 21
2.1. Treatments ... 21
2.2. Preharvest treatments ... 21
2.3. Postharvest treatments ... 22
2.4. Statistical analyses... 22
3. Results ... 23
3.1. Evaluation of natural decays ... 23
3.2. Preharvest treatments ... 23
3.3. Postharvest treatments ... 26
4. Discussion ... 27
References ... 29
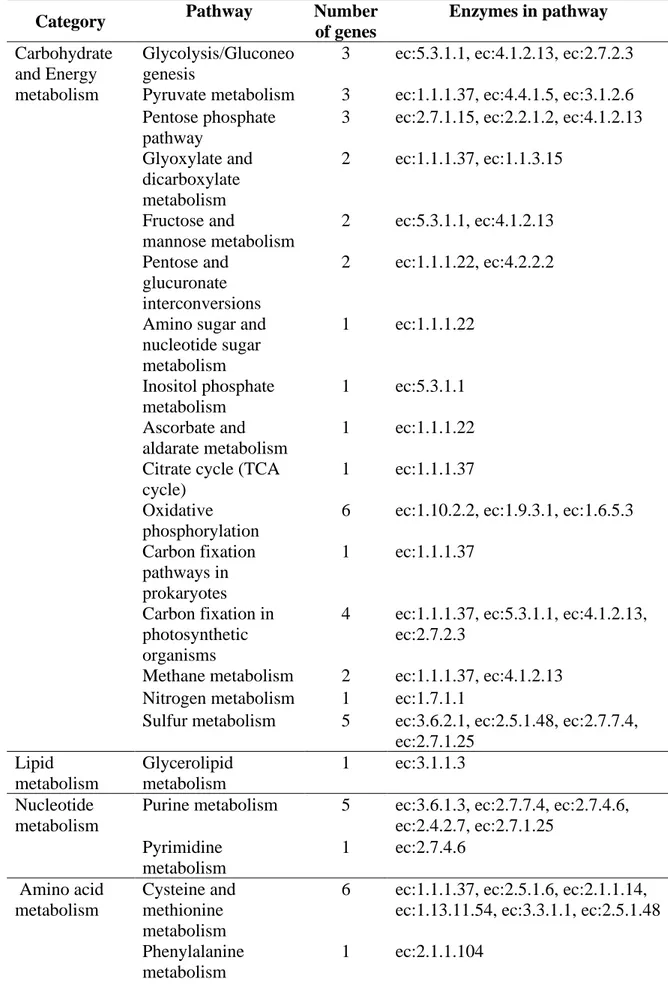
Chapter 3. Transcriptomic Analysis of Orange Fruit Treated with Pomegranate Peel Extract (PGE) ... 32
Abstract ... 32
1. Introduction ... 33
2. Materials and Methods ... 34
2
2.2. Data Analysis ... 34
3. Results ... 35
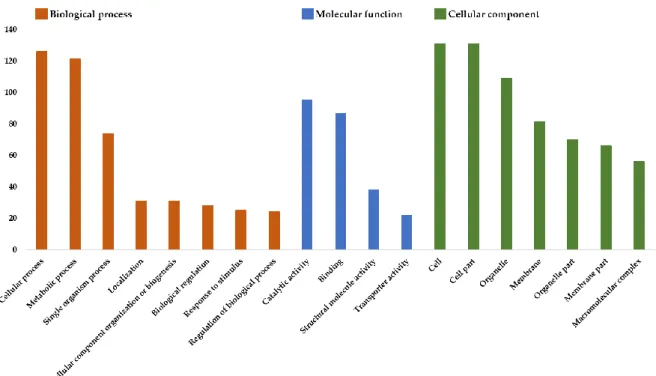
3.1. Gene Ontology Enrichment ... 37
3.2. KEGG Pathways ... 38
4. Discussion ... 40
5. Conclusions ... 43
References ... 44
Chapter 4. Effectiveness of a pomegranate peel extract (PGE) in reducing Listeria monocytogenes in vitro and on fresh-cut pear, apple and melon. ... 47
Abstract ... 47
1. Introduction ... 48
2. Material and Methods ... 49
2.1. Pomegranate peel extract (PGE) and Bacterial strains ... 49
2.2. In vitro assays ... 49 2.3. In vivo assays ... 50 2.4. Statistical analysis ... 51 3. Results ... 51 4. Discussion ... 56 References ... 59
Chapter 5. General discussion and conclusion ... 62
3
Abstract
A Pomegranate Peel Extract, named PGE, has been proposed as a natural antimicrobial substance with a wide spectrum of activity against several postharvest diseases. In the present project, the evaluation of the efficiency of the extract under large scale commercial conditions was conducted, together with a deep investigation on its mechanism of action and its antimicrobial activity against major foodborne pathogen. Under large-scale commercial conditions, the efficiency of PGE was evaluated against rots of Valencia orange and clementine. The extract, proved a significant higher level of protection compared to Imazalil (IMZ), a commonly used chemical fungicide for postharvest treatments. After cold storage and shelf life period, the incidences of decay on oranges sprayed just before harvest with PGE at 12, 6, and 3 g/l, was reduced by 78.9, 76.0, and 64.6%, respectively. Similarly, postharvest dipping treatments with PGE reduced rots by 90.2, 84.3, and 77.6%, respectively. Comparable levels of protection were also achieved on clementine treated before harvest. PGE treatments proved high antimicrobial activity with long persistence resulting in high reduction in losses, longer shelf life and enhancement of the fruit quality. Furthermore, the extract showed a strong antimicrobial activity against epiphytic bacterial and fungal population suggesting its possible use as sanitizers to reduce the microbial contamination of recirculated water in packinghouses.
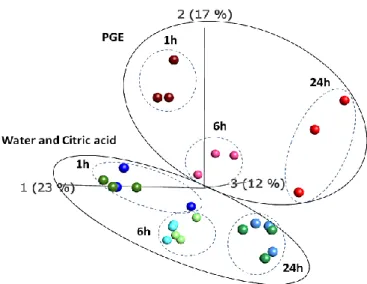
On the other hand, RNA-seq analyses, conducted on wounded orange fruit 0, 6, and 24 h after PGE applications, showed a significantly different transcriptome in treated oranges as compared to control samples. Our analysis showed a very quick response of gene expression (after 1h post treatment) accompanied by high up-regulation of genes (273 deferentially expressed gene). Gene Ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis showed the involvement of 1233 gene ontology (GO) terms and 35 KEGG metabolic pathways. Among these, important defense pathways were induced and antibiotic biosynthesis was the most enriched one. These findings may explain the previously documented preventive and curative activity of PGE against plant diseases.
Finally, the evaluation of the potential use of PGE as natural antimicrobial treatment to reduce the growth of foodborne pathogens using Listeria
monocytogenes as a model pathogen in vitro and on fresh-cuts of melon, apple
and pear, revealed high bactericidal and bacteriostatic activities of PGE.The in
vitro results revealed that regardless of the tested concentration, PGE exerted a
quick and high significant inhibitory activity against all the tested L.
4
antibacterial activity of the extract that significantly reduced the bacterial load on fresh-cut fruit and maintained the population at low levels throughout the storage period. The findings of the present study will incorporate new knowledge on the potential use of PGE as potent alternative control mean against wide range of pathogens and will particularly contribute to the already ongoing process to register a commercial formulation of PGE.
Keywords: Pomegranate peel extract; Citrus rots; Alternative control methods
5
Riassunto
Il progetto di ricerca riguarda lo studio di un estratto di buccia di melograno, denominato PGE (acronimo del nome inglese “PomeGranate peel Extract”), come mezzo di lotta alternativo contro svariati agenti di marciumi dei frutti nella fase post-raccolta. Nel corso dello studio vengono valutate sia le potenzialità applicative dell’estratto, saggiandolo in differenti condizioni commerciali di larga scala, sia i meccanismi di azione e l’attività antimicrobica nei confronti dei principali patogeni del post-raccolta. I risultati di questo studio mostrano l’elevata efficacia del PGE nella difesa post-raccolta di arance Valencia e clementine in condizioni commerciali di larga scala. Infatti, l’estratto ha mostrato un’azione protettiva significativamente superiore all’Imazalil, un fungicida largamente adoperato per la difesa degli agrumi in post-raccolta. L’incidenza dei marciumi sui frutti trattati immediatamente prima della raccolta e sottoposti a frigoconservazione e successiva shelf life è stata ridotta del 78.9, 76.0, e 64.6% , dal PGE rispettivamente alle concentrazioni di 12, 6 e 3 g/litro. Analogamente per quanto riguarda i trattamenti per immersione dei frutti in post-raccolta sono stati raggiunti livelli di riduzione dei marciumi pari al 90.2, 84.3, e 77.6%. Simili risultati sono stati ottenuti dall’applicazione dell’estratto sulle clementine prima della raccolta. La spiccata antività antimicrobica del PGE, associata alla sua lunga persistenza, sortisce oltre ad un prolungamento della shelf life, una forte riduzione delle perdite di prodotto ed un miglioramento della qualità dei frutti. Un’ulteriore caratteristica molto interessante dal punto di vista applicativo è l’abbattimento della carica microbica superficiale conseguente al trattamento dei frutti, che prospetta delle possibilità di utilizzo dell’estratto come sanitizzante delle acque di lavaggio e degli ambienti di stockaggio e lavorazione dei frutti. Gli effetti dei trattamenti con il PGE possono essere attribuiti all’induzione di vie metaboliche sui frutti trattati che ne influenzano la suscettibilità ai marciumi e la qualità. Infatti, dalle analisi di sequenziamento del m-RNA condotto su arance 0, 6 e 24 ore dopo il trattamento con PGE, i profili trascrittomici dei frutti trattati sono significativamente differenti rispetto al controllo non trattato. Dalle analisi si osserva l’attivazione immediata dell’espressione genica (1 ora dopo il trattamento) accompagnata da una diffusa sovrespressione genica (273 geni espressi in maniera differenziale fra trattati e non trattati). Secondo le analisi di genomica funzionale eseguite mediante Gene Ontology (GO) e Kyoto
encyclopedia of genes and genomes (KEGG) per individuare i pathways
metabolici attivati (pathway enrichment analysis) si rileva il coinvolgimento di 1233 termini GO e di 35 vie metaboliche KEGG. Nell’ambito di tali vie metaboliche sono incluse importanti vie deputate alla difesa, fra cui le vie preposte alla sintesi di antibiotici sono le più rappresentate. I risultati di cui sopra spiegano l’azione preventiva, oltre che curativa, dei trattamenti dei frutti con PGE nella lotta contro i patogeni. Inoltre l’azione antimicrobica del PGE è stata
6
valutata nei confronti di Listeria monocytogenes, patogeno dell’uomo di origine alimentare. Il PGE applicato in prove in vitro e per il trattamento di frutta porzionata (meloni, mele e pere) ha mostrato un forte effetto battericida e batteriostatico su questo patogeno-modello. Nelle prove in vitro è stata esercitata una forte azione battericida del PGE significativa per tutte le concentrazioni saggiate. Analogamente i risultati delle prove in vivo confermano la capacità dell’estratto di ridurre significativamente la carica batterica sulla frutta fresca porzionata, mantenendo la popolazione batterica a livelli contenuti durante la frigoconservazione. Le conoscenze acquisite dalla ricerca esposta nella presente tesi costituiscono un importante contributo per la valorizzazione del PGE come efficace metodo di lotta alternativo contro un’ampia gamma di patogeni ed in particolare per favorire il processo di registrazione di un formulato commerciale basato sull’estratto.
Parole chiave: estratto di melograno; marciumi dei frutti di agrumi; metodi di
lotta alternativi; trascrittomica; difesa delle piante; Listeria monocytogenes; frutta pronta al consumo (IV gamma)
7
Chapter 1. General Introduction
Over the past 50 years, the world population has increased to reach more than 7 billion people in 2017. To meet the food demand of such rapid growing population, the food production is estimated to increase by 70% by 2050 (Crist et
al., 2017). This increased demand for food production has in turn increased the
disease pressure on crop plants (Gill and Garg, 2014). Thus, food losses are more and more a matter of serious concern. In particular, fruit and vegetables have the highest wastage rates of any other food product; almost half of all the produced fruit and vegetables are being lost mainly due to pre and post-harvest diseases, poor management techniques, and bad conservation methods (Kitinoja et al., 2018; Rosegrant et al., 2018). Therefore, a major effort is conducted to control these losses.
For decades, chemical fungicides have been the main post harvest disease control mean because of the their high level of efficiency, easy application and relative low cost (Barzman et al., 2015). However, there is an increasing concern about their use because of the potential risks for human health, the negative impact on the environment and non-target microorganisms, and the increasing selection of pathogen resistance strains (Sanzani et al., 2010; Gill and Garg, 2014). This, together with the rise of consumer awareness in food safety and healthy living, is promoting an increasing interest to safe and environmentally friendly alternative control means. Currently, the development of effective and sustainable control means for postharvest diseases is one of the main focus of modern agriculture (Wisniewski et al., 2016). Therefore, the development of new biocontrol strategies is a well investigated line of research that aims to reduce or eliminate the use of chemical pesticides. These strategies are usually applied alone or as a part of integrated pest management program replacing, thereby, chemical pest control (Spadaro and Gullino, 2004; van Lenteren et al., 2018). To date, several alternative methods have been applied, mainly, biocontrol products e.g. Bacillus spp and Candida oleophila (van Lenteren et al., 2018; Mari et al., 2016); disinfecting agents e.g. chlorine, ethanol, and organic acids (Feliziani et
al., 2016); physical treatments e.g. UV radiation, cold, heat, radiofrequency, and
modified atmosphere (Usall et al., 2016; Yao et al., 2018); and more importantly Generally Regarded As Safe (GRAS) plant derivates including natural components, plant volatiles and, in particular, plant extracts (Palou et al., 2016; Mari et al., 2016).
8 1. Plant extracts
Plant extracts have recently emerged as very promising alternatives to chemicals. The high demand for environmentally sound and biodegradable products grabbed a special attention for more profound research on these substances. In fact, the use of plant extracts as antimicrobial agents has been known for centuries. They were used as remedies in folk medicine (Petereit et al., 1991; Ehrhardt et al., 2007). Therefore, the activity of several plant extracts has been intensively investigated. They showed different mechanism of action including direct antimicrobial activity against pathogens and/or induction of resistance in the plant host.
1.1. Plant extracts antimicrobial activity
Many plant extracts have been investigated as natural compounds to control plant diseases or to prevent fruit spoilage proving effectiveness against a wide range of plant and food borne pathogens such as Penicillium digitatum,
Penicillium italicum, Rhizopus sp., Alternaria solani, Escherichia coli, Staphylococcus aureus, Pseudomonas spp., Salmonella spp., etc. (Betoni et al.,
2006; Mostafa et al., 2018; Latha et al., 2009). The richness of these natural extracts in secondary metabolites, such as phenols, quinones, flavonoids, saponins and tannins, are reported to be the major active components of their antimicrobial activity (Gurjar et al., 2012). For instance, allicin (diallyl thio sulphinate) is a volatile antimicrobial component synthetized by garlic when the tissues are damaged. Extracts of garlic showed high antimicrobial efficiency against a range of plant and food pathogens such as Alternaria spp. Phytophthora
spp., Escherichia coli, and Salmonella, spp., (Ekwenye and Elegalam, 2005).
Other active components including curcumin and gingerol, highly present in turmeric (Curcuma longa Linn.) and ginger (Zingiber officinale Rosc.), respectively, were reported to also possess high antibacterial and antifungal properties against Phytopthora infestans, Fusarium solani, Pyricularia oryzae, E.
coli and Staphylococcus aureus, etc. (Gurjar et al., 2012; Jakribettu et al., 2016).
Notably, other extensively investigated and commercialized plant extracts are neem extracts (Azadirachta indica). They demonstrated strong and rapid antifungal, antibacterial and insecticidal activity due to the presence of important active components presented by Azadirachtin terpenoides (Lokanadhan et al., 2012; Girish and Shankara, 2008). Although the main antimicrobial active components of plant extracts are generally identified, reports showed that some more components might also be responsible for the antimicrobial effect of nature substances and plant extracts (Rauha et al., 2000). Furthermore, although the antimicrobial activity of these extracts is variable and relative to the extract composition, various extraction solvents such as acetone, methanol, ethanol, diethyl ether, chloroform, and water, have been investigated for better yield and quality of the extract (Jones and Kinghorn, 2006; Azmir et al., 2013). In
9
particular, the choice of the solvent depends on the target active components to be extracted, in order to guarantee a consistent antimicrobial activity (Parekh et
al., 2006). A good solvent should have a high efficiency in solubilizing
antimicrobial components, elevated extraction rate, fast ability to evaporate at low temperature, and low toxicity (Gurjar et al., 2012). The most commonly used solvents for plant material extraction are methanol, ethanol and water (Turkmen
et al., 2006). Among these, water is generally less effective in solubilizing
compound and its evaporation is not as fast as alcohols. On the other hand, methanol may pose toxicological issues. This latter aspect is very important since most of the antimicrobial plant extracts are used to control food and post-harvest pathogens and, therefore, the choice of the solvent is critical in order to respect the human health and the environment.
1.2. Plant induced resistance
Several natural alternative control substances and, particularly, plant extracts haven’t only showed a direct antimicrobial activity against major plant pathogens but, interestingly, they induce resistance in the host plant similar to the one induced by pathogen infection (Oostendorp et al., 2001; Burketova et al., 2015). This is an important feature that is very valuable as pest control strategy especially in organic agriculture and also in integrated pest management program that aims to reduce the use of chemical pesticides. These natural treatments are considered plant resistance inducers or so-called plant resistance activators. They induce the plant’s own defense mechanisms through the activation of battery of defense genes protecting, therefore, the plant against biotic and abiotic stresses (Burketova et al., 2015; Conrath, 2011). The protection level depends mainly on the type of the elicitor, the way and timing of its application as well as the plant genotype and developmental stage (Alexandersson et al., 2016). There are two forms of induced resistance: systemic acquired resistance (SAR), and induced systemic resistance (ISR). Both forms are triggered by prior infection or artificial treatment, and they are efficient against broad spectrum of pathogens, and they can be differentiated by the nature of the elicitor and the metabolic pathways involved (Burketova et al., 2015). SAR is triggered by local pathogen infection or artificial treatments such as BTH, probenazole. It relies on pathways regulated by salicylic acid (SA) and it induces the accumulation of pathogenesis-related proteins (Bernsdorff et al., 2016). Whereas ISR is initiated by the colonization of the roots by rhizobacteria or fungi and it is triggered by mobile signals consisting of jasmonic acid (JA) and ethylene (ET) and, unlike SAR, it doesn’t involve the accumulation of pathogenesis-related proteins, but instead it activates the production of antimicrobial peptides (defensins) (Oostendorp et al., 2001). Therefore, the level of efficiency of plant resistance elicitors usually depends on their ability to enhance plant resistance mechanism. The plant’s response to resistance inducers is generally associated with the activation of signal
10
transduction pathways leading to the alterations in cell wall composition, production of phytoalexins and anti-microbial protein, deposition of callose, production of reactive oxygen and nitric oxide, accumulation of pathogenesis-related (PR) proteins, etc (Alexandersson et al., 2016; Oliveira et al., 2016). The efficiency of resistance elicitors in higher plants is well documented and the advances in research is accompanied by the commercialization of products that respect the human health and the environment and, in the same time, prove high efficiency in controlling plant pathogens. For instance, one of the widely known and effective plant extracts is the ethanolic extract of knotweed (Reynoutria
sacchalinensis (F. Schmidt) Nakai) commercialized under the name of Milsana®.
It is categorized as resistance inducer against powdery mildew of cucumber, wheat and roses (Vechet et al., 2009; Burketova et al., 2015; Pasini et al., 1997; Fofana et al., 2002). It induces the expression of genes responsible of the biosynthesis of flavonoids and the accumulation of phytoalexins and hydrogen peroxide. Many other plant extracts were also tested as possible resistance inducers such as the aqueous extracts of neem leaves (Azadirachta indica Juss.) which triggered the activation of defense related genes such as phenylalanine ammonia lyase (PAL) and tyrosine ammonia lyase (TAL) with rapid accumulation of phenolic compounds (Paul and Sharma, 2002). Similarly, extracts of Hedera helix also proved implication in inducing defense responses to control Erwinia amylovora in apple rootstock (Baysal and Zeller, 2004). Another interesting example demonstrating that plant wastes could also serve as plant inducers, is extracts of sugar beet waste. These extracts showed, under greenhouse conditions, high efficiency in controlling Phytophthora infestans. The treated potatoes showed induction of the pathogenesis-related protein (PR-1 and PR-2) (Moushib et al., 2013).
2. Pomegranate peel extracts
Although the global trend has shifted towards alternative control means and particularly plant extracts, finding good extract that provides at once several features such as high antimicrobial efficiency, long persistence, and ability to induce resistance in the host plant is essential to build up a potent integrated pest management strategy. In this regard, extracts from pomegranate peel (Punica
granatum L.) have emerged as very promising antimicrobial substances. Their
medical applications in ancient times have pushed researchers into more profound studies about the application of these extracts not only in the medical sector but also in other biology fields including plant protection. Serval studies started with phytochemical screening of different parts of the pomegranate fruit revealing high predominance of bioactive components in the peel part (Singh et al., 2002; Orak et al., 2012). These bioactive constituents are represented by phenolic components, mainly punicalagin, and ellagic and gallic acids. These molecules are reported to be very potent antioxidants components (Tehranifar et al., 2011;
11
Zahin et al., 2010). However, the concentration of these active components varies from an extract to another depending on many factors such as extraction method, fruit maturity stage, variety, etc (Shwartz et al., 2009; Romeo et al., 2015). Therefore, many studies have investigated the antimicrobial activity of different pomegranate peel extracts (PPEs) and evaluated their potential use as biocontrol method against major plant diseases. Different PPEs showed, in in vitro trials, high significant inhibitory activity against various major plant and food pathogens including Botrytis cinerea, Penicillium digitatum, Alternaria
alternata, Stemphylium botryosum, Fusarium oxysporum, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella spp., etc (Rongai et al.,
2015; Glazer et al., 2012; Rongai et al., 2018; Tayel et al., 2009; Nuamsetti et
al., 2012; Oraki et al., 2011). Although, in the agronomic sector, most of the
investigations have yet been in vitro trials, numerous studies were carried out under in vivo conditions. For instance, (Elsherbiny et al., 2016) demonstrated that a methanolic extract of pomegranate peel exerts both preventive and curative antimicrobial activity against dry rot of potato tubers caused by Fusarium
sambucinum. Similarly, another PPE showed high efficiency in reducing rots
caused by P. digitatum on citrus fruit (Tayel et al., 2009). While, other reports showed the effect of these extracts in preserving the quality and extending the shelf life of fruit when the extract is incorporated in the coating formulations (Rongai et al., 2018; Nair et al., 2018). This high antimicrobial activity of pomegranate peel extracts pushed for more pronounced investigations on its possible toxicological effects on the human health. Studies proved its safe use and suggested its potential application as food additive or as treatment against several human diseases (El-Desouky et al., 2015; Jahromi et al., 2015).
Recently a particularly promising extract from pomegranate peel has been identified as PGE (Romeo et al., 2015). It is a concentrated aqueous extract obtained from 80% ethanol/water mixture after evaporation of ethanol under vacuum at 40 °C. PGE extract solution is supplemented with 1% citric acid as antioxidant or acidifying agent, regarded as safe and widely accepted by the public opinion and authorities (Romeo et al., 2015). A chemical characterization of PGE showed that the extract is rich in polyphenols with 66.97g gallic acid equivalents/kg of total Phenolics presented mainly by gallic acid and punicalagin, and 21.64mg cyanidin 3-glucoside equivalents/kg of total anthocyanins. PGE proved highly effective against a wide range of major fungal diseases under both experimental and commercial conditions. It showed a wide spectrum of activity being effective against grey mold caused by Botrytis cinerea on table grapes and sweet cherries, anthracnose caused by Colletotrichum acutatum sensu stricto and
Colletotrichum godetiae on olives, brown rot caused by Monilinia spp. on sweet
cherries, green and blue molds caused by P. digitatum and P. italicum on citrus and blue mold caused by Penicillium expansum on apples (Li Destri Nicosia et
12
antimicrobial effect with preventive and curative activity, and a long persistence even when applied under commercial conditions (Pangallo et al., 2017b). Furthermore, recent investigations have demonstrated the induction of resistance in citrus and olive fruit treated with PGE, although specific studies to identify the involved genes in the induced resistance have not been yet conducted (Pangallo
13 Scope of the thesis
Considering the potential use of PGE as natural antimicrobial and plant protection preparation, the aim of the present study was to advance the current knowledge of this extract. In this context, specific investigations were conducted to: i) evaluate its efficacy, under large scale commercial conditions, against postharvest blue and green mold of citrus as pre and postharvest treatment. ii) investigate its mechanism of action using a transcriptomic approach to determine the genes and pathways activated in citrus tissues after the extract treatment; and iii) evaluate the potential use of PGE as natural antimicrobial sanitizer to control a major foodborne pathogen such as Listeria monocytogenes. Obtained results will significantly contribute to the already ongoing process to register a commercial formulation of PGE.
14 References
Alexandersson, E., Mulugeta, T., Lankinen, Å., Liljeroth, E. and Andreasson, E. (2016) Plant resistance inducers against pathogens in Solanaceae
species—from molecular mechanisms to field application. International
journal of molecular sciences 17 (10), 1673.
Azmir, J., Zaidul, I., Rahman, M., Sharif, K., Mohamed, A., Sahena, F., Jahurul, M., Ghafoor, K., Norulaini, N. and Omar, A. (2013) Techniques for
extraction of bioactive compounds from plant materials: A review.
Journal of Food Engineering 117 (4), 426-436.
Barzman, M., Bàrberi, P., Birch, A. N. E., Boonekamp, P., Dachbrodt-Saaydeh, S., Graf, B., Hommel, B., Jensen, J. E., Kiss, J. and Kudsk, P. (2015) Eight principles of integrated pest management. Agronomy for
sustainable development 35 (4), 1199-1215.
Baysal, Ö. and Zeller, W. (2004) Extract of Hedera helix induces resistance on apple rootstock M26 similar to Acibenzolar-S-methyl against Fire Blight (Erwinia amylovora). Physiological and Molecular Plant Pathology 65 (6), 305-315.
Bernsdorff, F., Döring, A.-C., Gruner, K., Schuck, S., Bräutigam, A. and Zeier, J. (2016) Pipecolic acid orchestrates plant systemic acquired resistance and defense priming via salicylic acid-dependent and-independent pathways. The Plant Cell 28 (1), 102-129.
Betoni, J. E. C., Mantovani, R. P., Barbosa, L. N., Di Stasi, L. C. and Fernandes Junior, A. (2006) Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Memórias do Instituto
Oswaldo Cruz 101 (4), 387-390.
Burketova, L., Trda, L., Ott, P. G. and Valentova, O. (2015) Bio-based resistance inducers for sustainable plant protection against pathogens.
Biotechnology advances 33 (6), 994-1004.
Conrath, U. (2011) Molecular aspects of defence priming. Trends in plant
science 16 (10), 524-531.
Crist, E., Mora, C. and Engelman, R. (2017) The interaction of human population, food production, and biodiversity protection. Science 356 (6335), 260-264.
Ehrhardt, C., Hrincius, E. R., Korte, V., Mazur, I., Droebner, K., Poetter, A., Dreschers, S., Schmolke, M., Planz, O. and Ludwig, S. (2007) A polyphenol rich plant extract, CYSTUS052, exerts anti influenza virus activity in cell culture without toxic side effects or the tendency to induce viral resistance. Antiviral research 76 (1), 38-47.
Ekwenye, U. and Elegalam, N. (2005) Antibacterial activity of ginger (Zingiber officinale Roscoe) and garlic (Allium sativum L.) extracts on Escherichia coli and Salmonella typhi. Int J Mol Adv Sci 1, 411-416.
15
El-Desouky, T., Sherif, M. R., Sherif, M. S. and Khayria, N. M. (2015) Protective effect of aqueous extract pomegranate peel against sterigmatocystin toxicity in rat. Journal of Drug Delivery and
Therapeutics 5 (5), 9-18.
Elsherbiny, E. A., Amin, B. H. and Baka, Z. A. (2016) Efficiency of pomegranate (Punica granatum L.) peels extract as a high potential natural tool towards Fusarium dry rot on potato tubers. Postharvest
Biology and Technology 111, 256-263.
Feliziani, E., Lichter, A., Smilanick, J. L. and Ippolito, A. (2016) Disinfecting agents for controlling fruit and vegetable diseases after harvest.
Postharvest Biology and Technology 122, 53-69.
Fofana, B., McNally, D. J., Labbé, C., Boulanger, R., Benhamou, N., Séguin, A. and Bélanger, R. R. (2002) Milsana-induced resistance in powdery
mildew-infected cucumber plants correlates with the induction of
chalcone synthase and chalcone isomerase. Physiological and molecular
plant pathology 61 (2), 121-132.
Gill, H. K. and Garg, H. (2014) Pesticides: environmental impacts and management strategies. Pesticides-toxic aspects. IntechOpen. Girish, K. and Shankara, B. S. (2008) Neem–a green treasure. Electronic
journal of Biology 4 (3), 102-111.
Glazer, I., Masaphy, S., Marciano, P., Bar-Ilan, I., Holland, D., Kerem, Z. and Amir, R. (2012) Partial identification of antifungal compounds from Punica granatum peel extracts. Journal of agricultural and food chemistry 60 (19), 4841-4848.
Gurjar, M. S., Ali, S., Akhtar, M. and Singh, K. S. (2012) Efficacy of plant extracts in plant disease management. Agricultural Sciences 3 (3), 425. Jahromi, S. B., Pourshafie, M. R., Mirabzadeh, E., Tavasoli, A., Katiraee, F.,
Mostafavi, E. and Abbasian, S. (2015) Punica granatum peel extract toxicity in mice. Jundishapur J Nat Pharm Prod 10 (4).
Jakribettu, R. P., Boloor, R., Bhat, H. P., Thaliath, A., Haniadka, R., Rai, M. P., George, T. and Baliga, M. S. (2016) Ginger (Zingiber officinale Rosc.) Oils. Essential Oils in Food Preservation, Flavor and Safety. Elsevier. 447-454.
Jones, W. P. and Kinghorn, A. D. (2006) Extraction of plant secondary metabolites. Natural products isolation. Springer. 323-351.
Kitinoja, L., Tokala, V. Y. and Brondy, A. (2018) Challenges and opportunities for improved postharvest loss measurements in plant-based food crops.
Journal of Postharvest Technology 6 (4), 16-34.
Latha, P., Anand, T., Ragupathi, N., Prakasam, V. and Samiyappan, R. (2009) Antimicrobial activity of plant extracts and induction of systemic
resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biological Control 50 (2), 85-93.
16
Li Destri Nicosia, M. G., Pangallo, S., Raphael, G., Romeo, F. V., Strano, M. C., Rapisarda, P., Droby, S. and Schena, L. (2016) Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biology and Technology 114, 54-61.
Lokanadhan, S., Muthukrishnan, P. and Jeyaraman, S. (2012) Neem products and their agricultural applications. Journal of Biopesticides 5, 72. Mari, M., Bautista-Baños, S. and Sivakumar, D. (2016) Decay control in the
postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biology and Technology 122, 70-81.
Mostafa, A. A., Al-Askar, A. A., Almaary, K. S., Dawoud, T. M., Sholkamy, E. N. and Bakri, M. M. (2018) Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi journal of
biological sciences 25 (2), 361-366.
Moushib, L. I., Witzell, J., Lenman, M., Liljeroth, E. and Andreasson, E. (2013) Sugar beet extract induces defence against Phytophthora infestans in potato plants. European journal of plant pathology 136 (2), 261-271. Nair, M. S., Saxena, A. and Kaur, C. (2018) Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food chemistry 240, 245-252.
Nuamsetti, T., Dechayuenyong, P. and Tantipaibulvut, S. (2012) Antibacterial activity of pomegranate fruit peels and arils. Science Asia 38 (3), 319-22. Oliveira, M., Varanda, C. and Félix, M. (2016) Induced resistance during the
interaction pathogen x plant and the use of resistance inducers.
Phytochemistry letters 15, 152-158.
Oostendorp, M., Kunz, W., Dietrich, B. and Staub, T. (2001) Induced disease resistance in plants by chemicals. European Journal of Plant Pathology 107 (1), 19-28.
Orak, H. H., Yagar, H. and Isbilir, S. S. (2012) Comparison of antioxidant activities of juice, peel, and seed of pomegranate (Punica granatum L.) and inter-relationships with total phenolic, tannin, anthocyanin, and flavonoid contents. Food Science and Biotechnology 21 (2), 373-387. Oraki, H. H., Demirci, A. Ş. and Gümüş, T. (2011) Antibacterial and antifungal
activity of pomegranate (Punica granatum L. cv.) peel. Electronic Journal
of Environmental, Agricultural & Food Chemistry 10 (3).
Palou, L., Ali, A., Fallik, E. and Romanazzi, G. (2016) GRAS, plant-and
animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce.
Postharvest Biology and Technology 122, 41-52.
Pangallo, S., Li Destri Nicosia, M., Raphael, G., Levin, E., Ballistreri, G., Cacciola, S., Rapisarda, P., Droby, S. and Schena, L. (2017a) Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant pathology 66 (4), 633-640.
17
Pangallo, S., Li Destri Nicosia, M. G., Agosteo, G. E., Abdelfattah, A., Romeo, F. V., Cacciola, S. O., Rapisarda, P. and Schena, L. (2017b) Evaluation of a Pomegranate Peel Extract (PGE) as Alternative Mean to Control Olive Anthracnose. Phytopathology (ja).
Parekh, J., Jadeja, D. and Chanda, S. (2006) Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity.
Turkish Journal of Biology 29 (4), 203-210.
Pasini, C., D'Aquila, F., Curir, P. and Gullino, M. L. (1997) Effectiveness of antifungal compounds against rose powdery mildew (Sphaerotheca pannosa var. rosae) in glasshouses. Crop Protection 16 (3), 251-256. Paul, P. and Sharma, P. (2002) Azadirachta indica leaf extract induces
resistance in barley against leaf stripe disease. Physiological and
molecular plant pathology 61 (1), 3-13.
Petereit, F., Kolodziej, H. and Nahrstedt, A. (1991) Flavan-3-ols and
proanthocyanidins from Cistus incanus. Phytochemistry 30 (3), 981-985. Rauha, J.-P., Remes, S., Heinonen, M., Hopia, A., Kähkönen, M., Kujala, T.,
Pihlaja, K., Vuorela, H. and Vuorela, P. (2000) Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic
compounds. International journal of food microbiology 56 (1), 3-12. Romeo, F. V., Ballistreri, G., Fabroni, S., Pangallo, S., Li Destri Nicosia, M. G.,
Schena, L. and Rapisarda, P. (2015) Chemical characterization of different sumac and pomegranate extracts effective against Botrytis cinerea rots. Molecules 20 (7), 11941-11958.
Rongai, D., Pulcini, P., Pesce, B. and Milano, F. (2015) Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sciences 10 (1).
Rongai, D., Sabatini, N., Pulcini, P., Di Marco, C., Storchi, L. and Marrone, A. (2018) Effect of pomegranate peel extract on shelf life of strawberries: computational chemistry approaches to assess antifungal mechanisms involved. Journal of food science and technology 55 (7), 2702-2711. Rosegrant, M. W., Magalhaes, E., Valmonte-Santos, R. A. and Mason-D’Croz,
D. (2018) Returns to investment in reducing postharvest food losses and increasing agricultural productivity growth. Prioritizing Development: A
Cost Benefit Analysis of the United Nations' Sustainable Development Goals, 322.
Sanzani, S. M., Schena, L., De Girolamo, A., Ippolito, A. and González-Candelas, L. (2010) Characterization of genes associated with induced resistance against Penicillium expansum in apple fruit treated with quercetin. Postharvest biology and technology 56 (1), 1-11.
Shwartz, E., Glazer, I., Bar-Ya’akov, I., Matityahu, I., Bar-Ilan, I., Holland, D. and Amir, R. (2009) Changes in chemical constituents during the
maturation and ripening of two commercially important pomegranate accessions. Food Chemistry 115 (3), 965-973.
18
Singh, R., Chidambara Murthy, K. and Jayaprakasha, G. (2002) Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. Journal of agricultural and food chemistry 50 (1), 81-86.
Spadaro, D. and Gullino, M. L. (2004) State of the art and future prospects of the biological control of postharvest fruit diseases. International journal
of food microbiology 91 (2), 185-194.
Tayel, A., El-Baz, A., Salem, M. and El-Hadary, M. (2009) Potential
applications of pomegranate peel extract for the control of citrus green mould. Journal of Plant Diseases and Protection 116 (6), 252-256. Tehranifar, A., Selahvarzi, Y., Kharrazi, M. and Bakhsh, V. J. (2011) High
potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Industrial
Crops and Products 34 (3), 1523-1527.
Turkmen, N., Sari, F. and Velioglu, Y. S. (2006) Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods.
Food chemistry 99 (4), 835-841.
Usall, J., Ippolito, A., Sisquella, M. and Neri, F. (2016) Physical treatments to control postharvest diseases of fresh fruits and vegetables. Postharvest
Biology and Technology 122, 30-40.
van Lenteren, J. C., Bolckmans, K., Köhl, J., Ravensberg, W. J. and Urbaneja, A. (2018) Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63 (1), 39-59.
Vechet, L., Burketova, L. and Sindelarova, M. (2009) A comparative study of the efficiency of several sources of induced resistance to powdery mildew (Blumeria graminis f. sp. tritici) in wheat under field conditions. Crop
protection 28 (2), 151-154.
Wisniewski, M., Droby, S., Norelli, J., Liu, J. and Schena, L. (2016) Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biology and Technology 122, 3-10.
Yao, L., Yi, L., Li, S. and Chen, Y. (2018) Efficacy of heat treatment in
controlling citrus Huanglongbing by different temperatures combinations in field. Journal of Southern Agriculture 49 (7), 1346-1350.
Zahin, M., Aqil, F. and Ahmad, I. (2010) Broad spectrum antimutagenic activity of antioxidant active fraction of Punica granatum L. peel extracts.
Mutation Research/Genetic Toxicology and Environmental Mutagenesis
19
Chapter 2. Pre- and postharvest applications of a
pomegranate peel extract to control decay of citrus fruit
during storage and shelf life
Abstract: Blue and green mold rots caused by Penicillium italicum and Penicillium digitatum are the major postharvest citrus diseases. These diseases
are commonly controlled with conventional chemical compounds but legislative restrictions, consumer concerns and the developments of resistant strains of the pathogens have increasingly led to the search for alternative methods of control. A pomegranate peel extract (PGE) was very effective in controlling postharvest rots of valencia orange and clementine, under large scale commercial conditions. The extract, proved a significant higher level of protection compared to Imazalil (IMZ), a commonly used fungicide for postharvest treatments. After cold storage and shelf life period, the incidence of decay on oranges sprayed before harvest with PGE at 12, 6 and 3 g/l was reduced by 78.9, 76.0, and 64.6%, respectively. Similarly, postharvest dipping treatments with PGE reduced rots by 90.2, 84.3, and 77.6%, respectively. Comparable levels of protection were also achieved on clementine treated before harvest. The high level of efficacy and the consistence of results on different fruit species (clementine and orange) and with different application methods (pre- and postharvest) was evidence of reliability and flexibility, making PGE a potent antimicrobial treatment against postharvest diseases of citrus. PGE also showed a strong antimicrobial activity against epiphytic fungal and bacterial populations suggesting its possible use as sanitizers to reduce the microbial contamination of recirculated water in packinghouses. The results of the present study encourage the replacement of chemical fungicides and sanitizers with PGE to control citrus postharvest rots. This may lead to significant reductions of losses, lower environmental impact and improved quality and safety of products without chemical residues.
Keywords: Citrus rots; Alternative control methods; Penicillium digitatum; Penicillium italicum; Pomegranate peel extract; Valencia orange; Clementine
20 1. Introduction
Citrus is one of the most significant agricultural crops worldwide and, particularly, in Italy which ranked as the second largest European citrus producer after Spain (Eurostat, 2017). Oranges (Citrus sinensis (L.) Osbeck) and clementine (Citrus clementina hort. ex Tanaka) are considered the leading grown citrus species in the world with a production reaching 73 and 33 Million tonnes in 2017, respectively (FAO, 2017). These citrus species are characterized by a longer shelf life compared to other tropical and sub-tropical fruits. However, post-harvest losses are of serious concern. Each year, 30 to 50% of the total citrus production is wasted due to postharvest diseases (Porat et al., 2000). These diseases include Sour rot, Brown rot, Alternaria rot, Stem-end rots, and Penicillium rot caused by Geotrichum candidum, Phytophthora spp., Alternaria
spp., Diplodia natalensis and Phomopsis, and Penicillium. ulaiense, respectively.
The incidence of these fungal pathogens on the fruit is generally low, but when the environmental conditions are convenient for the pathogen growth, serious damages are observed. However, blue and green mold rots caused by P. italicum and P. digitatum are the major postharvest citrus diseases (Youssef et al., 2012). Both pathogens require wounds to infect the fruit, therefore, fruit injuries caused during harvest, packaging house manipulation, transport or storage, help to initiate the pathogen infection (Palou, 2014). Accordingly, good harvest and post-harvest handling is critical to prevent fungal diseases. Although in most citrus industries, these preventions are already delicately considered, blue and green molds are still inducing high economic losses to the citrus sector. Accordingly, a major effort is dedicated to search for efficient control means. For decades, the application of chemical fungicides in packaging houses, before fruit storage, has been the main management technique to prevent postharvest diseases. For instance, Imazalil (IMZ), widely used chemical fungicide, proved high efficiency in controlling postharvest pathogens (Altieri et al., 2013). However, with the increase of consumer awareness about chemical residues together with the latest European legislative restrictions, a special attention has recently been given to the alternative control means (Erasmus et al., 2015; Palou, 2014; Wisniewski et
al., 2016). Up to date, several alternative control methods have been used
including the application of antagonists and the use of resistance inducers and natural fungicides, however, their application in the citrus sector is still limited (Janisiewicz and Korsten, 2002; Talibi et al., 2014). Their inconsistent activity under practical commercial conditions, low persistence as well as the risk of fruit injury are the main limitations of these alternative methods (Palou et al., 2008; Youssef et al., 2012). Therefore, a continuous effort is dedicated to this line of research in order to find effective alternatives to control plant diseases. Accordingly, some of plant extracts proved high efficiency and persistence as alternative fungicides to control postharvest rots. In particular, pomegranate peel extract, called PGE, has recently proved to efficiently control wide range of
21
postharvest fungal pathogens including P. digitatum and P. italicum (Romeo et
al., 2015; Li Destri Nicosia et al., 2016). Its composition is proved to be rich in
phenolics including gallic acid and particularly punicalagins which are phenolic components only found in pomegranate (Romeo et al., 2015). This extract is characterized by preventive and curative effect, wide spectrum of activity, high efficiency and long persistence (Pangallo et al., 2017b). Studies not only proved its direct antimicrobial activity but also its ability to induce resistance in treated fruit tissues (Pangallo et al., 2017a; Pangallo et al., 2017b; Belgacem et al., 2019).
The aim of the present study was to evaluate, under practical commercial conditions, the effect of PGE treatments against postharvest rots of clementine and orange fruit through pre and postharvest application. The effect of PGE was further compared to a chemical control product (IMZ), and two salts (potassium bicarbonate (KHCO3) and sodium bicarbonate (NaHCO3)) which have already been proposed as effective and safe alternative means.
2. Material and Methods 2.1. Treatments
A concentrated extract of pomegranate peel (PGE) (120 g/l of dry matter) was obtained according to Romeo et al. (2015) from ripe pomegranate (Punica
granatum L.) fruit cv. “Mollar De Elche”. The extract was stored at 4°C and
diluted before use with tap water to obtain three concentrations of 12, 6 and 3 g/l. KHCO3 and NaHCO3 (Sigma Aldrich S.r.l., Milan, Italy) were dissolved in tap water to get 2% (W/V) solutions. A commercial formulation of IMZ (Deccozil 50, Decco, Italy) was used with the recommended concentration of 0.1%. All solutions were prepared just before use.
2.2. Preharvest treatments
Experiments were conducted in commercial orchards of Valencia oranges (GPS coordinates: 38°13’06.24”N105 – 16°14’11.54”E) and clementines (GPS coordinates: 38°13’06.24”N – 16°14’11.54”E) in April 2016 and November 2017, respectively. Uniform, symptomless, and not damaged plants were selected for the experiments. In both years, treatments were conducted according to a completely randomized block design of 3 replicates, each consisting of 5 plants. Plants were sprayed with approximately 10 litters of solution containing PGE at three different concentrations, salts (NaHCO3 and KHCO3) or tap water (control), using a backpack atomizer. The following day, the treated fruit were harvested, put in plastic boxes and stored for 1 week at 4±1°C and 95-98% RH.
22
Fruit from untreated plants were stored without any additional treatment (field control), or dipped in IMZ solution (chemical control) as described above for postharvest treatments. All fruit were cold stored for 7 days and then subjected to other 7 days of shelf life at 20±2°C. The fruit incidence was controlled at the end of the cold storage and shelf life. For each treatment, 2400 oranges or 6000 clementines placed in 12 plastic boxes (three replicates of 4 boxes) were visually inspected to determine the incidence of rots.
For both Valencia oranges and clementine fruit, the epiphytic bacterial and fungal population was evaluated before and at the end of cold storage. From 5 fruit, five disks of flavedo and albedo were cut using a sterile cork borer of 10 mm diameter.
Disks were put into sterile cups containing 100 ml of sterile water and shacked with rotary shaker at 200 rpm for 30 min. A volume of 100 μl of the obtained solutions were serially diluted and plated on PDA media to determine the fungal and bacterial population as described above respectively. the number of colony forming units was recorded and converted to CFU/ fruit.
2.3. Postharvest treatments
Oranges (cv Valencia) were collected in April 2016 from a commercial orchard located in Calabria, Southern Italy (GPS coordinates: 38°13’06.24”N – 16°14’11.54”E). Experiments were conducted by fruit dipping for 30 s in an IMZ solution, used as chemical control, or for 5 min in PGE at 12, 6 and 3 g/l, KHCO3
or NaHCO3. Oranges dipped in tap water and untreated oranges were used as
controls. Fruit were dried and waxed on the commercial packing line. Treated fruit were cold-stored for 7 days at 4°±1°C and -98% RH, and then subjected to 7 days of shelf life at 20± 2°C. The disease incidence was determined at the end of the cold storage and shelf life. For each treatment, 2400 fruit arranged in 12 plastic boxes (three replicates of 4 boxes) were visually inspected to determine the disease incidence. To evaluate the sanitizing activity of the tested treatments, three subsamples of 50 ml were collected from each dipping solution and maintained for 3h at 5°C. After shaking, a volume of 100 μl of water suspensions were serially diluted and plated on triplicate plates of Potato Dextrose Agar (PDA) and PDA amended with ampicillin and streptomycin sulphate (250 mg/l each) to detect the bacterial and fungal load, respectively. After incubation at 22°C for 3–4 days, the number of colony forming units was recorded and converted to CFU/ml.
2.4. Statistical analyses
After analysis of variance (ANOVA), significant differences between treatments were determined according to Tukey's test at a significance level of
23
P < 0.05. Percentages were converted into Bliss angular values (arcsine √%) before analysis.
3. Results
3.1. Evaluation of natural decays
Clementine and orange fruit were evaluated once the rot softening symptoms appeared on the peel. The identification of the fungal mycelium and spores, on the fruit rots, showed that 80% of rots were caused by P. digitatum, followed by P. italicum (around 20%), and lastly by both fungal pathogens (around 10%). However, grey mold infections caused by Botrytis cinerea were detected only on clementine and represented around 5% of the detected rots.
3.2. Preharvest treatments
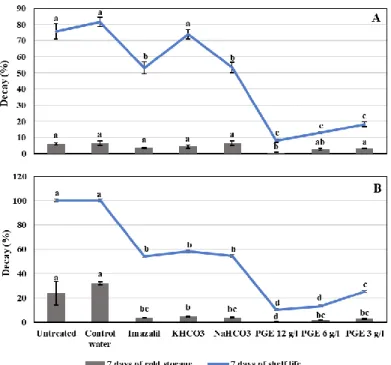
At the end of cold storage, low rot incidence was detected on all treated orange, before harvest, where the controls, orange sprayed with tap water and untreated, showed only 6.5% and 5.9% of rot symptoms, respectively (Fig.1, A). Among all treatments, only PGE (12g/l) showed significant rot reduction of 92.3 %. While, at the end of the shelf life, the disease incidence highly increased to reach 81.4% with control fruit. This was greatly reduced by PGE treatments to reach 90.2, 84.3, and 77.6%, when treated with PGE 12, 6, 3 g/l, respectively. Comparing to other treatments, PGE was significantly more effective than tested salts and IMZ.
A higher incidence of decay was observed with clementine fruit where the control treated fruit reached 32% of decay at the end of cold storage (Fig.1, B). PGE effectively reduced the rot incidence by 98.1, 94.1, and 91.3% at the three tested concentrations, respectively. While, lower efficiency was detected with IMZ, KHCO3 and NaHCO3 with rot incidence reduction of 89.1, 84.7, and 87.2%, respectively.
24
Fig 1. Incidence of decays after 7 days of cold storage and 7 days of shelf life of untreated and
treated Valencia orange (A) and clementine fruit (B), before harvest. Treatments include PGE (12, 6, and 3 g/l), 0.1% Imazalil, 2% NaHCO3 and KHCO3. Untreated fruit and fruit sprayed with tap water were used as controls. Imazalil was applied after harvest by dipping. Bars indicate standard errors of the means. For each time, different letters indicate significant differences (P < 0.05) among treatments.
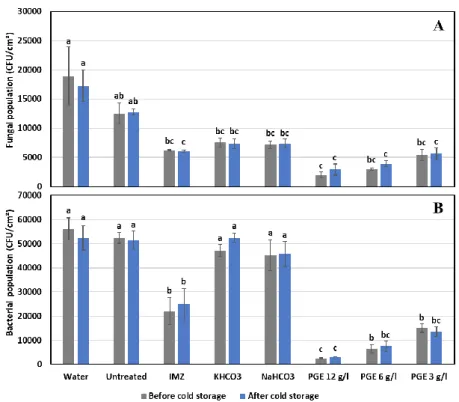
The microbiological analysis of the epiphytic fungal and bacterial population on Valencia orange (Fig 2) and clementine (fig 3) fruit revealed high microbial reductions, before and after cold storage, on fruit treated with PGE comparing to the controls (untreated and water dipped fruit). Overall, the antimicrobial activity of PGE was higher on bacteria than fungi, regardless of the fruit species or time of sampling. While, no significant differences were detected between orange control fruits, PGE treatments and in particular PGE 12g/l was the most effective treatment with a bacterial and fungal reductions varying between 94.3-97.7% and 83.1-89.5%, respectively. Significant but lower microbial reductions were also achieved by PGE at 6 and 3 g/l and, to a lesser extent, with IMZ. Salts, NaHCO3 and KHCO3 induced significant but much lower reductions only on the bacterial populations of clementine fruit.
25
Fig 2. Epiphytic population of Fungi (A) and bacteria (B) on Valencia oranges before and after
cold storage. Different letters indicate significantly different values according to Tukey's test (P ≤ 0.05).
Fig 3. Epiphytic population of fungi (A) and bacteria (B) on clementine before and after cold
storage. Different letters indicate significantly different values according to Tukey's test (P ≤ 0.05).
26 3.3. Postharvest treatments
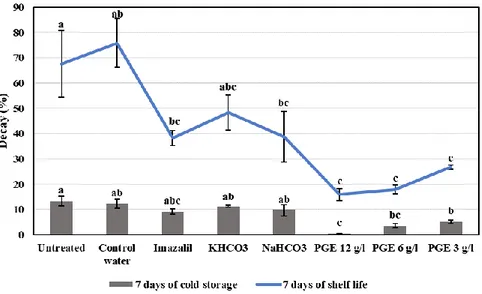
After 7 days of cold storage, untreated orange and oranges dipped in tap water (controls) showed low rot incidence of 12,29 and 13.3%, respectively (Fig. 4). This incidence was further reduced with PGE treatments to reach a reduction of 97.5, 71.3 and 58.2% when treated with PGE concentrations of 12, 6 and 3 g/l, respectively. However, no significant difference was detected between control fruit and IMZ treatment. After 7 days of shelf life, the disease incidence highly increased to reach 75.8% with fruit dipped in tap water. This was significantly reduced with fruit treated with PGE 12, 6 and 3 g/l, where the reductions reached 78.9, 76.0, and 64.6%, respectively. Lower but still significant reductions of rots were obtained with IMZ (49.6%) and NaHCO3 (48.8%).
Fig 4. Incidence of decays (%) after 7 days of cold storage and 7 days of shelf life of untreated
and treated Valencia oranges after harvest. Treatments include PGE (12, 6, and 3 g/l), 0.1% Imazalil, 2% NaHCO3 and KHCO3. Untreated oranges and oranges dipped in tap water were used as controls. Bars indicate standard errors of the means. For each time, different letters indicate significant differences (P < 0.05) among treatments.
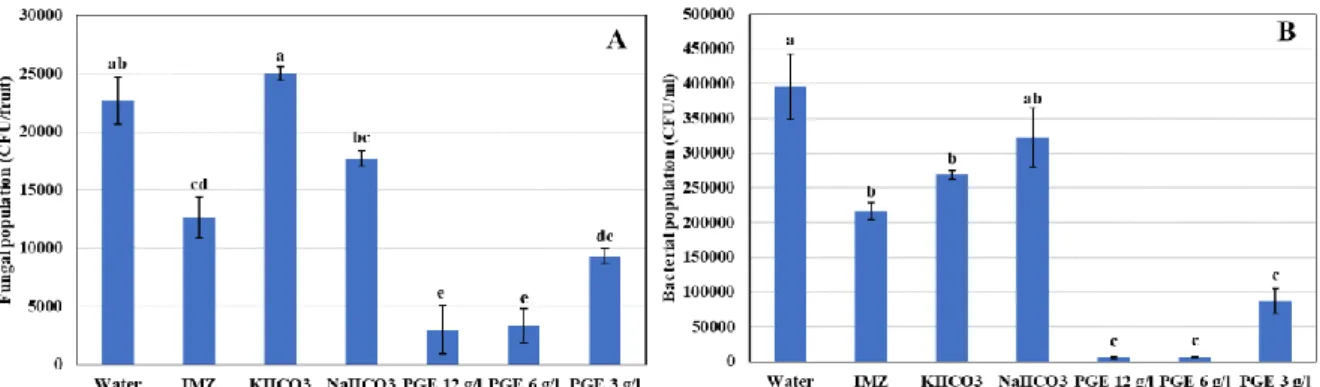
A microbiological analysis of the aqueous dipping treatment solutions revealed a strong antimicrobial activity of the extract against bacteria and fungi (Fig. 5). While, the bacterial population in tap water dipping solution was 4x109
CFU/ml, PGE significantly reduced it by 98.5 (PGE 12g/l), 98.4 (PGE 6g/l), and 78.0% (PGE 3g/l). A much lower but significant reduction was revealed in the dipping solutions containing KHCO3 and IMZ. The total fungal counts in tap water dipping solution reached 2.3x107 CFU/ml. This contamination was reduced by 44.1% in water containing IMZ and by 86.7, 85.3, and 58.8% in dipping water containing PGE at 12, 6 and 3 g/l, respectively. Overall, PGE showed high significant bactericidal and fungicidal activity comparing to all tested treatments (IMZ and salts), except with the lowest concentration of PGE (3g/l) where no significant fungicidal effect was observed comparing to IMZ.
27
Fig 5. Fungal (A) and bacterial (B) populations of postharvest dipping solutions on Valencia
oranges. bars are standard errors of the mean. For each group of microorganisms, different letters indicate significant differences (P < 0.05) among treatments.
4. Discussion
In the present study, different control substances were applied under large-scale commercial conditions as pre- and post-harvest treatments to control major citrus pathogens. Regardless of the concentration, PGE showed the highest efficiency, comparing to all tested substances, in controlling orange and clementine rots both before and after harvest. In particular, PGE showed higher efficiency comparing to IMZ which is a chemical treatment currently considered as the most effective fungicide to control postharvest citrus rots despite the identification of several resistant strains (Kinay et al., 2007; Pérez et al., 2011; Erasmus et al., 2015). This together with the consistency of PGE effect in controlling the epithetic microbial population on clementine and orange fruit, and in both pre and postharvest, makes the extract very potent reliable antibacterial substance. This is an important feature comparing to other alternative strategies particularly biocontrol agents which are considered as one of the most viable alternatives. However, their inconsistence efficiency mainly due to their sensibility to the external environmental conditions and host species, makes their application very limited (Sui et al., 2015; Ippolito et al., 2005). Furthermore, results of this study showed low but significant effect of salts in controlling citrus green and blue molds. This confirms previous studies reporting that the antimicrobial activity of salts in controlling citrus diseases is limited due to several restrictions such as limited persistence, inconsistent activity, risk of fruit injury, lack of preventive effect, etc (Youssef et al., 2012; Smilanick, 2010; Palou
et al., 2002). Therefore, the application of salts could be useful as an integrated
approach or in combination with other control means in integrated pest management systems, while PGE could be used as an alternative to chemicals. This together with the continuous growing public concerns about health and environmental risks concerning chemical residues, PGE could be considered an important control strategy particularly for cash crops such as citrus.
28
Noteworthy, the high level of protection achieved by the extract in both pre and post-harvest treatments makes the integration of PGE in control strategies more practical, flexible and particularly effective, regardless of the timing of disease appearance. In this case, the extract might be important as field treatment for citrus productions that don’t undergo, during packaging and commercialization, specific postharvest treatments e.g. washing and waxing. This is often the case in Italy where early varieties of clementine are quickly introduced into the distribution chain without any treatments or mechanical operations leaving the fruit with few leaves as an indication of product freshness. Similar case is found in Europe but with different fruit species that cannot be subjected to dip treatments such as strawberries and table grapes (Nigro et al., 2006). Furthermore, the field application of PGE could be considered easy, particularly for less technologically advanced countries where simple equipment including conventional fungicides sprayers could also be used for PGE field treatments. Moreover, postharvest dipping treatments can be easily and cheaply integrated into citrus packinghouse operations by adding the active ingredient to the washing water usually used to wash the fruit and/or reduce temperature (hydro-cooling).
PGE preharvest treatments showed strong antimicrobial activity, before and after cold storage, against epiphytic fungal and bacterial populations associated with oranges and clementine. This broad range of antimicrobial activity of the extract could negatively affect the microbiome of the carposphere which plays an important role in protecting the fruit (Chalutz and Wilson, 1990). This together with the persistent activity of PGE after 7 days of cold storage and 7 days of shelf life suggest the possible reduction of the fruit natural antagonists which could represent a relevant issue from a practical point of view. However, the capability of PGE in inducing resistance in fruit tissues could explain the antimicrobial persistence and efficiency of the extract overtime (Belgacem et al., 2019). This feature could be further exploit to protect washed citrus fruit where washing water may flash the fruit microorganisms and, therefore, affect the consistency of the carposphere microbiota. Furthermore, the application of PGE, as a postharvest dipping treatment, might be also strategic against water-borne microorganisms. The extract could be useful to reduce the microbial contamination of the recirculated water and substitute the use of chlorine and other sanitizers commonly used in citrus packinghouses.
In conclusion, results of this study demonstrated the potential use of PGE as an effective alternative control treatment to control pre and postharvest citrus rots. It is worth mentioning that the trials of this study were carried out under large scale commercial conditions where most of other alternative control means usually proved an antimicrobial activity under laboratory conditions but not under commercial conditions. Furthermore, the substitution of IMZ chemical treatments
29
with PGE could result in significant reduction in losses, enhancement of the fruit quality, and reduced environmental impact from the lack of chemical residues (Romeo et al., 2015). Therefore, the potential use of PGE is not restricted to organic production but also it could be incorporated in conventional and/or integrated farming systems. This together with the wide availability of PGE, as a by-product of processing industries, and the absence of phytotoxicity effect, might speed up the costly process for PGE registration as a natural fungicide (Li Destri Nicosia et al., 2016; Pangallo et al., 2017b).
References
Altieri, G., Di Renzo, G. C., Genovese, F., Calandra, M. and Strano, M. C. (2013) A new method for the postharvest application of imazalil fungicide to citrus fruit. Biosystems engineering 115 (4), 434-443. Belgacem, I., Pangallo, S., Abdelfattah, A., Romeo, F. V., Cacciola, S. O., Li
Destri Nicosia, M. G., Ballistreri, G. and Schena, L. (2019)
Transcriptomic Analysis of Orange Fruit Treated with Pomegranate Peel Extract (PGE). Plants 8 (4), 101.
Chalutz, E. and Wilson, C. (1990) Postharvest biocontrol of green and blue mold and sour rot of citrus fruit by Debaryomyces hansenii. Plant
Disease 74 (2), 134-137.
Erasmus, A., Lennox, C. L., Korsten, L., Lesar, K. and Fourie, P. H. (2015) Imazalil resistance in Penicillium digitatum and P. italicum causing citrus postharvest green and blue mould: Impact and options. Postharvest
Biology and Technology 107, 66-76.
Eurostat. (2017). available at: https://ec.europa.eu/eurostat/web/main/home Food and Agricultural Organization of the United Nations. (2017). Available at:
http://www.fao.org/economic/est/est-commodities/citrus-fruit/en/ Ippolito, A., Schena, L., Pentimone, I. and Nigro, F. (2005) Control of
postharvest rots of sweet cherries by pre-and postharvest applications of Aureobasidium pullulans in combination with calcium chloride or sodium bicarbonate. Postharvest Biology and Technology 36 (3), 245-252.
Janisiewicz, W. J. and Korsten, L. (2002) Biological control of postharvest diseases of fruits. Annual review of phytopathology 40 (1), 411-441. Kinay, P., Mansour, M. F., Gabler, F. M., Margosan, D. A. and Smilanick, J. L.
(2007) Characterization of fungicide-resistant isolates of Penicillium digitatum collected in California. Crop Protection 26 (4), 647-656. Li Destri Nicosia, M. G., Pangallo, S., Raphael, G., Romeo, F. V., Strano, M.
C., Rapisarda, P., Droby, S. and Schena, L. (2016) Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biology and Technology 114, 54-61.
30
Nigro, F., Schena, L., Ligorio, A., Pentimone, I., Ippolito, A. and Salerno, M. G. (2006) Control of table grape storage rots by pre-harvest applications of salts. Postharvest Biology and Technology 42 (2), 142-149.
Palou, L. (2014) Penicillium digitatum, Penicillium italicum (green mold, blue mold). Postharvest Decay. Elsevier. 45-102.
Palou, L., Smilanick, J. L. and Droby, S. (2008) Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds.
Stewart Postharvest Review 2 (2), 1-16.
Palou, L., Usall, J., Smilanick, J. L., Aguilar, M. J. and Vinas, I. (2002)
Evaluation of food additives and low‐toxicity compounds as alternative chemicals for the control of Penicillium digitatum and Penicillium italicum on citrus fruit. Pest management science 58 (5), 459-466. Pangallo, S., Li Destri Nicosia, M., Raphael, G., Levin, E., Ballistreri, G.,
Cacciola, S., Rapisarda, P., Droby, S. and Schena, L. (2017a) Elicitation of resistance responses in grapefruit and lemon fruits treated with a pomegranate peel extract. Plant pathology 66 (4), 633-640.
Pangallo, S., Li Destri Nicosia, M. G., Agosteo, G. E., Abdelfattah, A., Romeo, F. V., Cacciola, S. O., Rapisarda, P. and Schena, L. (2017b) Evaluation of a Pomegranate Peel Extract (PGE) as Alternative Mean to Control Olive Anthracnose. Phytopathology (ja).
Pangallo, S., Li Destri Nicosia, M. G., Agosteo, G. E., Abdelfattah, A., Romeo, F. V., Cacciola, S. O., Rapisarda, P. and Schena, L. (2017c) Evaluation of a pomegranate peel extract as an alternative means to control olive
anthracnose. Phytopathology 107 (12), 1462-1467.
Pérez, E., Blanco, O., Berreta, C., Dol, I. and Lado, J. (2011) Imazalil concentration for in vitro monitoring of imazalil resistant isolates of Penicillium digitatum in citrus packinghouses. Postharvest Biology and
Technology 60 (3), 258-262.
Porat, R., Daus, A., Weiss, B., Cohen, L., Fallik, E. and Droby, S. (2000)
Reduction of postharvest decay in organic citrus fruit by a short hot water brushing treatment. Postharvest Biology and Technology 18 (2), 151-157. Romeo, F. V., Ballistreri, G., Fabroni, S., Pangallo, S., Li Destri Nicosia, M. G.,
Schena, L. and Rapisarda, P. (2015) Chemical characterization of different sumac and pomegranate extracts effective against Botrytis cinerea rots. Molecules 20 (7), 11941-11958.
Smilanick, J. (2010) Integrated approaches to postharvest disease management in California citrus packinghouses. International Symposium on
Biological Control of Postharvest Diseases: Challenges and Opportunities 905.
Sui, Y., Wisniewski, M., Droby, S. and Liu, J. (2015) Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 81 (9), 2968-2975.
31
Talibi, I., Boubaker, H., Boudyach, E. and Ait Ben Aoumar, A. (2014)
Alternative methods for the control of postharvest citrus diseases. Journal
of applied microbiology 117 (1), 1-17.
Wisniewski, M., Droby, S., Norelli, J., Liu, J. and Schena, L. (2016) Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biology and Technology 122, 3-10.
Youssef, K., Ligorio, A., Sanzani, S. M., Nigro, F. and Ippolito, A. (2012) Control of storage diseases of citrus by pre-and postharvest application of salts. Postharvest Biology and Technology 72, 57-63.